Abstract
Calpains are Ca2+-activated proteases that are thought to be involved in muscle degenerative diseases such as Duchenne muscular dystrophy. Status and activity of calpains in adult muscle fibres are poorly documented. We report here in situ measurements of calpain activity in collagenase-isolated fibres from C57 mice and form two models of dystrophy: dystrophin-deficient mdx and calpain-3 knocked-out mice. Calpain activity was measured using a permeant, fluorogenic substrate and its Ca2+ dependence was studied. A 30-fold change of activity was observed between the lowest and the highest steady-state Ca2+ availability. Fast transient changes of [Ca2+]i induced by electrical stimulation or KCl-dependent depolarization were ineffective in activating calpain. Slow [Ca2+] transients, as elicited during depletion of Ca2+ stores, Ca2+ store repletion and hypo-osmotic swelling were able to activate calpain. On return to resting conditions, calpain activity recovered its basal rate within 10 min. In resting intact muscle, μ-calpain was predominantly in the 80 kDa native form, with a small fraction in the 78 kDa autolysed form. The latter is thought to be responsible for the activity measured in our conditions. Calpain activity in mdx fibres showed an average 1.5-fold increase compared to activity in C57 fibres. This activity was reduced by a 10-fold lowering of [Ca2+]o. Calpain-3-deficient fibres showed about the same increase, thus calpain-3 did not contribute to the activity measured here and calpain activation is not specific to dystrophin deficiency. In fibres from transgenic mice over-expressing calpastatin, a 40–50% reduction of calpain activity was observed, as with synthetic drugs (Z-Leu-Leu-CHO and SNT198438). We provide novel information on the physiological factors that control calpain activity in situ, particularly the effect of intracellular Ca2+ transients that occur in excitation–contraction coupling, Ca2+ store depletion and refilling, and activation of mechanosensitive Ca2+ channels.
Calpains are neutral, Ca2+-activated, cysteine proteases, that are essentially intracellular. Two ubiquitous forms of calpains have been identified: m-calpains and μ-calpains which are activated by millimolar and micromolar Ca2+ concentrations, respectively. Skeletal muscles contain both forms and a specific one, calpain-3. Most cells, including muscles, also contain calpastatin, which is a specific inhibitor of m- and μ-calpain. The concentration of calpastatin usually exceeds that of calpain and thus it can effectively regulate calpain activity. For a comprehensive review of the calpain–calpastatin system see Goll et al. (2003).
The physiological roles of the calpains are still largely unclear. Their involvement in fibroblast motility (membrane protusions and focal adhesions) and blood platelet activation (granule release and aggregation), together with the fact that several cytoskeletal proteins (e.g. talin, vinculin and spectrin) are quickly cleaved by calpains in in vitro assays, indicate a major role in ‘remodelling’ the cytoskeletal architecture and its interactions with the plasma membrane. Moreover, in addition to this structural role, calpain also seems to be involved in transmembrane signalling (Inomata et al. 1996). Interestingly, it was recently shown that calpain is also required for the Ca2+-dependent repair of wounded plasma membrane (Mellgren et al. 2006).
During the development of muscle fibres, the fusion of mononucleated myoblasts into multinucleated myotubes requires such an extensive remodelling and, accordingly, fusion stops if calpains are inhibited. By contrast, fully differentiated, adult fibres seem to possess a rather stable cytoskeletal architecture, made of structural proteins (e.g. γ-actin, α-actinin, titin and dystrophin) with a very low turnover rate and implicated into the longitudinal and radial transmission of mechanical forces, a function that precludes a labile structure, as shown by the dramatic loss of force when cytoskeleton proteolysis occurs (skeletal muscle, Verburg et al. 2005; smooth muscle, Haeberle et al. 1985). Considerable interest in the activity of calpains in adult muscle fibres arose from observations made on muscle from the mutant mdx mouse, which lacks the cytoskeletal protein dystrophin, as do muscles of patients suffering from Duchenne muscular dystrophy (DMD). The muscles of the mdx mouse present signs of increased Ca2+-dependent proteolysis (Turner et al. 1988) and increased concentrations and activation of calpain (Spencer & Tidball, 1992; Spencer et al. 1995). It was proposed that an abnormal activity of calpains, induced by an increased cytosolic [Ca2+], occurs in dystrophin-lacking muscles and initiates the pathological process leading to fibre necrosis in DMD. There is controversy concerning the question of a perturbed cell [Ca2+] as the initial event of the dystrophic process (see review by Gillis (1999), but the fact that muscle dystrophy symptoms are greatly reduced in transgenic mdx mice, which over-express the natural calpain inhibitor calpastatin (Spencer & Mellgren, 2002), or in mdx mice treated with a synthetic calpain inhibitor (Burdi et al. 2006), shows that calpains are involved at some step (possibly late) of the dystrophic process. However, increased calpain activity or concentration have been reported for other types of muscle diseases (e.g. polymyositis and denervation atrophy; Kumamoto et al. 1992, 1997), suggesting that involvement of calpain is a non-specific feature of various muscle pathologies.
As m-calpain requires millimolar levels of Ca2+ for activation, it is very unlikely that it is an active protease in vivo. Even the more Ca2+-sensitive μ-calpain requires a half-activating [Ca2+] as high as 34 μm (Kapprell & Goll, 1989), which is above the [Ca2+]i occurring within cells in physiological conditions. However, conditions that lower the Ca2+ requirement have been identified: the binding of calpain to membrane phosphatidyl-inositol and the autolysis that occurs in activated calpain (Kapprell & Goll, 1989). Moreover, muscle fibres contain as much calpastatin as the sum of the m- and μ-calpain contents, but the binding of calpastatin to calpain (the basis of its inhibitory effect) is itself Ca2+ dependent (Cottin et al. 1981).
In physiological conditions, however, practically nothing is known of the activity of calpains in muscle fibres and how the main factors that affect its activity in test-tube assays (i.e. [Ca2+], the native versus autolysed forms and calpastatin) affect the situation in situ. The activity of calpain has been shown to depend on [Ca2+]o in mdx myotubes (Alderton & Steinhardt, 2000) but the situation in adult fibres has not been investigated.
Recently, Murphy et al. (2006b) devised an ingenious assay for monitoring the proteolytic effect of Ca2+-activated calpain applied to skinned fibres. They established in which conditions calpain activity leads to structural damage with functional consequences (see Discussion).
However, skinned fibres are not suitable for studying the effect on calpain activity of Ca2+ influx mediated through membrane Ca2+ channels and of Ca2+ transients elicited by membrane-originated signals. Moreover, skinned fibres tend to lose most of their calpain content into the medium and are depleted of their membrane-bound calpain (Murphy et al. 2006b). Recently, a method to assess calpain activity in vivo has been developed in transgenic mice expressing the α-fodrin cleavage site flanked by cyan and yellow fluorescent proteins. The intensity of the Förster resonance energy transfer (FRET) is reduced when this compounded substrate is cleaved (Stockholm et al. 2005; Bartoli et al. 2006). Though very promising, this approach has not yet been developed to the stage of providing kinetic data on calpain activity, as reported here.
We report in situ measurements of calpain activity made on intact, collagenase-isolated fibres using a permeant, fluorogenic substrate; the rate of fluorescent increase direct reflects the enzymatic activity of calpain. Calpain was first studied in resting conditions. Various experimental protocols were designed to change the Ca2+ availability, either in steady-state conditions or during fast or slow Ca2+ transients. The inhibitory effect of calpastatin and various pharmacological compounds was also studied. In addition, we studied the differences in calpain activity between fibres from normal and dystrophic mice (dystrophin or calpain-3 deficiency) at rest. As calpain can undergo autolysis upon activation, we analysed fresh muscles to estimate the relative proportion of the native and autolysed forms of μ-calpain.
Methods
Isolation of adult skeletal muscle fibres
Adult, 60- to 120-day-old mice were killed by cervical dislocation, a procedure approved by the local animal ethics committee and applied to all strains used here (C57, NMRI, mdx, transgenic calpain-3-deficient and over-expressing calpastatin mice). In a few cases, NMRI white mice were also used to assess the state of μ-calpain (see Results and Fig. 7). Genetically modified C57 mice, either calpain-3 deficient (gift from Dr I. Richard, Généthon, France) or over-expressing calpastatin (gift from Dr M. Spencer, University of California Los Angeles, CA, USA) were compared with wild-type litter mates of the same genetic background. The flexor digitorum brevis (FDB) muscles were removed and incubated for 38 min at 37°C in an oxygenated Krebs solution (see composition below) containing 0.2% collagenase type IV. Muscles were then washed twice in Krebs buffer, suspended in Dulbecco Minimum Essential Medium with Ham F12 complement (DMEM/HAM F12) supplemented with 2% fetal bovine serum and mechanically dissociated by repeated passages through fire-polished Pasteur pipettes of progressively decreasing diameter. Dissociated fibres were plated onto tissue culture dishes coated with extracellular matrix basement membrane (Harbour Bio-products, Norwood, M1, USA) and allowed to adhere to the bottom of the dish for 2 h. For Ca2+ measurements, cells were plated on circular glass coverslips. Culture dishes were kept in an incubator, with 5% CO2 at 30°C.
