Abstract
Neurons of the preBötzinger complex (preBötC) form local excitatory networks and synchronously discharge bursts of action potentials during the inspiratory phase of respiratory network activity. Synaptic input periodically evokes a Ca2+-activated non-specific cation current (ICAN) postsynaptically to generate 10–30 mV transient depolarizations, dubbed inspiratory drive potentials, which underlie inspiratory bursts. The molecular identity of ICAN and its regulation by intracellular signalling mechanisms during inspiratory drive potential generation remains unknown. Here we show that mRNAs coding for two members of the transient receptor potential (TRP) family of ion channels, namely TRPM4 and TRPM5, are expressed within the preBötC region of neonatal mice. Hypothesizing that the phosphoinositides maintaining TRPM4 and TRPM5 channel sensitivity to Ca2+ may similarly influence ICAN and thus regulate inspiratory drive potentials, we manipulated intracellular phosphatidylinositol 4,5-bisphosphate (PIP2) and measured its effect on preBötC neurons in the context of ongoing respiratory-related rhythms in slice preparations. Consistent with the involvement of TRPM4 and TRPM5, excess PIP2 augmented the inspiratory drive potential and diminution of PIP2 reduced it; sensitivity to flufenamic acid (FFA) suggested that these effects of PIP2 were ICAN mediated. Inositol 1,4,5-trisphosphate (IP3), the product of PIP2 hydrolysis, ordinarily causes IP3 receptor-mediated ICAN activation. Simultaneously increasing PIP2 while blocking IP3 receptors intracellularly counteracted the reduction in the inspiratory drive potential that normally resulted from IP3 receptor blockade. We propose that PIP2 protects ICAN from rundown by interacting directly with underlying ion channels and preventing desensitization, which may enhance the robustness of respiratory rhythm.
Rhythmically active networks throughout the CNS generate activity patterns ranging from locomotor bursts (< 1 Hz) to gamma voltage oscillations (30–90 Hz) (Buzsáki, 2006). The characteristic features of these rhythms can often be attributed to specific ion channels and intracellular regulatory mechanisms that control the voltage trajectories of the constituent neurons and ultimately govern the form of the network output (Grillner, 2003, 2006; Bartos et al. 2007).
Breathing is a particularly important rhythmic behaviour in mammals that originates in the brainstem. Neurons within the ventral medullary site dubbed the preBötzinger complex (preBötC) synchronously discharge bursts of action potentials and drive inspiratory breathing movements (Smith et al. 1991; Rekling & Feldman, 1998; Feldman & Del Negro, 2006; Janczewski & Feldman, 2006). Although the rhythmogenic role of the preBötC is generally well accepted, the molecular mechanisms and intracellular signalling pathways that regulate inspiratory burst generation in preBötC neurons are not entirely clear.
Calcium-activated non-specific cation current (ICAN) contributes postsynaptically to inspiratory burst generation (Pace et al. 2007a). The ion channel(s) underlying ICAN may belong to the transient receptor potential (TRP) family of ion channels first identified in Drosophila (Minke, 1977). Members of the melastatin-like subfamily TRPM4 and TRPM5 are unique among TRP channels because they form flufenamic acid (FFA)-sensitive, Ca2+-impermeable monovalent cation channels gated by intracellular Ca2+ transients (Launay et al. 2002; Hofmann et al. 2003; Montell, 2005; Ullrich et al. 2005; Ramsey et al. 2006), which are properties expressed by ICAN at the whole-cell level in a variety of cell types (Teulon, 2000) including preBötC neurons (Pace et al. 2007a). If TRPM4 and TRPM5 give rise to ICAN in preBötC neurons, then their regulation could influence ICAN on a cycle-to-cycle basis and thus shape inspiratory bursts during respiratory rhythm generation.
The function of TRPM4 and TRPM5 is intimately coupled to phosphoinositide signalling (Fig. 1). For example, phosphatidylinositol 4,5-bisphosphate (PIP2) maintains the Ca2+ sensitivity required to activate TRPM4 and TRPM5 channels (Liu & Liman, 2003; Zhang et al. 2005; Nilius et al. 2006) and thus PIP2 depletion leads to TRPM4 and TRPM5 desensitization (Liu & Liman, 2003; Nilius et al. 2006). Phosphoinositide (PI) signalling also evokes TRPM4 and TRPM5 channel currents (as well as ICAN) because PIP2 hydrolysis forms inositol 1,4,5-trisphosphate (IP3) and causes IP3 receptor-mediated intracellular Ca2+ release (Clapham, 2003; Hofmann et al. 2003), which is important for inspiratory bursts in preBötC neurons (Pace et al. 2007a). If TRPM4 and TRPM5 underlie ICAN, then the cycling of PIP2 and IP3 could provide feedback mechanisms that regulate drive potential generation. PIP2 hydrolysis should deplete PIP2 stores and ultimately lead to TRPM4 and TRPM5 channel desensitization. Thus, determining how PI signalling regulates ICAN may be important to understand how respiratory rhythm, which must function unceasingly to sustain breathing, remains robust and stable.
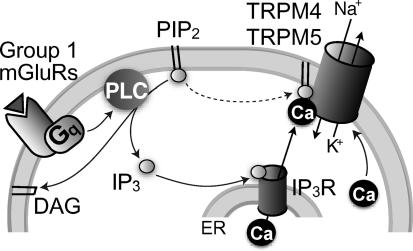
Figure 1. Model of ICAN activation with hypothesized channel identity and PIP2 regulation.
Glutamate (triangle) activates group 1 metabotropic glutamate receptors (mGluRs), which then cause Gq-proteins to stimulate phospholipase C (PLC). PLC hydrolyses PIP2 into diacylgylcerol (DAG) and IP3, which activates IP3 receptors (IP3R) causing Ca2+ release from the endoplasmic reticulum (ER). Intracellular Ca2+ gates TRPM4 and TRPM5 channels, causing Na+ influx and K+ efflux that results in depolarization from baseline membrane potentials of –60 mV. PIP2 also acts directly on TRPM4 and TRPM5 channels to maintain Ca2+ sensitivity (dotted arrow).
Based on the likelihood that TRPM4 and TRPM5 channels give rise to ICAN, here we test the two-part hypothesis that TRPM4 and TRPM5 channels are expressed within the preBötC region and that PIP2 regulates inspiratory burst generation. By limiting ICAN desensitization, we propose that PIP2 promotes periodic recruitment of ICAN and prevents the rundown of its inspiratory burst-generating function in preBötC neurons, which may help maintain robust breathing rhythms.
Methods
Slice preparation
We used neonatal C57BL/6 mice (postnatal day 1–7 (P1–7)) for in vitro electrophysiology as well as RT-PCR experiments. The Institutional Animal Care and Use Committee at The College of William and Mary approved all protocols.
