Abstract
During the response of vertebrate olfactory receptor cells to stimulation, Ca2+ enters the cilia via cyclic nucleotide-gated channels and is extruded by Na+–Ca2+ exchange. The rise in Ca2+ concentration opens a Ca2+-activated Cl− conductance which carries most of the inward receptor current. The dependence of Ca2+ extrusion upon external Na+ concentration was studied by using the falling phase of the Ca2+-activated Cl− current following a brief exposure to the phosphodiesterase inhibitor IBMX to monitor indirectly the decay in intraciliary Ca2+ concentration. External Na+ concentration was reduced by partial substitution with guanidinium, an ion which permeates the cyclic nucleotide-gated channel but does not support Na+–Ca2+ exchange. The time constant describing the decay in current following IBMX stimulation was surprisingly little affected by substitution of external Na+, being substantially retarded only when its concentration was reduced to a third or less of its normal value in Ringer solution. When the cilia were returned to Ringer solution after a period in reduced-Na+ solution, the time constant for the final decay of current was similar to that seen when returning immediately to IBMX-free Ringer solution. This observation suggests that Ca2+ extrusion via Na+–Ca2+ exchange dominates the falling phase of the response to IBMX, which can therefore be used to assess exchanger activity. Rate constants derived from the time constants for current decay at different external Na+ concentrations could be fitted by the Hill equation with a Kd of 54 ± 4 mm and Hill coefficient of 3.7 ± 0.4. The cooperativity of the dependence upon external Na+ concentration indicates that at least three Na+ ions enter for each exchanger cycle, while the high affinity for external Na+ contrasts with the photoreceptor and cardiac exchangers. The functional importance of this observation is that the relative insensitivity of the Na+–Ca2+ exchanger to external Na+ concentration allows normal response termination even following partial dilution or concentration of the olfactory mucus.
When a vertebrate olfactory receptor cell is exposed to odour, the G protein-coupled transduction cascade stimulates production of cAMP by adenylyl cyclase (reviewed by Schild & Restrepo, 1998; Firestein, 2001; Zufall & Munger, 2001), opening cyclic nucleotide-gated channels and allowing an influx of Ca2+ (Frings & Lindemann, 1991; Leinders-Zufall et al. 1997). The rise in Ca2+ concentration within the cilia opens a Ca2+-activated Cl− conductance which greatly augments the inward receptor current (Kleene & Gesteland, 1991; Kurahashi & Yau, 1993; Kleene, 1997). The Ca2+ which enters during stimulation is principally extruded by Na+–Ca2+ exchange, which lowers the Ca2+ concentration allowing closure of Ca2+-activated Cl− channels and termination of the response (Reisert & Matthews, 1998).
While the importance of Na+–Ca2+ exchange for the control of response dynamics and sensitivity has been clearly demonstrated in the olfactory receptors of both amphibia (Reisert & Matthews, 1999, 2001b,c) and mammals (Reisert & Matthews, 2001a), little is known about the detailed properties of the olfactory exchanger itself. The driving gradient for Ca2+ extrusion depends crucially on the Na+ concentration in the mucus surrounding the olfactory cilia. However, especially in an amphibious species the mucus composition is potentially under threat from dilution by water thereby decreasing ionic concentrations, while mucus desiccation and an increase in ionic strength are possible in terrestrial species. Cholinergic stimulation has been shown to decrease the concentration of Na+ in mouse tracheal surface fluid and increase its concentration in nasal fluid (Kozlova et al. 2005). In contrast, tracheal fluid Na+ concentration is increased in cystic fibrosis (Kozlova et al. 2005). If the resulting changes in Na+ gradient were to affect substantially the rate of exchange, changes in response kinetics might ensue. We have therefore investigated the effect of extracellular Na+ concentration on the extrusion of Ca2+ in frog olfactory receptors using the suction pipette technique. Our results demonstrate that the olfactory Na+–Ca2+ exchanger is only modestly affected until external Na+ concentration is reduced substantially below the normal value in Ringer solution, in contrast to the photoreceptor and cardiac exchangers. This high affinity for external Na+ defends the kinetics of olfactory response recovery against changes in Na+ concentration in the olfactory mucus. Preliminary results from this study have been reported to the Association for Chemoreception Sciences (Antolin & Matthews, 2006).
Methods
Preparation
Isolated olfactory receptor cells were obtained as previously described (Reisert & Matthews, 1998, 1999). Frogs (Rana temporaria) were killed according to Schedule 1 of the Animals (Scientific Procedures) Act 1986 by stunning by cranial concussion followed by rostral and caudal pithing. The olfactory epithelia were dissected and placed receptor side up on a layer of cured silicone rubber (Sylgard 184, Dow Corning, Wiesbaden, Germany) in a Petri dish filled with Ringer solution. Olfactory receptor cells were isolated mechanically by lightly cutting the olfactory epithelium with a piece of razor blade. The resulting cell suspension was collected with a 200 μl pipette, triturated several times in a microcentrifuge tube, and transferred to the recording chamber on the stage of an inverted microscope with phase contrast optics (Nikon TMS; Nikon Ltd, Kingston, UK). Cells were allowed to settle on the floor of the recording chamber for 30 min before bath perfusion commenced.
