Abstract
We examined the effects of hypoxia severity on peripheral versus central determinants of exercise performance. Eight cyclists performed constant-load exercise to exhaustion at various fractions of inspired O2 fraction (FIO2 0.21/0.15/0.10). At task failure (pedal frequency < 70% target) arterial hypoxaemia was surreptitiously reversed via acute O2 supplementation (FIO2 = 0.30) and subjects were encouraged to continue exercising. Peripheral fatigue was assessed via changes in potentiated quadriceps twitch force (ΔQtw,pot) as measured pre- versus post-exercise in response to supramaximal femoral nerve stimulation. At task failure in normoxia (haemoglobin saturation (SpO2) ∼94%, 656 ± 82 s) and moderate hypoxia (SpO2 ∼82%, 278 ± 16 s), hyperoxygenation had no significant effect on prolonging endurance time. However, following task failure in severe hypoxia (SpO2 ∼67%; 125 ± 6 s), hyperoxygenation elicited a significant prolongation of time to exhaustion (171 ± 61%). The magnitude of ΔQtw,pot at exhaustion was not different among the three trials (−35% to −36%, P = 0.8). Furthermore, quadriceps integrated EMG, blood lactate, heart rate, and effort perceptions all rose significantly throughout exercise, and to a similar extent at exhaustion following hyperoxygenation at all levels of arterial oxygenation. Since hyperoxygenation prolonged exercise time only in severe hypoxia, we repeated this trial and assessed peripheral fatigue following task failure prior to hyperoxygenation (125 ± 6 s). Although Qtw,pot was reduced from pre-exercise baseline (−23%; P < 0.01), peripheral fatigue was substantially less (P < 0.01) than that observed at task failure in normoxia and moderate hypoxia. We conclude that across the range of normoxia to severe hypoxia, the major determinants of central motor output and exercise performance switches from a predominantly peripheral origin of fatigue to a hypoxia-sensitive central component of fatigue, probably involving brain hypoxic effects on effort perception.
Whole-body exercise performance in aerobic activities is impaired in hypoxia. The physiological mechanisms underpinning this impairment are not fully understood (Knight et al. 1993; Fulco et al. 1998; Calbet et al. 2003a,b; Lundby et al. 2006). We have recently shown that decreases in endurance performance from hyperoxia to moderate hypoxia are associated with peripheral muscle fatigue and its subsequent effects on CNS motor output, probably acting via afferent feedback mechanisms (Amann et al. 2006a; Romer et al. 2007). Despite our proposed link between ‘peripheral’ and ‘central fatigue’ from hyperoxia to moderate hypoxia, we acknowledge that peripheral fatigue and its associated sensory feedback is not the only potential source of inhibitory influence on central neural output and thus exercise performance. On the contrary, multiple ‘peripheral’ and ‘central’ mechanisms have been proposed (Bigland-Ritchie & Vollestad, 1988; Fitts, 1994; Kjaer et al. 1999; Jones & Killian, 2000; Gandevia, 2001; Kayser, 2003; Amann et al. 2006a) and some of the causes of central fatigue have been shown to be independent of peripheral somatosensory feedback (Blomstrand et al. 1988; Meeusen & De Meirleir, 1995; Gandevia, 2001; Dalsgaard et al. 2002).
Studies in which the severity of hypoxia was increased beyond moderate levels have provided indirect evidence that severe CNS hypoxia may result in centrally mediated inhibitory effects on autonomic control and/or motor drive originating within the CNS itself (Kjaer et al. 1999; Boushel et al. 2001; Calbet et al. 2003a). For example, blunting of neural feedback from contracting limb muscles in acute severe hypoxia (arterial oxygen saturation (SaO2)53%; fraction of inspired oxygen (FIO2) 0.078 using epidural anaesthesia did not affect exercise time to exhaustion (Kjaer et al. 1999), suggesting that limitations in exercise performance in acute severe hypoxia are of central origin and independent of peripheral feedback mechanisms. Further support for this postulate stems from studies that have shown that increasing FIO2 at the point of task failure prolongs constant-load exercise in chronic severe hypoxia (SaO2 73–77%; altitude 5050 m) (Kayser et al. 1994) and increases maximal incremental exercise performance in acute severe hypoxia (SaO2 66–68%; FIO2 0.105) (Calbet et al. 2003a,b). The fact that exercise at the point of ‘exhaustion’ could be continued with hyperoxygenation argues against a peripheral mechanism as the main cause of fatigue in severe hypoxia and, by extension, suggests an independent effect of CNS hypoxia on regulating performance under such extreme conditions.
Collectively, these findings indicate that hypoxia-sensitive sources of inhibition of central motor drive exist outside any influences related to peripheral muscle fatigue and its associated afferent feedback. Thus, the purpose of the proposed study was to determine the relative effects of central and peripheral mechanisms of fatigue on whole-body exercise performance in normoxia, moderate hypoxia and severe hypoxia (induced via FIO2 0.15 and 0.10, respectively). We hypothesized that peripheral locomotor muscle fatigue would be important in limiting exercise performance in conditions of hyperoxia to moderate hypoxia, whereas central mechanisms of fatigue (i.e. as affected by CNS hypoxia) would become more dominant as the severity of hypoxia increases.
Methods
Subjects
Eight competitive male cyclists volunteered to participate in the study (mean ± s.e.m.; age 23.4 ± 1.5 years, body mass 72.8 ± 2.6 kg, stature 1.80 ± 0.03 m, maximal O2 consumption (V˙O2max) 67.2 ± 2.5 ml kg−1 min−1). Written informed consent was obtained from each participant. All procedures conformed to the standards set by the Declaration of Helsinki and the protocol was approved by the institution's human subjects committee (University of Wisconsin-Madison).
Exercise responses
Ventilation and pulmonary gas exchange were measured breath-by-breath at rest and throughout exercise using an open circuit system (Harms et al. 1998). Arterial O2 saturation was estimated (SpO2) using a pulse oximeter (Nellcor N-595, Pleasanton, CA, USA) with adhesive forehead sensors. We have previously found excellent agreement between directly measured arterial O2 saturation and SpO2 over the 70–100% range (Romer et al. 2006, 2007). Arterial O2 content (CaO2) was estimated assuming a haemoglobin concentration ([Hb]) of 15.0 g dl−1 and an alveolar (estimated via end-tidal partial pressure of O2 (PET,o2)) to arterial O2 difference of 20 mmHg:
Heart rate (HR) was measured from the R–R interval of an electrocardiogram (ECG) using a three-lead arrangement. Ratings of perceived exertion (RPE) for dyspnoea and limb discomfort were obtained at rest and every minute during exercise using Borg's modified CR10 scale (Borg, 1998). Arterialized (Finalgon, Boehringer Ingelheim, Germany) capillary blood samples were collected from an earlobe at rest and every minute during exercise (starting at 30 s) for determination of total whole-blood lactate concentration ([La−]B) using an electrochemical analyser (YSI 1500 Sport, OH, USA).
Near infrared spectroscopy
During all trials, subjects were instrumented with two pairs of near infrared (NIR) probes to monitor absorption of light across cerebral and muscle tissue (Oxymon, Artinis, The Netherlands). One NIR emitter and detector pair was placed over the left frontal cortex region of the forehead. Spacing between optodes was fixed at 4.5 cm using an optically dense plastic spacer. Optodes were held in place by a custom-made headset that was individually adjusted to each subject's head to ensure accurate replacement on subsequent visits. A second emitter and detector pair was affixed over the belly of the left vastus lateralis muscle (approximately 15 cm above the proximal border of the patella and 5 cm lateral to the midline of the thigh) using a spacer with optode distance of 5.18 cm. Probes were secured to the skin using double-sided tape and shielded from light using elastic bandages. An indelible pen was used to mark the position of the optodes for future visits. The Beer–Lambert Law was used to calculate micromolar changes in tissue oxygenation (oxyhaemoglobin (O2Hb) and deoxyhaemoglobin (HHb)) across time, using received optical densities from two continuous wavelengths of NIR light (780 and 850 nm) and fixed differential path length factor of 4.95 (Duncan et al. 1995) and 5.93 (van der Zee et al. 1992) for muscle and cerebral tissue, respectively. Total haemoglobin (THb) was calculated as the sum of O2Hb and HHb concentration changes, to give an index of change in regional blood volume (Van Beekvelt et al. 2001). Data were recorded continuously at 10 Hz, from the beginning of the normoxic rest period to the end of the exercise trial. Following collection, data were filtered using a Savitzky–Golay smoothing algorithm and normalized to reflect changes from a baseline reference point (0 μm) at the end of the normoxic rest period.
