Abstract
The signalling molecules that are involved in inflammatory pathways are now thought to play a part in many disorders of the central nervous system (CNS). In common with peripheral chronic inflammatory diseases such a rheumatoid arthritis and ulcerative colitis, evidence now exists for the involvement of inflammatory cytokines, for example tumour necrosis factor (TNF) and interleukins (IL), in neurological disorders. A common factor observed with the up-regulation of these cytokines in peripheral inflammatory diseases, is the increased expression of the proteinase-activated receptor (PAR) subtype PAR-2. Indeed, recent evidence suggests that targeting PAR-2 helps reduce joint swelling observed in animal models of arthritis. So could targeting this receptor prove to be useful in treating those CNS disorders where inflammatory processes are thought to play an intrinsic role? The aim of this review is to summarize the emerging data regarding the role of PAR-2 in neuroinflammation and ischaemic injury and discuss its potential as an exciting new target for the prevention and/or treatment of CNS disorders.
Inflammation and CNS disorders
Despite the CNS and the immune system formerly being thought of as separate entities, it is now well recognized that the communication between these two systems is a bidirectional process (Exton et al. 2001; Watkins & Maier, 2005). With the increased understanding of this interaction, it has now become clear that one of the fundamental defence mechanisms of the immune system, namely inflammation, is implicated to play a major role in certain CNS disorders. Indeed, the up-regulation of inflammatory cytokines, for example TNF-α, TNF-β, IL-1β and IL-6, are observed in acute CNS conditions such as brain trauma, as well as in chronic CNS diseases states, such as Alzheimer's disease, Parkinson's disease and multiple sclerosis (Campbell, 2004; Lucas et al. 2006). This increased expression of inflammatory cytokines in CNS disorders mirrors their well-documented role in peripheral inflammatory diseases, such as rheumatoid arthritis and inflammatory bowel disease (McInnes & Gracie, 2004; Gordon et al. 2005). Significantly, identification of cytokines in peripheral diseases has led to the development of novel therapies for the treatment of these inflammatory disorders (Olsen & Stein, 2004; Panaccione et al. 2005). However, other common mediators in these inflammatory diseases have now been identified, including the G-protein coupled receptor (GPCR) proteinase-activated receptor-2 (PAR-2).
Proteinase-activated receptors
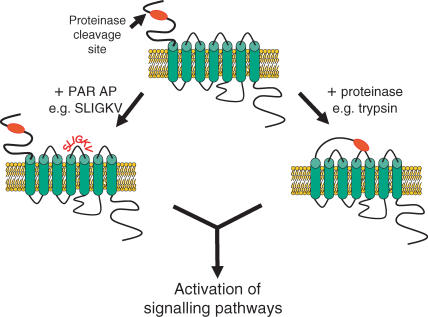
Proteinase-activated receptors (PARs) are a novel class of GPCRs that are unique in their activation, whereby the cleavage of the N-terminus by a serine proteinase unveils a sequence that acts as a ‘tethered-ligand’. The ‘tethered-ligand’ then binds to the second extracellular loop of the receptor, leading to the activation of the receptor (MacFarlane et al. 2001; Ossovskaya & Bunnet, 2004; Fig. 1). To date, four members of the PAR family have been cloned, namely PAR-1 to -4. Of these, PAR-1, -3 and -4 are preferentially activated by thrombin, whereas trypsin and trypsin-like proteinases are proposed to preferentially activate PAR-2 (MacFarlane et al. 2001; Ossovskaya & Bunnet, 2004). However, it should be noted that in astrocytes in which PAR-2 has been desensitized as well as in astrocytes derived from PAR-2 knockout mice, trypsin still elicits a robust, albeit reduced, increase in intracellular calcium (Ca2+) (Hamill 2006; T. Bushell et al. unpublished data), indicating that trypsin can act on multiple receptors. Several selective PAR-activating peptides (APs), based on the tethered ligand sequence for each receptor, e.g. TFLLR-NH2 (PAR-1; Hollenberg et al. 1997), SLIGRL-NH2 (PAR-2; al-Ani et al. 1995) and 2f-LIGKV-OH (PAR-2; Kawabata et al. 2004) and AYPGKF-NH2 (PAR-4; Hollenberg & Saifeddine, 2001), have been developed to probe the distinct functions of each receptor, although evidence suggests diligence is required when using these and other peptide agonists (Stenton et al. 2002; Hollenberg et al. 2004; Moffatt, 2004; Fig. 1). This approach, in addition to studies utilizing PAR-deficient mice, have indicated that, whilst thrombin-sensitive PARs seem to be involved in wound healing (MacFarlane et al. 2001; Strukova, 2001), thrombogenesis (MacFarlane et al. 2001; Strukova, 2004) and inflammation (Moller et al. 2000; Asokananthan et al. 2002; Suo et al. 2002; Vergnolle, 2004; Nicole et al. 2005). PAR-2, is thought primarily to play a key role in conditions associated with inflammation. Recent studies show roles in the mediation of inflammatory pain (Hoogerwerf et al. 2001; Vergnolle et al. 2001; Fiorucci & Distrutti, 2002; Dai et al. 2004), arthritis (Ferrell et al. 2003; Kelso et al. 2006) and a number of skin disorders (Steinhoff et al. 2003; Namazi, 2005), whilst other work has provided evidence for a protective, anti-inflammatory role in airways (Cocks et al. 1999; Kawabata & Kawao, 2005; Morello et al. 2005; Henry, 2006) and intestine (Kawabata et al. 2001; Fiorucci et al. 2001). These studies indicate that PAR-2 exhibits a duality of function depending upon the tissue and the disease context and, as such, considerable attention is being directed to the development of selective PAR-2 agonists and antagonists as therapeutic agents for peripheral inflammatory diseases (Scarborough, 2003; Kanke et al. 2005; Kelso et al. 2006).
Figure 1. Schematic representation of PAR-2 activation.
Cleavage of the N-terminus by a serine proteinase such as trypsin, reveals a ‘tethered ligand’ which binds to the second extracellular loop of the receptor leading to receptor activation of signalling pathways. The identification of the tethered ligand amino acid sequence has led to the development of PAR-2-activating peptides, e.g. SLIGKV for PAR-2 that has enhanced our understanding of the physiological roles of the receptor.
A role for PAR-2 in the brain?
