Abstract
Ca2+-stimulated adenylyl cyclases (AC) are known to play important roles in neurons but have not previously been reported in the heart. Here we present the first evidence for selective expression of Ca2+-stimulated AC in the sino-atrial node (SAN) but not in ventricular muscle of the guinea-pig heart. The AC1 isoform of Ca2+-stimulated AC was shown to be present in SAN, both as mRNA using RT-PCR and as protein using immuno-blotting with a specific antibody. Confocal immuno-fluorescence studies detected membrane localization of AC1 in SAN cells, but no AC1 in ventricular muscle. Ca2+-stimulated AC8 may also be present in SAN. The functional importance of AC activity was investigated by monitoring activation of If (gated by hyperpolarization and regulated by cAMP, which shifts activation to more depolarized voltages). Basal activity of AC in isolated SAN myocytes was demonstrated by the observations that an inhibitor of AC activity (MDL 12330A, 10 μm) shifted activation in the hyperpolarizing direction, while inhibition of phosphodiesterases (IBMX, 100 μm) shifted If activation in the depolarizing direction. Buffering cytosolic Ca2+ with the Ca2+ chelator BAPTA (by exposure to BAPTA-AM) shifted activation of If in the hyperpolarizing direction, and under these conditions the AC inhibitor MDL had little or no further effect. The actions of BAPTA were overcome by exposure to forskolin (10 μm), a direct stimulator of all AC isoforms, to restore cAMP levels. These effects are consistent with the functional importance of Ca2+-stimulated AC, which is expected to be fundamental to initiation and regulation of the heartbeat.
The second messenger cAMP has a fundamental role in cellular function. Cytosolic cAMP levels are in dynamic equilibrium between production by adenylyl cyclase (AC) and breakdown by phosphodiesterases. Elevated cAMP shifts the voltage dependence of If, increasing the contribution of this current to pacemaking (DiFrancesco & Tortora, 1991).
Acetylcholine activates an inhibitory G protein that suppresses AC activity. Acetylcholine has been found to inhibit If in sino-atrial node (SAN), but not in Purkinje fibres in the absence of β-adrenoceptor stimulation; however, upon β-adrenoceptor stimulation, acetylcholine reduces If in both preparations (DiFrancesco & Tromba, 1988a, b; Chang et al. 1990). The greater effect of acetylcholine in the SAN suggests higher basal activity of AC in the SAN than in Purkinje fibres. By a similar mechanism, acetylcholine inhibits ICaL in SAN in the absence of β-adrenoceptor stimulation, whereas in ventricular myocytes acetylcholine only reduces ICaL following β-adrenoceptor stimulation (Hescheler et al. 1986; Petit-Jacques et al. 1993). Again the observations indicate a significant resting production of cAMP by AC in SAN cells, but not in ventricular myocytes.
Both If in cardiac tissue and Ih in neurones are mediated by HCN proteins, being activated by hyperpolarization and regulated by cAMP (Beaumont & Zucker, 2000; Wang & Storm, 2003). Ih plays important roles in the control of pacemaker or bursting activity of the neurons in which the channel is expressed. All HCN channel proteins contain a carboxy terminal amino-acid motif which directly interacts with cAMP (Wainger et al. 2001), and hence If and Ih are regulated by cAMP without involvement of PKA. A substantial body of evidence has accumulated over many years showing that Ih can be regulated by Ca2+–calmodulin stimulated AC which in turn controls the cytosolic levels of cAMP; the Ca2+ concentration for half-maximal activation is 100 nm for AC1 and 500 nm for AC8 (Fagan et al. 1996). This study investigates whether a similar Ca2+-stimulated AC is present in cardiac tissue and if it regulates If via cAMP.
We have recently shown that If is regulated by cytosolic Ca2+ in guinea-pig SAN cells and speculated that a Ca2+-sensitive AC might be involved in this mechanism (Rigg et al. 2003). The effects of cytosolic Ca2+ on If involve calmodulin but not calmodulin-dependent protein kinase (CaMKII). In cardiac ventricular muscle, the predominant AC isoforms are AC5 and AC6 (Katsushika et al. 1992; Premont et al. 1992; Yoshimura & Cooper, 1992), with additional expression of some AC4 (Belevych et al. 2001). However, it is unknown whether different subtypes might be selectively expressed in SAN, although the differences in the resting activity of AC in SAN (Vinogradova et al. 2006) and ventricle (discussed above) suggest this as a possibility. The purpose of the experiments presented here was to test for selective expression of AC isoforms in SAN as compared with ventricular muscle, and to further investigate the function of this pathway in the control of If in SAN cells.
