Abstract
There is evidence that sympathetically evoked vasoconstriction in skeletal muscle is blunted in systemic hypoxia, but the mechanisms underlying this phenomenon are not clear. In Saffan-anaesthetized Wistar rats, we have studied the role of α2-adrenoceptors and neuropeptide Y (NPY) Y1 receptors in mediating vasoconstriction evoked by direct stimulation of the lumbar sympathetic chain by different patterns of impulses in normoxia (N) and systemic hypoxia (H: breathing 8% O2). Patterns comprised 120 impulses delivered in bursts over a 1 min period at 40 or 20 Hz, or continuously at 2 Hz. Hypoxia attenuated the evoked increases in femoral vascular resistance (FVR) by all patterns, the response to 2 Hz being most affected (40 Hz bursts: N = 3.25 ± 0.75 arbitrary resistance units (RU); H = 1.14 ± 0.29 RU). Yohimbine (Yoh, α2-adrenoceptor antagonist) or BIBP 3226 (Y1-receptor antagonist) did not affect baseline FVR. In normoxia, Yoh attenuated the responses evoked by high frequency bursts and 2 Hz, whereas BIBP 3226 only attenuated the response to 40 Hz (40 Hz bursts: N + Yoh = 2.1 ± 0.59 RU; N + BIBP 3226 = 1.9 ± 0.4 RU). In hypoxia, Yoh did not further attenuate the evoked responses, but BIBP 3226 further attenuated the response to 40 Hz bursts. These results indicate that neither α2-adrenoceptors nor Y1 receptors contribute to basal vascular tone in skeletal muscle, but both contribute to constrictor responses evoked by high frequency bursts of sympathetic activity. We propose that in systemic hypoxia, the α2-mediated component represents about 50% of the sympathetically evoked constriction that is blunted, whereas the contribution made by Y1 receptors is resistant. Thus we suggest the importance of NPY in the regulation of FVR and blood pressure increases during challenges such as systemic hypoxia.
The vasodilatation that occurs in skeletal muscle during systemic hypoxia represents a balance between the vasoconstrictor effects of increased muscle sympathetic nerve activity (MSNA) and the dilator influences of local mediators and circulating adrenaline (Marshall, 1994). Thus, in the rat, α-adrenoceptor blockade enhanced the decrease in muscle vascular resistance induced by systemic hypoxia and dilator responses in individual skeletal muscle arterioles, while converting arteriolar constrictor responses to dilator responses (Marshall & Metcalfe, 1988; Mian & Marshall, 1991). Similarly, in human subjects vasodilatation evoked in forearm by systemic hypoxia was augmented by α-adrenoceptor blockade (Weisbrod et al. 2001). Such observations raise the question of whether the vasoconstrictor influence of MSNA is blunted during systemic hypoxia, or there is summation of constrictor and dilator influences and the latter dominates.
There is some evidence that vasoconstriction evoked by increased MSNA is indeed blunted by systemic hypoxia. Reflex vasoconstriction evoked in forearm by lower body negative pressure (LBNP) was reduced by mild and moderate systemic hypoxia (breathing 12 and 10% O2, respectively; Heistad & Wheeler, 1970). Moreover, the increases in MSNA evoked by LBNP were preserved during mild and moderate hypoxia, but the forearm vasoconstrictor responses were reduced (Rowell & Seals, 1990). However, reflex vasoconstriction evoked by LBNP in muscle microcirculation, as assessed by near infra-red spectroscopy, was well maintained during moderate hypoxia (Hansen et al. 2000). Further, forearm vasoconstriction evoked by tyramine, which releases noradrenaline from sympathetic fibres, was preserved during mild and moderate hypoxia (Dinenno et al. 2003).
Such disparities may be attributable to differences in the levels of hypoxia and methods used to activate the sympathetic nerves. In anaesthetized rats, we stimulated the sympathetic chain with three different patterns that delivered the same number of impulses over 1 min (see Coney & Marshall, 2003): continuous stimulation at 2 Hz to represent a low, resting level of MSNA and bursts of impulses at 20 or 40 Hz to represent the high frequency bursts that occur when MSNA is raised, for example by systemic hypoxia (Macefield et al. 1994; Johnson & Gilbey, 1996; Hudson et al. 2002). The increases in femoral vascular resistance (FVR) evoked by bursts at 20 and 40 Hz were not affected by mild or moderate hypoxia, but were considerably blunted by severe hypoxia (8% O2): that evoked by continuous stimulation at 2 Hz was blunted by moderate, as well as severe, hypoxia (Coney & Marshall, 2003).
These blunting effects were not altered by adenosine receptor blockade, or inhibition of cyclooxygenase activity (Coney & Marshall, 2003; Tomlinson et al. 2005), even though the muscle vasodilatation of systemic hypoxia is largely mediated by adenosine with prostacyclin acting as an intermediate (Bryan & Marshall, 1999; Ray et al. 2002). Further, they could not be ascribed to NO generated by endothelial, or neuronal NOS (Coney et al. 2004), even though the hypoxia-induced vasodilatation is largely NO dependent (Skinner & Marshall, 1996).
In view of these findings, the question arises as to whether systemic hypoxia influences the action of the sympathetic cotransmitters. In vitro studies indicate that ATP makes the major contribution to vasoconstriction evoked in arteries by single impulses or short trains at low frequency, while noradrenaline becomes important when the impulse train is longer or the frequency increases (Kennedy et al. 1986; Sjöblom-Widfelt & Nilsson, 1990). On the other hand, in vivo studies on rat hindlimb suggest that ATP and noradrenaline are mutually facilitatory in producing vasoconstriction evoked by sympathetic stimulation with low and high frequencies (Johnson et al. 2001). Further, in vivo studies on skeletal muscle indicate that sympathetic stimulation at constant high frequency, or with irregular activity containing high frequency bursts releases neuropeptide Y (NPY), while in various arterial preparations, high frequency patterns evoke NPY-mediated contstiction (Pernow et al. 1989; Morris & Gibbins, 1992).
Considering these transmitters, the fact that A1 or A2A adenosine receptor blockade had no effect on hypoxia-induced blunting of sympathetically evoked vasoconstriction (see above, Coney & Marshall, 2003) indicates that it is not caused by pre- or postsynaptic actions of adenosine formed from sympathetically released ATP (Ralevic & Burnstock, 1990). Hypoxia may affect the postsynaptic actions of ATP, but this is difficult to test in vivo in the absence of selective P2X receptor antagonists. Severe systemic hypoxia inhibits vasoconstriction evoked by noradrenaline infusion in rats (Coney & Marshall, 2003) and humans (Heistad & Wheeler, 1970). Moreover, graded hypoxia (PO2 100–40 mmHg) produced graded depression of contraction evoked by stimulation of α1-adrenoceptors in rat iliac and other arteries (Ebeigbe, 1982; Pearce et al. 1992; Franco-Obregon & Lopez-Barneo, 1996; Bartlett & Marshall, 2002). However, sympathetically evoked vasoconstriction in skeletal muscle is partly mediated by α2-adrenoceptors (Ohyanagi et al. 1991; Dinenno et al. 2002) and α2-adrenoceptor-evoked arteriolar constriction is particularly vulnerable to local hypoxia (Tateishi & Faber, 1995). Whether the α2-adrenoceptor component of sympathetic vasoconstriction is blunted by systemic hypoxia has not been examined. Further, there is little pharmacological evidence on the contribution of NPY to sympathetically evoked vasoconstriction in skeletal muscle in vivo (see Jackson et al. 2004).