Our knowledge of PAR function in the brain is largely based on PAR-1 (for reviews see Gingrich & Traynelis, 2000; Noorbakhsh et al. 2003; Rohatgi et al. 2004); however, despite the extensive literature regarding the role of PAR-2 in the periphery, the extent of our knowledge regarding PAR-2 function in the brain is small in comparison. In situ hybridization and immunohistochemical localization studies reveal neuronal expression of PAR-2 in the rodent and human brain (D'Andrea et al. 1998; Striggow et al. 2001; Riek-Burchardt et al. 2002; Noorbakhsh et al. 2003, 2005, 2006; Jin et al. 2005; Bushell et al. 2006). Investigations into the consequence of PAR-2 activation on central neurones reveal that acute exposure of cultured hippocampal neurones to PAR-2-selective agonists elevates intracellular calcium (Ca2+) through the Gq/phospholipase C (PLC) (phospholipase C) pathway (Bushell et al. 2006). Furthermore, PAR-2 activation has been shown to be neurotoxic to cultured hippocampal neurones in a concentration-dependant manner (Smith-Swintosky et al. 1997). Interestingly, in both of these studies a heterogeneous expression of PAR-2 was observed in neurones, with a selective increased expression of PAR-2 on GABAergic neurones. Further investigation revealed that GABAergic neurones with higher levels of PAR-2 expression exhibited increased Ca2+ signalling upon exposure to agonists (Bushell et al. 2006). Although a similar heterogeneity in expression levels was not evident when investigated in slice preparations, the link between expression and function may be valuable in helping elucidate the consequence of PAR-2 up-regulation.
In addition to studies regarding neuronal PAR-2, it is now well established that the receptor is located in CNS glial cells. PAR-2 expression has been shown in astrocytes both in rodent primary cultures (Ubl et al. 1998; Wang et al. 2003; Bushell et al. 2006; Park et al. 2006) and acute brain slices (Pompilli et al. 2004; Bushell et al. 2006) as well in human white matter (Noorbakhsh et al. 2006). Similar to the findings with neuronal PAR-2, receptor activation in cultured astrocytes elicits rises in intracellular calcium levels, which has led to suggestions that astrocytic PAR-2 may play a neuroprotective and/or neurodegenerative role in the brain. In addition to astrocytic expression, PAR-2 has also recently been shown to be expressed in microglia (Goldshmidt & Traynelis, 2006; Noorbakhsh et al. 2006), cells which not only underlie the immune response within the brain but are also proposed to be involved in functions such as synaptogenesis and developmental apoptosis (Bessis et al. 2007).
PAR-2 up-regulation in disease conditions affecting the CNS
Clear evidence now exists for an up-regulation of PAR-2 expression in certain peripheral inflammatory disease states. However, is a similar up-regulation observed in experimental CNS disease models and in human tissue from patients suffering from CNS neurological disorders? The first evidence indicating PAR up-regulation was observed following exposure to severe experimental ischaemia (oxygen–glucose deprivation), which resulted in an increased expression of PAR-2, as well as other PARs, in organotypic hippocampal slice cultures (Striggow et al. 2001). A significant increase in PAR-2 was observed at 6 h which lasted until 24 h post-exposure to experimental ischaemia. However, the levels of PAR-2 exposure were not significantly altered when examined 72 h post-exposure. Despite the functional consequence of this up-regulation not being investigated, this study revealed that under experimental disease conditions, PAR-2 expression was indeed up-regulated, but over a specific time course. Similar to results observed in intestinal radiation damage (Wang et al. 2003), up-regulation of PAR-2 expression was also evident following experimental CNS radiation damage, with increased expression being observed for up to 40 days post-treatment (Olejar et al. 2002). This is significant because radiation injury is thought to occur via the activation of chronic inflammatory pathways (Monje et al. 2003). As inflammation is known to impair adult hippocampal neurogenesis (Ekdahl et al. 2003; Monje et al. 2003), which in itself may contribute to the cognitive dysfunction observed following cranial radiation therapy (Parent et al. 1999; Abayomi, 2002; Monje et al. 2002), the increase in PAR-2 expression exhibited under these conditions may contribute to the inhibition of neurogenesis. Taken together, these findings suggest a link between inflammation and PAR-2 function which appears to be common in both CNS and peripheral inflammation-induced disorders. However, the functional consequence of this up-regulation in the CNS remained unclear. The first indicator as to the role of PAR-2 was seen in in vivo models of acute focal ischaemic brain injury. In control experiments, PAR-2 was found to be significantly up-regulated up to 24 h following transient occlusion of the middle cerebral artery (tMCAO) (Jin et al. 2005), a finding similar to that previously observed following oxygen–glucose deprivation in organotypic slice cultures (Striggow et al. 2001). Furthermore, in PAR-2-deficient mice, the infarct volume following tMCAO was significantly increased when compared with wild-type controls (Jin et al. 2005) indicating a neuroprotective role for PAR-2 in this model of ischaemic brain injury.
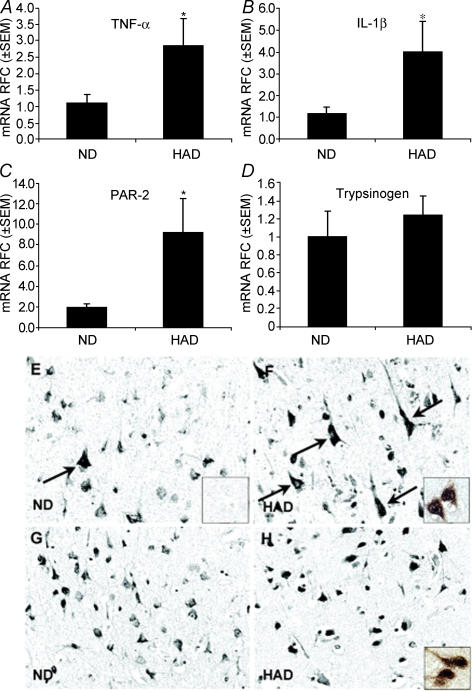
The notion that PAR-2 activation and up-regulation is neuroprotective in nature is also supported in a study that identified an increase in neuronal PAR-2 expression in a human CNS inflammatory disorder, HIV-associated dementia (HAD; Noorbakhsh et al. 2005; Fig. 2). Real time RT-PCR experiments revealed a significant up-regulation of PAR-2 mRNA in brain tissue when compared with non-dementia-associated HIV/AIDS patients (ND). Significantly, this was also associated with comparable elevations of the inflammatory cytokines TNF-α and IL-1β (Fig. 2). Immunohistochemical analysis revealed weak PAR-2 expression in neurones of ND tissue, similar to that reported previously in normal brain tissue (D'Andrea et al. 1998), but this was significantly increased in neurones from HAD patients (Fig. 2). To identify the role of PAR-2 in HAD, the consequence of PAR-2 activation was examined in a mouse model of HIV neuropathogenesis. Striatal implantation of PAR-2 APs significantly inhibited the neurotoxicity induced by the HIV-1 trans-activating protein, Tat, in control mice, whilst in PAR-2-deficient mice, an increased severity of neuroinflammation and neuronal damage occurred. This implies that PAR-2 plays a neuroprotective role in controlling HIV-induced neuropathogenesis, a finding in agreement with the experimental ischaemia study mentioned earlier.