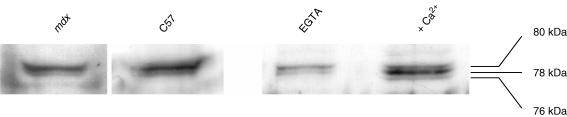
Figure 7. Immunodetection of calpain and its autolysed forms (78 and 76 kDa).
Left, comparison between C57 and mdx muscles (extraction performed in the presence of 1 mm EGTA). Right, illustration of Ca2+-induced autolysis: two muscles from the same NMRI mouse are compared (one extracted in the presence of 1 mm EGTA, the other extracted in the presence of 5 mm Ca2+).
In situ measurements of calpain activity
The synthetic substrate 7-amino-4-chloromethyl-coumarin - t - butoxycarbonyl - l - leucyl - l - methionine amide (Boc-Leu-Met-CMAC) was first developed by Rosser et al. (1993) for in situ measurements of combined m- and μ-calpain activities in hepatocytes. In its native form, this substrate is permeant but non-fluorescent. Upon penetration into cells, it is transformed into Boc-Leu-Met-MAC-SG by the gutathione S-transferase of the cell, which makes it impermeant. Cleavage of the Boc-Leu-Met moiety frees the fluorescent MAC-SG chromophore (7-amino-4-methylcoumarin glutathione conjugate; excitation and emission wavelengths, 380 and 480 nm, respectively) which, being impermeant, accumulates within the cell. The rate at which fluorescence increases reflects the intracellular accumulation rate of the cleavage product resulting from the enzymatic activity.
The substrate was added to the fibre chamber to obtain a final concentration of 10 μm in Krebs solution and fluorescence recording started within 30 s. Detection was made with a photon counter and restricted to an adjustable rectangular aperture of 100 μm × 35 μm, parallel to the long axis of the fibre and covering most of its width. The settings for fluorescence recordings were rigorously maintained constant. As soon as the shutter for photon counting was open (about 30 s after application of the Boc-Leu-Met-CMAC), we frequently observed a large initial fluorescence signal, followed by a linear increase that was recorded for at least 10 min. The amplitude of the initial fluorescence signal was not correlated to the subsequent steady increase. Moreover, in conditions that were meant to increase or inhibit calpain activity (see Results), it was not affected. We assumed that the initial signal was non-specific and that only the steady increase reflected the underlying calpain activity. For long-lasting measurements (several minutes), the fibre was illuminated for only 6 s every 1 min to avoid bleaching (as signal acquisition sampling was 2 s−1, every 6 s of data was the average of 12 individual measurements). Typical records of the steady fluorescence increase are given in Figs 4B, 5B and 6B. In some instances (see Results), fluorescence was recorded at a much higher sampling frequency (100 Hz) for several seconds to detect transient changes of calpain activity in response to [Ca2+]i transients. Through the paper, the use of ‘cleavage rate’ or of ‘calpain activity’ refers to the average slope of the fluorescence signals (photons counted per min) over the period of measurement. The quasi-linear increase of the signal (see Figs 4B, 5B and 6B) made the calculation of the slope straightforward. Slopes were usually expressed relative to a reference slope as explained below.
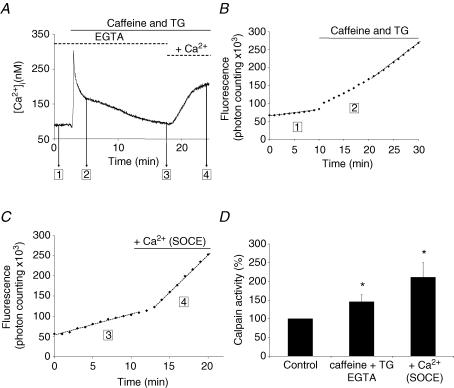
Figure 4. Effect of store depletion and store-operated entry of Ca2+ on calpain activity.
Fluorescence signals and [Ca2+]i transients were measured at rest in isolated C57 muscle fibres (1 in A and B) during stores depletion by application of caffeine and thapsigargin (TG) in the absence of external Ca2+ (2 in A and B). In another set of experiments, calpain activity and [Ca2+]i transients were measured in fibres previously depleted with caffeine and TG, and in which [Ca2+]i had returned to its basal value (3 in A and C). External Ca2+ (1.5 mm) was thereafter re-added to induce a store-dependent entry of Ca2+ (4 in A and C). Results are summarized in D. The calpain activity measured in resting conditions (1 and 3) is taken as 100%. * and **Significantly different (P < 0.05 and P < 0.01, respectively) from control conditions (paired Student's t test, n = 6). §Significantly different (P < 0.01) from C57 (unpaired t test).
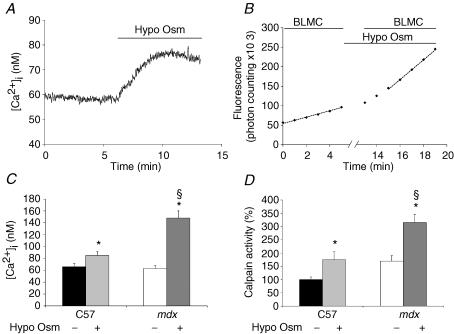
Figure 5. Calpain activity and [Ca2+]i transients in response to hypo-osmotic shocks.
Calpain activity was measured at rest and 10 min after bathing the fibres in a Krebs solution diluted to obtain an osmolarity of 160 mosm l−1 and containing 1.5 mm Ca2+. Note that fluorescence recording (and application of Boc-Leu-Met-CMAC (BLMC)) was interrupted during the first 10 min in hypo-osmotic solution. Results are illustrated for C57 fibres in A and B and summarized for C57 and mdx fibres in C and D. * and **Significantly different (P < 0.05 and P < 0.01, respectively) from control conditions (paired Student's t test). §Significantly different (P < 0.05) from C57 in the same conditions (unpaired t test); n = 8–14.
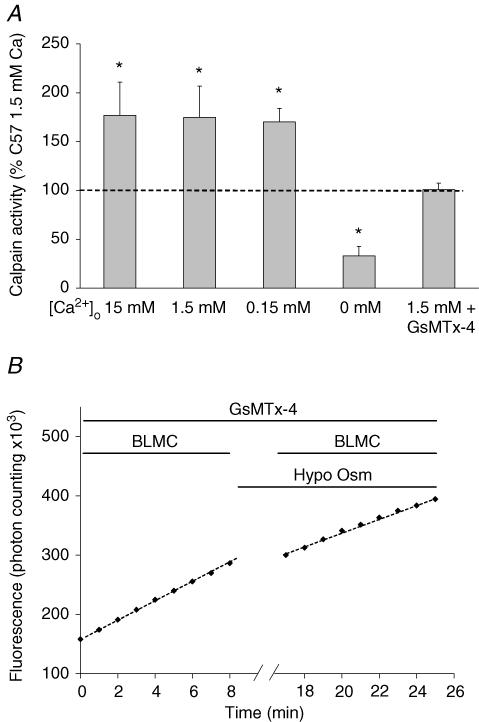
Figure 6. Role of extracellular [Ca2+] and the spider GsMTx-4 toxin in the activation of calpain by hypo-osmotic shock.
A, calpain activity was measured in resting conditions (average taken as 100%, dashed horizontal line) or after 10 min incubation in a hypo-osmotic solution containing 15 mm (n = 8), 1.5 mm (n = 5) or 0.1 mm[Ca2+]o (n = 8), or in the absence of [Ca2+]o (1 mm EGTA, n = 5). B, the effect of 5 μm GsMTx-4 was also tested in the presence of 1.5 mm[Ca2+]o (n = 6). *Significantly different from control conditions (P < 0.05), paired Student's t test.
Fluorescence measurements of MAC-SG are not ratiometric and depend on several factors, beside the cleavage rate, that are not under experimental control: the rate of cell penetration, the rate of the thiol conjugation, and the intracellular concentration of the substrate. Therefore, all measurements on treated, transgenic or dystrophic fibres were expressed relative to the calpain activity of untreated normal C57 fibres, measured in Krebs solution with 1.5 mm[Ca2+] (referred to here as ‘standard resting condition’) during the same experimental session (taken as 100%). The 1.5 mm[Ca2+]o will be maintained in all other experimental conditions, unless stated otherwise.
