Abstract
Previous work suggests that exercise-induced arterial hypoxaemia (EIAH), causing only moderate arterial oxygen desaturation (SaO2: 92 ± 1%), does not exaggerate diaphragmatic fatigue exhibited by highly trained endurance athletes. Since changes in arterial O2 tension have a significant effect on the rate of development of locomotor muscle fatigue during strenuous exercise, the present study investigated whether hypoxia superimposed on EIAH exacerbates the exercise-induced diaphragmatic fatigue in these athletes. Eight trained cyclists (V˙O2max: 67.0 ± 2.6 ml kg−1 min−1; mean ± s.e.m.) completed in balanced order four 5 min exercise tests leading to different levels of end-exercise SaO2 (64 ± 2, 83 ± 1, 91 ± 1 and 96 ± 1%) via variations in inspired O2 fraction (FIO2: 0.13, 0.17, 0.21 and 0.26, respectively). Measurements were made at corresponding intensities (65 ± 3, 80 ± 3, 85 ± 3 and 90 ± 3% of normoxic maximal work rate, respectively) in order to produce the same tidal volume, breathing frequency and respiratory muscle load at each FIO2. The mean pressure time product of the diaphragm did not differ across the four exercise tests and ranged between 312 ± 28 and 382 ± 22 cmH2O s min−1. Ten minutes into recovery, twitch transdiaphragmatic pressure (Pdi,tw) determined by bilateral phrenic nerve stimulation, was significantly (P = 0.0001) reduced after all tests. After both hypoxic tests (FIO2: 0.13, 0.17) the degree of fall in Pdi,tw (by 26.9 ± 2.7 and 27.4 ± 2.6%, respectively) was significantly greater (P < 0.05) than after the normoxic test (by 20.1 ± 3.4%). The greater amount of diaphragmatic fatigue in hypoxia at lower leg work rates (presumably requiring smaller leg blood flow compared with normoxia at higher leg work rates), suggests that when ventilatory muscle load is similar between normoxia and hypoxia, hypoxia exaggerates diaphragmatic fatigue in spite of potentially greater respiratory muscle blood flow availability.
Depending on the relationship between the magnitude of the work incurred by the diaphragm and the adequacy of its blood and/or oxygen supply, diaphragmatic fatigue occurs during high-intensity normoxic exercise in healthy subjects (Babcock et al. 2002). The contemporary view is that diaphragmatic and accessory respiratory muscle fatigue contributes importantly to exercise performance limitations through its adverse effects on limb vascular conductance and blood flow (Harms et al. 1998, 2000; Dempsey et al. 2006).
Highly fit athletes exhibiting exercise-induced arterial hypoxaemia (EIAH) also demonstrate diaphragmatic fatigue during normoxic exercise (Vogiatzis et al. 2006). However, elimination of EIAH during hyperoxic exercise, performed at a higher work rate than in normoxia, significantly amplified the degree of diaphragmatic fatigue (Vogiatzis et al. 2006). Since respiratory muscle load between normoxic and hyperoxic conditions was designed to be similar in that study, it was suggested that in this fit group of athletes, competition between the respiratory and locomotor muscles for the available blood flow might be more important in causing diaphragmatic fatigue than EIAH per se (Vogiatzis et al. 2006). This conclusion was, however, limited to the moderate levels of arterial oxygen desaturation (SaO2: 92 ± 1%) studied.
EIAH exhibited by endurance-trained subjects during high-intensity exercise has also been shown to contribute significantly towards quadriceps muscle fatigue. Fatigue was shown to be attenuated when EIAH was prevented and exacerbated when hypoxia was superimposed to EIAH (Romer et al. 2006). Thus, it appears reasonable to expect that reducing oxygen transport to the respiratory muscles by means of adding hypoxia to EIAH would also intensify the degree of diaphragmatic fatigue. Although EIAH in highly trained athletes is certainly recognized as an especially important determinant of performance in hypoxic environments (Dempsey & Wagner, 1999), the effect of hypoxia on diaphragmatic fatigue in this population has not yet been studied.
In subjects of average fitness, diaphragmatic force-generating capacity has been shown to be more impaired during cycling at high altitude compared with equivalent work rates at sea level (Cibella et al. 1996; Gudjonsdottir et al. 2001). However, in those studies environmental hypoxia caused severe arterial desaturation (SaO2: 87%) and potentiated the hyperventilatory response to exercise, thus markedly increasing the work of breathing. Hence, the effect of hypobaric hypoxia per se on diaphragmatic fatigue could not be addressed in those studies as respiratory muscle work is an important determinant of exercise-induced diaphragmatic fatigue (Babcock et al. 2002).
