Abstract
The objective of this study was to determine if load receptors contribute to the afferent-mediated enhancement of ankle extensor muscle activity during the late stance phase of the step cycle. Plantar flexion perturbations were presented in late stance while able-bodied human subjects walked on a treadmill that was declined by 4%, inclined by 4% or held level. The plantar flexion perturbation produced a transient, but marked, presumably spinally mediated decrease in soleus EMG that varied directly with the treadmill inclination. Similarly, the magnitude of the control step soleus EMG and Achilles' tendon force also varied directly with the treadmill inclination. In contrast, the ankle angular displacement and velocity were inversely related to the treadmill inclination. These results suggest that Golgi tendon organ feedback, via the group Ib pathway, is reduced when the muscle–tendon complex is unloaded by a rapid plantar flexion perturbation in late stance phase. The changes in the unload response with treadmill inclination suggest that the late stance phase soleus activity may be enhanced by force feedback.
Sensory feedback from peripheral afferents contributes to the control of walking by: modulating the basic motor programmes and/or the output from these programmes; controlling phase transitions; and reinforcing the locomotor muscle activity (e.g. Nielsen & Sinkjær, 2002a,b; Pearson, 2003; Donelan & Pearson, 2004). Feedback-mediated reinforcement of plantar flexor muscle activity contributes, together with supraspinal drive and possibly spinal drive (i.e. central pattern generator), to propel the body forward.
The role of force sense in the feedback-mediated reinforcement of the extensor muscles is still a matter of debate. Evidence from reduced cat preparations has shown that Golgi tendon organ feedback via the group Ib pathway is reversed from an inhibitory input to an excitatory input on the ankle extensor motoneurones during walking (Duysens & Pearson, 1980; Conway et al. 1987; Pearson & Collins, 1993; Gossard et al. 1994; McCrea et al. 1995). A similar mechanism has been proposed to exist in humans as a result of reduced gravity during standing (Dietz et al. 1992), body-load support treadmill training in spinal cord-injured patients (Harkema et al. 1997), and electrical stimulation during walking to evoke Ib inhibition (e.g. Stephens & Yang, 1996; Faist et al. 2006).
We have argued that the feedback component of the locomotor drive is best investigated by removing the feedback signal rather than enhancing the feedback signal (Sinkjær et al. 2000; Nielsen & Sinkjær, 2002a,b; Grey et al. 2004). This was first demonstrated by Sinkjær et al. 2000), who used a robotic actuator to rapidly plantar flex the ankle during the early mid stance phase of the step cycle. They observed a decrease in soleus EMG which they termed the ‘unload response’ because the decreased EMG was attributed to the presumed decrease in proprioceptive (i.e. spindle and Golgi tendon organ) feedback when the muscle–tendon complex was unloaded. The unload response is effectively the same as the ‘unload reflex’ that was extensively studied in muscles of the hand and arm for a variety of voluntary movement tasks (Merton, 1951; Angel & Iannone, 1966; Angel et al. 1973, 1987; Burke et al. 1978; Angel & Weinrich, 1986).
Angel et al. (1973) reported that the reduction in triceps brachii activity after its sudden release during a voluntary contraction was not affected by an anaesthetic block of the biceps brachii. Sinkjær et al. (2000) observed the same result in soleus when the ankle was plantar flexed while the tibialis anterior was anaesthetized with a common peroneal nerve block. These observations demonstrate that the unload response is not explained by reciprocal inhibition, and must be due to removal of afferent feedback from the plantar flexors.
Sinkjær et al. (2000) also showed that the unload response is unchanged when peripheral ischaemia is used to block transmission in the large-diameter group I afferents. Grey et al. (2004) extended these results with an anaesthetic block that suppressed feedback from the foot and ankle to rule out the possibility that cutaneous afferents or proprioceptive afferents from intrinsic muscles of the foot contribute to the unload response. They also showed that the onset latency of the unload response (54 ± 11 ms) was between the onset latencies of the group Ia-mediated short latency stretch reflex response (37 ± 5 ms) and the group II-mediated medium latency stretch reflex response (74 ± 6 ms), thus demonstrating that the unload response could not be attributed to the largest of the group Ia afferents.