In some instances, we measured calpain activity before and after a treatment, allowing direct comparisons between results obtained on the same fibre. This protocol required changes of solutions and rinsing. When the measurement of activity was resumed, the new solutions also contained the substrate. On the second admission of the substrate solution, no supplementary initial surge of fluorescence was observed (or a small increase due to the prolonged activity of calpain and the residual presence of intracellular MAC-SG during the treatment period). Depending on the experimental protocol, solution changes were achieved either by addition of 100 μl Krebs solution containing the drug of interest at 10 times the final concentration, or by a slow perfusion (to avoid fibre detachment) which completely exchanges the content of the chamber in about 1 min. We checked that the change of solution per se did not alter the rate of calpain activity.
When fibres were submitted to electrical stimulation or to protocols intended to increase [Ca2+]i, contractile activity had to be inhibited to prevent movement artifacts during fluorescence recordings. Therefore, fibres were incubated with 100 μm N-benzyl-p-toluene sulphonamide (BTS) which inhibits actin–myosin interaction (Cheung et al. 2002).
Electrical stimulation
Thin platinum wires were placed on both side of the fibre and the stimulus strength was increased to obtain vigorous twitching, which was detected visually. This ensured that only excitable fibres were studied. Then contraction was inhibited by BTS for at least 10 min before fluorescence recording. In these conditions, the mechanical response to electrical stimulation was completely abolished. It has been shown by others that BTS does not affect [Ca2+]i transients induced by action potentials (Pinniger et al. 2005).
Measurements of [Ca2+]i
Measurements of [Ca2+]i were performed on different batches of collagenase-isolated fibres submitted to the same experimental conditions as the fibres used for calpain activity measurements. Fibres were loaded with the diffusible Ca2+ indicator Fura-PE3-AM. Ratiometric fluorescence measurements and calibration parameters were obtained as reported previously (De Backer et al. 2002).
Analysis of muscle extracts for μ-calpain
We followed closely the procedure designed by Murphy et al. (2006b) for muscle extraction, electrophoresis and electrotransfer for Western blot analysis. Extensor digitorum longus (EDL) and tibialis anterior muscles of C57 and NMRI mice were homogenized with a Turax blendor, 3 × 20 s at low speed, 3 × 20 s at high speed in a solution containing (mm): KCl 126, NaCl 36, Hepes 60 and phenylmethylsulfonyl fluoride 0.5; pH 7.2. To assess the state (80 kDa native or 78 kDa autolysed) of the μ-calpain in resting muscles, extraction was performed in the presence of 1 mm EGTA to prevent activation of autolysis during the preparation procedures. Alternatively, to obtain the products of autolysis, extraction was made in the presence of 5 mm Ca2+ (Murphy et al. 2006b). After homogenization, SDS was added to a final concentration of 4%, muscles were extracted for 30 min at 0°C, and centrifuged at 3000 g for 10 min. Loading buffer 2x concentrated (containing 125 mm Tris, 4%SDS, 20% glycerol, 10%β-mercaptoethanol, 0.001% bromophenol and 4 m urea) was added to the supernatants. Samples were heated for 5 min at 95°C. Proteins were separated on 8% SDS-PAGE gels, transferred to nitrocellulose 0,22 μm for 1 h at 80 V at 4°C in transfer solution (containing 25 mm Tris, 200 mm glycine, 20%methanol and 0.037% SDS). Membranes were blocked with non-fat dry milk and incubated overnight with the mouse anti-μ-calpain (Sigma clone 15C 10 product C0355) diluted 1: 1000. After incubation with anti-mouse peroxidase (Sigma 1/80.000), peroxidase activity was detected with ECL plus (Amersham) on high-performance chemiluminescence film.
Reagents
The GsMTx-4 toxin, isolated from Grammostola spatulata spider (Suchyna et al. 2000), was obtained from PeptaNova (Sandhausen, Germany); Fura-PE3-AM was from Calbiochem (Darmstadt, Germany); Boc-Leu-Met-CMAC was from Molecular Probes; Z-Leu-Leu-CHO was from Bio Mol Laboratories and bromo-A27183 from Alexis Corp. and N-benzyl-p-toluene sulphonamide (BTS) was from Tocris. Inhibitors SNT198438 and bortezomid were gifts from Santhera Pharmaceuticals, Liestal, Switzerland. Collegenase type IV, DMEM/HAM F12 medium, fetal bovine serum and all other reagents (analytical grade) were purchased from Sigma. The Krebs solution contained (mm): NaCl 135.5, MgCl2 1.2, KCl 5.9, glucose 11.5, Hepes 11.5 and CaCl2 1.5; pH 7.3). When necessary, CaCl2 was omitted and replaced by 200 μm Na-EGTA or increased to 15 mm and osmolarity adjusted. Potassium aspartate solution contained (mm): potassium aspartate 150, MgCl2 5, EGTA 10 and Hepes 10; pH 7.3.
Statistical analysis
Data are presented as means ±s.e.m. The tests used to determine statistical significance are given in the figure legends.
Results
Measurements of calpain activity in isolated muscle fibres at rest
Over the entire course of the study, cleavage rates were measured in 173 individual fibres, distributed in 20 different experimental sessions of different size (from three to 22 fibres per session, but in most cases eight to 12 fibres per session). For each session the average rate was calculated and taken as 100%; individual results were expressed relative to that reference. Results obtained within a session were more consistent than between sessions. This is why comparisons between normal and modified fibres (either treated, transgenic or dystrophic) were always made relative to the average cleavage rate of normal, companion fibres studied in the same experimental session and taken as 100% as described above.
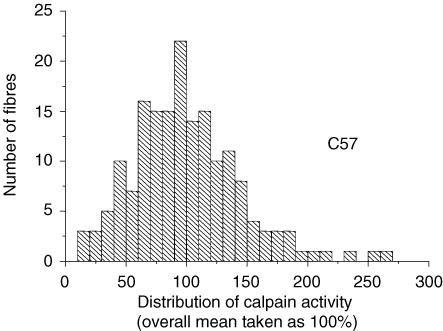
Figure 1 shows the histogram for the 173 individual results (class size, 10%) obtained in standard resting conditions (1.5 mm[Ca2+]o); this illustrates the large variation of the cleavage rate from fibre to fibre. The distribution appears reasonably symmetrical around the mean (100%, by definition) with apparent s.d.± 44%, and s.e.m± 3.3%. All the fibres studied displayed a detectable cleavage activity. The variability of calpain activity between individual fibres does not result from morphological heterogeneities: visual inspection of the fibres showed that shape, size, diameter and striation spacing were remarkably similar.
Figure 1. Histogram of distribution of calpain activity in isolated C57 muscle fibres.
Calpain activity was measured by using the fluorogenic substrate Boc-Leu-Met-CMAC. A total of 173 individual fibres were studied in 20 different sessions. For each session the mean activity was taken as 100%.
Analysis of the Ca2+ dependence of the calpain activity
Calpains are proteolytic enzymes, and thier activities are Ca2+ dependent. We examined this dependence through interventions aimed at reducing or increasing the intracellular Ca2+ availability. Steady-state and transient conditions were separately studied.
Cleavage rate in steady-state [Ca2+]i conditions
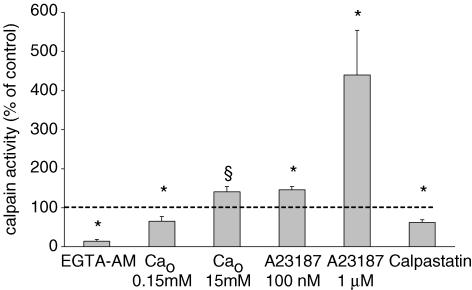
[Ca2+]i can be reduced by incubation in the presence of the diffusible Ca2+ chelator EGTA-AM (Gailly et al. 1993) and Ca2+ influx reduced by lowering [Ca2+]o. Both types of interventions were slow to affect the cleavage rate (see below). Routinely, fibres were incubated for 30 min in the modified medium before starting measurements. This precluded obtaining cleavage rate measurements before and after treatment on the same fibre. After application of EGTA-AM, the cleavage rate was reduced to 14% of the rate in untreated fibres. A 10-fold reduction of [Ca2+]o to 0.15 mm reduced the cleavage rate to 65% of control values (Fig. 2). Conversely, when [Ca2+]o was set to 15 mm, the cleavage rate slightly increased, so that a change of [Ca2+]o by a factor of 100 (0.15 to 15 mm) resulted in an increase of calpain activity by a factor of two. These results suggest that the Ca2+ dependence of calpain activity rests, at least partially, on an extracellular Ca2+ supply. This was confirmed when influx of Ca2+ ions from the external medium was increased by using the non-fluorescent calcium ionophore bromo-A23187. Cleavage rate quickly changed upon application of the ionophore and comparison before and after addition could be made on the same fibres. In the presence of 100 nm Br-A27183, the basal Ca2+ infux can be increased up to 10-fold without triggering unwanted contraction and increasing the bulk cytosolic [Ca2+] (De Backer et al. 2002). In this case, the cleavage rate increased to 146%. At the ionophore concentration of 1 μm, contraction occurred and had to be prevented by a 10 min incubation with 100 μm BTS to fully inhibit the actomyosin ATPase. Though Ca2+ influx and cytosolic [Ca2+]i were not measured at this high ionophore concentration, the occurrence of contraction suggests that they were both much larger than in the presence of the low concentration; this was accompanied by a 4- to 5-fold increase of calpain activity (Fig. 2).
