Abstract
The prevalence of central apnoea and periodic breathing is increased in the elderly. This implies that the chemical control of breathing might become less stable with ageing. To investigate this, we measured loop gain in healthy elderly individuals using proportional assist ventilation. Loop gain is an engineering term that describes the stability of a system controlled by feedback loops, such as the respiratory control system. A loop gain close to zero indicates a stable system, whereas a loop gain close to or greater than one indicates an unstable system. Eleven healthy elderly subjects were studied with a mean ± s.d. age and body mass index (BMI) of 71 ± 5 years and 25 ± 3 kg m−2, respectively. We also studied a small group of elderly individuals with obstructive sleep apnoea (OSA) for comparison (n = 3, age 68 ± 1 years, BMI 32 ± 11 kg m−2). Comparisons were made with previously studied young individuals (age 27 ± 4 years, BMI 23 ± 1 kg m−2). We found significantly lower loop gains in the healthy elderly group (loop gain ≤ 0.25) compared with the young group (loop gain ≤ 0.47, P = 0.001). Also, we found quite low loop gains in the elderly OSA group (loop gain ≤ 0.26). We conclude that the chemical control of breathing does not become unstable with ageing and is thus an unlikely cause of central (and possibly obstructive) apnoeas in this population.
Several features of the ventilatory pattern in elderly individuals suggest that the chemical control of breathing is unstable. For example, the proportion of central apnoeas increases in elderly patients with sleep apnoea, accounting for approximately one-third of all respiratory events (Carskadon & Dement, 1981; Krieger et al. 1983; Ancoli-Israel et al. 1985, 1991; Bixler et al. 1998). One study suggests that the higher prevalence of sleep apnoea in this population is due solely to an increase in the number of central events (Bixler et al. 1998). Moreover, among patients with congestive heart failure, age is an independent risk factor for central apnoeas and Cheyne–Stokes respiration (Sin et al. 1999). Even in healthy individuals without sleep apnoea, the ventilatory pattern during light sleep appears to be more oscillatory in the elderly (Webb, 1974; Shore et al. 1985; Pack et al. 1988; Hudgel et al. 1993; Hudgel & Hamilton, 1994). While frequent arousals (sleep state instability) (Pack et al. 1992) or fluctuations in upper airway resistance (upper airway instability) (Hudgel et al. 1993; Hudgel & Hamilton, 1994) might explain this oscillatory pattern, these systems per se are not intrinsically periodic. The chemical control system, however, is by nature periodic, which strongly suggests that chemical control instability contributes to the observed changes in ventilatory pattern with ageing.
While it is tempting to assume that chemical control becomes less stable with ageing, multiple studies show reduced chemosensitivity (tending to stabilize breathing) in this population (Kronenberg & Drage, 1973; Altose et al. 1977; Peterson et al. 1981; Brischetto et al. 1984; Naifeh et al. 1989; Mendez et al. 1996; Browne et al. 2003). However, chemosensitivity is only one of several components involved in the chemical regulation of breathing. Other major variables include plant gain (described in the next paragraph) and dynamic delays around the chemical feedback loop. Thus, whether overall chemical control stability is increased or decreased in the elderly is not known.
To investigate this, we applied a technique for measuring the total gain in the chemical feedback loop, i.e. loop gain (Younes et al. 2001). Loop gain is an engineering term that describes the stability of feedback-controlled systems. For the respiratory system, loop gain is defined by the ventilatory response to disturbance ratio. If the response (e.g. hyperpnoea) is greater than or equal in magnitude to the disturbance (e.g. hypopnoea), then loop gain is ≥1 and ventilation will become unstable. A loop gain close to zero, on the other hand, indicates a very stable chemical control system. Loop gain in the respiratory control system is primarily a product of controller gain (chemosensitivity) and plant gain. The plant is the lungs, blood and body tissues where carbon dioxide and oxygen are stored. Plant gain therefore is the effectiveness of ventilation at changing PCO2 and PO2. Loop gain is also dependent on the circulation delay between the lungs and the chemoreceptors, as well as the response dynamics of the controller and the plant. It thus quantifies the total stability of the respiratory chemical-feedback system.