Methods
Isolation of cells and tissues
Male guinea-pigs (400–500 g) were killed humanely in accordance with the Animals (Scientific Procedures) Act 1986, using cervical dislocation subsequent to stunning, and the heart was rapidly excised. For RT-PCR and immuno-blotting, cardiac tissues were carefully dissected, weighed and immediately frozen in liquid nitrogen (stored at −80°C). Alternatively for patch clamp and immuno-fluorescence, ventricular, atrial and SAN cells were isolated as previously described (Rigg et al. 2000). The translucent SAN region has been found to be located on the upper surface of the right atrium, in between the inferior and superior vena cava (Rigg et al. 2000), immediately inside of the crista terminalis.
RT-PCR
Total RNA was prepared from tissues using an RNeasy Mini Kit (Qiagen). The RNA concentration was determined by spectrofluorimetry. RNA (5 μg) from each tissue was reverse-transcribed into single stranded cDNA using a Cloned AMV First Strand Synthesis kit (Invitrogen). As guinea-pig AC sequences were not available, primers were derived from conserved regions in the AC1, AC5 and AC8 mouse, rat, human and dog DNA sequences. These sequences were aligned using clustal W, and primer sequences were selected that should selectively recognize AC1, AC5 or AC8 across a variety of mammalian species. Primers for β-actin were designed in the same way. Primer sequences were as follows (all 5′–3′ direction): AC1 (FOR) ACAAGATTTACATCCAGA(A/C)GC (REV) ATGAGCTGCACCAGCAG(A/G)TA; AC8 (FOR) TGGTGTGATTTTGACAAGTCG (REV) CCCCAAATGTCATACTGTGGT; AC5 (FOR) GGAAAGAAGAGAAGGCCATGA (REV) AGCAGCACGCTGTAGGTGAA; β-actin (FOR) TTGTTACCAACTGGGACGACA (REV) ATCCTTCTCCACGGTTGGCCTT. Primers were ordered from Invitrogen. RT-PCR was performed using the Expand High Fidelity PCR System (Roche Diagnostics). The DNA products were visualized by agarose gel electrophoresis. The resulting DNA products were subcloned by TOPO cloning (Invitrogen) and the AC1 clone was fully sequenced by MWG Biotech.
Immuno-blotting
Tissue for immuno-blotting was homogenized in 0.1 m phosphate buffered saline (PBS) containing complete protease inhibitors (Roche). Aliquots were stored at −80°C. Protein concentration was measured using the Bicinchoninic acid (BCA) protein assay. Protein samples were resolved by SDS-PAGE with 50 μg protein per lane, and then transferred to nitrocellulose membrane for immunolabelling in sodium phosphate buffer (20 mm), pH 6.8 for 60 min at 50 V. To ensure that the separated bands of proteins were properly transferred to the nitrocellulose membrane, the membrane was incubated in 0.5% Ponceau Red 1% acetic acid for 1 min on the plate rocker to visualize the separated protein bands, and then the dye removed by washing in PBS/Tween-20 (0.5%). Blots were preblocked by incubation in blocking buffer (PBS, 5% non-fat dry milk and 0.5% Tween-20) overnight at 4°C. Primary antibodies raised against AC1, AC5 and AC8 (Santa Cruz) were diluted in blocking buffer and applied to the blots overnight at 4°C or at RT for 60 min. The blots were then washed (three 10 min washes) in PBS/Tween-20 (0.5%). The secondary peroxidase-conjugated antibodies (anti-rabbit for AC1 and AC5, anti-goat for AC8) were diluted 1: 10000 in blocking buffer, and applied to the blot for 60 min at RT. Blots were washed in PBS/Tween-20 (0.5%). Signal detection was carried out with ECL (GE Healthcare) and exposed to Kodak X-Omat LS film.
Immuno-fluorescence
Isolated cardiac cells were plated onto flamed coverslips and left to adhere for 15 min. Cells were fixed in 4% paraformaldehyde/PBS for 15 min, washed in PBS (3 changes, 10 min each), permeabilized using 0.1% Triton X-100 (Sigma-Aldrich) for 10 min, washed in PBS, blocked with PBS/10% normal goat serum for 60 min before being incubated with the primary antibody at 4°C overnight. The next day, cells were first washed with PBS before being incubated with secondary antibody at RT for 60 min (either AlexaFluor 488 conjugated goat anti-rabbit or Rhodamine Red-X conjugated rabbit anti-goat (Invitrogen), 1: 200 and 1: 400 dilution, respectively) then washed. Finally, coverslips were mounted using Vectashield® and permanently sealed. Cells were stored in the dark at 4°C and visualized within 2 days. For control experiments the primary antibody stage was omitted. Primary antibodies against AC1, AC5/6 and AC8 were used at a 1: 100 dilution. Observations were carried out using a Leica DMIRB inverted microscope modified for confocal laser-scanning microscopy (×63 water objective) and Leica TCSNT software. For detection of AlexaFluor 488, fluorescence excitation was at 488 nm with emission collected > 515 nm. An excitation filter of 568 nm and an emission filter at 600 ± 15 nm were used to detect Rhodamine Red-X fluorescence.