Thus, we tested the hypotheses that severe systemic hypoxia blunts muscle vasoconstriction evoked by sympathetic chain stimulation with high and low frequency patterns chosen to mimic the range of MSNA that occur at rest and during reflex activation, by limiting the components that are mediated by α2-adrenoceptors and by NPY acting on Y1 receptors, the NPY receptors that are mainly responsible for inducing vasoconstriction (Modin et al. 1991).
Methods
Experiments were performed on two groups of male Wistar rats. All experiments were approved by UK legislation under the Home Office Animals (Scientific Procedures) Act 1986. Anaesthesia was induced with halothane (3.5% in O2) and judged to be of a surgical level when pedal withdrawal reflexes were absent. A jugular vein was then cannulated for continuous infusion of the anaesthetic Saffan (Schering-Plough Animal Health, Welwyn Garden City, UK) at 7–12 mg kg−1 h−1. Thereafter, the level of anaesthesia was judged to be adequate by the absence of withdrawal reflexes and stability of arterial blood pressure (ABP), as we have previously described (Coney & Marshall, 2003). At the end of the experiments all animals were killed by anaesthetic overdose.
The surgical preparation of the animal and the recording techniques were essentially as we have described before (Coney & Marshall, 2003). Briefly, the trachea was cannulated and the side-arm of the cannula was connected to a system of rotameters in a gas proportioner frame (CP Instruments, Hanwell, London, UK), allowing the rats to breathe either a normoxic (21% O2 in N2) or a hypoxic (8% O2 in N2) gas mixture, as described below. Both brachial arteries were cannulated, one being used to monitor arterial blood pressure (ABP), the other allowing 150 μl samples to be taken anaerobically for analysis by a blood gas analyser (IL1640; Instrumentation Laboratories, Warrington, Cheshire, UK): samples were taken in normoxia and during hypoxia following the period of sympathetic stimulation (see below). The caudal ventral tail artery was retrogradely cannulated to allow administration of drugs predominantly to the right hindlimb from which blood flow was recorded (see below). The left femoral vein was cannulated to allow administration of pharmacological antagonists.
A bipolar, silver-wire stimulating electrode was attached to the right lumbar sympathetic chain between L3 and L4 via a laparotomy, the great vessels being temporarily retracted to expose the sympathetic chain. The electrode tips were embedded in dental impression material (President, Light Body, Colténe, Switzerland) to both mechanically fix and electrically isolate them. The electrodes were used to deliver three different patterns of nerve stimulation at constant current via an isolated stimulator (DS2A; Digitimer, UK). The patterns delivered the same number of 1 ms pulses at a constant current of 1 mA in a 1 min period (see Coney & Marshall, 2003): (1) continuous stimulation at 2 Hz, (2) a 20 Hz burst for 1 s repeated every 10 s (bursts at 20 Hz), and (3) a 40 Hz burst for 0.5 s repeated every 10 s (bursts at 40 Hz). Each pattern resulted in 120 impulses being delivered over the 1 min stimulation period: they were delivered in random order.
Blood flow was recorded from the right femoral artery (FBF) via a perivascular flowprobe (0.7V; Transonic Systems, Ithaca, NY, USA) connected to a flowmeter (T106; Transonic Systems). ABP and FBF were acquired into Chart software (ADInstruments) via a MacLab/8 s (ADInstruments) at a sampling frequency of 100 Hz. Mean arterial pressure (MAP) and heart rate (HR) were derived on-line from the ABP signal, and femoral vascular resistance (FVR) was calculated on-line, by the division of ABP by FBF.
Protocols
Group 1: sympathetic stimulation before and after α2-adrenoceptor antagonism
The responses evoked by sympathetic stimulation with bursts at 40 and 20 Hz and by continuous stimulation at 2 Hz were tested in seven rats (body mass 218 ± 8 g: mean ±s.d.) during normoxia. The inspirate was then switched to hypoxia (8% O2) and at least 1 min after a new steady baseline had been achieved, the responses evoked by sympathetic stimulation were re-tested. The inspirate was then returned to normoxia and the animal allowed to recover for at least 10–15 min. The α2-adrenoceptor antagonist yohimbine (Sigma, UK) was administered intravenously at a dose of 0.5 mg kg−1 and following an equilibration period of 10–15 min, the responses to stimulation were re-tested under both normoxic and hypoxic conditions. In previous studies, this dose of yohimbine was shown to selectively and maximally inhibit α2-adrenoceptor-mediated sympathoinhibition (Szabo et al. 1993). In pilot studies this dose abolished the increase in ABP evoked by infusion of the α2-adrenoceptor agonist UK14304 (A. M. Coney & J. M. Marshall, unpublished observations).
Group 2: sympathetic stimulation before and during infusion of an NPY Y1 receptor antagonist
This protocol was performed in seven rats (mean ±s.d., body mass 213 ± 11 g). The protocol was essentially the same as for Group 1, except that when the responses to all three patterns of sympathetic stimulation had been tested in normoxia and during hypoxia, the animal was allowed to recover in normoxia for at least 10–15 min and then the NPY Y1 receptor antagonist BIBP 3226 (Sigma, UK) was infused through the caudal ventral artery at a rate of 10 μg kg−1 min−1. Responses evoked in normoxia, and in acute hypoxia were then re-tested as described above. This regime for administration of BIBP 3226 was shown in a previous study on the rat to selectively reduce the facilitation produced by NPY of peripheral vasoconstrictor responses evoked by α1-adrenoceptor stimulation (Bischoff et al. 1997). Further, in a pilot study on four rats, we found that administration of Leu-Pro NPY (a Y1 agonist) at 128 μg kg−1 increased ABP by 32 ± 3 mmHg. This was completely abolished by the above regime of BIBP 3226 administration.
Data analysis
All data are expressed as mean ±s.e.m. Data were analysed as described in Coney & Marshall (2003). Thus, FVR (mmHg ml−1 min) was computed on-line by the division of ABP by FBF and the size of the constrictor response to sympathetic stimulation was calculated by subtracting the integral of baseline FVR calculated from the 1 min preceding the stimulus, from the integral of FVR measured during the 1 min stimulus. The integrated constrictor response was expressed in arbitrary resistance units (RU). This allowed comparison between responses evoked by different patterns of stimulation as well as between responses evoked from different baseline values of FVR, i.e. during normoxia and hypoxia. Differences in baseline and in integrated FVR before and after each antagonist were determined by repeated measures ANOVA followed by Student–Newman–Keuls post hoc test if P < 0.05.
Results
Group 1
During normoxia, stimulation of the sympathetic chain with bursts at 40 Hz and 20 Hz and continuously at 2 Hz each evoked significant vasoconstriction in hindlimb muscle: integrated FVR increased by 3.25 ± 0.75, 3.71 ± 1.18 and 2.72 ± 0.83 RU, respectively. Continuous stimulation at 2 Hz tended to evoke smaller responses than bursts at 40 or 20 Hz, as previously reported, even though the same number of impulses was delivered in each case (see Fig. 1 and Coney & Marshall, 2003). These vasoconstrictor responses each resulted in a significant decrease in FBF (data not shown, see Coney & Marshall, 2003).
