Abstract
We examined the role of the concentrative nucleoside transporter CNT3 in the establishment of a transepithelial flux of natural nucleosides and their pharmacologically active derivatives in renal epithelial cell lines. Murine PCT cells grown on a transwell dish showed endogenous CNT3 activity at their apical membrane that was responsible for the sodium-dependent transepithelial flux of both purine and pyrimidine nucleosides. hCNT3 was also identified in human kidney and its role in the transport of nucleosides was tested. To this end, MDCK cells, lacking endogenous CNT3 activity, were genetically engineered to express the human orthologue of CNT3 (hCNT3-MDCK cells). In these cells, hCNT3 was inserted into the apical membrane, thus generating, as for PCT cells, a transepithelial flux of both nucleosides and nucleoside-derived drugs. Apical-to-basolateral transepithelial flux was present in all cells expressing a functional CNT3 transporter and was significantly higher than that found either in PCT cells in absence of sodium or in mock-transfected MDCK cells. Nevertheless in all cases a significant amount of the transported nucleoside was retained and transformed inside cells. However release to the opposite compartment was CNT3 dependent, not only in terms of absolute flux (much higher when an apical CNT3 transporter was active) but also regarding metabolic transformations of the apically absorbed nucleosides. These results underline a critical role of CNT3 in the renal reabsorption of nucleosides and their derivatives as well as in their intracellular metabolism.
One of the key functions in the physiology of absorptive epithelia is the generation of a transepithelial net flux of solutes. For this, epithelial cells have a large array of transporters, differentially distributed at the plasma membrane, often with concentrative carriers at the apical side and equilibrative transporters at the basolateral membrane (Mangravite et al. 2003). Thus, the perfect coupling between the two membrane compartments allows cells to perform either an active absorption/reabsorption or an active extrusion of solutes. Some of these transporters are also used by different drugs to enter and to leave the organism (Van Aubel et al. 2000).
Nucleoside transporter proteins (NTs) mediate the uptake of both natural nucleosides and most nucleoside-derived antitumoural drugs (Pastor-Anglada et al. 1998, 2005). Nucleoside-derived antiviral drugs, which seem to be modified in some critical parts of the nucleoside structure, are not so readily recognized or even not transported by NTs (Cano-Soldado et al. 2004). There are two families of nucleoside carriers, the equilibrative ENT/SLC29A transporters, with low affinity and wide selectivity, and the concentrative CNT/SLC28A transporters, with a higher affinity and more restricted selectivity (Griffith & Jarvis, 1996; Baldwin et al. 1999). Absorptive cells in the renal and intestinal epithelia express all members of the concentrative CNT family (Patil & Unadkat, 1997; Sundaram et al. 1998; Ritzel et al. 2001; Rodriguez-Mulero et al. 2005; Wu et al. 2005). In the intestine, a model has been put forward in which a transepithelial net flux relies on the consecutive action of the CNT transporters at the apical membrane and the ENT transporters at the basal (Ngo et al. 2001), and a similar model has also been proposed for the kidney (Gutierrez & Giacomini, 1993). Since CNT1 can transport drugs such as 5′-deoxy-5-fluoruridine (5′DFUR) or gemcitabine (Lostao et al. 2000; Mata et al. 2001; Smith et al. 2004), this net epithelial flux might be highly relevant in the pharmacokinetics of these molecules. Particularly relevant to this is the fact that nucleoside transporters are highly regulated in many organs including the intestine. Chronic deprivation of dietary nucleosides triggers an increase in the concentrative nucleoside transport in the intestine (Valdés et al. 2000), thus showing a nutritional regulation of these transporters. An endocrine regulation has also been shown, since in IEC-6 cells, derived from rat intestinal crypt cells, a well-known differentiation agent such as dexamethasone (Quaroni et al. 1999) up-regulates both the expression and activity of CNT1 and CNT2 (Aymerich et al. 2004). This increase can be envisaged as a result of the differentiation process, while proliferative agents such as EGF and TGF-α or the induction of an epithelial injury increase the mostly basally located ENT1 protein, presumably to draw nucleosides from the bloodstream to fulfil the requirements of the proliferative state (Aymerich et al. 2004).
Although CNT3 has also been described as being present along the rat nephron (Rodriguez-Mulero et al. 2005), little is currently known about its function in the uptake/reuptake of nucleoside-derived drugs. CNT3 is a concentrative nucleoside carrier with a wide substrate selectivity, accepting both purines and pyrimidines (Ritzel et al. 2001), and its role in drug transport is mostly unknown (Mangravite et al. 2003), being involved in the uptake of araC (Sarkar et al. 2005), and other non-nucleosidic drugs, such as the anthraycline pirarubicine (Nagai et al. 2005).
The aim of this study was to characterize the role of CNT3 in the transepithelial flux of natural nucleosides and some pharmacologically relevant derivatives in two different renal cell models, one of them endogenously expressing the murine orthologue of CNT3 and the other one ectopically expressing its human orthologue. Also, the accumulation and metabolism of the different substrates was analysed and the different routes of entry compared, thus enabling us to suggest that the apical localization of CNT3 was not only a key determinant of the transepithelial vectorial flux of nucleosides but also a major player in determining the metabolic fate of absorbed nucleosides and nucleoside-derived drugs.
Methods
Cell culture
Murine proximal convoluted tubule (PCT) cells were a kind gift from Prof Philippe Poujeol (Université de Nice-Sophia Antipolis, Nice, France). These cells were mantained at 37°C–5% CO2 in DMEM–F12 (1: 1) (Gibco) supplemented with 10% fetal bovine serum, 15 mm Hepes, 2 mm glutamine, 5 mg l−1 insulin, 50 nm dexamethasone, 10 μg l−1 EGF, 5 mg l−1 transferrin, 30 nm sodium selenite, 10 nm triiodotyronine (T3) and 250 μg ml−1 geneticin.
MDCK cells were also mantained at 37°C–5% CO2 in DMEM (BioWhittaker) supplemented with 10% fetal bovine serum. Cells were plated in transwell plates (Corning Costar, Cambridge, MA, USA; 12 mm diameter, 0.3 μm pore) and transfected with either hCNT3-pcDNA3.1 (for hCNT3-MDCK cells) or pcDNA3.1 (for mock-MDCK cells) as previously described (Harris et al. 2004).
Transport measurements were performed by incubating cell monolayers in an uptake buffer supplemented with either sodium or choline chloride, in which either [3H]uridine, [3H]guanosine or [3H]cytidine (Moravek Biochemicals, Brea, CA, USA) was added at a specific concentration of 1 μCi nmol−1. Incubations were stopped by rapidly aspirating the uptake buffer and immediate washing in a cold stop buffer, as previously described (del Santo et al. 1998).