Neuromuscular function
Electromyography
Quadriceps electromyograms (EMG) were recorded from the right vastus lateralis (VL), vastus medialis (VM) and rectus femoris (RF) using monitoring electrodes with full-surface solid adhesive hydrogel (Kendall H59P, Mansfield, MA, USA), with on-site amplification. Electrodes were placed in a bipolar electrode configuration over the middle of the respective muscle belly. The active electrode was placed over the motor point of the muscle. The recording electrode was moved along the muscle until a good configuration – confirmed by a ‘maximal’ M-wave shape – was achieved. The reference electrode was placed over an electrically neutral site. The position of the EMG electrodes was marked with indelible ink to ensure that they were placed in the same location at subsequent visits. Proper electrode configuration was checked before the beginning of every experiment. To minimize movement artifacts, electrode cables were fastened to the subject's quadriceps using medical adhesive tape, and wrapped in elastic bandage. The VL, VM and RF electrodes were used to record: (a) magnetically evoked compound muscle action potentials (M-waves) to evaluate changes in membrane excitability; and (b) EMG for VL throughout exercise to estimate fatigue. The M-wave properties included conduction time, peak amplitude and area (Caquelard et al. 2000; Sandiford et al. 2005; Amann et al. 2006b).
Raw EMG signals from VL, VM and RF corresponding to each muscle contraction during the exercise trials and the pre- and post-exercise MVC manoeuvres were recorded for later analysis. The EMG signals were amplified and filtered by a Butterworth band-pass filter (BMA –830, CWE, Inc., Ardmore, PA, USA) with a low-pass cut-off frequency of 10 Hz and a high-pass cut-off frequency of 1 kHz. The slope of the filters was −6 dB octave−1. The filtered EMG signals were sampled at 2 kHz by a 16 bit A/D converter (PCI-MIO-16XE-50, National Instruments, Austin, TX, USA) with custom software (Labview 6.0, National Instruments). A computer algorithm identified the onset of activity where the rectified EMG signals deviated by more than two standard deviations above the baselines for at least 100 ms. Each EMG burst was visually inspected to verify the timing identified by the computer. For data analysis, the integral of each burst (integrated EMG (iEMG)) was calculated using the formula
where m is the raw EMG signal.
A 1024-point fast Fourier transform (FFT) was used to compute a power spectrum periodogram. The mean power frequency (MPF) was calculated using the formula
where Sm(f) is the power density spectrum of the EMG signal.
Magnetic stimulation
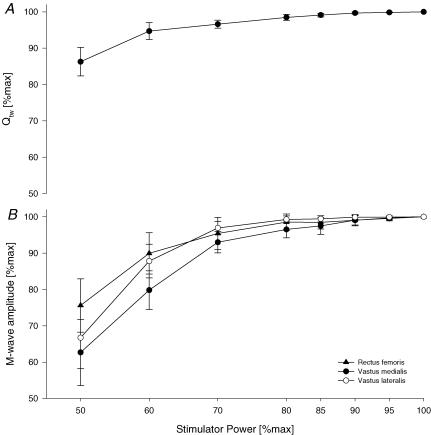
For a detailed description we refer the reader to previous studies from our laboratory (Amann et al. 2006a; Romer et al. 2006). Briefly, subjects lay semi-recumbent on a table with the right thigh resting in a preformed holder, the knee joint angle set at 1.57 rad (90 deg) of flexion and the arms folded across the chest. A magnetic stimulator (Magstim 200, The Magstim Company Ltd, Wales, UK) connected to a double 70 mm coil was used to stimulate the femoral nerve. The evoked quadriceps twitch force was obtained from a calibrated load cell (Interface, Model SM 1000, Scottsdale, AZ, USA) connected to a non-compliant strap, which was placed around the subject's right leg just superior to the ankle malleoli. To determine whether nerve stimulation was supramaximal, three single twitches were obtained every 30 s at 50, 60, 70, 80, 85, 90, 95 and 100% of maximal stimulator power output. A near-plateau in baseline quadriceps twitch force (Qtw) and M-wave amplitudes with increasing stimulus intensities was observed in every subject, indicating maximal depolarization of the femoral nerve (Fig. 1).
Figure 1.
Quadriceps twitch force (Qtw; A) and M-wave amplitudes (rectus femoris, vastus medialis, vastus lateralis; B) as a direct response to magnetic stimulation of the femoral nerve applying single twitches at increasing stimulator power settings. Means ± s.e.m., n = 8.
Activity-dependent muscle potentiation, affected by prior contractile activity, has been shown to enhance the contractile responses of skeletal muscle. Therefore, the co-existence of potentiation and fatigue – with one enhancing and one decreasing muscle performance – might attenuate the level of fatigue assessed immediately following exercise (Rassier & Macintosh, 2000). Various exercise trials in this study, characterized by significantly different levels of muscle activation and total exercise duration, could have induced diverse degrees of activity-dependent potentiation and thus might have affected the fatigue measurements. To circumvent this problem, we determined the pre- to post-exercise changes in the 1 Hz potentiated twitch (Qtw,pot). The Qtw,pot is more sensitive for detecting fatigue than the non-potentiated twitch, particularly when the degree of fatigue is small (Kufel et al. 2002). Accordingly, we measured quadriceps twitch force 5 s after a 5 s maximal voluntary contraction (MVC) of the quadriceps, and repeated this procedure six times such that six Qtw,pot were obtained. Like others (Kufel et al. 2002), we found that the degree of potentiation was slightly smaller after the first and, to a lesser extent, after the second MVC; therefore, we discarded the first two measurements. Activation of the quadriceps during the MVCs was assessed using a superimposed twitch technique (Merton, 1954; Strojnik & Komi, 1998). Briefly, the force produced during a superimposed single twitch on the MVC was compared to the force produced by the potentiated single twitch delivered 5 s afterward. The assessment procedure was performed before exercise (∼20 min) and at 2 min after exercise, which represented the maximum time needed to instrument the subjects. Peak force, contraction time (CT), maximal rate of force development (MRFD), one-half relaxation time (RT0.5), and maximal relaxation rate (MRR) were analysed for all Qtw,pot (Lepers et al. 2002; Sandiford et al. 2005).
Protocol
At a preliminary visit to the laboratory, subjects were thoroughly familiarized with the procedures used to assess neuromuscular function, and performed a maximal incremental exercise test (20 W + 25 W min−1; (Amann et al. 2004)) in ambient air on a computer-controlled electromagnetically braked cycle ergometer (Velotron, Elite Model, Racer Mate, Seattle, WA, USA) for the determination of peak power output (Wpeak) and V˙O2max On three separate occasions, the subjects performed constant-load exercise to task failure at 81.4 ± 0.4% of Wpeak, while breathing either ambient air (normoxia, FIO2 = 0.21) or a humidified gas mixture (moderate hypoxia, FIO2 = 0.15; severe hypoxia, FIO2 = 0.10). Subjects were exposed to the respective gas mixture 1 min prior to the beginning of exercise. The order of these three trials was randomized and the subjects were blinded to the respective FIO2. During the constant-load exercise trials, subjects used visual and verbal feedback in order to maintain a self-selected pedal cadence, as inferred from the maximal incremental exercise trials (95–110 rev min−1). When the pedal cadence dropped below 70% of the self-selected cadence for ≥5 s (task failure), the arterial hypoxaemia was rapidly removed by surreptitiously switching to an FIO2 of 0.30 (hyperoxygenation). The 70% threshold was chosen based on previous experience from our pilot work and other investigations in which most subjects were able to continue to exercise at a constant submaximal workload after reducing the pedal frequency down to about 70–75% of their self-selected target frequency (rev min−1). A further reduction in rev min−1 below 70% resulted in voluntary termination of exercise in most cases. Consequently, exercise was terminated by the investigators when pedal cadence dropped below 60% for ≥5 s of the self-selected cadence (exhaustion). Hyperoxygenation elicited a substantial increase in exercise time to exhaustion in the 0.10 FIO2 condition (severe hypoxia) (P < 0.01) and a slight, albeit non-significant, increase in 0.15 FIO2 (moderate hypoxia). Therefore, on separate days and in random order, we repeated these exercise trials but stopped the subjects at the time when the FIO2 was previously switched to 0.30. The repeat trial in severe hypoxia was also repeated in normoxia (isotime-severe hypoxia; FIO2 0.21). During the repeat trials, subjects used visual and verbal feedback to mimic their previously recorded target, and reduction in, pedal cadence. All of the exercise trials were preceded by a 5 min warm-up in normoxia at 1.5 W (kg body mass)−1, and the subjects remained seated throughout exercise. The time between warm-up and the beginning of exercise was fixed at 2 min.
The subjects were naive to the purpose of the study and the expected outcomes. Each exercise session was separated by at least 48 h and was completed at the same time of day. Subjects were instructed to refrain from caffeine for 12 h and stressful exercise for 48 h before each exercise trial. Ambient temperature and relative humidity were not different between conditions.
Reliability of magnetic stimulation and technical considerations
For between-day reliability, the subjects repeated the magnetic stimulation protocol at rest on separate visits to the laboratory. There was no systematic bias in the baseline measurements between days. Mean between-day, within-subject coefficients of variation for Qtw,pot were 4.4 ± 0.9 (range 0.6–7.0), 2.6 ± 1.0 (range 0.5–6.3) for MVC and 1.6 ± 0.7 (range 0.2–3.8) for voluntary muscle activation. Additional reliability measures regarding magnetic nerve stimulation, as well as technical considerations addressing the limitations of surface EMG and magnetic femoral nerve stimulation can be found in published reports (Enoka & Stuart, 1992; Amann et al. 2006a; b; Romer et al. 2006).