Electrophysiology
For electrophysiological recording, disturbance to the cytosol was minimized by using perforated patch techniques (with amphotericin in the pipette solution at 240 μg ml−1) and discontinuous voltage-clamp (Axoclamp 2B). Discontinuous (or ‘switched’) voltage-clamp involves rapid alternation of the function of a single microelectrode between voltage recording and current passing at a frequency of approximately 3 kHz. Since voltage is not measured at the same time that the current is passing through the electrode, this technique avoids problems that might arise if the ‘access resistance’ of the permeabilized patch electrode were to change during the experiment. Patch solutions contained (mm): KCl 150, MgCl2 5, K2ATP 1, Hepes 3, pH 7.2 with KOH. Superfusion solution contained (mm): NaCl 125, NaHCO3 25, KCl 5.4, NaH2PO4 1.2, MgCl2 1, CaCl2 1.8, glucose 5.5; gassed with 95% O2–5% CO2 to maintain a pH of 7.4 and heated to 36 ± 1°C. Conductance measurements were derived from If using a reversal potential of −19 mV (Rigg et al. 2003).
Data are expressed as means ± standard error of the mean. Statistical significance (P < 0.05) was assessed using Student's paired t test.
Results
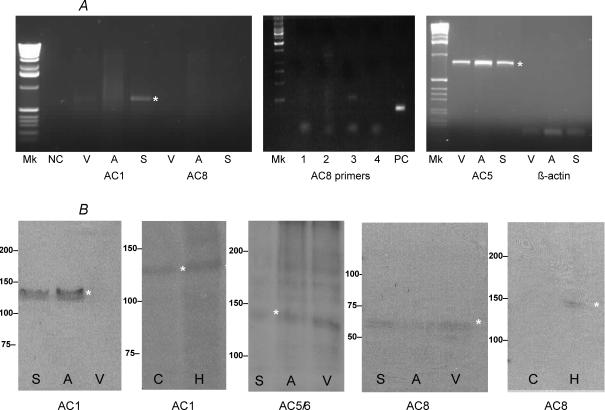
To determine whether the Ca2+-sensitive AC1 and AC8 subtypes are expressed in cardiac tissue at the mRNA level, we carried out RT-PCR with primers designed using sequence information derived from other species, since there were no guinea-pig AC sequences available. Primers were designed to recognize consensus regions common to a particular AC subtype from a variety of species, but that were not present in other AC subtypes. We also designed primers that would recognize guinea-pig AC5 and β-actin. RT-PCR was carried out on SAN, atrial and ventricular cardiac tissues. A PCR product of the expected size for AC1 was detected in the SAN (Fig. 1A). No clearly observable PCR product was detected in atria and ventricle. Other PCR primers (1–4) designed to recognize mammalian AC8 also failed to detect this subtype by RT-PCR (Fig. 1A), whereas β-actin acting as the positive control (PC) was detected. Products of the expected size for AC5 and β-actin were detected in SAN, atria and ventricle; however, none were detected corresponding to AC8 (Fig. 1A). The β-actin gene was used as a positive control in all experiments to check the mRNA produced for sample loss and equal loading of the wells. For the negative control (NC), the cDNA was replaced with nuclease-free water and no band was observed on the agarose gel. To confirm the identity of the AC1 PCR product, the DNA was subcloned and sequenced, and a BLAST search was undertaken which verified it as AC1. The accession number for the guinea-pig AC1 DNA sequence is EF184230.
Figure 1. RT-PCR and immuno-blot identify Ca2+ sensitive ACs in SAN cells.
A, RT-PCR of cardiac tissue: SAN (S), atrium (A) and ventricle (V), negative control (NC) and positive control (PC) with consensus primers designed to recognize AC1, AC8, AC5 and β-actin in other mammalian species. A range of different AC8 primers (1–4) were tested in the SAN without effect, despite PC finding the experiment to be effective. B, immuno-blot of cardiac tissue using polyclonal antibodies raised against peptide sequences in AC1, AC5/6 and AC8 common to other mammalian species. Brain extracts, cerebellum (C) and hippocampus (H), were also used to test for AC1 and AC8. Molecular mass markers are shown at the left. The asterisks draw attention to specific DNA or protein bands.
