Abstract
Clinical hypokalaemia is associated with acquired electrocardiographic QT prolongation and arrhythmic activity initiated by premature ventricular depolarizations and suppressed by lidocaine (lignocaine). Nevertheless, regular (S1) pacing at a 125 ms interstimulus interval resulted in stable waveforms and rhythm studied using epicardial and endocardial monophasic action potential (MAP) electrodes in Langendorff-perfused murine hearts whether under normokalaemic (5.2 mm K+) or hypokalaemic (3.0 mm K+) conditions, in both the presence and absence of lidocaine (10 μm). Furthermore, the transmural gradient in repolarization time, known to be altered in the congenital long-QT syndromes, and reflected in the difference between endocardial and epicardial MAP duration at 90% repolarization (ΔAPD90), did not differ significantly (P > 0.05) between normokalaemic (5.5 ± 4.5 ms, n = 8, five hearts), hypokalaemic (n = 8, five hearts), or lidocaine-treated normokalaemic (n = 8, five hearts) or hypokalaemic (n = 8, five hearts) hearts. However, premature ventricular depolarizations occurring in response to extrasystolic (S2) stimulation delivered at S1S2 intervals between 0 and 22 ± 6 ms following recovery from refractoriness initiated arrhythmic activity specifically in hypokalaemic (n = 8, five hearts) as opposed to normokalaemic (n = 25, 14 hearts), or lidocaine-treated hypokalaemic (n = 8, five hearts) or normokalaemic hearts (n = 8, five hearts). This was associated with sharp but transient reversals in ΔAPD90 in MAPs initiated within the 250 ms interval directly succeeding premature ventricular depolarizations, from 3.3 ± 5.6 ms to −31.8 ± 11.8 ms (P < 0.05) when they were initiated immediately after recovery from refractoriness. In contrast the corresponding latency differences consistently remained close to the normokalaemic value (−1.6 ± 1.4 ms, P > 0.05). These findings empirically associate arrhythmogenesis in hypokalaemic hearts with transient alterations in transmural repolarization gradients resulting from premature ventricular depolarizations. This is in contrast to sustained alterations in transmural repolarization gradients present on regular stimulation in long-QT syndrome models.
The pathophysiological state of hypokalaemia is associated both with acquired electrocardiographic T-wave inversion and marked prolongation of the QT interval reflecting increased ventricular action potential duration, and is known to predispose to ventricular arrhythmic activity (Zipes & Braunwald, 2005). In the murine heart, action potential prolongation under hypokalaemic conditions has recently been attributed to reductions in the repolarizing K+ currents Ito (transient outward current) and IK1 (inwardly rectifying current) (Killeen et al. 2007). Arrhythmic activity associated with increased ventricular action potential duration is also a characteristic physiological feature of the congenital long-QT syndromes (LQTS) (Moss & Kass, 2005). This heterogeneous group of disorders result from autosomal dominant mutations in cardiac ion channels and the disorders are characterized clinically by syncope and sudden cardiac death resulting from ventricular arrhythmic activity (Moss & Kass, 2005).
Arrhythmic activity in situations including thiazide-induced hypokalaemia (Whelton, 1984), LQTS (Noda et al. 2004), the Brugada syndrome (Yan & Antzelevitch, 1999) and myocardial ischaemia (El-Sherif et al. 1976; Naito et al. 1982) frequently follows premature ventricular depolarizations (PVDs). These are especially arrhythmogenic in both humans (Smirk, 1949; Ruberman et al. 1977; Ruberman et al. 1981) and animals (Naito et al. 1982; Kiryu et al. 1999) when they occur soon after the previous action potential and result in an electrocardiographic R-wave being superposed on the preceding T-wave. However, neither the electrophysiological basis for this phenomenon nor the antiarrhythmic effect of class 1b antiarrhythmic drugs, such as lidocaine (Rehnqvist et al. 1984), in such conditions has been explored in detail.
PVDs initiated by extrasystolic stimulation are known to exhibit altered repolarization kinetics (Boyett & Jewell, 1978) and to result in alterations in the repolarizaton kinetics of subsequent action potentials, part of the phenomenon of postextrasystolic potentiation (Bass, 1975; Schouten et al. 1990). Furthermore, PVDs display spatial heterogeneities in action potential repolarization (Allessie et al. 1976; Kuo et al. 1983). Such heterogeneities have been observed across the surfaces of both the epicardium (Laurita et al. 1998) and endocardium (Kobayashi et al. 1992), as well as between epicardial and endocardial cells in both isolated myocytes (Litovsky & Antzelevitch, 1989) and whole-heart preparations (Weissenburger et al. 2000; Wolk et al. 2002). This has been attributed variously to effects on Na+ or K+ channel activation (Schouten et al. 1990), as well as alterations in Ca2+ homeostasis whether through effects on L-type Ca2+ channels (Sun et al. 1997), the Na+–Ca2+ exchanger, possibly through effects on the Na+–K+-ATPase (Janvier et al. 1997), or sarcoplasmic reticular Ca2+ stores (Isenberg & Han, 1994). At all events the consequent PVDs are well established as triggers for arrhythmic activity (Naito et al. 1982).
Sustained increases in the magnitudes of transmural repolarization gradients have frequently been associated with arrhythmogenesis in the setting of action potential prolongation. This has been demonstrated in the congenital LQTS types 1 (Shimizu & Antzelevitch, 1998), 2 (Shimizu & Antzelevitch, 1997; Akar et al. 2002) and 3 (Shimizu & Antzelevitch, 1997; Milberg et al. 2005), as well as following exposure to sodium pentobarbital (Shimizu et al. 1999) and cisapride (Di Diego et al. 2003). Further, manoeuvres which reduce the magnitude of such repolarization gradients have been shown to exert an antiarrhythmic effect (Shimizu & Antzelevitch, 2000; Antzelevitch et al. 2004), even if they further increase action potential duration (Shimizu et al. 1999).
Prompted by these previous reports, we tested the hypothesis that transient alterations in transmural repolarization gradients following PVDs might be associated with the pro-arrhythmic effect of hypokalaemia and the antiarrhythmic effect of lidocaine in Langendorff-perfused murine hearts.
Methods
Experimental animals
Mice were housed in an animal facility at 21 ± 1°C with 12 h light/dark cycles. Animals were fed sterile chow (RM3 Maintenance Diet, SDS, Witham, Essex, UK) and had free access to water. Male wild-type 129 Sv mice aged 3–6 months were used in experiments. All procedures complied with UK Home Office regulations (Animals (Scientific Procedures) Act 1986).
