Abstract
M-channels are voltage-gated K+ channels that regulate the excitability of many neurons. They are composed of Kv7 (KCNQ) family subunits, usually Kv7.2 + Kv7.3. Native M-channels and expressed Kv7.2 + 7.3 channels are inhibited by stimulating Gq/11-coupled receptors – prototypically the M1 muscarinic acetylcholine receptor (M1-mAChR). The channels require membrane phosphatidylinositol-4,5-bisphosphate (PIP2) to open and the effects of mAChR stimulation result primarily from the reduction in membrane PIP2 levels following Gq/phospholipase C-catalysed PIP2 hydrolysis. However, in sympathetic neurons, M-current inhibition by bradykinin appears to be mediated through the release and action of intracellular Ca2+ by inositol-1,4,5-trisphosphate (IP3), a product of PIP2 hydrolysis, rather than by PIP2 depletion. We have therefore compared the effects of bradykinin and oxotremorine-M (a muscarinic agonist) on membrane PIP2 in sympathetic neurons using a fluorescently tagged mutated C-domain of the PIP2 binding probe, ‘tubby’. In concentrations producing equal M-current inhibition, bradykinin produced about one-quarter of the reduction in PIP2 produced by oxotremorine-M, but equal reduction when PIP2 synthesis was blocked with wortmannin. Likewise, wortmannin restored bradykinin-induced M-current inhibition when Ca2+ release was prevented with thapsigargin. Thus, inhibition by bradykinin can use product (IP3/Ca2+)-dependent or substrate (PIP2) dependent mechanisms, depending on Ca2+ availability and PIP2 synthesis rates.
M-channels are a species of low-threshold, non-inactivating voltage-gated potassium channels that play a crucial role in the regulation of neuronal excitability. The current generated by them was first identified in bullfrog sympathetic neurons (Brown & Adams, 1980) and subsequently in a variety of mammalian peripheral and central neurons, including human cortical neurons (reviewed by Brown, 1988; Marrion, 1997; Delmas & Brown, 2005). Progressive opening of the channels during depolarization from a threshold of around −60 mV serves to clamp the membrane potential (Fig. 1) and reduce excitability (see e.g. Fig. 2).
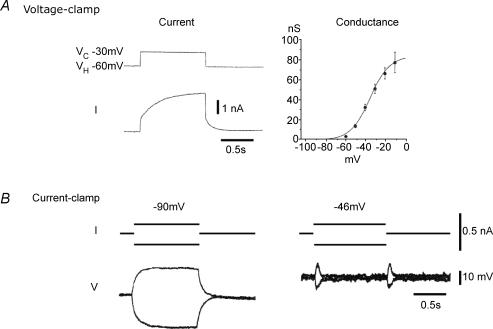
Figure 1. Basic properties of M-current as recorded in frog sympathetic neurons.
Twin-microelectrode recording from two different neurons. V, voltage; I, current. A, with a step-depolarization under voltage clamp, the current is seen as a slowly activated (τ≈ 150 ms) non-inactivating outward current with a threshold around −60 mV and half-activation at around −35 mV (from Adams et al. 1982). B, under current clamp the M-current acts as the neuron's own in-built ‘voltage clamp’. Thus, a ±0.2 nA current injection at −90 mV (where M-channels are shut) produces large (∼30 mV) voltage responses. In contrast, at −46 mV, where M-current is partly activated, the same current injections produce an initial transient voltage excursion, but the subsequent opening or closing of M-channels restores the membrane potential resulting in very little (< 5 mV) steady-state voltage change (from Brown, 1988). Figure adapted from Delmas & Brown (2005), with permission.
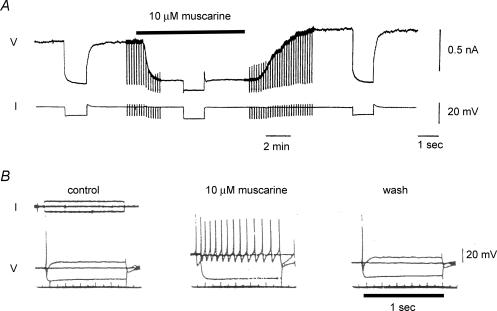
Figure 2. Inhibition of M-current in neurons from the rat superior cervical sympathetic ganglion by muscarine (a muscarinic acetylcholine-receptor stimulant).
