Abstract
During strenuous exercise, extracellular K+ ([K+]o) is increased, which potentially can reduce muscle excitability and force production. In addition, exercise leads to accumulation of lactate and H+ and increased levels of circulating catecholamines. Individually, reduced pH and increased catecholamines have been shown to counteract the depressing effect of elevated K+. This study examines (i) whether the effects of addition of lactic acid and adrenaline on the excitability of isolated muscles are caused by separate mechanisms and are additive and (ii) whether the effect of adding lactic acid or increasing CO2 is related to a reduction of intra- or extracellular pH. Rat soleus muscles were incubated at a [K+]o of 15 mm, which reduced tetanic force by 85%. Subsequent addition of 20 mm lactic acid or 10−5m adrenaline led to a small recovery of force, but when added together induced an almost complete force recovery. Compound action potentials showed that the force recovery was associated with recovery of muscle excitability. The improved excitability after addition of adrenaline was associated with increased Na+–K+ pump activity resulting in hyperpolarization and an increase in the chemical Na+ gradient. In contrast, addition of lactic acid had no effect on the membrane potential or the Na+–K+ pump activity, but most likely increased excitability via a reduction in intracellular pH. It is concluded that the protective effects of acidosis and adrenaline on muscle excitability and force took place via different mechanisms and were additive. The results suggest that circulating catecholamines and development of acidosis during exercise may improve the tolerance of muscles to elevated [K+]o.
It is well established that exercise causes a loss of potassium from the contracting muscles and during intense exercise, interstitial potassium may reach 12 mm or more (Juel et al. 2000; Nordsborg et al. 2003; Mohr et al. 2004; Street et al. 2005; for review, see Sejersted & Sjøgaard, 2000). Studies on isolated muscles have shown that because of the ensuing depolarization, increased extracellular potassium ([K+]o) may lead to a reduction in the amplitude of the action potentials and, eventually, to compromised muscle excitability and decreased force production (Cairns et al. 1995, 1997; Overgaard et al. 1999; for review, see Sejersted & Sjøgaard, 2000; Rich & Pinter, 2003). Based on these findings, it has been suggested that the increase in [K+]o contributes to muscle fatigue during strenuous exercise (for review, see Sejersted & Sjøgaard, 2000). Central to the evaluation of this hypothesis is knowledge about the tolerance of muscles to elevated [K+]o. Several studies have shown that a considerable safety margin exists (Cairns et al. 1995, 1997; Yensen et al. 2002; Nielsen et al. 2004). Thus, in isolated muscles at rest, [K+]o has to be increased to more than 8 mm for a depression of force to occur (Bouclin et al. 1995; Cairns et al. 1997; Pedersen et al. 2005). Furthermore, studies have shown that the tolerance of isolated muscles to elevated [K+]o is improved if adrenaline and other β2-agonists are added (Clausen et al. 1993; Cairns et al. 1995). Later it was shown that a similar increase in tolerance to elevated [K+]o could be induced by addition of lactic acid (Nielsen et al. 2001) and that this effect could be mimicked by an increase in CO2. Since intensive exercise is associated with acidification of the active muscles and with an increase in circulating adrenaline, these findings could suggest that the tolerance of muscles to elevated [K+]o is higher during exercise than during rest.
The relevance of the effect of addition of lactic acid to isolated muscles for the function of muscles during exercise has, however, been questioned (Kristensen et al. 2005; Lamb et al. 2006). It is argued that part of the effect of lactic acid on the K+ tolerance is conveyed via an increase in intracellular Na+, which causes an increase in the activity of the Na+–K+ pump (Kristensen et al. 2005). It is further argued that the effect of lactic acid is unlikely to be seen in muscles where the activity of the Na+–K+ pump is increased. Moreover, it was suggested by Kristensen et al. (2005) that the increase in K+ tolerance induced by lactic acid is not a general mechanism in the intact organism (Kristensen et al. 2005). This criticism was based on the observation that addition of lactic acid to isolated muscles leads to a larger reduction in extracellular pH (pHo) than in intracellular pH (pHi), which is in contrast to the acidosis induced by endogenous production of H+ during muscle contractions where the largest reduction is seen in pHi (Street et al. 2001; Juel et al. 2004). The identification of the mechanisms for the effects of β2-agonists and acidosis on K+ tolerance is further obscured by the observation that addition of catecholamines reduces pHi in rabbit ventricular myocytes (Guo et al. 1992).
Based on this, the aims of the present study were to test the following hypotheses: (1) That lactic acid and adrenaline (β-agonists) exert their effect on excitability and force in isolated muscles via two distinct mechanisms, and that the effects are additive; (2) That the effect of acidosis on excitability specifically is related to a reduction of intracellular pH and can be induced without a change in extracellular pH.
Using rat soleus muscles, we show here that when force was depressed by high [K+]o, addition of either lactic acid or adrenaline produced a pronounced recovery of excitability and force. In the case of lactic acid, the force recovery was strictly related to a reduction in pHi. No reduction in pHi was seen when adrenaline was added. Instead an increased ouabain-sensitive 86Rb+ uptake and a hyperpolarized resting membrane potential together with a decreased intracellular Na+ content (Na+i) were observed. It is concluded that the effect of acidosis is caused by a reduction in membrane Cl− conductance. Further, the effect of acidosis on muscle excitability is additive to the effect of adrenaline and no overlap between the mechanisms were seen. Part of the results has been presented in a preliminary form (de Paoli et al. 2002).
Methods
Animals and muscle preparation
All handling and use of animals complied with Danish animal welfare regulations. If not otherwise mentioned, experiments were carried out using soleus muscles from 4-week-old Wistar rats weighing 60–73 g (own breed). The rats were fed ad libitum and were kept at a constant temperature (21°C) and day length (12 h). The animals were killed by decapitation, and muscles were isolated with the proximal end attached to the bone and the distal end with an intact tendon. For experiments with stimulation via the motor nerve, approximately 10 mm of the nerve was left attached to the muscle. The standard incubation medium was Krebs–Ringer bicarbonate buffer (NKR) containing (mm): 122 NaCl, 25 NaHCO3, 2.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.3 CaCl2 and 5.0 d-glucose. If not otherwise noted, buffers were maintained at 30°C and equilibrated with a mixture of 95% O2 and 5% CO2 throughout the experiment (pH ≈ 7.4). In K+-enriched buffers an equivalent amount of Na+ was omitted to maintain iso-osmolarity. In one series of experiments either 45 mm NaHCO3 or sodium methanesulphonate (Sigma-Aldrich) was added and an equivalent amount of NaCl was omitted to maintain iso-osmolarity and a constant Cl− concentration in the buffer throughout the experiments. In another series of experiments 10.6 mm NaCl was removed or replaced with 21.2 mm sucrose to see the effects of an hypo-osmotic buffer on force. To avoid the exposure of the muscles to large and potentially damaging excursion in buffer pH, buffers in which l-lactic acid (Sigma-Aldrich, Fluka) or HCl was added were equilibrated for at least 30 min with 95% O2 and 5% CO2 before use. In all experiments, the muscles were mounted isometrically in the standard incubation medium and equilibrated for at least 30 min before starting the experiments.