Figure 2. Up-regulation of inflammatory cytokines and PAR-2 in brains of HAD patients.
A–C, up-regulation of mRNA for TNF-α, IL-1β and PAR-2 in brains from HAD patients when compared with ND patients. D, no change in mRNA levels of the potential endogenous activator of PAR-2, Trypsinogen. E, PAR-2 immunoreactivity in ND (inset shows Ab absorbed with immunogen peptide) brain. F, PAR-2 immunoreactivity is increased in HAD (inset shows co-localization with neuronal marker, Neu-N) compared with ND brains. G and H, similar trypsinogen IV immunoreactivity in both ND and HAD (inset shows co-localization with neuronal marker, Neu-N) patients. Reproduced with permission from J Immunol174, 7320–7329.
In contrast to the findings that neuronal PAR-2 up-regulation may be neuroprotective in nature, a degenerative role is proposed for the up-regulation of PAR-2 observed in astrocytes and macrophages in experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis (MS) (Noorbakhsh et al. 2006). In MS post-mortem tissue, PAR-2 expression is increased in CNS white matter compared with that of non-MS patients, with the increases being co-localized with astrocytic and macrophage markers in areas of demyelination. Importantly, PAR-2 expression was evident in neurones but no changes in expression levels were apparent between the MS and non-MS patients. Similar results were obtained in the EAE animal model which presents analogous features to MS in humans. The significance of PAR-2 in this disease is further evidenced by the fact that PAR-2-deficient mice show reduced neurobehavioural phenotype severity following the induction of EAE compared with wild-type litter mates.
In both HIV dementia and MS, a relationship emerges between PAR-2 expression and elevated inflammatory cytokines levels. However, it appears that depending on whether the PAR-2 up-regulation is neuronal or glial dictates if PAR-2 activation is either neuroprotective or neurodegenerative. These findings are further confirmed in experiments performed in human neuronal cell lines and cultured microglia. Exposure of the neuronal cell lines to TNF-α and IL-1β significantly increased PAR-2 expression. Furthermore, in Tat-induced neurotoxicity models performed in primary neuronal culture, TNF-α was neuroprotective in wild-type cultures, whereas it proved to be neurotoxic in PAR-2-deficient cultures (Noorbakhsh et al. 2005), indicating that an increase in PAR-2 expression is responsible for the neuroprotective effect of these cytokines. Although the effects of cytokines on PAR-2 expression was not studied in cultured glia, PAR-2 activation in microglia, but not astrocytes, is associated with an increase in the expression levels of TNF-α, IL-6, IL-12 and iNOS. Further, the supernatant taken from PAR-2-activated microglia is toxic to oligodendrocytes (Noorbakhsh et al. 2006), cells which are associated with demyelinating lesions in MS (Barnett & Prineas, 2004). These findings and, as mentioned earlier, the fact that PAR-2-deficient mice exhibit an decreased EAE phenotype suggest a link between PAR-2 and inflammatory cytokines in the neurodegeneration observed in MS and EAE animal models These findings are particular interesting as evidence exists for TNF-α being both neuroprotective or neurotoxic depending on the system utilized and the conditions investigated (Garcia et al. 1992; Barger et al. 1995; Herman et al. 2001; Zhao et al. 2001; Sitcheran et al. 2005; Zou & Crews, 2005; Sriram et al. 2006). The link between TNF-α and PAR-2 is further supported by studies which observed similar increases in infarct volume following tMCAO in mice deficient in TNF-R1 or both TNF-R1 and TNF-R2 (Bruce et al. 1996; Gary et al. 1998). In addition, mice with a genetic mutation in TNF-α were more susceptible to sodium nitroprusside-induced neurodegeneration (Turrin & Rivest, 2006). These findings strongly support a close interaction between TNF-α and PAR-2 and beg the question, could the potential link between either a neuroprotective or a neurotoxic effect of TNF-α be the capacity for PAR-2 up-regulation in the particular cell type investigated or neurological model used?
How does PAR-2 induce neuroprotection or neurodegeneration?
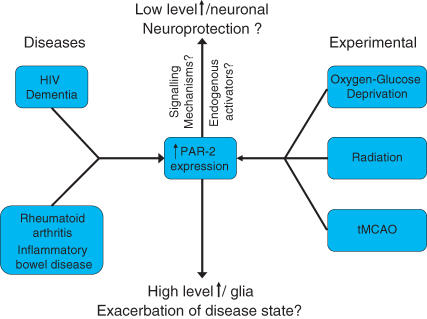
The available evidence indicates that activation of PAR-2 subsequent to its up-regulation is neuroprotective or neurodegenerative depending on the cell type in which it occurs (Fig. 3), yet there are still questions about the role of PAR-2 in the CNS which remain unanswered such as what are the endogenous PAR-2 activators and what are the signalling mechanisms that underlie its neuroprotective and/or neurodegenerative effects?
Figure 3. Flow chart illustrating the disease states and experimental conditions that have led to the increased expression of PAR-2.
It remains to be determined whether PAR-2 has a duality of function depending of the levels of expression and/or cell type in which the up-regulation is seen.