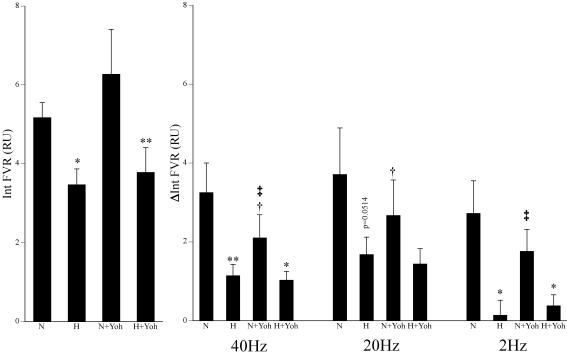
Figure 1. Effects of hypoxia and yohimbine on vasoconstrictor responses evoked in the hindlimb by different patterns of sympathetic stimulation.
Left panel shows baseline values of integrated femoral vascular resistance (FVR) over 1 min recorded immediately prior to sympathetic stimulation. Right panels indicate the responses evoked by the 3 patterns of sympathetic stimulation and show the mean ±s.e.m. of the change in integrated FVR (ΔInt FVR). Labels below columns indicate normoxia (N), hypoxia (H) and yohimbine (Yoh). Symbols indicate statistical significance of P < 0.05 (one symbol) and P < 0.01 (two symbols) where *=versus normoxia before or after yohimbine; †=versus before yohimbine in normoxia or hypoxia; and ‡=versus hypoxia before yohimbine.
Switching the inspirate to 8% O2 induced the expected fall in arterial PO2 (PaO2) with a fall in arterial PCO2 (PaCO2) and alkalosis, reflecting the hypoxia-induced hyperventilation (Table 1). Concomitantly, there was a significant fall in FVR indicating vasodilatation in hindlimb muscle, with FBF maintained despite the fall in ABP (Fig. 1, Table 2), as we have described before (see Coney & Marshall, 2003 and references therein). During hypoxia, the increase in FVR evoked by each pattern of sympathetic stimulation was attenuated as we have described before (Coney & Marshall, 2003), although the effect on the response evoked by bursts at 20 Hz just failed to reach statistical significance (P = 0.0514). The increase in FVR evoked by continuous stimulation at 2 Hz was reduced by a greater extent than those evoked by bursts at 20 and 40 Hz (by 96 versus 49% and 64%, respectively; see Coney & Marshall, 2003; Coney et al. 2004).
Table 1.
Arterial blood gas and pH values in all groups in different conditions
| PaO2 (mmHg) | PaCO2 (mmHg) | pH | ||
|---|---|---|---|---|
| Group 1 | Normoxia | 81.9 ± 3.2 | 38.4 ± 1.4 | 7.422 ± 0.013 |
| Hypoxia | 27.9 ± 1.0*** | 22.9 ± 1.3*** | 7.542 ± 0.014*** | |
| Normoxia + yohimbine | 93.3 ± 4.0 | 36.4 ± 2.2 | 7.428 ± 0.014 | |
| Hypoxia + yohimbine | 32.0 ± 1.8*** | 23.7 ± 1.8*** | 7.496 ± 0.035 | |
| Group 2 | Normoxia | 86.3 ± 4.1 | 39.3 ± 1.8 | 7.367 ± 0.023 |
| Hypoxia | 34 ± 1.3*** | 27.6 ± 1.1*** | 7.451 ± 0.015*** | |
| Normoxia + BIBP 3226 | 88.2 ± 3.1 | 38.2 ± 2.1 | 7.372 ± 0.031 | |
| Hypoxia + BIBP 3226 | 33.7 ± 1.6*** | 28.1 ± 1.3*** | 7.446 ± 0.012*** |
Values are mean ± s.e.m. Normoxia (air breathing) and hypoxia (breathing 8% O2) in the absence or presence of either yohimbine or BIBP 3226. Asterisks indicate statistical significance of P < 0.001 (***) compared to normoxia.
Table 2.
Baseline cardiovascular variables in all groups in different conditions
| ABP (mmHg) | HR (beats min−1) | FBF (ml min−1) | FVR (mmHg ml−1 min) | ||
|---|---|---|---|---|---|
| Group 1 | Normoxia | 106 ± 5 | 419 ± 19 | 1.26 ± 0.08 | 85.7 ± 6.1 |
| Hypoxia | 58 ± 6** | 407 ± 35 | 1.11 ± 0.20 | 57.8 ± 6.7* | |
| Normoxia + yohimbine | 100 ± 4 | 442 ± 17 | 1.25 ± 0.30 | 103.6 ± 18.6 | |
| Hypoxia + yohimbine | 62 ± 6** | 429 ± 29 | 1.24 ± 0.32 | 63.0 ± 10.6** | |
| Group 2 | Normoxia | 125 ± 5 | 451 ± 16 | 2.16 ± 0.37 | 69.6 ± 10.2 |
| Hypoxia | 74 ± 4*** | 488 ± 36 | 1.83 ± 0.18 | 42.4 ± 3.7* | |
| Normoxia + BIBP 3226 | 123 ± 6 | 481 ± 18 | 2.11 ± 0.18 | 61.6 ± 5.8 | |
| Hypoxia + BIBP 3226 | 77 ± 5** | 502 ± 24 | 2.22 ± 0.33 | 38.3 ± 4.2** |
Values are mean ± s.e.m. Normoxia (air breathing) and hypoxia (breathing 8% O2) in the absence or presence of either yohimbine or BIBP 3226. Asterisks indicate statistical significance of P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***) compared to normoxia. ABP, arterial blood pressure; HR, heart rate; FBF, femoral blood flow; FVR, femoral vascular resistance.
Administration of the α2-antagonist yohimbine had no effect on any of the cardiovascular baselines or blood gas values recorded in normoxia (Tables 1 and 2). Notably, baseline FVR did not change, suggesting no net role for α2-adrenoceptors in the maintenance of vascular tone in hindlimb (see Discussion). However, yohimbine significantly attenuated the vasoconstrictor responses evoked in normoxia by sympathetic stimulation with bursts at 20 Hz and 40 Hz, but not that evoked by continuous stimulation at 2 Hz.
When the inspirate was switched to 8% O2, the blood gas values were similar to those recorded in hypoxia before yohimbine (Table 1). Further, baseline ABP and FVR fell significantly to similar levels to those recorded in hypoxia before yohimbine (Table 2, Fig. 1). Under these conditions, the increases in FVR evoked by sympathetic stimulation with all three patterns were attenuated compared to those evoked in normoxia after yohimbine (Fig. 1). This did not reach statistical significance for the response to 20 Hz (P = 0.1) probably reflecting the variability of the responses evoked by bursts at 20 Hz in normoxia (see Fig. 1). Nevertheless the results indicated that yohimbine did not prevent an attenuating effect of hypoxia on the sympathetically evoked vasoconstrictor responses. There were no significant differences between the increase in FVR evoked by sympathetic stimulation in hypoxia after yohimbine compared to those evoked in hypoxia before yohimbine, i.e. hypoxia reduced the constrictor responses to the same levels before and after yohimbine (Fig. 1). On the other hand, the increases in FVR evoked by bursts at 40 Hz and continuous stimulation at 2 Hz in normoxia after yohimbine were significantly greater than those evoked during hypoxia before yohimbine (Fig. 1). In other words, hypoxia attenuated these vasoconstrictor responses to a greater extent than yohimbine.