To further study the pattern of expression of Ca2+-sensitive AC1 and AC8 subtypes and to assess whether these isoforms were present at the protein level, protein extracts from cardiac (SAN, atrial and ventricular) and brain (cerebellum and hippocampus) tissue were analysed by immuno-blotting, using polyclonal antibodies specific to these isoforms. For AC1, a protein of the expected size (∼125 kDa) was detected in SAN and atrium but could not be detected in ventricular tissue (Fig. 1B), despite being present in hippocampus brain protein extracts. With AC5/6 antibody (raised against a peptide sequence common to both subtypes), a protein of the expected size (∼140 kDa) was detected in SAN, atria and ventricular tissue, confirming the RT-PCR observations (Fig. 1B). In contrast, for AC8 we failed to detect a protein of the expected size, observing only a faint band of ∼65–70 kDa in all three cardiac tissue subtypes, which is half the predicted mass of AC8 of ∼140 kDa (Fig. 1B). To confirm the ability of the anti-AC8 antibodies to recognize a protein of the expected molecular mass in other tissues, we also carried out immuno-blotting studies with brain protein extracts and detected a protein of the expected size in the hippocampus (Fig. 1B) but not in the cerebellum, in line with previous studies (Wong et al. 1999).
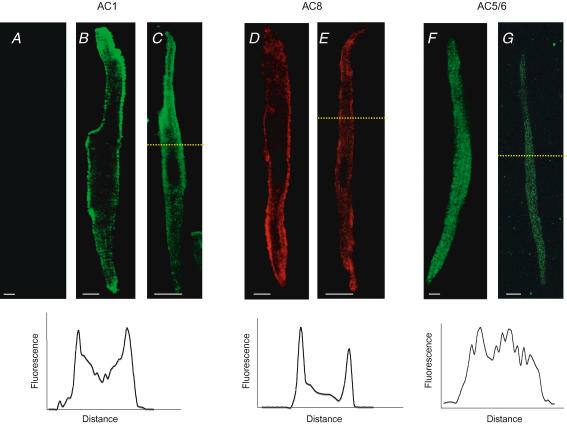
The same antibodies used to study AC1, AC5/6 and AC8 by immuno-blotting were used for immuno-fluorescence studies of SAN, and atrial and ventricular cardiomyocytes. Figure 2 shows the SAN cells (Fig. 2C, E and G) investigated to be very thin (the scale bar is 5 μm), with a central nucleus and tapered ends (spindle shaped). Immuno-fluorescence studies with anti-AC1 in ventricular cells found that AC1 was absent (Fig. 2A), reaffirming findings using RT-PCR (Fig. 1A) and immuno-blot (Fig. 1B). Unlike AC1 in ventricular cells, peripherally localized fluorescence was found in both atrial and SAN cells (Fig. 2B and C), with some diffuse cytosolic fluorescence as quantified in the profile (see inset). The distinct pattern of localization in SAN cells (similar to that of atrial cells) suggests association of AC1 with the plasma membrane. AC8 fluorescence was found to be similar to AC1 in atrial and SAN cells (Fig. 2D and E), localized to the periphery with diffuse cytosolic fluorescence. The immuno-fluorescence staining with anti-AC5/6 antibodies in SAN cells yielded a different pattern of localization (Fig. 2G).
Figure 2. Ca2+ sensitive AC1 and AC8 are present on SAN plasma membranes.
Localization of AC1, AC8 and AC5/6 in a single plane (z-axis resolution of 800 nm) of SAN cells using immuno-fluorescence and confocal microscopy. Ventricular, atrial and SAN cells were identified according to cellular morphology and size. Fluorescent labelling in ventricular (A), atrial (B, D and F) and SAN cardiac myocytes (C, E and G) and fluorescence intensity profile from SAN cells (at dashed yellow line, see inset below). Secondary antibody for AC1 and AC5/6 was AlexaFluor 488 while Rhodamine Red-X was used for AC8. Note the peripheral fluorescence in AC1 and AC8 in both atrial and SAN cells, presumably associated with the plasma membrane; however, no such staining was found in either cell type with AC5/6 or in ventricular cells with AC1. Scale bar is 5 μm.