Accordingly, the purpose of the present study was, by maintaining the respiratory muscle load similar during strenuous exercise while the fraction of inspired O2 gas mixture (FIO2) was changed, to investigate whether hypoxia superimposed on EIAH aggravates the exercise-induced diaphragmatic fatigue in endurance athletes with EIAH. To address this issue eight highly trained cyclists completed in a balanced ordering sequence, four 5 min exercise tests leading to different levels of end-exercise SaO2 via respective variations in FIO2 (ranging from 0.13 to 0.26). Tests were conducted at four different intensities relative to normoxic maximal work rate (ranging from 65 ± 3% at FIO2: 0.13 to 90 ± 3% at FIO2: 0.26) in order to produce the same tidal volume, breathing frequency and magnitude of diaphragmatic work throughout exercise at each FIO2. We reasoned that, as respiratory muscle work remained constant while varying FIO2, the lower leg work rate in hypoxia would presumably require lower leg blood flow than in normoxia such that the blood flow available to the respiratory muscles would be greater in hypoxia. Accordingly, it was hypothesized that hypoxia would not cause greater diaphragmatic fatigue than in normoxia as the presumable greater blood flow availability to respiratory muscles in hypoxia would compensate for the effects of arterial hypoxaemia. If diaphragmatic fatigue was, however, greater in hypoxia than in normoxia, this would be compatible with the hypothesis that diaphragmatic muscle blood flow compensation, if it occurred, would be insufficient to compensate for hypoxaemia.
Methods
Subjects
Eight male highly trained national team cyclists participated in the study which was approved by the authors' University Ethics Committee and was conducted in accordance with the guidelines of the Declaration of Helsinki. Prior to participation in the study all subjects (whose physical characteristics are given in Table 1) were informed of any risks and discomforts associated with the experiments and gave signed informed consent.
Table 1.
Pulmonary function and maximal exercise data of the study population
| Age (years) | 25 ± 2 |
| Height (cm) | 178 ± 2 |
| Weight (kg) | 70 ± 3 |
| FEV1 (l) | 5.0 ± 0.3 |
| FEV1 (% predicted) | 115 ± 5 |
| FVC (l) | 6.0 ± 0.2 |
| FVC (% predicted) | 110 ± 5 |
| SpO2 (%) at rest | 97 ± 1 |
| WRmax (W) | 397 ± 17 |
| V˙O2max (ml kg−1 min−1) | 67.0 ± 2.6 |
| HRmax (beats min−1) | 190 ± 3 |
| RER at WRmax | 1.19 ± 0.02 |
| V˙E,max (l min−1) | 151.2 ± 8.2 |
| VTmax (l min−1) | 2.67 ± 0.13 |
| fmax (breaths min−1) | 57 ± 3 |
| SpO2 (%) at WRmax | 92 ± 1 |
Values are means ± s.e.m. Exercise data depict the results of the incremental exercise test in room air. FEV, forced expiratory volume; FVC, forced vital capacity; SpO2, avfevial oxygen saturation.
Experimental design
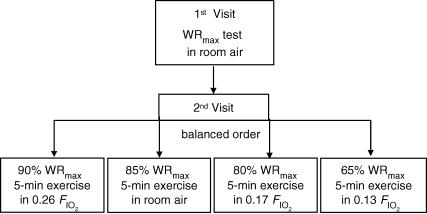
Experiments were conducted in two visits (Fig. 1). In visit 1, subjects underwent an incremental exercise test to the limit of tolerance (WRmax). This test was carried out in room air. In visit 2 (Fig. 1), subjects completed in a balanced ordering sequence four 5 min exercise tests, each separated by 90 min, at work rates corresponding to the following intensities: (i) 90% WRmax while breathing a high FIO2 (0.26: hyperoxia); (ii) 85% WRmax in room air (normoxia); (iii) 80% WRmax with FIO2 of 0.17 (mild hypoxia); and (iv) 65% WRmax with FIO2 of 0.13 (moderate hypoxia). During the tests, work rate was slightly adjusted to keep both tidal volume and breathing frequency the same at each FIO2 (Fig. 2).
Figure 1. Experimental design.
Experiments were conducted in 2 visits. In visit 1, subjects underwent an incremental exercise test in room air. In visit 2, subjects completed in balanced ordering sequence four 5 min exercise tests, separated by 90 min, breathing the following fractions of inspired O2 gas mixtures (FIO2): hyperoxic (0.26), normoxic (0.21) and two hypoxic (0.17 and 0.13).
Figure 2. Exercise load and O2 delivery responses during the four 5 min exercise tests.
Work rate (WR; A), oxygen uptake (V˙O2max; B), and heart rate (HR; C) throughout the hyperoxic (FIO2: 0.26; •), the normoxic (FIO2: 0.21; ○) and the two hypoxic exercise tests (FIO2: 0.17; ▪); FIO2: 0.13; □). Values are means ± s.e.m. for 8 subjects. †Differences between the four conditions, P < 0.05. WR changes during the course of each test reflect minor real-time adjustments needed to maintain comparable tidal volume and frequency at each FIO2.
Incremental exercise tests
The incremental exercise tests were performed on an electromagnetically braked cycle ergometer (Ergoline 800; Sensor Medics, Anaheim, CA, USA) starting at 20 W and increasing by 20 W every minute, with the subjects maintaining a pedalling frequency of 70–90 r.p.m. Tests were preceded by a 3 min rest period, followed by 3 min of unloaded pedalling. The following pulmonary gas exchange and ventilatory variables were recorded breath by breath (Vmax 229; Sensor Medics, Anaheim, CA, USA): oxygen uptake (V˙O2max), carbon dioxide elimination (V˙CO2max), respiratory exchange ratio (RER), minute ventilation (V˙E), tidal volume (VT), and breathing frequency (f). Heart rate (HR) was determined using the R–R interval from a 12-lead on line electrocardiogram (Marquette Max; Marquette Hellige GmbH, Germany).