Group 2
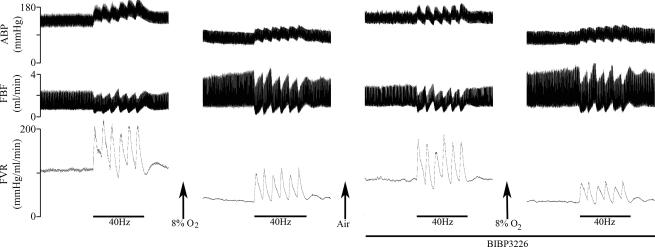
The blood gas values and baseline cardiovascular variables recorded during normoxia were similar to those of Group 1 (Tables 1 and 2). In normoxia, stimulation of the sympathetic chain with all three patterns of stimulation evoked increases in FVR that were similar to those evoked in Group 1. Moreover these responses were attenuated during hypoxia as in Group 1 (Fig. 3 cf. Fig. 1). Typical responses evoked by bursts at 40 Hz under the different experimental conditions are shown in Fig. 2.
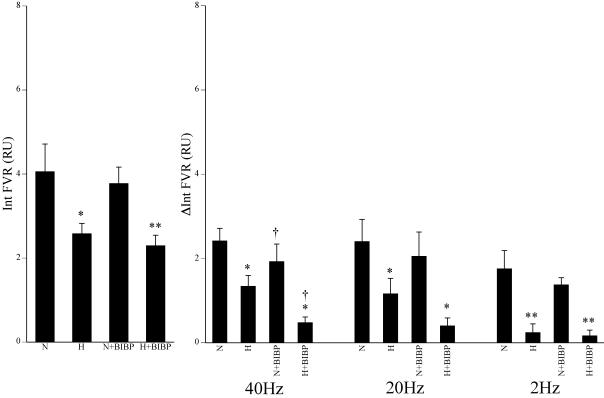
Figure 3. Effects of hypoxia and BIBP 3226 on vasoconstrictor responses evoked in the hindlimb by different patterns of sympathetic stimulation.
Left panel shows baseline values of integrated FVR over 1 min recorded immediately prior to sympathetic stimulation. Right panels indicate the responses evoked by the 3 patterns of sympathetic stimulation and show the mean ±s.e.m. of the change in integrated FVR (ΔInt FVR). Labels below the columns indicate normoxia (N), hypoxia (H) and BIBP 3226 (BIBP). Symbols indicate statistical significance of P < 0.05 (one symbol) and P < 0.01 (two symbols) where *=versus normoxia; †=versus before BIBP 3226 in normoxia or hypoxia.
Figure 2. Original recordings of responses evoked by the 40 Hz bursting pattern of sympathetic nerve stimulation during air breathing and systemic hypoxia in the absence and presence of the NPY antagonist BIBP 3226.
The periods of stimulation are indicated by bars under each response whilst the infusion of BIBP 3226 is indicated by the bar under the right panels. ABP, arterial blood pressure; FBF, femoral blood flow; FVR, femoral vascular resistance.
Infusion of the Y1 antagonist BIBP 3226 had no effect on the blood gas values or cardiovascular baselines (see Tables 1 and 2, Fig. 3). The constrictor responses evoked by the different patterns of sympathetic stimulation in normoxia were differentially affected by BIBP 3226. Thus, the increase in FVR evoked by bursts at 40 Hz was attenuated, while the increase in FVR evoked by bursts at 20 Hz and by continuous stimulation at 2 Hz was not different from that evoked before BIBP 3226 (Fig. 3).
Infusion of BIBP 3226 had no effect on the hypoxia-induced changes in blood gases, nor on the fall in baseline ABP and FVR (Tables 1 and 2, Fig. 3). Further, in the presence of BIBP 3226, hypoxia still reduced the increase in FVR evoked by each pattern of sympathetic stimulation (Fig. 3). However, the increase in FVR evoked by bursts at 40 Hz reached a lower level in hypoxia during BIBP 3226 infusion than it did in hypoxia before BIBP 3226 (Fig. 3). There was a trend for a similar effect of BIBP 3226 on the increase in FVR evoked in hypoxia by bursts at 20 Hz although this failed to reach statistical significance (P = 0.101). On the other hand the increase in FVR evoked by continuous stimulation at 2 Hz in hypoxia during BIBP 3226 infusion was similar to that evoked in hypoxia before the antagonist (Fig. 3).
Discussion
The present study confirmed that severe systemic hypoxia attenuates the increase in FVR evoked in the hindlimb by each of three different patterns of sympathetic stimulation: continuous stimulation at 2 Hz and bursts at 20 and 40 Hz (see Coney & Marshall, 2003; Coney et al. 2004). The new findings were (i) that the α2-adrenoceptor antagonist yohimbine had no effect on baseline FVR, but (ii) reduced the increases in FVR evoked by high frequency bursts of sympathetic stimulation in normoxia, whilst (iii) having no effect on the attenuation of the responses evoked by any of the three patterns of stimulation in hypoxia. On the other hand (iv) the Y1 receptor antagonist BIBP 3226 had no effect on baseline FVR, but (v) reduced the increase in FVR evoked in normoxia by high frequency bursts, and (vi) in hypoxia, these responses were reduced to a greater extent than they were in the absence of this antagonist.
Role of α2-adrenoceptors in hindlimb
A substantial component of the vasoconstrictor tone in rat hindlimb under conditions comparable to the normoxic condition of the present study is dependent on α-adrenoceptor stimulation (Johnson et al. 2001), in common with other studies on limb muscle (e.g. Kiowski et al. 1983; Jie et al. 1987; Taddei et al. 1988; Dinenno et al. 2002). Our new finding that the α2-adrenoceptor antagonist yohimbine had no effect on baseline FVR under normoxic conditions indicates that α2-adrenoceptor stimulation makes no overall contribution to resting tone in rat limb muscle in Saffan-anaesthetized rats. This may be because the noradrenaline that is tonically released from the sympathetic varicosities exerts a minimal vasoconstrictor effect by stimulating postjunctional α2-adrenoceptors. However, it may also be that the inhibitory effect of yohimbine on postjunctional α2-adrenoceptors is offset by its inhibitory effect on prejunctional α2-adrenoceptors whose stimulation inhibits noradrenaline release (Ruffolo et al. 1991). Another possibility concerns the location of the postjunctional receptors. Studies on the cremaster muscle of the rat, showed that the distal arterioles have α2- but not α1-adrenoceptors and constrictor responses evoked by sympathetic nerve stimulation were mediated entirely by α2-adrenoceptors; proximal arterioles have α1- and α2-adrenoceptors, but their responses to sympathetic stimulation were mediated by α1-adrenoceptors (Faber, 1988; Ohyanagi et al. 1991). Thus, if innervated α2-adrenoceptors are predominantly located on the distal arterioles of hindlimb muscle, it could be that tonically released noradrenaline constricts these arterioles, but removal of this influence has little effect on gross vascular resistance i.e. FVR.