RNA isolation and RT-PCR reaction
Total RNA was extracted from cultured PCT cells, kidney, liver and pancreas lysates using Rneasy Mini Kit (Qiagen, Barcelona, Spain). RNA was treated with DNase I from RNase-Free DNase Set (Qiagen) to eliminate contaminating DNA. In total, 1 μg RNA was retrotranscribed to cDNA using the TaqMan retrotranscription reagents as described by the manufacturer (Applied Biosystems, Foster City, CA, USA). The cDNA was used for PCR amplification. Different sets of primers were designed and synthesized for PCR analysis of each transporter. The primer pair used for amplifying mCNT1 was 5′-CAACACACAGAGGCAAAGAGAG-3′ and 5′-ACACCAGCAGCAAGGGCTAG-3′ which generated a 470 bp mCNT1 PCR product. For mCNT2, 5′-AAGTGACACAGGGACACAGCC-3′ and 5′-CTG-CACAAGGCCCAGGTAGTA-3′ generated a 734 bp mCNT2 PCR product. For mCNT3, 5′-GGACACGCCAAACAGGACGACAGGC-3′ and 5′-ATGATGGTATGGAGTTCCGACTTGG-3′ generated a 950 bp mCNT3 PCR product. Reactions were carried out under the following conditions: 1 min, 94°C; 1 min, 55°C (mCNT1 and mCNT2) and 57°C (mCNT3) and 3 min, 72°C for 40 cycles. Finally the PCR product was heated to 72°C for 15 min and cooled to 4°C. The amplified fragments were run in a 1% agarose gel.
Transwell transport experiments
PCT cells (4.2 × 105) were seeded on 12-well polycarbonate Transwell filter inserts (Corning Costar; 12 mm diameter, 0.3 μm pore) and cultured with regular changes of medium for 16–19 days after reaching confluence. To ensure that cells had polarized and formed tight junctions, transport experiments were conducted when the transepithelial electrical resistance (TEER) values (measured by millicell-ERS; Millipore, Bedford, MA, USA) reached 300–500 Ω cm2 in representative wells. MDCK were seeded as previously described. The Transwell filter inserts were washed three times in Na+-containing or Na+-free buffer, and then 1 μm, 1 μCi nmol−1[3H]cytidine or [3H]adenosine (Moravek Biochemicals) or 1 μm, 3 μCi nmol−1[3H]nucleoside-derived antineoplasic or antiviral drugs (gemcitabine, fludarabine, 5′DFUR, AZT and ribavirin; Moravek Biochemicals) was added to either the apical or the basal side. Phloridzin (200 μm) was added, simultaneously with the basolaterally added [3H]cytidine or adenosine, to the apical compartment of hCNT3-MDCK transfected cells, to analyse the role of hCNT3 in the apically released non-metabolized nucleoside reabsorption. Transport experiments were conducted with buffer (0.5 ml in the apical compartment and 0.5 ml in the basal compartment) containing sodium or choline on both sides of the Transwell filters. At various times (up to 20 min), 50 μl of buffer was collected from the opposite compartment, either apical or basal. The transport experiments were terminated by aspirating the buffer, and filters were washed with chilled buffer. The whole filter was wiped to remove any excess buffer, the filter was removed from the plastic support, and counted on a scintillation counter. The cells on the filters were solubilized by 0.1% SDS–100 mm NaOH.
HPLC analysis
To determine the possible metabolism of the assayed nucleoside or nucleoside derivative under the described conditions, buffer samples from the opposite compartment were collected. Samples of 200 μl were injected onto a C18 HPLC column (Kromasil 100 C18, 250 × 4 mm, 10 μm, Teknokroma, St Cugat, Barcelona, Spain.). Elution was performed by two gradient systems (1 ml min−1), using solvent A (ammonium acetate, 0.01 m) and solvent B (acetonitrile–H2O 1: 1) and the gradient programme was as follows: 0–10 min (99% A), 10–20 min (99% A to 50% A), 20–30 min (50% A to 99% A). The effluent was monitored at 260 nm. The column was equilibrated with the initial mobile phase for 20 min prior to the following injection. HPLC effluent fractions were collected and counted using a scintillation counter. Elution times of the different metabolites were confirmed by injection of cold standards and detection at 260 nm.
Gene construction
A 2.2 kb human renal CNT3 (hCNT3) fragment was amplified from kidney samples using primer pairs overlapping the start or end codons and cloned into pGem vector (BD Biosciences, Clontech, Palo Alto, CA, USA) This fragment was then subcloned into KpnI and PstI sites of the mammalian expression vector pcDNA3.1 (Invitrogen). Restriction sites were added using the following primers (in which restriction sites appear underlined): 5′-GGGGTACCGCATGGAGCTGAGGAG-3′ and 5′-AACTGCAGGGAGAAGAGGCTGACC-3′. To construct expression plasmids with tagged green fluorescent protein, pEGFP-C1 (Clontech) and hCNT3-pcDNA3.1 were double-digested with HindIII and PstI, resulting hCNT3 insert and pEGFP-C1 vector, purified, and ligated. The resulting constructs, hCNT3-pcDNA 3.1 and hCNT3-GFP, were used for transient transfection of the MDCK cell line.
Visualization of hCNT3 tagged with fluorescent protein
Cells (1.7 × 105) were grown in 12-well Corning Costar polycarbonate Transwell filter inserts for 24 h and then transfected as explained previously. Filters were washed in phosphate-buffered saline (PBS)–Ca2+–Mg2+, fixed with 3% paraformaldehyde (PFA) –0.06 m sucrose, excised and then loaded on a glass slide and coverslipped. Between the slide and the coverslip, an ∼1 mm gap was filled with aqua-poly/mount coverslipping medium (Polysciences, Inc., Warrington, PA). Actin filaments were stained using phalloidin-TRITC. Images were obtained using an Olympus Fluoview 500 laser-scanning confocal microscope equipped with He–Ne and Ar lasers as the light source. Images were captured by excitation at 488 nm and emission at 508 nm.
