Abstract
Animal studies suggest that the effects of fatty acids on gastric emptying and pancreatic secretion are both concentration and load dependent, while their suppressive effect on energy intake is only load dependent. We postulated that, in humans, the modulation of antropyloroduodenal pressure waves, plasma cholecystokinin (CCK) and peptide YY (PYY) concentrations and energy intake by intraduodenal lauric acid, a fatty acid with 12 carbon atoms (‘C12’) would be load, but not concentration, dependent. Two groups of 12 healthy males were each studied on three separate occasions in double-blind randomized fashion. Antropyloroduodenal pressure waves, plasma CCK and PYY, and appetite perceptions were measured during intraduodenal infusions of C12 at (1) different loads of (i) 0.2, (ii) 0.3 and (iii) 0.4 kcal min−1 (all 56 mm) for 90 min, and (2) different concentrations of (i) 40, (ii) 56 and (iii) 72 mm (all 0.4 kcal min−1) for 60 min. Energy intake at a buffet meal consumed immediately following each infusion was quantified. Suppression of antral and duodenal pressure waves, stimulation of pyloric pressure waves, stimulation of plasma CCK and PYY, and suppression of energy intake, were related to the load of C12 administered (r > 0.65, P < 0.05). In contrast, there were no concentration-dependent effects of C12 on any of these parameters. In conclusion, in humans, the effects of intraduodenal C12 on antropyloroduodenal motility, plasma CCK and PYY and energy intake appear to be related to load, but not concentration, at least at the loads and concentrations evaluated.
It is well established that free fatty acids are responsible for mediating the effects of fat on gastrointestinal (GI) motility, GI hormone secretion, including cholecystokinin (CCK) from the proximal, and peptide-YY (PYY) from the distal, small intestine (Lieverse et al. 1994; Lin & Chey, 2003), appetite and energy intake in humans (Hunt & Knox, 1968; McLaughlin et al. 1999; Matzinger et al. 2000; Feinle et al. 2003; Feltrin et al. 2004; Little et al. 2005). We have demonstrated (Little et al. 2005) that infusion of lauric acid, a fatty acid with 12 carbon atoms (‘C12’), into the human duodenum inhibits antral and duodenal pressure waves, stimulates pyloric motility, releases CCK and suppresses energy intake in a dose-related fashion. In this study C12 was infused at loads of 0.1, 0.2 and 0.4 kcal min−1, as 14, 28 and 56 mm solutions, respectively, and, while a concentration as low as 14 mm (at 0.1 kcal min−1) was shown to stimulate pyloric pressure waves and the release of CCK, as the load and concentration were varied in parallel, it was not possible to determine whether the load, or concentration, of C12 (or both) was responsible for the observed effects. In this context animal studies have demonstrated that increasing the load of C12 (or other nutrients) is associated with an increased length of the small intestine in contact with C12 (Meyer et al. 1998b), because, as the absorptive capacity is reached for a certain segment of the small intestine, C12 travels further along the small intestine until absorbed. While such studies have not been performed in humans, it is very likely that this is also the case in humans. In contrast, increasing the concentration of C12 is unlikely to modify the length of small intestinal contact, as the amount of C12 is constant.
In an earlier human study (Feltrin et al. 2004), C12 infused intraduodenally as a 106 mm solution at 0.4 kcal min−1 inhibited energy intake about twice as much, and released CCK some three times as much, as a 0.4 kcal min−1 load of C12 at 56 mm in the later study (Little et al. 2005). Because (a) 106 mm is more than twice the concentration of luminal fatty acids observed after a fatty meal (Borgstrom et al. 1957; Porter & Saunders, 1971; Porter et al. 1971; Ament et al. 1972), (b) cytotoxicity of fatty acids on gut mucosa is known to be concentration dependent (Velasquez et al. 1993), and (c) the 106 mm, but not the 56 mm, C12 solution induced nausea, it is unclear whether the responses observed were toxic, or physiological and, thus, whether the differential effects of a 0.4 kcal min−1 infusion at 106 and 56 mm reflected a physiological importance of concentration, independent of load, or not.
It is well established that nutrient-stimulated signals from the small intestine of experimental animals regulate pancreatic secretion (Meyer et al. 1970a,b, 1976; Meyer & Jones, 1974), gastric emptying (Lin et al. 1989, 1990) and energy intake (Meyer et al. 1998a) in a load-dependent fashion, independent of nutrient concentrations, over the ranges tested. Humans also sense caloric loads to regulate gastric emptying (Brener et al. 1983; Hunt et al. 1985) or energy intake (Rolls et al. 1991), and while it is clear that humans modulate gastric emptying in response to glucose loads, but not concentrations (Brener et al. 1983; Hunt et al. 1985), whether loads, or concentrations, of nutrients regulate human energy intake is controversial (Porikos et al. 1982; Rolls et al. 1998). The ability of dietary fats in the GI lumen to inhibit subsequent caloric intake with some, albeit varying, degrees of accuracy (Rolls et al. 1991; Shide et al. 1995) suggests that fat, specifically lipolytic products, must be sensed in a load-dependent, concentration-independent fashion. Determination of whether load and/or concentration of fatty acids modulates GI function, appetite and energy intake in humans, is important to an understanding of the mechanisms of action of fatty acids in the gut, which may be of relevance to the pathogenesis of obesity.
We have now examined in healthy humans the responses to intraduodenal loads of C12 of 0.2, 0.3 and 0.4 kcal min−1 at a fixed concentration (56 mm), and contrasted them with concentrations of 40, 56 and 72 mm at a fixed load (0.4 kcal min−1) to evaluate the effects of load, and concentration, of C12 on antropyloroduodenal motility, plasma CCK and PYY concentrations, appetite and energy intake. We hypothesized that if fatty acid concentration (above 40 mm) was the dominant stimulus, we should see no dose–response in the ‘load’ study, but a definite dose–response in the ‘concentration’ study, whereas, if load dominated we would observe no dose–response to concentration, but a definite load-dependent effect.
Methods
Subjects
A total of 21 healthy males were studied, 12 subjects in each of the two protocols described below, with three subjects participating in both protocols. The number of subjects was based on power calculations derived from our previous study (Little et al. 2005). We calculated that with 12 subjects we would be able to detect a 15% decrease in energy intake at α= 0.05, with a power of 80%. The subjects had a mean age of 23 ± 1 (range 18–36) years and were of normal body weight for their height (body mass index 22.3 ± 0.3 kg m−2). All subjects were unrestrained eaters (scoring <12 on the eating restraint part (Factor 1) of the Three Factor Eating questionnaire; Stunkard & Messick, 1985), had no GI disease or symptoms, and were not taking medication known to affect GI motility or appetite. No subject smoked or habitually consumed >20 g alcohol per day. The Royal Adelaide Hospital Research Ethics Committee approved the study protocol, and each subject provided written, informed consent prior to their inclusion. The experiments were carried out in accordance with the Declaration of Helsinki.