Five minute exercise tests
During these tests, recording of pulmonary gas exchange and ventilatory variables was performed as mentioned above, whilst gas of measured FIO2 was inspired by the subjects from a Douglas bag through a two-way non-breathing valve (model 2700, Hans Rudolph). Arterial blood was taken every minute throughout the exercise protocol, whereas swings in oesophageal pressure (Poes), gastric pressure (Pga), and transdiaphragmatic pressure (Pdi) were continuously monitored. Pdi was obtained from electronic subtraction of Poes from Pga. Tests were always preceded by 5 min of warm-up cycling at 50%V˙CO2max in room air.
Pressures and flow were displayed on a computer screen, and digitized at 60 Hz using an analog-to-digital converter connected to the same computer used for optoelectronic plethysmography (OEP system, BTS, Milan, Italy). Pdi was averaged over 30 s breath samples in every minute of the exercise tests. Mean Pdi was measured by integration of Pdi during inspiration divided by the inspiratory time. The pressure–time product of the diaphragm (mean Pdi × inspiratory time × respiratory frequency) was then calculated and expressed in cmH2O s min−1.
Poes and Pga were assessed by two commercially available thin-walled balloon catheters (Ackrad Laboratories, Inc., Crandford, NJ, USA) coupled to differential pressure transducers (MP-45, ±250 cmH2O; Validyne Corp., Northridge, CA, USA). The balloons were inserted by nasal intubation following the application of 2% lidocaine anaesthetic gel to the nose and with the assistance of continuous pressure monitoring. The two balloon tips were positioned in the middle third of the oesophagus and stomach, respectively. Flow was measured with a hot wire pneumotachograph (Vmax 229; Sensor Medics) near the mouthpiece, and lung volume changes were obtained by integrating the flow signal.
Assessment of diaphragmatic fatigue
To assess diaphragmatic fatigue, twitch transdiaphragmatic pressure (Pdi,tw) was measured before and 10, 30 and 60 min following the two exercise tests. At rest and during recovery (in which for all tests subjects breathed room air), Pdi,tw was recorded during stimulation of the phrenic nerves at the neck according to recommended techniques (Babcock et al. 2002). Bilateral supramaximal transcutaneous phrenic nerve single shocks (twitches) were performed with an electrical stimulator (Neuropack; Nichon-Kohden, Tokyo, Japan). The phrenic nerves were stimulated at the posterior border of the sternomastoid muscle at the level of the cricoid cartilage. Each nerve was separately stimulated by applying single square-wave electrical pulses (duration 100 μs). The stimulus voltage was progressively increased until there was no further increase in the amplitude of the ipsilateral peak-to-peak compound motor action potential of the diaphragm (Laghi et al. 1995). The intensity of the electrical stimulus was then increased by a further 20–50%. The position of stimulation of each phrenic nerve was marked, and the subsequent stimulations were performed at this point. Compound motor action potentials were recorded with surface electrodes placed at the seventh to eighth intercostals space and the anterior axillary line. The signals were amplified, band-pass filtered (bandwidth 10 Hz to 1 kHz), displayed on the screen, and printed by the stimulator-electromyograph (Neuropack, Nichon-Kohden, Tokyo, Japan). Chest wall volumes were continuously monitored throughout the stimulation tests by optoelectronic plethysmography (Vogiatzis et al. 2005) to subsequently ensure similar thoraco-abdominal configuration during twitches (Chen et al. 2000). The electrical stimulations were performed with the subject seated on the bicycle ergometer, with a nose clip on and with the mouth closed. Subjects were instructed to breathe quietly, then to perform a gentle expiratory effort to functional residual capacity and to hold their breath while the stimulation took place. We relied on Poes as a measure of position in the respiratory cycle relative to functional residual capacity. Hence, Poes immediately before stimulation was always carefully evaluated to ensure constant lung volume. Once relaxation was achieved (as judged by levelling off of Pdi, Poes, and Pga) the operator performed the stimulations. Criteria adopted for acceptance of Pdi,tw were those previously used (Laghi et al. 1995; Babcock et al. 2002). During each designated time point, 8–10 twitches were performed and the average value of the three best Pdi,tw measurements was used for the analysis.
Arterial blood gas measurements
Arterial tensions of O2 (PaO2), and CO2 (PaCO2), pH, and percentage arterial oxygen saturation (%SaO2) were measured from 2 ml blood samples using a blood gas electrode system combined with a co-oximeter (ABL 625, Radiometer, Copenhagen, Denmark) within 10 min of collection. Blood samples, taken every minute during the 5 min tests, were kept on ice prior to measurement. The blood gas analyser was auto-calibrated every 4 h throughout the day and calibrating gases of known concentrations were run before each set of measurements. Blood gas measurements were corrected to subject's tympanic temperature taken during withdrawal of each arterial blood gas sample.