Results
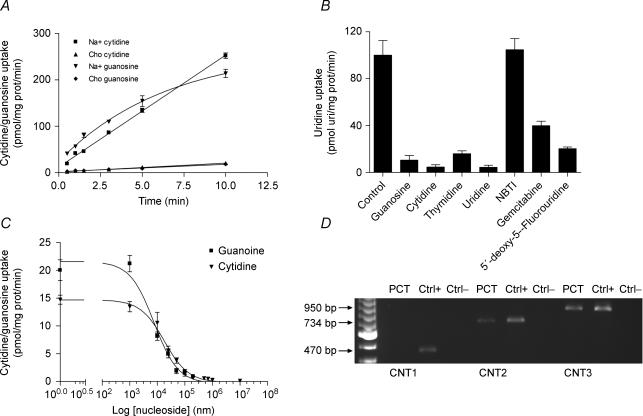
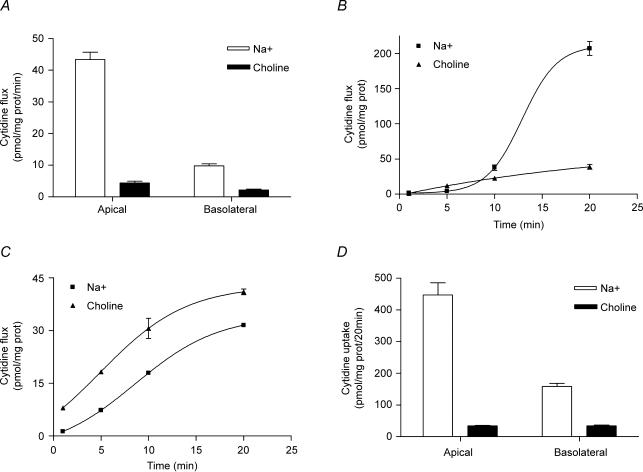
Nucleoside transport in PCT cells was characterized using either guanosine or cytidine as substrate and in both cases, a sodium-dependent transport component was evidenced (Fig. 1A). To analyse to which transport agency this component was attributable, sodium-dependent uridine transport was analysed and both guanosine and cytidine almost completely inhibited uridine transport (Fig. 1B), thus suggesting the presence of CNT3 as the only concentrative nucleoside transporter in PCT cells. This hypothesis was confirmed by the cross-inhibition of guanosine and cytidine sodium-dependent transport by the opposite nucleoside (Fig. 1C), which also enabled us to draw the respective Km values (7 μm and 16 μm for cytidine and guanosine, respectively) using the Cheng–Prusoff equation (Cheng & Prusoff, 1973). These kinetic data were consistent with molecular evidence generated by RT-PCR (Fig. 1D), showing no expression of CNT1, a faint band corresponding to CNT2-related mRNA, and a robust CNT3 fragment amplified from PCT cell-derived mRNA. To study the role of CNT3 in the transepithelial nucleoside flux across a layer of epithelial cells, PCT cells were grown on a transwell membrane to allow their polarization and traceable cytidine was added either to the apical or to the basal compartments, so that an apical-to-basal or a basal-to-apical flux was established. The sodium-dependent cytidine transport was higher when cytidine was added to the apical than to the basal compartment (Fig. 2A), although it was not negligible in this second case. In accordance, the net sodium-dependent, transepithelial flux of cytidine was higher in the apical-to-basal than in the opposite direction (Fig. 2B and C). All these results on transport and flux were corroborated by the evidence that intracellular accumulation of radioactive cytidine (or its metabolites) was also higher when cytidine was added to the apical than to the basal compartment (Fig. 2D).
Figure 1. Nucleoside transport characterization of PCT cell line.
A, time courses of [3H]cytidine and [3H]guanosine uptake by PCT cells. Uptake of 1 μm of cytidine and guanosine by differentiated PCT cells was measured. Total transport (Na+-containing medium) of cytidine (▪) and guanosine (▾) was compared with equilibrative transport (choline-containing medium) of both nucleosides (▴ and ♦, respectively). B, inhibition of Na+-dependent (mCNT3 mediated) [3H]uridine transport with 100 μm of guanosine, cytidine, thymidine, uridine, NBTI, gemcitabine and 5′-deoxy-5-fluorouridine (5′DFUR). C, cross-inhibition of guanosine transport with cytidine (▪) and cross-inhibition of cytidine transport with guanosine (▾). Km values were obtained using the Cheng–Prusoff equation. D, RT-PCR was used to amplify murine concentrative nucleoside transporter 1 (mCNT1), mCNT2 and mCNT3 mRNAs from PCT cell line. Kidney, liver and pancreas were used as positive controls, respectively. Representative agarose gel showing the amplification of cDNA fragments of the anticipated size is shown.
Figure 2. Vectorial flux of cytidine in PCT cell line.
A, [3H]cytidine uptake (1 min) into apical and basal compartments of polarized PCT cells, either in the presence (open bars) or in the absence (filled bars) of sodium. B and C, transepithelial flux of cytidine from the apical to the basolateral compartment (B) and from the basolateral to the apical compartment (C). D, intracellular accumulation of cytidine or its metabolites over a 20 min period. In all cases, results are expressed as the amount of nucleoside equivalents (in pmol) recovered in the opposite compartment to the one to which nucleoside was added (panels A, B and C) or accumulated in the cell monolayer (panel D). Data are expressed as the mean ±s.e.m. of uptake values obtained in three wells or filter inserts. Data are representative of three experiments carried out on different days on different cell batches.
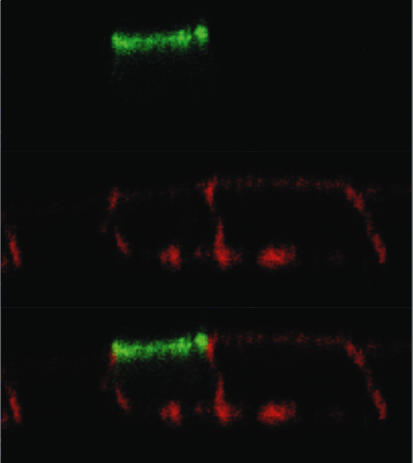
Due to the species origin of PCT cells, their endogenous CNT3 transporter was of murine origin. Thus, to examine whether the human CNT3 orthologue had a similar role in the transepithelial flux of nucleosides and nucleoside-derived drugs, hCNT3 was ectopically expressed in MDCK cells. Firstly, hCNT3 was cloned from human kidney and inserted into an expression vector. Secondly, a partial characterization of this clone in human HeLa cells resulted in the functional identification of a CNT3-type activity with kinetic parameters identical to those reported previously for CNT3 (data not shown) (Ritzel et al. 2001; Toan et al. 2003). Thirdly, hCNT3 was finally transfected into MDCK cells. MDCK mock-transfected cells do not express sodium-dependent activity and their transfection with hCNT3 resulted in the appearance of a CNT3 activity similar to that previously characterized in transfected HeLa cells (data not shown). By expressing an hCNT3-GFP construct, we also observed that hCNT3 was inserted at the apical membrane of polarized MDCK cells grown on a transwell dish (Fig. 3), thus indicating that hCNT3-MDCK cells are a suitable model to study the role of CNT3 in the transepithelial flux of nucleosides and nucleoside-derived drugs.
Figure 3. hCNT3 membrane insertion in polarized MDCK cell line.
MDCK cells were transfected with GFP-tagged hCNT3, and polarized by growth on permeable support. Cells were fixed, stained for actin with Texas Red-conjugated phalloidin and visualized by confocal microscopy. Red, actin; green, GFP.