Figure 2. Ca2+ dependence of calpain activity.
Calpain activity was measured in resting conditions (average taken as 100%, dashed horizontal line) or after 10 min incubation in the presence of 0.15 mm or 15 mm[Ca2+]o, after 30 min incubation in the presence of 10 μm EGTA-AM or after few minutes in the presence of 100 nm or 1 μm the ionophore bromo-A23187. Comparison is also presented between calpain activity measured in muscle fibres from calpastatin-over-expressing mice and control animals (measurements performed during the same session). *Significantly different from control conditions (P < 0.05), paired (0.15 mm[Ca2+]o and bromo-A23187) or unpaired Student's t test (15 mm[Ca2+]o and calpastatin). §Significantly different from in the presence of 0.15 mm[Ca2+]o (P < 0.05, unpaired t test); n = 6–14.
Altogether, the results summarized in Fig. 2 establish that the cleavage rate was Ca2+ dependent and displayed a 30-fold variation between the experimental conditions of ‘lowest’ (EGTA-AM) and ‘highest’ (1 μm bromo-A23187) Ca2+ availability tested here.
On four fibres, we studied the kinetics and the reversibility of the changes of calpain activity in response to changes of [Ca2+]o. By changing from 0.15 to 15 mm Ca2+, a higher (1.9-fold) steady-state rate of activity was attained within 3 min after the solution exchange. On return to 0.15 mm, the delay to reach the initial lower level of activity was systematically longer and variable: it ranged from 6 to 12 min.
Cleavage rate and transient increases of [Ca2+]i
The previous protocols were meant to measure the cleavage rate in response to long-lasting changes of Ca2+ availability. We next studied whether the cleavage rate was affected by transient changes of [Ca2+]i (e.g. as they occur in response to physiological signals). We tested four protocols where [Ca2+] transients differed in both amplitude and time course. Results obtained for protocols (1) to (4) are illustrated by typical signals (i.e. the cumulative photon counts reflect the total accumulated amounts of cleaved substrate). The cleavage rates or calpain activities are the derivative of these experimental curves, as stated in the Methods.
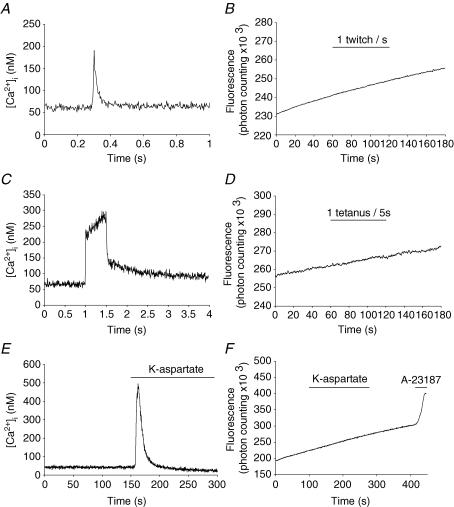
(1) Calpain activity and [Ca2+]i transients triggered by electrical stimulation. BTS-treated fibres were electrically stimulated (see Methods) by (i) a series of 60 single pulses, at 1 Hz, or by (ii) 12 trains of high-frequency pulses (100 Hz, for 500 ms) every 5 s. Either type of stimulation lasted 60 s during which fluorescence was continuously recorded. Fibres treated exactly the same way were loaded with the Ca2+ indicator Fura-PE3-AM to monitor the amplitudes and time courses of the associated [Ca2+]i transients (Fig. 3A and C). In these conditions, we did not detect any change of the basal cleavage rate (Fig. 3B and D). In a few cases, the acquisition sampling of fluorescence data was increased to 100 s−1, for 30 s, in order to detect a transient activation during the course of a tetanus, and just after. The latter records were noisy but, notwithstanding, did not reveal an increase of the cleavage rate.
Figure 3. Calpain activity during muscle stimulation.
Continuous fluorescence recordings (B, D and F) and [Ca2+]i transients (A, C and E) were measured during following stimulations: (i) single pulses (twitches) at 1 Hz (A and B; note the different timescale); (ii) trains of pulses (tetani) of 100 Hz frequency and 500 ms train duration (C and D); and (iii) complete depolarization with 100 mm potassium aspartate solution for 180 s.
(2) Calpain activity and [Ca2+]i transients in high-K+-dependent depolarized fibres. BTS-treated fibres were perfused with a 100 mm potassium aspartate solution for 180 s and calpain activity was continuously recorded before, during and after the perfusion. [Ca2+]i transients were recorded on fibres treated the same way (Fig. 3E). In spite of the fact that K+-dependent depolarization evoked a [Ca2+]i transient peaking at 500 nm, with a half-duration of 20 s, no detectable increase of the calpain activity was observed (Fig. 3F) As a positive control, a high concentration (1 μm) of the Ca2+ ionophore bromo-A23187 was added. This produced an immediate and sharp increase of calpain activity (Fig. 3F).
(3) Calpain activity and [Ca2+] transients during Ca2+ store depletion and subsequent refilling. The protocol was divided into two parts, illustrated in Fig. 4A, which gives a typical example of the [Ca2+] transients associated with each part.
Store depletion. Fibres were bathed for 5 min in 0 Ca Krebs solution plus EGTA (0.2 mm) and BTS, and the cleavage rate was measured (step 1 of Fig. 4A and B). Then caffeine (20 mm) and thapsigargin (1 μm) were added in order to deplete the intracellular Ca2+ stores. This was accompanied by a large but transient increase in [Ca2+]i (step 2 in Fig. 4A and B). Measurement of the cleavage rate started soon after addition of the drugs; however, as the duration of measurement lasted more than 10 min, fluorescence samplings were restricted to 6 s every minute. We systematically observed a progressive increase of the cleavage rate (Fig. 4B) which reached, after about 3 min, a maximal and stable value. Average values are shown in Fig. 4D.
Store refilling
Fibres were treated as in ‘Store depletion’ above. The first measurement of the cleavage rate (addition of Boc–Leu–Met–CMAC) started only when the [Ca2+] transient resulting from store depletion was completely over (step 3 of Fig. 4A). Then, fibres were returned to a 1.5 mm[Ca2+] external solution to induce the activation of the so-called ‘capacitative Ca2+ entry’ or ‘store-operated calcium entry’ (SOCE) that also produces a transient increase of [Ca2+]i (step 4 of Fig. 4A), and the cleavage rate was again measured in this situation. As seen in the example illustrated in Fig. 4C, a sharp increase of the cleavage rate (here 4-fold) followed the Ca2+ entry (and the [Ca2+]i rise) with a phase lag of about 2–3 min. On average, the cleavage rate, measured at step 4 of Fig. 4C, was 2.1-fold greater than (± 0.32, s.e.m., n = 6) the cleavage rate after store depletion (Fig. 4D).
(4) Calpain activity and [Ca2+] transients in response to hypo-osmotic shock. Muscles fibres possess a class of voltage-independent/mechanosensitive Ca2+ channels. We have previously shown that these channels possess similar biophysical and pharmacological properties to store-operated Ca2+ channels (see above), being inhibited by a toxin (GsMTx-4) isolated from the Grammostola spatulata spider, and possibly being composed of Transient Receptor Potential (TRP) channels (Suchyna et al. 2000; Vandebrouck et al. 2002; Ducret et al. 2006). These channels can be activated by applying mechanical pressure or hypo-osmotic swelling, obtained at 60% normal osmolarity. Figure 5A shows the elevation of [Ca2+]i that followed perfusion with the hypo-osmotic solution containing the standard 1.5 mm concentration of Ca2+; it took about 3–4 min to reach a stable value. Figure 5B shows the progressive increase in calpain activity that required 10–12 min to reach its steady rate. Figure 5C and D shows the steady values for [Ca2+]i and calpain activity, respectively, for normal (C57) and mdx fibres (see below). In normal fibres, the increased activity was 175% of that observed in standard control conditions.