In human forearm, an α2-adrenoceptor antagonist, whether given alone, or after an α1-adrenoceptor antagonist, suggested that α2-adrenoceptor stimulation is responsible for 50–60% of resting vascular tone (Dinenno et al. 2002). This apparent disparity between rat and human may reflect differences in the relative importance of pre- and postjunctional α2-adrenoceptors and/or in the contribution of the resistance of distal arterioles to gross vascular resistance. Alternatively, the patterning of MSNA may be different in resting human subjects and rats under Saffan anaesthesia and may influence the relative importance of α2-adrenoceptors.
That yohimbine reduced the increase in FVR evoked by sympathetic stimulation with high frequency bursts by ∼25–30% is consistent with this possibility. Assuming the innervated α2-adrenoceptors are located on the distal arterioles, our findings indicate that during high frequency bursts, noradrenaline causes substantial constriction of distal arterioles by stimulating α2-adrenoceptors and this exerts a substantial influence on FVR. Thus, it may be that the MSNA in the resting human subjects studied by Dinenno et al. (2002) was of a higher average frequency, or contained more high frequency bursts than in the Saffan-anaesthetized rat.
In a previous study on pithed rats with consequent low ABP, in which hindlimb muscle was perfused at constant flow, the increases in FVR evoked by sympathetic stimulation at frequencies of 2–4 Hz were potentiated by α2-adrenoceptor blockade, whereas those evoked by stimulation at higher frequencies up to 100 Hz were not affected (Medgett & Ruffolo, 1988). These results suggested a preferential influence of presynaptic α2-adrenoceptor stimulation at low frequencies, but revealed no constrictor role for postsynaptic α2-adrenoceptors at any frequency. It seems reasonable to suggest that the results of the present study on anaesthetized rats with an intact central nervous system supersede these earlier findings. Our finding that the depressive effect of yohimbine on the increase in FVR evoked by continuous stimulation at 2 Hz failed to reach statistical significance, may reflect the opposing influences of pre- and postsynaptic α2-adrenoceptor stimulation on this response. However, our findings on the effect of yohimbine on increases in FVR evoked by bursts add to the evidence that stimulation of postjunctional α2-adrenoceptors contributes to sympathetically evoked increases in muscle vascular resistance in humans (Bolli et al. 1983; Jie et al. 1984; Taddei et al. 1988), cats and dogs (Gardiner & Peters, 1982; Elsner et al. 1984).
Turning to systemic hypoxia, yohimbine had no effect on the hypoxia-induced fall in FVR. Moreover, in the presence of α2-adrenoceptor blockade, hypoxia still reduced the increases in FVR evoked by all three patterns of sympathetic stimulation relative to those evoked in normoxia, such that the remaining responses were comparable to those evoked in hypoxia in the absence of α2-adrenoceptor blockade (to ∼65, 50 and 15% of the control response for bursts at 40 and 20 Hz and continuous stimulation at 2 Hz, respectively). This finding clearly indicates that systemic hypoxia blunts a component of sympathetic vasoconstriction in muscle that is not mediated by α2-adrenoceptors. This may include the component mediated by α1-adrenoceptors, given this was blunted in rat iliac artery when PO2 was reduced below 70 mmHg (Bartlett & Marshall, 2002). However, our new finding also suggests that a substantial part of the blunting effect of systemic hypoxia on sympathetically evoked vasoconstriction is attributable to blunting of the α2-adrenoceptor-induced component in distal arterioles. If this were not the case, the vasodilatation induced by hypoxia per se would have been expected to be potentiated after yohimbine, for systemic hypoxia increases the average frequency of MSNA beyond 2 Hz and contains bursts of up to 50 Hz (Hudson et al. 2002). Moreover, the increases in FVR evoked by sympathetic stimulation in hypoxia would have been expected to be smaller than those evoked in hypoxia in the absence of α2-adrenoceptor blockade. In fact, the data suggest that blunting of the α2-adrenoceptor component explains ∼50% of the full effect of systemic hypoxia on the responses evoked by high frequency bursts and ∼30% of that evoked by continuous stimulation at 2 Hz (see Fig. 1). Clearly, this proposal contrasts with the conclusion of Dinenno et al. (2003) that systemic hypoxia does not blunt either the α1- or α2-adrenoceptor component of sympathetic vasoconstriction in human forearm. This disparity may be explained by the fact that the level of systemic hypoxia achieved in the present study was more severe (PaO2 35 versus 28 mmHg), or by the fact that Dinenno et al. (2003) stimulated the sympathetic fibres pharmacologically with tyramine which selectively releases noradrenaline from the varicosities: the effect of noradrenaline on its postsynaptic receptors may be very different to when electrical or reflex activation of the sympathetic fibres releases all three cotransmitters.
There is substantial evidence that the α2-adrenoceptor component of vasoconstriction is vulnerable to local factors. Thus, α2-mediated vasoconstriction of terminal arterioles in skeletal muscle in vivo was reported to be very sensitive to inhibition by acidosis and to reduced O2 delivery (McGillivray-Anderson & Faber, 1990; Muldowney & Faber, 1991), while α2-mediated constriction of muscle arterioles in vitro was attenuated by severe hypoxia (PO2: 10 mmHg, Tateishi & Faber, 1995). Further, the increase in vascular resistance evoked by infusion of an α2-adrenoceptor agonist in rat hindlimb was inhibited during muscle contraction (Thomas et al. 1994). Ours is the first study to indicate that α2-mediated vasoconstriction evoked by sympathetic nerve activity is blunted by systemic hypoxia.
The blunting of the α2-mediated constriction by hypoxia in muscle arterioles in vitro, and that caused by muscle contraction in vivo, has been attributed to the opening of ATP-sensitive K+ (KATP) channels on vascular smooth muscle (Tateishi & Faber, 1995; Thomas et al. 1997): KATP channel opening may impair α2-mediated influx of Ca2+ through voltage-sensitive Ca2+ channels (Ruffulo et al. 1991). We previously provided evidence that the opening of KATP channels, probably caused by locally released adenosine, contributes to the muscle vasodilatation of systemic hypoxia (Marshall et al. 1993). Thus, it is reasonable to propose that the opening of KATP channels underlies the blunting of the α2 component of sympathetic vasoconstriction in systemic hypoxia.
Role of NPY
That the selective NPY Y1 receptor antagonist BIBP 3226 had no effect on baseline FVR or ABP in normoxia indicates that NPY acting on Y1 receptors plays no significant role in maintaining resting vascular tone in hindlimb, or ABP in Saffan-anaesthetized rats. This is consistent with evidence that NPY does not contribute to maintenance of resting ABP in normotensive rats, or humans (Michel & Rascher, 1995; Zhao et al. 1997).