A, single microelectrode voltage-clamp recording from a dissociated neuron in short-term culture. The cell was held at −35 mV to pre-activate M-current and commanded to −50 mV for 1 s every 10 s to −55 mV to deactivate M-current. Muscarine (10 μm) suppressed outward M-current at the holding potential and eliminated current deactivation tails at −55 mV (see Fig. 1A). (N. V. Marrion & D. A. Brown, unpublished observations; see Marrion et al. 1989 for technical details). B, single microelectrode ‘current-clamp’ recording from a neuron in an intact isolated ganglion. Initial resting potential −53 mV. Muscarine depolarized the neuron, increased input resistance and promoted vigorous repetitive firing during the 1 s current injection, thereby converting the cell from its normal ‘phasic-firing’ discharge pattern to ‘tonic-firing. (Adapted from Brown & Constanti, 1980, with permission.)
The channels are composed of members of the Kv7 (KCNQ gene) family of K+ channels (Jentsch, 2000). There are five members of this family, Kv7.1 to Kv7.5. Kv7.1 is restricted primarily to the heart and epithelial tissues but all other members are expressed in the nervous system. Native M-channels in rat sympathetic neurons (the cells most intensively studied) comprise primarily a co-assembly of Kv7.2 and Kv7.3 subunits (Wang et al. 1998; Hadley et al. 2003). However, when expressed in cell lines, all Kv7 channels can generate ‘M-currents’ that are sensitive to the M-channel-blocking drug, linopirdine (Brown et al. 2002) and suppressed by M1-mAChRs (Selyanko et al. 2000; see further below); and there is evidence that Kv7.2 homomers (e.g. Hadley et al. 2003; Schwarz et al. 2006) or Kv7.5 + Kv7.3 heteromers (e.g. Shah et al. 2002; Yus-Najera et al. 2003) can also contribute to physiologically functional M-currents. This may have some significance concerning mechanisms of channel regulation (Li et al. 2005).
An important property of M-channels is that they can be closed by activating a number of Gq/11-coupled neurotransmitter receptors (Brown, 1988; Marrion, 1997) – prototypically (but far from exclusively) by muscarinic acetylcholine receptors (mAChRs; hence the name M-channel) (Fig. 2A). This leads to an enhanced excitability (Fig. 2B). When the receptors are activated by synaptically released acetylcholine, there is an accompanying slow depolarizing excitatory postsynaptic potential (Adams & Brown, 1982). The mechanisms for Gq/11-mediated inhibition are discussed further below.
Activation of Kv7/M-channels by PIP2
Though ‘gated’ by voltage, like many other ion channels (Suh & Hille, 2005), Kv7/M-channels require membrane PIP2 to open (Fig. 3A). Thus, Kv7.2 + 7.3 channels in membrane patches rapidly shut down on excision and then re-open on applying phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2 or ‘PIP2’) or dioctanoyl-PIP2 (diC8-PIP2) to the inner surface (Zhang et al. 2003; Li et al. 2005). Individual subunits vary widely in their sensitivity to diC8-PIP2 (Li et al. 2005), in a manner that correlates with their different maximal open probabilities in cell-attached mode (Li et al. 2004), suggesting that the latter is determined by the resting membrane level of PIP2. In support of this, Kv7.2/7.3 currents are reduced when PIP2 is depleted by targeted membrane insertion of a polyphosphate-5-phosphatase and Kv7 currents enhanced in a subunit-dependent manner by increased membrane phosphatidylinositol-4-5-kinase (PI(4)5-K) (Suh et al. 2006).
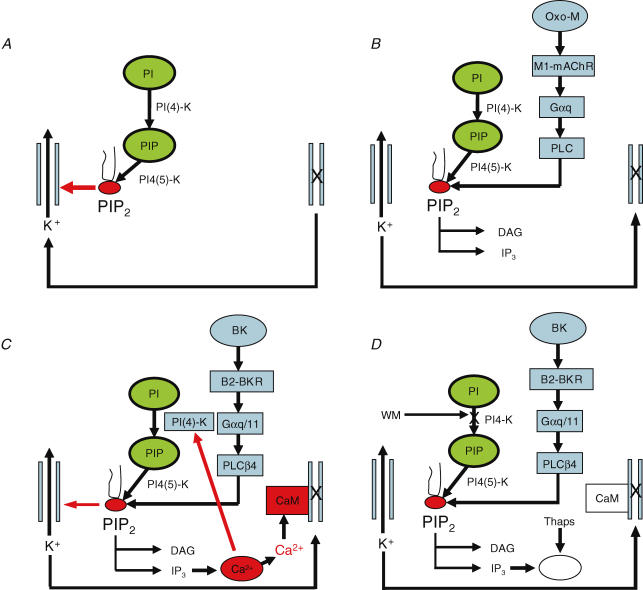
Figure 3. Pathways for M-channel activation and inhibition.