Neonatal mice were anaesthetized by hypothermia and rapidly decerebrated prior to dissection in normal artificial cerebrospinal fluid (ACSF) containing (mm): 124 NaCl, 3 KCl, 1.5 CaCl2, 1 MgSO4, 25 NaHCO3, 0.5 NaH2PO4 and 30 d-glucose, equilibrated with 95% O2 and 5% CO2 with pH = 7.4. Transverse slices (550 μm thick) containing the preBötC as well as hypoglossal (XII) motoneurons and premotoneurons were sectioned using a vibrating microslicer. The rostral surface was cut just above the XII nerve roots at the level of the dorsomedial cell column and principal lateral loop of the inferior olivary nucleus; the preBötC was at or near the rostral surface (Gray et al. 1999; Ruangkittisakul et al. 2006). The caudal cut captured the obex.
Electrophysiology
Slices were placed rostral surface up in a 0.5 ml recording chamber on a fixed-stage microscope equipped with Koehler illumination and perfused with 27°C ACSF at 4 ml min−1. ACSF K+ concentration was raised to 9 mm and respiratory motor output was recorded from XII nerve roots using suction electrodes and a differential amplifier.
Differential interference contrast videomicroscopy (Inoue & Spring, 1997) was used to visualize neurons and control micropipette movements. Patch-clamp recordings were performed on neurons visualized in the preBötC region ventrally adjacent to the semi-compact division of the nucleus ambiguus (Gray et al. 1999; Wang et al. 2001; Ruangkittisakul et al. 2006). Inspiratory neurons discharge spikes superimposed upon a 200–500 ms envelope of depolarization dubbed the inspiratory drive potential that is coincident with XII discharge (Pace et al. 2007a,b). All preBötC neurons with robust inspiratory activity were suitable for experiments; no attempt to identify neurons with voltage-dependent pacemaker properties was made and expiratory neurons were excluded. Current-clamp recordings were performed using a Dagan IX2-700 amplifier (Minneapolis, MN, USA). Intracellular pipettes were fabricated from capillary glass (O.D., 1.5 mm; I.D., 0.87 mm) and filled with patch solution (contents listed below). Access resistance was compensated with bridge balance. Data were digitally acquired at 4–20 kHz using a 16-bit A/D converter (Powerlab, ADInstruments, Colorado Springs, CO, USA) after low-pass filtering at 1 kHz to avoid aliasing.
We continuously monitored membrane potential and adjusted the bias current to maintain a consistent baseline of –60 mV, which provides a uniform standard for comparing inspiratory drive potentials among preBötC neurons. Inspiratory bursts were digitally smoothed to remove spikes and facilitate measurements of the underlying inspiratory drive potential (see Fig. 1 in Pace et al. 2007b) using Chart v5.4 (AD Instruments). The Peak Parameters extension in Chart software measured inspiratory drive potential amplitude and area.
The standard potassium gluconate patch solution contained (mm): 140 potassium gluconate, 5 NaCl, 1 EGTA, 10 Hepes, 2 Mg-ATP (excluded in some trials, e.g. Figs 3B, D–F and 5B and C) and 0.3 Na-GTP (pH = 7.3 with KOH). Pipette resistance was 3–4 MΩ and a liquid junction potential of 8 mV was corrected offline. One (or sometimes two, e.g. Fig. 5B) drugs that affect PIP2 or IP3 signalling pathways were added to the patch solution and applied intracellularly at the following concentrations: 10 μm PIP2–1,2-dicotanoyl-sn-glycerol (diC8-PIP2) (Echelon Biosciences, Salt Lake City, UT, USA), 30 μg ml−1 poly l-lysine (Sigma, St Louis, MO, USA), 50 μm wortmannin (Sigma), 1 μm Xestospongin-C (Sigma), or 10 μm PLC-resistant PIP2 synthesized by H. Zhang and G. Prestwich (Zhang et al. 2006; analog 4).
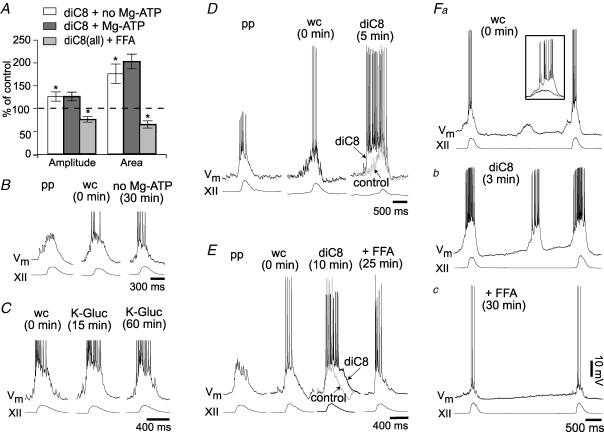
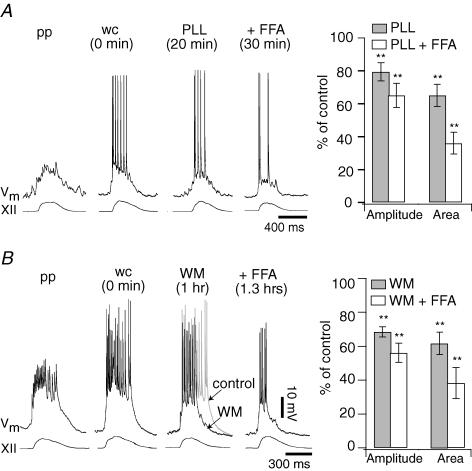
Figure 3. The effects of excess PIP2 on inspiratory drive potentials.
A, bar chart summarizing the effects of diC8-PIP2 (diC8) on mean inspiratory drive potentials (±s.e.m.). Inspiratory drive potential amplitude and area are plotted as a per cent of control for the following conditions: 10 μm diC8 with Mg-ATP (dark grey bars) or diC8 without Mg-ATP (white bars). The same measure is shown for the effects of flufenamic acid (FFA, 100 μm) in the presence of diC8-PIP2 (light grey bars). *Statistical significance at P < 0.05. B, representative traces showing the effects of Mg-ATP removal from the standard patch solution. Sequential traces represent perforated patch (pp), whole cell immediately after patch rupture (wc), and whole cell 30 min after patch rupture. Baseline membrane potential (Vm) was held at –60 mV. XII represents hypoglossal nerve motor output. Mg-ATP was excluded from all subsequent diC8 experiments in panels D–F. C, representative traces from a control experiment show drive potential stability and longevity under standard conditions in vitro. Standard patch solution was employed with Mg-ATP present. The drive potential did not change over 60 min. D, representative neuron showing diC8 enhancement of drive potential onset. Sequential traces show pp, wc and wc 5 min after diC8 dialysis. E, representative neuron showing diC8 prolongation of drive potentials and the effects of FFA. The sequence of traces are similar to D (above) with the additional FFA condition after 25 min of diC8 dialysis. Fa–c, the effects of diC8 and FFA on ectopic depolarizations in preBötC neurons. Fa, example of ectopic depolarizations observed immediately following patch rupture. Inset shows the superposition of ectopic depolarizations in control and diC8. Fb, the same cell 3 min after patch rupture. Fc, with diC8 still present, FFA was bath-applied and ectopic depolarizations disappeared entirely.