Statistical analysis
The minimum sample size was calculated based on 80% power and a two-sided 0.05 significance level (Dawson & Trapp, 2001). Sample size capable of detecting between-condition difference of 10% was estimated for the decrease in Pdi,tw using the standard deviation from our previous study (Vogiatzis et al. 2006). The critical sample size per group was estimated to be seven subjects. All data are reported as mean ± s.e.m. Two-way ANOVA with repeated measures was used to identify statistically significant differences across different time points among the four 5 min exercise tests for each variable. From previous work (Vogiatzis et al. 2006) showing that the greatest degree of fatigue occurs at 10 min post-exercise, we first examined whether the fall in Pdi,tw at 10 min of recovery was affected by FIO2. Then we explored whether the subsequent rate of recovery of Pdi,tw was affected by the FIO2 breathed during exercise. Two-way ANOVA with repeated measures was used to identify statistically significant differences between values recorded at baseline and at the 10th minute of recovery following the four exercise tests. When two-way ANOVA detected statistical significance, differences between the four conditions were identified with the LSD post hoc test. Pdi,tw values recorded at the 10th, 20th and 60th min of recovery for each subject in each condition were used to calculate the rate of recovery over time in Pdi,tw. Accordingly, recovery Pdi,tw slopes of the four tests over this time period were compared by one-way ANOVA. The level of significance for all analyses was set at P < 0.05.
Results
Incremental exercise tests
Table 1 shows the subjects' responses to the incremental exercise tests. All subjects demonstrated a reduction in percentage SaO2 ≥ 4% at the end of the incremental exercise tests in room air.
Normoxic and hypoxic exercise tests
Relative to the maximal workload achieved in the incremental exercise tests in room air, the mean workload achieved in the four 5 min tests was 90 ± 3% in hyperoxia, 85 ± 3% in normoxia, 80 ± 3% in mild hypoxia and 65 ± 3% in moderate hypoxia. Across the four tests mean work rate, V˙O2 and HR were significantly (P < 0.05) different (Fig. 2). Mean work rate throughout the normoxic test was significantly (P < 0.03) greater compared with both hypoxic tests (Fig. 2A). During the moderate hypoxia test (FIO2: 0.13) mean V˙O2 (48.7 ± 1.4 ml kg−1 min−1; 73 ± 2%V˙O2max) was significantly (P = 0.0001) lower compared with the normoxia (65.0 ± 2.2 ml kg−1 min−1; 97 ± 2%V˙O2max) and to the mild hypoxia [(FIO2: 0.17); 61.7 ± 1.4 ml kg−1 min−1; 92 ± 3%V˙O2max ] exercise tests (Fig. 2B). Mean HR was not significantly different between the normoxic and the two hypoxic tests (Fig. 2C) reaching values ranging from 93 ± 2 to 97 ± 1% of the maximum value recorded during the incremental exercise test.
Ventilatory response to the exercise
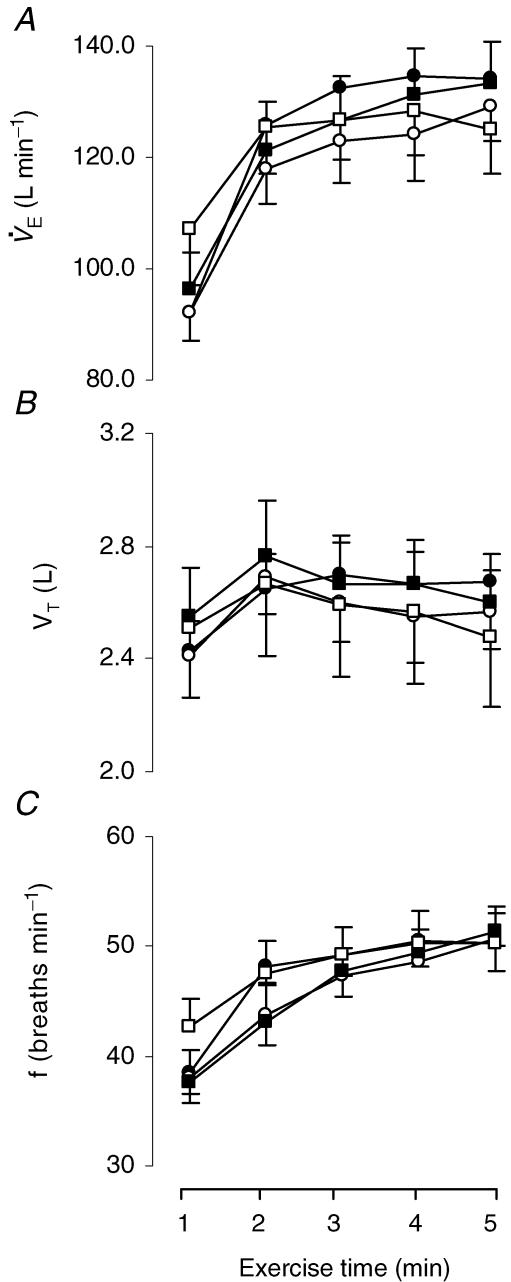
The ventilatory response to the exercise tests is shown in Fig. 3. V˙E increased similarly with exercise in all tests. Peak V˙E at the end of the four exercise tests ranged from 83 ± 5% to 88 ± 5% of the maximum value attained at the incremental test in room air (Fig. 3A). No significant differences were found in V˙E between the four tests. Neither VT nor f differed between the four tests (Fig. 3B and C).
Figure 3. Ventilatory responses during the four 5 min exercise tests.
Minute ventilation (V˙E; A), tidal volume (VT; B) and breathing frequency (f; C) throughout the hyperoxic (FIO2: 0.26; •), the normoxic [FIO2: 0.21; ○) and the two hypoxic exercise tests (FIO2: 0.17; ▪; FIO2: 0.13; □). Values are means ± s.e.m. for 8 subjects.