To further analyse the factors responsible for calpain activation by hypo-osmotic swelling, we modified the above protocol in two ways. (1) We explored the effects of three changes of [Ca2+]o: 15 mm (10 × normal), 0.15 mm (0.1 × normal) and nominal absence of Ca2+. Results are shown in Fig. 6A. Calpain activation by osmotic swelling was almost constant in the range 15–0.15 mm[Ca2+]o, suggesting that the influx of Ca2+ was rate-limiting in spite of the increase of the inward electrochemical gradient over the 0.15–15 mm concentration range. However, in the absence of external Ca2+, calpain activity was no longer increased by the swelling; instead it was inhibited down to 35% of the reference level in untreated fibres. Thus swelling by itself did not activate calpain (its effect entirely depended on an influx of external Ca2+) without contribution from the internal stores of Ca2+; this conclusion is confirmed by (2) below. (2) The importance of the mechanosensitive TRP-type Ca2+ channels was assessed on fibres pretreated with the specific GsMTx-4 toxin before being submitted to the hypo-osmotic shock. As illustrated in the example of Fig. 6B, the slope of the fluorescence signal did not change significantly upon application of the osmotic shock, in contrast to the sharp increase in the absence of the toxin (Fig. 5B), a result confirmed on all fibres tested (n = 6, Fig. 6A, far right). The same toxin completely inhibits Ca2+ flux through these mechanosensitive Ca2+ channels (Ducret et al. 2006).
Calpain activity in the presence of elevated concentrations of calpastatin
Cells contain a natural inhibitor of m- and μ-calpains called calpastatin. Its concentration usually exceeds that of calpains so that the enzymic activity of the latter is thought to be inhibited in basal conditions. Spencer & Mellgren (2002) have developed two murine transgenic lines that over-express very large concentrations of calpastatin. We measured the in situ calpain activity in fibres from the ‘74.1 Tg’ line where expression of calpastatin was over 300-fold greater than the normal level, which produced a complete inhibition of both m- and μ-calpain activities in assays on muscle extracts (Spencer & Mellgren, 2002). We observed that the cleavage rate was reduced to 62% of the value measured in fibres from normal littermates (Fig. 2, far right). Thus, although the calpain activity was significantly reduced by the high calpastatin concentration, the residual in situ activity was far from negligible, which is contrary to the expectations from the results of the in vitro assays. This puzzling observation will be examined in the Discussion.
Susceptibility of the calpain activity to pharmacological agents
We tested the effect on the cleavage rate of inhibitors of calpain activity, using the cell-permeant Z-Leu-Leu-CHO (Tsubuki et al. 1996) and SNT198438 (the latter combines inhibitory effects on calpains and the proteasome). Moreover, in order to evaluate the contribution of proteasome activity on the cleavage rate, we also tested the effect of bortezomib, a selective inhibitor of the proteasome. Each drug was applied 30 min before addition of the calpain substrate and the onset of the recording.
Z-Leu-Leu-CHO and SNT198438 reduced the cleavage rate to 49.4 ± 9.7% (n = 5) and 38.2 ± 3.2% (n = 4) of untreated fibres, respectively. As bortezomid had no effect at all, it can be concluded that the proteasome activity did not contribute to the cleavage of Boc-Leu-Met-CMAC. In the course of this pharmacological study, we checked that the use of BTS for preventing contraction (see above) did not affect the cleavage rate.
State of calpain in intact muscle fibres
Resting fibres maintain their bulk cytosolic [Ca2+] below 100 nm. Still a basal calpain activity could be measured, which proved to be Ca2+ sensitive (Fig. 2). We reported several experimental interventions that stimulated calpain activity while [Ca2+]i remained below 500 nm. In these conditions (rest and stimulated), a contribution of the m-calpain to the cleavage rate is extremely unlikely. Moreover, from the relationship between [Ca2+] and μ-calpain activity established by Kapprell & Goll (1989), the cleavage activity should also be very low because its half-maximal activation requires 34 μm Ca2+. The fact that a Ca2+-senstive calpain activity was measurable in our in situ conditions, suggests that some μ-calpain was present in the 78 kDa autolysed form which is much more Ca2+ sensitive (half-maximal activation at 600 nm Ca2+). Indeed, there are several reports that living muscles (from human and rat) at rest contain a small fraction of their μ-calpain in the 78 kDa autolysed form (see Murphy et al. 2006a,b). We wanted to confirm this for mouse muscle. Calpain analysis from intact muscles at rest (by electrophoresis and Western blot identification, see Methods) revealed that a small fraction of μ-calpain was present in the 78 kDa form. The Western blot pattern illustrated in Fig. 7 was systematically observed in all specimens analysed (n = 15), whatever the mouse strain (C57, n = 6; mdx, n = 6; NMRI, n = 3). If, before analysis, muscle extracts were treated with 5 mm[Ca2+] to activate autolysis, the 78 kDa form became the predominant form, and the further hydrolysed 76 kDa form was then clearly present, as previously reported (Murphy et al. 2006b).
Calpain activity in adult dystrophin-lacking fibres
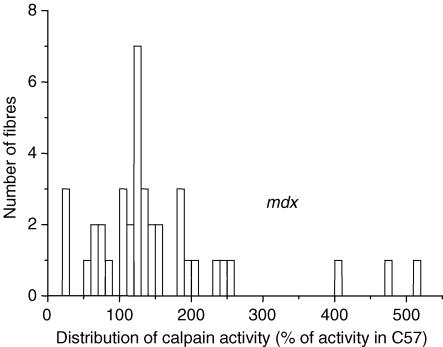
Fibres from the FDB muscles of adult mdx mouse were isolated and treated exactly as described above for normal fibres. Calpain activity was measured in 39 individual fibres. The histogram distribution (class size, 20%) is shown in Fig. 8. The mdx population appears unevenly distributed, with most results (36/39) ranging from 15 to 265% (the average activity of normal fibres taken as 100%); such a wide range was also observed in normal fibres (Fig. 1) and, indeed, there is a great deal of overlap of the two histograms (compare Figs 1 and 8). A second small group (3/39) showed much higher activities. The average of the first group is 128%± 9.7 (n = 36) and is significantly different from the average normal value of 100% (P < 0.01). When all values are taken together, the average is 154%± 17 (n = 39), which is also highly significantly different (P < 0.01) from normal average.
Figure 8. Histogram of distribution of calpain activity in dystrophic (mdx) muscle fibres.
Calpain activity observed in mdx fibres is normalized to average activity observed in C57 fibres (during the same session).
Extracts of freshly isolated muscles (tibialis anterior and EDL) from mdx mice showed, as in normal mice, the presence of μ-calpain, predominantly in the intact 80 kDa form, together with a small fraction as the autolysed 78 kDa form (Fig. 7). We could not find a significant difference of the 78/80 kDa proportion between mdx and normal mice.
Turner et al. (1988) observed that the tyrosine leakage from mdx soleus was ∼2-fold higher than from normal muscle, and was normalized by a 10-fold reduction of [Ca2+]o. However, strictly speaking, tyrosine leakage reflects the activity of the ubiquitin-proteasome and it was important to see whether the same influence of [Ca2+]o was observed when measuring calpain activity directly. The latter was first measured in normal conditions (1.5 mm[Ca2+]o), then after 10 min of perfusion with 0.15 mm[Ca2+]. We observed a significant (P < 0.05) reduction of 24 ± 7.8% (n = 5).
This elevated cleavage rate was also sensitive to the calpain inhibitor SNT 198438 (2 μm) which reduced it by about 50% (n = 6, P < 0.01). In dystrophin-lacking myotubes, the origin of the elevated calpain activity has been related to the increased activity of the so-called Ca2+ leak channels (Alderton & Steinhardt, 2000). The latter are sometimes considered as the developmental equivalent of the voltage-independent/mechanosensitive Ca2+ channels of the adult fibre (see above) because both are voltage-independent and show an increased activity in the absence of dystrophin (Franco-Obregón & Lansman, 1994). As for normal fibres, mdx fibres were submitted to the hypo-osmotic shock to activate these channels. Swelling produced a larger increase of [Ca2+]i than in normal fibres (Fig. 5C, right), as previously reported (Deconinck et al. 1997), and the associated increase of calpain activity was also larger, reaching 350% of the C57 reference value (Fig. 5D, right). However, when mdx fibres were pretreated with the GsMTx-4 toxin, the hypo-osmotic shock failed to produce any increase of the cleavage rate, as in normal fibres (Fig. 6A and B).
To determine whether the elevated calpain activity was a specific feature of dystrophinopathy, we measured the cleavage rate in fibres from mice suffering from a calpain-3 deficiency, a murine model of human limb-girdle muscular dystrophy type 2A (Richard et al. 1995), obtained by gene targeting (calp(−/−) mice; Richard et al. 2000). Compared with fibres from normal mice of the same genetic background (n = 13), the cleavage rate of calp(−/−) fibres (n = 14) was on average 143%± 17.2 of the normal rate (P = 0.04). Thus an elevated activity of ubiquitous calpains appears to be a non-specific feature of muscle dystrophy (at least in our conditions of study) whatever its causal origin (assuming that the content of ubiquitous calpains is normal in calpain-3-deficient mouse, as already observed for m-calpain by Dr Isabelle Richard, personal communication). This suggests that activation of calpain is a rather distant downstream consequence of the initialization of the dystrophic process. Moreover, this observation also shows that activity of calpain-3, the muscle-specific calpain isozyme (Sorimachi et al. 1989), did not contribute to the cleavage rates measured which reflected the activities of ubiquitous calpains (either μ alone or μ and m isozymes).