Study design
Each subject was studied on three occasions, separated by 3–10 days, to evaluate, in double-blind randomized fashion, the effects of intraduodenal infusion of C12 at varying loads or concentrations on antropyloroduodenal motility, plasma CCK and PYY, appetite and energy intake. For this purpose, solutions were designed to deliver (i) different loads (0.2–0.4 kcal min−1) of C12 at a constant concentration (56 mm) (‘C12 load’), and (ii) different concentrations (40–72 mm) of C12 at a constant load (0.4 kcal min−1) (‘C12 concentration’) to the small intestine. We expressed loads in kilocalories per minute, since it is well known that gastric emptying of nutrients is regulated on a kilocalorie per minute basis (Brener et al. 1983; Horowitz et al. 1996). It was a natural progression to initiate the C12 load protocol first, as this followed on directly from a previous study from our group (Little et al. 2005), and the C12 concentration protocol was initiated subsequently.
Preparation of C12 solutions
All solutions were prepared on the morning of the study and kept at 37°C to maintain the C12 in solution. The loads of the solutions were selected on the basis of previous studies in humans, indicating that fatty acids empty from the stomach into the small intestine at ∼0.2–0.4 kcal min−1 (Hunt & Knox, 1968) and that intraduodenal loads of C12 at 0.1–0.4 kcal min−1 for 90 min are well tolerated and do not induce nausea (Little et al. 2005). While the concentrations of the solutions were selected to be within the range of fatty acid concentrations that occur in the small intestine after triglyceride digestion (Borgstrom et al. 1957), we acknowledge that the range of fatty acid concentrations (40–72 mm) is quite narrow; however, as explained below, these concentrations could be tolerated by the subjects in the absence of adverse effects. The pH of all solutions was 8.4.
Experimental protocol 1: different loads of C12 at 56 mM (‘C12 load’)
C12 was delivered to the small intestine at loads of (i) 0.2 kcal min−1 (C12(0.2); total energy 18 kcal (75 kJ)), (ii) 0.3 kcal min−1 (C12(0.3); total energy 27 kcal (112.5 kJ)), and (iii) 0.4 kcal min−1 (C12(0.4); total energy 36 kcal (150 kJ)). The solution was prepared using 4.52 g of C12 (Sigma-Aldrich, Milwaukee, WI, USA) dissolved with 0.75 g of NaOH (Sigma-Aldrich, St Louis, MO, USA) in 0.9% saline, to a total volume of 400 ml, with a concentration of 56 mm. To achieve the different loads, the solution was infused at rates of (i) 2 ml min−1, (ii) 3 ml min−1, and (iii) 4 ml min−1, for 90 min; thus, the total volumes infused were 180, 270 and 360 ml, respectively.
Experimental protocol 2: different concentrations of C12 at 0.4 kcal min−1 (‘C12 concentration’)
C12 was delivered to the small intestine at concentrations of (i) 40 mm (C12(40)), (ii) 56 mm (C12(56)), and (iii) 72 mm (C12(72)). For this purpose 4.8, 4.52 and 3.6 g of C12 were dissolved with 0.67, 0.75 and 0.45 g of NaOH, respectively, in 0.9% saline, to total volumes of 600, 400 and 250 ml, respectively. Solutions were infused at rates of (i) 5.7 ml min−1 (40 mm), (ii) 4.0 ml min−1 (56 mm), and (iii) 3.1 ml min−1 (72 mm), for 60 min; thus, the total volumes infused were 342, 240 and 186 ml, respectively. Each infusion delivered a load of 0.4 kcal min−1; thus, the total amount of C12 infused was 24 kcal (100 kJ) with all three solutions.
We initially planned to evaluate a wider range of concentrations; however, in pilot studies it became apparent that concentrations less than 40 mm (e.g. 24 mm) induced adverse effects, including severe abdominal cramps and diarrhoea, most likely due to the large volume (9.4 ml min−1) required to deliver a load of 0.4 kcal min−1. We had also planned to infuse the C12 solutions in the C12 concentration protocol for 90 min, as was the case with the C12 load protocol; however, pilot studies demonstrated that the 40 mm C12 solution (5.7 ml min−1), when infused for 90 min, resulted in abdominal cramps or diarrhoea in some subjects (probably also because of the volume infused), but could be tolerated without adverse effects for 60 min; hence, the difference in the duration of the infusions between the two study protocols. None of the subjects who participated in the pilot studies was included in the final protocol.
Protocol
Each subject attended the laboratory at 08.30 h after fasting from 22.00 h the previous night from both solids and liquids. They were intubated with a 17-channel manometric catheter (Dentsleeve International Ltd, Ontario, Canada) that was inserted through an anaesthetized nostril and allowed to pass through the stomach and into the duodenum by peristalsis (Heddle et al. 1989). The catheter incorporated 16 side-holes, spaced at 1.5 cm intervals, to measure pressures in the antrum, pylorus and duodenum. Six side-holes (channels 1–6) were positioned in the antrum, a 4.5 cm sleeve sensor (channel 7), with two channels present on the back of the sleeve (channels 8 and 9), to measure pressure waves occurring over the entire pyloric region, was positioned across the pylorus, and seven side-holes (channels 10–16) were positioned in the duodenum. An additional channel, used for intraduodenal infusion, was positioned 11.75 cm distal to the end of the sleeve sensor. The correct positioning of the catheter, so the sleeve sensor straddled the pylorus, was maintained by continuous measurement of the transmucosal potential difference between the most distal antral (channel 6) (∼−40 mV) and the most proximal duodenal (channel 10) (∼0 mV), channel (Heddle et al. 1989). For this purpose, an intravenous cannula filled with sterile saline was placed subcutaneously in the left forearm and used as a reference electrode (Heddle et al. 1989). All manometric channels were perfused with degassed distilled water, except for the two transmucosal potential difference channels, which were perfused with degassed 0.9% saline at 0.15 ml min−1 (Heddle et al. 1989). An intravenous cannula was placed into a right forearm vein to obtain blood samples for subsequent measurement of plasma CCK and PYY concentrations.