Methods
Subjects
Healthy, independently living elderly individuals between the ages of 60 and 80 years were recruited from the community. A history and physical examination were performed on each subject prior to participation in the study. Exclusion criteria included cardiopulmonary disease, cerebrovascular disease, medications known to affect breathing, snoring, daytime sleepiness, and BMI ≥ 30 kg m−2. To assure ourselves that patients with obstructive sleep apnoea (OSA) were not fundamentally different from non-OSA subjects, we also recruited elderly OSA patients from our clinical sleep laboratory. OSA patients were required to be between 60 and 80 years of age, have a respiratory disturbance index >10 per hour, and be compliant on continuous positive airway pressure (CPAP) (>5 h per night for >2 months, as confirmed by CPAP machine readings). The study was approved by the institutional review board at Brigham and Women's Hospital, and each subject provided written consent to participate in the protocol. All experiments conformed to the Declaration of Helsinki.
Equipment
Proportional assist ventilation (PAV) was used to measure loop gain (Meza & Younes, 1996; Meza et al. 1998; Younes et al. 2001). PAV is a form of ventilatory support that provides airway pressure in proportion to ventilatory effort. Large efforts therefore receive more assist than smaller ones. ‘Effort’ is detected on a millisecond basis as the amount of airflow and volume passing from the ventilator to the subject. The airflow and volume (effort) signals are each amplified by adjustable gains (flow assist (FA) and volume assist (VA)) and used to drive ventilator pressure output. Increased levels of PAV can induce periodic breathing, which can be used to calculate loop gain.
Procedure
Subjects presented to the sleep laboratory approximately 1 h prior to their usual bedtime and were instrumented with the following equipment: paste-on electrodes for monitoring central and occipital electroencephalogram (EEG), left and right electro-oculogram (EOG), chin electromyogram (EMG), and continuous electrocardiogram (ECG). A pulse oximeter (BCI, Waukesha, WI, USA) for measuring arterial oxygen saturation and a nasal mask for delivering positive airway pressure and monitoring ventilatory variables were also attached. The mask was connected to a BiPAP Vision mechanical ventilator (Respironics, Murrysville, PA, USA) capable of delivering CPAP in combination with PAV. This ventilator is also equipped with a pneumotachometer for monitoring flow and a pressure transducer for measuring mask pressure. End-tidal PCO2 was monitored from a port in the mask using a calibrated CO2 analyser (BCI). All data were recorded and displayed on a computer using Spike 2 (Cambridge Electronic Design, Cambridge, UK) and Nihon Kohden (Tokyo, Japan) software.
Subjects slept in the supine position for the entire study. Once stable non-rapid eye movement (NREM) sleep was achieved, CPAP was adjusted to eliminate any flattening in the inspiratory flow pattern. A maximum tidal volume limit of twice the subject's basal tidal volume was used to prevent awakening from PAV amplification of occasional large efforts (e.g. sigh). We then incremented PAV by adjusting the VA and FA gains to successively higher levels every 2–3 min. At each level not associated with periodic breathing, the tidal volume amplification factor (VTAF) was measured 3–5 times by abruptly lowering PAV (VA = 0, FA = 0) for one breath (Younes et al. 2001). VTAF is the ratio of assisted tidal volume to unassisted tidal volume and is used for calculating loop gain (see Data analysis). The PAV manipulations ended after sustained periodic breathing or ‘runaway’ occurred. Runaway breathing (excessively large tidal volumes due to ventilator pressure exceeding pulmonary elastic and resistive pressures) precludes further increases in PAV. Thus, while not all subjects can be driven to periodic breathing due to runaway, the absence of periodic breathing at high PAV indicates a low loop gain as discussed in Data analysis.
If awakening occurred during the PAV increase, VA and FA gains were decreased back to zero until stable sleep resumed. Subsequent increases in PAV were usually done more rapidly (e.g. VA and FA were incremented every 30 s) until the VA and FA achieved prior to awakening were reached. Thereafter, PAV was increased more slowly as described above (with VTAFs being measured at each assist level not associated with periodic breathing). If at any time cycling ventilation occurred without an adequate number of preceding VTAF measurements, VA and FA were decreased slightly to measure several VTAFs during stable breathing. We then increased VA and FA back to the level associated with periodic breathing as confirmation.