Discussion
We report here in situ measurements of the cleavage rate of Boc-Leu-Met-CMAC, a synthetic, permeant substrate of calpains, in collagenase-isolated muscle fibres form normal mice. The cleavage rate varied from fibre to fibre and its distribution was documented in 173 individual measurements (Fig. 1). Calpain activity is needed for myoblast fusion and some residual activity was observed in myotubes (Alderton & Steinhardt, 2000). It could have been expected that in fully differentiated fibres, calpain activity would be negligible. This was not the case.
Ca2+ and calpain activity
In spite of the fact that the cytosolic [Ca2+] in these fibres is maintained within the 20–80 nm range (De Backer et al. 2002), a significant cleavage rate could be measured, suggesting either that this basal activity is Ca2+ insensitive, or, as already considered, the Ca2+ sensitivity of calpain is much higher because a significant proportion of μ-calpain is autolysed in living fibres. To test the first possibility, we studied several experimental conditions where the intracellular Ca2+ availability was either reduced or increased, in long-lasting, steady-state situations. We observed that the calpain activity could be changed by 30-fold within the range of experimental conditions studied (Fig. 2). Therefore, the calpain activity in situ was definitely Ca2+ sensitive. The second possibility was confirmed by the observation that a small but significant proportion of μ-calpain is present in the 78 kDa, autolysed form, in extracts from resting muscles (Fig. 7). Most probably, the basal calpain activity in resting fibres is due to this Ca2+-sensitive, autolysed form of μ-calpain. However, at [Ca2+]i of 100 nm, this activity would be, at most, 5% of its possible Vmax, as deduced from the activity–pCa2+ relationship established by Kapprell & Goll (1989). The observation by us and others that significant amount of the 78 kDa autolysed μ-calpain is present in resting condition is puzzling, as autolysis itself requires 30 μm Ca2+, whereas cytosolic [Ca2+] is about 50-fold lower. For the same reason, it seems unlikely that the Ca2+ stimulation of the cleavage rate could be due, even partially, to an increased proportion of the 78 kDa autolysed form, though we have no experimental way to exclude it.
In some experimental conditions tested here (change of [Ca2+]o to 15 mM, presence of a low concentration of a Ca2+ ionophore), calpain activity increased (Fig. 2) while it is documented that the bulk cytosolic [Ca2+] remained constant (De Backer et al. 2002). In the case of the ionophore, we previously showed that in spite of an increase of Ca2+ influx, the intracellular mechanisms of Ca2+ homeostasis were robust enough to maintain [Ca2+]i within the normal 20–80 nm range (De Backer et al. 2002). However, this bulk situation does not preclude the possibility that in microdomains, such as the submembranous space where Ca2+ channels open, the local [Ca2+] could be higher, in a dynamic equilibrium that depends on the size of the influx, on the one hand, and on the rate constants of the homeostatic mechanisms (diffusion, binding and active uptake), on the other hand. Thus, we propose that the observed changes of the cleavage rate reflected the activity of calpain located in the submembranous space where changes of Ca2+ influx could generate local changes of [Ca2+]. This is supported by the fact that the 78 kDa, autolysed form of μ-calpain is preferentially associated with the plasma membrane (Murphy et al. 2006b).
We further tested whether [Ca2+]i transients, as seen in excitation–contraction coupling, were able to increase calpain activity. In these experiments, [Ca2+]i peaked at a maximum of 500 nm with time courses ranging from tens of milliseconds to a few seconds. We found that neither series of action potentials, single (1 Hz) or in trains (100 Hz), nor K+-dependent depolarization affected the basal calpain activity, suggesting that the time-integral of these Ca2+ transients did not reach the threshold for further calpain activation. It must be recalled that fibres were treated with BTS so that no mechanical force was produced. The present results do not preclude the possibility that physiological contractions (when mechanical stress is developed) could activate the calpain system. However, it was reported that various types of exercise in humans did not increase autolysis of μ-calpain which requires [Ca2+] > 1 μm for several minutes (Murphy et al. 2006a).
We further examined the effect of [Ca2+]i transients of much slower time courses. The sequence of depletion and refilling of internal Ca2+ stores is associated with long-lasting [Ca2+]i transients evolving over several minutes, while [Ca2+]i peaks reached 250–350 nm. As our protocol allowed the separation in time of the depletion and the refilling, we observed that both phases were associated with a significant (1.5- to 2.5-fold) increase of the basal calpain activity. This increase occurred regardless of the origin of Ca2+ (i.e. the intracellular stores, in depletion and the extracellular milieu in refilling.
Activation of mechanosensitive Ca2+ channels offered another opportunity to increase Ca2+ availability and activate calpain. This was obtained by submitting the fibres to hypo-osmotic swelling that produced a 175% increase of calpain activity (Fig. 5D, left). This effect was directly related to an increased of [Ca2+]i, resulting from an increased influx of external Ca2+ (Fig. 5C, left) through the channels: it was suppressed when Ca2+ was removed from the external medium and when the channels were blocked by the specific GsMTx-4 toxin (Fig. 6A). Thus calpain activation was not produced by the osmotic swelling per se and the resulting membrane stress and deformation. Here again, [Ca2+]i transients evolved over several minutes and individual peaks rarely exceeded 120 nm (Fig. 5C)
Our results showed that [Ca2+]i transients must attain a certain amplitude and time course combination to activate calpain, suggesting the presence of some integrative mechanisms. This was indeed demonstrated in experiments on purified μ-calpain (from erythrocytes) subjected to repetitive (1–50 Hz) Ca2+ pulses producing 10 μm peaks of [Ca2+] (Tompa et al. 2001). Our results suggest that such an integrative mechanism might operate in situ for [Ca2+]i transients, the amplitudes of which remained well below 1 μm (i.e. similar to those attained by most Ca2+ signals in physiological conditions). Recent findings suggest that the ‘integrative mechanism’ proposed above might involve calpain phosphorylation by Ca2+ and/or by stress-activated kinases. Indeed, m- and μ-calpains can be phosphorylated and activated by protein kinase Cι and by extracellular signal-regulated kinases 1/2 (ERK1/2) in human lung cancer cells (Xu & Deng, 2004, 2006) It is interesting that in skeletal muscle these latter kinases are activated by mechanical stretch (hence the involvement of mechanosensitive Ca2+ channels) and by physical exercise (Kumar et al. 2004; Nakamura et al. 2005). Alternatively, calpain phosphorylation might provide an independent activation mechanism.
Is the basal calpain activity measured in resting fibres still sensitive to the calpastatin inhibition? The basis of this inhibitory effect is calpastatin binding to calpain, which is itself Ca2+ sensitive; it shows a much higher Ca2+ sensitivity for the autolysed forms (both m- and μ-calpain). At the cytosolic [Ca2+] of resting fibres, this binding would be ∼60% complete for autolysed μ-calpain (Kapprell & Goll, 1989). Thus the basal activity we measured most probably reflects that of the calpastatin-free and autolysed μ-calpain. In fibres from transgenic mice over-expressing calpastatin (300-fold increase), we observed that the basal calpain activity was further reduced by 40% (Fig. 2). Most probably the high concentration of calpastatin increased the relative importance of the calpain–calpastatin complex, but the very low [Ca2+]i prevented a complete inhibition. Moreover, one cannot exclude a differential calpain and calpastatin localization, as the latter has been observed to be confined to aggregates (in neuroblastoma; De Tullio et al. 1999), so that, in spite of an elevated calpastatin content, the formation of the calpain–calpasatin complex and the inhibition of calpain activity would be marginal or moderate in our resting conditions. This would not preclude an important inhibitory effect as [Ca2+]i increases by the combined effect of calpastatin solubilization (De Tullio et al. 1999) and increased binding of calpain to calpastatin. At 0.5 μm Ca2+, the calpain–calpastatin complex would amount to 90% (Kapprell & Goll, 1989). The fact that all our protocols intended to increase [Ca2+]i, produced, at most, a 3- to 5-fold increase of the basal calpain activity (Figs 2 and 4D) might reflect a self-limiting process, resulting from two antagonistic effects of Ca2+: activation of the enzyme and binding of its inhibitor calpastatin.
Calpain activity in situ was affected by several compounds documented as specific inhibitors that reduced the activity to 40–50% of the resting values, provided they were in contact with the fibres for at least 30 min before measurements. None completely suppressed calpain activity. They were less efficient than diffusible EGTA which reduced [Ca2+]i to undetectable levels (∼10−9m) and produced the more potent inhibition (Fig. 2). The absence of effect of bortezomid, a specific inhibitor of the proteasome, indicates that this proteolytic system did not contribute to the cleavage rate of Boc-Leu-Met-CMAC.