A, channels are normally activated by membrane PIP2. B, muscarinic receptor stimulation closes M-channels by reducing membrane PIP2. C, bradykinin closes channels through IP3-mediated Ca2+ release. D, when Ca2+ release is suppressed and PIP2 synthesis blocked, bradykinin can instead close channels by reducing PIP2. Abbreviations: PI, phosphatidylinositol; PIP, phosphatidylinositol-4-phosphate; PIP2, phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2); PI(4)-K, phosphatidylinositol-4-kinase; PI4(5)-K, phosphatidylinositol-4-phosphate; Oxo-M, oxotremorine-M; M1-mAChR, M1 muscarinic acetylcholine receptor; Gαq, α-subunit of the G proteins Gq; Gαq/11, α-subunit of the G proteins Gq and/or G11; PLC, phospholipase-C; PLCβ4, phospholipase-C β4; DAG, diacylglycerol; IP3, inositol-1,4,5-trisphosphate; BK, bradykinin; B2-BKR, B2-bradykinin receptor; CaM, calmodulin; WM, wortmannin; thaps, thapsigargin. See text for further details. (Adapted from Hughes et al. (2007), with permission.)
Further evidence that native ganglionic M-channels are sensitive to PIP2 stems from several sources. First, whole-cell M-currents require resynthesis of PIP2 to recover from inhibition by an mAChR agonist, and fail to recover when synthesis is inhibited by ATP deprivation or by inhibiting phosphatidylinositol-4-kinase (PI(4)-4K) with wortmannin (Suh & Hille, 2002; Ford et al. 2003). Second, diC8-PIP2 activates run-down M-channels in excised sympathetic neuron membrane patches (Zhang et al. 2003). Third, intracellular dialysis of PIP2 reduces M-current run-down in whole-cell recording mode and increases M-current after inhibition of PIP2 synthesis (Ford et al. 2003), Fourth, M-currents are inhibited by membrane-targeted lipidated basic peptides designed to ‘scavenge’ PIP2 by surface-charge screening (Robbins et al. 2006).
Mechanism of M-current inhibition by muscarinic receptor activation: PIP2 depletion
Muscarinic receptor activation clearly stimulates PIP2 hydrolysis in sympathetic neurons (see Brown, 1988; Marrion, 1997; Bofill-Cardona et al. 2000; Winks et al. 2005). Further, in cell-attached patch recording, both native M-channels (Selyanko et al. 1992; Marrion, 1993) and expressed Kv7.2 + Kv7.3 channels (Selyanko et al. 2000) enclosed within the patch are inhibited by stimulating muscarinic receptor outside the patch, suggestive of some form of ‘diffusible messenger’. Assuming (not unreasonably) that this messenger passes from the receptors to the channels, early experiments concentrated on the two principal products of PIP2 hydrolysis, inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), and on the respective subsequent events, Ca2+ release and protein kinase C (PKC) activation (see Marrion, 1997). However, it now seems unlikely that either pathway is primarily or solely responsible for muscarinic inhibition (Cruzblanca et al. 1998; Suh & Hille, 2006), although Kv7 channel phosphorylation by the DAG/PKC system appears to exert an essential ‘sensitizing’ role, facilitated by a channel-associated A-kinase anchoring protein (AKAP) that also binds PKC (Hoshi et al. 2003, 2005).
