Abstract
We describe a new assay to determine the fraction of cardiac Na+–Ca2+ exchangers (NCX1) in the surface membrane of cells (Fsurf). An extracellular NCX1 disulphide bond is rapidly reduced by tris(2-carboxyethyl)phosphine hydrochloride (TCEP), cysteines are ‘PEGylated’ by alkylation with an impermeable conjugate of maleimide and a 5000 MW polyethylene glycol (MPEG), and Fsurf is quantified from Western blots as the fraction of NCX1 that migrates at a higher molecular weight. Fsurf remains less than 0.1 when NCX1 is expressed via transient transfections. Values of 0.15–0.4 are obtained for cell lines with stable NCX1 expression, 0.3 for neonatal myocytes and 0.6–0.8 for adult hearts. To validate the assay, we analysed an intervention that promotes clathrin-independent endocytosis in fibroblasts. Using BHK cells, removal of extracellular potassium (K+) caused yellow fluorescent protein (YFP)-tagged NCX1 to redistribute diffusely into the cytoplasm within 30 min, Fsurf decreased by 35%, and whole-cell exchange currents decreased by > 50%. In both HEK 293 and BHK cell lines, expression of human hPIP5Iβ kinase significantly decreases Fsurf. In BHK cells expressing M1 receptors, a muscarinic agonist (carbachol) causes a 40% decrease of Fsurf in normal media. This decrease is blocked by a high wortmannin concentration (3 μm), suggesting that type III phosphatidylinositol-4-kinase (PI4K) activity is required. As predicted from functional studies, carbachol increases Fsurf when cytoplasmic Ca2 is increased by removing extracellular Na+. Phorbol esters are without effect in BHK cells. In intact hearts, interventions that change contractility have no effect within 15 min, but we have identified two long-term changes. First, we analysed the diurnal dependence of Fsurf because messages for cardiac phosphatidylinositol-4-phosphate (PIP) 5-kinases increase during the light phase in entrained mice (i.e. during sleep). Cardiac phosphatidylinositol-(4,5)-bis-phosphate (PIP2) levels increase during the light phase and Fsurf decreases in parallel. Second, we analysed effects of aortic banding because NCX1 currents do not mirror the increases of NCX1 message and protein that occur in this model. Fsurf decreases significantly within 10 days, and cardiac PIP and PIP2 levels are significantly increased. In summary, multiple experimental approaches suggest that PIP2 synthesis favours NCX1 internalization, that NCX1 internalization is probably clathrin-independent, and that significant changes of NCX1 surface expression occur physiologically and pathologically in intact myocardium.
Innumerable physiological processes are under the control of membrane trafficking processes. A few examples are water reabsorption in the kidney (Knepper & Inoue, 1997; Valenti et al. 2005), acid secretion in the stomach (Yao & Forte, 2003), and probably long-term potentiation and depression (Malenka, 2003; Malenka & Bear, 2004; Massicotte & Baudry, 2004). Furthermore, genetic diseases, such as cystic fibrosis (Bertrand & Frizzell, 2003; Amaral, 2005) and some long QT syndromes in the heart (Thomas et al. 2003), are caused at least in part by changes of membrane trafficking that occur when the trafficked cargos are mutated. Cardiac myocytes are known to contain internal membrane storage depots only for glucose (Becker et al. 2001) and fatty acid transporters (Chabowski et al. 2006). Nevertheless, the removal of transporters and channels from the sarcolemma will inevitably influence their abundance in the surface membrane. This article addresses the internalization of cardiac Na+–Ca2+ exchangers (NCX1) in the context of increasing interest in cardiac membrane trafficking, as highlighted briefly in this Introduction.
It was a great surprise to learn that cardiac connexin turnover happens within about an hour (Beardslee et al. 1998), rather than days. Accordingly, it can be expected that regulation of connexin trafficking will be equally important to regulation of connexin gating for physiology. While the abundance of cardiac glucose (GLUT) transporters in the sarcolemma increases with cardiac hypoxia and/or ischaemia (Becker et al. 2001), connexins undergo internalization and movement into a degradation pathway (Laing & Beyer, 1995; Vetterlein et al. 2006). Recent work suggests that cardiac KATP channels can be internalized in a protein kinase C (PKC)-dependent fashion in myocytes (Hu et al. 2003), possibly also in response to ischaemia that results in activation of several PKCs (Armstrong, 2004). The Kv4.3 channels appear to be internalized from myocytes in response to angiotensin, probably as a macromolecular complex with the receptor (Doronin et al. 2004). And finally, the HERG-type K+ channels appear to be internalized in response to cell signalling pathways activated by heart glycosides (Wang et al. 2006), perhaps coupling to their ubiquitin-dependent degradation (Chapman et al. 2005), and raising important new questions about the potential coupling of ion transporters to cell signalling.
Relatively little is known about the trafficking of cardiac ion transporters, although a rich literature suggests that Na+–K+ pumps are extensively regulated by trafficking in non-cardiac cells. Among the most impressive results, ischaemia causes extensive internalization of pumps and other membrane proteins, especially in polarized epithelial cells (Doctor et al. 2000; Woroniecki et al. 2003). In skeletal muscle, insulin appears to promote pump insertion (Al-Khalili & Chibalin, 2003) as does cyclic stretch (Yuan et al. 2006). Cell swelling results in large increases of pump activity in cardiac (Takeuchi et al. 2006) and skeletal (Venosa, 2003) myocytes, as well as epithelia (Vinciguerra et al. 2003). A cytoskeletal dependence of this effect and a large increase of maximal ouabain binding suggest that pumps are being inserted (Manery et al. 1977; Venosa, 2003). In alveolar epithelial cells, pumps can be inserted in response to PKA activation in a path that requires small G proteins (Lecuona et al. 2003). The importance of small G proteins for pump trafficking, Rho in particular, was indicated earlier in oocytes (Schmalzing et al. 1995), where phorbol esters cause large-scale internalization of Na+–K+ pumps (Khan et al. 1991). It remains to be established how and if these results are relevant to heart. But given the fundamental importance of cardiac Na+ homeostasis for Ca2+ homeostasis, via Na+–Ca2+ exchange, it seems unlikely that transporter internalization would not be subject to complex control mechanisms.
For cardiac Na+–Ca2+ exchangers very little is known. In cell cultures, cyclosporin (but not FK506) is reported to decrease surface expression (Rahamimoff et al. 2002). In myocytes, antisense RNA targeted for NCX1 results in loss of NCX1 activity before NCX1 protein is lost (Egger et al. 2005). These latter results suggest that different ‘pools’ of NCX1 exist in neonatal myocytes. The ‘functional’ NCX1 pool would appear to turn over more rapidly than the bulk NCX1 pool. Multiple groups have suggested that Na+–K+ pumps and Na+–Ca2+ exchangers interact physically in the cardiac sarcolemma, based on pull-downs (Dostanic et al. 2003; Mohler et al. 2005) and immunohistochemical co-localization (Moore et al. 1993). If such interactions indeed occur, it becomes of great interest to know when, during the transporter lifetime, such interactions form and how they may affect, possibly even control, trafficking of the interacting partners.
As a first step to begin to address these issues for NCX1, we have developed a new assay to determine the fraction of Na+–Ca2+ exchangers that are located to the surface of cells (Fsurf). The assay is based on the presence of an extracellular disulphide bond in NCX1 that can be readily reduced (Santacruz-Toloza et al. 2000). The free cysteines can then be rapidly reacted with a polyethylene glycol–maleimide reagent that is of high enough molecular weight, 5000, to induce a significant shift on Western blots of the reacted protein, while still small enough to readily permeate the walls of cardiac capillaries. Using this assay in parallel with other measurements, we present evidence that NCX1 internalization is promoted by PIP2 synthesis, high cytoplasmic Na+, activation of Gq-coupled receptors, and metabolic stress. Also, we demonstrate that in intact heart, changes of Fsurf are occurring physiologically in a diurnal fashion and pathologically during the cardiac response to aortic banding.
Methods
All animal protocols used in this study were approved by the University of Texas Southwestern Institutional Animal Care and Use Committee. Animals were killed by i.p. injection of Euthasol (Virbac AH, Inc., Fort Worth, TX, USA), 100 mg (kg body weight)−1.
Unless indicated otherwise, cell lines, myocyte preparation, patch clamp, and lipid analyses were as described in the preceding article (Yaradanakul et al. 2007). The DNA construct for NCX1-358-YFP fusion protein (Ottolia et al. 2007) was provided by Dr K. D. Philipson (UCLA, Los Angeles, CA, USA). Fluorescent NCX1 was expressed in BHK cells by transient transfection as described (Yaradanakul et al. 2007).