Calpain activity in dystrophin-lacking fibres (mdx)
Dystrophin-lacking fibres isolated from the mdx mouse, showed a 1.5-fold increase of calpain activity in resting conditions (Fig. 8), while the cytosolic [Ca2+] remained within normal values (Fig. 5C, right). We found no evidence that this effect reflected a significant increase of autolysed μ-calpain (Fig. 7). This elevated calpain activity could be normalized by a 10-fold reduction of [Ca2+]o as anticipated from previous results (Turner et al. 1988). As an elevated activity of the voltage-independent/mechanosensitive Ca2+ channels had been observed in mdx fibres, the increase of calpain activity seems directly related to the increased Ca2+ influx through these channels. When the latter are further stimulated by hypo-osmotic swelling, calpain activity was also increased (Fig. 5D, right), an effect that was suppressed by the specific channel blocker GsMTx-4. As discussed above, a higher Ca2+ influx is expected to increase the submembrane [Ca2+] and stimulate the autolysed μ-calpain preferentially located there, as already discussed for normal fibres. Submembrane [Ca2+] has been reported to be ∼3-fold higher in mdx fibres (Mallouk & Allard, 2000), but this observation was recently challenged using a membrane-bound Ca2+ indicator (Han et al. 2006) (for a detailed discussion of this controversial point, see Gillis, 2007). However, increased calpain phosphorylation could also contribute to the elevated cleavage rate in mdx fibres, as a higher level of activation of ERK1/2 has been observed in the mdx muscle in response to stretch, which is a Ca2+-dependent process (Kumar et al. 2004).
Voltage-independent/mechanosensitive Ca2+ channels display a higher activity in the absence of dystrophin. In resting conditions, the mechanisms of intracellular Ca2+ homeostasis are robust enough to cope with the increased Ca2+ influx and to maintain [Ca2+]i within normal values (Fig. 5C, right). Notwithstanding, the increased Ca2+ influx is able to stimulate some calpain activity (Fig. 5D, right) probably located near the plasma membrane. However, in response to stimulation of the channel activity by mechanical stress, [Ca2+]i exceeded normal values and further stimulated calpain activity (Fig. 5C and D, right). Assuming that fibre swelling imposes on the plasma membrane a stress that simulates the one generated by contraction, the present results suggest that mechanosensitive Ca2+ channels could be stimulated by contractile activity, and that this stimulation, amplified in the absence of dystrophin, could result in greater calpain activity in mdx fibres. In particular, his would be the case in eccentric contractions (Allen et al. 2005). The moderate calpain activation observed in mdx fibres is, however, not a specific feature of dystrophinopathy, as it was observed in calpain-3-deficient fibres (present results) and in δ-sarcoglycan-lacking fibres from the mutant hamster (Bartoli et al. 2006).
Calpain activation: a step towards fibre necrosis or a fibre protection mechanism?
A widely held view is that calpain activation contributes to muscle wasting in dystrophinopathy (Turner et al. 1988). This view is supported by the fact that calpain inhibition alleviates dystrophic disorders (Spencer & Mellgren, 2002). However, the only clear cause–effect relationship between calpain activation and a structural/functional defect comes from the recent work of Murphy et al. (2006b) on normal rat fibres. They showed that application of pre-activated exogenous μ-calpain to stretched (200%), skinned fibres produces a sharp decline in passive tension resulting from proteolysis of the tension-bearing filaments of titin. This required [Ca2+] of1 μm. The same effect was obtained by dipping the fibres in a 5 mm Ca2+ solution. These effects resulted from a massive activation of calpain and were predominantly due to the high proteolytic activity of the 76 kDa autolysed form (see Fig. 7 for the various forms of calpain). The possibility cannot be excluded that the experimental protocol artificially increased the susceptibility to calpain by exposing regions of the titin molecule that are recoiled at normal muscle length. By contrast, the situation in unstretched, resting muscles, normal and mdx, is far from these experimental conditions: [Ca2+]i is maintained at 100 nm, the 76 kDa form of μ-calpain is not detected and resting tension is negligible. Light microscopic observations of collagenase-isolated fibres do not reveal structural alterations (the striation pattern remained very sharp and regular) even after stimulation by Ca2+ of the calpain activity of the amplitude reported here.
Recently, it was shown that a localized wound of the plasma membrane of a muscle fibre reseals spontaneously in a matter of a few tens of seconds. This requires dysferlin and its activation by Ca2+ (Bansal et al. 2003). Moreover, resealing involves membrane fusion and a local remodelling of the cytoskeleton for which calpain activation by Ca2+ is essential for the degradation of talin and vimentin; resealing and thus cell survival is highly compromised in calpain-null mutant cells or in the presence of calpain inhibitors or EGTA (Mellgren et al. 2006). The presence of the highly Ca2+-sensitive 78 kDa form of μ-calpain close to the membrane would provide a ready-to-work system for membrane repair. In this context, the activation of Ca2+ leak channels in the surroundings of microlesions in myotubes (McCarter & Steinhardt, 2000) may be seen as a way to provide a channel-controlled influx of Ca2+ needed to activate both the dysferlin and the calpain systems. Loss-of-function mutations of dysferlin are responsible for limb girdle muscular dystrophy type 2B, suggesting unexpectedly that membrane wounds and repairs are common events in a healthy muscle fibre. Thus, instead of being seen as deleterious, calpain activation, at the level observed here, may be considered as playing an important function in maintaining fibre integrity. In these circumstances, calpain inhibition could have adverse effects. This view could be extended to mdx fibres where the slightly higher (∼1.5-fold) calpain activity may be seen as an adequate response to a higher occurrence of wounds in a plasma membrane made fragile by the loss of dystrophin and its associated glycoproteins. However, if membrane damage allows [Ca2+]i to rise and remain far above physiological values then a massive activation of calpain would occur with structural/functional damage as reported by Murphy et al. (2006b). They showed that the threshold [Ca2+]i for damage was in the 1–10 μm range, and full effect occurred at much higher Ca2+ concentrations. This probably occurs during eccentric contractions to which mdx fibres are highly susceptible (Moens et al. 1993). Indeed, a very recent study demonstrated that high calpain activity was specifically detected in fibres exhibiting structural damage after extensive downhill run (Bartoli et al. 2006). In these circumstances, calpain inhibition (e.g. by high levels of calpastatin or pharmacological compounds) would be beneficial.
Acknowledgments
We are specially grateful to Dr Melissa Spencer (University of California, Los Angeles, CA, USA) and Dr Isabelle Richard (Généthon, Evry, France) for providing us with mutated mice either over-expressing calpastatin or deficient in calpain-3, respectively. Inhibitors SNT198438 and bortezomid were gifts from Dr Thomas Meier (Santhera Pharmaceuticals, Liestal, Switzerland). This work was supported by the Association Belge contre les Maladies Neuromusculaire (ABMM), the Association Française contre les Myopathies (AFM) and by a grant ARC 05/10–328 from the General Direction of Scientific Research of the French Community of Belgium.