Instead, given the importance of PIP2 for channel activity (see above), the principal cause of channel closure is now thought to be the reduction in membrane PIP2 that accompanies Gq-PLC activation and subsequent PIP2 hydrolysis (Suh & Hille, 2002; Zhang et al. 2003; Suh et al. 2004) (Fig. 3B). Indeed, for expressed Kv7.2 + Kv7.3 channels, the dynamics and extent of channel inhibition by M1-mAChRs can be quantitatively accounted for in terms of changes in PIP2 (Suh et al. 2004; Horowitz et al. 2005), and the dependence of inhibition on PIP2 levels has recently been strengthened (Suh et al. 2006). Available evidence suggests that the same mechanism operates for M1-mAChR-induced inhibition of native M-channels in neurons (Suh & Hille, 2002; Zhang et al. 2003). For example, inhibition is reduced, rather than enhanced, when membrane PIP2 levels are increased by over-expressing the synthesizing enzyme PI4(5)-K (Winks et al. 2005), which would be expected if inhibition were due to the loss of PIP2 but not if it were due to a downstream product of PIP2 hydrolysis.
M-current inhibition by bradykinin: Ca2+ release
However, this scenario does not necessarily apply to all forms of receptor-mediated inhibition. Thus, in the case of at least two other receptor agonists – bradykinin and UTP – there is strong evidence that the primary cause of inhibition is the release of Ca2+ from intracellular stores by the IP3 generated by PIP2 hydrolysis (Cruzblanca et al. 1997; Bofill-Cardona et al. 2000; Shapiro & Zaika, 2007) (Fig. 3C). First, intracellular Ca2+ is known to inhibit M-channels, with an IC50 of ∼100 nm (Selyanko & Brown, 1996), an effect mediated through activation of channel-attached calmodulin (Gamper & Shapiro, 2003). Second (and unlike the muscarinic receptors), stimulation of bradykinin receptors produces a small but clear increase in intracellular Ca2+ (Cruzblanca et al. 1997). This is because the receptors are closely coupled to the IP3 receptors, whereas the muscarinic receptors are not (Delmas et al. 2002). Nevertheless, a door (or channel) is either open or shut (de Musset, 1845), so why is it not shut already by the loss of PIP2? The suggested answer (Gamper et al. 2004; Winks et al. 2005) is that bradykinin does not reduce PIP2 because it also stimulates PIP2 synthesis, possibly by Ca2+ activation of neuronal calcium sensor-1 (NCS-1) and consequent activation of PI(4)-K; such a stimulation has been detected in neuroblastoma cells (Loew, 2007).
Tracking changes in membrane PIP2 in neurons
The question then arises: can we measure receptor-induced changes in membrane PIP2 in the sympathetic neuron in such a manner that allows them to be related to the various forms of receptor-induced M-channel inhibition?
In previous experiments we attempted to do this in single dissociated sympathetic neurons by observing the membrane-to-cytosol translocation of the GFP-tagged PH-domain of PLCδ1 (PLCδ-PH-GFP) (Winks et al. 2005). This construct binds to the polar head-groups of PI(4,5)P2 in the membrane inner leaflet and then translocates to the cytosol when PIP2 is hydrolysed (Stauffer et al. 1998; Varnai & Balla, 1998) (Fig. 4A). In our experiments, muscarinic AChR activation induced a substantial membrane-to-cytosol translocation of PLCδ-PH-GFP that showed a close correlation with M-current inhibition in time-course (Fig. 4B and C) and also in agonist concentration dependence (Winks et al. 2005). However, since PLCδ-PH also binds to cytosolic IP3 (Hirose et al. 1999), PLCδ-PH-GFP translocation only provides a measure of overall PI(4,5)P2 hydrolysis, and is not a direct measure of the reduction in membrane PI(4,5)P2 concentration. We tried to correct for IP3 binding (and hence estimate changes in PIP2 concentration) using an intracellular IP3 displacement assay; our corrected data suggested a maximal depletion of ∼83%– in line with measurements in various cell lines (Willars et al. 1998; Li et al. 2005; Horowitz et al. 2005). As an indirect method, however, this estimate is subject to considerable uncertainty, and, importantly, does not make any allowance for changes in the rate of PIP2 synthesis during receptor-induced hydrolysis. Thus, in the experiments of Winks et al. (2005), bradykinin produced as great a translocation of PLCδ-PH-GFP as did the muscarinic agonist, oxotremorine-M, even though M-current inhibition by bradykinin is thought not to result from PIP2 depletion (see above).