Cell lines
T-REx 293 cells (Invitrogen) were cultured in DMEM supplemented with 10% FBS, 2 mm l-glutamine (Invitrogen), 100 U ml−1 penicillin, 100 μg ml−1 streptomycin (Invitrogen), and maintained with 15 μg ml−1 blasticidin (InvivoGen), and 100 μg ml−1 zeocin (Invitrogen).
Plasmid construction
The hPIP5KIβ cDNA was generated by PCR using pCMV5-PIP5KIβ (Dr Helen Yin, UTSouthwestern) as template and the following oligonucleotide primers: 5′-CTT AAG ATG GAG CAG AAG CTG AT-3′ and 5′-GAT ATC CTC GAG TTA TAA ATA GAC GTC C-3′. The primers incorporated Afl II and Xho I sites. The sequence of the hPIP5KIβ cDNA was confirmed. This PCR product was then cloned into inducible expression vector pcDNA5/FRT/TO (Invitrogen) to generate pcDNA5/FRT/TO-hPIP5KIβ plasmid. Human M1 muscarinic receptor was cloned into pcDNA3.1/Hygro(+) vector (Invitrogen), named pcDNA3.1/hg-hM1, by digestion of hM1pcDNA3.1 (Dr Mark Shapiro, UTSA, San Antonio, TX, USA) with BamH I and Xho I and then subcloned into pcDNA3.1/Hygro(+).
Double stable cell line production
T-REx 293 cells were transfected with a plasmid containing bovine cardiac NCX1 (Dr J. P. Reeves; UMDNJ, Newark, NJ, USA) using SuperFect transfection reagent (Qiagen), and followed by selection with 400 μg ml−1 of G418 (Sigma) to generate the T-REx 293 NCX1.1 cell line. The T-REx 293 NCX1.1 cells were then cotransfected with pcDNA5/FRT/TO-PIP5KIβ and pOG44 according to manufacturer-suggested procedures (Invitrogen Flp-In T-REx system). After transfection, cells were selected with 200 μg ml−1 of hygromycin B (InvivoGen) and 15 μg ml−1 of blasticidin (InvivoGen). Expression of hPIP5kIβ in the stable cell line was induced by addition of doxycycline (1 μg ml−1) to the culture medium for 24 h.
BHK-NCX-hM1 double cell line
The BHK-NCX cell line (Linck et al. 1998) was transfected with pcDNA3.1/hg-hM1 and selected with 400 μg ml−1 of hygromycin B (Sigma).
PEGylation of cultured cells
Baby-hamster kidney fibroblast (BHK) and human embryonic kidney (HEK 293) cells were grown in 6 cm culture dishes to near confluence. Immediately prior to the experiments serum-free Dulbecco's modified Eagle's medium (DMEM) solutions were prepared at 4°C with tris(2-carboxyethyl)phosphine hydrochloride (TCEP, 6 mm; pH adjusted to 7.0) and molecular weight 5000 PEG-maleimide (MPEG, 5 mm; Nektar Therapeutics, Inc., Huntsville, AL, USA). When ion or glucose concentrations were changed, a physiological saline solution was employed with 140 mm NaCl, 6 mm KCl, 1.0 mm CaCl, 1.0 mm MgCl2, 1.0 NaPO4 and 15 mm Hepes (pH 7.4). After treating cells as described in Results, the solution was aspirated, dishes were placed on ice, and the TCEP-containing solution (2 ml, 4°C) was applied for 12 min with intermittent agitation. Cells were then washed twice with cold serum-free DMEM, and the MPEG-containing solution (0.6 ml, 4°C) was applied for 30 min with intermittent agitation. Cells were again washed twice, lysis buffer (200 μl with protease inhibitor cocktail (Roche) and 5 mmN-ethylmaleimide (NEM) was applied, and cells were removed to microtubes. After agitation for 10 min on ice, the lysate was centrifuged for 10 min at 16 000 g and the supernatant was collected for Western blotting. We found that both the reduction of the NCX1 disulphide and the PEGylation were quite strongly temperature dependent. While reaction times of about 15 min are required at 4°C, as described in Results, 6 min reaction times for both reduction and PEGylation were adequate to achieve nearly complete reactions at room temperature.
PEGylation of perfused hearts
Murine hearts were isolated and perfused retrogradely at a flow rate of approximately 3 ml min−1 with solution containing 140 mm NaCl, 4 mm KCl, 10 mm Hepes, 0.5 mm Na2HPO4, 1 mm MgCl2, 15 mm glucose, and 1.5 mm CaCl2 at pH 7.4, saturated with 100% oxygen at 37°C. After a 10 min equilibration period, followed by a protocol described in Results, perfusion was switched to 23°C. Under these conditions, we determined that reaction times of 5 min were adequate to achieve nearly maximal PEGylation. Using the same perfusion solution, TCEP (6 mm) was perfused for 6 min followed by a 2 min wash and perfusion of MPEG (5 mm) for 6 min. To minimize chemical use, the MPEG solution was recycled twice during 6 min. Thereafter, the reaction was stopped with perfusion solution containing l-cysteine (5 mm) for 5 min. The hearts were then flash-frozen with aluminium block clamps that were pre-cooled in liquid nitrogen, and tissue samples were stored at −80°C before processing. The frozen tissue was powdered with a mortar and pestle that were pre-cooled in liquid nitrogen, and the powder was dissolved in RIPA high salt lysis buffer (150 mm NaCl, 1% NP40, 0.5% deoxycholate, 0.1% SDS, 50 mm Tris-HCl at pH 8.0, 5 mm NEM, protease inhibitor cocktail). After vortexing, the samples were placed on ice for 15 min, centrifuged at 16 000 g for 10 min, and the supernatants were collected and protein concentration determined. Note that the mouse line developed to overexpress PI4K2α in cardiac myocytes (Yaradanakul et al. 2007) could not be analysed because it was terminated for economic reasons before this assay was developed.
Western blotting
Protein concentrations were determined by MicroBCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA). Samples were prepared with SDS sample buffer without boiling. Proteins were separated by discontinuous 7% SDS-PAGE and then transferred onto nitrocellulose transfer membrane overnight. The running buffer contained 25 mm Tris, 192 mm glycine and 0.1% SDS (Bio-Rad). The transfer buffer contained 25 mm Tris, 192 mm glycine, 15% methanol. For immunodetection, proteins were probed with monocolonal R3F1 (1: 200) and/or anti-actin (Chemicon; 1: 1000). Peroxidase-conjugated anti-mouse IgG (Bio-Rad) was used as the secondary antibody at 1: 3000. Peroxidase-labelled proteins were visualized via chemiluminescence (PerkinElmer). Ca2+ buffers significantly affect the apparent molecular weight of NCX1 (Levitsky et al. 1994, 1996), and we found that PEGylated NCX1 bands were somewhat better resolved without Ca2+ buffers, as in Fig. 1. Figures 2 and 3 are with 2 mm EGTA and 2 mm EDTA, and all other results reported were obtained without Ca2+ buffers.
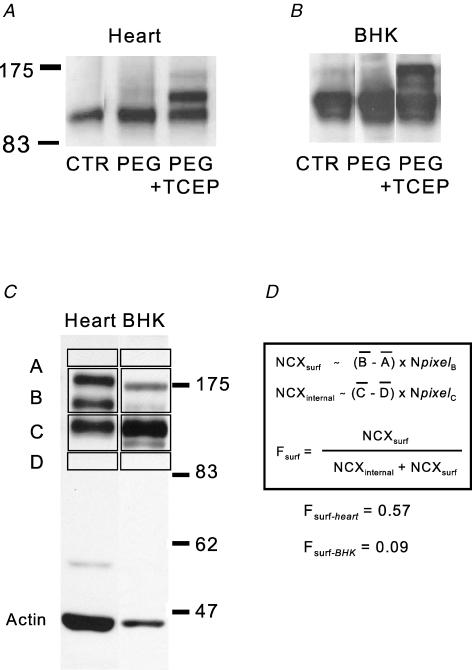
Figure 1. Determination of the NCX1 cell surface fraction (Fsurf) by extracellular cysteine PEGylation.
A, Western blots of murine cardiac lysates from a control (CTR) heart, a heart PEGylated for 15 min with MPEG, and a heart PEGylated for 15 min with MPEG after perfusion of TCEP for 5 min. B, Western blots of BHK cell lysates from control (CTR) cells, cells PEGylated for 15 min with MPEG, and cells PEGylated for 15 min with MPEG after application of TCEP for 5 min. C and D, densitometry procedure to determine Fsurf. Average densities of gel regions just below and above the NCX1 bands (‘D’ and ‘A’) are used for background subtraction of the NCX1 protein that does not shift with PEGylation (‘C’) and the two bands that result from PEGylation (‘B’). Protein amount is assumed to be proportional to the average background-subtracted densities (B – A) and (C – D), times the number of pixels in the selected region (Npixel). Cardiac Fsurf is typically about 0.6 whereas Fsurf in cell lines is severalfold lower, indicating the existence of large pools of internal NCX1.