Endogenous activators
One mechanism by which endogenous activators of PAR-2 may infiltrate the brain is via the blood–brain barrier, which is known to become ‘leaky’ under inflammatory conditions (Lossinsky & Shivers, 2004). Consequently, potential activators of PAR-2 such as coagulation factor VIIa and Xa and their cofactors (Bono et al. 2000; Camerer et al. 2000) may enter the brain, activate PAR-2 and so lead to the effects described earlier. Whether this is indeed a mechanism for potential endogenous activators of PAR-2 to enter the brain remains to be examined but such a pathway has been suggested previously for activators of PAR-1 (Gingrich & Traynelis, 2000). Alternatively, an endogenous activator of PAR-2 may exist within the CNS. Several serine proteinases have been shown to modulate neuronal activity and neurotoxicity in the CNS and, as such, could have the potential to be endogenous activators of PARs (Shimizu et al. 1998; Komai et al. 2000; Davies et al. 2001; Melchor & Strickland, 2005; Lochner et al. 2006). Focusing on PAR-2, several candidates could be proposed as potential endogenous activators. The trypsin-like serine proteinase, tryptase, which is known as an activator of PAR-2 in the periphery, has also been found in neurones, blood vessels, as well as mast cells located in the CNS. The proteinase is linked to angiogenesis in animal models of Duchenne muscular dystrophy and the pathogenesis associated with MS (Ibrahim et al. 1996; Nico et al. 2004). Another serine proteinase, trypsinogen IV, which was originally identified in epithelial cells lines (Cottrell et al. 2004), has recently been shown to be expressed in neurones and glia in the brain (Noorbakhsh et al. 2005, 2006). When cloned from PC-3 cells, trypsinogen IV was found to activate both PAR-2 and PAR-4 and thus is also a candidate as a potential endogenous activator of PAR-2 in the brain. Finally, neurotrypsin, whose primary protease domain is similar to that of the PAR-2 activator trypsin, has been found to be expressed in rodent and human CNS (Gschwend et al. 1997; Proba et al. 1998; Wolfer et al. 2001). Neurotrypsin is localized in the presynaptic bouton of both excitatory and inhibitory synapses in the hippocampus and is secreted following synaptic activity (Molinari et al. 2003). Neurotrypsin has been proposed to play an essential functional role in primate cognition throughout evolution (Xu & Su, 2005) and strikingly, a genetic mutation in neurotrypsin is thought to be responsible for autosomal recessive non-syndromic mental retardation (Molinari et al. 2002, 2003). However, despite the apparent similarity between neurotrypsin and the known activator of PAR-2, trypsin, it is still not known if neurotrypsin can indeed activate PAR-2. The proteinases highlighted here are by no means an exhaustive list of all potential endogenous activators of PAR-2; however, they are verification that until the activity of these and other serine proteinases on neuronal and glial PAR-2 are investigated, the identity of the physiological activators of PAR-2 will remain conjecture.
PAR-2 signalling pathways
PARs, including PAR-2, are known to mediate their cellular effects through the activation of heteromeric G-proteins. Studies have revealed that the predominant α subunit involved in mediating PAR effects is the pertussis-toxin-insensitive Gq/G11 and G12/G13 subunits (Babich et al. 1990; Offermanns et al. 1994, 1997; Vaidyula & Rao, 2003). The response to activation of these G-proteins is the elevation of intracellular Ca2+ via the phospholipase C/IP3 pathway as has been shown for PAR-2 in cultured hippocampal neurones (Smith-Swintosky et al. 1997; Bushell et al. 2006). Downstream of increases in intracellular Ca2+, PAR-2 activation stimulates numerous signalling pathways depending on the preparation utilized, for example protein kinase C (PKC), mitogen-activated protein kinases (MAPK) and stress-activated protein kinases (Belham et al. 1996; DeFea et al. 2000; Kanke et al. 2001). PAR-2 activation has also been shown to increase the activity of transient receptor potential vanilloid 1 (TRPV1) channels, a mechanism proposed to be involved in inflammatory pain (Amadesi et al. 2004, 2006; Dai et al. 2004). With TRPV1 being located in the brain (Steenland et al. 2006), its modulation by PAR-2 may be an intriguing avenue to explore with regard to TRPV1 function in relation to central pain processing and other non-pain-related functions. Recently, neuronal PAR-2 has been linked to the stimulation of the extracellular signal-regulated kinase (ERK) subfamily of MAPK following tMCAO (Jin et al. 2005). The increase in ERK activity was neurone specific and was significantly inhibited in PAR-2 KO mice. As an increase in ERK activity is proposed to be beneficial to neuronal survival (Hetman & Xia, 2000; Li et al. 2003; Mocchetti & Bachis, 2004), this suggests the neuroprotective role of PAR-2 observed in this study is directly linked to ERK activation. This raises an interesting issue as to PAR-2 signalling in the CNS. The activation of ERK by PAR-2 is thought to rely on the β-arrestin-dependant internalization of the receptor and the formation of a signalling complex (DeFea et al. 2000). Although the internalization and the formation of a signalling complex are not required for ERK activation per se (DeFea et al. 2000; Seatter et al. 2004; Stalheim et al. 2005), it may be required for appropriate subcellular localization and functioning of ERK. In contrast to the rapid PAR-2 internalization observed in heterologous expression systems and peripheral tissue (Bohm et al. 1996; Dery et al. 1999; DeFea et al. 2000; Seatter et al. 2004; Stalheim et al. 2005), prolonged exposure of cultured hippocampal neurones to trypsin does not result in receptor internalization (Bushell et al. 2006). Similar findings have also been reported for other GPCRs when investigated in neurones (Coutts et al. 2001; Bushell et al. 2002). This leads to further questions such as is PAR-2 internalization required for the observed ERK activation and it's neuroprotective effects or is the lack of internalization in cultured neurones an artefact of the preparation/neurone type used.
Similarities exist in the signalling pathways activated following neuronal and astrocytic PAR-2 activation. Astrocytic receptor activation induces the reversal of astrocytic stellation (Park et al. 2006), a change in astrocytic morphology which has been shown to occur under a variety of pathological conditions (Norton et al. 1992; Sofroniew, 2005). The reversal of stellation occurs in a Ca2+- and PKC-dependant manner, signalling pathways similar to those described earlier for neuronal PAR-2. However, a novel PAR-2 signalling pathway has recently been identified following astrocytic PAR-2 activation with receptor activation leading to the release of GRO/CINC1, a rat counterpart of human interleukin-8, from cultured astrocytes (Wang et al. 2007). This release is independent of PKC, phosphoinositide3-kinase and MAPK kinase (MEK) whereas c-Jun N-terminal kinase 1 (JNK1) plays a pivotal role. Under these conditions, the receptor-induced release of this chemokine is shown to be neuroprotective as astrocytes are protected from ceramide-induced cell death.