Data analysis
Loop gain was calculated from the average VTAF of the highest assist level achieved prior to the development of periodic breathing (Younes et al. 2001). VTAF is a measure of PAV amplification of the subject's intrinsic loop gain (LGintrinsic). Thus, the total loop gain on PAV (LGPAV) is the product of LGintrinsic and VTAF (LGPAV = LGintrinsic × VTAF). At the lowest PAV level associated with periodic breathing, LGPAV = 1. Thus, LGintrinsic is calculated as the reciprocal of VTAF. If LGPAV could not be brought to 1 due to runaway (LGintrinsic × VTAF < 1), LGintrinsic took on a ‘less than’ value, indicating that loop gain is at most less than the stated value.
Periodic breathing was defined as at least four cycles of crescendo–decrescendo breathing with a period between 20 and 100 s. The nadir tidal volume was required to be <30% of peak tidal volume. Cycles were excluded if they contained arousals (>3 s of >8 Hz EEG activity).
Statistics
Statistical comparisons were made with young subjects previously studied and reported from our laboratory (Wellman et al. 2003). Since some of the loop gain values were less than numbers, loop gain comparisons were analysed using survival analysis techniques for left censored data assuming a Weibull distribution. Other variables were compared using an unpaired t test. Results are presented as means ± standard deviation. A P < 0.05 was considered significant.
While it can be argued that loop gain might decrease with ageing (see Introduction), we determined sample size by calculating the number of elderly subjects needed to demonstrate an increase in loop gain, based on the previously stated concept that high loop gain might explain the central apnoeas and periodic breathing in the elderly. To do this, we estimated mean ± s.d. loop gain in young individuals to be 0.33 ± 0.1. We also estimated that a loop gain >0.45 would constitute a physiologically significant increase with ageing. This cut-off was chosen as an approximation for sample size calculation because it represents the upper quartile range of loop gain in patients with obstructive sleep apnoea from our lab (n = 35), and because an elevated loop gain may be important in the development of sleep related apnoeas in these patients (Younes et al. 2001; Wellman et al. 2004). With 80% power and a 5% level of significance, we determined that 11 elderly subjects would be needed to demonstrate a physiologically significant increase in loop gain.
Results
Data were analysed on 14 subjects (11 without OSA and 3 with OSA). Subject characteristics, respiratory variables, and loop gain for those without OSA are displayed in Table 1. Three males and eight females were studied. The subjects were 71 ± 5 years old, with an average BMI of 25 ± 3 kg m−2. Eupnoeic PCO2 and ventilation during sleep were 40 ± 4 mmHg and 5.4 ± 1.0 l min−1, respectively. The CPAP needed to stabilize the upper airway was 5 ± 2 cmH2O. ‘Mean’ loop gain, obtained by averaging loop gain in all subjects, including those with a less than value, was ≤ 0.25 ± 0.08. For the OSA subgroup (n = 3, age 68 ± 1 years, BMI was 32 ± 11 kg m−2), we found no systematic differences in respiratory variables (eupnoeic PCO2 = 39 ± 1 mmHg, ventilation 6.0 ± 0.5 l min−1) or loop gain (≤0.26 ± 0.03), although CPAP averaged 10 ± 3 cmH2O (see Table 2). Of note, slightly different CPAP levels were used in two of the three OSA patients compared with what they used at home. One subject used 10 cmH2O at home but was studied on 12 cmH2O. The other used 12 cmH2O at home but 10 cmH2O in the laboratory. All loop gain measurements were obtained during stage II sleep.
Table 1.