Statistical analyses
Repeated measures ANOVA was used to test for within-group effects across time. Following significant main effects, planned pairwise comparisons were made using the Holm's sequential Bonferroni procedure. Results are expressed as the mean ± s.e.m. Statistical significance was set at P < 0.05.
Results
Effects of hyperoxygenation on SpO2 and exercise performance (Tables 1 and 2)
Table 1.
Effects of hyperoxygenation on exercise performance following exercise to exhaustion at varying FIO2
| Normoxia | Moderate hypoxia | Severe hypoxia | ||||
|---|---|---|---|---|---|---|
| Normoxia FIO2 = 0.21 | →Hyperoxia →0.30 | Moderate hypoxia 0.15 | →Hyperoxia → 0.30 | Severe hypoxia 0.10 | →Hyperoxia →0.30 | |
| SpO2 (%) | 94 ± 1 | →97 ± 1 | 82 ± 1 | →93 ± 2 | 67 ± 1 | →99 ± 1 |
| Time (s) | 656 ± 82*† | 21 ± 10* | 278 ± 16*‡ | 18 ± 9* | 125 ± 6†‡ | 205 ± 70†‡ |
| Total time (s) | 677 ± 90*† | 296 ± 22‡ | 330 ± 68‡ | |||
| Prolongation (%) | 2.7 ± 1.1*§ | 5.9 ± 2.7*§ | 171 ± 61†‡ | |||
| (Range) | (0–8) | (0–24) | (51–483) | |||
P < 0.05 versus severe hypoxia
P < 0.05 versus moderate hypoxia
P < 0.05 versus normoxia
not significant.
Table 2.
Physiological response to the final minute of constant workrate exercise (333 ± 9 W) to task failure with various inspired O2 concentrations (F IO2 0.21/0.15/0.10) and at termination of exercise (final exhaustion) following re-oxygenation with a hyperoxic gas mixture (FIO2 0.30)
| Normoxia | Moderate hypoxia | Severe hypoxia | ||||
|---|---|---|---|---|---|---|
| FIO2 = 0.21 | → 0.30 | 0.15 | → 0.30 | 0.10 | → 0.30 | |
| Exercise time (s) | 656 ± 82*† | →+21 ± 10* | 278 ± 16*‡ | →+18 ± 9* | 125 ± 6†‡§ | →+205 ± 70††‡ |
| Pedal frequency (rev min−1) | 70 ± 1 | 60 ± 1 | 70 ± 1 | 60 ± 1 | 70 ± 1 | 60 ± 1 |
| CaO2(ml O2 dl−1) | 19.9 ± 0.2*†§ | 20.6 ± 0.2 | 17.4 ± 0.2*‡§ | 19.9 ± 0.3* | 14.0 ± 0.3†‡§ | 21.3 ± 0.0† |
| SpO2 (%) | 94.0 ± 0.7*†§ | 96.9 ± 1.0 | 82.4 ± 0.9*‡§ | 93.3 ± 1.6* | 66.6 ± 1.2†‡§ | 99.7 ± 0.1† |
| HR (beats min−1) | 192.1 ± 4.5*† | 192.3 ± 4.5 | 183.6 ± 4.5*‡ | 184.7 ± 4.9 | 175.3 ± 3.3†‡§ | 184.6 ± 4.4 |
| RPE (dyspnoea) | 8.6 ± 0.4* | 8.8 ± 0.4 | 8.3 ± 0.8* | 8.5 ± 0.8 | 6.3 ± 0.7†‡§ | 9.0 ± 0.5 |
| RPE (limb discomfort) | 8.7 ± 0.4* | 8.6 ± 0.5 | 8.4 ± 0.4* | 8.7 ± 0.4 | 6.8 ± 0.4†‡§ | 9.1 ± 0.4 |
| fR (breaths min−1) | 61.0 ± 2.9 | 60.6 ± 2.9 | 58.5 ± 2.9 | 57.9 ± 2.9 | 58.4 ± 3.9 | 58.0 ± 2.7 |
| V˙T (l) | 3.0 ± 0.2 | 3.0 ± 0.2 | 3.1 ± 0.2 | 3.1 ± 0.2 | 3.1 ± 0.3 | 3.0 ± 0.3 |
| V˙o2 (l min−1) | 181.8 ± 6.9 | 179.9 ± 6.2 | 176.5 ± 8.2 | 174.3 ± 6.8 | 179.6 ± 6.0 | 169.1 ± 7.0 |
| V˙O2 (l min−1) | 4.5 ± 0.1*† | — | 4.0 ± 0.1*‡ | — | 3.7 ± 0.1†‡§ | 4.3 ± 0.1 |
| V˙o2 (l min−1) | 4.8 ± 0.1a | 4.8 ± 0.1 | 4.7 ± 0.1 | 4.6 ± 0.2 | 4.5 ± 0.1c | 4.7 ± 0.1 |
| V˙E/V˙o2 | 39.4 ± 1.6 | — | 43.9 ± 1.6 | — | 60.6 ± 1.7 | 43.5 ± 2.3 |
| V˙E/V˙o2 | 35.4 ± 2.2 | 37.3 ± 1.3 | 37.1 ± 1.5 | 38.8 ± 1.6 | 39.4 ± 1.0 | 36.9 ± 1.6 |
| PET,o2 (mmHg) | 114.8 ± 1.0*†§ | 154.6 ± 9.0 | 77.2 ± 1.6*‡§ | 163.1 ± 2.8 | 50.6 ± 0.6†‡§ | 172.5 ± 0.9 |
| PET,co2 (mmHg) | 30.2 ± 1.2 | 30.7 ± 1.1 | 29.6 ± 1.4 | 30.1 ± 1.3 | 28.5 ± 1.2§ | 32.6 ± 0.8 |
| Capillary [La−]B (mmol l−1) | 8.1 ± 0.5* | 8.1 ± 0.5 | 8.1 ± 0.5* | 8.4 ± 0.6 | 6.2 ± 0.7†‡§ | 8.6 ± 0.7 |
Values are expressed as means ± s.e.m.
P < 0.05 versus severe hypoxia
P < 0.05 versus moderate hypoxia
P < 0.05 versus normoxia
P < 0.05 versus exhaustion in hyperoxia. n = 8. a, estimated values (see methods).
Reducing FIO2 from normoxia to moderate or severe hypoxia during exercise markedly reduced SpO2 and CaO2, and significantly affected the time to the limit of exhaustion. Subsequent to the switch to hyperoxia, SpO2 and CaO2 rapidly rose in various trials and were significantly increased (>130 mmHg pETO2) about 15 s after inhalation of 0.3 FIO2. A prompt and significant effect of the hyperoxic inspirate on cerebral and muscle tissue oxygenation was indicated by the NIR spectroscopy (NIRS) data (see NIRS section below).
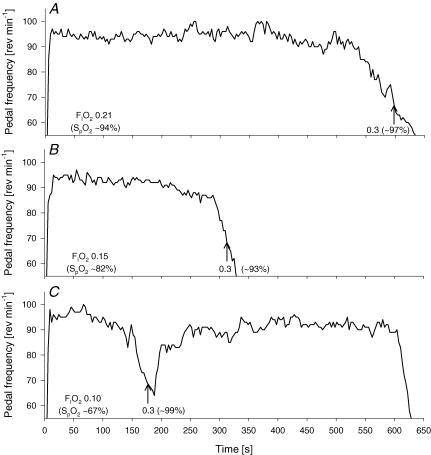
Effects of hyperoxygenation on pedal frequency and performance time are shown in Fig. 2 for an individual subject example, and mean values are shown in Table 2. Following hyperoxygenation at the point of task failure (defined as a drop in rev min−1 below 70% of self-selected cadence for more than 5 s) in severe hypoxia, all eight subjects were able to extend their performance time by 171 ± 61% (range, 51–483%; P < 0.01). Two out of eight subjects almost instantly increased their pedal frequency back to their individual target rev min−1 and maintained it towards the end of exercise in hyperoxia (exhaustion). The remaining six subjects increased their pedal frequency gradually to about 80–90% of their target rev min−1.
Figure 2. Representative example showing the effects of increases in arterial O2 content on pedal frequency and endurance time to exhaustion.
The arrow indicates the point at which the inspirate was switched from either normoxia (A; FIO2 0.21), moderate hypoxia (B; FIO2 0.15), or severe hypoxia (C; FIO2 0.10) to hyperoxia (FIO2 0.30). The criterion for increasing the FIO2 was a drop in pedal frequency below 70% of the respective individual target rev min−1 for ≥5 s (67 rev min−1 in this case). The exercise was terminated following a further reduction in pedal frequency below 60% of the respective individual target rev min−1 for ≥5 s (57 rev min−1).