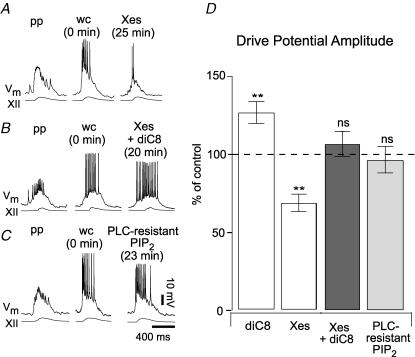
Figure 5. Excess PIP2 augments drive potentials through an IP3-independent mechanism.
A, intracellular Xestospongin (Xes, 1 μm) attenuates inspiratory drive potentials by blocking IP3 receptors. Perforated-patch (pp) and whole-cell (wc) control conditions are illustrated along with whole-cell Xes dialysis at steady state. B, typical data from an inspiratory neuron in response to co-application of Xes and diC8-PIP2 (diC8). The inspiratory burst changed shape somewhat but neither amplitude nor area changed significantly. C, typical response to 10 μm intracellular PLC-resistant PIP2. Again, neither the amplitude nor the area of the inspiratory burst changed significantly. D, bar chart plots the mean amplitude of inspiratory drive potentials (±s.e.m.) as per cent of control in the following conditions: diC8 alone (left white bar), Xes alone (right white bar), co-applied diC8 and Xes (dark grey bar), and PLC-resistant PIP2 (light grey bar). Here ** denotes P < 0.01 and ns denotes ‘not significant’ at P ≫ 0.05.
Control measurements of inspiratory drive potentials were obtained using nystatin-perforated patches. Nystatin (250 μg ml−1) was added to potassium gluconate patch solution immediately prior to use and discarded after 120 min. The amplitude and area of the underlying inspiratory drive potentials could be accurately measured after ∼20 min of exposure to nystatin, even though the high impedance of the perforated patch partially attenuated action potentials (Pace et al. 2007a). Subsequently, drugs that target PIP2 signalling pathways were delivered intracellularly via patch rupture, which dialyses the cytosol in the whole-cell configuration. Patch rupture also lowered access resistance and enabled action potentials to measure full amplitude. FFA (Sigma) was often bath-applied after intracellularly applied drugs achieved steady state.
The mean inspiratory drive potential and XII motor output were computed by averaging 10 consecutive inspiratory bursts. All samples were tested for normality using the Anderson–Darling test. Student's paired t tests and Wilcoxon signed ranks tests were applied to determine significance for normal and non-normal distributions, respectively, with minimum significance set at 0.05 or less. Experiments in which XII motor output changed significantly during the course of intracellular drug application were discarded.
RT-PCR
For reverse transcriptase polymerase chain reaction (RT-PCR) experiments, total RNA was purified from flash-frozen kidney tissue using RNeasy (Qiagen, Valencia, CA, USA). The preBötC kernel was dissected from bilateral regions of the slice preparation and immediately flash-frozen in liquid nitrogen. Total RNA from these samples was extracted with the RNaqueous Micro kit (Ambion, Austin, TX, USA). Possible genomic DNA contaminants were removed by incubating all RNA samples at 37°C with DNase (from RNAqueous kit or Promega, Madison, WI, USA) and either RNasin (Promega) or 1× Dnase I buffer (provided with RNAqueous kit). Using the iSCRIPT cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA), cDNA was reverse transcribed from 1 μg of total RNA. Following the manufacturer's instructions, samples were incubated for 5 min at 25°C, 60 min at 42°C, and 5 min at 85°C. For negative controls, no reverse transcriptase was added to the reaction.
PCR was carried out using standard conditions: 0.5 μm of each primer, 1.5 mm MgCl2, 0.2 mm deoxynucleoside triphosphates (dNTPs) and 1.25 U Supertaq polymerase (Ambion), although Supertaq buffer concentration was doubled (from 1×) for TRPM4 reactions to optimize the reaction. This conventional protocol was the basis for amplification: 5 min 94°C ‘hot start’ to prevent mis-priming, 40 cycles of 30 s at 94°C, 1–2 min primer annealing, and 1–2 min elongation at 72°C, followed by 7 min at 72°C, and finally holding at 4°C. These reaction conditions were modified as follows: TRPM4 reactions involved 1 min annealing at 60°C and 1 min elongation, TRPM5 reactions involved 2 min annealing at 57°C and 2 min elongation, and GADPH reactions involved 1 min annealing at 60°C and 2 min elongation. In order to assess the quality and presence of the cDNA, all samples were tested for a ubiquitously expressed gene, GADPH (accession no. NM_008084), using the following primers: Forward, 5′-ACCACAGTCCATGCCATCAC-3′; reverse, 5′-TCCACCACCCTGTTGCTGTA-3′ (Kunert-Keil et al. 2006). To test preBötC samples for the presence of TRPM4 (accession no. NM_175130) and TRPM5 (accession no. NM_020277), the primer sets: forward, 5′-GGCCCAAGATTGTCATAGTG-3′; reverse, 5′-TTGGCATACTGGGACACACA-3′ (Guinamard et al. 2004a; note: forward and reverse primers were switched in this report); and forward, 5′-TCCTGTTCATTG-TGGGAGTCAC-3′; reverse, 5′-TGGCGATCAGAAGG-TTCATG-3′ (Paulsen et al. 2000), were used, respectively. All primers were designed to cross at least one intron as an additional control to assess the unintended amplification of residual genomic DNA; amplification of genomic DNA would result in a recognizably higher molecular weight fragment. Fragments amplified by each set of primers were verified via direct sequencing of PCR products using the ABI BigDye 3.1 kit (Applied Biosystems, Foster City, CA, USA) and ABI 3100-Avant automated fluorescent sequencer.
Results
TRPM4 and TRPM5 expression
We used RT-PCR to detect the presence of TRPM4 and TRPM5 mRNA in the preBotC. Murine kidney tissue expressing TRPM4 and TRPM5 mRNA (Enklaar et al. 2000; Nilius et al. 2003; Kunert-Keil et al. 2006) served as positive controls for both genes.