Subject characteristics and measured variables for elderly individuals without obstructive sleep apnoea (OSA)
| Subject | Sex | BMI (kg m−2) | Age (years) | PCO2(mmHg)a | Ventilation(l min−1)a | CPAP (cmH2O) | Loop gain (dimensionless) |
|---|---|---|---|---|---|---|---|
| 1 | F | 27 | 65 | 43 | 5.0 | 5 | 0.15 |
| 2 | F | 28 | 71 | 40 | 5.1 | 8 | 0.23 |
| 3 | M | 28 | 73 | 35 | 7.8 | 9 | 0.23 |
| 4 | M | 22 | 72 | 39 | 5.2 | 6 | 0.26 |
| 5 | F | 23 | 65 | 38 | 4.2 | 4 | ≤0.17 |
| 6 | F | 27 | 72 | 38 | 5.1 | 4 | ≤0.26 |
| 7 | M | 24 | 75 | 39 | 6.2 | 4 | ≤0.26 |
| 8 | F | 26 | 78 | 37 | 5.0 | 4 | ≤0.27 |
| 9 | F | 20 | 60 | 47 | 5.9 | 6 | ≤0.28 |
| 10 | F | 26 | 75 | 46 | 3.9 | 5 | ≤0.29 |
| 11 | F | 28 | 71 | 42 | 5.7 | 4 | ≤0.44 |
| Mean | 3M/8F | 25 | 71 | 40 | 5.4 | 5 | ≤0.25 |
| s.d. | — | 3 | 5 | 4 | 1.0 | 2 | 0.08 |
Baseline levels during sleep on continuous positive airway pressure (CPAP).
Table 2.
Subject characteristics and measured variables for elderly individuals with OSA
| Subject | Sex | BMI (kg m−2) | Age (years) | PCO2(mmHg)a | Ventilation (l min−1)a | CPAP (cmH2O) | Loop gain (dimensionless) |
|---|---|---|---|---|---|---|---|
| 1 | M | 44 | 67 | 38 | 6.1 | 10 | ≤0.23 |
| 2 | M | 30 | 68 | 40 | 5.5 | 12 | 0.28 |
| 3 | M | 23 | 68 | 39 | 6.4 | 7 | 0.27 |
| Mean | 3M/0F | 32 | 68 | 39 | 6.0 | 10 | ≤0.26 |
| s.d. | — | 11 | 1 | 1 | 0.5 | 3 | 0.03 |
Baseline levels during sleep on CPAP.
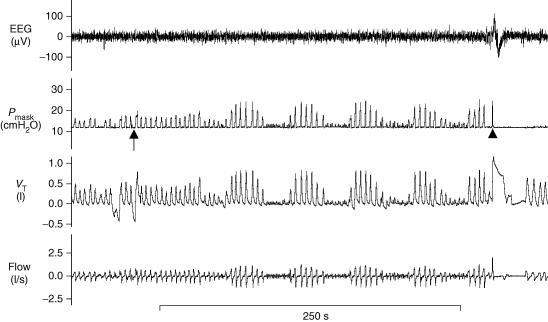
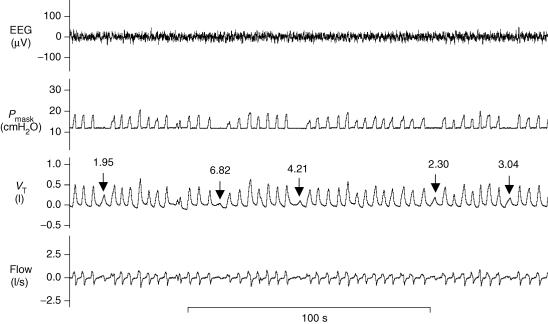
Raw data from a subject exhibiting periodic breathing on PAV is shown in Fig. 1. Approximately 1 min following an increase in PAV (arrow), crescendo–decrescendo periodic breathing occurs with a cycle length of 49 s. An arousal with awakening occurs near the end of the tracing (arrowhead) and PAV is decreased back to zero (i.e. CPAP only). In order to determine loop gain in this individual, several VTAFs were obtained at the highest PAV level not associated with periodic breathing. An example of how this was done is shown in Fig. 2. At the assist level one increment below the one that ultimately led to periodic breathing, PAV was decreased to zero for one breath (arrows in Fig. 2) and the ratio of the preceding assisted breaths to the unassisted breath was calculated. These ratios, which are the VTAFs, are displayed above the arrows. The average VTAF at the assist level just below the one associated with periodic breathing is 3.7. Thus, this subject's intrinsic loop gain is 0.27.
Figure 1. An example of periodic breathing induced by proportional assist ventilation.