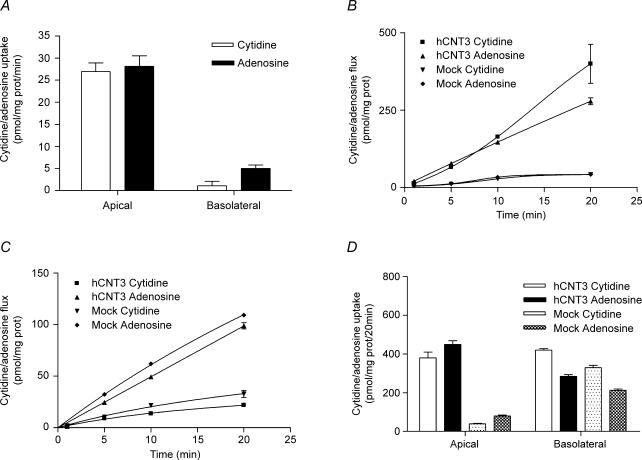
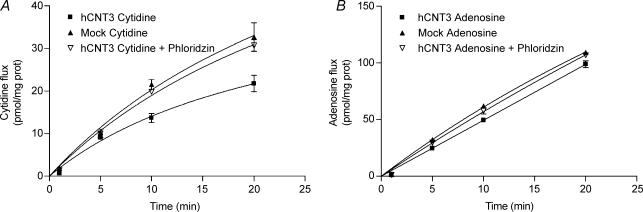
Once the functionality of hCNT3-MDCK cells was determined, a parallel study to that performed in PCT cells was carried out (Fig. 4). As expected, cytidine and adenosine transport in the presence of sodium was higher when analysed in the apical-to-basal than in the opposite direction (Fig. 4A), as it was the net transepithelial flux measured in a sodium medium (Fig. 4B and C) when compared with the mock-transfected cells. A net sodium-dependent nucleoside intracellular accumulation was only observed in hCNT3-MDCK cells, particularly when analysed in the apical-to-basal direction, but not in mock-MDCK cells (Fig. 4D). When studying nucleoside flux in the basal-to-apical direction, a lower flux was registered in the presence of active CNT3 compared with that in its absence (Fig. 2C for PCT cells and Fig. 4C for hCNT3- and mock-transfected MDCK cells). To determine if this effect was due to the presence of an active CNT3 at the apical membrane, the same experiment was repeated with hCNT3-MDCK cells with either cytidine (Fig. 5A) or adenosine (Fig. 5B) in the basal compartment, but adding phloridzin, an inespecific CNT inhibitor concentrative nucleoside transport inhibitor (Toan et al. 2003), to the apical compartment. The presence of phloridzin when assaying a sodium medium increased the ‘apparent basal-to-apical flux’ to identical values to those for the mock-transfected cells.
Figure 4. Vectorial flux of cytidine and adenosine in hCNT3- and mock-transfected MDCK cell line.
A, sodium-dependent [3H]cytidine and [3H]adenosine uptake (1 min) into apical and basal compartments of polarized hCNT3-MDCK cells. B and C, transepithelial flux of cytidine and adenosine, measured in a sodium medium, from the apical to the basolateral compartment (B) and from the basolateral to the apical compartment (C) in hCNT3-MDCK and mock-transfected cells. D, intracellular accumulation measured after 20 min in hCNT3-MDCK and mock-MDCK cells. In all cases, data are expressed as described in the legend of Fig. 2.
Figure 5. Characterization of the basal-to-apical transepithelial flux of natural nucleosides in hCNT3- and mock transfected MDCK cells.
Effect of phloridzin on the basal-to-apical transepithelial flux of cytidine (A) or adenosine (B) in hCNT3-MDCK cells, measured in a sodium medium. Data data are expressed as described in the legend of Fig. 2.
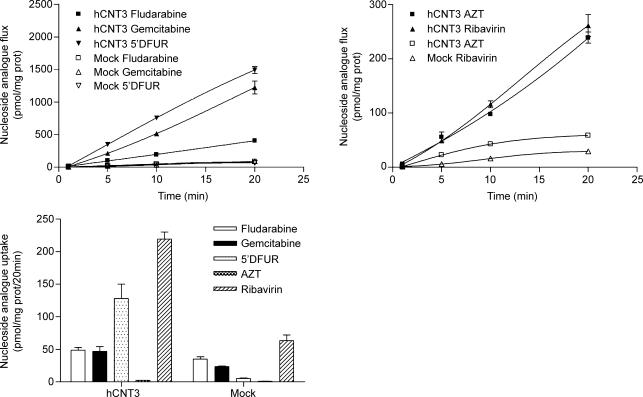
Since these cells expressed the human CNT3 orthologue, the net transepithelial flux of some nucleoside-derived drugs with antitumoural (Fig. 6A) or antiviral (Fig. 6B) activity was also determined. In the first case, the pyrimidine-derived drugs gemcitabine and 5′DFUR were readily transferred in a sodium medium from the apical to the basal compartment, at a higher rate than cytidine and adenosine, compared to the mock-transfected cells, while the purine-derived drug fludarabine was transferred at a similar rate to cytidine. The antiviral drugs ribavirin or AZT were also transferred in the apical-to-basal direction in the presence of sodium, but to a lesser extent than any of the antitumoural drugs tested.
Figure 6. Vectorial flux of nucleoside derived drugs in hCNT3- and mock-transfected MDCK cell line.
Transepithelial flux of antineoplasic (A) and antiviral (B) nucleoside derived drugs, measured in a sodium medium, from apical to basolateral compartment in hCNT3-MDCK and mock-transfected cells. Results are expressed as the amount of drug equivalents (in pmol) recovered in the opposite compartment to the one to which drug was added. Data are expressed as the mean ±s.e.m. of uptake values obtained in three wells or filter inserts. Data are representative of three experiments carried out on different days on different cell batches.
Although we have referred so far to the secreted compounds as ‘nucleosides’, we don't know the chemical nature of the tracer molecules appearing at the opposite side. When these compounds were analysed in terms of the chemical species derived from the traceable substrate, a different profile of labelled molecules was obtained when substrates were transported either through CNT3 or through the equilibrative endogenous transporters (Table 1 and Supplemental Fig. 1). Both in PCT and in hCNT3-MDCK, cytidine radioactivity was recovered mostly as uridine and uracil when the transepithelial flux was analysed in a sodium medium, and also adenosine was transformed mostly to nucleobases in these conditions. In constrast, cytidine and adenosine taken up through equilibrative transporters were released mostly unaltered to the opposite compartment. Pharmacologically active nucleoside derivatives, however, were transferred virtually unaltered regardless of the transporter responsible for their uptake. Obviously, this preferential metabolic fate of nucleosides depending on the carrier is not strict, since almost all species can be derived from any single nucleoside regardless of the participating transporter. However, the relative abundance of some species makes it clear that some relevant metabolic differences take place depending on the transporter that has internalized the substrate nucleoside. In this sense, levels of nucleobases are about 5-fold higher with an active hCNT3 than in its absence, with nucleoside levels decreased in a similar proportion. Similar results were found using MDCK cells expressing either the human concentrative nucleoside transporter 1 (hCNT1) or the rat concentrative nucleoside transporter 2 (rCNT2) (data not shown), thus showing that this can be a general feature common to the concentrative nucleoside transporters known to be present at the epithelial cells, but not to the equilibrative nucleoside transporters.
Table 1.