Suction pipette recording
Receptor current responses to stimulation were recorded using the suction pipette technique as previously described (Reisert & Matthews, 1998, 1999). Typical suction pipette diameter ranged from 5 to 7 μm, corresponding to an open pipette resistance of 1–2 MΩ. The cell body of an isolated olfactory receptor cell was drawn into the suction pipette leaving the cilia exposed to the superfusing solution, elevating the seal resistance at the pipette tip (2–7 MΩReisert & Matthews, 1999) by at least 2–3-fold (Reisert & Matthews, 2001c), and allowing the receptor current to be recorded. The suction pipette current was recorded with a patch clamp amplifier (Warner PC-501A, Warner Instruments, Hamden, CT, USA) and digitized over a relatively low bandwidth (filtered DC–50 Hz, sampled at 200 Hz) by a PC equipped with an intelligent interface card (Cambridge Research Systems, Rochester, UK) in order to analyse the receptor current. Traces are plotted according to the convention that current flowing into the suction pipette is of negative sign, representing the inward receptor current flowing across the ciliary membrane.
Solutions and solution changes
Amphibian Ringer solution contained 111 mm NaCl, 2.5 mm KCl, 1.6 mm MgCl2, 1 mm CaCl2, 3 mm Hepes, 10 mm glucose, and its pH was adjusted to 7.7 with NaOH. The solution also included 0.01 mm EDTA to chelate impurity heavy metal ions (Lamb et al. 1986). Reduced Na+ solutions were prepared by partial substitution of guanidinium chloride for NaCl; the pH was adjusted to 7.7 with approximately 1.7 mm TMAOH. The phosphodiesterase inhibitor 3-isobutyl-1 methylxanthine (IBMX; Sigma, Gillingham, UK) was dissolved in Ringer solution when required at the concentrations indicated in the text. Solutions with elevated Na+ concentration were prepared by addition of Na-gluconate (see Fig. 6).
Figure 6. Dependence of time constant for receptor current recovery on external [Na+].
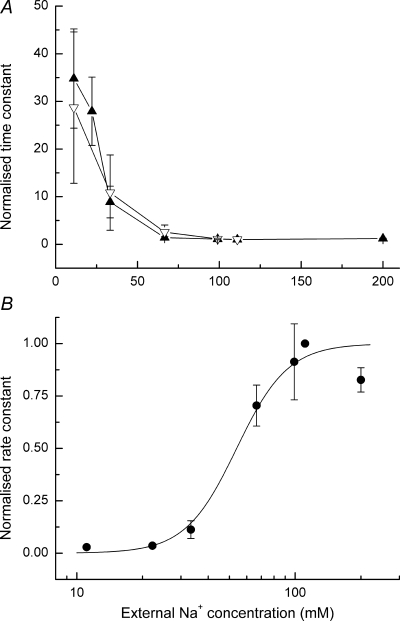
A, mean time constants for single exponential curves fitted to receptor current recovery in solutions with modified Na+ concentration following IBMX exposures of 1 s (▴, as in Fig. 4) or 2 s (▿) duration. Data have been normalized for each cell to the time constant measured in normal Ringer (111 mm[Na+]). Data points represent the means of 3–18 cells; error bars denote standard errors of the mean. B, rate constants, calculated as the reciprocal of the mean time constants for the 1 s IBMX exposures in A, normalized to the rate constant in normal Ringer solution, which was measured in each cell. Fitted curve represents the Hill equation with Kd= 62.3 mm and n = 3.24, with minimum rate constrained to zero and a maximum normalized rate of 1.15, fitted by a weighted Marquardt–Levenberg least squares algorithm.
Rapid solution changes were carried out by stepping the interface between parallel streams of flowing solution across the tip of the suction pipette under computer control using a miniature stepper motor (Reisert & Matthews, 1998). This will have served to rapidly exchange the solution bathing the exposed cilia; in contrast the cell body will have remained throughout in contact with the Ringer solution filling the suction pipette. Solutions were delivered by gravity, and selected by inert 6-way rotary valves (Rheodyne, Cotati, CA, USA). When changing to a solution of modified ionic composition, a liquid junction potential was set up between pipette and bath, causing a junction current to be added to the receptor current recorded by the suction pipette. This junction current artefact is illustrated in Fig. 1 (Uncorrected) for an olfactory receptor cell which was first stimulated with 500 μm of the phosphodiesterase inhibitor IBMX (see Results), then exposed for 1 s to an IBMX-free solution in which the Na+ concentration was reduced to 33 mm, and finally returned to normal Ringer. The resulting junction current was measured in isolation by exposure of the olfactory receptor cell to an equivalent solution change in the absence of IBMX stimulation (Fig. 1, Junction). The receptor current could then be obtained by subtraction of these two traces (Fig. 1, Corrected). The actual timings of solution changes were determined from the delay to 50% rise of the junction current artefact. The time taken for the solution change was assessed from the 10–90% rise time for the junction current, which typically fell within the range 50–90 ms.
Figure 1. Procedure for junction current subtraction.
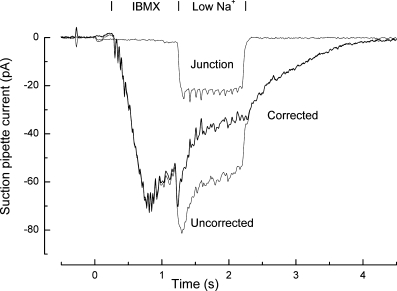
Suction pipette recordings from an isolated olfactory receptor cell in response to 1 s stimulation with Ringer solution containing 500 μm IBMX. Following IBMX stimulation the cilia were returned to Ringer solution either immediately (A) or after 1 s exposure to a solution in which the Na+ concentration had been reduced to 33 mm (low Na+) by equimolar substitution with guanidinium (B). Each trace is the average of 2 or 3 trials.