Preparation
A Langendorff-perfusion protocol previously adapted for murine hearts (Balasubramaniam et al. 2003) was used. The mice were killed by cervical dislocation (Schedule 1, UK Animals (Scientific Procedures) Act 1986). In brief, hearts were then quickly excised and placed in ice-cold bicarbonate-buffered Krebs–Henseleit solution (mm: NaCl 119, NaHCO3 25, KCl 4, KH2PO4 1.2, MgCl2 1, CaCl2 1.8, glucose 10 and sodium pyruvate 2; pH adjusted to 7.4) bubbled with 95% O2–5% CO2 (British Oxygen Company, Manchester, UK). A short section of aorta was cannulated under the surface of the solution and attached to a custom-made 21 gauge cannula filled with the same solution using an aneurysm clip. Fresh Krebs–Henseleit solution was then passed through 200 μm and 5 μm filters (Millipore, Watford, UK) and warmed to 37°C using a water jacket and circulator (Techne model C-85A; Cambridge, UK) before being used for constant-flow retrograde perfusion at 2–2.5 ml min−1 using a peristaltic pump (Watson-Marlow Bredel model 505S; Falmouth, Cornwall, UK). Hearts were regarded as suitable for experimentation if, on warming, they regained a healthy pink colour and began to contract spontaneously.
Electrophysiological measurements
A bipolar platinum stimulating electrode (1 mm interpole spacing) was placed on the basal surface of the right ventricular epicardium. Hearts were stimulated at an interstimulus interval of 125 ms, close to the expected in vivo electrocardiographic RR interval of 131.4 ± 32.8 ms (VanderBrink et al. 2000) and faster than the intrinsic rate, using 2 ms duration square-wave stimuli. Our experiments sought to study the effects of PVDs on the subsequent action potentials. This required a choice of stimulus parameters that would consistently produce PVDs and subsequent action potentials, all of which are all-or-none events. This was achieved by a applying stimuli at twice diastolic threshold which was in turn consistent with previous studies (Milberg et al. 2002, 2005; Papadatos et al. 2002; Kirchhof et al. 2003; Knollmann et al. 2006) using a Grass S48 stimulator (Grass-Telefactor, Slough, UK) for at least 10 min before recording began to allow a steady state to be established.
Epicardial monophasic action potential (MAP) electrodes (Hugo Sachs; Harvard Apparatus, Edenbridge, Kent, UK) were placed on the basal region of the left ventricular epicardium. In addition, a small access window was created in the interventricular septum in order to allow access to the left ventricular endocardium (Casimiro et al. 2001). A custom-made endocardial MAP electrode composing two twisted strands of high-purity Teflon-coated 0.25 mm diameter silver wire (Advent Research Materials Ltd, Eynsham, Oxford, UK) was constructed. The Teflon coat was removed from the distal 1 mm of the electrode which was then galvanically chlorided to eliminate DC offset, inserted and placed against the septal endocardial surface. MAPs were amplified, band-pass filtered (0.5 Hz to 1 kHz, Gould 2400S; Gould-Nicolet Technologies, Ilford, Essex, UK) and digitized at a sampling frequency of 5 kHz, giving a Nyquist frequency of 2.5 kHz (temporal resolution of 200 μs) using a 1401plus analog-to-digital converter (Cambridge Electronic Design, Cambridge, UK). Analysis of MAPs was performed using Spike II software (Cambridge Electronic Design).
Experimental protocol
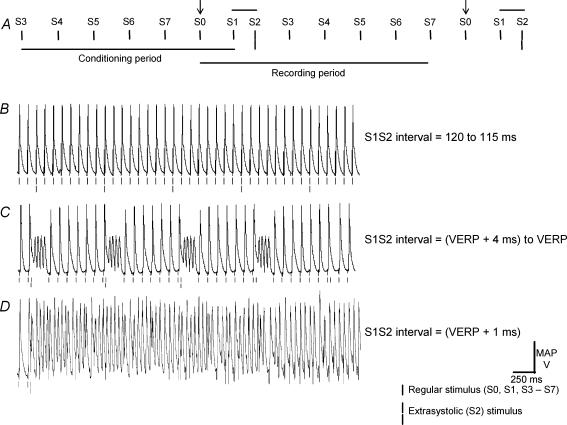
Electrical stimulation was carried out at an interstimulus interval of 125 ms and using an adaptation of previously described clinical techniques (Saumarez & Grace, 2000; Balasubramaniam et al. 2003; Head et al. 2005). This extrasystolic (S2) stimulation protocol imposed successive cycles beginning with two pacing stimuli (S0 and S1) separated by 125 ms, the S2 stimulus itself (open arrows), then five further pacing stimuli (S3–S7) again spaced by 125 ms (Fig. 1A) to investigate changes in the nature of the MAPs that succeeded the S2 stimulus, as well as to provide an 875 ms conditioning period between the delivery of successive S2 stimuli. The S1S2 interval was decremented by 1 ms with each successive cycle from an initial value of 120 ms. Cycles were continued until either the S2 stimulus appeared to initiate arrhythmic activity as confirmed by the demonstration of morphologically irregular waveforms during an imposed 250 ms pause, or the ventricular effective refractory period (VERP) following the previous action potential was reached whereupon the S2 stimulus failed to elicit a MAP.
Figure 1. Initiation of arrhythmic activity by premature ventricular depolarizations occurring in response to extrasystolic (S2) stimulation in hypokalaemic hearts.
A, extrasystolic (S2) stimulation protocol. Epicardial monophasic action potential (MAP) recordings from a heart exposed to hypokalaemic (3.0 mm K+) test solution for 20 min at S1S2 intervals that were decremented between 120 and 115 ms (B), and to intervals just greater and including the ventricular effective refractory period (VERP) (C).
Recordings were made after 10, 15 or 20 min exposure to test solutions. The constituents of normokalaemic (5.2 mm K+) test (Krebs–Henseleit) solution are described above. In hypokalaemic test solutions [K+] was lowered to 3.0 mm by reduction of KCl content. Both these solutions were also prepared with the addition of lidocaine (10 μm; Sigma-Aldrich, Poole, UK).
Data analysis
Data are expressed as means ± s.e.m., with n being the number of repetitions. Comparisons were made using ANOVA (StatsPlus 3.5; Analystsoft) with a significance threshold set at P ≤ 0.05.
Results
The experiments investigated the effect of hypokalaemia, proarrhythmic in clinical situations, and its modification by the class 1b antiarrhythmic agent lidocaine in isolated Langendorff-perfused murine hearts. Hearts were initially paced at a regular 125 ms interstimulus interval using bipolar platinum electrodes placed against the right ventricular epicardial surface while exposed for 20 min to normokalaemic (5.2 mm K+), hypokalaemic (3.0 mm K+), normokalaemic lidocaine-containing (10 μm) or hypokalaemic lidocaine-containing test solutions, respectively. Monophasic action potentials (MAPs) were recorded as regular stimulation was continued over the subsequent 20 min using basal left ventricular epicardial and endocardial electrodes. Action potential duration was quantified at 90% repolarization (APD90), APD0 being defined as the peak upstroke potential difference and APD100 as the diastolic potential difference. Inclusion or exclusion of the upstroke phase did not make a significant difference (P > 0.05) to results in any case. MAPs demonstrated stable durations with each stimulus eliciting a single MAP which showed a stable baseline, rapid upstroke phase that reached a consistent amplitude and a smooth repolarization phase (cf. Knollmann et al. 2001; Fabritz et al. 2003). Neither epicardial nor endocardial APD90 differed significantly between the first and last 5 min of the recording period after exposure to any test solution, confirming stable recording conditions.