In normoxia, hyperoxygenation at the point of task failure did not prolong exercise time (2.7 ± 1.1%; range, 0–8%; P = 0.13). Six out of eight subjects continued to exercise for ≤3% of their time to task failure; however, no effect of hyperoxygenation on rev min−1 was observed and subjects continued to drop their pedal frequency until the trial was terminated by the investigator (rev min−1 below 60% of self-selected cadence for more than 5 s). Nevertheless, two of the eight were able to momentarily return their pedal frequency to 65–70% of control, and thus prolonged exercise time to exhaustion by an additional 6% and 8%.
A similar, and again non-significant prolongation of exercise time was observed following exhaustion in moderate hypoxia (5.9 ± 2.7%; range, 0–24%; P = 0.10). Seven of eight subjects continued to exercise for ≤5% – the rev min−1 profile was similar compared to the normoxic trial. One subject prolonged his exercise time by 24% and was able to increase his rev min−1 back to about 80% of his initial pedal frequency.
Effects of exercise in various FIO2levels on lactate, effort perception and HR (Table 2, Fig. 3)
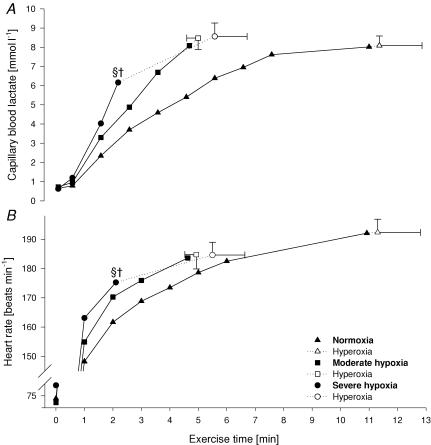
Figure 3. Capillary blood lactate (A) and heart rate (B) during various exercise trials.
Filled symbols represent values obtained in the respective FIO2 condition (0.21/0.15/0.10), open symbols indicate values at exhaustion in hyperoxia (FIO2 0.30). §P < 0.05 versus exhaustion in normoxia and moderate hypoxia. †P < 0.05 versus exhaustion in hyperoxia.
At the point of task failure at each FIO2 (immediately preceding the switch to the hyperoxic inspirate), the values for HR, dyspnoea, limb discomfort, and blood lactate were significantly lower in severe hypoxia versus normoxia and moderate hypoxia. Following the switch to hyperoxia, no further increase in any of these variables was observed over the brief additional time following the normoxia and moderate hypoxia trials, whereas following hyperoxygenation in severe hypoxia, effort perceptions, HR and blood lactate all increased further during the extended (205 ± 70 s) work period to exhaustion. However, hyperoxygenation at task failure in severe hypoxia had no direct effect on HR as evident by similar values over the 30 s period following the switch in FIO2 from 0.10 to 0.30. At the termination of exercise in hyperoxia, values for lactate (∼11 times rest), heart rate (HR) (185–192), rating of perceived exertion (RPE) (∼9), and V˙E/Vco2 were similar across trials at all FIO2 values.
Magnetic nerve stimulation (Table 3 and Fig. 4)
Table 3.
Effects of constant workrate exercise (333 ± 9 W) on fatigue variables
| Normoxia | Moderate hypoxia | Severe hypoxia | Isotime-severe hypoxia | ||||
|---|---|---|---|---|---|---|---|
| FIo2 = 0.21 | → 0.30 | 0.15 | → 0.30 | 0.10 | → 0.30 | 0.21 | |
| Time (s) | 656 ± 82 | →+21 ± 10 | 278 ± 16 | →+18 ± 9 | 125 ± 6 | →+205 ± 70 | 125 ± 6 |
| Magnetic femoral nerve stimulation, % change from pre- to 2 min post-exercise | |||||||
| Qtw,pot (N) | — | −35.9 ± 2.0* | −36.7 ± 2.6* | −34.7 ± 2.7* | −23.2 ± 2.4 | −36.0 ± 2.5* | −12.3 ± 2.4* |
| MRFD‡ (N s−1) | — | −35.5 ± 1.8* | −36.3 ± 3.1* | −34.8 ± 3.0* | −22.0 ± 2.0 | −36.7 ± 2.0* | −9.0 ± 2.1* |
| MRR‡ (N s−1) | — | −33.8 ± 1.4* | −34.5 ± 4.1* | −33.5 ± 2.8* | −20.7 ± 2.9 | −35.1 ± 2.4* | −9.6 ± 3.2* |
| CT (s) | — | −4.2 ± 1.2 | −2.6 ± 1.3† | −2.7 ± 1.3† | −3.5 ± 0.8 | −2.0 ± 1.6† | −3.9 ± 0.4 |
| RT0.5 (s) | — | 4.8 ± 1.0* | 5.9 ± 1.3* | 5.5 ± 1.8* | 1.6 ± 1.9† | 5.1 ± 1.2* | −1.0 ± 1.9*† |
| MVC peak force (N) | — | −13.9 ± 1.6* | −12.9 ± 2.9* | −12.6 ± 1.7* | −2.4 ± 2.1† | −13.2 ± 2.0* | 1.7 ± 1.1† |
| % Muscle activation | — | −1.1 ± 0.6† | −1.7 ± 1.1† | −1.3 ± 0.7† | −0.9 ± 0.8† | −1.8 ± 1.2† | 0.2 ± 0.5† |
| EMG, % change from 1st to last minute of exercise | |||||||
| iEMG (V) | 27.3 ± 5.6 | 27.7 ± 5.6 | 24.7 ± 3.0 | 25.9 ± 3.2 | 15.5 ± 3.1 | 28.6 ± 2.3* | — |
| MPF (Hz) | −9.7 ± 1.4 | −9.1 ± 1.4 | −9.9 ± 1.5 | −10.3 ± 1.8 | −7.9 ± 0.9 | −11.1 ± 1.4 | — |
Exercise was performed in three different gas concentrations (Fio20.21/0.15/0.10) to voluntary exhaustion, whereupon the Fio2 was increased to 0.30 and the subjects were encouraged to continue to exercise. Exercise performances in moderate and severe hypoxia were repeated in the respective Fio2 but without switching to hyperoxia at exhaustion. Exercise performance in severe hypoxia was also repeated in 0.21 Fio2 (isotime-severe hypoxia). Values are expressed as means ± s.e.m. MRFD, maximal rate of force development; MRR, maximal rate of relaxation; CT, contraction time; RT0.5, one-half relaxation time; MVC, maximal voluntary contraction.
When adjusted for the reduction in potentiated single twitch force (Qtw,pot), neither MRFD nor MRR was significantly different from pre-exercise baseline. % Muscle activation is based on superimposed twitch technique. Integrated EMG (iEMG) and mean power frequency (MPF) are based on myoelectrical activity of the vastus lateralis. The majority of variables changed significantly compared with baseline 2 min after exercise (P < 0.01).
P < 0.05 versus severe hypoxia ( Fio20.10).
Not significantly different from pre-exercise baseline. Pre-exercise, resting mean values for potentiated single twitch, MRFD, MRR, CT, RT0.5, MVC and % muscle activation were 165 ± 2 N, 1533 ± 23 N s−1, 1059 ± 13 N s−1, 0.26 ± 0.00 s, 0.12 ± 0.00 s, 623 ± 5 N and 97.3 ± 0.6%, respectively. n = 8.
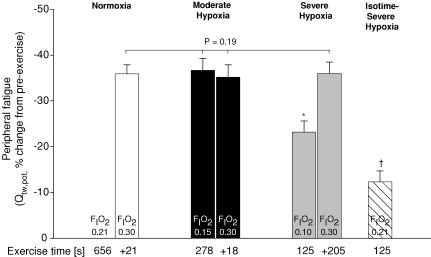
Figure 4. Peripheral quadriceps fatigue (Qtw,pot, potentiated twitch force) assessed 2 min following various trials with different values for FIO2.
Time performed in the respective FIO2 (0.21/0.15/0.10) and the additional exercise time following reoxygenation via a hyperoxic inspirate (FIO2 0.30) are indicated below the x-axis. No fatigue data are available for the point of exhaustion in normoxia (immediately prior to switch to hyperoxia). The trial in severe hypoxia (125 ± 6 s; FIO2 0.10) was also repeated in normoxia (isotime-severe hypoxia) to reveal the effects of severe hypoxia on locomotor muscle fatigue. *P < 0.05 versus FIO2 0.15 and 0.30; †P < 0.05 versus FIO2 0.10, 0.15 and 0.30.
M-waves
As a measure of membrane excitability we examined pre- versus post-exercise M-wave characteristics in conjunction with the muscle mechanical properties for VL, VM, and RF in all conditions. Although there was a similar trend in all trials towards an increased M-wave amplitude, increased area, and decreased conduction time post-exercise, none of these changes were significant. This suggests that the observed changes in Qtw,pot are mainly due to changes within the quadriceps and that peripheral failure of electrical transmission can be excluded.