Results
The extrusion of Ca2+ by Na+–Ca2+ exchange was studied using the approach illustrated in Fig. 2A. First the cilia were exposed for 1 s to 500 μm of the phosphodiesterase inhibitor IBMX, which elevates the level of cAMP by slowing its hydrolysis, and leads to the opening of the cyclic nucleotide-gated channels (Frings & Lindemann, 1991). Then the cilia were returned to IBMX-free solution, leading to a decay in the receptor current with monoexponential kinetics. During IBMX stimulation Ca2+ entering through the cyclic nucleotide-gated channels will have opened Ca2+-activated Cl− channels, which will have carried the majority of the receptor current at the time of withdrawal of IBMX (Kleene & Gesteland, 1991; Kurahashi & Yau, 1993). This notion was tested in the experiments of Fig. 2B–D by instead returning the cilia to an IBMX-free solution with a reduced Na+ concentration for 1, 2 or 3 s before the final return to normal Ringer solution. External Na+ was reduced to 33 mm by partial replacement with guanidinium. This ion permeates cyclic nucleotide-gated channels in rod photoreceptors with a permeability similar to that of sodium but does not support Na+–Ca2+ exchange (Fain et al. 1989); we have assumed that it displays similar properties with respect to the cyclic nucleotide-gated channel and Na+–Ca2+ exchanger of olfactory receptor cells. This solution was designed partially to inhibit Na+–Ca2+ exchange by reducing the driving Na+ gradient, and thereby to maintain the intraciliary Ca2+ concentration at an elevated level until the external Na+ concentration was restored. This manipulation delayed the final decay of receptor current until the cilia were returned to normal Ringer solution; however, the time constant of the final relaxation was little affected. Since cAMP levels will have fallen rapidly following the withdrawal of IBMX due to a high basal phosphodiesterase activity (Borisy et al. 1992), leading to rapid closure of the cyclic nucleotide-gated conductance, the invariant decay following the subsequent restoration of external Na+ is likely instead to reflect predominantly the fall in Ca2+ concentration due to its extrusion by Na+–Ca2+ exchange, leading to the progressive closure of Ca2+-activated Cl− channels. In contrast, the initial current decay seen during exposure to the sodium-containing IBMX solution will have reflected the combined effects of Ca2+ extrusion by the exchanger, and suppression of further Ca2+ entry by progressive Ca2+-dependent inhibition of the cyclic nucleotide gated channel.
Figure 2. The time constant for receptor current recovery reflects Ca2+ extrusion by Na+–Ca2+ exchange.
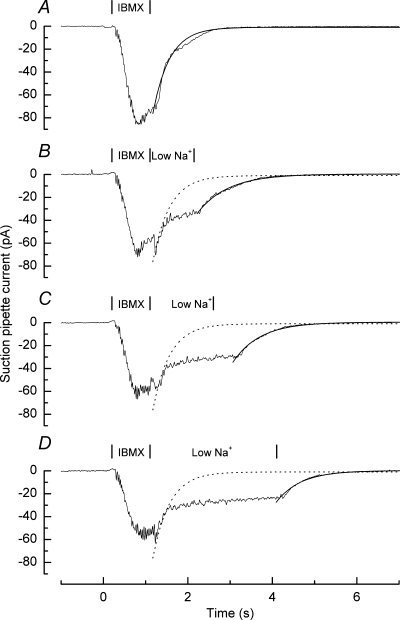
Suction pipette recordings from an isolated olfactory receptor cell in response to 1 s stimulation with Ringer solution containing 500 μm IBMX. Same cell as Figure 1. Following IBMX stimulation the cilia were returned to Ringer solution either immediately (A) or following exposure for 1 s (B), 2 s (C), or 3 s (D) to a solution in which the Na+ concentration had been reduced to 33 mm (low Na+) by equimolar replacement with guanidinium. In each case the rapid decay of receptor current upon the return to Ringer solution has been fitted with a single exponential of time constant A: 0.521 s; B: 0.692 s; C: 0.561 s; D: 0.498 s. Interrupted curves in B–D reproduce the exponential fit to A for comparison. Each trace is the average of 2 or 3 trials and has been junction corrected.
The dominant role of Na+–Ca2+ exchange in determining the time course of current decay was investigated further by attempting to inhibit the exchanger directly. Although more recently a number of specific pharmacological inhibitors of the Na+–Ca2+ exchanger have become available (Iwamoto & Shigekawa, 1998; Watanabe et al. 2006), rapid and reversible block can be achieved by heavy metals such as La3+, Mn2+ or Ni2+ ions (Cervetto & McNaughton, 1986; Brommundt & Kavaler, 1987; Kimura et al. 1987). Exposure of the cilia to 5 mm Ni2+ was used to inhibit the exchanger in a protocol similar to that described above.
Figure 3 shows results from such an experiment, in which the effect of 5 mm Ni2+ on receptor current decay was examined following IBMX stimulation. As with reduced external Na+ concentration, exposure of the cilia to Ni2+ retarded response recovery until the cilia were returned to normal Ringer solution, without any substantial effect on the time constant of the subsequent current decay.
Figure 3. Receptor current recovery is retarded when Na+–Ca2+ exchange is inhibited by .