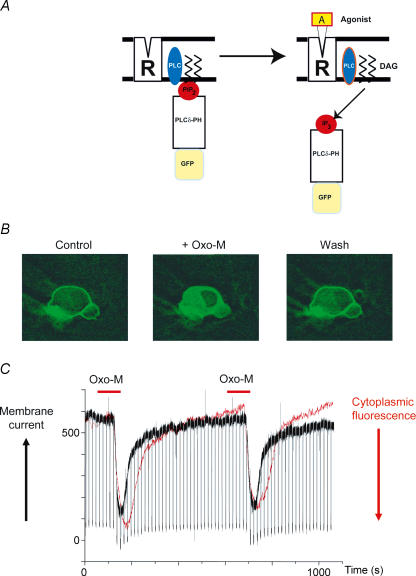
Figure 4. Oxotremorine-induced PIP2 hydrolysis in a sympathetic neuron monitored with the fluorescent probe GFP-PLCδ-PH.
A, the probe binds to membrane PIP2 (left) then translocates to the cytosol and binds to IP3 when PIP2 is hydrolysed (right). B, images showing membrane-to-cytosol translocation of GFP-PLCδ-PH during application of 10 μm oxotremorine-M (Oxo-M) and its recovery following washout. (J. Winks and S. J. Marsh, unpublished observations). C, simultaneous recording of increase in cytosolic fluorescence (red trace, increase downwards) and inhibition of outward M-current (black trace) during two applications of oxotremorine-M to a neuron voltage clamped through a perforated-patch electrode at −30 mV. Downward deflections in the current trace are responses to 1 s hyperpolarizing steps to −50 mV. (Recording by J. Winks and S. J. Marsh; figure reproduced from Delmas & Brown, 2005; with permission.)
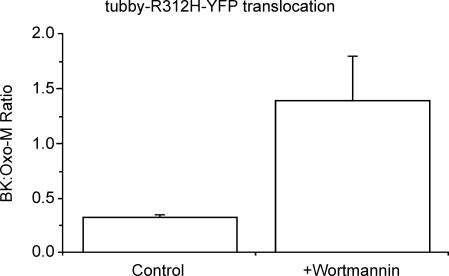
Accordingly, we have recently (Hughes et al. 2007) tried to follow receptor-induced membrane PIP2 changes in single sympathetic neurons more directly using a fluorescently tagged mutated C-terminal domain of the PIP2-binding construct ‘tubby’ (Santagata et al. 2001; Quinn & Tinker, 2004 and in prep.). This has the great advantage over the PLCδ-PH-GFP construct that it binds to PIP2 but not to IP3 or other downstream products of PIP2 hydrolysis such as DAG or Ca2+, and hence can be used to monitor PIP2 changes alone. Although it also binds to several membrane phosphatidyl polyphosphates, including PI(4,5)P2, PI(3,4)P2 and PI(3,4,5)P3 (but not to PI(3,5)P2, PI(4)P or PI), changes in PI(4,5)P2 are by far the most substantial on stimulating Gq-coupled receptors (Willars et al. 1998; McLaughlin et al. 2002). We found that the yellow fluorescent protein (YFP)-tagged tubby construct tubby-R332H-cYFP co-localized in the outer membrane with the PLCδ-PH construct, and that the two co-translocated to the cytosol with a similar time-course on stimulating mAChRs with oxotremorine-M (thus verifying that mAChR-stimulation does indeed reduce membrane PIP2 levels in these cells). However, there was a major difference between the response of the two indicators to stimulating bradykinin receptors: although bradykinin produced as large a translocation of PLCδ-PH-GFP as did oxotremorine-M, it only produced about one-quarter of the tubby-R332H-cYFP translocation signal induced by oxotremorine-M (Fig. 5). This would accord with previous suggestions that bradykinin might stimulate PIP2 synthesis, thus limiting PIP2 depletion. Indeed, when synthesis was blocked with wortmannin, then the tubby translocation signals by bradykinin and oxotremorine-M were comparable (Fig. 5).
Figure 5. The PIP2-binding probe ‘tubby’ differentiates the actions of oxotremorine-M and bradykinin.
Sympathetic neurons were labelled with a YFP-tagged mutated C-terminal domain of tubby (tubby-R312H-cYFP). This bound to the membrane and translocated to the cytosol following application of oxotremorine-M (Oxo-M), like PLCδ-PH (Fig. 4). Bars show the increase in cytosolic fluorescence produced by 100 nm bradykinin (BK) expressed as a fraction of that produced by 10 μm Oxo-M. In control neurons, BK produced less translocation than Oxo-M but an equivalent or greater translocation after 20 min application of 20 μm wortmannin to inhibit the PIP2-synthesizing enzyme PI(4)-kinase. (See Hughes et al. 2007 for technical details.)
