Abstract
The labelling pattern of cellular phosphoinositides (PtdInsPn) was studied in Ehrlich ascites cells labelled in vivo for 24 h with myo-[2-3H]- or l-myo-[1-3H]inositol and exposed to anisotonic or isosmotic volume perturbations. In parallel experiments the cell volume ([14C]3-OMG space) was monitored. In hypotonic media the cells initially swelled osmotically and subsequently as expected showed a regulatory volume decrease (RVD) response. Concurrently, the cell content of PtdInsP2 showed a marked, transient decrease and the content of PtdInsP a small, transient increase. The changes in PtdInsP2 and PtdInsP content increased progressively with the extent of hypotonicity (in the range 1.00–0.50 relative osmolarity). No evidence was found for either hydrolysis of PtdInsP2 or formation of PtdInsP3. In hypertonic medium (relative osmolarity 1.50), cells initially shrank osmotically and subsequently as expected showed a small regulatory volume increase (RVI) response. Concurrently, the cell content of PtdInsP2 showed a marked increase and the content of PtdInsP a small decrease, i.e. changes in the opposite direction of those seen in hypotonic media. In isosmotic media with high (100 mm) or low (0.8 mm) K+ concentration, cells slowly swelled or shrank due to uptake or loss of isosmotic KCl. Under these conditions, with largely unchanged intracellular ionic strength, the cell content of PtdInsP2 and PtdInsP remained constant. Our results show that PtdInsP2 is not volume sensitive per se, and moreover that the regulatory volume adjustments in Ehrlich ascites cells are not mediated by PtdInsP2 hydrolysis and its subsequent production of second messengers. The simplest interpretation of the observed effects would be that PtdInsP2 is controlled by ionic strength, probably via activation/inhibition of phosphoinositide-specific phosphatases/kinases. In Ehrlich ascites cells, as shown previously, the opposing ion channels and transporters activated during RVD and RVI, respectively, are controlled with tight negative coordination by a common cell volume ‘set-point’ that is shifted in anisotonic media, but unchanged during cell swelling in isosmotic high K+ medium. We hypothesize that PtdInsP2 might orchestrate this ‘set-point’ shift.
Phosphatidylinositol (4,5)bisphosphate (PtdInsP2) has attracted considerable interest for many years. It is the precursor for the second messengers inositol(1,4,5)trisphosphate (InsP3), diacylglycerol (DAG) and phosphatidylinositol(3,4,5)trisphosphate (PtdInsP3), and has also itself important signalling roles. It is seen as a multifunctional regulator and adaptor, modulating many cellular functions. It plays key roles in receptor-mediated signal transduction, translocation of cytoplasmic proteins containing PtdInsP2-binding domains, membrane trafficking, and reorganization of the actin cytoskeleton (see, e.g. Toker, 1998; Czech, 2000; Downes et al. 2005; Santarius et al. 2006; and references therein). Moreover, it modulates directly or indirectly the function of a wide range of ion channels and transporters (see, e.g. Hilgemann et al. 2001; Suh & Hille, 2005), including inward rectifier K+ channels, as studied among others by Logothetis and coworkers (see Du et al. 2004), and Na+/H+-exchangers (see Aharonovitz et al. 2000).
The effect of extracellular tonicity and cell volume on cellular phosphoinositide levels has only been addressed in a limited number of studies. Hyperosmotic stress has been shown to induce increased levels of PtdInsP2 and other phosphoinositides in several cell types (Van der Kaay et al. 1999; Nasuhoglu et al. 2002; Yamamoto et al. 2006), including plant cells (see Zonia & Munnik, 2004; and references therein). Hypotonic stress has attracted less interest, although a rapid phosphoinositide breakdown with production of cellular inositol phosphates has been demonstrated in MDCK cells (Fujii et al. 1999).
We have studied a conceivable role of phosphoinositides in cell volume regulation in Ehrlich ascites cells exposed to volume perturbations in anisotonic and isosmotic media. During cell swelling and shrinkage in hypotonic and hypertonic media, respectively, the cell PtdInsP2 content is markedly changed, and changed in opposite directions under the two conditions. In striking contrast, the PtdInsP2 content is unchanged during similar cell volume perturbations attained in isosmotic high and low K+ media. The results show that the PtdInsP2 content is not volume sensitive per se, but rather, might be controlled by ionic strength, probably via activation/inhibition of phosphoinositide-specific phosphatases/kinases. During cell volume regulation in anisotonic media the ‘set-point’ for volume regulation is shifted (Harbak & Simonsen, 1999), whereas this is not the case during cell swelling in isosmotic high K+ medium (Harbak, Jensen & Simonsen, 2001). The ‘set-point’ shift in anisotonic media involves a tight coordination between the opposing volume-regulatory transport systems, and it is proposed that the multifunctional regulator PtdInsP2 might perhaps orchestrate the setting of the volume regulatory ‘set point’. Some of the present data have previously been presented in abstract form (Nielsen et al. 1995; Harbak et al. 1995).