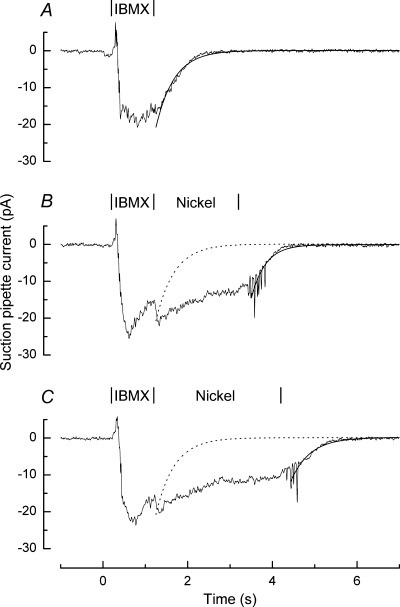
Suction pipette recordings from an isolated olfactory receptor cell in response to 1 s stimulation with Ringer solution containing 500 μm IBMX. Following IBMX stimulation the cilia were returned to Ringer either immediately (A) or following exposure for 2 s (B) or 3 s (C) to a solution which included 5 mm . In each case the rapid decay of receptor current upon the return to Ringer solution has been fitted with a single exponential of time constant A: 0.434 s; B: 0.335 s; C: 0.454 s. Interrupted curves in B and C reproduce the exponential fit to A for comparison. Each trace is the average of 2–3 trials and has been junction corrected.
Results from a number of such experiments are summarized in the bar graph of Fig. 4, which presents the time constants for current decay upon the final return to Ringer solution following IBMX stimulation from 20 cells exposed for progressively increasing durations to low Na+ solution, and 11 cells exposed to 5 mm . The data have been normalized to the time constant for current decay when the cilia were returned immediately to Ringer solution following IBMX stimulation. In both cases, when extrusion of the Ca2+ which entered during stimulation was delayed by preventing Na+–Ca2+ exchange, the time constant for current decay once this inhibition was withdrawn was little affected. The modest increase in time constant observed following 3 s Ni2+ superfusion may have reflected a slow reversal of exchanger inhibition upon this prolonged exposure to the blocker.
Figure 4. The time constant for receptor current recovery upon the return to Ringer solution is little affected by the duration of prior inhibition of Na+–Ca2+ exchange.
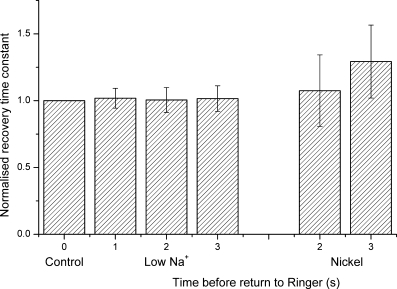
Collected data from experiments in which the cilia of an isolated olfactory receptor were exposed, following IBMX stimulation, to reduced Na+ concentration (as in Fig. 2; mean of 19–20 cells) or Ni2+ (as in Figure 3; mean of 10–11 cells) for the durations indicated before the return to Ringer solution. In each case time constants have been normalized for each cell to the value obtained upon the immediate return to Ringer solution. Error bars represent standard errors of the mean.
The ability of both Ni2+ and a reduction in external Na+ to retard the recovery of the response to IBMX without affecting the time constant of the final current decay upon the return to Ringer solution is consistent with the notion that in both cases this relaxation represented the closure of the Ca2+-activated Cl− conductance as the Ca2+ which entered during stimulation was extruded by Na+–Ca2+ exchange (Reisert & Matthews, 1998). This interpretation is reinforced by the observation that at the peak of the IBMX response in frog olfactory receptor cells 77 ± 8% of the total receptor current can be suppressed by 2 mm niflumic acid (Antolin, 2006), a saturating concentration of this specific blocker of Ca2+-activated Cl− channels. At the later time of response recovery this proportion is likely to be even larger due to a decreasing contribution to the total current by the cyclic nucleotide gated conductance. The time constant for current decay after stimulation by IBMX can therefore be used as an indirect monitor of the kinetics of the exchanger.
The dependence of Ca2+ extrusion upon external Na+ concentration was studied by using the decay of the receptor current to monitor indirectly the decay in intraciliary Ca2+ concentration following a brief exposure to IBMX. Figure 5A shows results from such an experiment in which the cilia were first exposed for 1 s to 100 μm IBMX, and then returned to an IBMX-free solution of reduced Na+ concentration. The final decay of current proved to be surprisingly little affected by partial replacement of external Na+, being substantially retarded only when its concentration was reduced to a third or less of its normal value in Ringer solution. These data have been normalized and replotted on an expanded time base in Fig. 5B. The decline in current following the reduction in Na+ concentration has been fitted by a single decaying exponential function. The time constant for the fitted exponential decay increased monotonically in a graded manner as Na+ was progressively replaced with guanidinium, being retarded for this cell by a factor of 17.4 for a 10-fold fall in [Na+].
Figure 5. Effect of external Na+ concentration on receptor current recovery.