Conclusion
Recent findings have increased our understanding of PAR-2 function in the CNS; however, questions still remain. Evidence pertaining to the endogenous activators of the receptor and the potential signalling mechanisms involved in the observed neuroprotection and/or degeneration is discussed in this review. However, whether PAR-2 displays a duality of function depending on (1) the levels of receptor up-regulation, with low level up-regulation being neuroprotective whilst high level up-regulation exacerbates disease conditions, and/or (2) the cell type in which PAR-2 up-regulation occurs, remains to be investigated. These are important questions, the answer to which will further our appreciation of the role of PAR-2 in the CNS. The link between PAR-2 and the numerous inflammatory cytokines also requires further investigation as the interactions between them are likely to be complex in nature and may underlie the contrasting neuroprotective and neurotoxic effects observed with TNF-α. However, despite the emerging evidence linking PAR-2 with CNS disorders, it is important to realize that we are at an early stage in fully understanding its role in the CNS, and further investigation is required before we can unequivocally state that PAR-2 plays a role in CNS disorders. Nevertheless, the finding that PAR-2 potentially plays a neuroprotective and/or neurodegenerative role in the CNS presents the receptor as a theoretical novel target for the treatment of CNS disorders particularly those in which inflammation is thought to play an intrinsic role.
Acknowledgments
Many thanks to R. Plevin and C. Kennedy for providing helpful comments on earlier versions of this manuscript.
References
- Abayomi OK. Pathogenesis of cognitive decline following therapeutic irradiation for head and neck tumors. Acta Oncológica. 2002;41:346–351. doi: 10.1080/028418602760169389. [DOI] [PubMed] [Google Scholar]
- al-Ani B, Saifeddine M, Hollenberg MD. Detection of functional receptors for the proteinase-activated-receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can J Physiol Pharmacol. 1995;73:1203–1207. doi: 10.1139/y95-172. [DOI] [PubMed] [Google Scholar]
- Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cε- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neuroscience. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, Thompson PJ, Stewart GA. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- Babich M, King KL, Nissenson RA. Thrombin stimulates inositol phosphate production and intracellular free calcium by a pertussis toxin-insensitive mechanism in osteosarcoma cells. Endocrinology. 1990;126:948–954. doi: 10.1210/endo-126-2-948. [DOI] [PubMed] [Google Scholar]
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurones against amyloid β-peptide toxicity: evidence for involvement of a κ B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci U S A. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- Belham CM, Tate RJ, Scott PH, Pemberton AD, Miller HR, Wadsworth RM, Gould GW, Plevin R. Trypsin stimulates proteinase-activated receptor-2-dependent and -independent activation of mitogen-activated protein kinases. Biochem J. 1996;320:939–946. doi: 10.1042/bj3200939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Bohm SK, Khitin LM, Grady EF, Aponte G, Payan DG, Bunnett NW. Mechanisms of desensitisation and resensitisation of proteinase-activated receptor-2. J Biol Chem. 1996;271:22003–22016. doi: 10.1074/jbc.271.36.22003. [DOI] [PubMed] [Google Scholar]
- Bono F, Schaeffer P, Herault JP, Michaux C, Nestor AL, Guillemot JC, Herbert JM. Factor Xa activates endothelial cells by a receptor cascade between EPR-1 and PAR-2. Arterioscler Thromb Vasc Biol. 2000;20:E107–E112. doi: 10.1161/01.atv.20.11.e107. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nature Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Bushell T, Endoh T, Simen AA, Ren D, Bindokas VP, Miller RJ. Molecular components of tolerance to opiates in single hippocampal neurones. Mol Pharmacol. 2002;61:55–64. doi: 10.1124/mol.61.1.55. [DOI] [PubMed] [Google Scholar]
- Bushell TJ, Plevin R, Cobb S, Irving A. Characterization of proteinase-activated receptor 2 signalling and expression in rat hippocampal neurones and astrocytes. Neuropharmacology. 2006;50:714–725. doi: 10.1016/j.neuropharm.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci U S A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. Inflammation, neurodegenerative diseases, and environmental exposures. Ann New York Acad Science. 2004;1035:117–132. doi: 10.1196/annals.1332.008. [DOI] [PubMed] [Google Scholar]
- Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, Henry PJ, Carr MJ, Hamilton JR, Moffatt JD. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Amadesi S, Grady EF, Bunnett NW. Trypsin IV, a novel agonist of protease-activated receptors 2 and 4. J Biol Chem. 2004;279:13532–13539. doi: 10.1074/jbc.M312090200. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Anavi-Goffer S, Ross RA, MacEwan DJ, Mackie K, Pertwee RG, Irving AJ. Agonist-induced internalisation and trafficking of cannabinoid CB1 receptors in hippocampal neurones. J Neuroscience. 2001;21:2425–2433. doi: 10.1523/JNEUROSCI.21-07-02425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade-Gordon P. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem. 1998;46:57–64. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B, Kearns IR, Ure J, Davies CH, Lathe R. Loss of hippocampal serine protease BSP1/neuropsin predisposes to global seizure activity. J Neurosci. 2001;21:6993–7000. doi: 10.1523/JNEUROSCI.21-18-06993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dery O, Thoma MS, Wong H, Grady EF, Bunnett NW. Trafficking of proteinase-activated receptor-2 and β-arrestin-1 tagged with green fluorescent protein. β-arrestin-dependent endocytosis of a proteinase receptor. J Biol Chem. 1999;274:18524–18535. doi: 10.1074/jbc.274.26.18524. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton MS, Herklotz J, Westermann J, Schedlowski M. Conditioning in the rat: an in vivo model to investigate the molecular mechanisms and clinical implications of brain-immune communication. Immunol Rev. 2001;184:226–235. doi: 10.1034/j.1600-065x.2001.1840120.x. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, Smith AJ, Hunter GD, McLean JS, McGarry F, Ramage R, Jiang L, Kanke T, Kawagoe J. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S, Distrutti E. Role of PAR2 in pain and inflammation. Trends Pharmacol Sci. 2002;23:153–155. doi: 10.1016/s0165-6147(00)01932-5. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Mencarelli A, Palazzetti B, Distrutti E, Vergnolle N, Hollenberg MD, Wallace JL, Morelli A, Cirino G. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci U S A. 2001;98:13936–13941. doi: 10.1073/pnas.241377298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JE, Jr, Nonner D, Ross D, Barrett JN. Neurotoxic components in normal serum. Exp Neurol. 1992;118:309–316. doi: 10.1016/0014-4886(92)90188-v. [DOI] [PubMed] [Google Scholar]