Electrical stimulation, isometric force measurement and recordings of compound action potentials (M-wave)
Muscles were mounted on isometric force transducers (Grass FT03) and adjusted to optimal length for force production. Tetanic force development was recorded with a chart recorder. In general, contractions were evoked via field stimulation using constant voltage pulses applied through two platinum wire electrodes passing current across the central part of the muscle. If not otherwise noted pulses of 1 ms duration and supramaximal voltage (24–30 V cm−1) were used.
In experiments where extracellular compound action potentials (M-waves) were measured, contractions were evoked via nerve stimulation through a stimulus isolator (ISU 165, Cibertec, Spain). The pulses were fixed current pulses that were supramaximal for stimulation of the motor nerve without producing any direct stimulation of muscle fibres (Overgaard & Nielsen, 2001). Contractions were elicited every 10 min throughout the experiments using 30 Hz pulse trains of 1.5 s duration which assured full development of tetanic force in all types of buffers. In experiments with adrenaline (Unikem, Denmark), a supramaximal concentration of 10−5m was used (Clausen & Flatman, 1977).
Unipolar M-wave signals were recorded from a circular silver electrode with a recording area of 0.79 mm2 placed in close contact with the muscle between the innervation zone and the tendon. The diameter of the electrode was approximately half of the diameter of the muscle, allowing a relatively large number of fibres to contribute to the M-wave recordings. The conduction velocity was estimated from the time from the stimulus to the peak of the M-wave.
Na+–K+ pump activity and intracellular Na+ content
The activity of the Na+–K+ pump was expressed as the ouabain-sensitive 86Rb+ (tracer for K+) uptake. Ouabain was used to block the activity of the Na+–K+ pump which allowed the calculation of the ouabain-sensitive 86Rb+ uptake by subtraction of the ouabain-insensitive 86Rb+ uptake from the total 86Rb+ uptake. In short, muscles were incubated for 10 min in buffer with or without 10−3m ouabain followed by 10 min of incubation in buffer containing 0.1 μCi 86Rb+ ml−1 (specific activity 0.5 mCi mmol−1). Finally, the muscles were washed for 4 × 15 min at 0°C in Na+-free non-radioactive Tris-sucrose buffer to remove extracellular 86Rb+, as previously described (Buchanan et al. 2002). Afterwards the muscles were blotted, weighed and counted for 86Rb+ activity by Cerenkov radiation in a β-counter while soaking in 2 ml of a 0.3 m TCA solution.
Intracellular Na+ content was calculated from determinations of the Na+ concentration in the TCA solution using flame photometry (Everts & Clausen, 1992). The study by Everts & Clausen (1992) showed that part of the intracellular Na+, but no intracellular K+, was lost during the washout and that this loss could be corrected for by multiplying the Na+ content of the muscles at the end of the washout by 1.46. In the present study a correction factor of 1.46 was therefore used for the calculation of intracellular Na+ content in all muscles undergoing washout.
Intracellular pH (pHi)
Muscles were mounted on a myograph adapted for use on a microscope (Leica DM-IRB) and were loaded with 20 μm 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF-AM) for 30 min and then washed to remove any extracellular indicator. To measure pHi, the preparation was excited alternately with wavelengths of 495 and 450 nm from a 75 W xenon lamp that led into a monochromator (Zeiss, M4, GII). The emission from the muscle was collected by the microscope, led through a bandpass filter (530–585 nm) and detected by a photomultiplier system (PTI). Data were stored on a computer, and the ratio of emission at the two excitation wavelengths (495/450) was calculated after subtracting the background fluorescence. The emission ratio was calibrated to pHi by the K+–nigericin technique (Thomas et al. 1979) and the data were fitted by linear regression (r2 > 0.96). The presented values show intracellular pH of the muscle in the various buffers recorded after a stable value was obtained.
Recordings of membrane potentials and membrane conductance
The resting membrane potential (Em) of individual fibres was measured by a standard electrophysiological technique using glass microelectrodes filled with 3 m KCl. Briefly, a microelectrode (10–20 MΩ) was placed in an individual fibre in the middle third region of the muscle. For each measurement of membrane potential in a muscle, 10 insertions were made over a 5 to 10 min period and the electrode was moved a small distance across the muscle between each insertion. The mean coefficient of variation of these 10 insertions was 5.0 ± 0.5% (n = 60).
The membrane conductance was measured as previously described (Pedersen et al. 2005). Briefly, two glass microelectrodes (10–20 MΩ) were inserted into the same fibre of isolated soleus muscles from adult female rats (12–14 weeks, 230–300 g). One electrode was used for injecting square current pulses, the other for measuring the membrane potential. The membrane potential deflections in response to the current pulses were measured at three to four inter-electrode distances in each fibre and the membrane conductance was calculated using the linear cable theory first applied to skeletal muscle by Boyd & Martin (1959). The myoplasmic resistivity was assumed constant under all circumstances and set to 180 Ω cm (Albuquerque & Thesleff, 1968).
Statistical analysis
All data are expressed as means ±s.e.m. The statistical significance of any difference between groups was ascertained using a two-way ANOVA followed by Student's two-tailed t test for non-paired observations. The statistical significance of the difference between groups for resting membrane potential were analysed using a one-way ANOVA. Differences were located with a Student–Newman–Keuls post hoc test.
Results
Effect of addition of lactic acid and adrenaline on excitability and force in muscles at high [K+]o
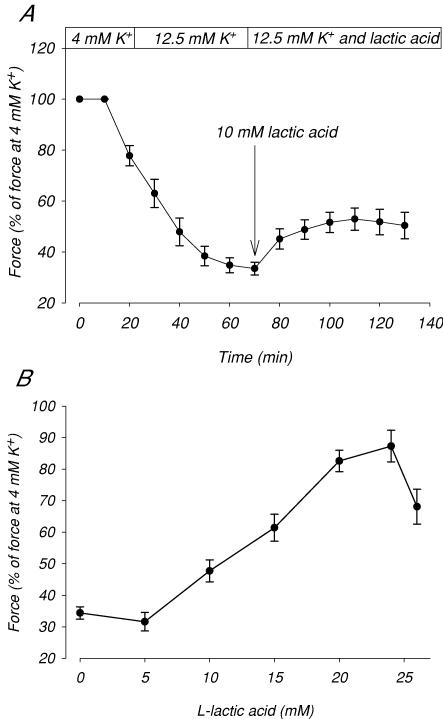
Figure 1A shows the effect of addition of lactic acid on the tetanic force production in muscles exposed to 12.5 mm[K+]o. The exposure to 12.5 mm[K+]o reduced force to 34% of the control force determined at 4 mm K+. Subsequent addition of 10 mm lactic acid recovered force to 45% of the control level. To study the dose–response relation for this effect of lactic acid the experiment depicted in Fig. 1A was repeated using concentrations of lactic acid from 5 to 26 mm. The maximum force obtained at each concentration of lactic acid is presented in Fig. 1B, which shows that the recovery of force increased sigmoidally with the concentration of lactic acid in the range from 5 to 24 mm. A decrease in force recovery was seen when lactic acid concentration was increased from 24 to 26 mm. In a few experiments, lactic acid was added to a concentration higher than 26 mm. In these experiments, pH in the buffer dropped markedly resulting in a loss of force, which could not be reversed upon return to normal pH.