The objective of the present study was to determine if load receptors contribute to the afferent-mediated enhancement of ankle extensor muscle activity during the late stance phase of the step cycle. Late stance phase is chosen for this study because it is this part of the step cycle when force feedback from Golgi tendon organ output should be greatest. In the present study, force feedback in able-bodied human subjects was modulated by changing the inclination of a treadmill. With small changes in treadmill inclination, the Achilles' tendon tension may be modulated with only small changes in ankle kinematics. In this way, Ib feedback may be modulated differently to spindle feedback. Plantar flexion perturbations of the ankle joint were presented in late stance phase while the subjects walked on an inclined, level, or declined treadmill. If load receptors contribute to the afferent-mediated component of the locomotor EMG, it may be hypothesized that the magnitude of the unload response should be modulated with the inclination of the treadmill.
Methods
Twenty-one healthy subjects (13 male, 8 female) with no history of neuromuscular disorders participated in this study. Subjects provided informed written consent prior to their participation. All experimental procedures were approved by the local ethics review board (Nordjyllands Amt, project VN 99/100) and the experiments were conducted in accordance with the Declaration of Helsinki.
Apparatus and instrumentation
The subjects walked on a treadmill for the duration of the experiment. The left leg was attached to a semiportable robotic actuator capable of rotating the ankle joint in dorsiflexion and plantar flexion. Full details of the device are presented elsewhere (Andersen & Sinkjær, 1995). Briefly, the device consists of a functional joint aligned with the ankle of the subject and attached to the foot and leg with a polypropylene plaster cast. The actuator is connected to an AC servomotor that applies torque to the functional joint through flexible Bowden cables. Ankle angular position was measured with an optical encoder incorporated within the functional joint and ankle angular velocity was determined on-line by numerical differentiation of the angular position record.
Electromyographic activity was recorded by surface EMG electrodes placed over the soleus (SOL), medial gastrocnemius (MG) and tibialis anterior (TA) muscles of the left leg according to guidelines suggested by the SENIAM project (Hermens et al. 2000). The EMG signals were amplified and bandpass filtered from 10 to 500 Hz. In 10 subjects, the Achilles' tendon force (ATF) was estimated by clamping a custom-made E-buckle transducer to the tendon. The design of this device was based on the in vivo buckle transducer (Salmons, 1969; Komi et al. 1987) and an external tendon clamp transducer (Berger et al. 1982). The kinematic, kinetic and electromyographic signals were sampled at 2.5 kHz and stored for off-line analysis. Data acquisition was triggered with a force-sensitive resistor placed in the insole of the left shoe.
Experimental protocol
Prior to data collection, each subject walked on a level treadmill at a comfortable self-selected speed (typically 3.5–4 km h−1) for an adaptation period of approximately 5 min. Following this adaptation period, the robotic actuator was programmed to deliver rapid plantar flexion ramp-and-hold perturbations (4–5 deg, 300 deg s−1, 200 ms hold time) in the later half of the stance phase. The delivery of the perturbations was timed so that the desired electromyographic responses occurred at about the time that the soleus muscle activity was greatest, 60–80% into the stance phase. Data were recorded for 1200 ms starting 600 ms before the perturbation. Perturbations and control steps were presented pseudo-randomly (every four to seven steps) until 25–30 trials were recorded for each condition. The treadmill was then inclined or declined by 4% and the procedure was repeated. The 4% change in treadmill inclination was chosen because it produces a small change in the late stance phase SOL and MG muscle activity with only very minor changes in the ankle kinematics (see Results).
Data analysis
Signal processing and analysis were carried out off-line. The EMG records were rectified and filtered with a 40 Hz first-order low-pass filter to extract an amplitude envelope. Individual records for a particular trial were then ensemble averaged to produce a single record for each subject and ramp inclination. The rapid plantar flexion perturbations produced a transient drop, or unload response, in the SOL EMG. An unload response was also measured in the MG EMG; however, the response was not present in most subjects and, when present, it was typically of very short duration compared with that of the SOL response. Consequently, unload responses in the MG muscle were not analysed in the present study.