Arterial blood gas variables
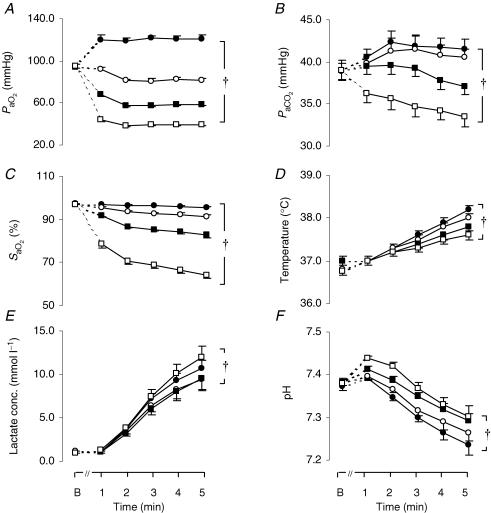
Throughout the exercise tests PaO2, PaCO2 and SaO2 were significantly (P < 0.0001) higher in the normoxic than the hypoxic tests (Fig. 4A–C), whereas pH was significantly (P = 0.006) lower during the normoxic test when compared with the moderately hypoxic (FIO2: 0.13) test (Fig. 4F). Although ventilation did not differ among the four conditions (Fig. 3A), PaCO2 in moderate hypoxia was significantly lower compared with the rest of the conditions as a result of the significantly lower metabolic demand reflected by the lower V˙O2 (Fig. 2A) and V˙CO2max. In addition, end-exercise arterial lactate in moderate hypoxia (FIO2: 0.13 = 11.9 ± 1.4 mmol l−1) was significantly (P = 0.001) higher compared with that in normoxia (9.5 ± 1.3 mmol l−1) and in mild hypoxia (FIO2: 0.17 = 9.5 ± 1.3 mmol l−1) (Fig. 4E).
Figure 4. Blood variables during the four 5 min exercise tests.
Arterial O2 pressure (PaO2; A), arterial CO2 pressure (PaCO2; B), arterial O2 saturation (SaO2%; C), tympanic temperature (°C; D), arterial lactate concentration (Lactate; E) and pH (F), at baseline (B) and throughout the hyperoxic (FIO2: 0.26; •), the normoxic (FIO2: 0.21; ○) and the two hypoxic exercise tests (FIO2: 0.17; ▪; FIO2: 0.13; □). Values are means ± s.e.m. for 8 subjects. †Differences between the four conditions, P < 0.05.
Diaphragmatic fatigue in recovery
Figure 5 shows the course of the pressure–time product of the diaphragm (that is indicative of the total effort of the diaphragm) during the four exercise tests. No significant (P = 0.16) differences were found between the four exercise tests. The mean pressure–time product of the diaphragm during the four exercise tests ranged between 312 ± 28 and 382 ± 22 cmH2O s min−1.
Figure 5. Diaphragmatic work during exercise.
Changes in pressure–time product of the diaphragm throughout the hyperoxic (FIO2: 0.26; •), the normoxic (FIO2: 0.21; ○) and the two hypoxic exercise tests (FIO2: 0.17; ▪; FIO2: 0.13; □). Values are means ± s.e.m. for 8 subjects.
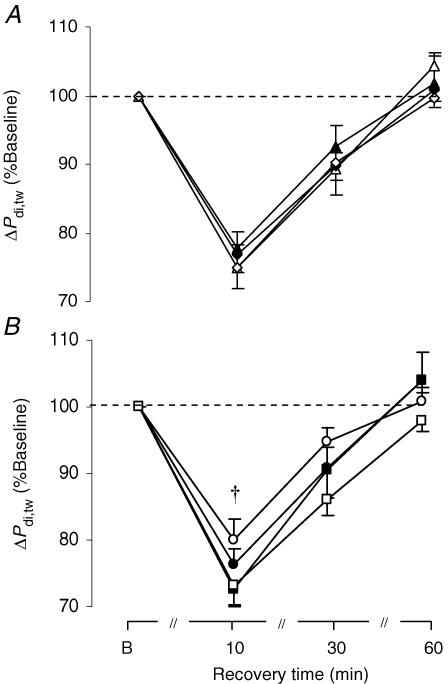
The time course of the percentage drop in Pdi,twafter each exercise test (irrespective of which workload was undertaken first) is shown in Fig. 6A, showing lack of significant ordering effect. Note that 60 min after completion of each exercise test regardless of the experimental condition, mean Pdi,tw had fully recovered to the pre-testing levels (Fig. 6A). As a result, Pdi,tw at the baseline of each subsequent test was at similar resting levels as for the first test.
Figure 6. Response of twitch transdiaphragmatic pressure during recovery.
Percentage fall in twitch diaphragmatic pressure (ΔPdi,tw) during recovery after A: the 1st (▴), 2nd (▵), 3rd (♦) and 4th (⋄) exercise tests (irrespective of FIO2); B: the hyperoxic (FIO2: 0.26; •), the normoxic ((FIO2: 0.26; •), the normoxic : 0.21; ○) and the two hypoxic exercise tests ((FIO2: 0.26; •), the normoxic : 0.17; ▪; (FIO2: 0.26; •), the normoxic : 0.13; □). Values are means ± s.e.m. for 8 subjects. †Significant difference between the normoxic and both hypoxic tests, P < 0.05.