We dissected the preBötC region bilaterally from slice preparations. Following total RNA extraction, all preBötC samples (n = 4) tested positive for the presence of TRPM4, TRPM5 and GADPH mRNA, whereas mRNA for these genes was absent in negative controls (Fig. 2). Sequencing and comparison to the National Center for Biotechnology Information (NCBI) database confirmed the identity of all RT-PCR products, which indicates that cells in the preBötC region express mRNA for TRPM4 and TRPM5 ion channels. These data are consistent with the proposal that TRPM4 and/or TRPM5 give rise to ICAN in preBötC neurons (Pace et al. 2007a).
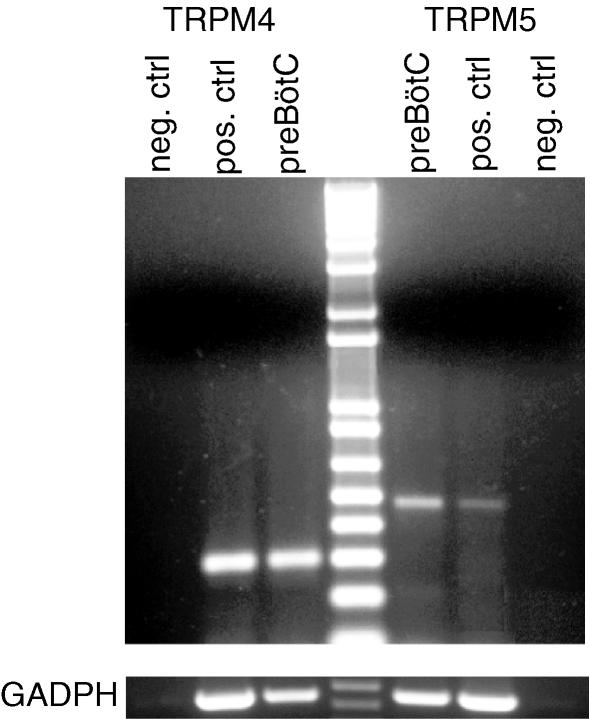
Figure 2. The mRNA coding for TRPM4 and TRPM5 is expressed in the preBötC region.
Total RNA was extracted from preBötC and positive control tissues and reverse transcribed. Amplified products of the expected sizes were obtained for TRPM4 (301 bp), TRPM5 (483 bp) and GADPH (452 bp). Negative control reactions were performed without reverse transcriptase and amplified nothing.
It is important to note that this technique does not precisely quantify mRNA levels, but rather reliably detects only its presence or absence. For example, in many cases, taking a broad region of kidney tissue as a positive control diluted TRPM5 mRNA levels, so its band appeared weaker than the TRPM5 band for preBötC tissue (e.g. Fig. 2, compare TRPM5 preBötC to positive control). Intensity of bands should not be taken to indicate levels of expression, but rather the presence or absence of expression. Nevertheless, these data clearly demonstrate that TRPM4 and TRPM5 mRNA are present in the preBötC.
PIP2 manipulation affects inspiratory burst generation in preBötC neurons
Synaptic excitation during the inspiratory phase involves metabotropic glutamate receptors (see Fig. 1) that stimulate IP3 production and trigger intracellular Ca2+ release to evoke ICAN (Pace et al. 2007a). In addition to its role as a precursor to IP3, we posit that PIP2 prevents ICAN desensitization by regulating its Ca2+ sensitivity, because PIP2 has this effect on TRPM4 and TRPM5 ion channels (Liu & Liman, 2003; Zhang et al. 2005; Nilius et al. 2006). Therefore, we predicted that augmenting the supply of PIP2 intracellularly would promote ICAN activation via increasing IP3 production and simultaneously preventing desensitization; the net effect would be to enhance inspiratory drive potentials.
We increased the concentration of intracellular PIP2 in a single inspiratory neuron using patch solution containing 10 μm of the water-soluble PIP2 analogue diC8-PIP2. The area and amplitude of the inspiratory drive potentials measured in control via perforated patch matched those observed during the first few cycles of whole-cell recording before diC8-PIP2 dialysis occurred (e.g. Fig. 3D and E). Therefore, control inspiratory drive potentials may be measured either during perforated-patch conditions (with attenuated spikes) or from the first few cycles in whole cell (with spikes full amplitude). After 10 min of diC8-PIP2 dialysis, inspiratory drive potential amplitude and area increased to 126 ± 10% and 203 ± 16% of control, respectively (n = 3).
Mg-ATP restores Ca2+ sensitivity to TRPM4 in excised patches (Nilius et al. 2005), in much the same way as PIP2 (Nilius et al. 2006), and thus we could not differentiate whether diC8-PIP2 or Mg-ATP caused drive potential enhancement. Therefore, we removed Mg-ATP from the patch solution to isolate the putative effects of PIP2 on ICAN desensitization during inspiratory bursts and repeated the experiment.
As a control we removed Mg-ATP from the patch solution without adding diC8-PIP2, which caused significant rundown in the inspiratory drive potential: amplitude and area decreased to 66 ± 12% and 58 ± 9% of control, respectively (P < 0.05, n = 8, Fig. 3B). In contrast, inspiratory drive potentials measured with potassium gluconate patch solution containing Mg-ATP did not change: amplitude and area were 100 ± 4% and 104 ± 6% of control, respectively (P ≫ 0.05, n = 6, Fig. 3C). These data show that Mg-ATP prevents drive potential rundown but does not, on its own, enhance inspiratory drive potentials.
Dialysing preBötC neurons with diC8-PIP2 in the absence of Mg-ATP nevertheless augmented inspiratory drive potentials: the amplitude and area significantly increased to 126 ± 7% and 177 ± 15% of perforated-patch control (P < 0.05, n = 6, Fig. 3A, D and E). Therefore, augmentation conferred by diC8-PIP2 is an independent effect unrelated to Mg-ATP.
Of the six preBötC neurons dialysed with diC8-PIP2 in the absence of Mg-ATP, four neurons activated more rapidly and attained maximum amplitude earlier during the inspiratory phase compared with control (Fig. 3D) whereas two neurons extended inspiratory burst duration (Fig. 3E). Three of the six exhibited spontaneous ectopic subthreshold depolarizations in the interval between XII discharge within the first minute of whole-cell recording (0 min, Fig. 3Fa) that were greatly enhanced by diC8-PIP2 after reaching steady state (= 3 min, Fig. 3Fb). This enhancement of the ectopic burst is emphasized in the inset in Fig. 3Fa, which overlays the control and diC8-PIP2 responses for comparison.