Figure 1. Effect of lactic acid on tetanic force in muscles at 12.5 mM K+.
Tetanic contractions were elicited by applying brief trains (2 s) of pulses at 30 Hz trains were given every 10 min. After control contractions in 4 mm K+, [K+]o was increased to 12.5 mm. After a futher 70 min at 12.5 mm K+, 5 to 26 mm lactic acid was added and the incubation was continued steady-state force was obtained. A, time course of the effect of 10 mm lactic acid on force in muscles at 12.5 mm K+. B, maximum force obtained after addition of the indicated concentrations of lactic acid. Symbols show means ±s.e.m. of 6–10 muscles.
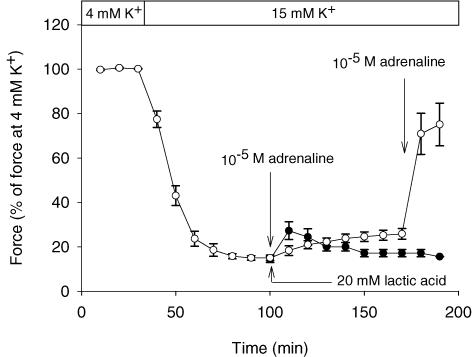
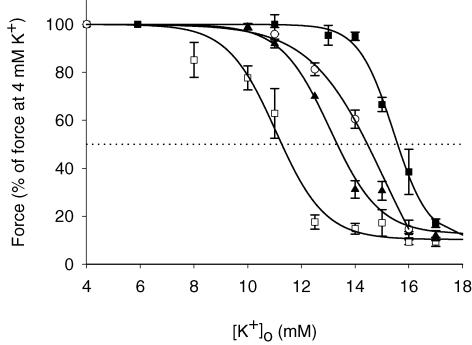
It has previously been demonstrated that a force recovery, similar to the one induced by addition of lactic acid, can be obtained by addition of adrenaline (Andersen & Clausen, 1993). To explore the combined effect of lactic acid and adrenaline on tetanic force production of muscles incubated at high [K+]o, a series of experiments like the one depicted in Fig. 1A was performed using concentrations of lactic acid (20 mm) and adrenaline (10−5m) that produces recoveries of force that are close to the maximal force recovery that can be obtained by the compounds (see Fig. 1B and Clausen & Flatman, 1977). Here, 15 mm K+ was used to depress force, ensuring that the addition of only one of the compounds only led to partial recovery of force. The data are presented in Fig. 2, which shows that the increase in [K+]o to 15 mm reduced tetanic force to 15% of the control level. Subsequent addition of 20 mm lactic acid or 10−5m adrenaline led to a recovery of force to 25% and 27% of control force, respectively. For adrenaline the increase in force was only temporary with maximum effect observed after 10 min. When adrenaline was added to muscles already exposed to lactic acid, a much larger increase in force to 75% of control level took place, indicating that the effects of lactic acid and adrenaline on force were additive. This conclusion is supported by Fig. 3, which shows the relation between [K+]o and force production in control muscles and in muscles exposed to either 20 mm lactic acid, 10−5m adrenaline or to both compounds at the same time. Since the effect of adrenaline was temporary, the curve for adrenaline was in this figure constructed using the maximum values for force achieved 10 min after addition of the compound. Figure 3 shows that in control muscles, force started to become depressed when [K+]o was increased to ∼8 mm, and at a [K+]o of ∼11.3 mm, force was reduced to 50% of control level (IC50= 11.3 mm). In muscles where 20 mm lactic acid was added, a [K+]o of ∼11.0 mm was needed to produce any reduction in force and IC50 was increased to 13.3 mm. Similar protection of force against the depressing effect of elevated [K+]o was seen in muscles exposed to 10−5m adrenaline. In muscles exposed to both 10−5m adrenaline and 20 mm lactic acid at the same time, force was fully maintained up to a [K+]o of ∼14.0 mm and IC50 was increased to 15.6 mm.
Figure 2. Effects of lactic acid and adrenaline on tetanic force in muscles incubated at 15 mM K+.
Tetanic contractions were elicited every 10 min by applying brief trains (2 s) of pulses at 30 Hz. After control contractions in 4 mm K+, [K+]o was increased to 15 mm. At t = 100 min, 20 mm lactic acid (buffer pH ∼6.8; ^ or 10−5m adrenaline (buffer pH ∼7.4; •) was added. At t = 150 min adrenaline was added to the muscles already exposed to lactic acid. Symbols show means ±s.e.m. of 3–8 muscles.
Figure 3. Effect of lactic acid and adrenaline on the relation between [K+]o and tetanic force.
Experiments were done as depicted in Fig. 2, using concentrations of extracellular K+ from 4 to 17 mm. For control muscles and muscles exposed to lactic acid, the forces shown are steady-state forces at the indicated [K+]o. For adrenaline the recovery of force was temporary (Fig. 2). For that reason the largest force production which was observed 10 min after addition adrenaline was used. □, control muscles, buffer pH ∼7.4 (n = 4–6); ▴, 20 mm lactic acid added, buffer pH ∼6.8 (n = 8–10); ^, 10−5m adrenaline added, buffer pH ∼7.4 (n = 6); ▪, 20 mm lactic and 10−5m adrenaline added, buffer pH ∼6.8 (n = 8). Continuous lines represent Bolzmann curves fitted to data. Symbols represent means ±s.e.m.
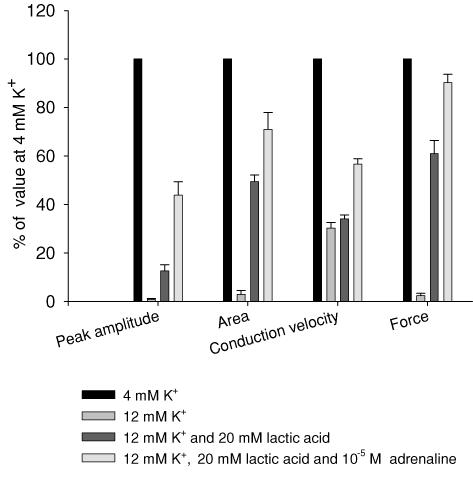
Figure 4 shows that the effects of high [K+]o, lactic acid and adrenaline on force production were closely mirrored by changes in muscle excitability, as judged from changes in M-wave parameters. To minimize stimulation artefacts in the M-wave recordings, the muscles were in these experiments excited via the motor nerve. Since this leads to more exacerbated effects of elevated [K+]o on force than direct field stimulation with 1 ms pulses, concentrations of 12 mm K+ were used in these experiments, which suppressed force to around 4% of the control level. In Fig. 4 the average tetanic force and the average peak amplitude, area and conduction velocity of the M-wave are shown before and after elevation of [K+]o. When [K+]o was elevated from 4 mm to 12 mm, M-wave peak amplitude, area and conduction velocity were reduced to 1%, 3% and 30% of control level, respectively. When 20 mm lactic acid was subsequently added, the peak amplitude and area of the M-waves recovered to 13% and 50% of control level, respectively (P < 0.003 and P < 0.001) whereas the conduction velocity was unchanged (P = 0.26). When 10−5m adrenaline was added in addition to lactic acid a further recovery of M-wave peak amplitude and area to 44% and 71% were seen (P < 0.001 and P = 0.03), and the conduction velocity recovered to 57% of the control level (P < 0.001). Figure 4 also shows that at all conditions, the changes in force closely followed the changes in M-wave area and peak amplitude.