Once the catheter was positioned correctly, fasting motility was monitored until the occurrence of a phase III of the interdigestive migrating motor complex (Cook et al. 1997). Immediately after the cessation of phase III activity (at t =−15 min), a baseline venous blood sample was taken, and a visual analogue scale questionnaire (see Measurements) (Parker et al. 2004), assessing appetite-related sensations, nausea and bloating, was administered. At t = 0 min (i.e. during phase I of the migrating motor complex), duodenal infusion of C12 was commenced. Antropyloroduodenal pressures were monitored throughout the infusion period, and blood samples were taken, and visual analogue scale questionnaires administered, every 15 min from t = 0–90 min for C12 load, or from t = 0–60 min for C12 concentration. At t = 90 min (C12 load), or t = 60 min (C12 concentration), the infusion was terminated, and the subject was immediately extubated and provided with a cold buffet-style meal. The amount of food offered was in excess of what the subject was expected to consume. The subject was given 30 min (i.e. t = 90–120 min for C12 load, or t = 60–90 min for C12 concentration) to consume the meal and instructed to eat until comfortably full. The types of food, as well as the macronutrient composition and energy content of the meal, have been described in detail previously (Feltrin et al. 2004). After ingestion of the meal the intravenous cannula was removed and the subject was allowed to leave the laboratory.
Measurements
Appetite perceptions and energy intake
Perceptions of appetite, including hunger and fullness, were assessed using validated visual analogue scale questionnaires (Parker et al. 2004). Nausea and bloating were also quantified. Each visual analogue scale questionnaire evaluated a sensation on a 100 mm horizontal line, where 0 represented ‘sensation is not felt at all’ and 100 represented ‘sensation is felt the greatest’. Subjects were asked to place a vertical stroke on the 100 mm line in relation to what they were feeling at that particular point in time. Energy intake from the buffet meal (energy consumption (kJ) and macronutrient distribution (% energy)) was analysed using commercially available software (Food works 3.0; Xyris Software, Highgate Hill, Queensland, Australia) (MacIntosh et al. 1999).
Antropyloroduodenal pressures
Manometric pressures were digitized and recorded on a computer-based system (PowerMac 7100/75; Apple Computer, Cupertino, CA, USA) running commercially available software (HAD; Associate Professor G. S. Hebbard, Melbourne, Australia), written in Labview 3.1.1 (National Instruments), and stored for subsequent analysis. Antropyloroduodenal pressures were analysed for the number and amplitude of isolated pyloric pressure waves and pressure waves in the antrum and duodenum using custom-written software (Gastrointestinal Motility Unit, Utrecht, The Netherlands; Samsom et al. 1998), modified to our requirements. Basal pyloric pressure (‘tone’) was also calculated for each minute by subtracting the mean basal pressure (excluding phasic pressures) recorded at the most distal antral side hole from the mean basal pressure recorded at the sleeve (Heddle et al. 1988b), using custom-written software (MAD; Professor Charles Malbert, INRA, Rennes, France). Phasic pressure waves in the antrum and isolated pyloric pressure waves were defined by an amplitude ≥10 mmHg, with a minimum interval of 15 s between peaks. Phasic duodenal pressure waves were defined by an amplitude ≥10 mmHg, with a minimum interval of 3 s between peaks (Heddle et al. 1988a).
Plasma CCK and PYY concentrations
Venous blood samples (10 ml) were collected in ice-chilled EDTA-treated tubes containing 400 KIU aprotinin (Trasylol; Bayer Australia Ltd, Pymble, Australia) per millilitre of blood. Plasma was separated by centrifugation (1491 g, 15 min, 4°C) within 30 min of collection and stored at −70°C until assayed.
Plasma CCK concentrations (picomoles per litre) were determined after ethanol extraction using a previously described radioimmunoassay (MacIntosh et al. 2001). A commercially available antibody (C258; Sigma) raised in rabbits against the synthetic sulphated CCK-8 was employed. This antibody binds to all CCK peptides containing the sulphated tyrosine residue in position 7, shows a 26% cross-reactivity with unsulphated CCK-8, less than 2% cross-reactivity with human gastrin, and does not bind to structurally unrelated peptides. The intra-assay coefficient of variation (CV) was 9%, and the interassay CV was 27%, with a detection limit of 2.5 pmol l−1.
Plasma PYY concentrations (picomoles per litre) were measured by radioimmunoassay using an antiserum (kindly donated by Dr B. Otto, Medizinische Klinik, Klinikum Innenstadt, University of Munich, Munich, Germany) raised in rabbits against human PYY-(1–36) (Sigma), as described (Pilichiewicz et al. 2005). The intra-assay CV was 12.3%, and the interassay CV was 16.6%, with a detection limit of 4 pmol l−1.
Data and statistical analysis
Baseline (‘0’) values were calculated as the means of values obtained at t =−15 and 0 min for visual analogue scale questionnaire scores and hormones, and between t =−15 to 0 min for basal pyloric pressures, number and amplitude of isolated pyloric pressure waves, and antral and duodenal pressure waves. Basal pyloric pressures and the number and amplitude of isolated pyloric pressure waves were expressed as means over 15 min segments during the infusion period. Numbers and amplitudes of antral and duodenal pressure waves were expressed as total number and mean values, respectively, during the infusion period. To enable a direct comparison between the C12 load and C12 concentration protocols, the area under the curve for basal pyloric pressure and the total number of isolated pyloric pressure waves and antral and duodenal pressure waves, were calculated between t = 0 and 60 min for C12(0.4) and compared with C12(56), as both infusions delivered the same load (0.4 kcal min−1) and concentration (56 mm).
Visual analogue scale questionnaire scores, basal pyloric pressures, number and amplitude of isolated pyloric pressure waves and plasma hormone concentrations were analysed by repeated-measures analysis of variance (ANOVA), with time and treatment as within-subject factors. One-way ANOVA was used to assess the effect of treatment on energy intake, macronutrient distribution, total numbers and mean amplitudes of antral and duodenal pressure waves. One-way ANOVA was also used to compare areas under the curves for basal pyloric pressures, the total number of isolated pyloric pressure waves and antral and duodenal pressure waves (between t = 0 and 60 min), plasma CCK and PYY (at t = 60 min) and energy intake, between C12(0.4) and C12(56). Post hoc comparisons, adjusted for multiple comparisons by Bonferroni's correction, were performed if ANOVAs revealed significances. To evaluate load- or concentration-dependent responses, correlations were determined between numbers and amplitudes of antral and duodenal pressure waves, basal pyloric pressure, number and amplitude of isolated pyloric pressure waves, plasma CCK and PYY concentrations and energy intake, with the natural logarithm (ln) of each load and concentration. The slopes of the linear regressions were then tested to see if they were greater, or less, than zero (Elashoff, 1981). Statistical significance was accepted at P < 0.05, and data are presented as means ±s.e.m.
Results
All subjects completed the three randomized study days, and both experimental conditions were well tolerated.