If the effects of diC8-PIP2 are attributable to ICAN, then they should be sensitive to the ICAN antagonist FFA. In steady-state diC8-PIP2 conditions, bath application of FFA reduced inspiratory drive potentials to below control levels: the amplitude and area decreased to 78 ± 6% and 68 ± 7% of control, respectively (P < 0.05, n = 5, e.g. Fig. 3A, E and Fc). These data are commensurate with the attenuation caused by bath-applied FFA in prior experiments that did not involve PIP2 manipulations (65–70% of control (their Fig. 6), Pace et al. 2007a). The ability of FFA to reverse the burst-augmenting effects of diC8-PIP2 indicates that ICAN is the primary current affected by excess PIP2.
Whereas excess PIP2 enhanced inspiratory burst generation (Fig. 3), we next sought to test whether PIP2 depletion would diminish the ability of preBötC neurons to generate inspiratory bursts (Fig. 4). We included Mg-ATP in the potassium gluconate patch solution so that any change in inspiratory drive potentials would be attributable to PIP2 reduction, and not the removal of Mg-ATP (e.g. Fig. 3C).
Figure 4. The effects of PIP2 removal on inspiratory drive potentials.
A, sequential traces show the response of a representative inspiratory neuron to intracellular 30 μg ml−1 poly l-lysine (PLL) and FFA. Perforated patch (pp) and whole cell immediately following patch rupture (wc) represent controls. PLL reached steady state after 20 min of whole-cell dialysis. Then, FFA was applied; the illustrated trace was taken at 30 min. The bar graph plots the mean drive potential amplitude and area (±s.e.m.) as per cent of control in response to PLL applied alone (grey bars) and in combination with FFA (white bars). B, sequential traces show the effects of intracellular application of 50 μm wortmannin (WM) and FFA on a representative inspiratory neuron. The control trace is superimposed (in grey) on top of the WM trace for comparison. The bar graph plots the mean drive potential amplitude and area (±s.e.m.) as per cent of control after application of WM alone (grey bars) and co-application of WM and FFA (white bars). For both graphs, ** denote statistical significance at P < 0.01 compared with pp or wc control.
Poly l-lysine (PLL) acts as a scavenger that reduces the amount of free PIP2 in the plasma membrane (Suh & Hille, 2005) and attenuates TRPM4 currents in excised patches (Zhang et al. 2005; Nilius et al. 2006). Applied to preBötC neurons intracellularly with Mg-ATP present, 30 μg ml−1 PLL significantly decreased inspiratory drive potential amplitude and area to 79 ± 6% and 70 ± 7% of perforated-patch control (amplitude P < 0.05, area P < 0.01, n = 7); a representative experiment is shown in Fig. 4A. Subsequent FFA application significantly reduced the amplitude and area of inspiratory drive potentials to 65 ± 7% and 36 ± 7% of control (P < 0.01, n = 7, Fig. 4A). These data suggest that PIP2 depletion diminishes PI-sensitive mechanisms that contribute to drive potential generation, including (but not limited to) ICAN. PLL scavenging does not completely block ICAN because the effects of FFA were not occluded.
As a complementary experiment, we depleted PIP2 using wortmannin, an inhibitor of PI 4-kinase that hinders PIP2 production by preventing the synthesis of its precursor phosphatidylinositol 4-monophosphate from PI (Nakanishi et al. 1995) and causes TRPM4 channel desensitization (Zhang et al. 2005; Nilius et al. 2006). Applied intracellularly with Mg-ATP in the patch solution and a perforated-patch control, 50 μm wortmannin significantly decreased inspiratory drive potential amplitude and area to 65 ± 4% and 61 ± 6% of control, respectively (P < 0.01, n = 8, Fig. 4B). FFA application further attenuated inspiratory drive potential amplitude and area to 56 ± 6% and 38 ± 11% of control (P < 0.01, n = 7, Fig. 4B). These data suggest that inhibiting the synthesis of PIP2 reduces the inspiratory burst-generating capabilities of preBötC neurons. However, wortmannin does not completely block the FFA-sensitive intrinsic current consistent with ICAN, similar to an analogous experiment with PLL.
We showed that excess PIP2 augments inspiratory drive potentials and that diminution of PIP2 attenuates them. However, these effects may be explained in several ways. For example, PIP2 may affect ICAN by promoting IP3-mediated Ca2+ release and ICAN activation, or by interacting directly with the underlying channels to affect sensitivity to Ca2+, or through some combination of both pathways (Fig. 1). Additionally, excess PIP2 may increase diacylgylcerol (DAG) levels, which regulates TRPM4 in cardiac myocytes (Guinamard et al. 2004a,b). Therefore, we designed experiments to differentiate the PIP2-mediated effects on ICAN desensitization from IP3- and DAG-dependent ICAN activation.
IP3 receptor-mediated ICAN activation is prevented by intracellularly applying the IP3 receptor antagonist Xestospongin-C (Xes; 1 μm) (Gafni et al. 1997). In a prior study we reported that Xes reduced inspiratory drive potential amplitude and area to 67 ± 5% and 64 ± 6% of perforated-patch control (P < 0.01, n = 16) by acting to prevent IP3-mediated Ca2+ release involved in ICAN activation (Pace et al. 2007a). Original data are shown in Fig. 5A.
To test directly the role of PIP2 in ICAN desensitization, we coupled intracellular dialysis of diC8-PIP2 with Xes (in the absence of Mg-ATP) to prevent IP3 receptor-mediated Ca2+ release. If exogenous PIP2 acts exclusively through the IP3 pathway, and not by preventing ICAN desensitization, then Xes should still attenuate inspiratory drive potentials, even in the presence of excess PIP2 (see Fig. 5D (white bars) and Pace et al. 2007a). However, the combined dialysis of Xes and diC8-PIP2 caused no statistically significant change compared with perforated-patch control (Fig. 5B): inspiratory drive potential amplitude and area measured 110 ± 8% and 130 ± 14% of control, respectively (P >> 0.05, n = 10, Fig. 5D, dark grey bars). These results suggest that the effects of excess PIP2 (i.e. to augment inspiratory drive potentials as shown in Fig. 3A and D–F) are not solely attributable to increased IP3 levels.