Qtw,pot
At the point of task failure at each FIO2. Following task failure in all FIO2 conditions (immediately preceding the switch to the hyperoxic inspirate), Qtw,pot was significantly reduced from pre-exercise baseline (P < 0.01). The loss in quadriceps twitch force was about one-third less in severe hypoxia than in normoxia or moderate hypoxia (P < 0.01). However, quadriceps fatigue was not significantly different between the normoxia and moderate hypoxia trial. When the exercise performance time achieved in severe hypoxia was repeated in normoxia (isotime-severe hypoxia), exercise-induced quadriceps fatigue was only about one-half of that in severe hypoxia (P < 0.01). At the point of exhaustion with superimposed hyperoxia. The substantial 35–37% decrease in Qtw,pot at the point of task failure in moderate hypoxia was not alleviated via hyperoxygenation, as exercise continued for an additional 18–21 s. Quadriceps twitch force further decreased with the extended exercise time achieved with hyperoxia following the severe hypoxia trial, so that at termination of exercise in hyperoxia, the magnitude of reduction in Qtw,pot from pre- to post-exercise (ΔQtw,pot) was similar following all three trials (−35 to −36%; P = 0.24).
Within-twitch measurements
Various measurements, namely MRFD, MRR and RT0.5 complement the findings reported for Qtw,pot. At the point of task failure at each FIO2. The changes in within-twitch measurements, namely MRFD, MRR and RT0.5, were significantly smaller in severe hypoxia versus normoxia and moderate hypoxia. However, these changes were not significantly different between the normoxia and moderate hypoxia trial. At the point of exhaustion in hyperoxia. No further changes in the various within-twitch measurements were induced by the brief additional time in hyperoxia following the normoxia and moderate hypoxia trials. MRFD, MRR and RT0.5 changed further with the extended exercise time following the severe hypoxia trial, so that at termination of exercise in hyperoxia, the magnitude of changes in these variables were similar following all three trials (Table 3).
MVC force and voluntary muscle activation
The results for peak voluntary force output mirror the twitch data. Percentage voluntary quadriceps activation was 97 ± 0% at rest and was not affected by the preceding exercise regardless of the FIO2.
EMG during exercise (Fig. 5)
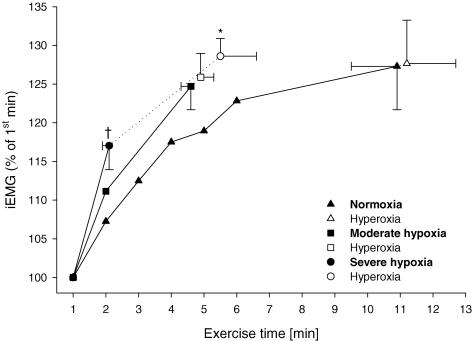
Figure 5. Myoelectrical activity (integrated EMG (iEMG)) of vastus lateralis during exercise at 333 ± 9 W in normoxia and two levels of hypoxia.
Values are normalized to the mean of first minute of each trial. The mean value for iEMG during each muscle contraction (cycle revolution) was calculated and averaged over each 60 s period. Filled symbols represent values obtained in the respective FIO2 condition (0.21/0.15/0.10), open symbols indicate iEMG values obtained at the termination of exercise (final exhaustion) after the switch to the hyperoxic inspirate (FIO2 0.30). *P < 0.05 from previous value. †P < 0.05 from normoxia at isotime.
iEMG
Integrated EMG of VL rose significantly from the first minute of exercise to the point of task failure (immediately prior to the switch to the hyperoxic inspirate) in all eight subjects at all FIO2 values (P < 0.01). The brief additional time in hyperoxia following the normoxia and moderate hypoxia trials did not cause a further significant increase in iEMG; however, the extended exercise time following the severe hypoxia trial resulted in a further rise in iEMG in all eight subjects (P < 0.01). Accordingly, at termination of exercise in hyperoxia, the percent increase in iEMG from the first to the last minute of exercise was similar at all FIO2 values (P = 0.33). Finally, iEMG rose significantly more from the first minute of exercise to the point of exhaustion in severe hypoxia as compared to iEMG during the same time (2.1 ± 0.1 min) in the normoxic trial (Fig. 5).
In summary, in most instances the effect of exercise or of FIO2 across the range of severe hypoxia to normoxia and upon reoxygenation to hyperoxia on ΔQtw,pot (pre- to post-exercise) was also reflected in the changes in quadriceps EMG observed during exercise under comparable experimental conditions.
MPF
Mean power frequency for VL decreased from the first to the second minute (P < 0.01) and continued to fall towards the end of exercise in all trials (Table 3). The magnitude of reduction in MPF was similar in all trials (P = 0.32).
Effects of FIO2 on tissue oxygenation: NIRS responses (Table 4, Fig. 6)
Table 4.
Micromolar (μm) changes in cerebral and muscle tissue oxygenation at the point of task failure with various inspired O2 concentrations (FIO20.21/0.15/0.10) and at termination of exercise (exhaustion) following re-oxygenation with a hyperoxygenation
| Cerebral | Muscle | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normoxia | Moderate hypoxia | Severe hypoxia | Normoxia | Moderate hypoxia | Severe hypoxia | |||||||
| FIO2 = 0.21 | → 0.30 | 0.15 | → 0.30 | 0.10 | → 0.30 | 0.21 | → 0.30 | 0.15 | → 0.30 | 0.10 | → 0.30 | |
| O2Hb | −6.3 | −6.4 | −17.7 | −14.4 | −23.9 | −7.2 | −15.6 | −12.9 | −17.3 | −12.3 | −19.0 | −12.1 |
| ± 3.2 | ± 3.5 | ± 1.8† | ± 1.7* | ± 1.7†‡ | ± 2.9* | ± 1.4 | ± 1.3* | ± 2.7 | ± 2.2* | ± 1.2† | ± 1.5* | |
| HHb | 15.5 | 13.5.5 | 21.8 | 17.4 | 26.9 | 10.7 | 17.9 | 16.8 | 18.7 | 16.8 | 17.9 | 14.1 |
| ± 1.9 | ± 2.2* | ± 1.7† | ± 2.5* | ± 1.6†‡ | ± 1.6* | ± 2.2 | ± 2.0 | ± 3.8 | ± 3.7 | ± 2.6 | ± 2.3*† | |
| THb | 9.2 | 7.1 | 4.0 | 3.0 | 3.0 | 3.5 | 2.3 | 3.9 | 1.4 | 4.5 | −1.2 | 2.0 |
| ± 1.8 | ± 1.5* | ± 0.8† | ± 0.6† | ± 1.0† | ± 1.4† | ± 2.0 | ± 1.7 | ± 2.1 | ± 2.1* | ± 2.1† | ± 1.4* | |
Values are expressed as means ± s.e.m. Measurements represent change from normalized, normoxic, resting values of 0 μm (P < 0.05). Values are statistically different from rest, except for cerebral O2Hb values during normoxia and all muscle THb values. O2Hb, oxyhaemoglobin; HHb, deoxyhaemoglobin; THb, total haemoglobin.
Different from value obtained immediately prior to the switch to hyperoxia.
Different from normoxia.
Different from moderate hypoxia. n = 8.
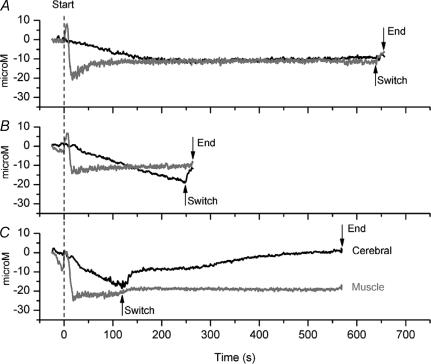
Figure 6. Micromolar changes in oxyhaemoglobin (O2Hb) from normoxic rest period (0 μm) to exhaustion for a single subject.
A, normoxia; B, moderate hypoxia; C, severe hypoxia. Dark lines represent cerebral [O2Hb] and light lines represent vastus lateralis [O2Hb]. The arrows indicate the points at which the inspirate was switched from either normoxia (A; FIO2 0.21), moderate hypoxia (B; FIO2 0.15), or severe hypoxia (C; FIO2 0.10) to hyperoxia (FIO2 0.30), and the end termination of exercise (exhaustion).
Changes in muscle oxygenation during exercise
Muscle oxygenation increased during the first few seconds of exercise in each FIO2, yet fell rapidly thereafter to reach steady-state levels within ∼30 s (Fig. 6). In general, the decrease in muscle oxygenation at the point of task failure was remarkably similar between trials (Table 4), although muscle oxygenation during severe hypoxia tended to be less than during normoxia. Following the switch to hyperoxia, muscle oxygenation increased slightly. At exhaustion, the extent of muscle oxygenation was again similar between trials.
Changes in cerebral oxygenation during exercise
Cerebral oxygenation fell progressively during the first few minutes of exercise (Fig. 6). In normoxia, steady-state levels were achieved after ∼180 s. Steady states were not achieved during moderate or severe hypoxia before the point of task failure. In contrast to muscle oxygenation, the extent of cerebral deoxygenation at task failure was different between all trials, with the largest changes observed during severe hypoxia (Table 4). Following the switch to hyperoxia, cerebral oxygenation increased rapidly in all trials. At exhaustion, changes in cerebral oxygenation were similar between trials.