Effects of load of C12
Antropyloroduodenal pressures
Antral pressures. There was a significant effect of treatment on the number, but not the amplitude, of antral pressure waves (P < 0.01) (Table 1). Both C12(0.3) and C12(0.4) reduced the number of antral pressure waves compared with C12(0.2) (P < 0.01), with no difference between C12(0.3) and C12(0.4). There was an inverse relationship between the number (r =−0.76, P < 0.05), but not amplitude, of antral pressure waves with the load of C12 administered.
Table 1.
Total number and mean amplitude of antral and duodenal pressure waves during intraduodenal infusion of C12 at different loads and concentrations
| C12 loada | C12 concentrationb | |||||
|---|---|---|---|---|---|---|
| C12(0.2) | C12(0.3) | C12(0.4) | C12(40) | C12(56) | C12(72) | |
| Antral pressure waves | ||||||
| Number | 61 ± 20 | 11 ± 4* | 11 ± 6* | 1 ± 4 | 5 ± 3 | 1 ± 5 |
| Amplitude (mmHg) | 18 ± 4 | 12 ± 2 | 10 ± 4 | 14 ± 4 | 15 ± 3 | 15 ± 3 |
| Duodenal pressure waves | ||||||
| Number | 734 ± 71 | 531 ± 78* | 362 ± 48† | 318 ± 44 | 302 ± 65 | 311 ± 67 |
| Amplitude (mmHg) | 25 ± 1 | 26 ± 1 | 23 ± 2 | 25 ± 1 | 24 ± 1 | 25 ± 2 |
Data are means ±s.e.m. (n = 12).
C12 infused at 0.2 (C12(0.2)), 0.3 (C12(0.3)) and 0.4 (C12(0.4)) kcal min−1 (all at 56 mm) for 90 min.
C12 infused at 40 (C12(40)), 56 (C12(56)), 72 (C12(72)) mm (all at 0.4 kcal min−1) for 60 min.
P < 0.05, versus C12(0.2)
P < 0.05, versus C12(0.2)/C12(0.3).
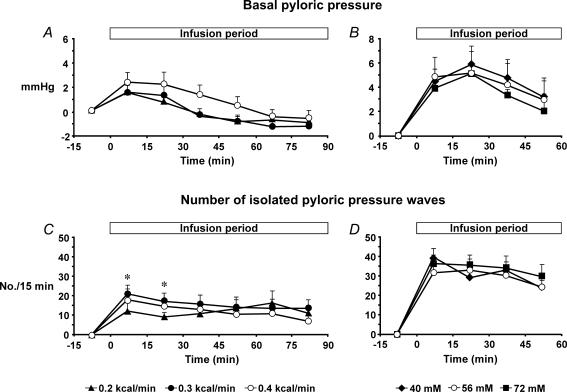
Pyloric pressures. (i) Basal pyloric pressure. There was no effect of treatment on basal pyloric pressure (Fig. 1A). However, there was an effect of time (P < 0.001). C12(0.2) increased basal pyloric pressure in the first 15 min compared with baseline (P < 0.05), and both C12(0.3) and C12(0.4) increased basal pyloric pressure over the first 30 min of the infusion compared with baseline (P < 0.05). There was no relationship between basal pyloric pressure and the load of C12 administered. (ii) Phasic pressures. All treatments increased both the number and amplitude of isolated pyloric pressure waves throughout the 90 min infusion period compared with baseline (P < 0.05, for all). There was a significant treatment–time interaction for the number of isolated pyloric pressure waves (P < 0.01) (Fig. 1C). Both C12(0.3) and C12(0.4) increased the number of isolated pyloric pressure waves compared with C12(0.2) between t = 0 and 30 min (P < 0.01), with no difference between C12(0.3) and C12(0.4). There was no effect of treatment on the amplitude of isolated pyloric pressure waves (data not shown); however, there was a significant effect of time (P < 0.001). There was a direct relationship between the number (r = 0.70, P < 0.05) and amplitude (r = 0.66, P < 0.05) of isolated pyloric pressure waves with the load of C12 administered.
Figure 1. Basal pyloric pressure and isolated pyloric pressure waves.
Basal pyloric pressure during intraduodenal infusion of C12 at different loads (0.2, 0.3 and 0.4 kcal min−1, all at 56 mm) for 90 min (A), and different concentrations (40, 56 and 72 mm, all at 0.4 kcal min−1) for 60 min (B). Number of isolated pyloric pressure waves during intraduodenal infusion of C12 at different loads (0.2, 0.3 and 0.4 kcal min−1, all at 56 mm) for 90 min (C), and different concentrations (40, 56 and 72 mm, all at 0.4 kcal min−1) for 60 min (D). *C12(0.4) and C12(0.3) versus C12(0.2), P < 0.001. Data are means ±s.e.m. (n = 12).
Duodenal pressures. There was a significant effect of treatment on the number, but not the amplitude, of duodenal pressure waves (P < 0.001) (Table 1). C12(0.3) decreased the number of duodenal pressure waves compared with C12(0.2) (P < 0.01), and C12(0.4) decreased the number compared with C12(0.2) (P < 0.01) and C12(0.3) (P < 0.05). There was an inverse relationship between the number (r =−0.70, P < 0.001), but not the amplitude, of duodenal pressure waves with the load of C12 administered.
Plasma hormone concentrations
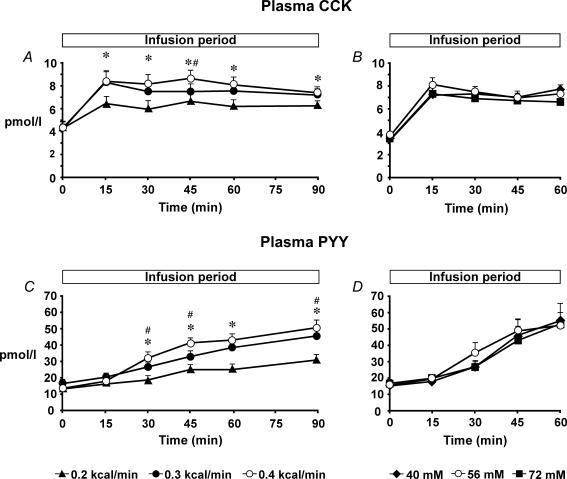
Plasma CCK. Baseline plasma CCK concentrations did not differ among study days. There was a significant treatment–time interaction for plasma CCK (P < 0.001) (Fig. 2A). Both C12(0.3) and C12(0.4) increased plasma CCK compared with C12(0.2) between t = 15 and 90 min (P < 0.05), and C12(0.4) when compared with C12(0.3) at t = 45 min (P < 0.01). There was also a significant effect of time for plasma CCK (P < 0.001). All treatments increased plasma CCK between t = 15 and 90 min compared with baseline (P < 0.001, for all). There was a direct relationship between plasma CCK concentrations with the load of C12 administered (r = 0.91, P < 0.001).