Methods
Reagents
All reagents were of analytical grade and unless otherwise indicated obtained from Sigma-Aldrich, Denmark or Merck Eurolab Norden. myo-[2-3H]Inositol (with PTK-271 stabilizer, 1 mCi ml−1) was from Amersham Biosciences, UK. l-myo-[1-3H]Inositol was obtained from New England Nuclear (NEN), Boston MA, USA on special request. 3-O-[14C]Methyl-d-glucose and 51Cr(III)EDTA complex were purchased from NEN, Boston, MA, USA.
Media
The standard incubation medium contained (mm): 150 Na+, 150 Cl−, 5 K+, 1 Ca2+, 1 Mg2+, 1 HPO42−, 1 SO42−, 3.3 Mops, 3.3 Tes, and 5 Hepes (pH 7.4; 300 mosmol l−1). All media were nominally bicarbonate free. Bovine serum albumin (fraction V, from Sigma; extensively dialysed against distilled water) was included in all media at 10 mg ml−1. The tonicity was reduced by addition of buffered water and increased by adding extra NaCl. The reduced Ca2+ concentration in the hypotonic media was not considered significant, as the RVD response in Ehrlich ascites cells is unaffected in Ca2+-free media (see, e.g. Hoffmann et al. 1993). The K+ concentration in isosmotic medium was varied by exchanging KCl and NaCl mole by mole.
In vivo labelling of cellular phosphoinositides and inositol phosphates
Ehrlich mouse ascites tumour cells were maintained by weekly inoculation (100 μl) into the abdominal cavity of female NMRI mice. In experiments with measurements of cellular phosphoinositides and inositol phosphates the cells were labelled in vivo with myo-[2-3H]inositol or l-myo-[1-3H]inositol by intraperitoneal injection of 0.3 mCi in tumour-bearing mice 24 h before harvesting the cells. The handling of the mice followed the guidelines of the institute and the procedures used in earlier work (Christensen et al. 1994, 2003).
Cell suspensions
The mice were killed by cervical dislocation 7 days after inoculation, and the ascites fluid collected into a large volume of standard medium. The cells were packed by gentle centrifugation, washed twice, and resuspended in standard medium. The fractional cell volume (cytocrit) of the cell suspension was measured by centrifugation of duplicate samples of the cell suspension in haematocrit tubes, and adjusted to 4–8%. The suspension of washed cells was preincubated for 20 min at 37°C in an Erlenmeyer flask on a shaking water bath in order to reach a steady state for ion content and cell volume. Aliquots of the preincubated cell suspension were transferred to an incubator with multihead magnetic stirring for ease of sampling. Silanized glass vials were used throughout. Experimental groups to be directly compared were incubated in parallel, with only a short delay between groups. An isotonic control group was included in all experiments.
Measurement of cell water volume (3-OMG space)
Cell water volume was measured using the 14C-labelled non-metabolizable glucose analogue 3-O-methyl-d-glucose (3-OMG) (0.05–0.1 μCi (ml cell suspension)−1) (Kletzien et al. 1975). [14C]3-OMG equilibrates across the plasma membrane of Ehrlich ascites cells within seconds. Assuming that the concentration of 3-OMG in cell water is the same as the concentration in the medium, the cellular volume in which 3-OMG distributes (the ‘cell 3-OMG space’) is about 0.70 ml per ml original cell volume (as determined from the cytocrit value, see above) (data not shown), consistent with 3-OMG distributing in most of the water phase of the cells. The cell content of [14C]3-OMG responds to tonicity changes as expected for a simple equilibration of 3-OMG across the cell membrane, and accordingly we have used cell 3-OMG space as a measure of cell volume. As 3-OMG equilibrates so rapidly, cell 3-OMG space reflects cell volume even during quite steep volume changes. 51Cr(III) EDTA complex (0.5–1 μC (ml cell suspension)−1) was used as an extracellular marker, in order to correct for [14C]3-OMG in medium trapped in the cell pellet below the oil during the sampling procedure (see below).
Sampling from cell suspensions
From the cell suspension serial 600 μl samples were taken for determination of 14C and 51Cr radioactivity in cells and in extracellular medium. The cells were separated from the medium within seconds by centrifugation through an oil layer (phthalate ester or silicone oil mixture with appropriate density). Aliquots (200 μl) of the supernatant (the extracellular medium) above the oil were saved for analysis. The rest of the supernatant and most of the oil layer was removed by suction, the walls of the microfuge tube carefully wiped with a cotton swab, and the cell pellets suspended in distilled water by vortexing and left to lyse for an hour. The protein in cell lysate and in samples of the medium was precipitated using trichloroacetic acid (TCA; final concentration 5%, w/v). Tracer radioactivities in the TCA supernatants were measured by dual channel liquid scintillation counting with Ultima-Gold (Packard BioScience). The calculated 3-OMG spaces of the cells in any given experiment were normalized to the mean of the values of isotonic control cells, either initial values recorded in isotonic medium (see Figs. 1 and 2) or the values in a parallel control group in that same experiment (see Fig. 3).