Onset latencies of the soleus unload responses were determined by visual inspection, defined as the first major deflection from the ensemble-averaged record of the control step EMG within a 30–80 ms window immediately following the onset of the ankle perturbation. Each unload response was quantified by calculating the area between the ensemble-averaged EMG of the control and perturbed steps. Typically, the duration of the unload response was just greater than 100 ms; therefore, the area of the response was quantified in a 100-ms window. The start of the analysis window was placed to coincide with the onset of the response. The transient depression in the Achilles' tendon force was quantified in a similar manner. The ATF recordings were first normalized with the level-walking peak force at the end of the stance phase and then quantified in a 100-ms window placed at the onset of the ATF decline. One-way repeated measures anovas were used to test for the effect of treadmill inclination on the unload response, control step SOL EMG, ankle kinematics, and Achilles' tendon force. Geisser–Greenhouse adjustments were made when the covariance matrix sphericity assumption was violated (denoted by GG following the F test). All statistical tests were conducted with a significance level of 0.05 and all results are shown as mean ± s.d.
Results
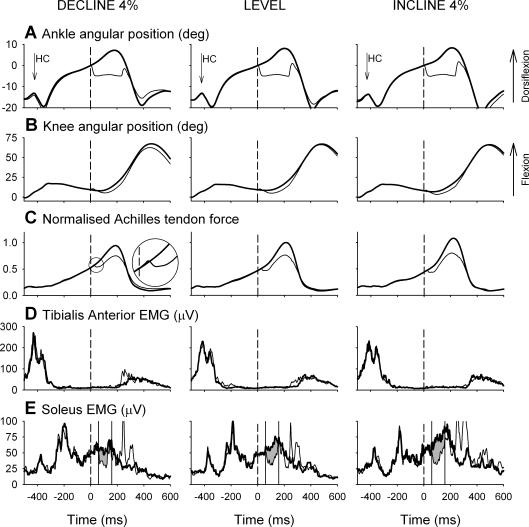
A typical set of ensemble-averaged data for one subject is shown in Fig. 1 with the control step (thick line) superimposed over the perturbed step (thin line). In this case the subject walked at 3.6 km h−1 with perturbations applied 350 ms after heel contact. The perturbation onset is defined as time zero and indicated with a vertical dashed line through each record. In all cases the ankle was perturbed for 5 deg at 300 deg s−1, held for 200 ms and then released, returning to its natural position within the swing phase of the same step cycle. The perturbation was followed by a small increase in knee extension with an approximate delay of 25 ms. Shortly after the perturbation, the Achilles' tendon force markedly decreased and then rose to a level in the late stance phase that was decreased compared with the control step (see Fig. 1C inset).
Figure 1. Example of ensemble-averaged data records for a single subject walking on a treadmill that was declined by 4% (n = 27), level (n = 30), or inclined by 4% (n = 30).
In each case the control step (thick line) is shown superimposed over the perturbed step (thin line). Data are shown for 1.1 s starting approximately 50 ms before heel contact to highlight the stance phase. Time zero corresponds to the onset of the perturbation and is indicated in each panel with a dashed line. The ankle angular position (A) is offset such that zero corresponds to the angle at the onset of the perturbation. The knee angular position (B) has not been offset, i.e. zero corresponds to an extended knee. The Achilles' tendon tension (C) is shown normalized to the peak tension recorded during level treadmill walking. The plantar flexion perturbation does not produce a short-latency stretch reflex response in the tibialis anterior EMG (D). The perturbation produces a marked unload response in the soleus EMG (E) at approximately 60 ms that is modulated with the treadmill inclination.
The electromyographic responses to the plantar flexion perturbation were similar to that described in our earlier studies (Sinkjær et al. 2000; Grey et al. 2004). The soleus EMG in the perturbed step was matched to the control step until approximately 50–60 ms after the onset of the perturbation (60 ms in Fig. 1E). At this point, a marked decrease in the soleus EMG activity can be seen that lasts for approximately 100 ms. This depression is followed by a rapid rise in EMG that can most likely be attributed to a supraspinal, and possibly transcortical, corrective response following the unexpected perturbation (Christensen et al. 2000). It is notable that the duration of the unload response was variable between subjects, ranging from about 50 to 200 ms. In two subjects, the duration of the unload response was only 50–60 ms; and in these cases, the unload response was quantified in a 50-ms window. In the example shown in Fig. 1D, the tibialis anterior did not exhibit a stretch reflex response following the perturbation. Small tibialis anterior stretch reflex responses were observed in a few subjects, although Sinkjær et al. (2000) have shown that such responses do not affect the soleus unload response.