Figure 4. Effects of high [K+]o, lactic acid and adrenaline on tetanic force and M-wave parameters.
M-wave recordings obtained from a muscle at 4 mm K+, after 70 min incubation at 12 mm K+, 50 min following the addition of lactic acid and 10 min after further addition of adrenaline. Values obtained during the experiment are average of 23 M-wave recordings obtained during a 1.5 s, 30 Hz tetanic train. n = 5. Bars show means ±s.e.m.
The effect of addition of lactic acid and adrenaline on excitability was further investigated by measurements of Em. When [K+]o was elevated from 4 mm K+ to 10 mm K+ a depolarization of 14.6 mV was observed (−79.2 ± 0.7 to −64.6 ± 0.6, P < 0.001, n = 6 muscles/60 fibres). Subsequent addition of 20 mm lactic acid did not produce any significant change in the Em (−64.6 ± 0.6 to −63.8 ± 0.7, P = 0.42, n = 6 muscles/60 fibres). However, when 10−5m adrenaline was added in addition to 20 mm lactic acid a small but significant hyperpolarization of 3.8 mV was seen (−63.8 ± 0.7 to −67.6 ± 0.6, P < 0.001, n = 6 muscles/60 fibres).
Mechanisms for the effect of lactic acid and adrenaline on excitability and force
Figures 2 and 4 suggest that the effect on excitability and force of lactic acid and adrenaline are additive indicating that their effects are caused by distinct mechanisms with no or very little overlap. Previously, it has been shown that the effect of adding lactic acid and adrenaline, respectively, is at least partly related to a reduction in muscle pH and to a stimulation of muscle Na+–K+ pumps, respectively (Andersen & Clausen, 1993; Nielsen et al. 2001). The improved excitability after Na+–K+ pump stimulation is probably related to a hyperpolarization of the muscle fibres and an increase in the chemical gradient for Na+ (Nielsen & Clausen, 2000) whereas the effect of reduced pH is related to a reduction in the Cl− conductance of the muscle fibres (Pedersen et al. 2005). Since it has been suggested, however, that there may be a considerable overlap between the mechanisms for the effect of lactic acid and adrenaline on force and excitability (Kristensen et al. 2005), the effects of the two compounds on pH and the Na+–K+ pump was determined:
Effects on the Na+–K+ pump
Table 1 shows that lactic acid per se had no effect on either the ouabain-sensitive 86Rb+ uptake or the intracellular Na+ content of the muscles (P > 0.1), indicating that the activity of the Na+–K+ pump was unaffected. In contrast, adrenaline increased the ouabain-sensitive 86Rb+ uptake by 147%, and caused a 49% decrease in the intracellular Na+ content of the muscle. Importantly, similar effects of adrenaline were obtained in muscles incubated with lactic acid (Table 1).
Table 1.
Effect of lactic acid and adrenaline on 86Rb+ uptake, intracellular Na+ content and membrane potential in rat soleus muscles incubated in high K+
| Ouabain-insensitive 86Rb+ uptake (nmol (g wet wt)−1 min−1) | Ouabain-sensitive 86Rb+ uptake (nmol (g wet wt)−1 min−1) | Intracellular Na+ content (μmol (g wet wt)−1) | |
|---|---|---|---|
| Control | 533 ± 17 | 222 ± 87 | 11.0 ± 0.2 |
| Lactic acid | 431 ± 15† | 210 ± 40 | 12.4 ± 0.7 |
| Adrenaline | 540 ± 17 | 547 ± 17† | 5.6 ± 0.2† |
| Lactic acid + adrenaline | — | 416 ± 26* | 5.0 ± 0.3† |
Soleus muscles were pre-incubated for 20 min at 12.5 mm K+ in buffer without (control) or with the addition of 20 mm lactic acid, 10−5m adrenaline or both 20 mm lactic acid and 10−5m adrenaline. After pre-incubation, 0.1 μCi 86Rb+ ml−1 was added and the incubation was continued for 10 min. Following incubation, the muscles were washed 4 × 15 min in ice-cold Na+-free Tris-sucrose buffer, blotted and 86Rb+ activity counted and intracellular Na+ content determined. Significantly different from control
P < 0.05
P < 0.001. Data show means ±s.e.m. of 5–6 muscles.
Effects on pH
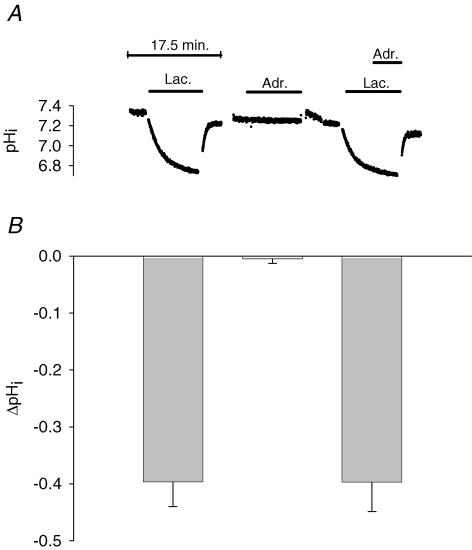
Addition of 20 mm lactic acid reduced buffer pH by 0.64 ± 0.03 units (from 7.44 ± 0.02 to 6.80 ± 0.02, n = 8) whereas addition of 10−5m adrenaline either alone or in addition to lactic acid had no effect on buffer pH (data not shown). As shown in Fig. 5A, addition of lactic acid also led to a significant reduction in pHi. Control experiments (see also Nielsen et al. 2001) demonstrated that the reduction in pHi was maintained as long as the acid was present (data not shown). On average, the reduction in pHi after addition of 20 mm lactic acid was 0.40 ± 0.04 units (Fig. 5B, P < 0.001). In contrast, addition of 10−5m adrenaline was without significant effect on pHi (Fig. 5, P = 0.46). This was also the case when adrenaline was added to muscles already exposed to lactic acid (Fig. 5, P = 0.83).
Figure 5. Effect of lactic acid and adrenaline on pHi in muscles at 11 mM K+.