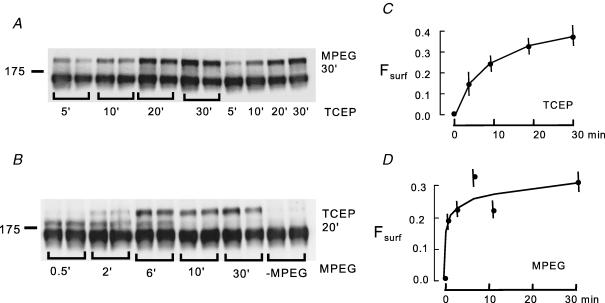
Figure 2. Time courses of NCX1 disulphide reduction by TCEP (6 mm) and PEGylation by MPEG (5 mm) at 4°C.
A, Western blots of lysates from BHK cells exposed to TCEP (6 mm) for 5, 10, 20 and 30 min followed by PEGylation with MPEG (5 mm) for 30 min. B, Western blots of lysates from BHK cells exposed to TCEP (6 mm) for 20 min, followed by PEGylation with MPEG for 0.5, 2, 6, 10 and 30 min. C, time course of NCX1 reduction by TCEP, as reflected in an increase of Fsurf. D, time course of NCX1 PEGylation by MPEG as reflected in an increase of Fsurf.
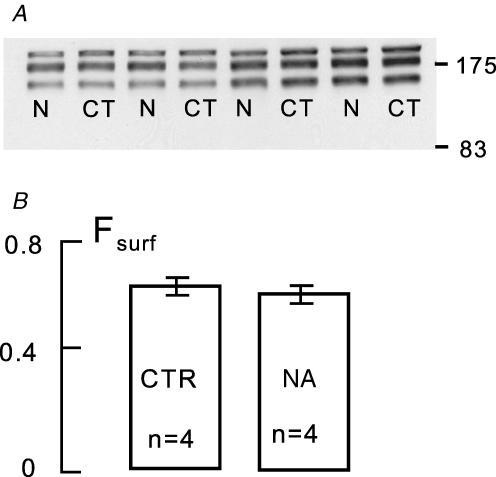
Figure 3. Lack of effect of 10 min arterial perfusion of noradrenaline (0.5 μm) on Fsurf in murine hearts.
A, Western blots of 2 control (CT) and 2 noradrenaline-treated hearts (N). B, analysis of Fsurf from the Western blots in A: CTR, control; NA, noradrenaline.
Pressure-overload hypertrophy models
Male C57BL6 mice (6–8 weeks old) were subjected to pressure overload by thoracic aortic banding (Wang et al. 2001). Constriction to a 27G stenosis induces moderate hypertrophy (40% increases in heart mass) without clinical signs of heart failure or malignant ventricular arrhythmia (Hill et al. 2000). Severe, decompensated hypertrophy was induced by banding the thoracic aorta to a 28G diameter.
Light–dark entrainment of mice
Wild-type (WT) mice were housed for 8–14 days in 12 h light: 12 h dark-controlled boxes in individual cages equipped for voluntary wheel-running (Dudley et al. 2003). At selected times, animals were removed from cages and killed by i.p. injection of 100 mg Euthasol (kg body weight)−1. Hearts were isolated, perfused retrogradely for 30 s with 1 mm Ca2+-containing physiological saline, and flash-frozen in liquid nitrogen-cooled aluminium clamps for lipid analysis. For PEGylation, procedures were as described above.
Materials
Unless indicated otherwise, all reagents were from Sigma and were the highest grade available. We point out that the MPEG used (Nektar Therapeutics, 2E2M0H01) does not contain a ‘linker’, and those products employing a linker group between maleimide and the polyethylene glycol did not effectively PEGylate transporters.
Statistical analysis
Observation numbers given in parentheses are the number of hearts for biochemical measurements and the number of myocytes for fluorescence measurements. Symbols indicating significance levels using Student's t test are as follows: *P < 0.05, **P < 0.01 and ***P < 0.001.
Results
NCX1 PEGylation assay
Figure 1 illustrates the analysis of NCX1 Western blots from murine heart and BHK lysates to determine Fsurf. Figure 1A and B shows blots of lysates from hearts and BHK cells to illustrate that upper bands are obtained by PEGylation with MPEG (‘PEG’) only if hearts and cells are pretreated with TCEP to reduce the extracellular NCX1 disulphide (‘PEG + TCEP’). Figure 1C illustrates the analysis of blots by densitometry. Actin is blotted together with NCX1 as an internal control for the amount of protein that is loaded in different samples. Protein amounts, development of the chemiluminescence, and digitization of the blots were carefully adjusted to avoid pixel saturation, while the major densities were at least 25% of saturation. Under the conditions chosen, we found that the calculated Fsurf changed by only a few per cent when the amount of protein loaded doubled or decreased by a factor of 3. While the cardiac blots show typically three distinct and well-defined bands, Western blots from BHK cells are typically more complex in that NCX1 bands show some spreading toward lower molecular weights. However, upper bands in BHK lysates depend absolutely on the sequential application of both TCEP and MPEG, and are therefore assumed to represent NCX1 on the surface of cells. Note that multiple still-higher molecular weight bands were obtained if cells were disrupted or made permeable, for example with detergents. Thus, the measurements have internal controls for cell integrity. As will be illustrated in Fig. 2, extension of the PEGylation times, as expected, shifts all NCX1 protein from the middle band to the uppermost band as both cysteines of the disulphide bond are reacted.
To calculate Fsurf, blots are scanned and average densities are determined in four rectangles as described in Fig. 1C. The two upper bands are included in one rectangle, the lower band in another, and regions on both sides of the bands are used as backgrounds. The nearer background is subtracted from each rectangle with NCX1 protein, and the total density is then calculated as the average density of the rectangle multiplied by the number of pixels. Finally, Fsurf is calculated from the total density of bands that were labelled (NCXsurf) and not labelled (NCXinternal), as indicated in Fig. 1D: Fsurf= NCXsurf/(NCXinternal+ NCXsurf).
Figure 2 shows time courses for the two sequential reactions in BHK cells. Figure 2A shows duplicate Western blots of BHK cell lysates from cells that were treated with TCEP for 5, 10, 20 and 30 min and then reacted with MPEG for 30 min. Figure 2B shows duplicate Western blots of BHK cell lysates from cells that were treated with TCEP for 20 min and then reacted with MPEG for 0.5, 2, 6, 10 and 30 min. The Fsurf values obtained are plotted in Fig. 2C and D, respectively. Reduction of the disulphide bond (Fig. 2C) takes place with a time constant of about 8 min, while the PEGylation reaction is faster. At 2 min, substantial amounts of NCX1 have been PEGylated at one site; at 6 min the majority of protein is already PEGylated at two sites (i.e. shifted to the uppermost molecular weight), and at 10 min the reaction is almost complete. We used 15 min TCEP reaction times and 30 min PEGylation times for routine experiments with cell lines.
Fsurf in different cell types and with different expression systems
One practical use of the NCX1 PEGylation assay is to determine the status of NCX1 in different cell systems used in NCX1 studies. As summarized in Table 1, Fsurf values of 0.6–0.7 are typical for intact heart, while stable NCX1-expressing cell lines (BHK and Chinese hamster ovary (CHO)) range from 0.1 to 0.3. BHK and HeLa cells transiently transfected with NCX1 via Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) typically gave Fsurf values of less than 0.05, even after 48 h of expression. In other words, almost all NCX1 protein is presumably intracellular. Using a neonatal murine myocyte cell culture (Cunha & Mohler, 2006), Fsurf was 0.26, substantially less than intact heart. This result is in good agreement with immunohistochemical results for NCX1 expression in neonatal myocytes (Egger et al. 2005) and BHK cells (Secondo et al. 2007).
Table 1.
Summary of Fsurf values in intact murine heart and different cell systems
| Fsurf | |
|---|---|
| BHK with stable NCX1 | ∼0.25 |
| HEK 293 with stable NCX1 | ∼0.2 |
| Intact murine hearts | ∼0.7 |
| Neonatal myocyte culture | ∼0.3 |
| BHK transient transfection | ∼0.1 |
| HeLa transient transfection | <0.1 |
Using intact hearts, we initially tested whether significant changes of Fsurf might occur rapidly (i.e. within 10 min) with large changes of cardiac excitation–contraction coupling that are readily induced by calcium channel blockers (verapamil, 1 μm) and catecholamines (noradrenaline (norepinephrine), 0.5 μm). As illustrated for noradrenaline in Fig. 3, no rapid changes were detected. The Western blots shown are duplicates for two control hearts and two hearts perfused with noradrenaline for 10 min prior to TCEP and PEGylation. The average Fsurf is 0.63 ± 0.04. Verapamil at the concentration employed caused complete heart block and similarly was without effect. On the one hand, these results indicate clearly that large changes of cardiac calcium transients and heart rate have no immediate influence on NCX1 surface expression. On the other hand, these results provide an important control for results presented subsequently, namely that large changes of cardiac contraction strength, frequency, and probably coronary flow have no effect on the determination of Fsurf.