Metabolic profile of natural nucleosides and nucleoside derived drugs
| Molecular species | PCT (NaCl) | PCT (ChoCl) | MDCK-hCNT3 | MDCK-mock |
|---|---|---|---|---|
| A, cytidine | ||||
| Apical to basolateral (% of recovered from basolateral) | ||||
| Phosphorylated | 17.1 ± 3.2 | 15.2 ± 2.2 | 16.6 ± 5.1 | 24.5 ± 6.3 |
| Uracil | 35.2 ± 2.3*** | 9.7 ± 1.1 | 67.4 ± 3.4*** | 11.8 ± 2.3 |
| Cytidine | 8.9 ± 2.2*** | 58.7 ± 4.4 | 13.6 ± 2.1** | 60.9 ± 5.3 |
| Uridine | 37.8 ± 3.3** | 16.4 ± 1.8 | 2.4 ± 0.8 | 2.8 ± 1.1 |
| Basolateral to apical (% of recovered from apical) | 6.6 ± 3.1 | 4.1 ± 1.1 | 22.4 ± 4.5 | 25.7 ± 5.2 |
| Phosphorylated | 6.6 ± 3.1 | 4.1 ± 1.1 | 22.4 ± 4.5 | 25.7 ± 5.2 |
| Uracil | 13.2 ± 4.2 | 10.5 ± 3.2 | 10.6 ± 2.2 | 5.0 ± 4.3 |
| Cytidine | 66.9 ± 5.3 | 73.1 ± 6.5 | 54.3 ± 5.5 | 63.7 ± 4.6 |
| Uridine | 13.1 ± 3.2 | 12.3 ± 4.1 | 12.7 ± 3.3 | 5.6 ± 4.3 |
| B, adenosine | ||||
| Apical to basolateral (% of recovered from basolateral) | ||||
| Phosphorylated | — | — | 21.3 ± 2.2 | 24.2 ± 3.2 |
| Nucleobases | — | — | 57.3 ± 3.4*** | 10.2 ± 1.1 |
| Adenosine | — | — | 11.2 ± 3.1*** | 59.8 ± 4.4 |
| Uric acid | — | — | 10.2 ± 2.5 | 5.8 ± 1.4 |
| Basolateral to apical (% of recovered from apical) | 27.4 ± 3.3 | 23.2 ± 2.5 | ||
| Phosphorylated | — | — | 27.4 ± 3.3 | 23.2 ± 2.5 |
| Nucleobases | — | — | 12.1 ± 2.1 | 9.1 ± 1.6 |
| Adenosine | — | — | 55.2 ± 3.5 | 60.2 ± 4.3 |
| Uric acid | — | — | 5.3 ± 1.3 | 7.5 ± 2.2 |
| C, antiviral and antineoplasic drugs | ||||
| Apical to basolateral (% of recovered from basolateral) | ||||
| Fludarabine | — | — | 75.2 ± 8.6 | 90.2 ± 10.1 |
| Other | — | — | 24.8 ± 3.3 | 9.8 ± 5.3 |
| Gemcitabine | — | — | 93.1 ± 7.3 | 98.2 ± 9.8 |
| Other | — | — | 6.9 ± 3.2 | 1.8 ± 0.8 |
| 5′DFUR | — | — | 95.1 ± 5.5 | 99.2 ± 7.2 |
| Other | — | — | 4.9 ± 2.2 | 0.8+/0.5 |
| AZT | — | — | 98.2 ± 9.4 | 99.5 ± 10.2 |
| Other | — | — | 1.8 ± 0.2 | 0.5 ± 0.09 |
| Ribavirin | — | — | 62.2 ± 5.6 | 85.2 ± 7.7 |
| Other | — | — | 37.8 ± 5.4 | 14.8 ± 4.1 |
Metabolism of exogenous [3H] labelled cytidine (A) adenosine (B) or antiviral and antineoplasic drugs (C) during transport by polarized PCT, hCNT3-MDCK and mock-transfected cells. Each metabolite is expressed as the percentage of radioactivity appearing in the medium. Statistical analysis by Student's t test: PCT NaCl versus PCT ChoCl and hCNT3-MDCK cells versus mock-transfected cells
P < 0.01
P < 0.001. Data are expressed as the mean ±s.e.m. of radioactivity values obtained in three wells or filter inserts. Data are representative of three experiments carried out on different days on different cell batches.
Discussion
Concentrative nucleoside transporters are difficult to study since they are usually absent or poorly expressed in most cell lines. This lack of good models hinders many biochemical and pharmacological studies on the internalization of nucleoside-derived drugs. Such studies are particularly relevant in the kidney, since transport of molecules across the apical membrane of kidney epithelial cells is a major regulator of their blood concentrations. We had previously detected CNT3 along the rat nephron, especially in the proximal convoluted tubule and the cortical collector duct. In the present study, we also identified CNT3 in human kidney. Although previous reports did not detect CNT3-related mRNA in kidney (Ritzel et al. 2001), those studies were performed by using a Northern blot analysis of whole organ RNA. Here, we identified CNT3-related mRNA in human renal samples by real time PCR, cloned it from the same source, and sequenced it, thus providing further evidence for CNT3 expression in the kidney. The low levels of its mRNA and its expression restricted to a few cell types may explain why it was not detected in previous studies. On the other hand, as for CNT1 in liver parenchyal cells (Felipe et al. 1998), low mRNA levels do not necessarily imply low expression of the protein, nor low CNT3-related activity. In a previous study (Rodríguez-Mulero et al. 2005), rCNT3 mRNA was detected in regions of the renal tubule showing nucleoside transport activity in which adenosine (actively transported by CNT3 as shown in the present report) has very relevant physiological functions. These two studies support a role of CNT3 in renal physiology.
In this study, we describe a cell model derived from murine kidney cells, PCT cells, which retain many proximal convoluted tubule features and express substantial amounts of endogenous mCNT3 activity. Since these cells are easily polarizable when grown on transwell plates, we have also characterized the role played by mCNT3 in the establishment of a transepithelial flux of nucleosides. However, since from a pharmacological point of view the relevant studies concern the human orthologues of these transporters, we have also generated a suitable model for the study of the pharmacological properties of hCNT3 by transfecting it into MDCK cells.
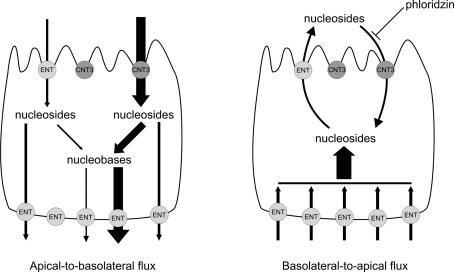
Concentrative cytidine transport in polarized PCT cells and in MDCK cells transfected with human CNT3 is mostly apical, in accordance with previous studies with CNT1 and CNT2 in different renal cell lines (Lai et al. 2002; Mangravite et al. 2003). In fact, a significant sodium-dependent cytidine flux is observed only in an apical-to-basal direction in both cell lines. A similar result was obtained in MDCK cells expressing hCNT1 and hENT1 using adenosine as a substrate (Lai et al. 2002). When analysing the basolateral-to-apical flux, a significant sodium-independent nucleoside flux is detected, about 20% that of the apical-to-basolateral flux. This flux has been shown to be generated by the presence of ENT transporters at the basal and at the apical membrane, thus creating a physiological pathway that can be used for excretion of nucleosides and nucleoside derivatives such as deoxynucleosides or pharmacological derivatives (Lai et al. 2002), although the excretion rate is much more favourable for nucleosidic drugs than for natural nucleosides. The different amount of ENTs expressed at the basal and apical membranes, much higher in the former than in the latter in MDCK cells (Mangravite et al. 2003), may confer some concentrative capacity to epithelial cells if nucleoside uptake is coupled to metabolism, exactly as described in the present study. The sodium-dependent component of the basal-to-apical flux is lower than the sodium-independent part, and this could be attributed to the presence of an active CNT3 in the apical membrane re-internalizing some of the nucleosides released by the cells to the apical compartment. It is remarkable that this effect takes place very quickly once the basolateral uptake of nucleosides has begun, since from the first time points analysed there is a difference between the assays in the presence and in the absence of an active concentrative transporter. The addition of the CNT activity inhibitor phloridzin to the apical compartment clearly shows this role of CNT3 in the re-absorption of released nucleosides, a role that can have physiological relevance. These results pinpoint the importance of CNT3 in establishing a concentrative net flux of nucleosides from the tubule lumen to the circulation and are summarized in the diagram in Fig. 7.