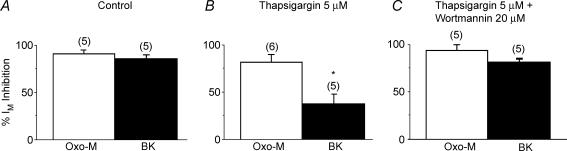
This raised the interesting question whether, if PIP2 synthesis is inhibited in this way, bradykinin might deplete PIP2 to a sufficient extent to lead to M-channel closure through this mechanism, rather than through IP3-induced Ca2+ release. To test this, we first inhibited Ca2+ release with thapsigargin; this did not affect bradykinin-induced tubby translocation, but reduced M-current inhibition by bradykinin (but not by oxotremorine-M) by ∼60% (Fig. 6A and B), in line with previous reports (Cruzblanca et al. 1997; Bofill-Cardona et al. 2000). Subsequent addition of wortmannin then restored full inhibition by bradykinin, to a level matching that produced by oxotremorine-M (Fig. 6C). This recapitulates the experiments of Gamper et al. (2004) who showed that wortmannin sensitized the normally insensitive N-type Ca2+ channels to bradykinin, but with the difference that the Ca2+ channels are not inhibited by Ca2+, whereas M-channels are. Thus, in the latter case, we have not simply enhanced a PIP2 depletion-dependent inhibition, but have switched the mechanism of inhibition entirely, from one produced primarily by a product of PIP2 hydrolysis (Fig. 3C) to one dependent on PIP2 itself (Fig. 3D). We say ‘primarily’ because bradykinin clearly does produce some PIP2 depletion, as judged from its effect on tubby translocation – probably enough to generate some 10–20% inhibition of M-current, even in the absence of a Ca2+ signal; this may account for the incomplete effect of thapsigargin in preventing inhibition. Hence, it seems that the two mechanisms co-exist, and indeed their proportionate significance might well vary under different metabolic conditions or between different cell types.
Figure 6. Wortmannin restores M-current inhibition by bradykinin after its reduction with thapsigargin.
Bar charts show mean percentage inhibition of sympathetic neuron M-currents produced by 10 μm oxotremorine-M (Oxo-M) and 100 nm bradykinin (BK) under control conditions (A), after 10 min pre-exposure to 5 μm thapsigargin (B), and after pre-exposure to thapsigargin followed by 5 min addition of 20 μm wortmannin (C). (Figure adapted from Hughes et al. (2007) with permission.)
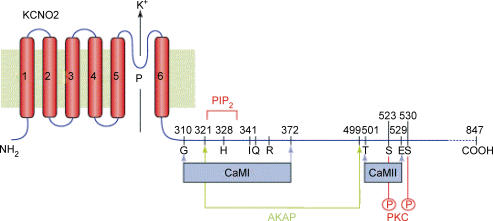
Up to this point, we have regarded PIP2-dependent and PIP2 product-dependent inhibition (by Ca2+ or DAG), as independent mechanisms. However, their precise molecular mechanisms have not fully been elucidated, and indeed they might well be inter-dependent, rather than independent. Thus, bearing in mind that (in so far that they have been defined) the target sites for PIP2, Ca2+–calmodulin and AKAP-bound PKC appear to lie within overlapping domains in the Kv7 channel C-terminal (Fig. 7), all effects might coincide on one final common path, such as a change in the sensitivity of the channel to PIP2 (Delmas & Brown, 2005). This is in line with some evidence regarding the apparent modulation of PIP2-sensitive Kir channels by multiple ‘messengers’ (Du et al. 2004; Brown et al. 2005; Keselman & Logothetis, 2007), thereby putting PIP2 in the ultimate ‘driving seat’.
Figure 7. Putative overlapping binding sites for PIP2, AKAP and calmodulin (CaM), and PKC phosphorylation sites (P) in the proximal C-terminus of Kv7.2 (KCNQ2).
Reproduced from Delmas et al. 2004, with permission.