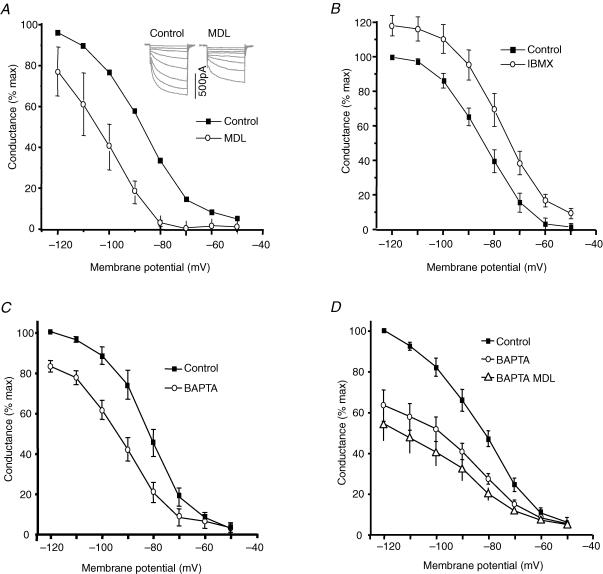
The functional importance of Ca2+-stimulated AC was investigated by testing for effects of changing cytosolic cAMP on If activated by hyperpolarization. Isolated SAN cells were voltage clamped and If was activated by a series of hyperpolarizations. Conductance was calculated using the reversal potential recorded under these experimental conditions (Rigg et al. 2003). Figure 3A shows that an inhibitor of AC (MDL 12330A (Merck Biosciences), 10 μm) shifted the activation curve for If by 17 mV in the hyperpolarizing direction (V1/2 shifted from −88.4 ± 1.8 to −105.2 ± 1.9 mV, n = 6, P < 0.05). Figure 3B shows that exposure to 100 μm 3-isobutyl-1-methylxanthine (IBMX) (Sigma Aldrich) shifted activation of If in the depolarizing direction (V1/2 shifted from −84.2 ± 0.4 to −77.7 ± 0.2 mV, n = 6 P < 0.05). Taken together, the observations in Fig. 3A and B are consistent with the suggestion that there is a resting activity of AC in these SAN cells, with the resting cAMP levels being increased (when IBMX inhibits the breakdown of cAMP) or decreased (when MDL reduces AC activity). We have previously shown that buffering cytosolic Ca2+ at a low level with BAPTA reduced If and shifted the activation curve in the hyperpolarizing direction (Rigg et al. 2003). These findings were repeated (Fig. 3C) and BAPTA was found to cause a 10 mV shift in If activation (5 min exposure to 5 μm BAPTA-AM (Invitrogen); V1/2 shifted from −81.3 ± 0.8 to −91.8 ± 0.9 mV, n = 10, P < 0.05). When the AC inhibitor MDL was added to cells that had been previously loaded with BAPTA (Figs 3D, n = 6), there was little or no further change in the V1/2 of If; −82.6 ± 0.3 (control), −87.9 ± 1.8 (BAPTA, P < 0.05) and −89.3 ± 1.5 mV (BAPTA and MDL, n.s. compared to BAPTA, P < 0.05 compared to control). These observations are consistent with the suggestion that both MDL and BAPTA have their primary effects through inhibition of AC and their effects are therefore not additive.
Figure 3. Effects of MDL and IBMX on basal AC activity: BAPTA and MDL inhibition of If are not additive.
Example If currents are shown inset. MDL (10 μm, ^) shifted activation (V1/2) in the hyperpolarizing direction (A); IBMX (100 μm, ^) shifted activation in the depolarizing direction (B); BAPTA (5 μm BAPTA-AM for 5 min, ^) shifted activation in the hyperpolarizing direction (C) and MDL (▵) had little or no further effect after BAPTA (D).
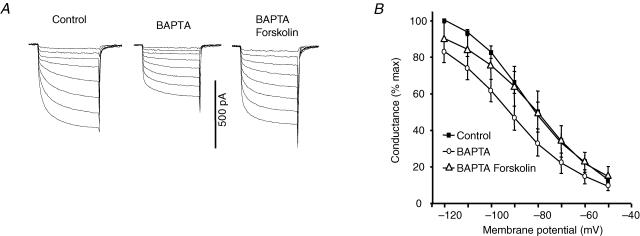
If the effects of BAPTA are indeed via inhibition of a Ca2+-stimulated AC when cytosolic Ca2+ is buffered at a low level, the effects on If would be due to lack of cAMP and these effects should be overcome by restoration of cAMP levels. We tested this using forskolin to directly activate AC (Seamon et al. 1983). Figure 4A shows example currents and Fig. 4B shows activation curves for If both in SAN cells loaded with BAPTA and in the same cells after exposure to forskolin (Sigma Aldrich). It can be seen that forskolin increased If in BAPTA loaded cells and shifted the activation curve nearly 10 mV in the depolarizing direction (−92.3 ± 0.4 to −84.6 ± 0.9 mV, n = 6, P < 0.05), so that the V1/2 with forskolin in the presence of BAPTA was similar to that under control conditions. Exposure to forskolin therefore overcame the effects of loading with BAPTA, consistent with the proposal that the effects of BAPTA resulted from inhibition of a Ca2+-sensitive AC.