Figure 7. Diagram representing the role of CNT3 and equilibrative transporters (ENTs) in the transepithelial flux of nucleosides.
In the apical-to-basolateral flux (left side), the presence of an active CNT3 at the plasma membrane allows a quantitatively much more significant uptake of nucleosides through CNT3 than through ENTs, both for its higher abundance as well as, particularly, for its concentrative capacity. Once inside the cell, nucleosides carried by CNT3 are more readily metabolized to nucleobases than those transported by ENTs, and this can be seen both in the intracellular contents in each case and in the proportions of each released to the basolateral side. Regarding the basolateral-to-apical flux (right side), entrance of nucleosides is granted by the large amount of ENTs hosted at the basal membrane. Most of these nucleosides are addressed to the metabolic machinery of the cell and readily converted into different chemical species (mainly phosphorylated derivatives), thus allowing a large accumulation of nucleoside derivatives in the epithelial cell. Some of the internalized nucleosides, however, are released to the apical compartment by the few ENTs present at the apical membrane. These released nucleosides are then re-internalized by CNT3 when it is in an active state, thus revealing an ‘apparent sodium-dependent flux’ lower than the ‘apparent sodium-independent flux’. This phenomenon can be abolished by the presence of phloridzin, an inhibitor of concentrative nucleoside transport, in the apical compartment, which renders both sodium-dependent and sodium-independent fluxes identical.
hCNT3 also mediates the transepithelial flux of all pharmacologically active nucleoside-derived drugs tested (AZT, ribavirin, gemcitabine, fludarabine and 5′-DFUR). In all cases (except with ribavirin), intracellular accumulation is quite low and most of the internalized drug is released to the basal compartment. Since it is a broad selectivity concentrative nucleoside transporter, these results depict hCNT3 as a key player in the renal reabsorption of natural nucleosides and pharmacologically active derivatives, maybe even affecting their bioavailability. Of particular interest is the fact that antiviral nucleoside-derived drugs such as AZT or ribavirin are readily transferred from the apical to the basal compartment in the presence of CNT3. AZT is not easily transported by other nucleoside transporters, such as hCNT1 (Cano-Soldado et al. 2004), so hCNT3 could be of key importance in their pharmacokinetics.
A second relevant aspect of our study is the importance of the entry pathway in the metabolism of the transported nucleoside. The chemical species in which a substrate nucleoside is accumulated intracellularly or released to the opposite compartment is crucially dependent on the transporter through which the substrate has been previously internalized. In all three cell models assayed (PCT, mock-MDCK and hCNT3-MDCK), the nucleoside transported through equilibrative carriers is released almost completely unaltered to the opposite compartment, regardless of the direction of the flux. When there is an active CNT3, however, the main chemical species released to the basal compartment in a sodium medium are nucleobases (and also uridine for PCT) with only a minor fraction being non-metabolized nucleoside. These results suggest a functional relationship between the CNT3 carrier and some nucleoside metabolizing enzymes, while the equilibrative transporters would either direct nucleoside towards its phosphorylation or leave it unmetabolized. This effect seems not particularly restricted to CNT3 since other concentrative nucleoside carriers such as hCNT1 and rCNT2 yielded similar results (data not shown). In a previous study (He et al. 1994) using CaCo2 human intestinal cells, similar differences in nucleoside metabolism results were found, although that study was done at very high nucleoside concentrations, while we used more physiological concentrations of cytidine and adenosine (Saito et al. 1999; Zou et al. 1999). Nucleoside-derived drugs, in general, are much less metabolized than natural nucleosides.
In agreement with our results, Bronk & Hastewell (1988) also recovered high levels of uracil and little cytidine in rat jejunum when cytidine was added to the apical compartment. In jejunal cells, there are reports of the presence of CNT1, CNT2 (Patil & Unadkat, 1997), CNT3 (Ritzel et al. 2001), ENT1 (Sundaram et al. 1998) and ENT2 (Wu et al. 2005). One possible explanation for the almost complete metabolism of the cytidine taken up by CNT3 is the existence of a complex constituted by this carrier and some of the enzymes of cytidine metabolism. In support of this hypothesis, Bose & Yamada (1977) showed that a fraction of uridine phosphorylase is associated with the plasma membrane, and this fraction could be part of this putative complex. An alternative explanation is that CNT3 allows higher intracellular concentrations of nucleoside, and thus some metabolic pathways could be favoured by a mass law effect. However, our results preclude such a hypothesis, since in mock-MDCK cells (which show no CNT3 activity), the basal-to-apical flux reaches high intracellular nucleoside levels due to the asymmetric distribution of equilibrative transporters in both membranes and little metabolism of cytidine to uridine or uracil is seen.
On the other hand, nucleoside taken up by equilibrative transporters seems to be directed primarily towards its phosphorylation, since high intracellular levels of phosphorylated nucleotides are found whenever ENTs are the major carriers functioning in the cells. These nucleotides could be used for nucleic acid synthesis, both in the nucleus and in the mitochondria, as recently reported (Zhang et al. 2006). This relatively high phosphorylation rate could also be responsible for the intracellular nucleos(t)ide levels found in mock-MDCK cells when analysing the basal-to-apical vectorial flux, almost as high as in hCNT3-MDCK cells apical-to-basal flux measured in sodium medium. Although the more abundant equilibrative transport activity in the basal membrane of MDCK cells compared to the apical membrane plays a role in this accumulation, such intracellular levels could hardly be explained if the retained nucleosides were not phosphorylated. This equilibrative activity in mock-MDCK and hCNT3-MDCK cells is mostly mediated by ENT1, since it is the main equilibrative transporter in these cells (Hammond et al. 2004) and the minor ENT2 transporter shows a much higher Km for cytidine (Ward et al. 2000), so its contribution to the total transepithelial cytidine flux would be almost negligible. However, ENT2 plays an important role in the release of the uracil formed in the cell, since this molecule cannot be transported by ENT1 (Baldwin et al. 2004).