Initiation of arrhythmic activity by extrasystolic stimulation
Experiments began by establishing steady initial conditions with 25 s of regular stimulation at a 125 ms cycle length. PVDs were then initiated by extrasystolic (S2) stimuli in a protocol adapted from a programmed electrical stimulation procedure previously used to assess arrhythmic tendency in clinical situations (Saumarez & Grace, 2000; Balasubramaniam et al. 2003; Head et al. 2005) and described in the Methods (Fig. 1A).
Figure 1B, C and D shows typical results. Single vertical lines indicate the timing of regular stimuli and double lines indicate S2 stimuli. Hearts exposed to hypokalaemic test solution for 10 or 15 min maintained a stable rhythm whether on regular stimulation at an interstimulus interval of 125 ms or on application of the S2 stimulation protocol in 36 out of 36 cases (26 out of 26 hearts). After 20 min exposure to hypokalaemic test solution, hearts maintained a stable rhythm on regular stimulation and on S2 stimulation at long S1S2 intervals (Fig. 1B). However, when S2 stimuli were delivered at S1S2 intervals of between the ventricular effective refractory period (VERP, filled arrow) and VERP + (14 ± 5) ms, arrhythmic activity was initiated in 15 out of 15 cases (in seven out of seven hearts, Fig. 1C) and became sustained in 13 out of 15 cases (in six out of seven hearts, Fig. 1D). This was seen under hypokalaemic conditions only.
Arrhythmogenicity is specific to hypokalaemic hearts
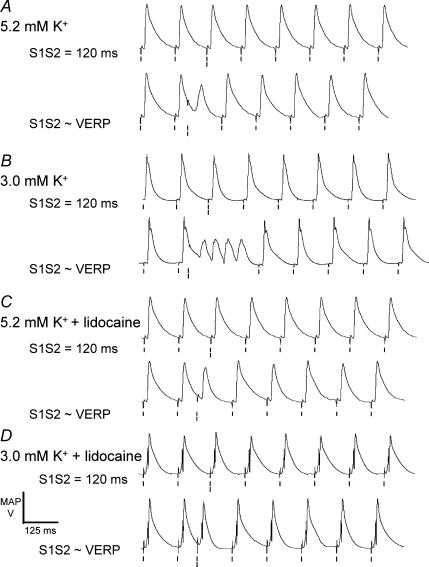
The arrhythmogenic effect of PVDs occurring in response to S2 stimulation soon after recovery from refractoriness was specific to hypokalaemic hearts and could be prevented by exposure to lidocaine. Figure 2 shows typical results obtained at a long S1S2 interval (115 ms, upper traces in each pair) and at an S1S2 interval resulting in the delivery of an S2 stimulus immediately after recovery from refractoriness (early S2 stimulation, lower traces in each pair). When this stimulation protocol was also applied to hearts exposed to normokalaemic solution a stable rhythm was maintained regardless of S1S2 interval (n = 25, 13 hearts, Fig. 2A). In contrast, hearts exposed to hypokalaemic test solution showed arrhythmic activity following PVDs initiated by early S2 stimuli in 15 out of 15 cases (seven out of seven hearts, Fig. 2B). Normokalaemic hearts exposed to lidocaine never showed arrhythmic activity (n = 8, four hearts, Fig. 2C). Furthermore, arrhythmic activity was prevented by addition of lidocaine even with hypokalaemic test solution (n = 15, seven hearts, Fig. 2D).
Figure 2. Specificity of arrhythmogenicity to hypokalaemic hearts.
Epicardial MAP recordings resulting from application of the S2 stimulation protocol at S1S2 intervals of 120 ms (upper traces in each pair) and close to the ventricular effective refractory period (VERP) (lower traces in each pair) in hearts exposed to normokalaemic (5.2 mm K+, A) and hypokalaemic (3.0 mm K+, B) test solutions, and normokalaemic (C) and hypokalaemic (D) test solutions containing lidocaine (10 μm), for 20 min. Single vertical lines indicate the timing of S0–S1 and S3–S7 stimuli and double lines indicate the timing of S2 stimuli.
PVDs occurring in response to early S2 stimuli alter transmural repolarization gradients
Arrhythmic activity in models showing QT prolongation has been associated with large transmural repolarization gradients even during regular stimulation (Shimizu & Antzelevitch, 1997, 1998; Shimizu et al. 1999; Akar et al. 2002; Di Diego et al. 2003; Milberg et al. 2005). The possibility that altered transmural repolarization gradients of this kind could be involved in arrhythmogenesis in hypokalaemic murine hearts and the detailed circumstances in which such alterations might occur were investigated.
We proceeded to examine transmural repolarization gradients by determining stimulation to depolarization latencies and APD90 values in the epicardia and endocardia of hearts exposed to the four test solutions. The time taken for depolarization to propagate across the myocardial wall, Δ(latency), was calculated as endocardial latency minus epicardial latency. Similarly, the time taken for repolarization to spread across the myocardial wall, ΔAPD90, was calculated as endocardial APD90 minus epicardial APD90. Summed together, ΔAPD90 and Δ(latency) provided a measure of the difference in time between stimulation and action potential repolarization between endocardium and epicardium.
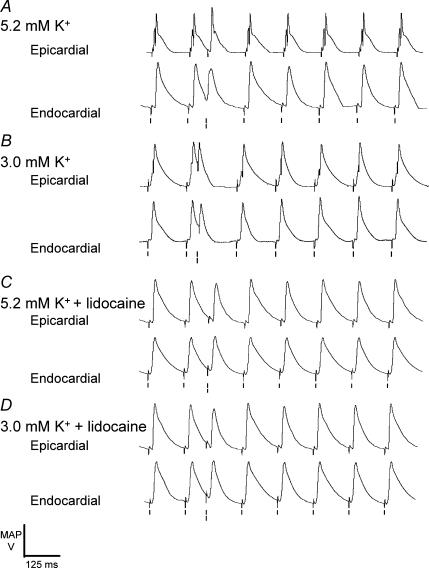
Application of the S2 stimulation protocol to hearts exposed to hypokalaemic test solution for 20 min reliably precipitated arrhythmic activity and thus precluded quantitative analysis of transmural repolarization gradients. Accordingly, recordings were made after 15 min rather than 20 min exposure to test solutions. At this time a stable rhythm still persisted following the application of both regular and S2 stimulation protocols in all cases. There was no significant difference (P > 0.05) in Δ(latency) or ΔAPD90 between hearts exposed to normokalaemic (−0.7 ± 2.3 and 5.5 ± 4.5 ms, respectively) and other test solutions when subjected to regular stimulation at an interstimulus interval of 125 ms. However, contrasting results were obtained when hearts were subjected to the S2 stimulation protocol. Figure 3 illustrates typical examples of the resulting epicardial (upper traces in each pair) and endocardial (lower traces in each pair) recordings. MAPs occurring in response to S2 and S3 stimuli showed noticeably different waveforms that contrasted with the consistent waveforms obtained in response to other stimuli. Nevertheless, latency and Δ(latency) values obtained from hearts exposed to the four test solutions and subjected to such stimulation were statistically indistinguishable (P > 0.05) from values obtained with regular stimulation.