A, typical recordings of pHi from a muscle exposed to first lactic acid, then adrenaline and finally to both lactic acid and adrenaline. Bars indicate time of presence of the indicated compounds. In the last experiment, lactic acid was added 4 min before the addition of adrenaline. Between each treatment, muscles were washed 3 times in control buffer with 11 mm K+. B, change in pHi (mean ±s.e.m., n = 4–7) induced by addition of lactic acid, adrenaline or both lactic acid and adreline, as illustrated in A. The reduction in pHi was calculated from the difference between the lowest value for pHi obtained during 10–30 min exposure to the indicated compound and the average of the values for pHi obtained before and after the exposure.
Importance of intracellular pH and the H+ gradient for the effect of lactic acid on muscle excitability
In resting muscles, pHi is lower than extracellular pH, creating a chemical H+ gradient from inside-out. During acidosis induced by endogenous production of H+ in exercising muscles, this chemical H+ gradient is normally increased (Street et al. 2001; Juel et al. 2004; Lindinger et al. 2005). Since this reduction in the chemical gradient for H+ lowers the inward electrochemical gradient for the ion, this is likely to facillitate the maintenace of intracellular pH homeostasis. In contrast to this, addition of 20 mm lactic acid to resting muscles resulted in a decrease in the chemical H+ gradient (Nielsen et al. 2001), which will increase the inward electrochemical gradient for the ion. Based on this difference, Kristensen et al. (2005) argued that the increase in K+ tolerance induced by addition of lactic acid to resting muscles is not a general mechanism that is active during normal muscle activity. We therefore evaluated the role of intracellular pH and the importance of the H+ gradient for the effect on force of lactic acid on the K+ tolerance of the muscles by examining the effect of specifically reducing intra- or extracellular pH.
Reduction in pHi
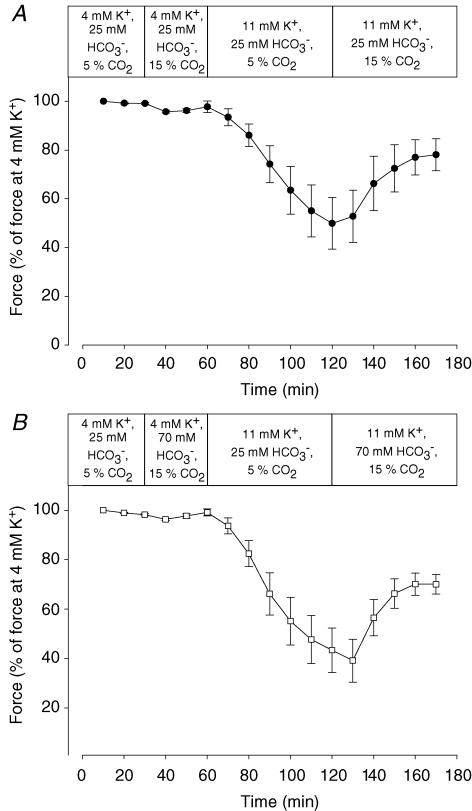
A decrease in pHi was obtained by increasing CO2 from 5 to 15% while at the same time increasing the concentration of buffer HCO3− from 25 to 70 mm. Table 2 shows that when this was done, extracellular pH remained unchanged but pHi was reduced by 0.21 units (P < 0.04). The effect of these changes in CO2 and HCO3− on force production is shown in Fig. 6B. In muscles at 4 mm K+, these changes had very little effect on tetanic force. In muscles depressed by exposure to 11 mm K+, however, the changes led to a significant recovery of force. Importantly, the recovery of force was very similar to the recovery of force induced when CO2 was increased from 5 to 15% at a constant HCO3− concentration of 25 mm (Fig. 6A), which in addition to a reduction of pHi of 0.22 units (P < 0.04) also led to a reduction of extracellular pH by 0.48 units (P < 0.001, Table 2).
Table 2.
Effect of CO2, HCO3− and HCl on intra- and extracellular pH
| Condition | pHo | ΔpHo relative to control | pHi | ΔpHi relative to control |
|---|---|---|---|---|
| 5% CO2 + 25 mm HCO3− | 7.44 ± 0.01 (3) | — | 7.16 ± 0.07 (4) | — |
| 15% CO2 + 25 mm HCO3− | 6.96 ± 0.01 (3) | 0.48 ± 0.01 | 6.94 ± 0.04 (4) | 0.22 ± 0.05 |
| 15% CO2 + 70 mm HCO3− | 7.44 ± 0.01 (3) | −0.01 ± 0.01 | 6.95 ± 0.04 (4) | 0.21 ± 0.04 |
| 5% CO2 + 25 mm HCO3− | 7.40 ± 0.01 (3) | — | 7.17 ± 0.05 (6) | — |
| + 20 mm HCl | 6.72 ± 0.01 (2) | 0.68 ± 0.01 | 7.09 ± 0.05 (6) | 0.08 ± 0.03 |
Effect on pHi in muscles incubated at 11 mm K+ and increasing CO2 from 5 to 15% with or without a simultaneous increased in HCO3− from 25 to 70 mm. Buffers with 70 mm HCO3− were made by substituting 45 mm of NaCl with 45 mm NaHCO3−. Since this reduced the buffer Cl− concentration by 45 mm, 45 mm NaCl was replaced by 45 mm sodium methanesulphate in the control buffers with only 25 mm NaHCO3− whereby the concentration of buffer Cl− was kept constant throughout all the experiments. ( ) indicates n-values.
Figure 6. Effects of increased HCO3− and CO2 on force in muscles depressed by high K+.
Effect on tetanic force in muscles incubated at 4 or 11 mm K+ of increasing CO2 from 5 to 15% with (A) or without (B) a simultaneous increased in HCO3− from 25 to 70 mm. Tetanic contractions were elicited every 10 min by applying 2 s trains of pulses at 30 Hz. Muscles were incubated in KR buffer with the modifications indicated in the bars. Buffers with 70 mm HCO3− were made by substituting 45 mm of NaCl with 45 mm NaHCO3−. Since this reduced the buffer Cl− concentration by 45 mm, 45 mm NaCl was replaced with 45 mm of sodium methanesulphonate in the control buffers with only 25 mm NaHCO3− whereby the concentration of buffer Cl− was kept constant throughout all the experiments.
To examine whether the recovery of force induced by a decrease in pHi was related to an increase in the activity of the Na+–K+ pump, the ouabain-sensitive 86Rb+ uptake rate and the intracellular Na+ content were measured in control muscles incubated in KR buffer with 25 mm HCO3− and 5% CO2 and compared with values obtained from muscles incubated KR buffer with 70 mm HCO3− and 15% CO2. In pairs of contralateral muscles pre-incubated in these buffers for 10 min, the Na+ content of the control muscles was 11.3 ± 0.3 μmol (g wet wt)−1 and the ouabain-sensitive 86Rb+ uptake rate was 261 ± 17 nmol (g wet wt)−1 min−1. In muscles at low pH, the Na+ content was sligthly lower (10.2 ± 0.4 μmol (g wet wt)−1, n = 6, P = 0.05) whereas the ouabain-sensitive 86Rb+ uptake rate was the same (251 ± 16 nmol (g wet wt)−1 min−1, n = 6, P = 0.66). After 50 min of pre-incubation, the Na+ content of control muscles was decreased to 8.2 μmol (g wet wt)−1 which was not significantly different from the Na+ content of muscles with low intracellular pH (8.3 μmol (g wet wt)−1). In these muscles, the ouabain-sensitive 86Rb+ uptake rate was 292 nmol (g wet wt)−1 min−1 (n = 6) in control muscles and slightly lower in muscles at low pH (198 nmol (g wet wt)−1 min−1, n = 6).