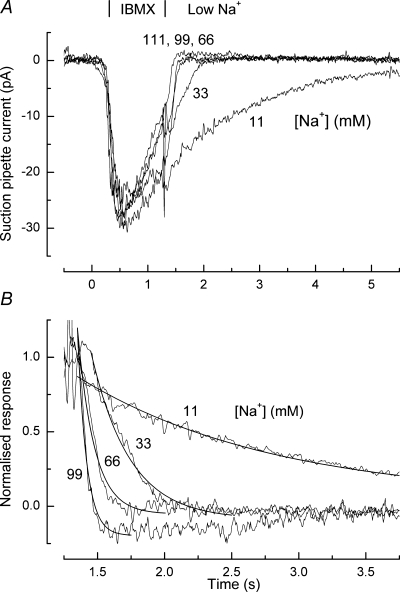
A, suction pipette recordings from an isolated olfactory receptor cell in response to 1 s stimulation with Ringer solution containing 100 μm IBMX. Following IBMX stimulation the cilia were returned either to Ringer solution, or to partially guanidinium-substituted solutions of reduced Na+ concentration as indicated beside each trace. B, final decay of receptor currents in low-Na+ solution from A, normalized to the value just following the solution change. Individual traces have been zeroed as in Fig. 5A to the initial baseline before the IBMX stimulus; note slight baseline drift. In each case the decay of receptor current has been fitted with a single exponential of time constant 99 mm: 0.067 s; 66 mm: 0.125 s; 33 mm: 0.285 s; 11 mm: 1.46 s. Time constant in normal Ringer solution 0.084 s. Each trace is the average of 2–3 trials and has been junction corrected.
Results from a number of such experiments examining the current decay after 1 s IBMX stimulation, as in Fig. 5, are collected as the filled symbols in Fig. 6A, normalized for each cell to the time constant for current decay in normal Ringer solution. Again, it can be seen that the time constant for current decay varied only weakly with external sodium concentration, slowing by only around 50% when the external sodium concentration was approximately halved. The open symbols, obtained after 2 s IBMX stimulation, show a similar dependence on external Na+, supporting again the notion that the kinetics of current decay are dominated by the rate of Ca2+ extrusion by Na+–Ca2+ exchange rather than by the transduction cascade itself. At still-lower concentrations of Na+ only incomplete relaxation of current was observed during the period of superfusion, precluding the fitting of a decaying exponential function, followed by a normal decay of the residual current upon the return to Ringer solution.
The filled symbols for 1 s IBMX stimulation have been transformed to rate constants in Fig. 6B, calculated as the reciprocal of the time constants, and normalized to the rate constant in normal Ringer solution. It can be seen that increasing the sodium concentration above that in normal Ringer solution by addition of sodium gluconate did not further elevate the rate constant for current decay. The data have been fitted with the Hill equation with a Kd of 54 ± 4 mm and Hill coefficient of 3.7 ± 0.4, yielding a characteristic sigmoidal variation with Na+ concentration when plotted in semilogarithmic coordinates. These results indicate that the olfactory exchanger possesses a relatively high affinity for Na+ at its external face.
Discussion
Calcium ions are known to play a crucial role in olfactory transduction, not only as an excitatory messenger by opening Ca2+-activated Cl− channels, but also through feedback actions on cyclic nucleotide-gated channels and other components of the transduction cascade (reviewed by Matthews & Reisert, 2003). Ca2+ enters the cilia during stimulation as the main component of the current through cyclic nucleotide-gated channels, and is believed to be extruded principally by Na+–Ca2+ exchange (Jung et al. 1994; Reisert & Matthews, 1998), which lowers the Ca2+ concentration during recovery (Reisert & Matthews, 1998). While complementary mechanisms may contribute to Ca2+ extrusion from dendrite (Jung et al. 1994) and soma (Leinders-Zufall et al. 1997), it seems unlikely that these contribute to ciliary Ca2+ homeostasis in the short term (Leinders-Zufall et al. 1997; Leinders-Zufall et al. 1998), since the cilia are diffusionally remote from these more proximal structures (Lindemann, 2001). While it might appear superficially attractive to study the kinetics of Ca2+ extrusion by recording the electrogenic currents carried by the exchanger itself, our previous work has demonstrated that these are of very small amplitude (< 2 pA, Antolin & Matthews, 2004), making such measurements unreliable. Direct measurement of calcium concentration is impractical in frog olfactory receptors, since their olfactory cilia fail to load with tetracarboxylate calcium indicators (S. Antolin, J. Reisert & H. R. Matthews, unpublished observations), most probably due to their extrusion by multidrug resistance anion transporters which have been shown to be present in the olfactory cilia of Xenopus (Manzini & Schild, 2003). We have therefore adopted instead an indirect approach.
We used the phosphodiesterase inhibitor IBMX to elevate the cAMP concentration within the olfactory cilia, thereby enabling Ca2+ entry through cyclic nucleotide-gated channels, and examined the subsequent decay of current upon its withdrawal. At the peak of the response to IBMX, nearly 80% of the receptor current can be inhibited by the Ca2+-activated Cl− channel blocker niflumic acid (Antolin, 2006), indicating that at the time of response recovery the vast majority of the current is likely to flow through this conductance. The final decline in receptor current could be delayed by either substantial reduction of the external Na+ concentration (Fig. 2) or by external application of Ni2+ (Fig. 3) without change in the final decay kinetics upon the return to normal Ringer solution (Fig. 4). Since either of these manipulations would be expected to inhibit Na+–Ca2+ exchange, these results imply that the recovery of the response to IBMX is dominated by the kinetics of Ca2+ extrusion, governing the decline in [Ca2+]i which leads to the progressive closure of Ca2+-activated Cl− channels. Since the magnitude of this conductance depends approximately on the square of the Ca2+ concentration (Kleene, 1997), the time constant for current decay is likely to reflect about half the time constant for the extrusion of Ca2+ by Na+–Ca2+ exchange. The kinetics of Ca2+ extrusion will depend not only upon the sodium gradient, but also on the number of exchanger molecules and the native calcium buffering properties of the cytoplasm (see e.g. Lagnado et al. 1992); however, these factors will normally remain constant for any given cell.