At the start of the tracing, the volume assist (VA) = 11 and the flow assist (FA) = 4. At the arrow, VA is increased to 12 (i.e. the next higher level of proportional assist ventilation (PAV)). One minute later, a few slightly larger than normal breaths produce several small breaths followed by five cycles of waxing and waning tidal volume. Central apnoea occurs in three of the five cycles. Towards the end of the tracing, arousal with awakening occurs (arrowhead) and PAV is decreased to zero. VT, tidal volume; Pmask, mask pressure.
Figure 2. Measurement of tidal volume amplification factor for the subject in Fig. 1.
Several tidal volume amplification factors (VTAFs; arrows) are measured at the highest PAV level achieved in which breathing was still stable (here, VA = 11 and FA = 4). To measure the VTAF, PAV was decreased to zero for one breath (i.e. VA = 0, FA = 0) and the ratio of assisted to unassisted VT was calculated. These values are displayed above the arrows. The VTAF is a measure of how much the patient's intrinsic loop gain is being amplified. For instance, the subject's loop gain in this figure is being amplified by a factor of 3.7 (obtained by averaging all VTAFs displayed).
Periodic breathing was produced in 4 out of 11 non-OSA subjects and 2 out of 3 OSA subjects. The cycle duration during periodic breathing in these individuals ranged between 60 and 79 s (mean 72.3 s). In the subjects who did not exhibit cycling (due to runaway breathing at high PAV), loop gain could be calculated to less than 0.30 in all but one subject (see Tables 1 and 2). Thus, we obtained sensitive measurements in all subjects.
Table 3 shows a comparison between healthy elderly and young individuals (OSA patients are not included in Table 3). Mean age in the young group was 27 ± 4 years. While BMI was statistically higher in the elderly group (P = 0.005), both groups were relatively thin. No differences were found in eupnoeic PCO2 (P = 0.68) or CPAP levels (P = 0.07). Using survival analysis to compare subjects (including those with a less than loop gain value), we found that loop gain was significantly lower in the elderly group (P = 0.001), and remained significant after adjusting for sex and BMI (P < 0.05).
Table 3.
Comparison of old versus young (non-obstructive sleep apnoea subjects)
| Subject | Sex | BMI (kg m−2) | Age (years) | PCO2(mmHg) | CPAP (cmH2O) | Loop gain (dimensionless) |
|---|---|---|---|---|---|---|
| Old | 3M/8F | 25 ± 3 | 71 ± 5 | 40 ± 4 | 5 ± 2 | ≤0.25 ± 0.08 |
| Young | 8M/9F | 23 ± 1* | 27 ± 4* | 41 ± 3 | 4 ± 1 | ≤0.47 ± 0.13* |
Values are means ±s.d.
P < 0.05 compared with the elderly.
Discussion
To determine if ageing is associated with an increase or decrease in chemical control stability, we measured loop gain in healthy elderly individuals and a subgroup of OSA patients during stable sleep and in the absence of changes in upper airway resistance. We found a significantly lower loop gain in healthy elderly individuals, suggesting that chemical control is quite stable in this population. This seems to be true for elderly OSA patients also, although more subjects are needed to confirm this. These data suggest that chemical control instability is unlikely to be a cause of central, and possibly obstructive, apnoeas in the elderly.
Comparison with previous studies
As mentioned, previous studies measuring stability characteristics in elderly individuals have focused on measurements of chemical responsiveness to hypercapnia and hypoxia, and these studies have generally shown reduced ventilatory responses to these stimuli (Kronenberg & Drage, 1973; Altose et al. 1977; Peterson et al. 1981; Brischetto et al. 1984; Naifeh et al. 1989; Mendez et al. 1996; Browne et al. 2003). Typically, these were performed using two techniques: (1) rebreathing studies, in which PCO2 increases progressively and stimulates ventilation (so-called progressive gain measurement), and (2) steady-state methods: manipulation of inspired gases to produce a quasi-steady-state chemical stimulus to the ventilatory control system. While these techniques are valuable in determining the characteristics of the chemical controller, i.e. controller gain, they do not assess the dynamics of the controller response or how differences in other variables such as plant gain (the change in PCO2 and PO2 due to a change in ventilation) or time delays might affect chemical stability. The advantage of the PAV technique is that these factors are incorporated in the loop gain measurement.