The initial ATF response was quite variable between subjects. In some cases, such as that illustrated in Fig. 1C (see inset), the ATF increased very slightly before decreasing. This effect was most likely due to lateral twisting of the buckle or to motion artefact as a result of contact with the functional joint's heel cup. In all cases the ATF dropped sharply as would be expected when the Achilles' tendon is unloaded. The ATF response was consistently delayed with respect to the ankle perturbation by approximately 10 ms. To test if this delay may have resulted from the mechanical coupling of the ankle with the functional joint, the ATF response was elicited in response to plantar flexion and dorsiflexion perturbations. The 10 ms delay was present in both cases, suggesting that the cause was mechanical (see Discussion).
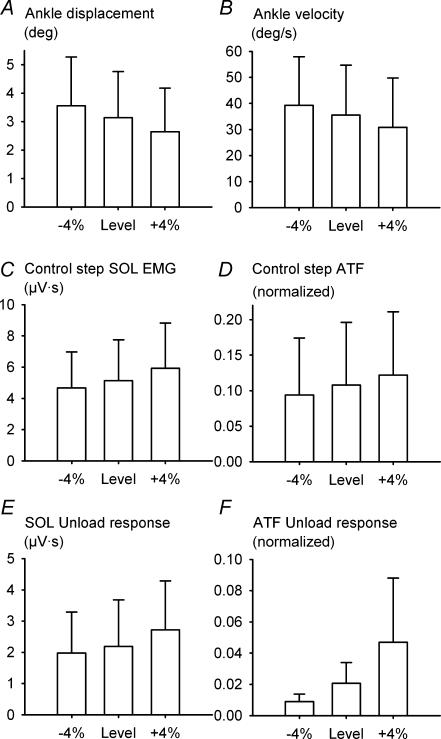
The effect of the treadmill inclination can also be seen in Fig. 1. The 4% change in grade produced almost no observable effect on the ankle kinematics. However, across all subjects, there was a small, but statistically significant, change in angular displacement and velocity with treadmill inclination (Fig. 2A and B). Over a 100-ms window placed at time zero (perturbation onset) the ankle angular displacement for the control step was larger when walking downhill (3.6 ± 1.7 deg) and smaller when walking uphill (2.6 ± 1.5 deg) compared with level walking (3.1 ± 1.6 deg; F2,20 = 42.37, GG; P < 0.001). Over the same window, the ankle angular velocity was faster for declined walking (39 ± 18 deg s−1) and slower for inclined walking (31 ± 19 deg s−1) compared with level walking (35 ± 19 deg s−1; F2,16 = 9.26, GG; P < 0.001). The control step Achilles' tendon force was normalized to the peak magnitude recorded during level treadmill walking and measured over a 100-ms window placed at the onset of the ATF decrease (Figs 1C and 2D). Due to technical difficulties with the ATF device, this measure was only possible for 8 of the 10 subjects with whom it was used. Across these subjects, the Achilles' tendon force decreased for downhill walking (0.09 ± 0.08) and increased for uphill walking (0.12 ± 0.09) compared with level walking (0.11 ± 0.08; F2,7 = 16.42, GG; P < 0.001). The magnitude of the ATF unload response was 0.009 ± 0.005, 0.021 ± 0.013 and 0.047 ± 0.041 for the decline, level and incline conditions, respectively (Fig. 2F), and the one-way ANOVA indicated that this difference was statistically significant (F2,7 = 4.76; P = 0.026, GG). Similarly, the late stance phase control step soleus EMG is clearly smaller for declined walking and larger for the inclined walking in Fig. 1E. Across all subjects, the area of the control step soleus EMG over the 100-ms analysis window was 4.8 ± 2.3 μV s, 5.1 ± 2.6 μV s and 5.9 ± 2.9 μV s for the decline, level and incline conditions, respectively, and this difference was statistically significant (F2,20 = 42.37, GG; P < 0.001; Fig. 2C). The effect of treadmill inclination on the magnitude of the soleus unload response can also be seen in Figs 1E, and 2E and F. Across all subjects, the magnitude of the soleus unload response was 2.0 ± 1.3 μV s, 2.2 ± 1.5 μV s and 2.7 ± 1.6 μV s for the decline, level and incline conditions, respectively, and this difference was statistically significant (F2,20 = 19.5; P < 0.001; Fig. 2E).
Figure 2. The effect of treadmill inclination on the control step ankle angular displacement (A), ankle angular velocity (B), control step soleus EMG (C), normalized Achilles' tendon force (D), soleus unload response (E) and Achilles' tendon unload response (F).