In contrast with these findings, Jackson et al. (2004) reported that BIBP 3226 caused an increase in baseline vascular conductance of hindlimb of anaesthetized rats that was equivalent to that induced by α1-adrenoceptor blockade, implying a major role for NPY in determining basal vascular tone. The discrepancy with the present results might be attributed to the fact that Jackson et al. (2004) used Sprague–Dawley rats whereas we used Wistars. However, it seems more likely that the explanation lies in the status of the cardiovascular system and this may, in turn, reflect the anaesthetic regime: Jackson et al. (2004) used barbiturate whereas we used the steroid anaesthetic Saffan (alphaxalone–alphadalone). The rats used by Jackson et al. (2004) were larger than those of the present study (256–426 g versus 218 and 213 g in Groups 1 and 2, respectively) and baseline levels of ABP were similar (103–106 versus 106 and 125 mmHg, respectively), and yet baseline FBF was considerably lower in the study of Jackson et al. (2004): ∼0.028–0.018 versus 1.26 and 2.16 ml min−1 in Groups 1 and 2, respectively. Certainly, recent evidence indicates that barbiturate anaesthesia induces a sympathetically mediated increase in hindlimb vascular resistance (Teranishi et al. 2000). This would be consistent with a higher average frequency of MSNA and/or more high frequency bursts under the baseline conditions of Jackson et al. (2004). If this were the case, their results would be consistent with the evidence that NPY becomes important as a vasoconstrictor in skeletal muscle and other vasculature when the frequency of sympathetic nerve activity is high (Pernow et al. 1989; Morris & Gibbins, 1992; Lundberg et al. 1994).
In further accord with this concept, BIBP 3226 reduced the increase in FVR evoked in normoxia by bursts at 40 Hz by ∼20%, tended to reduce that evoked by bursts at 20 Hz, but had no effect on the increase in FVR evoked by continuous stimulation at 2 Hz. This finding clearly agrees with evidence that exogenous NPY can produce vasoconstriction in cat and human skeletal muscle vasculature by stimulating Y1 receptors (Pernow & Lundberg, 1986; Pernow et al. 1987) and that NPY released by sympathetic stimulation evokes constriction in pig skeletal muscle by stimulating Y1 receptors (Lundberg & Modin, 1995). Studies on cat hindlimb muscle, involving intravascular pressure measurements at different levels of the vascular tree and on rat cremaster muscle by intravital microscopy, indicated that infused or topically applied NPY, respectively, evoked constriction of proximal and distal arterioles (Joshua, 1991; Ekelund & Erlinge, 1997). There has been no comparable study of the site(s) of action of NPY released from the sympathetic varicosities. Thus, the contribution of NPY we have identified in sympathetically evoked increases in FVR in normoxia may have reflected constrictor influences on proximal and/or distal arterioles.
Since in the presence of Y1 receptor blockade, systemic hypoxia still caused a further reduction in the increase in FVR evoked by high frequency bursts and continuous low frequency stimulation, this confirms that hypoxia blunts the non-NPY-mediated component of the vasoconstriction. However, the fact that in hypoxia, the increase in FVR evoked by bursts at 40 Hz was smaller in the presence of BIBP 3226 than in its absence, allows the novel proposal that the NPY-mediated component of this response is resistant even to the severe level of systemic hypoxia used in the present study. Since we have argued above that the constriction of distal arterioles that is evoked by high frequency bursts is mediated by α2-adrenoceptors and is blunted by systemic hypoxia, it seems likely that it was the NPY-induced constriction of the proximal arterioles that was resistant to the effects of hypoxia. However, it has to be acknowledged that our results may not fully describe the actions of NPY since it is possible that it may also act prejunctionally on Y2 receptors to inhibit neurotransmitter release.
Interactions between cotransmitters and functional implications
It is well established that the sympathetic cotransmitters facilitate the actions of one another (Ralevic & Burnstock, 1990; Morris & Gibbins, 1992). In accord with this, we recently showed that blockade of α1- and α2-adrenoceptors with phentolamine virtually abolished the increases in FVR evoked by a range of different patterns of sympathetic stimulation, while desensitization of the P2×1 purinergic receptors with αβ-methylene-ATP reduced these responses by as much as 40–60% (Johnson et al. 2001). Further, there is in vitro evidence that NPY given exogenously or released by sympathetic nerve stimulation facilitates the constrictor influence of both noradrenaline and ATP (Morris & Gibbins, 1992; Racchi et al. 1999). Thus, the inhibitory effects of yohimbine and BIBP 3226 on the increase in FVR evoked by high frequency bursts in normoxia cannot be taken to simply indicate the direct contributions of α2-adrenoceptor and Y1 receptor stimulation to these responses: either receptor antagonist may remove both the direct constrictor action of that receptor and the facilitatory influence of its stimulation on the response evoked by another transmitter. It is possible that yohimbine not only inhibited the α2-adrenoceptor component of distal arteriolar constriction, but also any constriction induced in these and proximal arterioles by nerve-released noradrenaline and/or ATP acting on α1-adrenoceptors and P2X receptors, respectively. Further, BIBP 3226 is likely to have inhibited the facilitatory influence of NPY on constriction evoked in proximal and distal arterioles by nerve-released noradrenaline acting on α1- and α2-adrenoceptors and by ATP acting on P2X receptors.
The fact that the effects of these two antagonists were differentially affected by severe systemic hypoxia allows important functional proposals. Thus, it seems likely that α2-adrenoceptor constriction of distal arterioles induced directly, or indirectly, by the high frequency bursts of sympathetic activity induced by systemic hypoxia per se and by additional inputs to the central nervous system, is blunted during severe systemic hypoxia. This would help to maximize the dilatation of distal arterioles caused by locally released adenosine and NO and accords with direct and indirect evidence that dilatation of distal arterioles of skeletal muscle during systemic hypoxia facilitates the distribution of the available O2 to the capillary network and allows muscle O2 consumption to be maintained (Mian & Marshall, 1991; Edmunds & Marshall, 2001a,b). On the other hand, if the direct and indirect influence of the NPY released by high frequency bursts of sympathetic activity on the proximal arterioles of muscle is preserved during severe systemic hypoxia, this would allow some sympathetic control over the vascular resistance of skeletal muscle to be retained. Indeed, in severe systemic hypoxia, as in sepsis, haemorrhage and cold stress (Zukowska-Grojec & Vaz, 1988; Qureshi et al. 1998), sympathetically released NPY may provide a crucial means of regulating muscle vascular resistance and therefore ABP.
Acknowledgments
This study was generously supported by the British Heart Foundation.