As shown in Figs 6B and 7, the major finding of our study was that all exercise tests induced a significant (P = 0.0001) fall in Pdi,tw that was greatest at 10 min into recovery (ranging from 20 ± 3 to 27 ± 3%). The degree of fall in Pdi,tw 10 min into recovery was significantly different (P = 0.039) across the four conditions (Fig. 6B). Post hoc analysis revealed significant differences in the percentage fall in Pdi,tw between the normoxic (by 20.1 ± 3.4%) and both mild (FIO2: 0.17) and moderate (FIO2: 0.13) hypoxic runs (by 27.4 ± 2.6%, P = 0.047; and by 26.9 ± 2.7%, P = 0.003, respectively). The fall in Pdi,tw at the 10th min of recovery after the moderate hypoxic test (FIO2: 0.13) was greater than after the normoxic test in six subjects, whereas in the remaining subjects the decrease in Pdi,tw was similar after those two conditions (Fig. 8A). Similarly, in six subjects the fall in Pdi,tw 10 min after the mild hypoxic test (FIO2: 0.17) was greater than after the normoxic test, whereas in one subject the decrease in Pdi,tw was similar between the two conditions (Fig. 8B). The slope of recovery in Pdi,tw across the 10th, 30th and 60th min of recovery was not significantly different (P = 0.24) across the four exercise tests (Fig. 6B).
Figure 7. Factors affecting twitch transdiaphragmatic pressure at the 10th min of recovery.
Effect of varying FIO2 and leg workrate (as percentage of maximal workrate) on the percentage fall in twitch diaphragmatic pressure (ΔPdi,tw) at the 10th minute of recovery after the hyperoxic (FIO2: 0.26; •), the normoxic (FIO2: 0.21; ○) and the two hypoxic exercise tests (FIO2: 0.17; ▪; FIO2: 0.13; □). Values are means ± s.e.m. for 8 subjects. †Significant difference between the normoxic and both hypoxic tests, P < 0.05.
Figure 8. Factors affecting twitch transdiaphragmatic pressure at the 10th min of recovery.
Effect of varying FIO2 on the individual fall in twitch transdiaphragmatic pressure (ΔPdi,tw) at the 10th min of recovery after the 4 exercise tests.
Discussion
EIAH constitutes an important determinant of maximal oxygen uptake during high-intensity exercise in hypoxic environments, particularly in highly trained endurance athletes who usually suffer the greatest decrement in systemic oxygen delivery and maximal exercise performance (Lawler et al. 1988). Since changes in arterial O2 tension have a significant effect on the rate of development of locomotor muscle fatigue (Amann et al. 2006) during strenuous exercise, the present study investigated the effects of hypoxia superimposed on EIAH on exercise-induced diaphragmatic fatigue in highly trained endurance athletes.
The experimental design aimed at comparing the degree of diaphragmatic fatigue when ventilatory load was similar under conditions where arterial oxygen tension and work rate varied significantly. The difference in work rate sustained during the exercise tests was not only essential in order to match ventilatory load between normoxia and hypoxia, but also realistic as the absolute power output is considerably reduced during exercise at high altitude (by approximately 5–10% for each 1000 m above sea level) (Saltin, 1996). Accordingly, the lower work rates sustained during the mild and moderately hypoxic runs in the present study (by 5 and 20% WRmax, respectively) were within the range expected for exercise at altitudes equivalent to ∼1500 and 3000 m, respectively (West, 1995).
We reasoned that if hypoxaemia is important to diaphragmatic fatigue during exercise, greater diaphragmatic fatigue in hypoxic conditions would suggest that the presumed higher respiratory muscle blood flow availability in hypoxia is insufficient to compensate for hypoxaemia. This was, in turn, based on the presumption that in hypoxia the lower leg work rate would require smaller leg blood flow than in normoxia sustained at higher work rates. We found that hypoxia aggravated the exercise-induced diaphragmatic fatigue and that diaphragmatic muscle blood flow compensation, if it occurred, was therefore insufficient to compensate for hypoxaemia.
Respiratory loading during the normoxic and hypoxic tests
The workload endured by the respiratory muscles is a critical determinant of the exercise-induced diaphragmatic fatigue since unloading the respiratory muscles with the use of a proportional assist ventilator prevents diaphragmatic fatigue (Babcock et al. 2002). Accordingly, the key design feature of the present study was to control for respiratory muscle load by matching tidal volume, frequency and ventilation in normoxia and hypoxia by adjusting leg work rate. Figure 5 shows that the pressure–time product of the diaphragm and thereby the energy requirement of the diaphragm did not differ between the experimental conditions. Accordingly, the strategy of matching ventilatory requirement between the tests allowed us to isolate the role of arterial hypoxaemia per se on diaphragmatic operation in highly trained athletes.
Cardiovascular adjustments at different FIO2
Although cardiac output was not measured in the present study it is known that during acute hypoxic exposure, cardiac output at a given power output is elevated reaching, however, maximal values similar to those in normoxia, albeit at lower work rates (Knight et al. 1993; Calbet et al. 2003). In addition, leg blood flow is related to leg work rate (Mortensen et al. 2005) and does not increase at maximal exercise with hypoxia since in hypoxia lower maximal work rates can be achieved compared with normoxia (Knight et al. 1993; Calbet et al. 2003). Therefore, assuming that cardiac output was not different between the hypoxic (FIO2: 0.13) and the normoxic exercise conditions, we may speculate that during the moderate hypoxic tests (FIO2: 0.13) there was greater respiratory muscle blood flow availability (consequent to the substantially lower leg blood flow requirement). Our results therefore show that in spite of the presumably greater respiratory blood flow availability, arterial hypoxaemia aggravated diaphragmatic fatigue, further suggesting that any increase in diaphragmatic blood flow that might have occurred was insufficient to fully compensate for the low PO2.