Data are illustrated as mean ± s.d. across all 21 subjects (8 subjects for ATF). Ankle displacement and velocity decrease with treadmill inclination (P < 0.001 in both cases). In contrast, the control step soleus EMG, normalized control step Achilles' tendon force, soleus unload response, and normalized Achilles' tendon force unload response increase with treadmill inclination (P < 0.001, P < 0.006, P < 0.001 and P = 0.026, respectively).
Discussion
The aim of the present study was to examine the contribution of proprioceptive feedback to the enhancement of the soleus muscle activity during the late stance phase of the step cycle. A rapid plantar flexion perturbation unloads the triceps surae muscle–tendon complex and produces a transient spinal-mediated drop in the soleus EMG as a result of the transient removal of proprioceptive homonymous and/or heteronymous feedback. The observation that the modulation of this feedback is directly with the Achilles' tendon force and inversely with the ankle kinematics suggests that force feedback contributes to the late stance phase enhancement of the locomotor muscle activity.
As pointed out by Duysens et al. (2000), load feedback may be obtained from Golgi tendon organs, muscle spindles, and cutaneous receptors. Grey et al. (2004) demonstrated that cutaneous afferents from the foot and ankle do not contribute to the unload response described in the present study. Whereas both ankle displacement and velocity decreased when walking uphill, the Achilles' tendon tension and soleus muscle activity increased. Similarly, when walking downhill, the ankle displacement and velocity increased while the Achilles' tendon tension and soleus muscle activity decreased. In contrast to the ankle displacement and length, the Achilles' tendon force is modulated in parallel with the background soleus muscle activity when the treadmill is inclined or declined. In addition to the parallel reduction of the soleus EMG with the Achilles' tendon force following the imposed rapid perturbation, these observations are consistent with the idea that tendon organ feedback may contribute to soleus muscle activity via positive force feedback in human walking, as has been shown in the cat (Pearson & Collins, 1993; Gossard et al. 1994; McCrea et al. 1995). While the small changes in ankle trajectory associated with ramp inclination are not consistent with the idea that spindle discharge due to the muscle lengthening during stance phase would contribute to the observed changes in soleus muscle activity, it should be noted that absolute changes in muscle fibre length can be different from the measured muscle–tendon length (Loram et al. 2004).
The delay observed between the onset of the perturbation and the onset of the ATF response remains unexplained. One possibility is that the stretch is taken up by the muscle fibres before the tendon tension is reduced. Another possibility is that the delay results from the mechanical coupling between the functional joint and the ankle joint. It is notable that a much shorter delay of 4 ms between the ankle perturbation and ATF response with this device has been observed during sitting, with the foot firmly connected to a robotic pedal (Grey MJ unpublished observations). Irrespective of the mechanism responsible for this delay, conclusions about changes in the Achilles' tendon force within a few milliseconds of the perturbation and, consequently, changes in proprioceptor firing rates, cannot be presumed based on the output of this device. Like all in vivo estimates of muscle–tendon tension (e.g. buckle, optic fibre, ultrasound) the tendon clamp transducer has limited accuracy. In the present study, the peak ATF was very well correlated with the treadmill inclination and control step soleus EMG and the ATF transducer was sufficiently accurate to provide a reliable estimate of the magnitude of the unloading response.
In all cases, a decrease in the Achilles' tendon force was observed following the rapid plantar flexion, but the magnitude of the decrease was sometimes small and it was frequently variable. It is possible that some of the decrease in tension observed with this transducer may relate to muscle properties, although it is not possible to discern this possibility with the present study. Further investigation with in vivo measures of the muscle–tendon mechanics during an unloading perturbation is required to address this issue. The variability in the AFT decline is most likely to reflect the mechanics of the coupling between the transducer and the Achilles' tendon rather than the real tension of the muscle–tendon complex. Nevertheless, the decrease in ATF following the perturbation was well correlated with the soleus unload response in most subjects.
The 4% change in treadmill inclination produced very small changes in the ankle trajectory and negligible changes in perceived effort; however, a supraspinal contribution to the changes in soleus EMG magnitude with treadmill inclination cannot be ruled out with the present protocol. This also means that spindle feedback cannot be conclusively ruled out as a contributor to the locomotor EMG. Changes in descending drive will produce changes in both α- and γ-motoneuronal excitation, therefore it is conceivable that changes in fusimotor excitability could compensate for the ankle kinematics such that spindle feedback could increase or decrease, respectively, when the ramp is inclined or declined.