Acknowledgments
Supported by grants from the UK Medical Research Council, the Wellcome Trust, the European Union and the British Heart Foundation. S.H. is an MRC Postgraduate Student.
References
- Adams PR, Brown DA, Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PR, Brown DA. Synaptic inhibition of the M-current: slow excitatory postsynaptic mechanism in bullfrog sympathetic neurones. J Physiol. 1982;332:263–272. doi: 10.1113/jphysiol.1982.sp014412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill-Cardona E, Vartian N, Nanoff C, Freissmuth M, Boehm S. Two different signaling mechanisms involved in the excitation of rat sympathetic neurons by uridine nucleotides. Mol Pharmacol. 2000;57:1165–1172. [PubMed] [Google Scholar]
- Brown DA. M currents. In: Narahashi T, editor. Ion Channels. Vol. 1. New York: Plenum Press; 1988. pp. 55–99. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+-current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown DA, Constanti A. Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol. 1980;70:593–608. doi: 10.1111/j.1476-5381.1980.tb09778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Selyanko AA, Hadley JK, Tatulian L. Some pharmacological properties of neural KCNQ channels. Neurophysiology. 2002;34:91–95. [Google Scholar]
- Brown SG, Thomas A, Dekker LV, Tinker A, Leaney J. Protein kinase C-δ sensitizes Kir3.1/3.2 to changes in membrane phospholipid levels following M3 receptor activation in HEK293 cells. Am J Physiol Cell Physiol. 2005;289:C543–C556. doi: 10.1152/ajpcell.00025.2005. [DOI] [PubMed] [Google Scholar]
- Cruzblanca H, Koh DS, Hille B. Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1998;95:7151–7156. doi: 10.1073/pnas.95.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Musset A. Il Faut Qu'une Porte Soit Ouverte Ou Fermée. Paris: Charpentier; 1845. [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Delmas P, Crest M, Brown DA. Functional organization of PLC signaling microdomains in neurons. Trends Neurosci. 2004;27:41–47. doi: 10.1016/j.tins.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Delmas P, Wanaverbecq N, Abogadie FC, Mistry M, Brown DA. Signaling microdomains define the specificity of receptor-mediated InsP3 pathways in neurons. Neuron. 2002;34:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J Biol Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- Ford CP, Stemkowski PL, Light PE, Smith PA. Experiments to test the role of phosphatidylinositol 4,5-bisphosphate in neurotransmitter-induced M-channel closure in bullfrog sympathetic neurons. J Neurosci. 2003;23:4931–4941. doi: 10.1523/JNEUROSCI.23-12-04931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Reznikov V, Yamada Y, Yang J, Shapiro MS. Phosphatidylinositol 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J Neurosci. 2004;24:10980–10992. doi: 10.1523/JNEUROSCI.3869-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels. J Gen Physiol. 2003;122:17–31. doi: 10.1085/jgp.200208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JK, Passmore GM, Tatulian L, Al-Qatari M, Ye F, Wickenden AD, Brown DA. Stoichiometry of expressed KCNQ2/KCNQ3 channels and subunit composition of native ganglionic M-channels deduced from block by tetraethylammonium (TEA) J Neurosci. 2003;23:5012–5019. doi: 10.1523/JNEUROSCI.23-12-05012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi N, Langeberg LK, Scott JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol. 2005;7:1066–1073. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, Langeberg LK, Yoneda Y, Scott JD, Brown DA, Higashida H. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6:564–571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Marsh SJ, Tinker A, Brown DA. PIP2-dependent inhibition of M-type (Kv7.2/7.3) potassium channels: direct on-line assessment of PIP2 depletion by Gq-coupled receptors in single living neurons. Pflugers Arch. 2007 doi: 10.1007/s00424-007-0259-6. DOI: 10.1007/500424-007-0259-6. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Keselman I, Logothetis D. Diverse signalling pathways regulate Kir3 activity through modulation of channel interactions with PI (4,5) P2. Biophys J Abstract. 2007 P-120-Plat. [Google Scholar]