At the mild hypoxic test (FIO2: 0.17), however, the subjects produced only slightly lower work rate (80% WRmax) compared with normoxia (85% WRmax). In light of the increase in leg blood flow observed during submaximal cycling in severe hypoxia (FIO2: 0.105; Calbet et al. 2003), one could argue that leg blood flow may have been elevated in mild hypoxia compared with normoxia (to compensate for the reduced O2 availability and maintain V˙O2), thus potentially competing with the diaphragm for the available blood flow. Although blood flow was not measured in the present study, the mild level of hypoxia used (FIO2: 0.17), the high exercise intensity (80% WRmax) implemented and the slightly lower V˙O2 (by 200 ml) in mild hypoxia compared with normoxia suggest that such a compensation with greater leg blood flow in hypoxia did not probably occur. However, it must be acknowledged that without direct measurement of both cardiac output and leg blood flow, these conclusions are speculative.
Effect of arterial hypoxaemia on diaphragmatic fatigue
Considering that the energy requirement of the diaphragm was comparable in hypoxia and in normoxia (shown by the similar breathing pattern and the pressure–time product of the diaphragm) and presuming that the reduced oxygen saturation in arterial blood perfusing the diaphragm in the hypoxic conditions was not adequately compensated by its potentially greater blood flow, it is reasonable to suggest that greater fatigue in hypoxia implies that the diaphragm's energy demand in hypoxia outgrew its supply. This would, in turn, force the diaphragm to use anaerobic energy-generating processes, thus producing lactate and partly contributing to the higher lactate values found after the moderately hypoxic (FIO2: 0.13) test. Note that the exercise induced low-frequency diaphragmatic fatigue, is a type of fatigue in which muscle ischaemia and reliance on anaerobic metabolism are important factors in its generation (Zakynthinos & Roussos, 2005).
Acidosis and/or accumulation of metabolic by-products, such as lactate, in the active musculature have been frequently proposed as possible contributors to diaphragm fatigue (Fitzgerald et al. 1984; Fregosi & Dempsey, 1986; Babcock et al. 1995). Previous work by Fregosi & Dempsey (1986) has shown that muscle lactate content in the diaphragm increases as blood lactate concentration increases after whole-body normoxic exercise, suggesting that the diaphragm may become progressively acidic, thus contributing to low-frequency fatigue.
Effect of leg work rate on diaphragmatic fatigue
Our finding that there was a trend towards a fall in Pdi,tw following hyperoxic exercise (Fig. 6B) is in line with our previous report (Vogiatzis et al. 2006) showing that elimination of EIAH during high-intensity exercise (90% WRmax) produced more diaphragmatic fatigue than normoxic exercise at 80% WRmax, thereby suggesting that competition between respiratory and locomotor muscles for the available blood flow might be a more important causative factor on diaphragmatic fatigue than EIAH per se. Lack of significant difference in the amount of diaphragmatic fatigue between normoxia and hyperoxia in the present study, may be attributed to the smaller difference in work rate sustained during the hyperoxic and the normoxic tests (i.e. 5% WRmax) as opposed to our previous protocol (Vogiatzis et al. 2006) where there was a 10% difference in work rate between those two conditions.
Potential limitations of the study
Performing all four 5 min exercise tests on the same day could have presented a limitation to the present study. However, the complexity of the experiment and the invasive techniques employed prevented us from testing the subjects on separate days. This is the reason why we performed the tests in a balanced ordering sequence to avoid any sequential effects.
Furthermore, we allowed a 90 min period for recovery between the tests because previous work (Vogiatzis et al. 2006) showed that the Pdi,tw values had recovered almost completely by an average time of 60 min in highly trained athletes exercising in normoxic and hyperoxic conditions. In the study of Babcock et al. (1995)Pdi,tw had recovered by approximately 90 min following hypoxic exercise. Indeed, in the present study, Pdi,tw had completely returned to baseline 60 min after each test (Fig. 6A).
Lack of blood flow measurements during the four exercise conditions also constitutes a limitation to the present study.
Significance of arterial O2 tension and exercise intensity on diaphragmatic fatigue
Our findings provide new insights into the functional effects of hypoxia superimposed on EIAH. The results of the present investigation expand those of our previous study which explored the effects of moderate arterial desaturation (SaO2: 92 ± 1%) on exercise-induced diaphragmatic fatigue in highly trained athletes (Vogiatzis et al. 2006). Accordingly, although naturally induced arterial oxygen desaturation was previously shown not to be important in causing diaphragmatic fatigue (Vogiatzis et al. 2006), severe arterial oxygen desaturation, caused by superimposing hypoxia on EIAH, exaggerated fatigue of the diaphragm despite the reduced leg power output and presumably the greater respiratory muscle blood flow availability. On the other hand, increasing leg power output to near-maximal levels (by preventing arterial desaturation with hyperoxia) also compromised diaphragmatic function as presumably the greater leg blood flow requirement restricted blood supply to the diaphragm, thus enhancing the degree of diaphragmatic fatigue. Taken together, our previous (Vogiatzis et al. 2006) and present findings suggest that breathing room air is the optimal FIO2, since normoxic exercise consistently yielded the lowest degree of diaphragmatic fatigue (Fig. 7). Accordingly, we suggest that training strategies exposing highly trained endurance athletes to either hypoxic or hyperoxic exercise conditions may compromise diaphragmatic function, the former because of low PaO2 and the latter because of diaphragmatic blood flow restrictions (Fig. 7).