Figure 4. Forskolin reverses BAPTA inhibition of If.
After superfusion of BAPTA-AM (5 μm for 5 min, ^), forskolin (10 μm) increased If (A, example records) and shifted activation in the depolarizing direction (▵), overcoming the effects of BAPTA (B).
Discussion
These observations provide the first evidence that Ca2+-stimulated AC is selectively expressed in SAN rather than ventricular muscle. AC1 mRNA was detected in SAN, but not ventricle, by RT-PCR and its identity verified by sequencing. The presence of AC1 protein in SAN was also demonstrated by immuno-blotting and immuno-fluorescence studies, with the latter approach indicating a localization of AC1 in SAN associated with the plasma membrane. Immuno-blotting also detected AC1 protein in atrial tissue. The discrepancy between this finding and the failure to detect AC1 mRNA in atrial tissue by RT-PCR remains to be explained, but could reflect the fact that mRNA and protein levels do not always correspond. Whether the other Ca2+-stimulated AC subtype (AC8) is also present in SAN is less clear. AC8 mRNA was not detected by RT-PCR (despite using a variety of primers) and immuno-blotting studies failed to detect the expected protein (although a 60–65 kDa protein was detected which could possibly be an AC8 breakdown product) with a range of AC8 antibodies. The consensus primers are derived from other mammalian species, so these primers may not be sufficiently specific to recognize guinea-pig AC8. However the AC8 antibody did bind to a protein in the plasma membrane of SAN. In the face of this contradictory evidence it remains to be shown whether AC8 is present in SAN. Further studies with more specific PCR primers and antibodies specifically designed to recognize guinea-pig AC8 will be required to address this issue. However, any uncertainty about the presence of AC8 does not detract from the very clear evidence supporting the presence of AC1 in SAN.
Experiments of Hagiwara & Irisawa (1989) first showed a role for cytosolic Ca2+ in regulating If: after rupture of the membrane beneath a patch electrode there was a reduction in If when the pipette Ca2+ concentration was 100 pm and an increase when the pipette contained 1 μm, consistent with exchange of the pipette solution for the cytosol and subsequent regulation of If. Our recent experiments (Rigg et al. 2003) provided further support for this hypothesis: If was reduced by chelating cytosolic Ca2+ with BAPTA and by calmodulin inhibitors (though these effects were not additive), but inhibition of CaMKII was without effect on If. We speculated that the effects of cytosolic Ca2+ on If might be mediated by calmodulin-dependent regulation of a Ca2+-sensitive AC (as in neurones; Wang & Storm, 2003), hence being independent of CaMKII regulation as described by Rigg et al. (2003). It seems that Ca2+-sensitive AC in the SAN provides a background production of cAMP even in the absence of β-adrenoceptor stimulation, since an inhibitor of AC (MDL) reduced If and an inhibitor of phosphodiesterase (IBMX) increased If, presumably by inhibiting the breakdown of basally produced cAMP.
Although the observations in BAPTA loaded myocytes are consistent with our proposal that the resting activity of AC in the absence of β-adrenoceptor stimulation is associated primarily with Ca2+-stimulated AC1 (though we cannot exclude AC8), we would expect other isoforms to be present that might contribute to the response to β-agonists. Our observation that forskolin can overcome the effects of BAPTA (in that foskolin reversed the shift in activation caused by BAPTA) is consistent with the suggestion that additional cAMP resulting from the actions of forskolin can overcome the effects of BAPTA. This argument does not depend on the subtypes of AC stimulated by forskolin. Forskolin was chosen as a suitable alternative to membrane permeant cAMP analogues since very high concentrations of these analogues have been found to be necessary to be effective in experiments on neurons (Beaumont & Zucker, 2000). We do not wish to imply any quantitative argument about contributions of subtypes under these conditions, but simply to show that the effects of BAPTA can be overcome with sufficient cAMP. In the presence of BAPTA, forskolin approximately returned If to its control level. However, it is likely that in the presence of forskolin there is a higher level of cAMP than under control conditions. This would be expected to have a greater effect on If than we observed. A potential explanation for this is that the cAMP produced in the presence of forskolin may be in a different microdomain and so may not have an effect on If. We cannot exclude additional unexpected actions of BAPTA, although our earlier observations concerning the non-additive effects of BAPTA and calmodulin anatagonists, and the non-additive effects of BAPTA and MDL reported here are consistent with a lack of major additional effects of BAPTA in this context, other than the predicted effect on AC1.