Figure 2. Plasma cholecystokinin and peptide YY concentrations.
Plasma concentrations of cholecystokinin (CCK) during intraduodenal infusion of C12 at different loads (0.2, 0.3 and 0.4 kcal min−1, all at 56 mm) for 90 min (A), and different concentrations (40, 56 and 72 mm, all at 0.4 kcal min−1) for 60 min (B). Plasma concentrations of peptide YY (PYY) during intraduodenal infusion of C12 at different loads (0.2, 0.3 and 0.4 kcal min−1, all at 56 mm) for 90 min (C), and different concentrations (40, 56 and 72 mm, all at 0.4 kcal min−1) for 60 min (D). *C12(0.4) and C12(0.3) versus C12(0.2), P < 0.01; #C12(0.4) versus C12(0.3), P < 0.05. Data are means ±s.e.m. (n = 12).
Plasma PYY. Baseline plasma PYY concentrations did not differ among study days. There was a significant treatment–time interaction for plasma PYY (P < 0.001) (Fig. 2C). Both C12(0.3) and C12(0.4) increased plasma PYY compared with C12(0.2) between t = 30 and 90 min (P < 0.01), and C12(0.4) compared with C12(0.3) at t = 30, 45 and 90 min (P < 0.05). There was also a significant effect of time for plasma PYY (P < 0.001). C12(0.2) increased plasma PYY between t = 45 and 90 min (P < 0.001), and both C12(0.3) and C12(0.4) between t = 30 and 90 min, compared with baseline (P < 0.01). There was a direct relationship between plasma PYY concentrations with the load of C12 administered (r = 0.79, P < 0.001).
Appetite perceptions and energy intake
There was no effect of treatment on perceptions of hunger, fullness, nausea or bloating (data not shown). There was a significant effect of treatment on energy intake (kilojoules) (P < 0.01) (Table 2). C12(0.4) decreased energy intake compared with C12(0.3) and C12(0.2) (P < 0.01), with no difference between C12(0.2) and C12(0.3). The percentage of energy from fat, carbohydrate or protein did not differ between treatments (Table 2), hence, the decrease in energy intake reflected a comparable reduction in all three macronutrients. There was an inverse relationship between energy intake with the load of C12 administered (r =−0.70, P < 0.05).
Table 2.
Energy intake and macronutrient distribution from buffet meal after intraduodenal infusion of C12 at different loads and concentrations
| C12 loada | C12 concentrationb | |||||
|---|---|---|---|---|---|---|
| C12(0.2) | C12(0.3) | C12(0.4) | C12(40) | C12(56) | C12(72) | |
| Energy intake (kJ) | 5610 ± 545 | 5488 ± 271 | 4510 ± 470* | 4946 ± 397 | 4483 ± 446 | 4398 ± 359 |
| Energy (%) | ||||||
| Fat | 31 ± 1 | 33 ± 2 | 32 ± 1 | 33 ± 2 | 32 ± 2 | 33 ± 2 |
| Carbohydrate | 48 ± 2 | 46 ± 2 | 47 ± 2 | 45 ± 2 | 46 ± 2 | 45 ± 3 |
| Protein | 22 ± 1 | 21 ± 1 | 21 ± 1 | 23 ± 1 | 22 ± 1 | 22 ± 1 |
Data are means ±s.e.m. (n = 12).
C12 infused at 0.2 (C12(0.2)), 0.3(C12(0.3)) and 0.4 (C12(0.4)) kcal min−1 (all at 56 mm) for 90 min.
C12 infused at 40 (C12(40)), 56 (C12(56)), 72 (C12(72)) mm (all at 0.4 kcal min−1) for 60 min.
P < 0.05 versus C12(0.2).
Effects of concentration of C12
Antropyloroduodenal pressures
Antral pressures. There was no effect of treatment on the number or amplitude of antral pressure waves (Table 1).
Pyloric pressures. (i) Basal pyloric pressure. There was no effect of treatment on basal pyloric pressure; however, there was a significant effect of time (P < 0.001) (Fig. 1B). Both C12(40) and C12(56) increased basal pyloric pressure between t = 15 and 60 min (P < 0.05 for all) and C12(72) between t = 15 and 45 min (P < 0.01) compared with baseline. (ii) Phasic pressures. There was no effect of treatment on the number or amplitude of isolated pyloric pressure waves; however, there was significant effect of time (P < 0.001, for both) (Fig. 1D). All three treatments increased the number and amplitude of isolated pyloric pressure waves (P < 0.001, for all) between t = 15 and 60 min compared with baseline.
Duodenal pressures
There was no effect of treatment on the number or amplitude of duodenal pressure waves (Table 1).
Plasma hormone concentrations
Plasma CCK. Baseline plasma CCK concentrations did not differ among study days. There was no effect of treatment on plasma CCK; however, there was a significant effect of time (P < 0.001) (Fig. 2B). All treatments increased plasma CCK between t = 15 and 60 min (P < 0.001, for all) compared with baseline.
Plasma PYY. Baseline plasma PYY concentrations did not differ among study days. There was no effect of treatment on plasma PYY; however, there was a significant effect of time (P < 0.001, for all) (Fig. 2D). C12(56) increased plasma PYY between t = 30 and 60 min (P < 0.001), and both C12(40) (P < 0.001) and C12(72) (P < 0.001) between t = 45 and 60 min, compared with baseline.
Appetite perceptions and energy intake
There was no effect of treatment on perceptions of hunger, fullness, nausea and bloating (data not shown). There was an effect of time on scores for bloating (P < 0.05). C12(40) increased bloating slightly between t = 15 and 30 min (P < 0.01) compared with baseline, while C12(56) and C12(72) had no effect, i.e. the effect of C12(40) may have been related to the larger volume administered. There was no effect of treatment on energy intake or percentage of fat, carbohydrate or protein consumed (Table 2).
There were no relationships between any parameter with the concentration of C12 administered.
Comparison between C12(0.4) (C12 load) and C12(56) (C12 concentration)
There were no statistically significant differences in basal pyloric pressures, numbers of isolated pyloric pressure waves, antral and duodenal pressure waves (between t = 0 and 60 min) or plasma CCK or PYY concentrations (at t = 60 min) between C12(0.4) and C12(56), although mean values for basal pyloric pressure and the number of isolated pyloric pressure waves tended to be greater during C12(56) compared with C12(0.4). There was also no difference in energy intake between the two conditions.