NCX1 internalization in reponse to lipid kinase overexpression
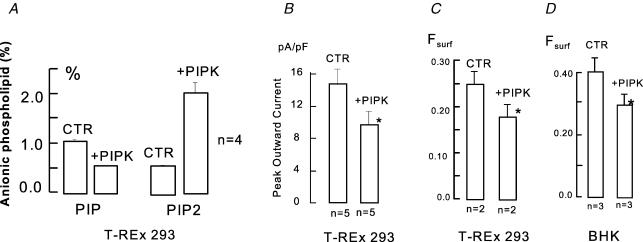
As described in the preceding article (Yaradanakul et al. 2007), an increase of PIP2 via pipette perfusion of lipid or via overexpression of lipid kinases appears to induce a loss of NCX1 activity that might involve internalization. Therefore, we analysed the effects of lipid kinase expression on Fsurf and Fig. 4 shows the effects of inducible expression of the hPIP5KIβ kinase in HEK 293 cells and of transient Lipofectamine-mediated expression of the same kinase in BHK cells. To induce the kinase, doxycycline (1 μg ml−1) was applied for 20 h, and Fig. 4 verifies the expected changes of phosphoinositides. Expression of the PIP5-kinase causes a 3-fold increase of PIP2 and a nearly 50% fall of PIP. As shown in Fig. 4B, the average peak outward exchange currents were decreased by 35% in cells treated identically. The NCX1 Fsurf was reduced by 31% in cells treated identically. Since these effects, while significant, were smaller than expected from functional results for transient transfection of the kinase in BHK cells (Yaradanakul et al. 2007), we also determined the effects of transient kinase expression in BHK cells. Comparing transfected and mock-transfected BHK cells, Fsurf was reduced by 28%. On the basis of green fluorescent protein (GFP) coexpression, we estimate that the percentage of transfected cells was more than 35% and less than 50%. Thus, we can project (but not prove) that in the BHK cells transient lipid kinase expression results in still larger changes of Fsurf in the cells transfected. We attempted to define NCX1 location changes with hPIP5KIβ overexpression using the NCX1-358-YFP construct, expressed with or without the lipid kinase. From the fluorescence, we did not detect a significant loss of surface membrane NCX1, although we did detect in some cells large fluorescent intracellular vesicles with kinase expression.
Figure 4. Effects of overexpressing hPIP5Iβ on phosphoinositides and Fsurf.
A, PIP and PIP2 as percentage of total anion phospholipid in T-REx 293 cell line with doxycyclin-inducible hPIP5Iβ. With 24 h application of 1 μg ml−1 doxycyclin, PIP levels decrease by 40% and PIP2 levels are tripled. B, peak outward exchange currents in T-REx 293 cells without and with induction of hPIP5Iβ. C, Fsurf determinations in T-REx 293 cells without and with induction of hPIP5Iβ. D, Fsurf determinations in BHK cells expressing NCX1 without and with transient transfection of hPIP5Iβ.
Dual effects of M1 receptor activation: PIP dependence of receptor-activated internalization
In the preceding article (Yaradanakul et al. 2007), opposing effects of M1 receptor activation were described on NCX1 activity at low and high cytoplasmic Ca2+. In the former case, a decrease of membrane capacitance is observed to occur over the course of 3 min. In the latter case, an increase of capacitance occurs over a time course of 1–2 min with an increase of NCX1 activity. As described in Fig. 5 we have determined that Fsurf changes occur in equivalent protocols in the direction expected from capacitance changes and that internalization with receptor activation requires continued synthesis of PIP.
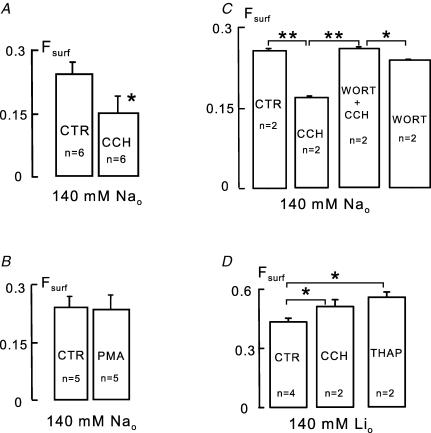
Figure 5. Effects of carbachol and lack of effect of phorbol ester on Fsurf in BHK cells expressing both NCX1 and M1 receptors.
A, reduction of Fsurf by carbachol (CCH, 0.2 mm) application for 10 min in serum-free DMEM solution. B, lack of effect of phorbol ester (PMA, 0.5 μm) on Fsurf. C, complete block of carbachol-induced reduction of Fsurf by wortmannin (WORT, 3 μm). Note that in the presence of wortmannin, carbachol causes a small increase of Fsurf.D, increase of Fsurf by carbachol (0.2 mm) and by thapsigargin (1 μm) applied for 10 min simultaneously with replacement of extracellular Na+ by lithium.
Figure 5A shows the effect of carbachol in NCX1-expressing BHK cells that also express M1 receptors under G418. Cells were initially incubated in serum-free Tyrode solution, and carbachol (0.2 mm) was applied for 15 min. Fsurf decreased by 42% (P = 0.02) at 15 min, and we note that there were no significant changes at 5 min. Since PKC activation is a potentially important mechanism in these results we tested whether phorbol ester (0.5 μm) could mimic the effect of carbachol in these experiments. As shown in Fig. 5B, phorbol ester is without effect on Fsurf in BHK cells (n = 5), suggesting that mechanisms besides PKC activation are important to evoke the decrease of Fsurf.
It seems contradictory in these experiments that NCX1 internalization appears to be PIP2 dependent, and yet internalization is strongly activated by carbachol when PIP2 is depleted. However, it is also known that PIP and PIP2 rebound substantially during continued activation of M1 receptors (Zaika et al. 2006), raising the question, is the activation of phosphoinositide synthesis essential to support endocytosis? Since the synthesis of phosphoinositides during and after receptor activation can be largely blocked by high concentrations of wortmannin (Balla et al. 2005; Yaradanakul et al. 2007), we tested whether wortmannin (3 μm) might block internalization of NCX1 in this protocol. As shown in Fig. 5C, the reduction of Fsurf by carbachol was completely blocked. Wortmannin itself, which caused a 35% decrease of PIP and a 5.2% decrease of PIP2 in BHK cells (not shown), had no significant effect on Fsurf. In the presence of wortmannin, carbachol caused a small but significant increase of Fsurf, and in this condition PIP2 remained below our standard level of detection for mass measurements of anion lipids.
In order to generate an increase of cytoplasmic free Ca2+, that might be comparable to conditions under which inward current increased with carbachol (Yaradanakul et al. 2007), we removed extracellular Na+ completely with Li+ as replacement. Dishes of cells were compared in which Na+ was removed, and in which Na+ was removed and carbachol (0.2 mm) was applied simultaneously. In this protocol, it may be expected that carbachol will release Ca2+ from internal stores, that Ca2+ extrusion by NCX1 will be inhibited, and that NCX1 will tend to load cells with Ca2+. As shown in Fig. 5D, Fsurf increased by 20% (P < 0.05) within 10 min. To induce an even higher cytoplasmic free Ca2+, we applied thapsigargin (1 μm) to deplete intracellular Ca2+ stores together with Na+ removal to block Ca2+ extrusion by NCX1. After 10 min, Fsurf is increased by 27% (P < 0.05) in reasonable quantitative agreement with effects observed on inward NCX1 currents (Yaradanakul et al. 2007).
NCX1 internalization in response to metabolic stress and cytoplasmic Na+ accumulation
Next, we tested several putative means to modulate endocytosis, and from those studies we describe here that modest metabolic stress and cellular K+ depletion have substantial additive effects on Fsurf. A role for metabolic stress was suggested by work outlined in the Introduction, indicating that multiple transporters and channels are internalized in response to ischaemia. We examined the effects of glucose removal because this intervention is well studied in BHK cells and is known to cause activation of some stress-dependent kinases without causing significant depletion of the total ATP (Lefebvre & Rosen, 2005). K+ depletion is well known to block clathrin-dependent endocytosis (Larkin et al. 1983), but it is also established that clathrin-independent endocytic processes can be strongly enhanced (Altankov & Grinnell, 1993). Fibronectin is rapidly internalized when extracellular K+ is removed, whereby K+ is largely depleted in fibroblasts within 30 min (Altankov & Grinnell, 1995) and presumably is replaced by cytoplasmic Na+.