The present results provide compelling, albeit indirect, evidence that it is the removal of force feedback that is primarily responsible for the decrease in the late stance phase soleus EMG following the plantar flexion perturbation. This suggests that tendon organ feedback via an excitatory group Ib pathway contributes to the late stance phase enhancement of the soleus muscle activity.
References
- Andersen JB, Sinkjær T. An actuator system for investigating electrophysiological and biomechanical features around the human ankle joint during gait. IEEE Trans Rehabil Eng. 1995;3:299–306. [Google Scholar]
- Angel RW. Unloading reflex of a hand muscle. Electroencephalogr Clin Neurophysiol. 1987;67:447–451. doi: 10.1016/0013-4694(87)90008-3. [DOI] [PubMed] [Google Scholar]
- Angel RW, Garland H, Moore W. Unloading reflex during blockade of antagonist muscle nerves. Electroencephalogr Clin Neurophysiol. 1973;34:303–307. doi: 10.1016/0013-4694(73)90256-3. [DOI] [PubMed] [Google Scholar]
- Angel RW, Iannone AM. Analysis of check reflex. Neurology. 1966;16:345–350. doi: 10.1212/wnl.16.4.345. [DOI] [PubMed] [Google Scholar]
- Angel RW, Weinrich M. Stretch and unloading reflexes in a human hand muscle. Exp Neurol. 1986;94:348–358. doi: 10.1016/0014-4886(86)90108-1. [DOI] [PubMed] [Google Scholar]
- Berger W, Quintern J, Dietz V. Pathophysiology of gait in children with cerebral palsy. Electroencephalogr Clin Neurophysiol. 1982;53:538–548. doi: 10.1016/0013-4694(82)90066-9. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L. Muscle spindle activity in man during shortening and lengthening contractions. J Physiol. 1978;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen LO, Petersen N, Andersen JB, Sinkjær T, Nielsen JB. Evidence for transcortical reflex pathways in the lower limb of man. Prog Neurobiol. 2000;62:251–272. doi: 10.1016/s0301-0082(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Dietz V, Gollhofer A, Kleiber M, Trippel M. Regulation of bipedal stance: dependency on ‘load’ receptors. Exp Brain Res. 1992;89:229–231. doi: 10.1007/BF00229020. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of sensory feedback to ongoing ankle extensor activity during the stance phase of walking. Can J Physiol Pharmacol. 2004;82:589–598. doi: 10.1139/y04-043. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Faist M, Hoefer C, Hodapp M, Dietz V, Berger W, Duysens J. In humans Ib facilitation depends on locomotion while suppression of Ib inhibition requires loading. Brain Res. 2006;1076:87–92. doi: 10.1016/j.brainres.2005.12.069. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Mazzaro N, Nielsen JB, Sinkjær T. Ankle extensor proprioceptors contribute to the enhancement of the soleus EMG during the stance phase of human walking. Can J Physiol Pharmacol. 2004;82:610–616. doi: 10.1139/y04-077. [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Komi PV, Salonen M, Jarvinen M, Kokko O. In vivo registration of Achilles tendon forces in man. I. Methodological development. Int J Sports Med. 1987;8(Suppl. 1):3–8. doi: 10.1055/s-2008-1025697. [DOI] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Paradoxical muscle movement in human standing. J Physiol. 2004;556:683–689. doi: 10.1113/jphysiol.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol. 1995;487:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. The silent period in a muscle of the human hand. J Physiol. 1951;114:183–198. doi: 10.1113/jphysiol.1951.sp004610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Sinkjær T. Afferent feedback in the control of human gait. J Electromyogr Kinesiol. 2002a;12:213–217. doi: 10.1016/s1050-6411(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Sinkjær T. Reflex excitation of muscles during human walking. Adv Exp Med Biol. 2002b;508:369–375. [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res. 2003;143:123–129. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol. 1993;70:1009–1017. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Salmons S. The 8th international conference on medical and biological engineering – meeting report. Bio med Eng. 1969;4:467–474. [Google Scholar]
- Sinkjær T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MJ, Yang JF. Short latency, non-reciprocal group I inhibition is reduced during the stance phase of walking in humans. Brain Res. 1996;743:24–31. doi: 10.1016/s0006-8993(96)00977-8. [DOI] [PubMed] [Google Scholar]