Figure 3. Epicardial and endocardial MAPs following application of the S2 stimulation protocol at S1S2 intervals just greater than the ventricular effective refractory period.
Epicardial (upper traces in each pair) and endocardial (lower traces in each pair) monophasic action potential recordings from hearts exposed to normokalaemic (A) and hypokalaemic (B) test solutions, and normokalaemic (C) and hypokalaemic (D) test solutions containing lidocaine, for 15 min. Single vertical lines indicate the timing of S0–S1 and S3–S7 stimuli, and double lines indicate the timing of S2 stimuli.
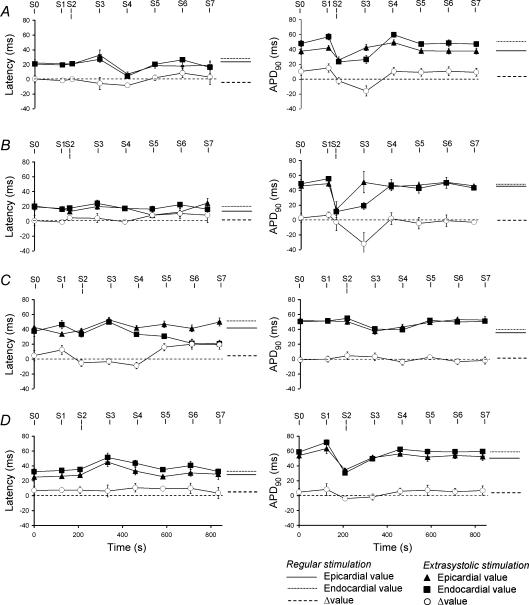
Transmural repolarization gradients alter with S1S2 interval in hypokalaemic hearts
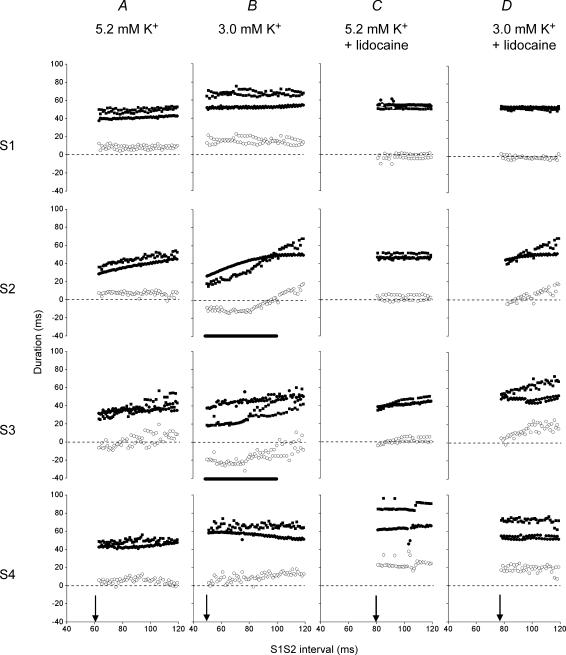
Figure 4A–D gives typical results of a closer, quantitative exploration of changes in these kinetics of MAPs evoked by S1–S4 stimuli. The figure shows epicardial APD90 (filled circles), endocardial APD90 (filled squares) and ΔAPD90 (open circles) at S1S2 intervals ranging between the VERP (arrows) and 120 ms, a positive ΔAPD90 indicating spread of repolarization from epicardium to endocardium and a negative ΔAPD90 indicating the reverse. ΔAPD90 was consistently positive in hearts subjected to regular stimulation irrespective of the test solution, as reflected in the ΔAPD90 values at the longest S1S2 interval in Fig. 4. In hearts exposed to normokalaemic test solution, progressively shortening the S1S2 interval proportionally decreased epicardial and endocardial APD90 values, leaving ΔAPD90 constant and positive. However, in MAPs following the subsequent S3 stimulus endocardial APD90 decreased more sharply than did epicardial APD90 with decreasing S1S2 interval. The two sets of points crossed to give negative ΔAPD90 values at S1S2 intervals between the VERP and (VERP + 18 ± 6 ms) (n = 8, five hearts, VERP = 61 ms in the heart shown). Finally after the S4 stimulus, epicardial and endocardial APD90 values and ΔAPD90 had recovered to their values obtained with regular stimulation. Perfusion with hypokalaemic test solution (Fig. 4B) accentuated these changes, S2 and S3 stimulation resulting in steeper decreases in endocardial as compared to epicardial APD90 with decreasing S1S2 interval. The two sets of points intersected to give a negative ΔAPD90 at a wider range of S1S2 intervals between the VERP and (VERP + 22 ± 6 ms) (n = 8, five hearts, VERP = 50 ms in the heart shown), that agreed with the range of S1S2 intervals that resulted in arrhythmic activity after longer durations (20 min) of hypokalaemia (horizontal thick lines in Fig. 4B). Nevertheless, after the S4 stimulus both epicardial and endocardial APD90 values and ΔAPD90 again had fully recovered.
Figure 4. Transient alterations in transmural repolarization gradients at varying S1S2 intervals.
Epicardial APD90 (filled circles), endocardial APD90 (filled squares) and ΔAPD90 (open circles) of monophasic action potentials obtained after control (S1) extrasystolic (S2) and subsequent (S3, S4) stimulation at S1S2 intervals progressively from 120 ms to the ventricular effective refractory period (filled arrows) in hearts exposed to normokalaemic (A) and hypokalaemic (B) test solutions, and normokalaemic (C) and hypokalaemic (D) test solutions containing lidocaine. Thick horizontal lines indicate significantly negative ΔAPD90 in B (S2 and S3).
In parallel with its abolition of arrhythmic activity (Fig. 2), exposure to lidocaine resulted in ΔAPD90 remaining positive throughout the S2 stimulation protocol in both normokalaemic (n = 8, five hearts, Fig. 4C) and hypokalaemic (n = 8, five hearts, Fig. 4D) hearts despite its decreasing at S1S2 intervals just greater than the VERP (81 ms in Fig. 4C, and 78 ms in Fig. 4D).
PVDs resulting from extrasystolic stimulation produce transient alterations in ΔAPD90
These findings prompted a further, quantitative, analysis of Δ(latency) and ΔAPD90 in a population of hearts specifically studied at the shortest S1S2 interval compatible with the generation of a MAP, the point at which both maximal changes in ΔAPD90 and arrhythmogenesis were observed as illustrated in Fig. 2. Figure 5 indicates mean epicardial latency and APD90 obtained with regular pacing by continuous lines, endocardial latency and APD90 by dotted lines, and Δ(latency) and ΔAPD90 by dashed lines; the corresponding mean values obtained following the S0–S7 stimuli of the final cycle of the S2 stimulation protocol in which early S2 stimuli were delivered are indicated by triangles, squares and circles, respectively. This demonstrates for the first time transient changes in transmural repolarization gradients following PVDs occurring in response to extrasystolic simulation, taking place in the absence of alterations in latencies.
Figure 5. Transient alterations in ΔAPD90 following S2 stimulation close to refractoriness.