- Gary DS, Bruce-Keller AJ, Kindy MS, Mattson MP. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J Cereb Blood Flow Metab. 1998;18:1283–1287. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Gingrich MB, Traynelis SF. Serine proteases and brain damage – is there a link? Trends Neurosci. 2000;23:399–407. doi: 10.1016/s0166-2236(00)01617-9. [DOI] [PubMed] [Google Scholar]
- Goldshmidt A, Traynelis SF. Four-dimensional imaging reveals effects of PAR-2 agonists on cell morphology and process motility in primary microglia. Abstr Soc Neurosci. 2006:386.9. [Google Scholar]
- Gordon JN, Di Sabatino A, Macdonald TT. The pathophysiologic rationale for biological therapies in inflammatory bowel disease. Current Opinion Gastroenterol. 2005;21:431–437. [PubMed] [Google Scholar]
- Gschwend TP, Krueger SR, Kozlov SV, Wolfer DP, Sonderegger P. Neurotrypsin, a novel multidomain serine protease expressed in the nervous system. Mol Cellular Neurosci. 1997;9:207–219. doi: 10.1006/mcne.1997.0616. [DOI] [PubMed] [Google Scholar]
- Hamill CE, Goldshmidt A, Traynelis SF. Pharmacological characterization of protease-activated receptor-2 agonists. Abstr Soc Neurosci. 2006:791.9. [Google Scholar]
- Henry PJ. The protease-activated receptor2 (PAR2)–prostaglandin E2–prostanoid EP receptor axis: a potential bronchoprotective unit in the respiratory tract? Eur J Pharmacol. 2006;533:156–170. doi: 10.1016/j.ejphar.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Tumor necrosis factor-α induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis. 2001;8:590–599. doi: 10.1006/nbdi.2001.0414. [DOI] [PubMed] [Google Scholar]
- Hetman M, Xia Z. Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol Exp. 2000;60:531–545. doi: 10.55782/ane-2000-1374. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M. Proteinase-activated receptor 4 (PAR4): activation and inhibition of rat platelet aggregation by PAR4-derived peptides. Can J Physiol Pharmacol. 2001;79:439–442. [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M, al-Ani B, Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can J Physiol Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M, Sandhu S, Houle S, Vergnolle N. Proteinase-activated receptor-4: evaluation of tethered ligand-derived peptides as probes for receptor function and as inflammatory agonists in vivo. Br J Pharmacol. 2004;143:443–454. doi: 10.1038/sj.bjp.0705946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerwerf WA, Zou L, Shenoy M, Sun D, Micci MA, Lee-Hellmich H, Xiao SY, Winston JH, Pasricha PJ. The proteinase-activated receptor 2 is involved in nociception. J Neurosci. 2001;21:9036–9042. doi: 10.1523/JNEUROSCI.21-22-09036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MZ, Reder AT, Lawand R, Takash W, Sallouh-Khatib S. The mast cells of the multiple sclerosis brain. J Neuroimmunol. 1996;70:131–138. doi: 10.1016/s0165-5728(96)00102-6. [DOI] [PubMed] [Google Scholar]
- Jin G, Hayashi T, Kawagoe J, Takizawa T, Nagata T, Nagano I, Syoji M, Abe K. Deficiency of PAR2 gene increases acute focal ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:302–313. doi: 10.1038/sj.jcbfm.9600021. [DOI] [PubMed] [Google Scholar]
- Kanke T, MacFarlane SR, Seatter MJ, Davenport E, Paul A, McKenzie RC, Plevin R. Proteinase-activated receptor-2-mediated activation of stress-activated protein kinases and inhibitory κ B kinases in NCTC 2544 keratinocytes. J Biol Chem. 2001;276:31657–31666. doi: 10.1074/jbc.M100377200. [DOI] [PubMed] [Google Scholar]
- Kanke T, Takizawa T, Kabeya M, Kawabata A. Physiology and pathophysiology of proteinase-activated receptors (PARs): PAR-2 as a potential therapeutic target. J Pharmacol Sci. 2005;97:38–42. doi: 10.1254/jphs.fmj04005x7. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kanke T, Yonezawa D, Ishiki T, Saka M, Kabeya M, Sekiguchi F, Kubo S, Kuroda R, Iwaki M, Katsura K, Plevin R. Potent and metabolically stable agonists for protease-activated receptor-2: evaluation of activity in multiple assay systems in vitro and in vivo. J Pharmacol Exp Ther. 2004;309:1098–1107. doi: 10.1124/jpet.103.061010. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kawao N. Physiology and pathophysiology of proteinase-activated receptors (PARs): PARs in the respiratory system: cellular signaling and hysiological/pathological roles. J Pharmacol Sci. 2005;97:20–24. doi: 10.1254/jphs.fmj04005x4. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kinoshita M, Nishikawa H, Kuroda R, Nishida M, Araki H, Arizono N, Oda Y, Kakehi K. The protease-activated receptor-2 agonist induces gastric mucus secretion and mucosal cytoprotection. J Clin Invest. 2001;107:1443–1450. doi: 10.1172/JCI10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, Hollenberg MD, Sommerhoff CP, McLean JS, Ferrell WR. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther. 2006;316:1017–1024. doi: 10.1124/jpet.105.093807. [DOI] [PubMed] [Google Scholar]
- Komai S, Matsuyama T, Matsumoto K, Kato K, Kobayashi M, Imamura K, Yoshida S, Ugawa S, Shiosaka S. Neuropsin regulates an early phase of schaffer-collateral long-term potentiation in the murine hippocampus. Eur J Neurosci. 2000;12:1479–1486. doi: 10.1046/j.1460-9568.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- Li F, Omori N, Jin G, Wang SJ, Sato K, Nagano I, Shoji M, Abe K. Cooperative expression of survival p-ERK and p-Akt signals in rat brain neurons after transient MCAO. Brain Res. 2003;962:21–26. doi: 10.1016/s0006-8993(02)03774-5. [DOI] [PubMed] [Google Scholar]
- Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, Scalettar BA. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol. 2006;66:564–577. doi: 10.1002/neu.20250. [DOI] [PubMed] [Google Scholar]
- Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood–brain barrier during inflammatory conditions. Histol Histopathol. 2004;19:535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl. 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- McInnes IB, Gracie JA. Targeting cytokines beyond tumor necrosis factor-alpha and interleukin-1 in rheumatoid arthritis. Current Rheumatol Rep. 2004;6:36–42. doi: 10.1007/s11926-004-0007-2. [DOI] [PubMed] [Google Scholar]
- Melchor JP, Strickland S. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb Haemost. 2005;93:655–660. doi: 10.1160/TH04-12-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A. Brain-derived neurotrophic factor activation of TrkB protects neurones from HIV-1/gp120-induced cell death. Crit Rev Neurobiol. 2004;16:51–57. doi: 10.1615/critrevneurobiol.v16.i12.50. [DOI] [PubMed] [Google Scholar]
- Moffatt JD. Proteinase-activated receptor pharmacology: trickier and trickier. Br J Pharmacol. 2004;143:441. doi: 10.1038/sj.bjp.0705949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari F, Meskanaite V, Munnich A, Sonderegger P, Colleaux L. Extracellular proteases and their inhibitors in genetic diseases of the central nervous system. Human Mol Genet. 2003;12:R195–R200. doi: 10.1093/hmg/ddg276. [DOI] [PubMed] [Google Scholar]
- Molinari F, Rio M, Meskenaite V, Encha-Razavi F, Auge J, Bacq D, Briault S, Vekemans M, Munnich A, Attie-Bitach T, Sonderegger P, Colleaux L. Truncating neurotrypsin mutation in autosomal recessive nonsyndromic mental retardation. Science. 2002;298:1779–1781. doi: 10.1126/science.1076521. [DOI] [PubMed] [Google Scholar]
- Moller T, Hanisch UK, Ransom BR. Thrombin-induced activation of cultured rodent microglia. J Neurochem. 2000;75:1539–1547. doi: 10.1046/j.1471-4159.2000.0751539.x. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nature Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morello S, Vellecco V, Roviezzo F, Maffia P, Cuzzocrea S, Cirino G, Cicala C. A protective role for proteinase activated receptor 2 in airways of lipopolysaccharide-treated rats. Biochem Pharmacol. 2005;71:223–230. doi: 10.1016/j.bcp.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Namazi MR. Possible molecular mechanisms to account for the involvement of tryptase in the pathogenesis of psoriasis. Autoimmunity. 2005;38:449–452. doi: 10.1080/08916930500246289. [DOI] [PubMed] [Google Scholar]
- Nico B, Marzullo A, Corsi P, Vacca A, Roncali L, Ribatti D. A possible role of tryptase in angiogenesis in the brain of mdx mouse, a model of Duchenne muscular dystrophy. Neuroscience. 2004;123:585–588. doi: 10.1016/j.neuroscience.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, Hepler JR, McKeon RJ, Traynelis SF. Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. J Neurosci. 2005;25:4319–4329. doi: 10.1523/JNEUROSCI.5200-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh F, Tsutsui S, Vergnolle N, Boven LA, Shariat N, Vodjgani M, Warren KG, Andrade-Gordon P, Hollenberg MD, Power C. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med. 2006;203:425–435. doi: 10.1084/jem.20052148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh F, Vergnolle N, Hollenberg MD, Power C. Proteinase-activated receptors in the nervous system. Nat Rev Neurosci. 2003;4:981–990. doi: 10.1038/nrn1255. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F, Vergnolle N, McArthur JC, Silva C, Vodjgani M, Andrade-Gordon P, Hollenberg MD, Power C. Proteinase-activated receptor-2 induction by neuroinflammation prevents neuronal death during HIV infection. J Immunol. 2005;174:7320–7329. doi: 10.4049/jimmunol.174.11.7320. [DOI] [PubMed] [Google Scholar]
- Norton WT, Aquino DA, Hozuni I, Chiu FC, Brosnan CF. Quantitative aspects of reactive gliosis: a review. Neurochem Res. 1992;17:877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Laugwitz KL, Spicher K, Schultz G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc Natl Acad Sci U S A. 1994;91:504–508. doi: 10.1073/pnas.91.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S, Toombs CF, Hu Y-H, Simon MI. Defective platelet activation in Gαq-deficient mice. Nature. 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- Olejar T, Matej R, Zadinova M, Pouckova P. Proteinase-activated receptor-2 expression on cerebral neurones after radiation damage: immunohistochemical observation in Wistar rats. Int J Tissue React. 2002;24:81–88. [PubMed] [Google Scholar]
- Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350:2167–2179. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- Panaccione R, Ferraz JG, Beck P. Advances in medical therapy of inflammatory bowel disease. Curr Opin Pharmacol. 2005;5:566–572. doi: 10.1016/j.coph.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Parent JM, Tada E, Fike JR, Lowenstein DH. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci. 1999;19:4508–4519. doi: 10.1523/JNEUROSCI.19-11-04508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GH, Ryu JR, Shin CY, Choi MS, Han BH, Kim WK, Kim HC, Ko KH. Evidence that protease-activated receptor-2 mediates trypsin-induced reversal of stellation in cultured rat astrocytes. Neurosci Res. 2006;54:15–23. doi: 10.1016/j.neures.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Pompili E, Nori SL, Geloso MC, Guadagni E, Corvino V, Michetti F, Fumagalli L. Trimethyltin-induced differential expression of PAR subtypes in reactive astrocytes of the rat hippocampus. Brain Res Mol Brain Res. 2004;122:93–98. doi: 10.1016/j.molbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Proba K, Gschwend TP, Sonderegger P. Cloning and sequencing of the cDNA encoding human neurotrypsin. Biochim Biophys Acta. 1998;1396:143–147. doi: 10.1016/s0167-4781(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Riek-Burchardt M, Striggow F, Henrich-Noack P, Reiser G, Reymann KG. Increase of prothrombin-mRNA after global cerebral ischemia in rats, with constant expression of protease nexin-1 and protease-activated receptors. Neurosci Lett. 2002;329:181–184. doi: 10.1016/s0304-3940(02)00645-6. [DOI] [PubMed] [Google Scholar]