We found that the kinetics of current decay following IBMX stimulation depended only rather weakly on external Na+ concentration (Fig. 5). In order to prolong substantially the time constant for recovery, it was necessary to reduce the external Na+ concentration to a third or less of its value in normal Ringer. We infer that the rate constant for Na+–Ca2+ exchange must exhibit a similarly shallow dependence upon Na+ concentration, implying a high affinity for Na+ at its external site. Since our experiments were not carried out under clamped conditions, the voltage experienced by the exchanger will have varied continuously during response recovery. This might be expected to affect the electrogenic exchanger, not only through a change in the driving electrochemical gradient, but also perhaps through voltage dependence of external Na+ binding (Lagnado et al. 1988). However, in our experiments the external Na+ concentration would not be expected to affect the intraciliary voltage directly, since only the cilia were exposed to reduced Na+ concentration and their conductance is dominated by the opening of Ca2+-actived Cl− channels during response recovery (Fig. 2 and Antolin, 2006). In contrast, the cell body was in contact throughout the experiment with the normal Ringer solution within the pipette. Since the receptor current decayed exponentially back towards zero, the voltage would be expected to have followed a common trajectory during extrusion of the Ca2+ load at each Na+ concentration, with only a corresponding variation in time scale. Therefore, the perturbing effect of voltage changes would be expected to be equivalent in each case, allowing direct comparison between the time constants for current decay.
Consequently, comparison of the time constants for current recovery allows the dependence of exchanger rate upon external Na+ concentration to be inferred. The power-law dependence of Ca2+-activated Cl− channel opening upon Ca2+ concentration implies a fixed scaling relationship between the time constants for current decay and exchange extrusion. The Hill analysis of the normalized reciprocal time constant as a function of Na+ concentration (Fig. 6) therefore represents the effect of Na+ on the rate constant of Ca2+ extrusion by olfactory Na+–Ca2+ exchange.
Although the photoreceptor exchanger (NCKX1) and the cardiac exchanger (NCX) appear to have evolved independently (Nicoll et al. 1990; Reilander et al. 1992), both of these two Ca2+ extrusion mechanisms are more strongly affected by variations of Na+ concentration in the external medium than is the olfactory exchanger. Our results show that for the frog olfactory exchanger, a reduction in external Na+ concentration to 66 mm increased the time constant by only 1.5-fold, while a reduction to 33 mm increased it by approximately 6-fold. In contrast, in salamander photoreceptors, reduction of external Na+ to 55 mm by substitution with Li+ slows Na+–Ca2+,K+ exchange approximately 5-fold, while at an external Na+ concentration of 35 mm a 16-fold slowing ensues (Hodgkin & Nunn, 1987). This greater sensitivity to reduction in external Na+ concentration corresponds to a dissociation constant for the photoreceptor exchanger of 139 mm at depolarized potentials, decreasing to 109 mm at potentials closer to the neuronal resting potential (Lagnado et al. 1988). This is approximately twice the Na+ dissociation constant of 54 ± 4 mm which we obtain for the olfactory exchanger. Since the voltage was free to vary in our experiments, we cannot assess whether the external Na+ binding site experiences a fraction of the membrane field, as is the case for the photoreceptor exchanger (Lagnado et al. 1988). If this were the case, our value would represent the weighted mean for the voltage range over which response recovery takes place. In cardiac myocytes, the cardiac exchanger is believed to operate near thermodynamic equilibrium, to establish a cytoplasmic Ca2+ concentration which depends upon the membrane potential and the Na+ gradient (reviewed by Hilgemann, 2004). In consequence a striking effect on the excitation–contraction cycle in the heart occurs with only small changes in Na+ concentration. Measurement of the Na+ affinity at the cardiac Na+–Ca2+ exchanger's external site reveals a dissociation constant of 87.5 mm (Kimura et al. 1987), a value rather higher than we observe for the olfactory exchanger. In contrast to the photoreceptor and cardiac exchangers, neuronal Na+–Ca2+ exchangers of both the NCX (Sanchez-Armass & Blaustein, 1987) and NCKX2 (Dong et al. 2001) families exhibit considerably lower dissociation constants and hence higher affinities for external Na+ (reviewed by Blaustein & Lederer, 1999; Schnetkamp, 2004). Consequently, the comparatively high affinity of the olfactory exchanger for external Na+ does not place it unequivocally in one or the other family.
The Hill coefficient of 3.7 ± 0.4 which we obtained from the olfactory exchanger for the binding of Na+ at its external site suggests that at least three Na+ ions are likely to enter the cilia for each exchanger cycle. However, this value does not allow the olfactory exchanger to be assigned unequivocally to the NCX rather than the NCKX family of Na+–Ca2+ exchangers, since the photoreceptor NCKX1 exchanger also exhibits a similar Hill coefficient of around three for external Na+, suggesting that the fourth Na+ binding site is not equivalent to the other three (Lagnado et al. 1988). Earlier studies unfortunately do not help to resolve this issue. Olfactory cilia are labelled by an antibody raised against the bovine rod Na+–Ca2+,K+ exchanger, suggesting that the olfactory exchanger might be a member of the NCKX family also (Noe et al. 1997). However, the olfactory exchanger can be reversed without a requirement for external K+ (Reisert et al. 2003), in contrast to the photoreceptor exchanger (Cervetto et al. 1989), a result more consistent with membership of the NCX family. But even this cannot be regarded as conclusive, since the requirement for external K+ can be removed by a single point mutation in the NCKX2 exchanger (Kang et al. 2005). Consequently, conclusive identification of the identity of the olfactory exchanger is likely to require a more molecular approach. However, a recent in situ hybridization study has suggested that multiple isoforms of both NCX and NCKX may be present in multiple splice variants within mammalian olfactory receptor cells (Pyrski et al. 2007), further complicating the understanding of the functional role of these exchangers in olfactory transduction.