References
- Alderton JM, Steinhardt RA. Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem. 2000;275:9452–9460. doi: 10.1074/jbc.275.13.9452. [DOI] [PubMed] [Google Scholar]
- Allen DG, Whitehead NP, Yeung EW. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: role of ionic changes. J Physiol. 2005;567:723–735. doi: 10.1113/jphysiol.2005.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- Bartoli M, Bourg N, Stockholm D, Raynaud F, Delevacque A, Han Y, Borel P, Seddik K, Armande N, Richard I. A mouse model for monitoring calpain activity under physiological and pathological conditions. J Biol Chem. 2006;281:39672–39680. doi: 10.1074/jbc.M608803200. [DOI] [PubMed] [Google Scholar]
- Burdi R, Didonna MP, Pignol B, Nico B, Mangieri D, Rolland JF, Camerino C, Zallone A, Ferro P, Andreetta F, Confalonieri P, De Luca A. First evaluation of the potential effectiveness in muscular dystrophy of a novel chimeric compound, BN 82270, acting as calpain-inhibitor and anti-oxidant. Neuromuscul Disord. 2006;16:237–248. doi: 10.1016/j.nmd.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–88. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- Cottin P, Vidalenc PL, Ducastaing A. Ca2+-dependent association between a Ca2+-activated neutral proteinase (CaANP) and its specific inhibitor. FEBS Lett. 1981;136:221–224. doi: 10.1016/0014-5793(81)80622-9. [DOI] [PubMed] [Google Scholar]
- De Backer F, Vandebrouck C, Gailly P, Gillis JM. Long-term study of Ca2+ homeostasis and of survival in collagenase-isolated muscle fibres from normal and mdx mice. J Physiol. 2002;542:855–865. doi: 10.1113/jphysiol.2002.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tullio R, Passalacqua M, Averna M, Salamino F, Pontremoli S, Melloni E. Changes in intracellular localization of calpastatin during calpain activation. Biochem J. 1999;343:467–472. [PMC free article] [PubMed] [Google Scholar]
- Deconinck N, Tinsley J, De Backer F, Fisher R, Kahn D, Phelps S, Davies K, Gillis JM. Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nat Med. 1997;3:1216–1221. doi: 10.1038/nm1197-1216. [DOI] [PubMed] [Google Scholar]
- Ducret T, Vandebrouck C, Cao ML, Lebacq J, Gailly P. Functional role of store-operated and stretch-activated channels in murine adult skeletal muscle fibres. J Physiol. 2006;575:913–924. doi: 10.1113/jphysiol.2006.115154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Obregón AJ, Lansman JB. Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. J Physiol. 1994;481:299–309. doi: 10.1113/jphysiol.1994.sp020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly P, Boland B, Himpens B, Casteels R, Gillis JM. Critical evaluation of cytosolic calcium determination in resting muscle fibres from normal and dystrophic (mdx) mice. Cell Calcium. 1993;14:473–483. doi: 10.1016/0143-4160(93)90006-r. [DOI] [PubMed] [Google Scholar]
- Gillis JM. Understanding dystrophinopathies: an inventory of the structural and functional consequences of the absence of dystrophin in muscles of the mdx mouse. J Muscle Res Cell Motil. 1999;20:605–625. doi: 10.1023/a:1005545325254. [DOI] [PubMed] [Google Scholar]
- Gillis JM. The functional consequences of dystrophin deficiency in skeletal muscles. In: Uversky VN, Fink A, editors. Protein Misfolding, Aggegation, and Conformational Diseases. Part B: Molecular Mechanisms of Conformational Diseases. New York: Spinger; 2007. pp. 409–433. [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Haeberle JR, Coolican SA, Evan A, Hathaway DR. The effects of a calcium dependent protease on the ultrastructure and contractile mechanics of skinned uterine smooth muscle. J Muscle Res Cell Motil. 1985;6:347–363. doi: 10.1007/BF00713174. [DOI] [PubMed] [Google Scholar]
- Han R, Grounds MD, Bakker AJ. Measurement of sub-membrane [Ca2+] in adult myofibers and cytosolic [Ca2+] in myotubes from normal and mdx mice using the Ca2+ indicator FFP-18. Cell Calcium. 2006;40:299–307. doi: 10.1016/j.ceca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Inomata M, Hayashi M, Ohno-Iwashita Y, Tsubuki S, Saido TC, Kawashima S. Involvement of calpain in integrin-mediated signal transduction. Arch Biochem Biophys. 1996;328:129–134. doi: 10.1006/abbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- Kapprell HP, Goll DE. Effect of Ca2+ on binding of the calpains to calpastatin. J Biol Chem. 1989;264:17888–17896. [PubMed] [Google Scholar]
- Kumamoto T, Kleese WC, Cong JY, Goll DE, Pierce PR, Allen RE. Localization of the Ca2+-dependent proteinases and their inhibitor in normal, fasted, and denervated rat skeletal muscle. Anat Rec. 1992;232:60–77. doi: 10.1002/ar.1092320108. [DOI] [PubMed] [Google Scholar]
- Kumamoto T, Ueyama H, Sugihara R, Kominami E, Goll DE, Tsuda T. Calpain and cathepsins in the skeletal muscle of inflammatory myopathies. Eur Neurol. 1997;37:176–181. doi: 10.1159/000117430. [DOI] [PubMed] [Google Scholar]
- Kumar A, Khandelwal N, Malya R, Reid MB, Boriek AM. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J. 2004;18:102–113. doi: 10.1096/fj.03-0453com. [DOI] [PubMed] [Google Scholar]
- McCarter GC, Steinhardt RA. Increased activity of calcium leak channels caused by proteolysis near sarcolemmal ruptures. J Membr Biol. 2000;176:169–174. doi: 10.1007/s00232001086. [DOI] [PubMed] [Google Scholar]
- Mallouk N, Allard B. Stretch-induced activation of Ca 2+-activated K+ channels in mouse skeletal muscle fibers. Am J Physiol Cell Physiol. 2000;278:C473–C479. doi: 10.1152/ajpcell.2000.278.3.C473. [DOI] [PubMed] [Google Scholar]
- Mellgren RL, Zhang W, Miyake K, McNeil PL. Calpain is required for the rapid, calcium-dependent repair of wounded plasma membrane. J Biol Chem. 2006;282:2567–2575. doi: 10.1074/jbc.M604560200. [DOI] [PubMed] [Google Scholar]
- Moens P, Baatsen PH, Maréchal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J Muscle Res Cell Motil. 1993;14:446–451. doi: 10.1007/BF00121296. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Snow RJ, Lamb GD. μ-Calpain and calpain-3 are not autolyzed with exhaustive exercise in humans. Am J Physiol Cell Physiol. 2006a;290:C116–C122. doi: 10.1152/ajpcell.00291.2005. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Verburg E, Lamb GD. Ca2+-activation of diffusible and bound pools of μ-calpain in rat skeletal muscle. J Physiol. 2006b;576:595–612. doi: 10.1113/jphysiol.2006.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Yoshida K, Ueda H, Takeda S, Ikeda S. Up-regulation of mitogen activated protein kinases in mdx skeletal muscle following chronic treadmill exercise. Biochim Biophys Acta. 2005;1740:326–331. doi: 10.1016/j.bbadis.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Pinniger GJ, Bruton JD, Westerblad H, Ranatunga KW. Effects of a myosin-II inhibitor (N-benzyl-p-toluene sulphonamide, BTS) on contractile characteristics of intact fast-twitch mammalian muscle fibres. J Muscle Res Cell Motil. 2005;26:135–141. doi: 10.1007/s10974-005-2679-2. [DOI] [PubMed] [Google Scholar]
- Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, et al. Mutations in the proteolytic enzyme calpain-3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]
- Richard I, Roudaut C, Marchand S, Baghdiguian S, Herasse M, Stockholm D, et al. Loss of calpain-3 proteolytic activity leads to muscular dystrophy and to apoptosis-associated IκBα/nuclear factor κB pathway perturbation in mice. J Cell Biol. 2000;151:1583–1590. doi: 10.1083/jcb.151.7.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser BG, Powers SP, Gores GJ. Calpain activity increases in hepatocytes following addition of ATP. Demonstration by a novel fluorescent approach. J Biol Chem. 1993;268:23593–23600. [PubMed] [Google Scholar]
- Sorimachi H, Imajoh-Ohmi S, Emori Y, Kawasaki H, Ohno S, Minami Y, Suzuki K. Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and μ-types. Specific expression of the mRNA in skeletal muscle. J Biol Chem. 1989;264:20106–20111. [PubMed] [Google Scholar]
- Spencer MJ, Croall DE, Tidball JG. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem. 1995;270:10909–10914. doi: 10.1074/jbc.270.18.10909. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Mellgren RL. Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum Mol Genet. 2002;11:2645–2655. doi: 10.1093/hmg/11.21.2645. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Tidball JG. Calpain concentration is elevated although net calcium-dependent proteolysis is suppressed in dystrophin-deficient muscle. Exp Cell Res. 1992;203:107–114. doi: 10.1016/0014-4827(92)90045-a. [DOI] [PubMed] [Google Scholar]
- Stockholm D, Bartoli M, Sillon G, Bourg N, Davoust J, Richard I. Imaging calpain protease activity by multiphoton FRET in living mice. J Mol Biol. 2005;346:215–222. doi: 10.1016/j.jmb.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol. 2000;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompa P, Toth-Boconadi R, Friedrich P. Frequency decoding of fast calcium oscillations by calpain. Cell Calcium. 2001;29:161–170. doi: 10.1054/ceca.2000.0179. [DOI] [PubMed] [Google Scholar]
- Tsubuki S, Saito Y, Tomioka M, Ito H, Kawashima S. Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine. J Biochem (Tokyo) 1996;119:572–576. doi: 10.1093/oxfordjournals.jbchem.a021280. [DOI] [PubMed] [Google Scholar]
- Turner PR, Westwood T, Regen CM, Steinhardt RA. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988;355:735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburg E, Murphy RM, Stephenson DG, Lamb GD. Disruption of excitation-contraction coupling and titin by endogenous Ca2+-activated proteases in toad muscle fibres. J Physiol. 2005;564:775–790. doi: 10.1113/jphysiol.2004.082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induces phosphorylation of μ- and m-calpain in association with increased secretion, cell migration, and invasion. J Biol Chem. 2004;279:53683–53690. doi: 10.1074/jbc.M409889200. [DOI] [PubMed] [Google Scholar]
- Xu L, Deng X. Protein kinase Cι promotes nicotine-induced migration and invasion of cancer cells via phosphorylation of μ- and m-calpains. J Biol Chem. 2006;281:4457–4466. doi: 10.1074/jbc.M510721200. [DOI] [PubMed] [Google Scholar]