Exposure of SAN cells to 5 μm BAPTA-AM would be expected to result in accumulation of at least 150 μm cytosolic BAPTA. This estimate is based on our experience comparing Ca2+ probes loaded via the patch pipette with application of the AM-ester; in the case of fluo-3, fluo-4, indo-1 and indo-5F, approximately the same level of fluorescence observed with a known concentration of free acid in the patch pipette is achieved with a much lower concentration of AM-ester in the extracellular solution, so that an accumulation of cytosolic free acid of at least 30-fold greater than the concentration of AM-ester is expected under the conditions of our experiments. This concentration of BAPTA would be expected to reduce the cytosolic Ca2+ level in resting SAN cells from approximately 200 nm (Sanders et al. 2006) to 5.9 nm (calculated using Max C assuming cytosolic free Mg2+ of 0.7 mm). Reduction of cytosolic Ca2+ to this level would be expected to greatly reduce AC1 activity close to its minimal level since the Ca2+ concentration for half activation is approximately 100 nm (Fagan et al. 1996). Approaching this argument another way, similar calculations show that cytosolic Ca2+ would be kept below 10 nm (and therefore AC1 activity would be minimal) provided that at least 87 μm BAPTA were accumulated under the conditions of our experiments. It seems very likely that at least this much BAPTA is accumulated under the conditions of our experiments, and the observation that MDL had little or no further effect when If was first reduced with BAPTA provides further support for the proposal that reducing cytosolic Ca2+ suppressed AC activity. These data also show that the effects of MDL were unlikely to result from simple channel block.
As a further test that BAPTA was achieving the falls in cytosolic Ca2+ that we expect, we carried out a series of experiments on atrial myocytes loaded with the Ca2+ probe, indo-5F. Exposure of atrial cells to BAPTA-AM under similar conditions to those for the SAN experiments reported here caused the expected fall in indo-5F fluorescence ratio (n = 5 cells, P < 0.05). In addition, the minimum level for the fluorescence ratio after exposure to BAPTA-AM was not significantly different (P > 0.05) from Rmin (the minimal fluorescence ratio under calibration conditions when Ca2+ was buffered with EGTA). These observations show that under the conditions of the experiments reported here exposure of myocytes to BAPTA-AM caused a fall in cytosolic Ca2+ to levels which are as low as can be detected with indo-5F (see also Sanders et al. 2006).
The observations reported here also shed light on previous controversy concerning the role of If for pacemaker activity in the absence of β-adrenoceptor stimulation. Voltage-clamp experiments have generally found that the amounts of If at the most negative potential during pacemaker activity in SAN cells (approximately −60 to −65 mV) are very small, leading some to question whether If plays a significant role. However, inhibitors of If do reduce pacemaker activity in atrial preparations in the absence of β-adrenoceptor stimulation, and in isolated SAN cells under action potential clamp conditions block of If gives rise to a difference current that is consistent with a significant contribution of If (Zaza et al. 1997). It seems likely that the conventional voltage-clamp protocols used to record If (held at −40 mV and hyperpolarizing pulses are applied) may create conditions in which the resting level of Ca2+ is lower than it would be in a beating cell (see Sanders et al. (2006) for evidence that cytosolic Ca2+ may be higher in a beating than in a quiescent SAN cell). The levels of If may therefore be underestimated if the activity of a Ca2+-sensitive AC were reduced under these conditions. It should also be noted that during β-adrenoreceptor stimulation Ca2+ regulation of AC would be important, as ICaL would be increased, leading to enhanced Ca2+ entry and elevated cytosolic Ca2+, and hence Ca2+ stimulated AC enhancement of If. The focus of the present paper is regulation of If, but the presence of Ca2+ stimulated AC would also be expected to be important for other currents in the SAN. In particular, Ca2+ regulation of AC would be expected to influence the activity of PKA activated by cAMP. Targets of PKA include ICaL, IKs channels, ryanodine receptors, phosholamban and perhaps Na+–Ca2+ exchange (NCX). These possibilities are beyond the scope of the present investigation but will be the subject of future studies.