The protocol in Fig. 5B tests the direct effects of PIP2 on ICAN desensitization but does not preclude DAG signalling since Xes only blocks IP3 receptors. Our subsequent test (Fig. 5C) precludes both IP3 and DAG production. We intracellularly dialysed preBötC neurons with a water-soluble PIP2 analogue that lacks the scissile P–O bond normally cleaved by PLC and thus cannot be hydrolysed into IP3 and DAG. This PLC-resistant PIP2 at high intracellular concentrations functions normally in maintaining Ca2+ sensitivity of TRPM4 (Zhang et al. 2006), and is predicted to outcompete with endogenous PIP2 for PLC binding sites (W. Huang and G. D. Prestwich, unpublished molecular simulations) and thus occlude IP3-dependent ICAN activation by preventing IP3 synthesis. Finally, if DAG does not contribute to the diC8-PIP2 effects, then we predict commensurate results between PLC-resistant PIP2 and co-application of Xes with diC8-PIP2 (e.g. Fig. 5B).
The PLC-resistant PIP2 (10 μm) caused no significant change in the inspiratory drive potential amplitude or area compared with perforated-patch control; these measures remained at 95 ± 8% and 97 ± 9% of control, respectively (P ≫ 0.05, n = 8). Figure 5C shows a representative experiment; mean data for amplitude are shown in part D. These data are consistent with PIP2 directly influencing the Ca2+ sensitivity of ICAN.
Discussion
Here we demonstrate that PIP2 directly regulates inspiratory drive potentials during endogenous respiratory-related rhythmic activity. We propose that PIP2 prevents long-term desensitization of ICAN through interactions with underlying TRPM4 and TRPM5 channels.
Experimental approach to study ICAN regulation in the context of inspiratory bursts
PIP2 regulates TRPM4 and TRPM5 ion channels that give rise to ICAN-like whole-cell currents (Liu & Liman, 2003; Zhang et al. 2005; Nilius et al. 2006). We now show that manipulating PIP2 can both enhance as well as diminish inspiratory drive potentials in the context of endogenous respiratory-related behaviour in vitro.
Our objective is to understand the mechanisms that regulate inspiratory bursts, so we cannot study ICAN using excised patch or whole-cell experiments from cell lines expressing only TRPM4 or TRPM5 ion channels. These experimental approaches are divorced from respiratory function in vitro. In addition, excised patch (Liu & Liman, 2003; Zhang et al. 2005; Nilius et al. 2006) and Ca2+ uncaging experiments (Ullrich et al. 2005) employ Ca2+ concentrations of 1 μm to 10 mm to evoke TRPM4 and TRPM5 currents, which may exaggerate Ca2+-dependent rundown of channel activity (Liu & Liman, 2003; Nilius et al. 2004). We do not yet know how these experimentally induced Ca2+ levels compare to the physiologically relevant Ca2+ fluctuations in preBötC neurons. Therefore we aimed to study PI regulation of inspiratory drive potentials, and by inference the role of ICAN desensitization, with the endogenous preBötC rhythm intact.
ICAN is the major charge carrier of the drive potential (Pace et al. 2007a), but it is not the only contributor pre- or postsynaptically. We used 100 μm FFA to ensure that our PIP2 manipulations modified drive potentials via effects on ICAN and not other intrinsic currents that contribute to drive potentials. FFA (100 μm) significantly attenuates ICAN (Pace et al. 2007a). In various other neurons, this dose of FFA also affects: (i) gap junctions, (ii) NMDA receptors, (iii) Ca2+-activated Cl− channels, and (iv) Ca2+-dependent K+ currents (IK-Ca) (Ottolia & Toro, 1994; Greenwood & Large, 1995; Teulon, 2000; Harks et al. 2001; Srinivas & Spray, 2003; Wang et al. 2006). However, in preBötC neurons, 100 μm FFA had no effect on inspiratory drive potentials following intracellular blockade of ICAN accomplished with the rapid intracellular Ca2+ buffer BAPTA (Pace et al. 2007a). These results indicate that the first three FFA side-effects (listed above) do not significantly influence inspiratory drive potential properties, and thus do not confound our use of FFA to test for the involvement of ICAN in the current study.
We do not yet know whether FFA affects IK-Ca in preBötC neurons (cf. Wang et al. 2006). Ca2+-dependent K+ currents contribute to burst termination in adult mammals, as shown in cats in vivo (Richter et al. 1993; Pierrefiche et al. 1995), but IK-Ca does not significantly contribute to inspiratory drive potentials in preBötC neurons of neonatal mice (see Supplementary Fig. 1). Therefore, even if 100 μm FFA affects IK-Ca, this side-effect would not be relevant to the periodic regulation of inspiratory drive potentials.
PIP2 regulation of inspiratory drive potentials probably involves ICAN
If excess PIP2 augments drive potentials by enhancing ICAN, then the ICAN antagonist FFA should be equally effective if applied with diC8-PIP2 as when applied alone. The combined application of diC8-PIP2 and FFA decreased drive potentials to 65–75% of control; this is the same level of attenuation reported by Pace et al. (2007a) in experiments where FFA was applied alone. We conclude that excess intracellular PIP2 enhances the burst-generating contribution of ICAN.
Conversely, if PIP2 removal acts principally to diminish ICAN, then drive potential amplitude and area should decline in the presence of PLL or wortmannin, which we observed. One might predict that the inspiratory burst-generating role of ICAN in preBötC neurons would be abolished entirely during PLL or wortmannin trials because the absence of PIP2 has been shown to completely block TRPM4 and TRPM5 channel currents (Liu & Liman, 2003; Zhang et al. 2005; Nilius et al. 2006). However, the presence of Mg-ATP prevents complete TRPM4 channel desensitization after PIP2 removal (Zhang et al. 2005), which is probably attributable to the necessary role of Mg-ATP in maintaining PI 4-kinases (Balla, 1998). Based on these findings, we expect a fraction of ICAN in preBötC neurons to remain available following PIP2 depletion and thus able to activate in response to intracellular Ca2+ attributable to NMDA receptor- or Ca2+ channel-mediated fluxes.
However, we are left with the conundrum that co-application of FFA with PLL or wortmannin decreased inspiratory drive potentials to a greater extent (area measured ∼36% of control, see Fig. 4) than FFA alone (∼65% of control, see Pace et al. 2007a). This suggests that PIP2 removal might decrease the drive potential by affecting synaptic and intrinsic currents other than ICAN, that are also sensitive to PIP2 levels.
PIP2 promotes activation of several ion channel types including HCN, TRPM7 and TRPM8 (Runnels et al. 2002; Rohacs et al. 2005; Suh & Hille, 2005; Zolles et al. 2006) so we cannot rule out the possibility that PIP2 removal influences the burst-generating function of preBötC neurons via effects on multiple intrinsic membrane properties besides ICAN. However, HCN channels that underlie the mixed cationic current Ih appear to influence respiratory frequency but not inspiratory burst generation (Mironov et al. 2000; Thoby-Brisson et al. 2000). It is currently unknown whether TRPM7 and/or TRPM8 channels are expressed in preBötC neurons and is beyond the scope of the present investigation. In addition, PIP2 might be required for mGluR1-mediated mechanisms of inspiratory drive potential generation that involve K+ currents, not ICAN (Pace et al. 2007a).