The significance of our data in relation to previous studies showing reduced controller gain with ageing (Kronenberg & Drage, 1973; Altose et al. 1977; Peterson et al. 1981; Brischetto et al. 1984; Naifeh et al. 1989; Mendez et al. 1996; Browne et al. 2003) is that we have demonstrated a low total loop gain, suggesting that changes in plant factors and/or dynamic delays with ageing do not appear to offset the influence of a reduced controller gain on total loop gain.
Potential consequences of a low loop gain
While a low loop gain would tend to stabilize ventilatory dynamics, a significant reduction in loop gain may not necessarily be favourable. In general, feedback-controlled systems require a certain amount of gain to function properly. Too little gain, and the system will not respond appropriately to disturbances, leading to errors between the desired and existing output variables (e.g. ventilation does not keep up with changing ventilatory requirements). Such a system is highly susceptible to perturbations from external sources, since these perturbations go uncorrected. Thus, an important and somewhat paradoxical question we raise is whether or not the low loop gain found in elderly subjects predisposes the ventilatory system to instability originating from ‘external’ sources (e.g. perturbations due to sleep state changes and/or upper airway resistance fluctuations).
Changes in sleep–wake state (e.g. sleep onset) are a strong perturbation to the chemoreflexes. During wakefulness, there is an additional stimulus to breath that is unrelated to blood gas homeostasis (Fink, 1961) (hence, we consider this wakefulness stimulus external to the chemical control system per se). With a relatively abrupt loss in this added drive (sleep onset), the integrity of the chemical control system is tested since any compensatory ventilation must be provided primarily by the chemoreflexes. If the underlying chemoreflexes are weak (low loop gain), there may be insufficient drive to breath, leading to a reduction in ventilation and possibly hypopnoea or apnoea during sleep. This is supported by recent experimental evidence in which partial destruction of preBötzinger neurons leads to apnoeas during sleep with relative preservation of ventilation during wakefulness (McKay et al. 2005). To the extent that preBötzinger neurons are influenced by blood-gas chemistry, age-related neural degeneration of the preBötzinger complex might be a possible mechanism for the observed ventilatory changes seen in the elderly.
Reduced loop gain during sleep might also decrease ventilatory drive to the pharyngeal muscles, leading to airway narrowing and possibly further reductions in ventilation (beyond that induced by reduced drive to the ventilatory pump muscles). This is consistent with data showing that ageing is associated with greater reductions in ventilation and genioglossus and diaphragm EMG activity at sleep onset (Worsnop et al. 1998; Browne et al. 2001). We speculate that a slightly higher loop gain, if it does not lead to instability, might provide a smoother transition to sleep and better ventilatory compensation.
Increased pharyngeal resistance due to an anatomically narrow upper airway may also perturb the chemical control system, and the ventilatory response to such a perturbation is mediated in part by loop gain. It is reasonable to think that a low loop gain system would not compensate as well for an added resistive load. Consider the response to partial upper airway narrowing during sleep in a subject with reduced controller and plant gains. If airway narrowing leads to a reduction in ventilation, then there will be less rise in PCO2 (and less reduction in PO2) due to the low plant gain (since low plant gain means there will be less change in blood gases for a given change in ventilation). Likewise, the controller response will be diminished due to both the reduced controller gain and the smaller changes in PCO2 and PO2. Consequently, ventilatory drive to the diaphragm and upper airway muscles might not be sufficient to reverse the partial obstruction and maintain ventilation at a reasonable level. Hudgel demonstrated this in elderly individuals by showing that airway narrowing had a substantial limiting effect on ventilation (Hudgel et al. 1993; Hudgel & Hamilton, 1994). We believe that a low loop gain may be contributing to this phenomenon, and that perhaps a somewhat higher loop gain might render ventilation less susceptible to partial airway obstruction.