It is well accepted that changes in cell volume can affect the force production of muscle fibres. Since fibre volume is sensitive to intracellular acidification (Fraser et al. 2005; Usher-Smith et al. 2006), we determined the intracellular water content of the muscle fibres acidified by incubation in KR buffer with 70 mm HCO3− and 15% CO2. After 50 min of incubation, the cellular water content of control muscles was 2.72 ± 0.04 g water (g dry wt)−1 whereas the water content of acidified muscles was 2.99 ± 0.02 g water (g dry wt)−1, corresponding to a 7% increase in volume. In another series of experiments, in which muscles force was depressed to 31 ± 9% of control force by incubation at 11 mm K+, subsequent incubation for 50 min in a buffer where osmolarity was reduced by 7% by withdrawal of 21.2 mm sucrose, completely failed to improve force production (data not shown).
Reduction in extracellular pH
To reduce extracellular pH, 20 mm HCl was added to the buffer. As shown in Table 2, this reduced extracellular pH by 0.68 units but had only a small and insignificant effect on intracellular pH (P = 0.07). In muscles depressed by 11 mm K+, addition of 20 mm HCl only led to a small and transient recovery of force and M-waves with a maximum effect after 20 min (tetanic force increased from 16 ± 3 to 37 ± 9%, P = 0.06, and M-wave area from 17 ± 5 to 31 ± 11%, P = 0.29, of the control levels). Recently, Pedersen et al. (2005) concluded that the large force recovery obtained when both intra- and extracellular pH were reduced by increased CO2, was caused by a reduction in the total membrane conductance (Gm) of the muscle fibres, which specifically was caused by a reduction in fibre Cl− conductance. We examined therefore whether the reduction in extracellular pH by addition of HCl was without effect on Gm, as suggested by the almost complete absence of force recovery. Table 3 shows that in muscles at 11 mm K+ the addition of 20 mm HCl was without significant effect on Gm whereas an increase of CO2 to 24% reduced Gm by 35%. Addition of HCl did, however, cause a small but significant hyperpolarization of the muscle fibres (Table 3).
Table 3.
Effect of pHo on membrane conductance of muscle fibres at 11 mM K+
| [K+]o (mm) | pHo | Vm (mV) | Gm (μS cm−2) | n (muscles/fibres) |
|---|---|---|---|---|
| 11 | 7.4 | −51.7 ± 0.4# | 2136 ± 150# | 10/23 |
| 11 | 6.8 (20 mm HCl) | −53.2 ± 0.4* | 2365 ± 119 | 6/15 |
| 11 | 6.8 (24% CO2) | −52.1 ± 0.4# | 1393 ± 56†# | 7/29 |
Hyperpolarizing current pulses of 75 ms duration were injected through the current electrode, and the voltage electrode recorded the membrane responses at three to five locations in each fibre. ΔVm/I ratios were plotted on a log scale against inter-electrode distance and fitted to a two-parameter exponentially decaying function, giving a straight line. The slope of the line was used to calculate the length constant (λ) and the ordinate intercept gave the input resistance, Rin (Fig. 2). Conductance was calculated from λ and Rin according to Boyd & Martin (1959).
Data reproduced from Pedersen et al. (2005) and were made with similar technique as the HCl data. n is muscles/fibres used in each group. Values are means ±s.e.m.
P < 0.05 (significantly different from corresponding value at the same [K+]o and normal pH).
Discussion
Additive effects of lactic acid and adrenaline on force and excitability
This study shows that in the range from 5 to 26 mm, addition of lactic acid causes a dose-dependent recovery of force in muscles at high [K+]o, with a tendency for the force recovery to become lower if the concentration of lactic acid was increased to above 24 mm (Fig. 1). Figure 3 shows that at 20 mm lactic acid the effect on force corresponded to an increase in the K+ tolerance of the muscles by 1.9 mm (IC50). The reduction in the protective effect at lactic acid concentrations above 24 mm indicates that at these concentrations the adverse effects of acidification were beginning to overwhelm the protective ones. Measurements of M-waves (Fig. 4) clearly showed that the recovery of force was related to a recovery of muscle excitability. These results agree with previously reported effects of acidosis on excitability in muscles at elevated [K+]o (Nielsen et al. 2001; Pedersen et al. 2005), and indicate that acidosis may provide muscles with some protection against the possible loss of excitability caused by the increase in extracellular K+ during strenuous exercise.
Several studies have shown that a similar protection of excitability can be induced by stimulation of the Na+–K+ pump via β2-adrenergic agonists. Since this may indicate that the exposure of muscles to circulating catecholamines during exercise makes the muscles even more tolerant to elevated [K+]o, it was important to determine if the effects of acidosis and catecholamines on the function of muscles at elevated [K+]o were additive. Pedersen et al. 2003) demonstrated that the addition of 10 mm lactic acid in combination with the β2-agonist salbutamol produces a larger recovery of force in muscles incubated at high [K+]o than the addition of 10 mm lactic acid alone. Since, however, 10 mm lactic acid was submaximal with respect to recovery of force at elevated [K+]o (Fig. 1), it was not possible from these experiments to firmly establish whether the effects of the two compounds were additive. In agreement with this, it was argued by Kristensen et al. (2005) that the effect of lactic acid on force was unlikely to be seen in muscles where the Na+–K+ pump was already stimulated. Figure 2 in the present study shows, however, that when lactic acid and adrenaline were used at the concentrations that produced the largest force recovery, the effect on force was almost twice as large when the compounds were added together than when added individually. Similar results were obtained when the effects of lactic acid and adrenaline on muscle excitability was examined (Fig. 4). Together these findings show that the effects of lactic acid and β2-adrenergic agonists on force and excitability in muscles at elevated [K+]o are fully additive. This conclusion is strongly supported by the observation that the two compounds caused their effects via two distinct mechanisms. Thus, the addition of adrenaline had no effect on either intra- or extracellular pH (Fig. 5) but in agreement with previous studies examining the effect of β2-agonists on muscle (for review see Clausen, 2003), the activity of muscle Na+–K+ pumps was increased as judged from the increase in the ouabain-sensitive 86Rb+ uptake. The increased Na+–K+ pump activity was associated with a decrease in the intracellular Na+ and a hyperpolarization of 3.8 mV, which most probably improve muscle excitability via the ensuing increase in the electrochemical gradient for Na+ and the relief of some of the inactivation of Na+ channels (Ruff, 1997; Ruff et al. 1988; Rich & Pinter, 2003). In contrast, acidification of the muscles by addition of lactic acid had no effect on the ouabain-sensitive 86Rb+ uptake, the intracellular Na+ content or the membrane potential. Likewise, when only intracellular pH was reduced by increasing CO2 to 15% in combination with 70 mm HCO3−, there was no increase in the ouabain-sensitive 86Rb+ uptake and only a very small reduction in intracellular Na+ content after 10 min, which could not be detected after 50 min of incubation. Together, these results demonstrate that the Na+–K+ pump was not involved in the effect of low pH on muscle excitability. Both lactic acid and increased CO2, however, caused a decrease in pHi, which previously has been shown to convey at least part of the effect of lactic acid on muscle excitability (Nielsen et al. 2001; Kristensen et al. 2005).