Effect of regular stimulation at a 125 ms interstimulus interval and of S2 stimulation at an S1S2 interval just greater than the ventricular effective refractory period on the difference between endocardial and epicardial latencies, Δ(latency) and on the difference between endocardial and epicardial action potential duration at 90% repolarization, ΔAPD90 after 15 min exposure to normokalaemic (A) and hypokalaemic (B) test solutions, and normokalaemic (C) and hypokalaemic (D) test solutions containing lidocaine. Epicardial ΔAPD90 on regular stimulation (continuous lines) and on S2 stimulation (triangles), endocardial APD90 on regular stimulation (dotted lines) and on S2 stimulation (squares), and ΔAPD90 on regular stimulation (dashed lines) and on S2 stimulation (circles). Lines indicate the timing of stimuli.
First, latencies and Δ(latency) were consistent throughout both types of stimulation (Fig. 5, left). Regular stimulation after exposure to normokalaemic test solution initiated MAPs with a 21.0 ± 0.8 ms epicardial and a 19.4 ± 1.2 ms endocardial latency giving a Δ(latency) of −1.6 ± 1.4 ms (n = 25, 14 hearts). The latter did not change significantly (P > 0.05) after exposure to hypokalaemic test solution (n = 8, five hearts), lidocaine-containing normokalaemic (n = 8, five hearts) or hypokalaemic (n = 8, five hearts) test solutions. Furthermore, the S2 stimulation protocol gave statistically identical MAP latencies following S0–S7 stimulation, all of which were indistinguishable from those obtained on regular stimulation (P > 0.05).
Second, a statistical analysis of changes in ΔAPD90 (Fig. 5, right) following extrasystolic stimulation confirmed and extended the findings presented in Fig. 4. When normokalaemic hearts were subjected to regular stimulation, epicardial APD90 (42.0 ± 3.6 ms) was significantly shorter than endocardial APD90 (57.0 ± 4.3 ms, P < 0.05) giving a baseline ΔAPD90 of 15.0 ± 5.6 ms (Fig. 5A, right), all in close agreement with values previously reported in mice (Knollmann et al. 2001; Liu et al. 2004). Both S0 and S7 stimulation gave similar results, reflecting stable APD90 at the beginning and end of each cycle. However, when PVDs were elicited by early S2 stimuli (S1S2 interval = 45 ± 6 ms) both epicardial and endocardial APD90 decreased significantly (P < 0.05), epicardial APD90 falling to 25.7 ± 4.3 ms and endocardial APD90 falling to 23.4 ± 9.9 ms, giving a ΔAPD90 of −2.3 ± 4.4 ms. This change was accentuated in MAPs following subsequent S3 stimulation, epicardial APD90 recovering to its value after S0 stimulation (42.5 ± 4.2 ms) while endocardial APD90 remained significantly shortened (26.6 ± 5.3 ms), resulting in the reversal of ΔAPD90 to −15.9 ± 6.8 ms (P < 0.05). After S4 and subsequent stimulation, all MAP parameters had fully recovered to their steady-state values, as indicated in Fig. 4A.
Hypokalaemia accentuated these effects. On regular stimulation, epicardial APD90 had increased to 52.4 ± 1.7 ms, not differing significantly from endocardial APD90 (55.7 ± 5.3 ms, P > 0.05), giving a ΔAPD90 of 3.3 ± 5.6 ms (Fig. 5B, right). Early S2 stimuli delivered at an S1S2 interval of 35 ± 4 ms resulted in PVDs with reduced epicardial (14.1 ± 3.9 ms) and endocardial APD90 values (11.5 ± 5.2 ms), giving a ΔAPD90 of −2.7 ± 4.2 ms. In contrast, MAPs obtained in response to subsequent S3 stimulation showed an epicardial APD90 that had fully recovered (50.9 ± 10.5 ms). However, endocardial APD90 remained significantly shortened (24.1 ± 4.5 ms), resulting in a significant and negative ΔAPD90 (−31.8 ± 11.8 ms, P < 0.01). Once again, APD90 values and ΔAPD90 after S4 and subsequent stimulation had fully recovered, as indicated in Fig. 4B. Thus exposure to hypokalaemic test solution significantly increased the magnitude of the negative ΔAPD90 obtained on such S3 stimulation, in fitting with the initiation of arrhythmic activity on early S2 stimulation after 20 min hypokalaemia.
These delayed effects of PVDs resulting from early S2 stimulation on ΔAPD90 were entirely abolished by exposure to lidocaine. On regular stimulation addition of lidocaine to the normokalaemic test solution resulted in proportionate increases in epicardial (50.3 ± 4.0 ms) and endocardial (54.9 ± 5.2 ms) APD90 (P < 0.05) and had no effect on ΔAPD90 (4.6 ± 5.2 ms, Fig. 5C, right). APD90 and ΔAPD90 values of MAPs were unchanged during the S2 stimulation protocol (S1S2 interval for early S2 stimuli = 89 ± 6 ms). On regular stimulation, addition of lidocaine to the hypokalaemic test solution again increased both epicardial (50.3 ± 4.0 ms) and endocardial (54.9 ± 5.2 ms) APD90 but had no effect on ΔAPD90 (4.6 ± 5.2 ms, Fig. 5D, right). These values remained unchanged during the S2 stimulation protocol (S1S2 interval for early S2 stimulation = 84 ± 8 ms), again compatible with the antiarrhythmic effect of lidocaine in hearts exposed to hypokalaemic test solution for 20 min.
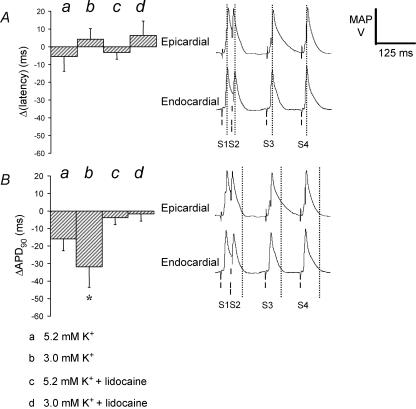
Reversals in ΔAPD90 are reflected in altered epicardial and endocardial MAP waveforms
Figure 6 compares in detail Δ(latency) (Fig. 6Aa–d, left) and ΔAPD90 (Fig. 6Ba–d, left) values in hypokalaemic hearts (b, n = 8, five hearts) where the transient transmural repolarization gradients were at their most prominent with corresponding findings from hearts exposed to normokalaemic (a, n = 25, 14 hearts), and lidocaine-containing normokalaemic (c, n = 8, five hearts) and hypokalaemic (d, n = 8, five hearts) test solutions. Δ(latency) values were neither significantly different from zero nor from each other (Fig. 6A, left). In contrast, the asterisk indicates that the ΔAPD90 value in hypokalaemic hearts is significantly more negative (P < 0.05) than those obtained in each of the remaining cases a, c and d. These latter findings correlate with the comparison of the corresponding epicardial and endocardial MAP waveforms (Fig. 6A and B, right). Thus the vertical lines pass through not only peak amplitudes of the endocardial MAPs evoked by S1–S4 stimuli, but also those of the epicardial MAPs (Fig. 6A, right). In contrast the vertical lines drawn through the APD90 values of endocardial MAPs evoked by S3, but not S2 or S4, stimuli transect the epicardial MAPs while the epicardium was still significantly depolarized.