Figure 6A shows the effects of glucose removal and K+ reduction from 6 to 0.1 mm for 30 min on Fsurf in physiological saline solution without serum. Important control measurements are also shown. From left to right, it is shown first that removal of extracellular Ca2+ does not significantly affect Fsurf. This is important because it is expected that cytoplasmic Ca2+ will change with extracellular K+ removal in NCX1-expressing cells. As shown by the third bar, removal of glucose for 30 min in the presence of extracellular Ca2+ results in a 25% reduction of Fsurf (P < 0.05). Removal of extracellular K+ for 30 min in the presence of extracellular Ca2+ and glucose causes a 38% reduction in Fsurf, and the reduction is somewhat smaller when extracellular Ca2+ is removed together with K+. Removal of glucose and K+ together causes a 62% reduction of Fsurf. Thus, the effects of glucose and K+ removal are additive in nature. Finally, we tested whether Na+ loading of cells upon reduction of extracellular K+ from 6 to 0.1 mm plays a role in the reduction of Fsurf. When extracellular NaCl (140 mm) was replaced by NMG-Cl (140 mm), the decrease of Fsurf upon reduction of extracellular K+ was almost abolished. Thus, Na+ loading may indeed play an important role in the effect of low extracellular K+.
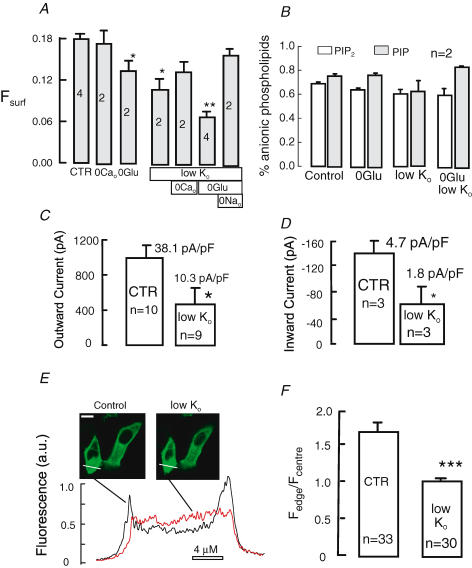
Figure 6. Additive reductions of Fsurf, NCX1 currents and NCX1-358-YFP surface expression by glucose removal and extracellular K+ reduction in BHK cells.
A, Fsurf in BHK cells in serum-free physiological saline (37°C). From left to right the vertical bars give Fsurf for control (CTR) cells, cells incubated with Ca2+-free saline for 30 min, cells incubated without glucose for 30 min, cells incubated without K+ for 30 min, cell incubated without K+ and Ca2+ for 30 min, cells incubated without glucose and K+ for 30 min, and finally cells without K+ and glucose and with extracellular Na+ replaced by N-methylglucamine. B, PIP and PIP2 levels in NCX1-expressing BHK cells as indicated with and without glucose and with reduced (0.1 mm) K+. C, average peak outward NCX1 currents before and after 30 min treatment with 0.1 mm K+ in nominally Ca2+-free solution. D, average peak outward NCX1 currents before and after 30 min treatment with 0.1 mm K+ in nominally Ca2+-free saline solution. E, images and line scans of BHK cells' expression of NCX1-YFP fusion protein before and after incubation with 0.1 mm K+ solution in nominally Ca2+-free saline solution. Calibration bar corresponds to 10 μm. F, composite line scan results for 33 cells before and 30 cells after incubation in low-K+ saline. ‘1.0’ indicates that fluorescence at the surface of cells is equal to average fluorescence in the cell interior. Symbols (*, **, ***) indicate significance with respect to the control results (CTR).
As shown in Fig. 6B, we tested whether glucose removal and K+ reduction might affect phosphoinositides. There was no significant effect of either intervention, although PIP tended to rise and PIP2 tended to fall with the combined removal of glucose and reduction of extracellular K+. Figure 6C and D shows results for exchange currents in cells that were incubated in 6 mm K+versus 0.1 mm K+ for 30 min prior to measurements. Both solutions were nominally Ca2+- and glucose-free. Seals were formed in these same solutions and currents were monitored as described (Yaradanakul et al. 2007: solutions C1 and X1 for outward current and solutions C4 and X4 were used for inward currents with dibromo-BAPTA instead of EGTA for 3 μm free Ca2+). There was a 65% reduction of both outward (Fig. 6C) and inward (Fig. 6D) exchange currents, approximately as would be predicted from the Fsurf measurements. Figure 6E shows typical fluorescence line scans of BHK cells that were transiently transfected with NCX1-358-YFP. The cytoplasm and intracellular compartments show substantial NCX1 fluorescence, as expected from the Fsurf determinations for BHK cells. Just before the ‘control’ scan was taken, cells were shifted into glucose-free solution with 6 mm K+. Then, after approximately 5 min, the solution was exchanged for glucose-free solution with 0.1 mm K+. Over the course of 30 min, fluorescence at the edges of cells decreased to approximately the average value in the cytoplasm. Figure 6F shows composite results for cells that were incubated in Ca2+- and glucose-free solution with 6 mm K+versus the same solution with 0.1 mm K+. With these solutions, identical to those used prior to the current recordings in Fig. 6C and D, the ratio of fluorescence at the edge to the centre of cells decreases effectively to ‘1’. To summarize, the same conditions that we have found to induce a strong reduction of Fsurf have also been found to cause a reduction of NCX1 currents and NCX1-358-YFP at the edges of BHK cells.
Inverse diurnal changes of cardiac PIP2 and Fsurf
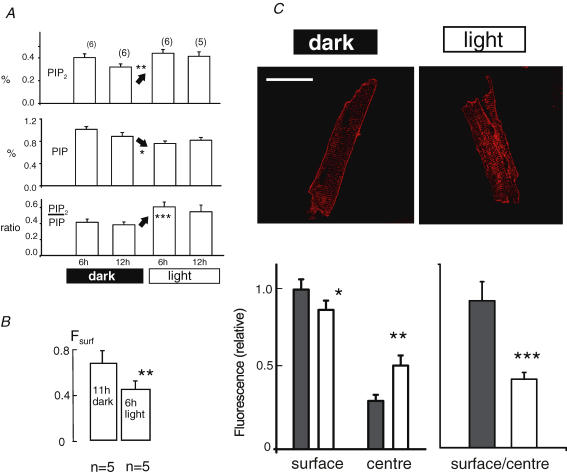
As already presented, we did not detect any short-term changes of the NCX1 Fsurf in murine hearts with several interventions that strongly affect cardiac calcium homeostasis and contractility. Therefore, we proceeded to test whether changes of Fsurf might occur on a longer time scale not readily analysed in isolated hearts. First, we chose to investigate diurnal influences because message for mouse PIP5KIα-kinase (homologue to human PIPKIβ) was reported in two studies to increase significantly during light exposure in light–dark-entrained mice (Storch et al. 2002; Martino et al. 2004). As shown in Fig. 7A, global cardiac PIP2 decreased between the 6th and 12th hour of the dark phase, and then increased by 27% at the 6th hour of the light phase (P < 0.01). In the same time frame, PIP decreased as expected for an increase of PIP5 kinase activity (see Fig. 4). Thus, the ratio of PIP2 to PIP increased by 60% (P < 0.001). As shown in Fig. 7B, Fsurf decreased from 0.67 at the 11th hour of the dark cycle (i.e. during running) to 0.45 at the 6th hour of the light cycle (P < 0.01). Figure 7C shows our immunohistochemical analysis of NCX1 distribution in myocytes from two hearts isolated at the end of the dark phase and at the 6th hour of the light phase. Fluorescence at the outer surface of myocytes is decreased (P < 0.05) while average fluorescence between the edges is increased (P < 0.01) with the surface-to-centre ratio being decreased by > 50% (P < 0.001).
Figure 7. Diurnal changes of phosphoinositides and Fsurf in murine hearts.
A, PIP2 and PIP levels as percentage of total anionic phospholipids, and their ratio, in flash-frozen hearts from light–dark-entrained mice at the given times. B, Fsurf values determined from hearts at the times that presented the largest difference in PIP2/PIP ratios. C, NCX1 immunohistochemical labelling of myocytes at the same times (11 h dark and 6 h light). Cells were fixed immediately upon isolation (i.e. within 15 min of mice being killed). Line scans were taken at a middle depth of the myocytes. ‘Surface’ is the relative fluorescence intensity at the outer edge of a myocyte in the line scan. ‘Centre’ fluorescence is the average fluorescence value across the central portion of the scan (n = 12; results are from completely relaxed myocytes from two cell batches). Calibration bar corresponds to 40 μm.