Furthermore, PLL and wortmannin undoubtedly cause rundown of intrinsic currents that depend on PKC phosphorylation because PIP2 hydrolysis produces DAG, which in turn activates protein kinase C (PKC) (Hille, 2001). However, since the hydrolysable compound diC8-PIP2 in the presence of Xes compared with the PLC-resistant PIP2 analogue yielded similar results, we concluded that DAG is not a major factor in drive potential generation.
In addition to group I mGluRs, other important metabotropic receptors in preBötC neurons, including neurokinin 1 receptors (NK1Rs) and subtype 1 of the purinergic P2Y receptors (P2Y1R) may activate the PLC pathway via Gq-protein and therefore utilize PIP2 (Li et al. 1997; North & Barnard, 1997). However, the signalling pathways underlying NK1R and P2Y1R actions in preBötC neurons remain to be determined; for example, NK1Rs can be coupled to a wide variety of G-proteins (Roush & Kwatra, 1998). Moreover, previous studies have shown that NK1Rs and P2Y1Rs primarily regulate respiratory frequency (Gray et al. 1999; Pena & Ramirez, 2004; Lorier et al. 2007). In contrast, inspiratory drive potentials depend on metabotropic glutamate receptors coupled to PLC signalling, and this process is largely independent of frequency modulation (Pace et al. 2007a). Nevertheless, P2Y1R and NK1R activation may both diminish available PIP2 stores leading to less IP3 production and ICAN desensitization, which would attenuate the inspiratory drive potential. This is consistent with attenuations observed in the amplitude of preBötC field recordings during applications of agonists for both P2Y1Rs and NK1Rs (Pena & Ramirez, 2004; Lorier et al. 2007).
TRPM4 and TRPM5 channels probably underlie ICAN expressed by inspiratory neurons
We found mRNA coding for TRPM4 and TRPM5 channels in the preBötC region of the neonatal murine brainstem, despite previous reports that the brain is deficient in these genes (Perez et al. 2002; Nilius et al. 2003; Kunert-Keil et al. 2006). Our extraction methods provide a more sensitive test because we focused on a limited region of the ventral respiratory brain stem including the preBötC. Sampling the whole brain – even the whole brainstem – may dilute preBötC mRNA and thus increase the likelihood of a false negative.
Observing these genes in the preBötC is not sufficient to conclude that inspiratory neurons express functional TRPM4 and TRPM5 channels. Additionally, our protocol did not limit RNA extraction to preBötC inspiratory neurons but most probably sampled expiratory and non-respiratory neurons, as well as non-neural cells. It is also possible that post-transcriptional processing precludes the translation of one or both mRNAs. However, TRPM4 and TRPM5 are the only known channels that give rise to whole-cell currents with the biophysical properties of ICAN present in preBötC neurons (Pace et al. 2007a) with respect to pharmacology, monovalent cation permeability and activation mechanism (Launay et al. 2002; Hofmann et al. 2003; Montell, 2005; Ullrich et al. 2005; Ramsey et al. 2006). While TRPM4 and TRPM5 differ in sensitivity to Ca2+ and FFA, fundamental properties of the two channels overlap substantially (Ullrich et al. 2005). We also documented that PIP2 regulates ICAN-dominated inspiratory drive potentials in a manner consistent with its effects on TRPM4 and TRPM5 channels. Therefore, we conclude that TRPM4 and TRPM5 channels are the ion channels underlying ICAN in preBötC neurons.
Physiological role of PIP2 in preBötC neurons
We propose that excess PIP2 augments inspiratory drive potentials by counteracting ICAN desensitization. Excess PIP2 has no effect on non-desensitized TRPM5 channels (Liu & Liman, 2003) and pre-treatment with PIP2 prevented TRPM4 desensitization from occurring (Nilius et al. 2006). Thus, PIP2 counteracts desensitization but does not appear to augment other aspects of channel function. Our results with diC8-PIP2 imply that ICAN normally functions in a partially desensitized state in preBötC neurons during periodic respiratory activity.
TRPM4 and TRPM5 channels are mostly (or completely) deactivated at steady state when held at negative membrane potentials, which gives rise to their characteristic outwardly rectifying current–voltage curves (Hofmann et al. 2003; Liu & Liman, 2003; Nilius et al. 2003; Ullrich et al. 2005). PIP2 reverses this desensitization for TRPM4 such that large inward currents gated by Ca2+ can flow at hyperpolarized membrane potentials (Nilius et al. 2006), and the same may be true for TRPM5 although the mechanism has not been fully documented (Liu & Liman, 2003). We propose that PIP2 plays a similar role in preBötC neurons by alleviating (at least in part) ICAN desensitization thus promoting its activation at negative voltages. This would enable ICAN to participate to a greater extent in inspiratory burst generation, because inspiratory bursts initiate from baseline membrane potentials in the range –70 to –50 mV.
Consistent with this mechanism, diC8-PIP2 frequently (4 cells out of 6 tested with diC8-PIP2 and no Mg-ATP) augmented drive potentials at the onset of inspiratory bursts (see Fig. 3D). This suggests that bursts initiate at lower voltages (and possibly with smaller excitatory postsynaptic potentials as well) in the presence of excess PIP2. Also supporting this interpretation, diC8-PIP2 enabled small ectopic depolarizations to reach burst threshold (see Fig. 3Fa and b) and cause full-fledged inspiratory-like bursts. We conclude that the addition of exogenous PIP2 augments inspiratory drive potential generation by lowering or removing the threshold of excitatory input necessary to activate ICAN.
How might PIP2-regulated ICAN desensitization operate on a cycle-to-cycle basis to shape inspiratory bursts? If periodic PIP2 depletion is involved in burst termination, then excess exogenous PIP2 should stave off desensitization and delay inspiratory burst termination. However, this effect was only observed in 2 of the 6 neurons treated with diC8-PIP2 (see Fig. 3E). We conclude that PIP2 depletion (during IP3 production) is not a major factor that governs inspiratory burst termination.
ICAN in preBötC neurons normally functions in a partially desensitized state during rhythmic activity in vitro. Moreover, PIP2 does not act on a cycle-to-cycle basis to cause burst termination nor promote burst onset. These two points are not in conflict; we theorize that PIP2 maintains ICAN functionality for the ‘long haul’, i.e. respiratory oscillations are meant to continue without lapse, conferring stability and longevity to inspiratory burst generation in a critical physiological system that necessitates both.