Discussion
Summary of findings
The purpose of this study was to investigate the relative importance of peripheral locomotor muscle fatigue versus ‘central’ effects of hypoxia on exercise performance across a range of severity of arterial hypoxaemia (end-exercise SpO2 94% to 66%). At task failure in normoxia and moderate hypoxia, increasing SpO2 via a hyperoxic inspirate did not result in a continuation of exercise despite an instantaneous muscle/cerebral hyperoxygenation (as indicated by the NIRS data), whereas in severe hypoxia, muscle/cerebral hyperoxygenation enabled the subjects to significantly extend their exercise time. The level of end-exercise peripheral muscle fatigue (ΔQtw,pot, pre- versus post-exercise) was substantial and identical at exhaustion in normoxia and moderate hypoxia despite marked differences in exercise time, and superimposed hyperoxia did not alleviate peripheral fatigue. In severe hypoxia, peripheral fatigue at task failure was significant despite the relatively brief exercise duration, but substantially less than fatigue at task failure in normoxia and moderate hypoxia. Hyperoxygenation at task failure in severe hypoxia enabled the subjects to continue exercising, and at exhaustion locomotor muscle fatigue rose to the same level as in normoxia and moderate hypoxia following superimposition of hyperoxia. These reductions in Qtw,pot from pre- to post-exercise corresponded with the relative magnitude of time-dependent increases in quadriceps EMG (reflecting additional motor unit recruitment) during the various conditions of exercise and ΔFIO2. Furthermore, heart rate, capillary blood lactate, and effort perceptions shared similar time-dependent increases during exercise, and near-identical absolute values at end-exercise following hyperoxygenation, in all conditions of varying FIO2. Our findings support the postulate that the mechanisms underlying hypoxia-induced curtailment of exercise performance will differ depending on the severity of the hypoxaemia, with hypoxic-induced peripheral locomotor muscle fatigue being a dominant influence in moderate hypoxia (SaO2 > 70%) and primarily – but not exclusively – dependent upon non-peripheral influences (such as CNS hypoxia) in more severe hypoxaemia.
Dominance of peripheral effects on exercise performance in mild and moderate hypoxaemia
Evidence from the current investigation argues in favour of a dominant role for peripheral fatigue development in determining exercise performance under conditions of normoxia and moderate hypoxia. The key evidence supporting this conclusion is provided by the observation that at the point of task failure in normoxia (SpO2 94%) and in moderate hypoxia (SpO2 82%), the participants were unable to prolong their exercise following hyperoxygenation of systemic and cerebral tissues to hyperoxic levels. We propose that the degree of quadriceps fatigue developed, as represented by the greater than 35% reduction in quadriceps twitch force, was sufficient to prevent further exercise upon hyperoxygenation, primarily through its feedback effect on central motor output. The observation that peripheral fatigue was nearly identical at exhaustion in normoxia and moderate hypoxia, despite markedly different performance times and levels of arterial and cerebral oxygenation, provides strong correlative evidence that peripheral fatigue was a ‘regulated variable’. Apparently the CNS ‘allowed’ the development of locomotor muscle fatigue until, as suggested by Gandevia (2001), a critical threshold or ‘sensory tolerance limit’ was achieved, which in turn curtailed central motor output. We previously observed similar effects of moderate hypoxia through hyperoxia on central motor output, force output and quadriceps fatigue, employing a time trial test during which the cyclist was allowed to continually ‘select’ the desired power output throughout the performance (Amann et al. 2006a).
There are two types of evidence that speak against the capability of the substantially fatigued locomotor muscle to continue exercise despite the superimposition of systemic and tissue hyperoxia. First, during exercise, tissue hyperoxygenation would not be expected to instantly alleviate the already developed substantial level of peripheral muscle fatigue. Using magnetic resonance spectroscopy during submaximal constant work rate plantar flexion exercise in hypoxia, Haseler et al. (1998, 1999) observed a time-dependant accumulation of inorganic phosphate (Pi), and also showed that substantial hyperoxygenation of the hypoxic fatiguing muscle tissue via a hyperoxic inspirate attenuated the rate accumulation of muscle Pi. Inorganic phosphate has been shown to be a major determinant of muscle fatigue (Fitts, 1994; Fryer et al. 1995; Dahlstedt et al. 2001; Dutka et al. 2005). However, the resulting reduction in muscular accumulation of (cytosolic) Pi via hyperoxygenation during exercise was apparently insufficient to significantly reduce the level of peripheral quadriceps fatigue, as we observed identical levels of fatigue (ΔQtw,pot) in normoxia/moderate hypoxia at task failure and 18–21 s later at exhaustion following hyperoxygenation (Table 3). Furthermore, when Qtw,pot is reduced substantially, as it was in these experiments at task failure in normoxia and moderate hypoxia, our work and that of others shows that even if hypoxaemia is removed via increased FIO2 and the exercise completely terminated, the Qtw,pot does not return to control even when the muscle is held normoxic and at rest for more than 70 min (Edwards et al. 1977; Amann et al. 2006a; Romer et al. 2007). These observations in humans using functional measures of peripheral fatigue are supported by Dutka et al. (2005) who showed in isolated rat muscle fibres that force-generating capacity was reduced after exposure to increased Pi in a concentration-dependent manner, and that this reduction in force output was still present after all Pi had been subsequently washed out of the intracellular space.
Combined, these findings show that the increase in tissue oxygenation of the quadriceps at task failure, i.e. at a point when substantial peripheral muscle fatigue has been incurred in normoxia and moderate hypoxia, was not capable of alleviating the magnitude of peripheral fatigue, and we suggest that exercise was therefore terminated via a reduction in central motor output in order to prevent further development of peripheral fatigue beyond a critical threshold.
Support for a hypoxia-sensitive, ‘non-peripheral’ source of inhibition of central neural drive and exercise performance limitation in severe hypoxia
In severe hypoxia our evidence suggests that the major determinant of exercise performance switches from one of a predominantly peripheral origin (as it was in normoxia and moderate hypoxia – see above) to a hypoxia-sensitive ‘central’ component of fatigue, probably involving brain hypoxia. This proposal is based on the observation that exercise beyond the point of task failure in severe hypoxia could be continued for a substantial period following hyperoxygenation. Furthermore we propose that the exercise in severe hypoxia was terminated before peripheral fatigue development reached a critical level (as observed at task failure in normoxia and moderate hypoxia).
The level of peripheral locomotor muscle fatigue was significant at task failure in severe hypoxia, as indicated by significant exercise-induced ΔQtw,pot, and this reduction in Qtw,pot was shown to be due about equally to the exercise, per se, and to the severe hypoxia (note normoxia versus severe hypoxia comparisons at isotime of exercise, Table 3). However, ΔQtw,pot at task failure in severe hypoxia was only about two-thirds of the quadriceps fatigue obtained at task failure in normoxia and moderate hypoxia, suggesting a relatively minor involvement of peripheral fatigue in the decision to terminate exercise in severe hypoxia. In addition, capillary blood lactate concentration, heart rate and ratings of perceived exertion for dyspnoea and leg discomfort were substantially lower at task failure in severe hypoxia, as compared to the two other conditions (Table 2), which supports earlier findings (Green et al. 1989; Boushel et al. 2001; Lundby et al. 2001). Finally, very quickly following hyperoxygenation at the point of task failure in severe hypoxia, pedal frequency was restored and exercise could be continued. This ability to continue high-intensity exercise upon hyperoxygenation also suggests that the observed degree of development of peripheral fatigue induced via exercise in severe hypoxia is clearly not a significant deterrent to continued high levels of central motor output and muscular energy production. Furthermore, with hyperoxygenation, peripheral quadriceps fatigue (ΔQtw,pot and quadriceps EMG activity), blood lactate, RPE, and heart rate all continued to rise significantly and, at exhaustion in hyperoxia, were nearly identical to the values observed at task failure in normoxia and moderate hypoxia (Table 2).
Combined, these data suggest that the decision for the termination of exercise in severe hypoxia was, at least in part, independent of peripheral feedback from the locomotor muscles, and indicates the existence of a hypoxia-sensitive source of inhibition of central motor drive outside the contracting muscle. This situation in severe hypoxia contrasts with that during exercise in normoxia and moderate hypoxia (see above), where the level of CNS hypoxia was apparently not sufficient to restrain a continued high level of central motor output; accordingly the CNS ‘allowed’ exercise to continue to the point of development of a critical threshold of locomotor muscle fatigue, resulting in task failure.