Mechanisms for the effect of acidosis on muscle excitability
In a previous study on muscles at elevated [K+]o, we showed that a recovery of force similar to the recovery induced by addition of lactic acid could be obtained by addition of propionic acid or increased CO2. This indicates that the effect of lactic acid is related to an acidification of the muscle rather than to an effect of the lactate ion per se (Nielsen et al. 2001). Since addition of propionic acid, increased CO2 and lactic acid all reduce both the intra- and extracellular pH it was, therefore, not possible from these experiments to conclude whether the effect on force was related to the intra- or extracellular acidification. Another problem with these experiments was that the addition of the acids almost completely abolished the chemical H+ gradient of the muscle fibres (Nielsen et al. 2001). Since this is in contrast to the change in the H+ gradients seen in fatiguing muscle (Juel et al. 2004), this led Kristensen et al. (2005) to suggest that the protective effect of lactic acidosis against the depressing effect of increased K+ is not a general mechanism. The present study shows, however, that when a reduction in pH was induced specifically in the intracellular compartment by combining an increase in CO2 to 15% with an increase in extracellular HCO3− to 70 mm, it led to a recovery of force that was similar to the recovery induced by 15% CO2 at normal buffer HCO3− levels where both intra- and extracellular pH were reduced. These observations show that the recovery of force induced by lowered pH was not related to a decrease in the chemical H+ gradient, but was rather caused by the reduction in pHi. This conclusion is supported by a study on mechanically skinned extensor digitorum longus fibres of the rat which showed a similar enhancement of T-tubular excitability in depolarized fibres with intracellular acidosis (Pedersen et al. 2004). Moreover, in agreement with Kristensen et al. 2005), the addition of HCl to muscles at high [K+]o only produced an insignificant and transient recovery of force and M-wave area. In contrast to the effect of increased CO2 (Pedersen et al. 2005), HCl did not lead to a reduction in the Gm of the muscle fibres (Table 3). Since addition of HCl led to a large reduction in pHo without changing pHi, this demonstrates that a reduction of the chemical H+ gradient is without effect on muscle excitability and that such a mechanism therefore does not contribute to the recovery of muscle function seen after addition of lactic acid or increased CO2. The transient and insignificant recovery of force produced by addition of HCl could be related to the small hyperpolarization (Table 3), which most likely arises from the transient change in the chemical Cl− gradient when HCl was added (Hodgkin & Horowicz, 1957).
Previously it has been shown that the hyperosmotic addition of lactate to buffers or intracellular acidification can cause a reduction in fibre volume while these manoeuvres have very little effect on the membrane potential of the muscles (Fraser et al. 2005; Usher-Smith et al. 2006). In agreement with this, we observed no change in the membrane potential when an intracellular acidification was induced by increasing lactic acid or by increasing CO2. However, when an intracellular acidification was induced by increased CO2, we found a 7% increase in fibre volume. Since such an increase in fibre volume may increase maximal force production because of the decreased mechanical friction within the fibre, the effect on force of exposing muscles to hypotonic buffer was examined. These experiments failed, however, to show any recovery of force with decreased buffer osmolarity.
Role of intracellular lactic acidosis and circulating catecholamines in maintenance muscle excitability during exercise
Skeletal muscle fatigue, where the force-generating ability of the muscle is compromised, is a complex phenomenon that, depending on the type of work, may be caused by failure at multiple sites in the activation and force generation steps involved in muscle contractions. Based on a comparison of the elevation in extracellular K+ during exercise with the effect of elevated K+ on the function of isolated, resting muscle it has been argued that loss of excitability caused by accumulation of extracellular K+ contributes to these fatigue mechanisms during intense exercise. The present study shows that intracellular acidosis and β2-adrenergic stimulation elicits additive protective effects on the excitability of resting muscles against the depressing effects of elevated [K+]o.
When added exogenously in the present study, 20 mm lactic acid reduced intracellular pH to 6.8, which is comparable to the pH values measured in working muscle after intense exercise (for review, see Cairns, 2006). The concomitant intracellular accumulation of lactate was not determined but assuming a 1: 1 entry of lactate and protons (Juel) and an intracellular buffer capacity of 33 μmol pH−1 (kg wet wt)−1 (Sahlin & Henriksson, 1984), the reduction in intracellular pH from 7.2 to 6.8 corresponds to an increase in the intracellular concentrations of lactate of 13.2 mm. This value is far lower than the up to 40 mm that has been measured in the muscle fibres in human subjects during intense exercise. This suggests that the protective mechanism on excitability induced in resting muscles by the addition of lactic acid or by increasing CO2 is likely to be activated during exercise. Together with the similar effect of catecholamines, this effect should therefore be taken into account when the role of elevated K+ in muscle fatigue is evaluated from data on isolated muscles.
Acknowledgments
This study was supported by The Danish Medical Research Council (22-01-0096 and 22-02-0188). The technical assistance of Marianne Stürup-Johansen, Tove Lindahl Andersen and Vibeke Uhre are gratefully acknowledged. Also we thank Professor Christian Aalkjær and Dr John Flatman for technical assistance and the use of their equipment.