Our finding that the rate of Ca2+ extrusion by Na+–Ca2+ exchange was only modestly affected by extracellular Na+ until its concentration fell to 30% of its value in Ringer solution has important functional implications for the recovery of the olfactory response. This range of relative Na+ insensitivity included the value of 55 mm, the Na+ concentration reported to be present in both frog and rat olfactory mucus (Joshi et al. 1987; Reuter et al. 1998). Consequently, although the recovery phase of the olfactory receptor cell response appears to be dominated by the rate of Ca2+ extrusion from the cilia, response kinetics would be expected to be only marginally affected by modest increases or decreases in mucus Na+ concentration from this value, as might arise through desiccation or dilution.
Acknowledgments
This work was supported by the Wellcome Trust and by a studentship to S.A. from the Fundação para a Ciência e a Tecnologia, Portugal.
References
- Antolin S. University of Cambridge; 2006. Sodium-calcium exchange in amphibian olfactory receptor cells. PhD Thesis. [Google Scholar]
- Antolin S, Matthews HR. Characterisation of the electrogenic Na+-Ca2+ exchange current in frog olfactory receptor cells. J Physiol. 2004;555.P:C160. [Google Scholar]
- Antolin S, Matthews HR. Effect of external Na+ on Na-Ca exchange-mediated current recovery in frog ORNs. p. A37. Association for Chemoreception Sciences meeting abstract.
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Borisy FF, Ronnett GV, Cunningham AM, Juilfs D, Beavo J, Snyder SH. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. J Neurosci. 1992;12:915–923. doi: 10.1523/JNEUROSCI.12-03-00915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommundt G, Kavaler F. La3+, Mn2+, and Ni2+ effects on Ca2+ pump and on Na+-Ca2+ exchange in bullfrog ventricle. Am J Physiol Cell Physiol. 1987;253:C45–C51. doi: 10.1152/ajpcell.1987.253.1.C45. [DOI] [PubMed] [Google Scholar]
- Cervetto L, Lagnado L, Perry RJ, Robinson DW, McNaughton PA. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989;337:740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Cervetto L, McNaughton PA. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. J Physiol. 1986;370:91–109. doi: 10.1113/jphysiol.1986.sp015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Light PE, French RJ, Lytton J. Electrophysiological characterization and ionic stoichiometry of the rat brain K+-dependent Na+/Ca2+ exchanger, NCKX2. J Biol Chem. 2001;276:25919–25928. doi: 10.1074/jbc.M103401200. [DOI] [PubMed] [Google Scholar]
- Fain GL, Lamb TD, Matthews HR, Murphy RLW. Cytoplasmic calcium concentration as the messenger for light adaptation in salamader rods. J Physiol. 1989;416:215–243. doi: 10.1113/jphysiol.1989.sp017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- Frings S, Lindemann B. Current recording from sensory cilia of olfactory receptor cells in situ. I. The neuronal response to cyclic nucleotides. J Gen Physiol. 1991;97:1–16. doi: 10.1085/jgp.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW. New insights into the molecular and cellular workings of the cardiac Na+/Ca2+ exchanger. Am J Physiol Cell Physiol. 2004;287:C1167–C1172. doi: 10.1152/ajpcell.00288.2004. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Nunn BJ. The effect of ions on sodium-calcium exchange in salamander rods. J Physiol. 1987;391:371–398. doi: 10.1113/jphysiol.1987.sp016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Shigekawa M. Differential inhibition of Na+/Ca2+ exchanger isoforms by divalent cations and isothiourea derivative. Am J Physiol Cell Physiol. 1998;275:C423–C430. doi: 10.1152/ajpcell.1998.275.2.C423. [DOI] [PubMed] [Google Scholar]
- Joshi H, Getchell ML, Zielinski B, Getchell TV. Spectrophotometric determination of cation concentrations in olfactory mucus. Neurosci Lett. 1987;82:321–326. doi: 10.1016/0304-3940(87)90276-x. [DOI] [PubMed] [Google Scholar]
- Jung A, Lischka FW, Engel J, Schild D. Sodium/calcium exchanger in olfactory receptor neurons of Xenopus laevis. Neuroreport. 1994;5:1741–1744. doi: 10.1097/00001756-199409080-00013. [DOI] [PubMed] [Google Scholar]
- Kang KJ, Shibukawa Y, Szerencsei RT, Schnetkamp PP. Substitution of a single residue, Asp575, renders the NCKX2 K+-dependent Na+/Ca2+ exchanger independent of K+ J Biol Chem. 2005;280:6834–6839. doi: 10.1074/jbc.M412933200. [DOI] [PubMed] [Google Scholar]