Discussion
This study has demonstrated that, in healthy humans, the load, but not the concentration, of C12 modulates antropyloroduodenal motility, plasma CCK and PYY concentrations and energy intake, at least at the loads and concentrations administered. Specifically, the greater the load of C12, the greater the (i) suppression of antral and duodenal pressure waves, (ii) increase in the number and amplitude of isolated pyloric pressure waves, (iii) secretion of CCK and PYY, and (iv) suppression of energy intake. In contrast, there was no significant difference in these responses when the concentration was altered from 40 to 72 mm, at a fixed load of 0.4 kcal min−1.
We believe that this study is the first to directly compare the effects of variations in load and concentration of intraduodenal fatty acids, specifically C12, in humans. It has been well established that although nutrient receptors in the small intestine do not detect calories per se, these receptors are tuned in such a way as to optimize caloric delivery, i.e. gastric emptying of nutrients is regulated on a kilocalorie per minute basis (Brener et al. 1983; Horowitz et al. 1996). Load dependence, with concentration independence, of responses evoked by luminal stimuli in the small intestine has been demonstrated in a variety of situations in animal experiments, including stimulation of pancreatic secretion by hydrogen ions infused at concentrations of ≥1 mm (Meyer et al. 1970b), stimulation of pancreatic enzyme secretion, by luminal l-phenylalanine infused at concentrations between 8 and 128 mm (Meyer et al. 1976) and luminal oleic acid infused between 5 and 80 mm (Meyer & Jones, 1974), inhibition of gastric emptying by luminal glucose at concentrations between 250 and 1000 mm (Lin et al. 1989) and inhibition of energy intake by either luminal oleic, or lauric, acid infused at concentrations between 20 and 80 mm (Meyer et al. 1998a). In each case, load dependence was ultimately shown in these animal models to be related to the length of small intestine exposed to each stimulus and, thus, presumably to the number of sensors excited. Our previous dose–response study of C12 in humans (Little et al. 2005) suggested that the threshold for concentration dependence, at least for inhibition of GI pressures and hormone release, was ≤14 mm. Accordingly, it is not altogether surprising that, at considerably higher concentrations (40–72 mm), we would observe load dependence, but concentration independence, for these responses. In the current study, the effect of ‘load’ of C12 could not be discriminated from that of ‘length’ of small intestinal contact; however, a previous study in our laboratory supports the concept that ‘load’ and ‘length’ are synonymous (Little et al. 2006), as is the case in animals (Meyer et al. 1998b).
There are only a few reports in humans from which the concentrations of free fatty acids in the postcibal duodenum or jejunum can be calculated and the majority of these were conducted more than 30 years ago (Borgstrom et al. 1957; Hofmann & Borgstroem, 1964; Porter & Saunders, 1971; Porter et al. 1971; Ament et al. 1972). Observations derived from these studies indicate that fatty acid concentrations range from ∼29–67 mm in the duodenum after fatty meals. More recent observations in animals and humans (Meyer et al. 1996) have demonstrated that the rate of duodenal entry of dietary fat (and, thus, the rate of release and luminal concentrations of free fatty acids) varies with the amount of fat ingested, as well as the state of emulsification of fat in the meal, so the above estimates were likely to be conditioned by the circumstances of the test meals. The range of fatty acid concentrations we utilized in the present study (40–72 mm) overlaps physiological postprandial concentrations in the human duodenum. It is appropriate to note that because lauric acid is a less prevalent dietary fatty acid, its use herein in these concentrations is atypical of luminal conditions. Nevertheless, our results clearly demonstrate load-dependent, but concentration-independent, responses to this particular fatty acid. Because lauric acid and the most prevalent dietary fatty acid, oleic acid, exhibited similar load-dependent, concentration-independent, effects on satiety in rats (Meyer et al. 1998a), we speculate that the present results with lauric acid in humans can be generalized to all fatty acids with chain lengths of 12 or more carbon atoms.
There are a number of limitations in our study design that need to be recognized. Even in previously reported animal experiments (Meyer et al. 1970a,b, 1976, 1998b), there were restrictions as to how much loads could be manipulated by altering volume rates at low concentrations, because as volume rates were increased, the animals frequently developed diarrhoea and, eventually, vomiting. This operational problem makes it difficult, if not impossible, except in unusual circumstances (Meyer et al. 1970a; Lin et al. 1990), to examine sensor responsiveness of luminal stimuli at lower concentrations. The same problem compromised the present design, where in a pilot study we aimed to assess C12 at concentrations of 24, 40 and 56 mm for 90 min. However, the rate required to deliver 0.4 kcal min−1 for the 24 mm solution was 9.4 ml min−1 and frequently resulted in severe abdominal cramps, and then diarrhoea, before the end of the infusion. Consequently, we were obliged to accept a study design in which C12 solutions were infused at 40, 56 and 72 mm, and at the shorter duration of 60 min, which decreased the total volume of C12 solution received by each subject. The variations in the infusion periods (90 min for C12 load and 60 min for C12 concentration) make it somewhat difficult to directly compare the two protocols, and it may be argued that the reason why only a load-, but not a concentration-dependent, effect was seen, was as a result of the longer infusion period. However, both studies had a common infusion, which delivered C12 at 0.4 kcal min−1 and 56 mm (C12(0.4) and C12(56)). When areas under the curves for basal pyloric pressure, numbers of isolated pyloric pressure waves and antral and duodenal pressure waves, as well as CCK and PYY, were determined for the first 60 min of the C12(0.4) infusion and compared with the C12(56) infusion, no differences were found in these parameters, although mean values for the number of isolated pyloric pressure waves and basal pyloric pressure were greater during the C12(56) infusion compared with the C12(0.4) infusion. This most probably reflects intersubject variability, as only three subjects participated in both study parts, and it is well recognized that there is substantial interindividual variability for measures of GI motility, including gastric emptying and intestinal transit (Collins et al. 1983; Degen & Phillips, 1996). Indeed when data from the two days were compared informally in these three subjects, they were found to be very similar (data not shown). Furthermore, there were no differences in energy intake, despite the C12(0.4) infusion delivering ∼30% more energy (12 kcal), when compared with the C12(56) infusion. These comparisons demonstrate that both infusions of C12 at 0.4 kcal min−1 and 56 mm, in the different conditions, overall had comparable effects on motility, plasma hormone secretion and energy intake, also making it doubtful that an additional 30 min of the C12 infusion in the C12 concentration protocol would have revealed an effect of concentration. In addition, as all of the C12 infusions in the C12 load protocol were at 56 mm, we would have been unable to demonstrate a ‘load-dependent’ effect if this concentration had evoked a maximal effect on GI function and energy intake, which was clearly not the case.