Limitations of the study
The limitations of our methodology for measuring loop gain have been discussed extensively in previous publications (Younes et al. 2001; Wellman et al. 2003, 2004) and are not repeated here. We only point out that positive airway pressure may actually influence loop gain (effects on lung volume, oxygen stores, cardiac output, etc.) and could thus potentially confound the results. However, the CPAP used in this study was low (5 ± 2 cmH2O in the non-OSA group, and 10 ± 3 cmH2O in the OSA group) and was not likely to significantly change the gas exchange properties or pulmonary mechanics (other than upper airway resistance) to the point that loop gain was appreciably altered.
In this study, we measured loop gain in a selective group of healthy elderly individuals. The major factors predisposing to chemical control instability, such as cardiovascular and cerebrovascular disease, increase with ageing. It is possible that the greater prevalence of ventilatory irregularities observed in this population is related to the greater prevalence of these disorders. However, as mentioned, even healthy elderly individuals tend to have a more oscillatory breathing pattern than young individuals (Webb, 1974; Shore et al. 1985; Pack et al. 1988; Hudgel et al. 1993; Hudgel & Hamilton, 1994), and ageing has been shown to be an independent risk factor for Cheyne–Stokes respiration (Sin et al. 1999). Thus, we believe it was appropriate for us to study healthy individuals without risk factors for chemical control instability if we wished to determine whether ageing alone is associated with a destabilization in chemical control.
Lastly, due to the limitations of precisely quantifying loop gain in the low range (below ∼0.30) with PAV, we were required to make comparisons between young and old individuals using ‘less than’ numbers in several subjects. Nevertheless, we found very low loop gains in virtually all elderly subjects, which was not the case in the younger group. Thus, we believe it is reasonable to conclude that ageing is not associated with an increase in chemical control instability, and may very well be associated with a reduction in loop gain.
Conclusion
Loop gain is quite low in both healthy elderly individuals and those with OSA, although we concede that the latter is preliminary due to the small number of subjects studied. This suggests that chemical control instability is unlikely to be a mechanism for the high prevalence of sleep disordered breathing in this population. On the other hand, a significant reduction in loop gain may indicate a poorly responsive or over-damped control system, which itself may lead to problems in integrated ventilatory control. We propose that such a low gain system might be more susceptible to perturbations from systems interacting with the chemical control system, such as alterations in sleep–wake state or upper airway resistance changes. Consequently, instability in these interactive systems might alter ventilation substantially and give the spurious appearance that chemical control is unstable as well. On the contrary, a higher, more normal loop gain system, so long as it does not become unstable, may diminish the effect of these perturbating influences and lead to a more regular breathing pattern.
Acknowledgments
This work was supported by grants from the National Institutes of Health (F32 HL072560-01, RO1 HL48531, P50 HL60292, MO1-RR01032) and the National Institute of Aging (Beeson Award AG024837-01).
References
- Altose MD, McCauley WC, Kelsen SG, Cherniack NS. Effects of hypercapnia and inspiratory flow-resistive loading on respiratory activity in chronic airways obstruction. J Clin Invest. 1977;59:500–507. doi: 10.1172/JCI108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Kripke DF, Mason W, Kaplan OJ. Sleep apnea and periodic movements in an aging sample. J Gerontol. 1985;40:419–425. doi: 10.1093/geronj/40.4.419. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men. I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- Brischetto MJ, Millman RP, Perterson DD, Silage DA, Pack AI. Effect of aging on ventilatory response to exercise and CO2. J Appl Physiol. 1984;56:1143–1150. doi: 10.1152/jappl.1984.56.5.1143. [DOI] [PubMed] [Google Scholar]