Inverse changes of cardiac phosphoinositides and Fsurf in response to aortic banding
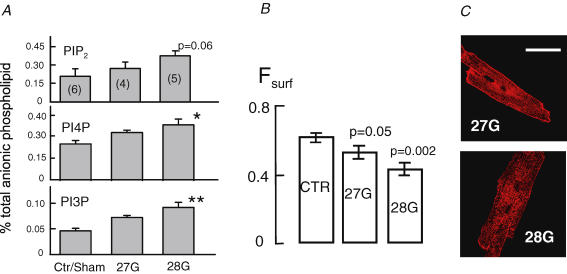
Finally, we performed a similar analysis of hearts from mice subjected to aortic banding in which NCX1 message and protein are increased, while NCX1 currents are reported to be little changed (Wang et al. 2001). Cardiac phosphatidylinositides were measured in hearts subjected to banding to two different degrees for 2 weeks, and in hearts from sham-operated animals (Hill et al. 2000). We report results for phosphatidylinositol-4-phosphate (PI4PK) and phosphatidylinositol-3-phosphate (PI3P) separately, because PI(3)P changes were significant in this sole case. As shown in Fig. 8A, the mean PIP2 level increased by 33 and 83% in the mild and severe overload, respectively, but these changes remained at the edge of significance with our sample size (P = 0.06); PI(4)P increased by 33 and 68% (P < 0.05), respectively; and PI(3)P increased by 55 and 99% (P < 0.01), respectively. It should be noted that we found no increase of phosphoinositides in hypertrophied hearts of mice with overexpression of calcineurin (Molkentin et al. 1998). As shown in Fig. 8B, Fsurf values decreased by 14 and 33% in the 27G- and 28G-banded hearts (P = 0.05 and 0.002, respectively). As shown in the micrographs in Fig. 8C, we found that myocytes from banded mice often showed NCX1 staining of internal membranes, especially perinuclear membranes, and in many cases NCX1 staining appeared rather granular and disorganized compared to the usual crisp T-tubule labelling in control hearts (Fig. 7C).
Figure 8. Changes of cardiac phosphoinositides and Fsurf in hearts from animals subjected to aortic banding for 12 days with 27G and 28G restrictions.
A, PIP2, PI(4)P and PI(3)P levels as percentage of total anionic phospholipid. B, Fsurf values from equivalent animals. C, NCX1 immunohistochemical labelling of myocytes from banded hearts. In comparison to control myocytes (see Fig. 7), myocytes from banded hearts often showed staining of perinuclear regions and more granular NCX1 localization. Calibration bar corresponds to 40 μm.
Discussion
The cysteine PEGylation assay described in this article allows relatively rapid determination of the fraction of NCX1 transporters on the surface of cells, Fsurf, including cell lines and intact myocardium. Using this assay, we have attempted to ask whether Fsurf is a significant variable in the cellular regulation of NCX1. We discuss first the method itself and then the experimental results in relation to previous studies of NCX1, in particular results consistent with significant NCX1 trafficking described in the preceding article (Yaradanakul et al. 2007).
Determination of Fsurf by cysteine PEGylation: attractions and dangers
The PEGylation assay of the NCX1 cell surface fraction has several attractions. Compared to biotinylation of extracellular lysines on NCX1 (Rahamimoff et al. 2002), the reaction of cysteines, as described in Fig. 1, is faster and does not require a ‘pull-down’. Thus, it may be more reliable. The labelling assay can distinguish between transporters in the surface membrane and transporters in small vesicles attached to the membrane, which is not possible with fluorescent tags on cytoplasmic transporter domains. The assay can be used in both cell lines and intact myocardium with wild-type NCX1, and thus has allowed us to probe questions that could not be addressed by any other available technique. As with any labelling technique there are admittedly multiple limitations that we have tried to address by performing parallel experiments with other approaches. Two clear dangers are that NCX1 labelling might be conformationally sensitive and/or sensitive to the quality of arterial perfusion and capillary permeability in intact hearts. From the additional measurements and other information available, we will outline reasonable independent arguments that support the validity of each result and conclusion.
First, the Fsurf values obtained are largely as expected from other types of measurements. From immunohistochemical studies, it is known that the large majority of NCX1 is located to the sarcolemma of adult cardiac myocytes (Frank et al. 1992; Li et al. 1993), while the majority of exchangers in neonatal myocytes (Egger et al. 2005) and the BHK cell line employed (Secondo et al. 2007) are not. Second, large changes of contractility and presumably arterial perfusion, induced by verapamil and noradrenaline, were without effect on the myocardial Fsurf values obtained (Fig. 3). Third, the reduction of Fsurf by overexpression of lipid kinases (Fig. 4) correlates reasonably with changes of NCX1 current densities, when one takes into account that our transient transfections are only about 50% efficient in BHK cells (Yaradanakul et al. 2007). Fourth, the decrease of Fsurf induced by muscarinic receptor activation under control conditions, and the increase of Fsurf induced under conditions to elevate cytoplasmic Ca2+, correlate with capacitance changes previously described (Yaradanakul et al. 2007). Fifth, the reductions of Fsurf caused by reductions of extracellular K+ are correlated in Fig. 6 to changes of NCX1 currents as well as to long-distance changes of the location of the NCX1-YFP fusion in the same cell type. Sixth, the reductions of Fsurf determined in intact myocardium during the ‘light phase’ (i.e. sleep) of light–dark-entrained mice and in response to aortic banding have reasonable correlations to immunohistochemical analyses. At minimum, the immunohistochemical images provided proof that NCX1 can redistribute from the outer surface membrane to other places in the myocytes (Figs 7 and 8).
Control of NCX1 internalization: roles of PIP2, PI4-kinases and ions
As noted in the Introduction, cardiac myocytes do not contain obvious intracellular pools of most transporters and ion channels. Thus, the insertion of most cardiac transporters and ion channels into the sarcolemma can ultimately be under the control of transcriptional and translational processes. However, it seems inevitable that removal of transporters and channels from the sarcolemma will be a significant determinant of their abundance in the sarcolemma. and it therefore seems likely that internalization processes will be subject to significant cellular regulation. In the simplest case, phosphorylation or ubiquitination of an individual residue of a transporter (Robinson, 2002) or channel (Staub et al. 2000; Hu et al. 2003; Snyder, 2005) will serve as an all-or-none switch to initiate removal from the membrane. However, it seems unlikely that this will be the case in general. For NCX1, its inactivation/activation state, cytoskeletal interactions, and interactions with other membrane proteins can all be expected to influence its ‘availability’ for endocytosis. Furthermore, the endocytic machinery itself will be dependent on multiple cellular processes besides protein kinase phosphorylation, among them multiple PIP2-dependent processes including the recruitment of adapters (Czech, 2003). Our experimental results may be viewed best from this cellular perspective.
First, we have not been able to influence the fraction of NCX1 at the cell surface of cardiac myocytes by any means in the time frame of 10 min. Thus, the average ‘dwell time’ of NCX1 at the cardiac surface is presumably longer. Second, we have verified that PIP2 synthesis, via PIP5-kinase overexpression, can decrease the fraction of NCX1 in the cell surface (Fig. 4), and we have determined that maintained PIP synthesis is needed to internalize NCX1 in response to M1 receptor activation (Fig. 5). It is at least possible therefore that the regulation of lipid kinases will be used by cardiac myocytes to regulate transporter density. In this connection, we have presented two cases in which phosphoinositides are increased in hearts while the fraction of NCX1 at the myocyte surface is decreased (Figs 7 and 8). Whether or not there is a causal relation between PIP2 changes and Fsurf changes will require more sophisticated experimental models, in particular models in which NCX1 trafficking can be monitored in real time and in which lipid kinases can be specifically activated or inactivated.
Our further studies with cell lines provide insights that in the long term may be relevant to the internalization of NCX1 in myocytes. First, our data related to NCX1 internalization during M1 muscarinic receptor activation is largely comparable to data on receptor internalization (Sorensen et al. 1998). The internalization of both muscarinic and β2 receptors requires maintained PIP2 synthesis and is blocked by wortmannin (Sorensen et al. 1999). That PIP synthesis could be an important local factor for NCX1 endocytosis is supported by the fact that a phorbol ester does not cause significant internalization of NCX1 (Fig. 5). Thus, the process does not seem to be primarily PKC-activated. Further, this conclusion is supported by the loss of NCX1 activity in myocytes over-expressing a PI4-kinase activity (Yaradanakul et al. 2007). We suggest therefore that the inhibition of PIP2-sensitive transporters and channels, caused in some cells by receptor-activated PIP2 depletion, may be supported by endocytosis over the longer time frames in which PIP2 is resynthesized. The effect of muscarinic receptor activation to promote an increase of Fsurf, when cytoplasmic Ca2+ is high, can in principle reflect these same processes. The effect of high cytoplasmic Ca2+per se to promote a higher Fsurf and override the effect of receptor activation (Fig. 5D) is also explained if activation of phospholipase C (PLC) favours NCX1 insertion and disfavours NCX1 internalization.