Figure 6. Reversals in ΔAPD90 as reflected in altered epicardial and endocardial MAP waveforms.
Relationship between changes in the difference between endocardial and epicardial latencies, Δ(latency) (A, left) and the difference between endocardial and epicardial action potential durations at 90% repolarization, ΔAPD90 (B, left) in hearts subjected to S2 stimulation after 15 min exposure to normokalaemic (a) and hypokalaemic (b) test solutions, and normokalaemic (c) and hypokalaemic (d) test solutions containing lidocaine, and corresponding MAP waveforms. The asterisk indicates that the value in hearts exposed to hypokalaemic test solution is significantly (P < 0.05) more negative than that recorded in hearts exposed to all the other test solutions. In the right-hand panels, lines indicate the timings of S1–S4 stimuli. Vertical dotted lines are drawn through peak endocardial action potential amplitudes (A, right) and endocardial ΔAPD90 (B, right) in a single set of waveforms recorded from a heart exposed to hypokalaemic test solution for 15 min.
Taken together, these findings thus demonstrate that PVDs occurring in response to early S2 stimuli result in the transient reversal of ΔAPD90. The resulting altered transmural repolarization gradients otherwise resemble those described in the LQTS models cited above.
Discussion
Clinical hypokalaemia predisposes to ventricular arrhythmias associated with T-wave inversion and prolongation of the electrocardiographic QT interval reflecting increased ventricular action potential duration (Zipes & Braunwald, 2005). The latter has already been attributed to decreased [K+]o reducing repolarizing currents through altered ion channel gating, despite increased outward K+ gradients that would otherwise have been expected to increase these currents (Yang & Roden, 1996; Killeen et al. 2007).
Arrhythmogenicity in the context of QT prolongation has previously been associated with sustained increases in the magnitude of transmural repolarization gradients (Shimizu & Antzelevitch, 1997, 1998; Shimizu et al. 1999; Akar et al. 2002; Di Diego et al. 2003; Milberg et al. 2005). Manipulation of such gradients where they occur across the thickness of the myocardium by warming or cooling the epicardium has correspondingly been shown to modify T-wave polarity in dogs (Higuchi & Nakaya, 1984), in fitting with T-wave changes being an important indicator of arrhythmic tendency in humans (Zipes & Braunwald, 2005).
PVDs are implicated in the initiation of arrhythmic activity in a variety of situations including both hypokalaemia (Whelton, 1984) and the congenital LQTS (Noda et al. 2004). The present experiments explored the possibility that transient alterations in transmural repolarization gradients following PVDs initiated by extrasystolic (S2) stimuli might be associated with arrhythmogenesis in the hypokalaemic murine heart and further whether the clinically effective antiarrhythmic drug lidocaine (Rehnqvist et al. 1984) influenced such gradients. Hearts were studied following exposure times just shorter than those producing frank arrhythmogenesis that would otherwise preclude the quantitative measurement of monophasic action potential parameters.
First, these experiments established that any arrhythmogenicity does not reflect alterations in conduction velocity. This contrasts with findings in a mouse model of the Brugada syndrome where arrhythmogenicity is attributed to slowed conduction (Papadatos et al. 2002). Thus the difference between endocardial and epicardial stimulation to depolarization latency (Δ(latency)) did not differ significantly whether hearts were exposed to hypokalaemic test solution, lidocaine, or both, when compared to normokalaemic controls.
Second, on regular stimulation under the conditions investigated, hearts did not show the transmural repolarization gradients described in LQTS (Shimizu & Antzelevitch, 1997, 1998; Akar et al. 2002; Milberg et al. 2005). Thus the difference between endocardial and epicardial depolarization to repolarization time (quantified at 90% repolarization, ΔAPD90) was not statistically different from normokalaemic hearts.
Third, PVDs initiated by S2 stimuli delivered soon after recovered from refractoriness were shorter than action potentials evoked by regular stimuli. The possibility that these events rather represented motion artefacts is unlikely since they were reproducible between hearts, appeared identical in both electrodes and similar findings have been reported in previous studies (reviewed in Franz, 1999). This has previously been explained in terms of a combination of reduced inward currents owning to incomplete recovery of L-type Ca2+ channels (Sun et al. 1997), reduced Ca2+-induced-Ca2+-release from sarcoplasmic recticular stores (Isenberg & Han, 1994) and decreased currents through the Na+–Ca2+ exchanger (Janvier et al. 1997). Regional heterogeneities in the degree of action potential shortening resulted in alterations in transmural repolarization gradients, reflected in the negative ΔAPD90 seen specifically in hypokalaemic hearts. This extends to isolated rodent hearts findings from canine heart preparations (Litovsky & Antzelevitch, 1989) and further correlates these with the initiation of arrhythmic activity observed after longer exposure times. In addition the finding that ΔAPD90 became most negative when PVDs were initiated by S2 stimuli delivered immediately after recovery from refractoriness is in fitting with the especially arrhythmogenic effect of PVDs which interrupt electrocardiographic R waves reported elsewhere (Smirk, 1949; Naito et al. 1982; Kiryu et al. 1999). In hypokalaemic hearts PVDs could be elicited by S2 stimuli delivered at shorter S1S2 intervals than in normokalaemic hearts. As predicted by the Nernst equation, experimental work confirms that decreasing extracellular [K+] hyperpolarizes feline ventricular myocyte membranes (Sperelakis et al. 1970). Such hyperpolarization increases the proportion of Na+ channels available for activation in guinea pig ventricular myocytes (Gettes & Reuter, 1974), potentially explaining this finding.
Fourth, the present study went on to demonstrate for the first time in the intact murine heart that PVDs result in changes in the duration of subsequent (S3-evoked) action potentials. This observation has previously been reported in excised canine papillary muscle (Bass, 1975) and human ventricular trabeculae (Schouten et al. 1990) as a part of the phenomenon of postextrasystolic potentiation. In the ventricular trabeculae of the ferret such changes have been attributed to changes in the diastolic interval preceding the initiation of the S3-evoked action potential modifying Ca2+-induced Ca2+ release with consequences for inward Na+–Ca2+ exchanger activity and therefore action potential duration (Urthaler et al. 1994). Differences in Ca2+ handling between murine epicardial and endocardial myocytes may thus contribute to changes in ΔAPD90 following S3 stimulation (Dilly et al. 2006). Ultimately responses to S3 stimuli unmasked significant alterations in ΔAPD90.
Fifth, exposure to lidocaine abolished all changes in ΔAPD90 resulting from PVDs, correlating with its antiarrhythmic effect both in these preparations and in clinical situations (Rehnqvist et al. 1984). This effect of Na+ channel block is in fitting with a previous report that increases in action potential duration in response to S3 stimulation are attenuated by reduction of extracellular [Na+] (Schouten et al. 1990). Finally this is emphasized by comparison of ΔAPD90 values with corresponding MAP waveforms.