In summary, CNT3 plays a crucial role in the transepithelial flux of natural nucleosides and their pharmacologically active derivatives, thus affecting their bioavailability. We have demonstrated that heterologous expression of hCNT3 in MDCK cells reproduces the transepithelial flux obtained in PCT cells, a model expressing its own endogenous CNT3 transporter. Besides, the entrance pathway of nucleosides in a cell has a determinant influence in the metabolism of these molecules and, thus, in their metabolic fates. Our results indicate that nucleoside transporters and nucleoside metabolizing enzymes act in a coordinate way to establish the metabolic destination of these molecules. The adaptive advantages of such a coordination between concentrative transport and metabolism must be related to the function of these transporters. Equilibrative transporters seem to act as the main suppliers of nucleosides to the cell in many cell types, but concentrative transporters play additional roles to the elementary nucleoside supply function. They allow higher concentrative power (this is particularly relevant in the case of CNT3 with its 2 Na+: 1 nucleoside coupling ratio), thus making them the best suited for absorption/reabsorption of nucleosides by epithelial cells, but they also play additional roles mostly related to the metabolic regulation of the cell. In this sense, CNT2 has been related to both purinergic regulation (Duflot et al. 2004) and AMP-dependent kinase activity (Aymerich et al. 2006) and a close relationship between transporter function and metabolism may help to fine tune this regulatory processes.
Acknowledgments
This work was supported by grants PI-020934 from Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo, to F.J.C. and SAF2005–01259 from Dirección General de Ciencia y Tecnología, Ministerio de Educación y Ciencia, to M.P.-A., and 2005SGR00315 from Direcció General de Recerca, DURSI, Generalitat de Catalunya, to M.P.-A. The Regulation of Transport Systems Research Group (RST) is a member of the Biomedical Research Institute Network (CIBER) of Liver and Gastrointestinal Diseases. E.E.-M. was the recipient of a FPU fellowship from Ministerio de Educación y Ciencia. The authors want to thank Dr Philippe Poujeol for kindly providing PCT cells for this study and his critical reading of the manuscript and suggestions.
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.130138/DC1 and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.130138
References
- Aymerich I, Foufelle F, Ferre P, Casado FJ, Pastor-Anglada M. Extracellular adenosine activates AMP-dependent protein kinase (AMPK) J Cell Sci. 2006;119:1612–1621. doi: 10.1242/jcs.02865. [DOI] [PubMed] [Google Scholar]
- Aymerich I, Pastor-Anglada M, Casado FJ. Long term endocrine regulation of nucleoside transporters in rat intestinal epithelial cells. J Gen Physiol. 2004;124:505–512. doi: 10.1085/jgp.200409086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Mackey JR, Cass CE, Young JD. Nucleoside transporters: molecular biology and implications for therapeutic development. Mol Med Today. 1999;5:216–224. doi: 10.1016/S1357-4310(99)01459-8. [DOI] [PubMed] [Google Scholar]
- Bose R, Yamada EW. Uridine phosphorylase activity of isolated plasma membranes of rat liver. Can J Biochem. 1977;55:528–533. doi: 10.1139/o77-075. [DOI] [PubMed] [Google Scholar]
- Bronk JR, Hastewell JG. The transport and metabolism of naturally occurring pyrimidine nucleosides by isolated rat jejunum. J Physiol. 1988;395:349–361. doi: 10.1113/jphysiol.1988.sp016923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Soldado P, Larrayoz IM, Molina-Arcas M, Casado FJ, Martinez-Picado J, Lostao MP, Pastor-Anglada M. Interaction of nucleoside inhibitors of HIV-1 reverse transcriptase with the concentrative nucleoside transporter-1 (SLC28A1) Antivir Ther. 2004;9:993–1002. [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- del Santo B, Valdés R, Mata J, Felipe A, Casado FJ, Pastor-Anglada M. Differential expression and regulation of nucleoside transport systems in rat liver parenchymal and hepatoma cells. Hepatology. 1998;28:1504–1511. doi: 10.1002/hep.510280609. [DOI] [PubMed] [Google Scholar]
- Duflot S, Riera B, Fernandez-Veledo S, Casado V, Norman RI, Casado FJ, Lluis C, Franco R, Pastor-Anglada M. ATP-sensitive K+ channels regulate the concentrative adenosine transporter CNT2 following activation by A1 adenosine receptors. Mol Cell Biol. 2004;24:2710–2719. doi: 10.1128/MCB.24.7.2710-2719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felipe A, Valdés R, Santo B, Lloberas J, Casado J, Pastor-Anglada M. Na+-dependent nucleoside transport in liver: two different isoforms from the same gene family are expressed in liver cells. Biochem J. 1998;330:997–1001. doi: 10.1042/bj3300997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith DA, Jarvis SM. Nucleoside and nucleobase transport systems of mammalian cells. Biochim Biophys Acta. 1996;1286:153–181. doi: 10.1016/s0304-4157(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Gutierrez MM, Giacomini KM. Substrate selectivity, potential sensitivity and stoichiometry of Na+-nucleoside transport in brush border membrane vesicles from human kidney. Biochim Biophys Acta. 1993;1149:202–208. doi: 10.1016/0005-2736(93)90202-b. [DOI] [PubMed] [Google Scholar]
- Hammond JR, Stolk M, Archer RG, McConnell K. Pharmacological analysis and molecular cloning of the canine equilibrative nucleoside transporter 1. Eur J Pharmacol. 2004;491:9–19. doi: 10.1016/j.ejphar.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Kagawa T, Dawson PA, Arias IM. Taurocholate transport by hepatic and intestinal bile acid transporters is independent of FIC1 overexpression in Madin-Darby canine kidney cells. J Gastroenterol Hepatol. 2004;19:819–825. doi: 10.1111/j.1440-1746.2004.03347.x. [DOI] [PubMed] [Google Scholar]
- He Y, Sanderson IR, Walter WA. Uptake, transport and metabolism of exogenous nucleosides in intestinal epithelial cell cultures. J Nutr. 1994;124:1942–1949. doi: 10.1093/jn/124.10.1942. [DOI] [PubMed] [Google Scholar]
- Lai Y, Bakken AH, Unadkat JD. Simultaneous expression of hCNT1-CFP and hENT1-YFP in Madin-Darby canine kidney cells. Localization and vectorial transport studies. J Biol Chem. 2002;277:37711–37717. doi: 10.1074/jbc.M204986200. [DOI] [PubMed] [Google Scholar]