Finally, the pronounced effects of glucose removal and extracellular K+ reduction to promote NCX1 internalization (Fig. 6) are intriguing because they might be relevant to cardiac ischaemia in which stress kinases become activated (Armstrong, 2004; Russell, 2006) and cytoplasmic Na+ rises (Avkiran et al. 2001). The effects of glucose removal and K+ reduction evidently involve different mechanisms because they are ‘additive’ effects (Fig. 6A). PIP and PIP2 do not change with either intervention (Fig. 6B). Since K+ removal promotes clathrin-independent endocytic processes in fibroblasts that do not express NCX1 (Altankov & Grinnell, 1995) the presence of NCX1 is not per se essential to initiate the endocytic processes. In short, we have no real clues at this time about underlying mechanisms. The effect of K+ removal requires Na+ loading but not Ca2+ loading of cells (Fig. 6A). Since clathrin-dependent endocytosis is blocked by K+ removal (Larkin et al. 1983), NCX1 must be internalized by a clathrin-independent mechanism. This conclusion is consistent with our finding of enhanced cholera toxin but not transferrin uptake in myocytes overexpressing PI4-kinases with decreased NCX1 current densities (Yaradanakul et al. 2007).
In summary, we have supported by a direct method our hypothesis that NCX1 is removed from the surface membrane of cells by processes that are activated by increasing cellular PIP and PIP2. The results and approaches described represent a starting point to analyse the molecular mechanisms that remove NCX1 from the cardiac sarcolemma in relation to physiological and pathological cardiac function.
Acknowledgments
We gratefully acknowledge Dr Paul P. Schnetkamp (U. Calgary) for suggesting the use of extracellular cysteine PEGylation of NCX1 and for critical discussions of the method. We thank Cem Nasuhoglu (UTSW) for help with initial experiments and Kenneth D. Philipson (UCLA) and Helen L. Yin (UTSW) for reagents and critical discussions. This work was supported by NIH grants HL0679420 and HL051323 (D.W.H) and by the Donald W. Reynolds Cardiovascular Clinical Research Center at UT Southwestern (J.A.H).
References
- Al-Khalili L, Yu M, Chibalin AV. Na+,K+-ATPase trafficking in skeletal muscle: insulin stimulates translocation of both α1- and α2-subunit isoforms. FEBS Lett. 2003;536:198–202. doi: 10.1016/s0014-5793(03)00047-4. [DOI] [PubMed] [Google Scholar]
- Altankov G, Grinnell F. Depletion of intracellular potassium disrupts coated pits and reversibly inhibits cell polarization during fibroblast spreading. J Cell Biol. 1993;120:1449–1459. doi: 10.1083/jcb.120.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altankov G, Grinnell F. Fibronectin receptor internalization and AP-2 complex reorganization in potassium-depleted fibroblasts. Exp Cell Res. 1995;216:299–309. doi: 10.1006/excr.1995.1038. [DOI] [PubMed] [Google Scholar]
- Amaral MD. Processing of CFTR: traversing the cellular maze – how much CFTR needs to go through to avoid cystic fibrosis? Pediatr Pulmonol. 2005;39:479–491. doi: 10.1002/ppul.20168. [DOI] [PubMed] [Google Scholar]
- Armstrong SC. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:427–436. doi: 10.1016/j.cardiores.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Avkiran M, Gross G, Karmazyn M, Klein H, Murphy E, Ytrehus K. Na+/H+ exchange in ischemia, reperfusion and preconditioning. Cardiovasc Res. 2001;50:162–166. doi: 10.1016/s0008-6363(01)00228-0. [DOI] [PubMed] [Google Scholar]
- Balla A, Tuymetova G, Tsiomenko A, Varnai P, Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol Biol Cell. 2005;16:1282–1295. doi: 10.1091/mbc.E04-07-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- Becker C, Sevilla L, Tomas E, Palacin M, Zorzano A, Fischer Y. The endosomal compartment is an insulin-sensitive recruitment site for GLUT4 and GLUT1 glucose transporters in cardiac myocytes. Endocrinology. 2001;142:5267–5276. doi: 10.1210/endo.142.12.8555. [DOI] [PubMed] [Google Scholar]
- Bertrand CA, Frizzell RA. The role of regulated CFTR trafficking in epithelial secretion. Am J Physiol Cell Physiol. 2003;285:C1–C18. doi: 10.1152/ajpcell.00554.2002. [DOI] [PubMed] [Google Scholar]
- Chabowski A, Gorski J, Calles-Escandon J, Tandon NN, Bonen A. Hypoxia-induced fatty acid transporter translocation increases fatty acid transport and contributes to lipid accumulation in the heart. FEBS Lett. 2006;580:3617–3623. doi: 10.1016/j.febslet.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Chapman H, Ramstrom C, Korhonen L, Laine M, Wann KT, Lindholm D, Pasternack M, Tornquist K. Downregulation of the HERG (KCNH2) K+ channel by ceramide: evidence for ubiquitin-mediated lysosomal degradation. J Cell Sci. 2005;118:5325–5334. doi: 10.1242/jcs.02635. [DOI] [PubMed] [Google Scholar]
- Cunha SR, Mohler PJ. Cardiac ankyrins: Essential components for development and maintenance of excitable membrane domains in heart. Cardiovasc Res. 2006;71:22–29. doi: 10.1016/j.cardiores.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Czech MP. Dynamics of phosphoinositides in membrane retrieval and insertion. Annu Rev Physiol. 2003;65:791–815. doi: 10.1146/annurev.physiol.65.092101.142522. [DOI] [PubMed] [Google Scholar]
- Doctor RB, Dahl RH, Salter KD, Fouassier L, Chen J, Fitz JG. ATP depletion in rat cholangiocytes leads to marked internalization of membrane proteins. Hepatology. 2000;31:1045–1054. doi: 10.1053/he.2000.5983. [DOI] [PubMed] [Google Scholar]
- Doronin SV, Potapova IA, Lu Z, Cohen IS. Angiotensin receptor type 1 forms a complex with the transient outward potassium channel Kv4.3 and regulates its gating properties and intracellular localization. J Biol Chem. 2004;279:48231–48237. doi: 10.1074/jbc.M405789200. [DOI] [PubMed] [Google Scholar]
- Dostanic I, Lorenz JN, Schultz JJ, Grupp IL, Neumann JC, Wani MA, Lingrel JB. The a2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem. 2003;278:53026–53034. doi: 10.1074/jbc.M308547200. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Egger M, Porzig H, Niggli E, Schwaller B. Rapid turnover of the ‘functional’ Na+-Ca2+ exchanger in cardiac myocytes revealed by an antisense oligodeoxynucleotide approach. Cell Calcium. 2005;37:233–243. doi: 10.1016/j.ceca.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Frank JS, Mottino G, Reid D, Molday RS, Philipson KD. Distribution of the Na+-Ca2+ exchange protein in mammalian cardiac myocytes: an immunofluorescence and immunocolloidal gold-labeling study. J Cell Biol. 1992;117:337–345. doi: 10.1083/jcb.117.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron. 2003;38:417–432. doi: 10.1016/s0896-6273(03)00256-3. [DOI] [PubMed] [Google Scholar]
- Khan NA, Quemener V, Moulinoux JP. Exogeneous diacylglycerols downregulate the activity of Na+-K+ pump in Xenopus laevis oocytes. Exp Cell Res. 1991;194:248–251. doi: 10.1016/0014-4827(91)90361-w. [DOI] [PubMed] [Google Scholar]
- Knepper MA, Inoue T. Regulation of aquaporin-2 water channel trafficking by vasopressin. Curr Opin Cell Biol. 1997;9:560–564. doi: 10.1016/s0955-0674(97)80034-8. [DOI] [PubMed] [Google Scholar]
- Laing JG, Beyer EC. The gap junction protein connexin43 is degraded via the ubiquitin proteasome pathway. J Biol Chem. 1995;270:26399–26403. doi: 10.1074/jbc.270.44.26399. [DOI] [PubMed] [Google Scholar]
- Larkin JM, Brown MS, Goldstein JL, Anderson RG. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Lecuona E, Ridge K, Pesce L, Batlle D, Sznajder JI. The GTP-binding protein RhoA mediates Na,K-ATPase exocytosis in alveolar epithelial cells. Mol Biol Cell. 2003;14:3888–3897. doi: 10.1091/mbc.E02-12-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre DL, Rosen CF. Regulation of SNARK activity in response to cellular stresses. Biochim Biophys Acta. 2005;1724:71–85. doi: 10.1016/j.bbagen.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Levitsky DO, Fraysse B, Leoty C, Nicoll DA, Philipson KD. Cooperative interaction between Ca2+ binding sites in the hydrophilic loop of the Na+-Ca2+ exchanger. Mol Cell Biochem. 1996;160–161:27–32. doi: 10.1007/BF00240027. [DOI] [PubMed] [Google Scholar]