Mechanisms of ‘non-peripheral fatigue’: CNS hypoxia
Accumulation and depletion of various brain neurotransmitters induced by normoxic exercise have been implicated in central fatigue and in the constraint of endurance exercise performance (Meeusen & De Meirleir, 1995; Davis & Bailey, 1997). Of particular interest is the ‘central fatigue hypothesis’, which is based on the increase in brain serotonin (5-hydroxytryptamine, 5-HT) (Newsholme et al. 1987; Blomstrand et al. 1988) following exercise-induced increases in the plasma level of its precursor tryptophan (Chaouloff et al. 1985; Newsholme et al. 1987; Blomstrand, 2006; Newsholme & Blomstrand, 2006). A different model of central fatigue proposes the local depletion of brain glycogen during normoxic exercise. Dalsgaard et al. (2002) found that during high-intensity exercise, ‘central motor command’ increases brain metabolism and that this intense activity in cerebral regions causes energy demand to exceed production. Nevertheless, while both of these models of central fatigue might well be involved in normoxic and both levels of hypoxic exercise, neither of them is capable of explaining the instant prolongation of exercise following hyperoxygenation at task failure in severe hypoxia. It seems unlikely that hyperoxygenation results in (a) an instant reduction in the plasma level of the 5-HT precursor tryptophan and (b) an instant restoration of brain glycogen.
The most appealing explanation for our finding that exercise in severe hypoxia was terminated well before the critical threshold of peripheral muscle fatigue was reached is the observation that hypoxia per se – even in the absence of exercise – is capable of altering the turnover of several CNS neurotransmitters including dopamine, noradrenaline and serotonin (Koob & Annau, 1974; Gibson & Duffy, 1981; Olson et al. 1983). These neurotransmitters play an important role in the limbic-to-motor link within the basal ganglia (Nauta, 1986), goal-directed behaviour (limbic processing, i.e. motivation: Schultz et al. 1997, 1998; motor processing (preparation and execution of movement): Chaudhuri & Behan, 2000; and cognitive functions: Harik et al. 1982) involving both prefrontal cortex and the basal ganglia. The latter are particularly vulnerable to hypoxia, and alterations of neurotransmitter balance can cause reversible basal ganglia dysfunction (Chaudhuri & Behan, 2000). Consequently, hypoxia-induced alterations in neurotransmitter turnover have been suggested as significant determinants of the pathophysiology of behavioural abnormalities and central fatigue in severe hypoxia (Chaudhuri & Behan, 2000; Raichle & Hornbein, 2001).
We did not address the relative importance of reduced PaO2 versus CaO2 (achieved via ΔFIO2); however, there is indirect evidence from studies utilizing experimental changes in [Hb] in acute and chronic severe hypoxia to support the concept that the effects of severe hypoxia on exercise performance and presumably CNS oxygenation are likely to be modified via differences in PaO2 rather than CaO2 (or [Hb]), per se (Horstman et al. 1980; Calbet et al. 2002; Lundby & Damsgaard, 2006).
Other potential factors affecting central motor drive in severe hypoxia
Our findings only suggest that in severe acute hypoxia exercise performance is limited by influences other than peripheral locomotor muscle fatigue. Independent of severe cerebral hypoxia it has been suggested that central motor output may also be constrained in severe hypoxia in response to inhibitory reflexes originating in the respiratory muscles (Bigland-Ritchie & Vollestad, 1988; Kayser, 2003) and the myocardium (Alexander et al. 1967; Richalet et al. 1988), apparently in order to avoid severe respiratory muscle fatigue and/or hypoxic myocardial dysfunction. However, both of these hypotheses have been challenged (Reeves et al. 1987; Suarez et al. 1987; Savard et al. 1996; Kjaer et al. 1999; Wagner, 2000). Evidence against an effect on performance imposed by hypoxia-induced cardiac limitations is provided by the observation that in severe acute hypoxia no changes in cardiac output were observed at the same absolute workload after the subjects were switched to breathe normoxic or hyperoxic gas mixtures (Calbet et al. 2003a,b). We also observed no change in heart rate upon superimposition of hyperoxia at task failure in severe acute hypoxia. However, in chronic severe hypoxia, a markedly elevated parasympathetic tone limits the exercise-induced tachycardia, and hyperoxygenation during exercise causes an immediate increase in heart rate and cardiac output (Boushel et al. 2001). So, chronic hypoxia might impose additional types of cardiovascular limitations on exercise performance as well as those experienced in acute hypoxia.
Proposal: a threshold of arterial oxygenation determines central over peripheral determinants of exercise performance
Our findings are consistent with the hypothesis that peripheral muscle fatigue is the major determinant of central motor output and therefore of exercise performance in normoxia and acute moderate hypoxia (Amann et al. 2006a; Romer et al. 2007); whereas in acute severe hypoxia, CNS hypoxia precedes the development of significant peripheral muscle fatigue and dominates the decision to reduce central motor output and to terminate exercise. Furthermore, the present findings obtained at FIO2 0.21, 0.15 and 0.10, together with recent data obtained at FIO2 0.12 (Romer et al. 2007) point to a threshold of SaO2 for our proposed switch from a predominant effect of peripheral fatigue to a predominant effect of CNS hypoxia on central motor output and exercise performance. In total, these data show that critical (and near-identical) levels of peripheral muscle fatigue (ΔQtw,pot about −35%) were reached at the point of task failure when SaO2 averaged 94%, 82%, or 76% (FIO2 0.21–0.12), but not at SaO2 67% (FIO2 0.10). Accordingly, we propose that the dominance of CNS hypoxia over peripheral muscle fatigue in influencing central motor output and therefore exercise performance occurs below a level of acutely compromised O2 transport represented by a range of 70–75% SaO2.
Acknowledgments
This research was supported by a National Heart, Lung, and Blood Institute (NHLBI) RO1 grant (HL-15469). We thank Mr Andrew C. Dimmen and Mrs Rose E. Voelker for valuable assistance with the NIRS and with analysing the EMG data, respectively. We also thank Dr Robert Roach at the Colorado Centre for Altitude Medicine and Physiology for generously allocating his NIRS during the course of the study.
References
- Alexander JK, Hartley LH, Modelski M, Grover RF. Reduction of stroke volume during exercise in man following ascent to 3100 m altitude. J Appl Physiol. 1967;23:849–858. doi: 10.1152/jappl.1967.23.6.849. [DOI] [PubMed] [Google Scholar]
- Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue. J Physiol. 2006a;575:937–952. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Romer LM, Pegelow DF, Jacques AJ, Hess CJ, Dempsey JA. Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J Appl Physiol. 2006b;101:119–127. doi: 10.1152/japplphysiol.01596.2005. [DOI] [PubMed] [Google Scholar]
- Amann M, Subudhi A, Foster C. Influence of testing protocol on ventilatory thresholds and cycling performance. Med Sci Sports Exerc. 2004;36:613–622. doi: 10.1249/01.mss.0000122076.21804.10. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Vollestad N. Hypoxia and Fatigue. How are they Related? Indianapolis IL USA: Benchmark; 1988. [Google Scholar]
- Blomstrand E. A role for branched-chain amino acids in reducing central fatigue. J Nutr. 2006;136:544S–547S. doi: 10.1093/jn/136.2.544S. [DOI] [PubMed] [Google Scholar]
- Blomstrand E, Celsing F, Newsholme EA. Changes in plasma concentrations of aromatic and branched-chain amino acids during sustained exercise in man and their possible role in fatigue. Acta Physiol Scand. 1988;133:115–121. doi: 10.1111/j.1748-1716.1988.tb08388.x. [DOI] [PubMed] [Google Scholar]
- Borg G. Borg's Perceived Exertion and Pain Scales. Champaign IL USA: Human Kinetics; 1998. [Google Scholar]
- Boushel R, Calbet JA, Radegran G, Sondergaard H, Wagner PD, Saltin B. Parasympathetic neural activity accounts for the lowering of exercise heart rate at high altitude. Circulation. 2001;104:1785–1791. doi: 10.1161/hc4001.097040. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003a;284:R291–R303. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Why is V˙o2 after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol. 2003b;284:R304–R316. doi: 10.1152/ajpregu.00156.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Radegran G, Boushel R, Sondergaard H, Saltin B, Wagner PD. Effect of blood haemoglobin concentration on V˙o2 and cardiovascular function in lowlanders acclimatised to 5260 m. J Physiol. 2002;545:715–728. doi: 10.1113/jphysiol.2002.029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caquelard F, Burnet H, Tagliarini F, Cauchy E, Richalet JP, Jammes Y. Effects of prolonged hypobaric hypoxia on human skeletal muscle function and electromyographic events. Clin Sci. 2000;98:329–337. [PubMed] [Google Scholar]