- Li Y, Gamper N, Hilgemann DW, Shapiro MS. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:9825–9835. doi: 10.1523/JNEUROSCI.2597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gamper N, Shapiro MS. Single-channel analysis of KCNQ K+ channels reveals the mechanism of augmentation by a cysteine-modifying reagent. J Neurosci. 2004;24:5079–5090. doi: 10.1523/JNEUROSCI.0882-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew LM. Where does all the PIP2 come from? J Physiol. 2007;582:945–951. doi: 10.1113/jphysiol.2007.132860. DOI: 10.1113/jphysiol.2007.132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Wang J, Gambhir A, Murray D. PIP2 and proteins: interactions, organization and information flow. Ann Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- Marrion NV. Selective reduction of one mode of M-channel gating by muscarine in sympathetic neurons. Neuron. 1993;11:77–84. doi: 10.1016/0896-6273(93)90272-s. [DOI] [PubMed] [Google Scholar]
- Marrion NV. Control of M-current. Ann Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Smart TG, Marsh SJ, Brown DA. Muscarinic suppression of the M-current in the rat sympathetic ganglion is mediated by receptors of the M1-subtype. Br J Pharmacol. 1989;98:557–573. doi: 10.1111/j.1476-5381.1989.tb12630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KV, Tinker AW. Using domains from the protein tubby to report changes in PIP2 concentration in living cells. J Physiol. 2004;557P:PC92. [Google Scholar]
- Robbins J, Marsh SJ, Brown DA. Probing the regulation of M (Kv7) potassium channels in intact neurons with membrane-targeted peptides. J Neurosci. 2006;26:7950–7961. doi: 10.1523/JNEUROSCI.2138-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L. G protein signaling through tubby proteins. Science. 2001;292:2041–2050. doi: 10.1126/science.1061233. [DOI] [PubMed] [Google Scholar]
- Schwarz JR, Glassmeier G, Cooper E, Kao T, Nodera H, Tabuena D, Kaji R, Bostock H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the node of Ranvier. J Physiol. 2006;573:17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko AA, Brown DA. Intracellular calcium directly inhibits potassium M channels in excised membrane patches from rat sympathetic neurons. Neuron. 1996;16:151–162. doi: 10.1016/s0896-6273(00)80032-x. [DOI] [PubMed] [Google Scholar]
- Selyanko AA, Hadley JK, Wood IC, Abogadie FC, Jentsch TJ, Brown DA. Inhibition of KCNQ1-4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors. J Physiol. 2000;522:349–355. doi: 10.1111/j.1469-7793.2000.t01-2-00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko AA, Stansfeld CE, Brown DA. Closure of potassium M-channels by muscarinic acetylcholine-receptor stimulants requires a diffusible messenger. Proc Roy Soc Series B. 1992;250:119–125. doi: 10.1098/rspb.1992.0139. [DOI] [PubMed] [Google Scholar]
- Shah MM, Mistry M, Marsh SJ, Brown DA, Delmas P. Molecular correlates of the M-current in cultured rat hippocampal neurons. J Physiol. 2002;544:29–37. doi: 10.1113/jphysiol.2002.028571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M, Zaika O. Purinergic modulation of neuronal M-current involves IP3-mediated Ca2+ release. Biophys Soc Abstract. 2007 2183-Pos/B399. [Google Scholar]
- Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Does diacylglycerol regulate KCNQ channels? Pflugers Arch. 2006;453:293–301. doi: 10.1007/s00424-006-0092-3. [DOI] [PubMed] [Google Scholar]
- Suh BC, Horowitz LF, Hirdes W, Mackie K, Hille B. Regulation of KCNQ2/KCNQ3 current by G protein cycling: the kinetics of receptor-mediated signaling by Gq. J Gen Physiol. 2004;123:663–683. doi: 10.1085/jgp.200409029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5),P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium and agonist-induced dynamic changes and relationship to myo-[3H] inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Willars GB, Nahorski SR, Challis RAJ. Differential regulation of muscarinic acetylcholine receptor-sensitive polyphosphoinositide pools and consequences for signaling in human neuroblastoma cells. J Biol Chem. 1998;273:5037–5046. doi: 10.1074/jbc.273.9.5037. [DOI] [PubMed] [Google Scholar]
- Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, Brown DA, Marsh SJ. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25:3400–3413. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yus-Najera E, Munoz A, Salvador N, Jensen BS, Rasmussen HB, Defelipe J, Villarroel A. Localization of KCNQ5 in the normal and epileptic human temporal neocortex and hippocampal formation. Neuroscience. 2003;120:353–364. doi: 10.1016/s0306-4522(03)00321-x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, Logothetis DE. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]