- Kimura J, Miyamae S, Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene SJ. High-gain, low-noise amplification in olfactory transduction. Biophys J. 1997;73:1110–1117. doi: 10.1016/S0006-3495(97)78143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene SJ, Gesteland RC. Calcium-activated chloride conductance in frog olfactory cilia. J Neurosci. 1991;11:3624–3629. doi: 10.1523/JNEUROSCI.11-11-03624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlova I, Nilsson H, Phillipson M, Riederer B, Seidler U, Colledge WH, Roomans GM. X-ray microanalysis of airway surface liquid in the mouse. Am J Physiol Lung Cell Mol Physiol. 2005;288:L874–L878. doi: 10.1152/ajplung.00303.2004. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Yau KW. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363:71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L, Cervetto L, McNaughton PA. Ion transport by the Na-Ca exchange in isolated rod outer segments. Proc Natl Acad Sci U S A. 1988;85:4548–4552. doi: 10.1073/pnas.85.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L, Cervetto L, McNaughton PA. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Matthews HR, Torre V. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J Physiol. 1986;372:315–349. doi: 10.1113/jphysiol.1986.sp016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Greer CA, Shepherd GM, Zufall F. Imaging odor-induced calcium transients in single olfactory cilia: specificity of activation and role in transduction. J Neurosci. 1998;18:5630–5639. doi: 10.1523/JNEUROSCI.18-15-05630.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Rand MN, Shepherd GM, Greer CA, Zufall F. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics. J Neurosci. 1997;17:4136–4148. doi: 10.1523/JNEUROSCI.17-11-04136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. Predicted profiles of ion concentrations in olfactory cilia in the steady state. Biophys J. 2001;80:1712–1721. doi: 10.1016/S0006-3495(01)76142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini I, Schild D. Multidrug resistance transporters in the olfactory receptor neurons of Xenopus laevis tadpoles. J Physiol. 2003;546:375–385. doi: 10.1113/jphysiol.2002.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR, Reisert J. Calcium, the two-faced messenger of olfactory transduction and adaptation. Curr Opin Neurobiol. 2003;13:469–475. doi: 10.1016/s0959-4388(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Longoni S, Philipson KD. Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+ exchanger. Science. 1990;250:562–565. doi: 10.1126/science.1700476. [DOI] [PubMed] [Google Scholar]
- Noe J, Tareilus E, Boekhoff I, Breer H. Sodium/calcium exchanger in rat olfactory neurons. Neurochem Int. 1997;30:523–531. doi: 10.1016/s0197-0186(96)00090-3. [DOI] [PubMed] [Google Scholar]
- Pyrski M, Koo JH, Polumuri SK, Ruknudin AM, Margolis JW, Schulze DH, Margolis FL. Sodium/calcium exchanger expression in the mouse and rat olfactory systems. J Comp Neurol. 2007;501:944–958. doi: 10.1002/cne.21290. [DOI] [PubMed] [Google Scholar]
- Reilander H, Achilles A, Friedel U, Maul G, Lottspeich F, Cook NJ. Primary structure and functional expression of the Na/Ca,K-exchanger from bovine rod photoreceptors. EMBO J. 1992;11:1689–1695. doi: 10.1002/j.1460-2075.1992.tb05219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Bauer PJ, Yau KW, Frings S. The Ca-activated Cl channel and its control in rat olfactory receptor neurons. J Gen Physiol. 2003;122:349–363. doi: 10.1085/jgp.200308888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. J Gen Physiol. 1998;112:529–535. doi: 10.1085/jgp.112.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Adaptation of the odour-induced response in frog olfactory receptor cells. J Physiol. 1999;519:801–813. doi: 10.1111/j.1469-7793.1999.0801n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Response properties of isolated mouse olfactory receptor cells. J Physiol. 2001a;530:113–122. doi: 10.1111/j.1469-7793.2001.0113m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Responses to prolonged odour stimulation in frog olfactory receptor cells. J Physiol. 2001b;534:179–191. doi: 10.1111/j.1469-7793.2001.t01-1-00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Simultaneous recording of receptor current and intraciliary Ca2+ concentration in salamander olfactory receptor cells. J Physiol. 2001c;535:637–645. doi: 10.1111/j.1469-7793.2001.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter D, Zierold K, Schroder WH, Frings S. A depolarizing chloride current contributes to chemoelectrical transduction in olfactory sensory neurons in situ. J Neurosci. 1998;18:6623–6630. doi: 10.1523/JNEUROSCI.18-17-06623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Armass S, Blaustein MP. Role of sodium-calcium exchange in regulation of intracellular calcium in nerve terminals. Am J Physiol Cell Physiol. 1987;252:C595–C603. doi: 10.1152/ajpcell.1987.252.6.C595. [DOI] [PubMed] [Google Scholar]
- Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev. 1998;78:429–466. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- Schnetkamp PP. The SLC24 Na+/Ca2+-K+ exchanger family: vision and beyond. Pflugers Arch. 2004;447:683–688. doi: 10.1007/s00424-003-1069-0. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Koide Y, Kimura J. Topics on the Na+/Ca2+ exchanger: pharmacological characterization of Na+/Ca2+ exchanger inhibitors. J Pharmacol Sci. 2006;102:7–16. doi: 10.1254/jphs.fmj06002x2. [DOI] [PubMed] [Google Scholar]
- Zufall F, Munger SD. From odor and pheromone transduction to the organization of the sense of smell. Trends Neurosci. 2001;24:191–193. doi: 10.1016/s0166-2236(00)01765-3. [DOI] [PubMed] [Google Scholar]