In summary, the present experiments demonstrate the presence of Ca2+-stimulated AC1 in SAN but not ventricular muscle and the observations are consistent with the functional importance of this Ca2+-stimulated AC in regulating If. The Ca2+-stimulated AC is expected to be a major new player in initiation and regulation of the heartbeat.
Acknowledgments
Contributors to this research were funded by The Wellcome Trust, British Heart Foundation and the Medical Research Council.
References
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3:133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- Belevych AE, Sims C, Harvey RD. ACh-induced rebound stimulation of L-type Ca2+ current in guinea-pig ventricular myocytes, mediated by Gβγ-dependent activation of adenylyl cyclase. J Physiol. 2001;536:677–692. doi: 10.1111/j.1469-7793.2001.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Gao J, Tromba C, Cohen I, DiFrancesco D. Acetylcholine reverses effects of β-agonists on pacemaker current in canine cardiac Purkinje fibers but has no direct action. A difference between primary and secondary pacemakers. Circ Res. 1990;66:633–636. doi: 10.1161/01.res.66.3.633. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Tromba C. Inhibition of the hyperpolarization-activated current (If) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol. 1988a;405:477–491. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D, Tromba C. Muscarinic control of the hyperpolarization-activated current (If) in rabbit sino-atrial node myocytes. J Physiol. 1988b;405:493–510. doi: 10.1113/jphysiol.1988.sp017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan KA, Mahey R, Cooper DM. Functional co-localization of transfected Ca2+-stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J Biol Chem. 1996;271:12438–12444. doi: 10.1074/jbc.271.21.12438. [DOI] [PubMed] [Google Scholar]
- Hagiwara N, Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J, Kameyama M, Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986;407:182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Katsushika S, Chen L, Kawabe J, Nilakantan R, Halnon NJ, Homcy CJ, Ishikawa Y. Cloning and characterization of a sixth adenylyl cyclase isoform: types V and VI constitute a subgroup within the mammalian adenylyl cyclase family. Proc Natl Acad Sci U S A. 1992;89:8774–8778. doi: 10.1073/pnas.89.18.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Jacques J, Bois P, Bescond J, Lenfant J. Mechanism of muscarinic control of the high-threshold calcium current in rabbit sino-atrial node myocytes. Pflugers Arch. 1993;423:21–27. doi: 10.1007/BF00374956. [DOI] [PubMed] [Google Scholar]
- Premont RT, Chen J, Ma HW, Ponnapalli M, Iyengar R. Two members of a widely expressed subfamily of hormone-stimulated adenylyl cyclases. Proc Natl Acad Sci U S A. 1992;89:9809–9813. doi: 10.1073/pnas.89.20.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigg L, Heath BM, Cui Y, Terrar DA. Localisation and functional significance of ryanodine receptors during β-adrenoceptor stimulation in the guinea-pig sino-atrial node. Cardiovasc Res. 2000;48:254–264. doi: 10.1016/s0008-6363(00)00153-x. [DOI] [PubMed] [Google Scholar]
- Rigg L, Mattick PA, Heath BM, Terrar DA. Modulation of the hyperpolarization-activated current (If) by calcium and calmodulin in the guinea-pig sino-atrial node. Cardiovasc Res. 2003;57:497–504. doi: 10.1016/s0008-6363(02)00668-5. [DOI] [PubMed] [Google Scholar]
- Sanders L, Rakovic S, Lowe M, Mattick PA, Terrar DA. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J Physiol. 2006;571:639–649. doi: 10.1113/jphysiol.2005.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon KB, Daly JW, Metzger H, de Souza NJ, Reden J. Structure-activity relationships for activation of adenylate cyclase by the diterpene forskolin and its derivatives. J Med Chem. 1983;26:436–439. doi: 10.1021/jm00357a021. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 2001;411:805–810. doi: 10.1038/35081088. [DOI] [PubMed] [Google Scholar]
- Wang H, Storm DR. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol Pharmacol. 2003;63:463–468. doi: 10.1124/mol.63.3.463. [DOI] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Cooper DM. Cloning and expression of a Ca2+-inhibitable adenylyl cyclase from NCB-20 cells. Proc Natl Acad Sci U S A. 1992;89:6716–6720. doi: 10.1073/pnas.89.15.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaza A, Micheletti M, Brioschi A, Rocchetti M. Ionic currents during sustained pacemaker activity in rabbit sino-atrial myocytes. J Physiol. 1997;505:677–688. doi: 10.1111/j.1469-7793.1997.677ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]