- Browne H, Adams L, Simonds A, Morrell M. Impact of age on breathing and resistive pressure in people with and without sleep apnea. J Appl Physiol. 2001;90:1074–1082. doi: 10.1152/jappl.2001.90.3.1074. [DOI] [PubMed] [Google Scholar]
- Browne H, Adams L, Simonds A, Morrell M. Ageing does not influence the sleep-related decrease in the hypercapnic ventilatory response. Eur Respir J. 2003;21:523–529. doi: 10.1183/09031936.03.00039002. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Respiration during sleep in the aged human. J Gerontol. 1981;36:420–423. doi: 10.1093/geronj/36.4.420. [DOI] [PubMed] [Google Scholar]
- Fink BR. Influence of cerebral activity in wakefulness on regulation of breathing. J Appl Physiol. 1961;16:15–20. doi: 10.1152/jappl.1961.16.1.15. [DOI] [PubMed] [Google Scholar]
- Hudgel DW, Devadatta P, Hamilton H. Pattern of breathing and upper airway mechanics during wakefulness and sleep in healthy elderly humans. J Appl Physiol. 1993;74:2198–2204. doi: 10.1152/jappl.1993.74.5.2198. [DOI] [PubMed] [Google Scholar]
- Hudgel DW, Hamilton HB. Respiratory muscle activity during sleep-induced periodic breathing in the elderly. J Appl Physiol. 1994;77:2285–2290. doi: 10.1152/jappl.1994.77.5.2285. [DOI] [PubMed] [Google Scholar]
- Krieger J, Turlot JC, Mangin P, Kurtz D. Breathing during sleep in normal young and elderly subjects: hypopneas, apneas, and correlated factors. Sleep. 1983;6:108–120. doi: 10.1093/sleep/6.2.108. [DOI] [PubMed] [Google Scholar]
- Kronenberg RS, Drage CW. Attenuation of the ventilatory and heart rate responses to hypoxia and hypercapnia with aging in normal men. J Clin Invest. 1973;52:1812–1819. doi: 10.1172/JCI107363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R, De Oca MM, Rassulo J, Celli B. Effects of age on ventilatory drive response to CO2. Chest. 1996;110:61S. [Google Scholar]
- Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol. 1998;85:1929–1940. doi: 10.1152/jappl.1998.85.5.1929. [DOI] [PubMed] [Google Scholar]
- Meza S, Younes M. Ventilatory stability during sleep studied with proportional assist ventilation (PAV) Sleep. 1996;19:S164–S166. doi: 10.1093/sleep/19.suppl_10.164. [DOI] [PubMed] [Google Scholar]
- Naifeh KH, Severinghaus JW, Kamiya J, Krafft M. Effect of aging on estimates of hypercapnic ventilatory response during sleep. J Appl Physiol. 1989;66:1956–1964. doi: 10.1152/jappl.1989.66.4.1956. [DOI] [PubMed] [Google Scholar]
- Pack AI, Cola MF, Goldszmidt A, Ogilvie MD, Gottschalk A. Correlation between oscillations in ventilation and frequency content of the electroencephalogram. J Appl Physiol. 1992;72:985–992. doi: 10.1152/jappl.1992.72.3.985. [DOI] [PubMed] [Google Scholar]
- Pack AI, Silage DA, Millman RP, Knight H, Shore ET, Chung DC. Spectral analysis of ventilation in elderly subjects awake and asleep. J Appl Physiol. 1988;64:1257–1267. doi: 10.1152/jappl.1988.64.3.1257. [DOI] [PubMed] [Google Scholar]
- Peterson DD, Pack AI, Silage DA, Fishman AP. Effects of aging on ventilatory and occlusion pressure responses to hypoxia and hypercapnia. Am Rev Respir Dis. 1981;124:387–391. doi: 10.1164/arrd.1981.124.4.387. [DOI] [PubMed] [Google Scholar]
- Shore ET, Millman RP, Silage DA, Chung DC, Pack AI. Ventilatory and arousal patterns during sleep in normal young and elderly subjects. J Appl Physiol. 1985;59:1607–1615. doi: 10.1152/jappl.1985.59.5.1607. [DOI] [PubMed] [Google Scholar]
- Sin D, Fitzgerald F, Parker J. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- Webb P. Periodic breathing during sleep. J Appl Physiol. 1974;37:899–903. doi: 10.1152/jappl.1974.37.6.899. [DOI] [PubMed] [Google Scholar]
- Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz E, Schory KE, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman A, Malhotra A, Fogel R, Schory KE, Edwards JK, White DP. Respiratory system loop gain in norman men and women measured with proportional assist ventilation. J Appl Physiol. 2003;94:205–212. doi: 10.1152/japplphysiol.00585.2002. [DOI] [PubMed] [Google Scholar]
- Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]