References
- Bartlett IS, Marshall JM. Analysis of the effects of graded levels of hypoxia on noradrenaline-evoked contraction in the rat iliac artery in vitro. Exp Physiol. 2002;87:171–184. doi: 10.1113/eph8702341. [DOI] [PubMed] [Google Scholar]
- Bischoff A, Freund A, Michel MC. The Y-1 antagonist BIBP 3226 inhibits potentiation of methoxamine-induced vasoconstriction by neuropeptide Y. Naunyn-Schmiedebergs Arch Pharmacol. 1997;356:635–640. doi: 10.1007/pl00005100. [DOI] [PubMed] [Google Scholar]
- Bolli P, Erne P, Kiowski W, Ji BH, Block LH, Buhler FR. Adrenaline-induced α2 adrenoceptor mediated vasoconstrictor response in normotensive subjects and in patients with essential hypertension. Clin Res. 1983;31:A843. [Google Scholar]
- Bryan PT, Marshall JM. Adenosine receptor subtypes and vasodilatation in rat skeletal muscle during systemic hypoxia: a role for A1 receptors. J Physiol. 1999;514:151–162. doi: 10.1111/j.1469-7793.1999.151af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coney AM, Bishay M, Marshall JM. Influence of endogenous nitric oxide on sympathetic vasoconstriction in normoxia, acute and chronic hypoxia in the rat. J Physiol. 2004;555:793–804. doi: 10.1113/jphysiol.2003.058156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coney AM, Marshall JM. Contribution of adenosine to the depression of sympathetically evoked vasoconstriction induced by systemic hypoxia in the rat. J Physiol. 2003;549:613–623. doi: 10.1113/jphysiol.2003.042267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional α-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002;540:1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ, Halliwill JR. Failure of systemic hypoxia to blunt α-adrenergic vasoconstriction in human forearm. J Physiol. 2003;549:985–994. doi: 10.1113/jphysiol.2003.042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeigbe AB. Influence of hypoxia on contraction and calcium uptake in rabbit aorta. Experimentia. 1982;38:935–937. doi: 10.1007/BF01953662. [DOI] [PubMed] [Google Scholar]
- Edmunds NJ, Marshall JM. Vasodilatation, oxygen delivery and oxygen consumption in rat hindlimb during systemic hypoxia: roles of nitric oxide. J Physiol. 2001a;532:251–259. doi: 10.1111/j.1469-7793.2001.0251g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds NJ, Marshall JM. Oxygen delivery and oxygen consumption in rat hindlimb during systemic hypoxia: role of adenosine. J Physiol. 2001b;536:927–935. doi: 10.1111/j.1469-7793.2001.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U, Erlinge D. In vivo receptor characterization of neuropeptide Y-induced effects in consecutive vascular sections of cat skeletal muscle. Br J Pharmacol. 1997;120:387–392. doi: 10.1038/sj.bjp.0700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner D, Saeed M, Sommer O, Holtz J, Bassenge E. Sympathetic vasoconstriction sensitive to α2-adrenergic receptor blockade – no evidence for preferential innervation of α1-adrenergic receptors in the canine femoral bed. Hypertension. 1984;6:915–925. doi: 10.1161/01.hyp.6.6.915. [DOI] [PubMed] [Google Scholar]
- Faber JE. In situ analysis of α-adrenoceptors on arteriolar and venular smooth muscle in rat skeletal muscle microcirculation. Circ Res. 1988;62:37–50. doi: 10.1161/01.res.62.1.37. [DOI] [PubMed] [Google Scholar]
- Franco-Obregon A, Lopez-Barneo J. Low PO2 inhibits calcium channel activity in arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 1996;271:H2290–H2299. doi: 10.1152/ajpheart.1996.271.6.H2290. [DOI] [PubMed] [Google Scholar]
- Gardiner JC, Peters CJ. Postsynaptic α1-adrenoceptor and α2-adrenoceptor involvement in the vascular responses to neuronally released and exogenous noradrenaline in the hindlimb of the dog and cat. Eur J Pharmacol. 1982;84:189–198. doi: 10.1016/0014-2999(82)90201-1. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sander M, Hald CF, Victor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J Physiol. 2000;527:387–396. doi: 10.1111/j.1469-7793.2000.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Wheeler RC. Effect of acute hypoxia on vascular responsiveness in man. J Clin Invest. 1970;49:1252–1263. doi: 10.1172/JCI106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson S, Johnson CD, Coney AM, Marshall JM. Changes in sympathetic nerve activity recorded from skeletal muscle arteries of the anaesthetized rat during graded levels of systemic hypoxia. J Physiol. 2002;544:28P. [Google Scholar]
- Jackson DN, Noble EG, Shoemaker JK. Y1- and α1-receptor control of basal hindlimb vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;287:R228–R233. doi: 10.1152/ajpregu.00723.2003. [DOI] [PubMed] [Google Scholar]
- Jie K, Vanbrummelen P, Vermey P, Timmermans PBMWM, Vanzwieten PA. Identification of vascular postsynaptic α1-adrenoceptors and α2-adrenoceptors in man. Circ Res. 1984;54:447–452. doi: 10.1161/01.res.54.4.447. [DOI] [PubMed] [Google Scholar]
- Jie K, Vanbrummelen P, Vermey P, Timmermans PBMWM, Vanzwieten PA. Postsynaptic α1-adrenoceptor and α2-adrenoceptor in human blood vessels – interactions with exogenous and endogenous catecholamines. Eur J Clin Invest. 1987;17:174–181. doi: 10.1111/j.1365-2362.1987.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Coney AM, Marshall JM. Roles of norepinephrine and ATP in sympathetically evoked vasoconstriction in rat tail and hindlimb in vivo. Am J Physiol Heart Circ Physiol. 2001;281:H2432–H2440. doi: 10.1152/ajpheart.2001.281.6.H2432. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Gilbey MP. On the dominant rhythm in the discharges of single postganglionic sympathetic neurons innervating the rat tail artery. J Physiol. 1996;497:241–259. doi: 10.1113/jphysiol.1996.sp021764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua IG. Neuropeptide Y-induced constriction in small resistance vessels of skeletal muscle. Peptides. 1991;12:37–41. doi: 10.1016/0196-9781(91)90163-j. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Saville VL, Burnstock G. The contributions of noradrenaline and ATP to the responses of the rabbit ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur J Pharmacol. 1986;122:291–300. doi: 10.1016/0014-2999(86)90409-7. [DOI] [PubMed] [Google Scholar]