References
- Albuquerque EX, Thesleff S. A comparative study of membrane properties of innervated and chronically denervated fast and slow skeletal muscles of the rat. Acta Physiol Scand. 1968;73:471–480. doi: 10.1111/j.1365-201x.1968.tb10886.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Clausen T. Calcitonin gene-related peptide stimulates active Na+-K+ transport in rat soleus muscle. Am J Physiol. 1993;268:C1528–C1536. doi: 10.1152/ajpcell.1993.264.2.C419. [DOI] [PubMed] [Google Scholar]
- Bouclin R, Charbonneau E, Renaud JM. Na+ and K+ effect on contractility of frog sartorius muscle: implication for the mechanism of fatigue. Am J Physiol. 1995;268:C1528–C1536. doi: 10.1152/ajpcell.1995.268.6.C1528. [DOI] [PubMed] [Google Scholar]
- Boyd IA, Martin AR. Membrane constants of mammalian fibres. J Physiol. 1959;147:450–457. doi: 10.1113/jphysiol.1959.sp006255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan R, Nielsen OB, Clausen T. Excitation- and β2-agonist-induced activation of the Na+-K+ pump in rat soleus muscle. J Physiol. 2002;545:229–240. doi: 10.1113/jphysiol.2002.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns SP. Lactic acid and exercise performance – Culprit or friend? Sports Med. 2006;36:279–291. doi: 10.2165/00007256-200636040-00001. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Flatman JA, Clausen T. Relation between extracellular [K+]o on membrane potential and contraction in rat soleus muscle: modulation by Na+-K+ pump. Pflugers Arch. 1995;430:909–915. doi: 10.1007/BF01837404. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Hing WA, Slack JR, Mills RG, Loiselle DS. Different effects of raised [K+]o on membrane potential and contraction in mouse fast- and slow-twitch muscle. Am J Physiol. 1997;273:C598–C611. doi: 10.1152/ajpcell.1997.273.2.C598. [DOI] [PubMed] [Google Scholar]
- Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- Clausen T, Andersen SL, Flatman JA. Na+–K+ pump stimulation elicits recovery of contractility in K+-paralysed rat muscle. J Physiol. 1993;472:521–536. doi: 10.1113/jphysiol.1993.sp019960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Flatman JA. The effect of catecholamines on Na–K transport and membrane potential in rat soleus muscle. J Physiol. 1977;270:383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paoli F, Overgaard K, Nielsen OB. Protective effects of acidosis and Na+,K+-pump activations on force in K+-depressed skeletal muscle. J Physiol. 2002;544:82P. [Google Scholar]
- Everts ME, Clausen T. Activation of the Na-K pump by intracellular Na in rat slow- and fast-twitch muscle. Acta Physiol Scand. 1992;145:353–362. doi: 10.1111/j.1748-1716.1992.tb09375.x. [DOI] [PubMed] [Google Scholar]
- Fraser JA, Middlebrook CE, Usher–Smith JA, Schwiening CJ, Huang CL. The effect of intracellular acidification on the relationship between cell volumes and membrane potential in amphibian skeletal muscle. J Physiol. 2005;563:745–764. doi: 10.1113/jphysiol.2004.079657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Wasserstrom JA, Rosenthal JE. Effect of catecholamines on intracellular pH in sheep cardiac Purkinje fibres. J Physiol. 1992;458:289–306. doi: 10.1113/jphysiol.1992.sp019418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, Hororwicz Effects of K and Cl on the membrane potential of isolated muscle fibres. J Physiol. 1957;137:30P. [PubMed] [Google Scholar]
- Juel C. Lactate-proton cotransport in skeletal muscle. Physiol Rev. 1997;77:321–358. doi: 10.1152/physrev.1997.77.2.321. [DOI] [PubMed] [Google Scholar]
- Juel C, Klarskov C, Nielsen JJ, Krustrup P, Mohr M, Bangsbo J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E245–E251. doi: 10.1152/ajpendo.00303.2003. [DOI] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Comp Physiol. 2000;278:R400–R406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Kristensen M, Albertsen J, Rentsch M, Juel C. Lactate and force production in skeletal muscle. J Physiol. 2005;562:521–526. doi: 10.1113/jphysiol.2004.078014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson G, Bangsbo J, Juel C. Point: Counterpoint: Lactic acid accumulation is an advantage/disadvantage during muscle activity. J Appl Physiol. 2006;100:1410–1412. doi: 10.1152/japplphysiol.00023.2006. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, Kowalchuk JM, Heigenhauser GJF. Applying physicochemical principles to skeletal muscle acid-base status. Am J Physiol Regul Comp Physiol. 2005;289:R891–R894. doi: 10.1152/ajpregu.00225.2005. [DOI] [PubMed] [Google Scholar]
- Mohr M, Nordsborg N, Nielsen JJ, Pedersen LD, Fischer C, Krustrup P, Bangsbo J. Potassium kinetics in human muscle interstitium during repeated intense exercise in relation to fatigue. Pflugers Arch. 2004;448:452–456. doi: 10.1007/s00424-004-1257-6. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, Clausen T. The Na+/K+-pump protects muscle excitability and contractility during exercise. Exerc Sports Sci Rev. 2000;28:159–164. [PubMed] [Google Scholar]
- Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, Ortenblad N, Lamb GD, Stephenson DG. Excitability of the T-tubular system in rat skeletal muscle: roles of K+ and Na+ gradients and Na+–K+ pump activity. J Physiol. 2004;557:133–146. doi: 10.1113/jphysiol.2003.059014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J. Muscle interstitial potassium kinetics during intense exhaustive exercise: effects of previous arm exercise. Am J Physiologi Regul Integr Comp Physiol. 2003;285:R143–R148. doi: 10.1152/ajpregu.00029.2003. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Nielsen OB. Activity-induced recovery of excitability in K+-depressed rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2001;280:R48–R55. doi: 10.1152/ajpregu.2001.280.1.R48. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Nielsen OB, Flatman JA, Clausen T. Relations between excitability and contractility in rat soleus muscle: role of the Na+–K+ pump and Na+/K+ gradient. J Physiol. 1999;518:215–225. doi: 10.1111/j.1469-7793.1999.0215r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Clausen T, Nielsen OB. Loss of force induced by high extracellular [K+] in rat muscle: effect of temperature, lactic acid and β2-agonist. J Physiol. 2003;551:277–283. doi: 10.1113/jphysiol.2003.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, de Paoli F, Nielsen OB. Increased excitability of acidified skeletal muscle: role of chloride conductance. J Gen Physil. 2005;25:237–246. doi: 10.1085/jgp.200409173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- Rich MM, Pinter MJ. Crucial role of sodium channel fast inactivation in muscle fibre inexcitability in a rat model of critical illness myopathy. J Physiol. 2003;547:555–566. doi: 10.1113/jphysiol.2002.035188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL. Sodium channel regulation of skeletal muscle membrane excitability. Ann N Y Acad Sci. 1997;835:64–76. doi: 10.1111/j.1749-6632.1997.tb48618.x. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Simoncini L, Stühmer W. Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle Nerve. 1988;11:502–510. doi: 10.1002/mus.880110514. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Henriksson J. Buffer capacity and lactate accumulation in skeletal muscle of trained and untrained men. Acta Physiol Scand. 1984;122:331–339. doi: 10.1111/j.1748-1716.1984.tb07517.x. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Street D, Bangsbo J, Juel C. Interstitial pH in human skeletal mscle during and after dynamic graded exercise. J Physiol. 2001;537:993–998. doi: 10.1111/j.1469-7793.2001.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street D, Nielsen JJ, Bangsbo J, Juel C. Metabolic alkalosis reduces exercise-induced acidosis and potassium accumulation in human skeletal muscle interstitium. J Physiol. 2005;566:481–489. doi: 10.1113/jphysiol.2005.086801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochem. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Usher-Smith JA, Fraser JA, Bailey PS, Griffin JL, Huang CL. The influence of intracellular lactate and H+ on cell volume in amphibian skeletal muscle. J Physiol. 2006;573:799–818. doi: 10.1113/jphysiol.2006.108316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yensen C, Matar W, Renaud JM. K+-induced twitch potentiation is not due to longer action potential. Am J Physiol Cell Physiol. 2002;283:C169–C177. doi: 10.1152/ajpcell.00549.2001. [DOI] [PubMed] [Google Scholar]