In conclusion, in well trained athletes, the greater amount of diaphragmatic fatigue in hypoxia at lower leg work rates (presumably requiring smaller leg blood flow compared with normoxia at higher leg work rates) suggests that when ventilatory muscle load is similar between normoxia and hypoxia, hypoxia exaggerates diaphragmatic fatigue in spite of potentially greater respiratory muscle blood flow availability.
Acknowledgments
This work was supported by the Thorax Foundation.
References
- Amann M, Romer LM, Pegelow DF, Jacques AJ, Joel Hess C, Dempsey JA. Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J Appl Physiol. 2006;101:119–127. doi: 10.1152/japplphysiol.01596.2005. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Johnson BD, Pegelow DF, Suman OE, Griffin D, Dempsey JA. Hypoxic effects on exercise-induced diaphragmatic fatigue in normal healthy humans. J Appl Physiol. 1995;78:82–92. doi: 10.1152/jappl.1995.78.1.82. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragmatic fatigue. J Appl Physiol. 2002;93:201–206. doi: 10.1152/japplphysiol.00612.2001. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003;287:R996–R999. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
- Chen R, Kayser B, Macklem PT. Twitch transdiaphragmatic pressure depends critically on thoracoabdominal configuration. J Appl Physiol. 2000;88:54–60. doi: 10.1152/jappl.2000.88.1.54. [DOI] [PubMed] [Google Scholar]
- Cibella F, Cuttitta G, Kayser B, Narici M, Romano S, Saibene F. Respiratory mechanics during exhaustive submaximal exercise at high altitude in healthy humans. J Physiol. 1996;494:881–890. doi: 10.1113/jphysiol.1996.sp021540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson B, Trapp RG. Basic and Clinical Biostatistics. 3. New York: McGrow-Hill; 2001. pp. 125–1. 26. [Google Scholar]
- Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol. 2006;151:242–250. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol. 1999;87:1997–2006. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Hauer MX, Bierkamper GG, Raff H. Responses of in vitro rat diaphragm to changes in acid base environment. J Appl Physiol. 1984;57:1202–1210. doi: 10.1152/jappl.1984.57.4.1202. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Dempsey JA. Effects of exercise in normoxia and acute hypoxia on respiratory muscle metabolites. J Appl Physiol. 1986;60:1274–1283. doi: 10.1152/jappl.1986.60.4.1274. [DOI] [PubMed] [Google Scholar]
- Gudjonsdottir M, Appending L, Baderna P, Purro A, Patessio A, Vilianis G, et al. Diaphragm fatigue during exercise at high altitude: the role of hypoxia and workload. Eur Respir J. 2001;17:674–680. doi: 10.1183/09031936.01.17406740. [DOI] [PubMed] [Google Scholar]
- Harms CA, Thomas A, Wetter J, McClaran SR, Pegelow DF, Nickele GA, et al. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Harms GA, Wetter TJ, Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- Laghi F, D'Alfonso N, Tobin MJ. Pattern of recovery from diaphragmatic fatigue over 24 hours. J Appl Physiol. 1995;79:539–546. doi: 10.1152/jappl.1995.79.2.539. [DOI] [PubMed] [Google Scholar]
- Lawler JS, Powers K, Thompson D. Linear relationship between V˙O2max and V˙O2max decrement during exposure to acute hypoxia. J Appl Physiol. 1988;64:1486–1492. doi: 10.1152/jappl.1988.64.4.1486. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, et al. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol. 2005;566:273–285. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer LM, Haverkamp HC, Lovering AT, Pegelow DF, Dempsey JA. Effect of exercise-induced arterial hypoxemia on quadriceps muscle fatigue in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2006;290:R365–R375. doi: 10.1152/ajpregu.00332.2005. [DOI] [PubMed] [Google Scholar]
- Saltin B. Exercise and the environment: focus on altitude. Res Quart Exer Sport. 1996;67:1–10. doi: 10.1080/02701367.1996.10608849. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Aliverti A, Golemati S, Georgiadou O, LoMauro A, Kosmas E, et al. Respiratory kinematics by optoelectronic plethysmography during exercise in men and women. Eur J Appl Physiol. 2005;93:581–587. doi: 10.1007/s00421-004-1249-4. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Georgiadou O, Giannopoulou I, Koskolou M, Peraki E, Kostikas K, et al. Effects of exercise-induced arterial hypoxaemia and work rate on diaphragmatic fatigue in highly trained endurance athletes. J Physiol. 2006;572:539–549. doi: 10.1113/jphysiol.2005.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB. Respiratory Physiology – the Essentials. Hagerstown, MD: Williams & Wilkins; 1995. Respiratory system under stress; pp. 133–150. [Google Scholar]
- Zakynthinos S, Roussos C. Respiratory muscle fatigue. In: Hamid Q, et al., editors. Physiologic Basis of Respiratory Disease. 1. Hamilton: BC Decker; 2005. pp. 289–307. [Google Scholar]