- Kiowski W, Hulthen UL, Ritz R, Buhler FR. α2-Adrenoceptor-mediated vasoconstriction of arteries. Clin Pharmacol Ther. 1983;34:565–569. doi: 10.1038/clpt.1983.216. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Franco-Cereceda A, Lou YP, Modin A, Pernow J. Differential release of classical transmitters and peptides. Adv Second Messenger Phosphoprotein Res. 1994;29:223–234. doi: 10.1016/s1040-7952(06)80018-2. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Modin A. Inhibition of sympathetic vasoconstriction in pigs in vivo by the neuropeptide Y Y1 receptor antagonist BIBP3226. Br J Pharmacol. 1995;116:2971–2982. doi: 10.1111/j.1476-5381.1995.tb15952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurons in human muscle nerves. J Physiol. 1994;481:799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillivray-Anderson KM, Faber JE. Effect of reduced blood flow on α1-adrenoceptor and α2-adrenoceptor constriction of rat skeletal-muscle microvessels. Circ Res. 1990;69:165–173. doi: 10.1161/01.res.69.1.165. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Metcalfe JD. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol. 1988;407:385–403. doi: 10.1113/jphysiol.1988.sp017422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM, Thomas T, Turner L. A link between adenosine, ATP-sensitive K+ channels, potassium and muscle vasodilatation in the rat in systemic hypoxia. J Physiol. 1993;472:1–9. doi: 10.1113/jphysiol.1993.sp019931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medgett IC, Ruffolo RR. α2-Adrenoceptor-mediated vasoconstriction in rat hindlimb – innervated α2 adrenoceptors in the saphenous arterial bed. J Pharmacol Exp Ther. 1988;246:249–254. [PubMed] [Google Scholar]
- Mian R, Marshall JM. The roles of catecholamines in responses evoked in arterioles and venules of rat skeletal muscle by systemic hypoxia. J Physiol. 1991;436:499–510. doi: 10.1113/jphysiol.1991.sp018563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Rascher W. Neuropeptide Y – a possible role in hypertension. J Hypertens. 1995;13:385–395. [PubMed] [Google Scholar]
- Modin A, Pernow J, Lundberg JM. Evidence for two neuropeptide Y receptors mediating vasoconstriction. Eur J Pharmacol. 1991;203:165–171. doi: 10.1016/0014-2999(91)90711-x. [DOI] [PubMed] [Google Scholar]
- Morris JL, Gibbins IL. Co-transmission and neuromodulation. In: Burnstock G, Hoyle CHV, editors. Autonomic Neuroeffector Mechanisms. Switzerland: Harwood, Chur; 1992. pp. 33–119. [Google Scholar]
- Muldowney SM, Faber JE. Preservation of venular but not arteriolar smooth muscle α-adrenoceptor sensitivity during reduced blood flow. Circ Res. 1991;69:1215–1225. doi: 10.1161/01.res.69.5.1215. [DOI] [PubMed] [Google Scholar]
- Ohyanagi M, Faber JE, Nishigaki K. Differential activation of α1- and α2-adrenoceptors on microvascular smooth muscle during sympathetic nerve stimulation. Circ Res. 1991;68:232–244. doi: 10.1161/01.res.68.1.232. [DOI] [PubMed] [Google Scholar]
- Pearce WJ, Ashwal S, Long DM, Cuevas J. Hypoxia inhibits calcium influx in rabbit basilar and carotid arteries. Am J Physiol Heart Circ Physiol. 1992;262:H106–H113. doi: 10.1152/ajpheart.1992.262.1.H106. [DOI] [PubMed] [Google Scholar]
- Pernow J, Lundberg JM. Neuropeptide-Y constricts human skeletal muscle arteries via a nifedipine-sensitive mechanism independent of extracellular calcium. Acta Physiol Scand. 1986;128:655–656. doi: 10.1111/j.1748-1716.1986.tb08028.x. [DOI] [PubMed] [Google Scholar]
- Pernow J, Ohlen A, Hokfelt T, Nilsson O, Lundberg JM. Neuropeptide Y – presence in perivascular noradrenergic neurons and vasoconstrictor effects on skeletal muscle blood vessels in experimental animals and man. Regul Pept. 1987;19:313–324. doi: 10.1016/0167-0115(87)90173-x. [DOI] [PubMed] [Google Scholar]
- Pernow J, Schwieler J, Kahan T, Hjemdhal P, Oberle J, Wallin BG, Lundberg JM. Influence of sympathetic nerve discharge pattern on norepinephrine and neuropeptide Y-release. Am J Physiol Heart Circ Physiol. 1989;257:H866–H872. doi: 10.1152/ajpheart.1989.257.3.H866. [DOI] [PubMed] [Google Scholar]
- Qureshi NU, Dayao EK, Shirali S, Zukowska-Grojec Z, Hauser GJ. Endogenous neuropeptide Y mediates vasoconstriction during endotoxic and hemorrhagic shock. Regul Pept. 1998;75–76:215–220. doi: 10.1016/s0167-0115(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Racchi H, Irarrazabal MJ, Howard M, Moran S, Zalaquett R, Huidobro-Toro JP. Adenosine 5′-triphosphate and neuropeptide Y are co-transmitters in conjunction with noradrenaline in the human saphenous vein. Br J Pharmacol. 1999;126:1175–1185. doi: 10.1038/sj.bjp.0702396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Postjunctional synergism of noradrenaline and adenosine 5′-triphosphate in the mesenteric arterial bed of the rat. Eur J Pharmacol. 1990;175:291–299. doi: 10.1016/0014-2999(90)90567-p. [DOI] [PubMed] [Google Scholar]
- Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol. 2002;544:195–209. doi: 10.1113/jphysiol.2002.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB, Seals DR. Sympathetic activity during graded central hypovolemia in hypoxemic humans. Am J Physiol Heart Circ Physiol. 1990;259:H1197–H1206. doi: 10.1152/ajpheart.1990.259.4.H1197. [DOI] [PubMed] [Google Scholar]
- Ruffolo RR, Nichols AJ, Stadel JM, Hieble JP. Structure and function of α-adrenoceptors. Pharmacol Rev. 1991;43:475–505. [PubMed] [Google Scholar]
- Sjöblom-Widfeldt N, Gustafsson H, Nilsson H. Transmitter characteristics from small mesenteric arteries in the rat. Acta Physiol Scand. 1990;138:203–212. doi: 10.1111/j.1748-1716.1990.tb08834.x. [DOI] [PubMed] [Google Scholar]
- Skinner MR, Marshall JM. Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. J Physiol. 1996;495:553–560. doi: 10.1113/jphysiol.1996.sp021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Urban R, Starke K. Sympathoinhibition by rilmenidine in conscious rabbits – involvement of α2-adrenoceptors. Naunyn-Schmiedebergs Arch Pharmacol. 1993;348:593–600. doi: 10.1007/BF00167235. [DOI] [PubMed] [Google Scholar]
- Taddei S, Salvetti A, Pedrinelli R. Further evidence for the existence of α2-mediated adrenergic vasoconstriction in human vessels. Eur J Clin Pharmacol. 1988;34:407–410. doi: 10.1007/BF00542444. [DOI] [PubMed] [Google Scholar]
- Tateishi J, Faber JE. Inhibition of arteriole α2-adrenoceptor but not α1-adrenoceptor constriction by acidosis and hypoxia in vitro. Am J Physiol Heart Circ Physiol. 1995;268:H2068–H2076. doi: 10.1152/ajpheart.1995.268.5.H2068. [DOI] [PubMed] [Google Scholar]
- Teranishi Y, Tsuru H, Shimomura H, Amano T, Matsubayashi H. Compensatory vasoconstrictor effects of sodium pentobarbital on the hindquarters of conscious normotensive control and lumbar-sympathectomized Wistar rats. Auton Neurosci. 2000;82:130–136. doi: 10.1016/S0165-1838(00)00102-8. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of α2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol Heart Circ Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest. 1997;99:2602–2609. doi: 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson C, Coney AM, Marshall JM. Do prostanoids modulate sympathetically-evoked vasoconstriction in skeletal muscle in normoxia or hypoxia. FASEB J. 2005;19:A713. [Google Scholar]
- Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol. 2001;537:613–621. doi: 10.1111/j.1469-7793.2001.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XH, Sun XY, Edvinsson L, Hedner T. Does the neuropeptide Y Y1-receptor contribute to blood pressure control in the spontaneously hypertensive rat? J Hypertens. 1997;15:19–27. doi: 10.1097/00004872-199715010-00002. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Vaz AC. Role of neuropeptide Y (NPY) in cardiovascular responses to stress. Synapse. 1988;2:293–298. doi: 10.1002/syn.890020319. [DOI] [PubMed] [Google Scholar]