- Chaouloff F, Elghozi JL, Guezennec Y, Laude D. Effects of conditioned running on plasma, liver and brain tryptophan and on brain 5-hydroxytryptamine metabolism of the rat. Br J Pharmacol. 1985;86:33–41. doi: 10.1111/j.1476-5381.1985.tb09432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci. 2000;179:34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Westerblad H. Role of myoplasmic phosphate in contractile function of skeletal muscle: studies on creatine kinase-deficient mice. J Physiol. 2001;533:379–388. doi: 10.1111/j.1469-7793.2001.0379a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard MK, Ide K, Cai Y, Quistorff B, Secher NH. The intent to exercise influences the cerebral O2/carbohydrate uptake ratio in humans. J Physiol. 2002;540:681–689. doi: 10.1113/jphysiol.2001.013062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997;29:45–57. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol. 1995;40:295–304. doi: 10.1088/0031-9155/40/2/007. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Cole L, Lamb GD. Calcium phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C1502–C1512. doi: 10.1152/ajpcell.00273.2005. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. J Physiol. 1995;482(1):123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco CS, Rock PB, Cymerman A. Maximal and submaximal exercise performance at altitude. Aviat Space Environ Med. 1998;69:793–801. [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Duffy TE. Impaired synthesis of acetylcholine by mild hypoxic hypoxia or nitrous oxide. J Neurochem. 1981;36:28–33. doi: 10.1111/j.1471-4159.1981.tb02373.x. [DOI] [PubMed] [Google Scholar]
- Green HJ, Sutton J, Young P, Cymerman A, Houston CS. Operation Everest II: muscle energetics during maximal exhaustive exercise. J Appl Physiol. 1989;66:142–150. doi: 10.1152/jappl.1989.66.1.142. [DOI] [PubMed] [Google Scholar]
- Harik SI, Busto R, Martinez E. Norepinephrine regulation of cerebral glycogen utilization during seizures and ischemia. J Neurosci. 1982;2:409–414. doi: 10.1523/JNEUROSCI.02-04-00409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol. 1998;507:619–628. doi: 10.1111/j.1469-7793.1998.619bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol. 1999;86:2013–2018. doi: 10.1152/jappl.1999.86.6.2013. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Richardson RS, Videen JS, Hogan MC. Phosphocreatine hydrolysis during submaximal exercise: the effect of FIO2. J Appl Physiol. 1998;85:1457–1463. doi: 10.1152/jappl.1998.85.4.1457. [DOI] [PubMed] [Google Scholar]
- Horstman D, Weiskopf R, Jackson RE. Work capacity during 3-wk sojourn at 4300 m: effects of relative polycythemia. J Appl Physiol. 1980;49:311–318. doi: 10.1152/jappl.1980.49.2.311. [DOI] [PubMed] [Google Scholar]
- Jones NL, Killian KJ. Exercise limitation in health and disease. N Engl J Med. 2000;343:632–641. doi: 10.1056/NEJM200008313430907. [DOI] [PubMed] [Google Scholar]
- Kayser B. Exercise starts and ends in the brain. Eur J Appl Physiol. 2003;90:411–419. doi: 10.1007/s00421-003-0902-7. [DOI] [PubMed] [Google Scholar]
- Kayser B, Narici M, Binzoni T, Grassi B, Cerretelli P. Fatigue and exhaustion in chronic hypobaric hypoxia: influence of exercising muscle mass. J Appl Physiol. 1994;76:634–640. doi: 10.1152/jappl.1994.76.2.634. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Hanel B, Worm L, Perko G, Lewis SF, Sahlin K, Galbo H, Secher NH. Cardiovascular and neuroendocrine responses to exercise in hypoxia during impaired neural feedback from muscle. Am J Physiol Regul Integr Comp Physiol. 1999;277:R76–R85. doi: 10.1152/ajpregu.1999.277.1.R76. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- Koob GF, Annau Z. Behavioral and neurochemical alterations induced by hypoxia in rats. Am J Physiol. 1974;227:73–78. doi: 10.1152/ajplegacy.1974.227.1.73. [DOI] [PubMed] [Google Scholar]
- Kufel TJ, Pineda LA, Mador MJ. Comparison of potentiated and unpotentiated twitches as an index of muscle fatigue. Muscle Nerve. 2002;25:438–444. doi: 10.1002/mus.10047. [DOI] [PubMed] [Google Scholar]
- Lepers R, Maffiuletti NA, Rochette L, Brugniaux J, Millet GY. Neuromuscular fatigue during a long-duration cycling exercise. J Appl Physiol. 2002;92:1487–1493. doi: 10.1152/japplphysiol.00880.2001. [DOI] [PubMed] [Google Scholar]
- Lundby C, Damsgaard R. Exercise performance in hypoxia after novel erythropoiesis stimulating protein treatment. Scand J Med Sci Sports. 2006;16:35–40. doi: 10.1111/j.1600-0838.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- Lundby C, Moller P, Kanstrup IL, Olsen NV. Heart rate response to hypoxic exercise: role of dopamine D2-receptors and effect of oxygen supplementation. Clin Sci. 2001;101:377–383. [PubMed] [Google Scholar]
- Lundby C, Sander M, Van Hall G, Saltin B, Calbet JAL. Maximal exercise and muscle oxygen extraction in acclimatizing lowlanders and high altitude natives. J Physiol. 2006;573:535–547. doi: 10.1113/jphysiol.2006.106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20:160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJH. The relationship of basal ganglia to the limbic system. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. Vol. 49. Amsterdam: Elsevier Science; 1986. pp. 19–32. [Google Scholar]
- Newsholme EA, Acworth I, Blomstrand E. Amino Acids, Brain Neurotransmitters and a Functional Link between Muscle and Brain that is Important in Sustained Exercise. London: John Libby Eurotext; 1987. [Google Scholar]
- Newsholme EA, Blomstrand E. Branched-chain amino acids and central fatigue. J Nutr. 2006;136:274S–276S. doi: 10.1093/jn/136.1.274S. [DOI] [PubMed] [Google Scholar]
- Olson EB, Jr, Vidruk EH, McCrimmon DR, Dempsey JA. Monoamine neurotransmitter metabolism during acclimatization to hypoxia in rats. Respir Physiol. 1983;54:79–96. doi: 10.1016/0034-5687(83)90115-9. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Hornbein TF. The high-altitude brain. In: Hornbein TF, Schoene RB, editors. High Altitude. New York: Marcel Dekker, Inc.; 2001. pp. 377–423. [Google Scholar]
- Rassier DE, Macintosh BR. Coexistence of potentiation and fatigue in skeletal muscle. Braz J Med Biol Res. 2000;33:499–508. doi: 10.1590/s0100-879x2000000500003. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Groves BM, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM, Houston CS. Operation Everest II: preservation of cardiac function at extreme altitude. J Appl Physiol. 1987;63:531–539. doi: 10.1152/jappl.1987.63.2.531. [DOI] [PubMed] [Google Scholar]
- Richalet JP, Larmignat P, Rathat C, Keromes A, Baud P, Lhoste F. Decreased cardiac response to isoproterenol infusion in acute and chronic hypoxia. J Appl Physiol. 1988;65:1957–1961. doi: 10.1152/jappl.1988.65.5.1957. [DOI] [PubMed] [Google Scholar]
- Romer LM, Haverkamp HC, Amann M, Lovering AT, Pegelow DF, Dempsey JA. Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R598–R606. doi: 10.1152/ajpregu.00269.2006. [DOI] [PubMed] [Google Scholar]
- Romer LM, Haverkamp HC, Lovering AT, Pegelow DF, Dempsey JA. Effect of exercise-induced arterial hypoxemia on quadriceps muscle fatigue in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2006;290:R365–R375. doi: 10.1152/ajpregu.00332.2005. [DOI] [PubMed] [Google Scholar]
- Sandiford SD, Green HJ, Duhamel TA, Schertzer JD, Perco JD, Ouyang J. Muscle Na–K-pump and fatigue responses to progressive exercise in normoxia and hypoxia. Am J Physiol Regul Integr Comp Physiol. 2005;289:R441–R449. doi: 10.1152/ajpregu.00652.2004. [DOI] [PubMed] [Google Scholar]
- Savard GK, Areskog NH, Saltin B. Maximal muscle activation is not limited by pulmonary ventilation in chronic hypoxia. Acta Physiol Scand. 1996;157:187–190. doi: 10.1046/j.1365-201X.1996.493234000.x. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37:421–429. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Strojnik V, Komi PV. Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J Appl Physiol. 1998;84:344–350. doi: 10.1152/jappl.1998.84.1.344. [DOI] [PubMed] [Google Scholar]
- Suarez J, Alexander JK, Houston CS. Enhanced left ventricular systolic performance at high altitude during Operation Everest II. Am J Cardiol. 1987;60:137–142. doi: 10.1016/0002-9149(87)91000-9. [DOI] [PubMed] [Google Scholar]
- Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG. Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J Appl Physiol. 2001;90:511–519. doi: 10.1152/jappl.2001.90.2.511. [DOI] [PubMed] [Google Scholar]
- van der Zee P, Cope M, Arridge SR, Essenpreis M, Potter LA, Edwards AD, Wyatt JS, McCormick DC, Roth SC, Reynolds EO, et al. Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv Exp Med Biol. 1992;316:143–153. doi: 10.1007/978-1-4615-3404-4_17. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Reduced maximal cardiac output at altitude – mechanisms and significance. Respir Physiol. 2000;120:1–11. doi: 10.1016/s0034-5687(99)00101-2. [DOI] [PubMed] [Google Scholar]