While our data indicate clear load-dependent, but concentration-independent, effects on motility, hormones, appetite and energy intake, our results may have potentially also been confounded by the fact that both limbs of the study employed varying rates of volume inflows, from 2 to 4 ml min−1 in the C12 load part, and from 3.1 to 5.7 ml min−1 in the C12 concentration part. However, if increasing volume rates of flow would have increased responses, we should have observed a systematic effect in the C12 concentration study, but this did not occur over the range of 3.1–5.7 ml min−1. Nevertheless, we cannot conclude that with entire certainty, as our study, due to the number of study conditions, did not allow us to include a volume control for each nutrient infusion, which would almost certainly not have been logistically feasible, particularly in relation to subject recruitment. Previous intraduodenal studies from our laboratory, including those investigating the effects of fatty acid chain length (Feltrin et al. 2004) and increasing doses of C12 (Little et al. 2005), have included control infusions and demonstrated that changes in antropyloroduodenal motility and gut hormones, as well as appetite, over time are minimal.
In conclusion, our study demonstrated that, at the loads and concentrations administered and using the example of lauric acid, load, but not concentration, modulates antropyloroduodenal motility patterns, plasma CCK and PYY concentrations and energy intake in response to intraduodenal fatty acids.
Supplementary Material
Acknowledgments
Kate L. Feltrin was supported by a Dawes Postgraduate Research Scholarship provided by the Royal Adelaide Hospital, Tanya J. Little by a Postgraduate Research Scholarship from the University of Adelaide, and Christine Feinle-Bisset by a Career Development Award from the National Health & Medical Research Council (NHMRC) in Australia. The study was supported by a project grant provided by the Royal Adelaide Hospital in 2004 and a Research Development Award from the University of Adelaide in 2005.
References
- Ament ME, Shimoda SS, Saunders DR, Rubin CE. Pathogenesis of steatorrhea in three cases of small intestinal stasis syndrome. Gastroenterology. 1972;63:728–747. [PubMed] [Google Scholar]
- Borgstrom B, Dahlqvist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957;36:1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener W, Hendrix TR, McHugh PR. Regulation of the gastric emptying of glucose. Gastroenterology. 1983;85:76–82. [PubMed] [Google Scholar]
- Collins PJ, Horowitz M, Cook DJ, Harding PE, Shearman DJ. Gastric emptying in normal subjects – a reproducible technique using a single scintillation camera and computer system. Gut. 1983;24:1117–1125. doi: 10.1136/gut.24.12.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CG, Andrews JM, Jones KL, Wittert GA, Chapman IM, Morley JE, Horowitz M. Effects of small intestinal nutrient infusion on appetite and pyloric motility are modified by age. Am J Physiol Regul Integr Comp Physiol. 1997;273:R755–R761. doi: 10.1152/ajpregu.1997.273.2.R755. [DOI] [PubMed] [Google Scholar]
- Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut. 1996;39:299–305. doi: 10.1136/gut.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elashoff JD. Down with multiple t-tests. Gastroenterology. 1981;80:615–620. [PubMed] [Google Scholar]
- Feinle C, O'Donovan DG, Doran S, Andrews JM, Wishart J, Chapman I, Horowitz M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am J Physiol Gastrointest Liver Physiol. 2003;284:G798–G807. doi: 10.1152/ajpgi.00512.2002. [DOI] [PubMed] [Google Scholar]
- Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJ, Wishart J, Pilichiewicz AN, Rades T, Chapman IM, Feinle-Bisset C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol. 2004;287:R524–R533. doi: 10.1152/ajpregu.00039.2004. [DOI] [PubMed] [Google Scholar]
- Heddle R, Collins PJ, Dent J, Horowitz M, Read NW, Chatterton B, Houghton LA. Motor mechanisms associated with slowing of the gastric emptying of a solid meal by an intraduodenal lipid infusion. J Gastroenterol Hepatol. 1989;4:437–447. doi: 10.1111/j.1440-1746.1989.tb01741.x. [DOI] [PubMed] [Google Scholar]
- Heddle R, Dent J, Read NW, Houghton LA, Toouli J, Horowitz M, Maddern GJ, Downton J. Antropyloroduodenal motor responses to intraduodenal lipid infusion in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 1988a;254:G671–G679. doi: 10.1152/ajpgi.1988.254.5.G671. [DOI] [PubMed] [Google Scholar]
- Heddle R, Dent J, Toouli J, Read NW. Topography and measurement of pyloric pressure waves and tone in humans. Am J Physiol Gastrointest Liver Physiol. 1988b;255:G490–G497. doi: 10.1152/ajpgi.1988.255.4.G490. [DOI] [PubMed] [Google Scholar]
- Hofmann AF, Borgstroem B. The intraluminal phase of fat digestion in man: The lipid content of the micellar and oil phases of intestinal content obtained during fat digestion and absorption. J Clin Invest. 1964;43:247–257. doi: 10.1172/JCI104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Cunningham KM, Wishart JM, Jones KL, Read NW. The effect of short-term dietary supplementation with glucose on gastric emptying of glucose and fructose and oral glucose tolerance in normal subjects. Diabetologia. 1996;39:481–486. doi: 10.1007/BF00400681. [DOI] [PubMed] [Google Scholar]
- Hunt JN, Knox MT. A relation between the chain length of fatty acids and the slowing of gastric emptying. J Physiol. 1968;194:327–336. doi: 10.1113/jphysiol.1968.sp008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JN, Smith JL, Jiang CL. Effect of meal volume and energy density on the gastric emptying of carbohydrates. Gastroenterology. 1985;89:1326–1330. doi: 10.1016/0016-5085(85)90650-x. [DOI] [PubMed] [Google Scholar]
- Lieverse RJ, Jansen JB, Masclee AA, Rovati LC, Lamers CB. Effect of a low dose of intraduodenal fat on satiety in humans: studies using the type A cholecystokinin receptor antagonist loxiglumide. Gut. 1994;35:501–505. doi: 10.1136/gut.35.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Chey WY. Cholecystokinin and peptide YY are released by fat in either proximal or distal small intestine in dogs. Regul Pept. 2003;114:131–135. doi: 10.1016/s0167-0115(03)00115-0. [DOI] [PubMed] [Google Scholar]
- Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by glucose depends on length of intestine exposed to nutrient. Am J Physiol Gastrointest Liver Physiol. 1989;256:G404–G411. doi: 10.1152/ajpgi.1989.256.2.G404. [DOI] [PubMed] [Google Scholar]
- Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by sodium oleate depends on length of intestine exposed to nutrient. Am J Physiol Gastrointest Liver Physiol. 1990;259:G1031–G1036. doi: 10.1152/ajpgi.1990.259.6.G1031. [DOI] [PubMed] [Google Scholar]
- Little TJ, Doran S, Meyer JH, Smout AJ, O'Donovan DG, Wu KL, Jones KL, Wishart J, Rayner CK, Horowitz M, Feinle-Bisset C. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am J Physiol Endocrinol Metab. 2006;291:E647–E655. doi: 10.1152/ajpendo.00099.2006. [DOI] [PubMed] [Google Scholar]
- Little TJ, Feltrin KL, Horowitz M, Smout AJ, Rades T, Meyer JH, Pilichiewicz AN, Wishart J, Feinle-Bisset C. Dose-related effects of lauric acid on antropyloroduodenal motility, gastrointestinal hormone release, appetite, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1090–R1098. doi: 10.1152/ajpregu.00290.2005. [DOI] [PubMed] [Google Scholar]
- MacIntosh CG, Andrews JM, Jones KL, Wishart JM, Morris HA, Jansen JB, Morley JE, Horowitz M, Chapman IM. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am J Clin Nutr. 1999;69:999–1006. doi: 10.1093/ajcn/69.5.999. [DOI] [PubMed] [Google Scholar]
- MacIntosh CG, Morley JE, Wishart J, Morris H, Jansen JB, Horowitz M, Chapman IM. Effect of exogenous cholecystokinin (CCK)-8 on food intake and plasma CCK, leptin, and insulin concentrations in older and young adults: evidence for increased CCK activity as a cause of the anorexia of aging. J Clin Endocrinol Metab. 2001;86:5830–5837. doi: 10.1210/jcem.86.12.8107. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Grazia-Luca M, Jones MN, D'Amato M, Dockray GJ, Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology. 1999;116:46–53. doi: 10.1016/s0016-5085(99)70227-1. [DOI] [PubMed] [Google Scholar]
- Matzinger D, Degen L, Drewe J, Meuli J, Duebendorfer R, Ruckstuhl N, D'Amato M, Rovati L, Beglinger C. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut. 2000;46:688–693. doi: 10.1136/gut.46.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Hlinka M, Kao D, Lake R, MacLaughlin E, Graham LS, Elashoff JD. Gastric emptying of oil from solid and liquid meals. Effect of human pancreatic insufficiency. Dig Dis Sci. 1996;41:1691–1699. doi: 10.1007/BF02088732. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Hlinka M, Tabrizi Y, DiMaso N, Raybould HE. Chemical specificities and intestinal distributions of nutrient-driven satiety. Am J Physiol Gastrointest Liver Physiol. 1998a;275:G1293–G1307. doi: 10.1152/ajpregu.1998.275.4.R1293. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Jones RS. Canine pancreatic responses to intestinally perfused fat and products of fat digestion. Am J Physiol. 1974;226:1178–1187. doi: 10.1152/ajplegacy.1974.226.5.1178. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Kelly GA, Spingola LJ, Jones RS. Canine gut receptors mediating pancreatic responses to luminal 1-amino acids. Am J Physiol. 1976;231:669–677. doi: 10.1152/ajplegacy.1976.231.3.669. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Tabrizi Y, DiMaso N, Hlinka M, Raybould HE. Length of intestinal contact on nutrient-driven satiety. Am J Physiol Regul Integr Comp Physiol. 1998b;275:R1308–R1319. doi: 10.1152/ajpregu.1998.275.4.R1308. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Way LW, Grossman MI. Pancreatic bicarbonate response to various acids in duodenum of the dog. Am J Physiol. 1970a;219:964–970. doi: 10.1152/ajplegacy.1970.219.4.964. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Way LW, Grossman MI. Pancreatic response to acidification of various lengths of proximal intestine in the dog. Am J Physiol. 1970b;219:971–977. doi: 10.1152/ajplegacy.1970.219.4.971. [DOI] [PubMed] [Google Scholar]
- Parker B, Sturm K, MacIntosh CG, Feinle C, Horowitz M, Chapman IM. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr. 2004;58:212–218. doi: 10.1038/sj.ejcn.1601768. [DOI] [PubMed] [Google Scholar]
- Pilichiewicz AN, Little TJ, Brennan IM, Meyer JH, Wishart JM, Otto B, Horowitz M, Feinle-Bisset C. Effects of load, and duration, of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol. 2005;290:R668–R677. doi: 10.1152/ajpregu.00606.2005. [DOI] [PubMed] [Google Scholar]
- Porikos KP, Hesser MF, van Itallie TB. Caloric regulation in normal-weight men maintained on a palatable diet of conventional foods. Physiol Behav. 1982;29:293–300. doi: 10.1016/0031-9384(82)90018-x. [DOI] [PubMed] [Google Scholar]
- Porter HP, Saunders DR. Isolation of the aqueous phase of human intestinal contents during the digestion of a fatty meal. Gastroenterology. 1971;60:997–1007. [PubMed] [Google Scholar]
- Porter HP, Saunders DR, Tytgat G, Brunser O, Rubin CE. Fat absorption in bile fistula man. A morphological and biochemical study. Gastroenterology. 1971;60:1008–1019. [PubMed] [Google Scholar]
- Rolls BJ, Castellanos VH, Halford JC, Kilara A, Panyam D, Pelkman CL, Smith GP, Thorwart ML. Volume of food consumed affects satiety in men. Am J Clin Nutr. 1998;67:1170–1177. doi: 10.1093/ajcn/67.6.1170. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Kim S, McNelis AL, Fischman MW, Foltin RW, Moran TH. Time course of effects of preloads high in fat or carbohydrate on food intake and hunger ratings in humans. Am J Physiol Regul Integr Comp Physiol. 1991;260:R756–R763. doi: 10.1152/ajpregu.1991.260.4.R756. [DOI] [PubMed] [Google Scholar]
- Samsom M, Roelofs JM, Akkermans LM, van Berge Henegouwen GP, Smout AJ. Proximal gastric motor activity in response to a liquid meal in type I diabetes mellitus with autonomic neuropathy. Dig Dis Sci. 1998;43:491–496. doi: 10.1023/a:1018894520557. [DOI] [PubMed] [Google Scholar]
- Shide DJ, Caballero B, Reidelberger R, Rolls BJ. Accurate energy compensation for intragastric and oral nutrients in lean males. Am J Clin Nutr. 1995;61:754–764. doi: 10.1093/ajcn/61.4.754. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Velasquez OR, Tso P, Crissinger KD. Fatty acid-induced injury in developing piglet intestine: effect of degree of saturation and carbon chain length. Pediatr Res. 1993;33:543–547. doi: 10.1203/00006450-199306000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.