Taken together, these findings suggest that PVDs initiated by S2 stimuli result in transient changes in transmural repolarization gradients in association with the initiation of arrhythmic activity in hypokalaemic murine hearts. During such arrhythmia the consequent rapid succession of depolarizations may then replicate further PVDs, contributing to its maintenance. At the very least, the possibility that transient changes in transmural repolarization gradients following PVDs might also be associated with arrhythmogenicity in LQTS now merits investigation.
Acknowledgments
We thank the James Baird Fund, the Frank Elmore Fund, the Medical Research Council, the Wellcome Trust, the British Heart Foundation, the Helen Kirkland Trust, and the Avrith Fund for their generous support.
References
- Akar FG, Yan GX, Antzelevitch C, Rosenbaum DS. Unique topographical distribution of M cells underlies reentrant mechanism of torsade de pointes in the long-QT syndrome. Circulation. 2002;105:1247–1253. doi: 10.1161/hc1002.105231. [DOI] [PubMed] [Google Scholar]
- Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. II. The role of nonuniform recovery of excitability in the occurrence of unidirectional block, as studied with multiple microelectrodes. Circ Res. 1976;39:168–177. doi: 10.1161/01.res.39.2.168. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam R, Grace AA, Saumarez RC, Vandenberg JI, Huang CL. Electrogram prolongation and nifedipine-suppressible ventricular arrhythmias in mice following targeted disruption of KCNE1. J Physiol. 2003;552:535–546. doi: 10.1113/jphysiol.2003.048249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BG. Restitution of the action potential in cat papillary muscle. Am J Physiol. 1975;228:1717–1724. doi: 10.1152/ajplegacy.1975.228.6.1717. [DOI] [PubMed] [Google Scholar]
- Boyett MR, Jewell BR. A study of the factors responsible for rate-dependent shortening of the action potential in mammalian ventricular muscle. J Physiol. 1978;285:359–380. doi: 10.1113/jphysiol.1978.sp012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro MC, Knollmann BC, Ebert SN, Vary JC, Jr, Greene AE, Franz MR, Grinberg A, Huang SP, Pfeifer K. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange–Nielsen Syndrome. Proc Natl Acad Sci U S A. 2001;98:2526–2531. doi: 10.1073/pnas.041398998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Diego JM, Belardinelli L, Antzelevitch C. Cisapride-induced transmural dispersion of repolarization and torsade de pointes in the canine left ventricular wedge preparation during epicardial stimulation. Circulation. 2003;108:1027–1033. doi: 10.1161/01.CIR.0000085066.05180.40. [DOI] [PubMed] [Google Scholar]
- Dilly KW, Rossow CF, Votaw VS, Meabon JS, Cabarrus JL, Santana LF. Mechanisms underlying variations in excitation–contraction coupling across the mouse left ventricular free wall. J Physiol. 2006;572:227–241. doi: 10.1113/jphysiol.2005.102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherif N, Myerburg RJ, Scherlag BJ, Befeler B, Aranda JM, Castellanos A, Lazzara R. Electrocardiographic antecedents of primary ventricular fibrillation. Value of the R-on-T phenomenon in myocardial infarction. Br Heart J. 1976;38:415–422. doi: 10.1136/hrt.38.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabritz L, Kirchhof P, Franz MR, Eckandt L, Monnig G, Milberg P, Breithardt G, Havercamp W. Prolonged action potential duration, increased dispersion of repolarization and polymorphic ventricular tachycandia in a mouse model of proarrhythmia. Basic Res Cardiol. 2003;98:25–32. doi: 10.1007/s00395-003-0386-y. [DOI] [PubMed] [Google Scholar]
- Franz MR. Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc Res. 1999;41:25–40. doi: 10.1016/s0008-6363(98)00268-5. [DOI] [PubMed] [Google Scholar]
- Gettes LS, Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol. 1974;240:703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head CE, Balasubramaniam R, Thomas G, Goddard CA, Lei M, Colledge WH, Grace AA, Huang CL. Paced electrogram fractionation analysis of arrhythmogenic tendency in ΔKPQ Scn5a mice. J Cardiovasc Electrophysiol. 2005;16:1329–1340. doi: 10.1111/j.1540-8167.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Nakaya Y. T wave polarity related to the repolarization process of epicardial and endocardial ventricular surfaces. Am Heart J. 1984;108:290–295. doi: 10.1016/0002-8703(84)90614-8. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Han S. Gradation of Ca2+-induced Ca2+ release by voltage-clamp pulse duration in potentiated guinea-pig ventricular myocytes. J Physiol. 1994;480:423–438. doi: 10.1113/jphysiol.1994.sp020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier NC, McMorn SO, Harrison SM, Taggart P, Boyett MR. The role of Na+–Ca2+ exchange current in electrical restitution in ferret ventricular cells. J Physiol. 1997;504:301–314. doi: 10.1111/j.1469-7793.1997.301be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen M, Thomas G, Gurung I, Goddard C, Fraser J, Mahaut-Smith M, Colledge H, Grace A, Huang C. Arrhythmogenic mechanisms in the isolated perfused hypokalemic murine heart. Acta Physiol. 2007;189:33–46. doi: 10.1111/j.1748-1716.2006.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof P, Degen H, Franz MR, Eckardt L, Fabritz L, Milberg P, Laer S, Neumann J, Breithardt G, Haverkamp W. Amiodarone-induced postrepolarization refractoriness suppresses induction of ventricular fibrillation. J Pharmacol Exp Ther. 2003;305:257–263. doi: 10.1124/jpet.102.046755. [DOI] [PubMed] [Google Scholar]
- Kiryu K, Machida N, Kashida Y, Yoshihara T, Amada A, Yamamoto T. Pathologic and electrocardiographic findings in sudden cardiac death in racehorses. J Vet Med Sci. 1999;61:921–928. doi: 10.1292/jvms.61.921. [DOI] [PubMed] [Google Scholar]
- Knollmann BC, Katchman AN, Franz MR. Monophasic action potential recordings from intact mouse heart: validation, regional heterogeneity, and relation to refractoriness. J Cardiovasc Electrophysiol. 2001;12:1286–1294. doi: 10.1046/j.1540-8167.2001.01286.x. [DOI] [PubMed] [Google Scholar]
- Knollmann BC, Schober T, Petersen AO, Sirenko SG, Franz M. Action potential characterization in intact mouse heart: steady-state cycle length dependence and electrical restitution. Am J Physiol Heart Circ Physiol. 2006;292:H614–H621. doi: 10.1152/ajpheart.01085.2005. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Gotoh M, Mandel WJ, Karagueuzian HS. Increased temporo-spatial dispersion of repolarization during double premature stimulation in the intact ventricle. Pacing Clin Electrophysiol. 1992;15:2194–2199. doi: 10.1111/j.1540-8159.1992.tb03046.x. [DOI] [PubMed] [Google Scholar]