- Levitsky DO, Nicoll DA, Philipson KD. Identification of the high affinity Ca2+-binding domain of the cardiac Na+-Ca2+ exchanger. J Biol Chem. 1994;269:22847–22852. [PubMed] [Google Scholar]
- Li ZP, Burke EP, Frank JS, Bennett V, Philipson KD. The cardiac Na+-Ca2+ exchanger binds to the cytoskeletal protein ankyrin. J Biol Chem. 1993;268:11489–11491. [PubMed] [Google Scholar]
- Linck B, Qiu Z, He Z, Tong Q, Hilgemann DW, Philipson KD. Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3) Am J Physiol Cell Physiol. 1998;274:C415–C423. doi: 10.1152/ajpcell.1998.274.2.C415. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manery JF, Dryden EE, Still JS, Madapallimattam G. Enhancement (by ATP, insulin, and lack of divalent cations) of ouabain inhibition of cation transport and ouabain binding in frog skeletal muscle; effect of insulin and ouabain on sarcolemmal (Na + K)MgATPase. Can J Physiol Pharmacol. 1977;55:21–33. doi: 10.1139/y77-004. [DOI] [PubMed] [Google Scholar]
- Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M, Sole MJ. Day/night rhythms in gene expression of the normal murine heart. J Mol Med. 2004;82:256–264. doi: 10.1007/s00109-003-0520-1. [DOI] [PubMed] [Google Scholar]
- Massicotte G, Baudry M. Brain plasticity and remodeling of AMPA receptor properties by calcium-dependent enzymes. Genet Eng (N Y) 2004;26:239–254. doi: 10.1007/978-0-306-48573-2_12. [DOI] [PubMed] [Google Scholar]
- Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ED, Etter EF, Philipson KD, Carrington WA, Fogarty KE, Lifshitz LM, Fay FS. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993;365:657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- Ottolia M, John S, Ren X, Philipson KD. Fluorescent Na+-Ca2+ exchangers: Electrophysiological and optical characterization. J Biol Chem. 2007;282:3695–3701. doi: 10.1074/jbc.M610425200. [DOI] [PubMed] [Google Scholar]
- Rahamimoff H, Ren X, Kimchi-Sarfaty C, Ambudkar S, Kasir J. NCX1 surface expression: a tool to identify structural elements of functional importance. Ann N Y Acad Sci. 2002;976:176–186. [PubMed] [Google Scholar]
- Robinson MB. Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- Russell R., III Stress signaling in the heart by AMP-activated protein kinase. Curr Hypertens Rep. 2006;8:446–450. doi: 10.1007/s11906-006-0021-z. [DOI] [PubMed] [Google Scholar]
- Santacruz-Toloza L, Ottolia M, Nicoll DA, Philipson KD. Functional analysis of a disulfide bond in the cardiac Na+-Ca2+ exchanger. J Biol Chem. 2000;275:182–188. doi: 10.1074/jbc.275.1.182. [DOI] [PubMed] [Google Scholar]
- Schmalzing G, Richter HP, Hansen A, Schwarz W, Just I, Aktories K. Involvement of the GTP binding protein Rho in constitutive endocytosis in Xenopus laevis oocytes. J Cell Biol. 1995;130:1319–1332. doi: 10.1083/jcb.130.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secondo A, Staiano RI, Scorziello A, Sirabella R, Boscia F, Adornetto A, Valsecchi V, Molinaro P, Canzoniero LM, Di RG, Annunziato L. BHK cells transfected with NCX3 are more resistant to hypoxia followed by reoxygenation than those transfected with NCX1 and NCX2: Possible relationship with mitochondrial membrane potential. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.01.006. doi 10.1016/j.ceca.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Snyder PM. Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology. 2005;146:5079–5085. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Linseman DA, McEwen EL, Heacock AM, Fisher SK. A role for a wortmannin-sensitive phosphatidylinositol-4-kinase in the endocytosis of muscarinic cholinergic receptors. Mol Pharmacol. 1998;53:827–836. [PubMed] [Google Scholar]
- Sorensen SD, Linseman DA, McEwen EL, Heacock AM, Fisher SK. Inhibition of b2-adrenergic and muscarinic cholinergic receptor endocytosis after depletion of phosphatidylinositol bisphosphate. J Pharmacol Exp Ther. 1999;290:603–610. [PubMed] [Google Scholar]
- Staub O, Abriel H, Plant P, Ishikawa T, Kanelis V, Saleki R, Horisberger JD, Schild L, Rotin D. Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int. 2000;57:809–815. doi: 10.1046/j.1523-1755.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Tatsumi S, Sarai N, Terashima K, Matsuoka S, Noma A. Ionic mechanisms of cardiac cell swelling induced by blocking Na+/K+ pump as revealed by experiments and simulation. J Gen Physiol. 2006;128:495–507. doi: 10.1085/jgp.200609646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Kiehn J, Katus HA, Karle CA. Defective protein trafficking in hERG-associated hereditary long QT syndrome (LQT2): molecular mechanisms and restoration of intracellular protein processing. Cardiovasc Res. 2003;60:235–241. doi: 10.1016/j.cardiores.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Minireview: aquaporin 2 trafficking. Endocrinology. 2005;146:5063–5070. doi: 10.1210/en.2005-0868. [DOI] [PubMed] [Google Scholar]
- Venosa RA. Hypotonic stimulation of the Na+ active transport in frog skeletal muscle: role of the cytoskeleton. J Physiol. 2003;548:451–459. doi: 10.1113/jphysiol.2002.036830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetterlein F, Muhlfeld C, Cetegen C, Volkmann R, Schrader C, Hellige G. Redistribution of connexin43 in regional acute ischemic myocardium: influence of ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2006;291:H813–H819. doi: 10.1152/ajpheart.01177.2005. [DOI] [PubMed] [Google Scholar]
- Vinciguerra M, Deschenes G, Hasler U, Mordasini D, Rousselot M, Doucet A, Vandewalle A, Martin PY, Feraille E. Intracellular Na+ controls cell surface expression of Na,K-ATPase via a cAMP-independent PKA pathway in mammalian kidney collecting duct cells. Mol Biol Cell. 2003;14:2677–2688. doi: 10.1091/mbc.E02-11-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Nolan B, Kutschke W, Hill JA. Na+-Ca2+ exchanger remodeling in pressure overload cardiac hypertrophy. J Biol Chem. 2001;276:17706–17711. doi: 10.1074/jbc.M100544200. [DOI] [PubMed] [Google Scholar]
- Wang L, Wible BA, Wan X, Ficker E. Cardiac glycosides as novel inhibitors of human ether-a-go-go-related gene channel trafficking. J Pharmacol Exp Ther. 2006 doi: 10.1124/jpet.106.113043. [DOI] [PubMed] [Google Scholar]
- Woroniecki R, Ferdinand JR, Morrow JS, Devarajan P. Dissociation of spectrin-ankyrin complex as a basis for loss of Na-K-ATPase polarity after ischemia. Am J Physiol Renal Physiol. 2003;284:F358–F364. doi: 10.1152/ajprenal.00100.2002. [DOI] [PubMed] [Google Scholar]
- Yao X, Forte JG. Cell biology of acid secretion by the parietal cell. Annu Rev Physiol. 2003;65:103–131. doi: 10.1146/annurev.physiol.65.072302.114200. [DOI] [PubMed] [Google Scholar]
- Yaradanakul A, Feng S, Shen C, Lariccia V, Lin M-J, Yang J, Dong TP, Yin HL, Albanesi JP, Hilgemann DW. Dual control of cardiac Na+–Ca2+ exchange by PIP2: electrophysiological analysis of direct and indirect mechanisms. J Physiol. 2007;XXX:xxx–xxx. doi: 10.1113/jphysiol.2007.132712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Luo S, Lin Z, Wu Y. Cyclic stretch translocates the α2-subunit of the Na pump to plasma membrane in skeletal muscle cells in vitro. Biochem Biophys Res Commun. 2006;348:750–757. doi: 10.1016/j.bbrc.2006.07.120. [DOI] [PubMed] [Google Scholar]
- Zaika O, Lara LS, Gamper N, Hilgemann DW, Jaffe DB, Shapiro MS. Angiotensin II regulates neuronal excitability via phosphatidylinositol 4,5-bisphosphate-dependent modulation of Kv7 (M-type) K+ channels. J Physiol. 2006;575:49–67. doi: 10.1113/jphysiol.2006.114074. [DOI] [PMC free article] [PubMed] [Google Scholar]