Acknowledgments
This work was supported by National Science Foundation IOB-0616099 (USA), the Suzann Wilson Matthews Faculty Research Award (The College of William and Mary, Williamsburg, VA, USA), the Jeffress Memorial Trust (Richmond, VA, USA), and grants in support of undergraduate research from the Howard Hughes Medical Institute Undergraduate Science Education Grant awarded to The College of William and Mary (M. S. Saha, Project director). G.D.P. thanks the NIH for NS29632 and W. Huang (University of Utah) for molecular simulations.
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.134577/DC1 and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.134577
References
- Balla T. Phosphatidylinositol 4-kinases. Biochim Biophys Acta. 1998;1436:69–85. doi: 10.1016/s0005-2760(98)00134-9. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the Brain. Oxford, New York: Oxford University Press; 2006. [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Enklaar T, Esswein M, Oswald M, Hilbert K, Winterpacht A, Higgins M, Zabel B, Prawitt D. Mtr1, a novel biallelically expressed gene in the center of the mouse distal chromosome 7 imprinting cluster, is a member of the Trp gene family. Genomics. 2000;67:179–187. doi: 10.1006/geno.2000.6234. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. Comparison of the effects of fenamates on Ca-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. Br J Pharmacol. 1995;116:2939–2948. doi: 10.1111/j.1476-5381.1995.tb15948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Chatelier A, Demion M, Potreau D, Patri S, Rahmati M, Bois P. Functional characterization of a Ca2+-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol. 2004a;558:75–83. doi: 10.1113/jphysiol.2004.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R, Chatelier A, Lenfant J, Bois P. Activation of the Ca2+-activated nonselective cation channel by diacylglycerol analogues in rat cardiomyocytes. J Cardiovasc Electrophysiol. 2004b;15:342–348. doi: 10.1046/j.1540-8167.2004.03477.x. [DOI] [PubMed] [Google Scholar]
- Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, van Zoelen EJ, Theuvenet AP. Fenamates: a novel class of reversible gap junction blockers. J Pharmacol Exp Ther. 2001;298:1033–1041. [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Inoue S, Spring KR. Video Microscopy: the Fundamentals. New York: Plenum Press; 1997. [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Li H, Leeman SE, Slack BE, Hauser G, Saltsman WS, Krause JE, Blusztajn JK, Boyd ND. A substance P (neurokinin-1) receptor mutant carboxyl-terminally truncated to resemble a naturally occurring receptor isoform displays enhanced responsiveness and resistance to desensitization. Proc Natl Acad Sci U S A. 1997;94:9475–9480. doi: 10.1073/pnas.94.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD, Funk GD. P2Y1 receptor modulation of the pre-Botzinger complex inspiratory rhythm generating network in vitro. J Neurosci. 2007;27:993–1005. doi: 10.1523/JNEUROSCI.3948-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B. Drosophila mutant with a transducer defect. Biophys Struct Mech. 1977;3:59–64. doi: 10.1007/BF00536455. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Langohr K, Richter DW. Hyperpolarization-activated current, Ih, in inspiratory brainstem neurons and its inhibition by hypoxia. Eur J Neurosci. 2000;12:520–526. doi: 10.1046/j.1460-9568.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;272:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci U S A. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25:467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 2003;278:30813–30820. doi: 10.1074/jbc.M305127200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Janssens A, Voets T, Droogmans G. Decavanadate modulates gating of TRPM4 cation channels. J Physiol. 2004;560:753–765. doi: 10.1113/jphysiol.2004.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. 2005;280:6423–6433. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Ottolia M, Toro L. Potentiation of large conductance KCa channels by niflumic, flufenamic, and mefenamic acids. Biophys J. 1994;67:2272–2279. doi: 10.1016/S0006-3495(94)80712-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cationic current linked to glutamate receptors in neonatal mice. J Physiol. 2007a;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Role of persistent sodium current in mouse preBotzinger complex neurons and respiratory rhythm generation. J Physiol. 2007b;580:485–496. doi: 10.1113/jphysiol.2006.124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen M, El-Maarri O, Engemann S, Strodicke M, Franck O, Davies K, Reinhardt R, Reik W, Walter J. Sequence conservation and variability of imprinting in the Beckwith–Wiedemann syndrome gene cluster in human and mouse. Hum Mol Genet. 2000;9:1829–1841. doi: 10.1093/hmg/9.12.1829. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Champagnat J, Richter DW. Calcium-dependent conductances control neurones involved in termination of inspiration in cats. Neurosci Lett. 1995;184:101–104. doi: 10.1016/0304-3940(94)11179-m. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to trp channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Richter DW, Champagnat J, Jacquin T, Benacka R. Calcium currents and calcium-dependent potassium currents in mammalian medullary respiratory neurones. J Physiol. 1993;470:23–33. doi: 10.1113/jphysiol.1993.sp019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI (4,5),P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Roush ED, Kwatra MM. Human substance P receptor expressed in Chinese hamster ovary cells directly activates Gαq/11, Gαs, Gαo. FEBS Lett. 1998;428:291–294. doi: 10.1016/s0014-5793(98)00553-5. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Spray DC. Closure of gap junction channels by arylaminobenzoates. Mol Pharmacol. 2003;63:1389–1397. doi: 10.1124/mol.63.6.1389. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Teulon J. Ca2+-activated nonselective cation channels. In: Endo M, Kurachi Y, Mishina M, editors. Pharmacology of Ionic Channel Function: Activators and Inhibitors. Berlin: Springer-Verlag; 2000. pp. 625–649. [Google Scholar]
- Thoby-Brisson M, Telgkamp P, Ramirez JM. The role of the hyperpolarization-activated current in modulating rhythmic activity in the isolated respiratory network of mice. J Neurosci. 2000;20:2994–3005. doi: 10.1523/JNEUROSCI.20-08-02994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium. 2005;37:267–278. doi: 10.1016/j.ceca.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wang D, Grillner S, Wallen P. Effects of flufenamic acid on fictive locomotion, plateau potentials, calcium channels and NMDA receptors in the lamprey spinal cord. Neuropharmacology. 2006;51:1038–1046. doi: 10.1016/j.neuropharm.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol. 2001;434:128–146. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xu Y, Zhang Z, Liman ER, Prestwich GD. Synthesis and biological activity of phospholipase C-resistant analogues of phosphatidylinositol 4,5-bisphosphate. J Am Chem Soc. 2006;128:5642–5643. doi: 10.1021/ja060621d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem. 2005;280:39185–39192. doi: 10.1074/jbc.M506965200. [DOI] [PubMed] [Google Scholar]
- Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, Fakler B. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron. 2006;52:1027–1036. doi: 10.1016/j.neuron.2006.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.134577/DC1 and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.134577