- Kuo CS, Munakata K, Reddy CP, Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983;67:1356–1367. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- Laurita KR, Girouard SD, Akar FG, Rosenbaum DS. Modulated dispersion explains changes in arrhythmia vulnerability during premature stimulation of the heart. Circulation. 1998;98:2774–2780. doi: 10.1161/01.cir.98.24.2774. [DOI] [PubMed] [Google Scholar]
- Litovsky SH, Antzelevitch C. Rate dependence of action potential duration and refractoriness in canine ventricular endocardium differs from that of epicardium: role of the transient outward current. J Am Coll Cardiol. 1989;14:1053–1066. doi: 10.1016/0735-1097(89)90490-7. [DOI] [PubMed] [Google Scholar]
- Liu G, Iden JB, Kovithavongs K, Gulamhusein R, Duff HJ, Kavanagh KM. In vivo temporal and spatial distribution of depolarization and repolarization and the illusive murine T wave. J Physiol. 2004;555:267–279. doi: 10.1113/jphysiol.2003.054064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberg P, Eckardt L, Bruns HJ, Biertz J, Ramtin S, Reinsch N, Fleischer D, Kirchhof P, Fabritz L, Breithardt G, Haverkamp W. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J Pharmacol Exp Ther. 2002;303:218–225. doi: 10.1124/jpet.102.037911. [DOI] [PubMed] [Google Scholar]
- Milberg P, Reinsch N, Wasmer K, Monnig G, Stypmann J, Osada N, Breithardt G, Haverkamp W, Eckardt L. Transmural dispersion of repolarization as a key factor of arrhythmogenicity in a novel intact heart model of LQT3. Cardiovasc Res. 2005;65:397–404. doi: 10.1016/j.cardiores.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115:2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M, Michelson EL, Kaplinsky E, Dreifus LS, David D, Blenko TM. Role of early cycle ventricular extrasystoles in initiation of ventricular tachycardia and fibrillation: evaluation of the R on T phenomenon during acute ischemia in a canine model. Am J Cardiol. 1982;49:317–322. doi: 10.1016/0002-9149(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Noda T, Shimizu W, Satomi K, Suyama K, Kurita T, Aihara N, Kamakura S. Classification and mechanism of Torsade de Pointes initiation in patients with congenital long QT syndrome. Eur Heart J. 2004;25:2149–2154. doi: 10.1016/j.ehj.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, Colledge WH, Grace AA. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene. Scn5a Proc Natl Acad Sci U S A. 2002;99:6210–6215. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehnqvist N, Ericsson CG, Eriksson S, Olsson G, Svensson G. Comparative investigation of the antiarrhythmic effect of propafenone (Rytmonorm) and lidocaine in patients with ventricular arrhythmias during acute myocardial infarction. Acta Med Scand. 1984;216:525–530. doi: 10.1111/j.0954-6820.1984.tb05041.x. [DOI] [PubMed] [Google Scholar]
- Ruberman W, Weinblatt E, Goldberg JD, Frank CW, Chaudhary BS, Shapiro S. Ventricular premature complexes and sudden death after myocardial infarction. Circulation. 1981;64:297–305. doi: 10.1161/01.cir.64.2.297. [DOI] [PubMed] [Google Scholar]
- Ruberman W, Weinblatt E, Goldberg JD, Frank CW, Shapiro S. Ventricular premature beats and mortality after myocardial infarction. N Engl J Med. 1977;297:750–757. doi: 10.1056/NEJM197710062971404. [DOI] [PubMed] [Google Scholar]
- Saumarez RC, Grace AA. Paced ventricular electrogram fractionation and sudden death in hypertrophic cardiomyopathy and other non-coronary heart diseases. Cardiovasc Res. 2000;47:11–22. doi: 10.1016/s0008-6363(00)00096-1. [DOI] [PubMed] [Google Scholar]
- Schouten VJ, ter Keurs HE, Quaegebeur JM. Influence of electrogenic Na/Ca exchange on the action potential in human heart muscle. Cardiovasc Res. 1990;24:758–767. doi: 10.1093/cvr/24.9.758. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade des pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997;96:2038–2047. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long-QT syndrome: effects of beta-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Effects of a K+ channel opener to reduce transmural dispersion of repolarization and prevent torsade de pointes in LQT1, LQT2, and LQT3 models of the long-QT syndrome. Circulation. 2000;102:706–712. doi: 10.1161/01.cir.102.6.706. [DOI] [PubMed] [Google Scholar]
- Shimizu W, McMahon B, Antzelevitch C. Sodium pentobarbital reduces transmural dispersion of repolarization and prevents torsades de pointes in models of acquired and congenital long QT syndrome. J Cardiovasc Electrophysiol. 1999;10:154–164. doi: 10.1111/j.1540-8167.1999.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Smirk FH. R waves interrupting T waves. Br Heart J. 1949;11:23–26. doi: 10.1136/hrt.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N, Mayer G, Macdonald R. Velocity of propagation in vertebrate cardiac muscles as functions of tonicity and [K+] Am J Physiol. 1970;219:952–963. doi: 10.1152/ajplegacy.1970.219.4.952. [DOI] [PubMed] [Google Scholar]
- Sun H, Leblanc N, Nattel S. Mechanisms of inactivation of L-type calcium channels in human atrial myocytes. Am J Physiol Heart Circ Physiol. 1997;272:H1625–H1635. doi: 10.1152/ajpheart.1997.272.4.H1625. [DOI] [PubMed] [Google Scholar]
- Urthaler F, Walker AA, Reeves RC, Hefner LL. Estimates of beat to beat handling of activator calcium using measurements of [Ca2+]i in aequorin loaded ferret cardiac muscle. Cardiovasc Res. 1994;28:40–46. doi: 10.1093/cvr/28.1.40. [DOI] [PubMed] [Google Scholar]
- VanderBrink BA, Sellitto C, Saba S, Link MS, Zhu W, Homoud MK, Estes NA, 3rd, Paul DL, Wang PJ. Connexin40-deficient mice exhibit atrioventricular nodal and infra-Hisian conduction abnormalities. J Cardiovasc Electrophysiol. 2000;11:1270–1276. doi: 10.1046/j.1540-8167.2000.01270.x. [DOI] [PubMed] [Google Scholar]
- Weissenburger J, Nesterenko VV, Antzelevitch C. Transmural heterogeneity of ventricular repolarization under baseline and long QT conditions in the canine heart in vivo: torsades de pointes develops with halothane but not pentobarbital anesthesia. J Cardiovasc Electrophysiol. 2000;11:290–304. doi: 10.1111/j.1540-8167.2000.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Whelton PK. Diuretics and arrhythmias in the Medical Research Council trial. Drugs. 1984;28(Suppl. 1):54–65. doi: 10.2165/00003495-198400281-00006. [DOI] [PubMed] [Google Scholar]
- Wolk R, Kane KA, Cobbe SM, Hicks MN. Apex-to-base dispersion of refractoriness underlies the proarrhythmic effect of hypokalaemia/hypomagnesaemia in the rabbit heart. J Electrocardiol. 2002;35:245–252. doi: 10.1054/jelc.2002.33764. [DOI] [PubMed] [Google Scholar]
- Yan G-X, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- Yang T, Roden DM. Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence. Circulation. 1996;93:407–411. doi: 10.1161/01.cir.93.3.407. [DOI] [PubMed] [Google Scholar]
- Zipes DP, Braunwald E. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia: Elsevier Saunders; 2005. [Google Scholar]