- Rohatgi T, Sedehizade F, Reymann KG, Reiser G. Protease-activated receptors in neuronal development, neurodegeneration, and neuroprotection: thrombin as signaling molecule in the brain. Neuroscientist. 2004;10:501–512. doi: 10.1177/1073858404269955. [DOI] [PubMed] [Google Scholar]
- Scarborough RM. Protease-activated receptor-2 antagonists and agonists. Curr Med Chem Cardiovasc Hematol Agents. 2003;1:73–82. doi: 10.2174/1568016033356698. [DOI] [PubMed] [Google Scholar]
- Seatter MJ, Drummond R, Kanke T, MacFarlane SR, Hollenberg MD, Plevin R. The role of the C-terminal tail in protease-activated receptor-2-mediated Ca2+ signalling, proline-rich tyrosine kinase-2 activation, and mitogen-activated protein kinase activity. Cell Signal. 2004;16:21–29. doi: 10.1016/s0898-6568(03)00095-0. [DOI] [PubMed] [Google Scholar]
- Shimizu C, Yoshida S, Shibata M, Kato K, Momota Y, Matsumoto K, Shiosaka T, Midorikawa R, Kamachi T, Kawabe A, Shiosaka S. Characterization of recombinant and brain neuropsin, a plasticity-related serine protease. J Biol Chem. 1998;273:11189–11196. doi: 10.1074/jbc.273.18.11189. [DOI] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-κB: a role for N-myc in TNFα-controlled repression. EMBO J. 2005;24:510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Swintosky VL, Cheo-Isaacs CT, D'Andrea MR, Santulli RJ, Darrow AL, Andrade-Gordon P. Protease-activated receptor-2 (PAR2) is present in the rat hippocampus and is associated with neurodegeneration. J Neurochem. 1997;69:1890–1896. doi: 10.1046/j.1471-4159.1997.69051890.x. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-α. FASEB J. 2006;20:670–682. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- Stalheim L, Ding Y, Gullapalli A, Paing MM, Wolfe BL, Morris DR, Trejo J. Multiple independent functions of arrestins in the regulation of protease-activated receptor-2 signaling and trafficking. Mol Pharmacol. 2005;67:78–87. doi: 10.1124/mol.104.006072. [DOI] [PubMed] [Google Scholar]
- Steenland HW, Ko SW, Wu LJ, Zhuo M. Hot receptors in the brain. Mol Pain. 2006;2:34. doi: 10.1186/1744-8069-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenton GR, Nohara O, Dery RE, Vliagoftis H, Gilchrist M, Johri A, Wallace JL, Hollenberg MD, Moqbel R, Befus AD. Proteinase-activated receptor (PAR) -1 and -2 agonists induce mediator release from mast cells by pathways distinct from PAR-1 and PAR-2. J Pharmacol Exp Ther. 2002;302:466–474. doi: 10.1124/jpet.302.2.466. [DOI] [PubMed] [Google Scholar]
- Striggow F, Riek-Burchardt M, Kiesel A, Schmidt W, Henrich-Noack P, Breder J, Krug M, Reymann KG, Reiser G. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14:595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- Strukova SM. Thrombin as a regulator of inflammation and reparative processes in tissues. Biochemistry. 2001;66:8–18. doi: 10.1023/a:1002869310180. [DOI] [PubMed] [Google Scholar]
- Strukova SM. Role of platelets and serine proteinases in coupling of blood coagulation and inflammation. Biochemistry. 2004;69:1067–1081. doi: 10.1023/b:biry.0000046880.91848.01. [DOI] [PubMed] [Google Scholar]
- Suo Z, Wu M, Ameenuddin S, Anderson HE, Zoloty JE, Citron BA, Andrade-Gordon P, Festoff BW. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J Neurochem. 2002;80:655–666. doi: 10.1046/j.0022-3042.2001.00745.x. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Rivest S. Tumor necrosis factor α but not interleukin 1 β mediates neuroprotection in response to acute nitric oxide excitotoxicity. J Neurosci. 2006;26:143–151. doi: 10.1523/JNEUROSCI.4032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubl JJ, Vohringer C, Reiser G. Co-existence of two types of [Ca2+]i-inducing protease-activated receptors (PAR-1 and PAR-2) in rat astrocytes and C6 glioma cells. Neuroscience. 1998;86:597–609. doi: 10.1016/s0306-4522(97)00686-6. [DOI] [PubMed] [Google Scholar]
- Vaidyula VR, Rao AK. Role of Gαq and phospholipase C-β2 in human platelets activation by thrombin receptors PAR1 and PAR4: studies in human platelets deficient in Gαq and phospholipase C-β2. Br J Haematol. 2003;121:491–496. doi: 10.1046/j.1365-2141.2003.04296.x. [DOI] [PubMed] [Google Scholar]
- Vergnolle N. Modulation of visceral pain and inflammation by protease-activated receptors. Br J Pharmacol. 2004;141:1264–1274. doi: 10.1038/sj.bjp.0705750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, Hollenberg MD, Wallace JL. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- Wang H, Ubl JJ, Reiser G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia. 2002;37:53–63. doi: 10.1002/glia.10012. [DOI] [PubMed] [Google Scholar]
- Wang J, Zheng H, Hollenberg MD, Wijesuriya SJ, Ou X, Hauer-Jensen M. Up-regulation and activation of proteinase-activated receptor 2 in early and delayed radiation injury in the rat intestine: influence of biological activators of proteinase-activated receptor 2. Radiat Res. 2003;160:524–535. doi: 10.1667/rr3080. [DOI] [PubMed] [Google Scholar]
- Wang Y, Luo W, Reiser G. Proteinase-activated receptor-1 and -2 induce the release of chemokine GRO/CINC-1 from rat astrocytes via differential activation of JNK isoforms, evoking multiple protective pathways in brain. Biochem J. 2007;401:65–78. doi: 10.1042/BJ20060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Lang R, Cinelli P, Madani R, Sonderegger P. Multiple roles of neurotrypsin in tissue morphogenesis and nervous system development suggested by the mRNA expression pattern. Mol Cell Neurosci. 2001;18:407–433. doi: 10.1006/mcne.2001.1029. [DOI] [PubMed] [Google Scholar]
- Xu HL, Su B. Genetic evidence of a strong functional constraint of neurotrypsin during primate evolution. Cytogenet Genome Res. 2005;108:303–309. doi: 10.1159/000081523. [DOI] [PubMed] [Google Scholar]
- Zhao X, Bausano B, Pike BR, Newcomb-Fernandez JK, Wang KK, Shohami E, Ringger NC, DeFord SM, Anderson DK, Hayes RL. TNF-α stimulates caspase-3 activation and apoptotic cell death in primary septo-hippocampal cultures. J Neurosci Res. 2001;64:121–131. doi: 10.1002/jnr.1059. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF α potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF κ B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]