- Lostao MP, Mata JF, Larrayoz IM, Inzillo SM, Casado FJ, Pastor-Anglada M. Electrogenic uptake of nucleosides and nucleoside-derived drugs by the human nucleoside transporter 1 (hCNT1) expressed in Xenopus laevis oocytes. FEBS Lett. 2000;481:137–140. doi: 10.1016/s0014-5793(00)01983-9. [DOI] [PubMed] [Google Scholar]
- Mangravite LM, Badagnani I, Giacomini KM. Nucleoside transporters in the disposition and targeting of nucleoside analogs in the kidney. Eur J Pharmacol. 2003;479:269–281. doi: 10.1016/j.ejphar.2003.08.076. [DOI] [PubMed] [Google Scholar]
- Mata JF, Garcia-Manteiga JM, Lostao MP, Fernandez-Veledo S, Guillen-Gomez E, Larrayoz IM, Lloberas J, Casado FJ, Pastor-Anglada M. Role of the human concentrative nucleoside transporter (hCNT1) in the cytotoxic action of 5′-deoxy-5-fluorouridine, an active intermediate metabolite of capecitabine, a novel oral anticancer drug. Mol Pharmacol. 2001;59:1542–1548. doi: 10.1124/mol.59.6.1542. [DOI] [PubMed] [Google Scholar]
- Nagai K, Nagasawa K, Fujimoto S. Uptake of the anthracycline pirarubicin into mouse M5076 ovarian sarcoma cells via a sodium-dependent nucleoside transport system. Cancer Chemother Pharmacol. 2005;55:222–230. doi: 10.1007/s00280-004-0861-7. [DOI] [PubMed] [Google Scholar]
- Ngo LY, Patil SD, Unadkat JD. Ontogenic and longitudinal activity of Na+-nucleoside transporters in the human intestine. Am J Physiol Gastrointest Liver Physiol. 2001;280:G475–G481. doi: 10.1152/ajpgi.2001.280.3.G475. [DOI] [PubMed] [Google Scholar]
- Pastor-Anglada M, Cano-Soldado P, Molina-Arcas M, Lostao MP, Larrayoz I, Martinez-Picado J, Casado FJ. Cell entry and export of nucleoside analogues. Virus Res. 2005;107:151–164. doi: 10.1016/j.virusres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Pastor-Anglada M, Felipe A, Casado FJ. Transport and mode of action of nucleoside derivatives used in chemical and antiviral therapies. Trends Pharmacol Sci. 1998;19:424–430. doi: 10.1016/s0165-6147(98)01253-x. [DOI] [PubMed] [Google Scholar]
- Patil SD, Unadkat JD. Sodium-dependent nucleoside transport in the human intestinal brush-border membrane. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1314–G1320. doi: 10.1152/ajpgi.1997.272.6.G1314. [DOI] [PubMed] [Google Scholar]
- Quaroni A, Tian JQ, Goke M, Podolsky DK. Glucocorticoids have pleiotropic effects on small intestinal crypt cells. Am J Physiol Gastrointest Liver Physiol. 1999;277:G1027–G1040. doi: 10.1152/ajpgi.1999.277.5.G1027. [DOI] [PubMed] [Google Scholar]
- Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, Ritzel RG, Mowles DA, Carpenter P, Chen XZ, Karpinski E, Hyde RJ, Baldwin SA, Cass CE, Young JD. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib) J Biol Chem. 2001;276:2914–2927. doi: 10.1074/jbc.M007746200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mulero S, Errasti-Murugarren E, Ballarin J, Felipe A, Doucet A, Casado FJ, Pastor-Anglada M. Expression of concentrative nucleoside transporters SLC28 (CNT1, CNT2, and CNT3) along the rat nephron: effect of diabetes. Kidney Int. 2005;68:665–672. doi: 10.1111/j.1523-1755.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- Saito H, Nishimura M, Shinano H, Makita H, Tsujino I, Shibuya E, Sato F, Miyamoto K, Kawakami Y. Plasma concentrations of adenosine during normoxia and moderate hypoxia in humans. Am J Respir Crit Care Med. 1999;159:1014–1018. doi: 10.1164/ajrccm.159.3.9803100. [DOI] [PubMed] [Google Scholar]
- Sarkar M, Han T, Damaraju V, Carpenter P, Cass CE, Agarwal RP. Cytosine arabinoside affects multiple cellular factors and induces drug resistance in human lymphoid cells. Biochem Pharmacol. 2005;70:426–432. doi: 10.1016/j.bcp.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Smith KM, Ng AM, Yao SY, Labedz KA, Knaus EE, Wiebe LI, Cass CE, Baldwin SA, Chen XZ, Karpinski E, Young JD. Electrophysiological characterization of a recombinant human Na+-coupled nucleoside transporter (hCNT1) produced in Xenopus oocytes. J Physiol. 2004;558:807–823. doi: 10.1113/jphysiol.2004.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram M, Yao SY, Ng AM, Griffiths M, Cass CE, Baldwin SA, Young JD. Chimeric constructs between human and rat equilibrative nucleoside transporters (hENT1 and rENT1) reveal hENT1 structural domains interacting with coronary vasoactive drugs. J Biol Chem. 1998;273:21519–21525. doi: 10.1074/jbc.273.34.21519. [DOI] [PubMed] [Google Scholar]
- Toan SV, To KW, Leung GP, de Souza MO, Ward JL, Tse CM. Genomic organization and functional characterization of the human concetrative nucleoside transporter-3 isoform expressed in mammalian cells. Pflugers Arch. 2003;447:195–204. doi: 10.1007/s00424-003-1166-0. [DOI] [PubMed] [Google Scholar]
- Valdés R, Ortega MA, Casado FJ, Felipe A, Gil A, Sanchez-Pozo A, Pastor-Anglada M. Nutritional regulation of nucleoside transporter expression in rat small intestine. Gastroenterology. 2000;119:1623–1630. doi: 10.1053/gast.2000.20183. [DOI] [PubMed] [Google Scholar]
- Van Aubel RA, Masereeuw R, Russel FG. Molecular pharmacology of renal organic anion transporters. Am J Physiol Renal Physiol. 2000;279:F216–F232. doi: 10.1152/ajprenal.2000.279.2.F216. [DOI] [PubMed] [Google Scholar]
- Ward JL, Sherali A, Mo ZP, Tse CM. Kinetic and pharmacological properties of cloned human equilibrative nucleoside transporters, ENT1 and ENT2, stably expressed in nucleoside transporter-deficient PK15 cells. Ent2 exhibits a low affinity for guanosine and cytidine but a high affinity for inosine. J Biol Chem. 2000;275:8375–8381. doi: 10.1074/jbc.275.12.8375. [DOI] [PubMed] [Google Scholar]
- Wu SK, Ann DK, Kim KJ, Lee VH. Fine tuning of rabbit equilibrative nucleoside transporter activity by an alternatively spliced variant. J Drug Target. 2005;13:521–533. doi: 10.1080/10611860500403099. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sun X, Smith KM, Visser F, Carpenter P, Barron G, Peng Y, Robins MJ, Baldwin SA, Young JD, Cass CE. Studies of nucleoside transporters using novel autofluorescent nucleoside probes. Biochemistry. 2006;45:1087–1098. doi: 10.1021/bi0520535. [DOI] [PubMed] [Google Scholar]
- Zou AP, Nithipatikom K, Li PL, Cowley AW. Role of medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol Reg Int Comp Physiol. 1999;45:R790–R798. doi: 10.1152/ajpregu.1999.276.3.R790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.130138/DC1 and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.130138