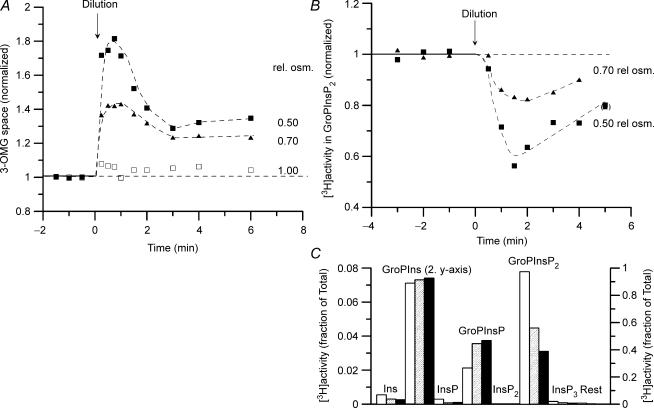
Figure 1. Effect of hypotonic exposure on cell water (3-OMG space) and on PtdInsP2 content in Ehrlich ascites cells.
At zero time (arrow) the cell suspensions were diluted with an equal volume of 2/5 strength incubation medium (diluted with buffered water) or of buffered water, to give final, relative osmolarity at 0.70 and 0.50, respectively. A parallel group (control, not illustrated) was diluted with the same volume of the standard incubation medium. A, the cell suspension was preincubated with [14C]3-O-methylglucose, and with 51Cr(III)EDTA as a marker of trapped volume in the cell pellet. For the serial aliquots of the cell suspension taken after the dilution, the radioactivity recorded was corrected such that all samples nominally represent the same number of cells. The calculated 3-OMG spaces of the cells are given normalized to the mean of the initial values in isotonic medium. Typical results from 4 and 3 replicate experiments, respectively. The small increase in 3-OMG space in the control group receiving isotonic dilution was found consistently (see Table 2 and Fig. 2A) and probably represents a small systematic error in the correction applied to compensate for the dilution of the cell suspension (see above). The 3-OMG data presented have not been corrected for this error. B, the cells were labelled in vivo with l-myo-[1-3H]inositol, and the water-soluble glycerophosphoinositols (GroPInsPn, derived from PtdInsPn) and inositol phosphates (InsPn) in the deacylated lipid extracts of the cell suspension separated by small-column anion-exchange chromatography. The cell content of [3H]GroPInsP2, representing PtdInsP2, is calculated as the fraction of the total 3H radioactivity eluted from the column and given normalized to the mean of the initial values in isotonic medium. Single experiment. Consistent results were obtained for cells labelled with myo-[2-3H]inositol in 4 and 3 experiments at 0.70 and 0.50 relative osmolarity, respectively. C, peak areas representing InsPn and GroPInsPn in elution profiles of deacylated lipid extracts for samples taken 1.5 min after exposure to isotonic medium (open columns) and to hypotonic medium at 0.70 (hatched columns) and 0.50 (filled columns) relative osmolarity, respectively. Note that the column for GroPIns refers to the second y-axis (right). Typical experiment.
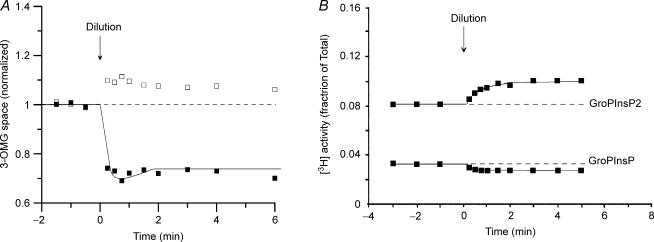
Figure 2. Effect of hypertonic exposure on cell water (3-OMG space) and on PtdInsP2 and PtdInsP content in Ehrlich ascites cells.
At zero time (arrow) the cell suspensions were diluted with an equal volume of double-osmolarity incubation medium, to give final, relative osmolarity at 1.50. Protocol otherwise as in Fig. 1. A, typical results from 5 and 2 replicate experiment at 1.0 (□) and 1.50 (▪) relative osmolarity, respectively. The small increase in 3-OMG space in the control group receiving isotonic dilution probably represents a small systematic error (see legend to Fig. 1). In the hypertonic medium the small RVI response indicated was genuine, as evidenced from a number of other experiments in hypertonic media, and is small in size, because the volume regulatory ‘set-point’ is markedly depressed in hypertonic media (see Harbak & Simonsen, 1999). B, typical experiment. One other experiment gave essentially identical results.
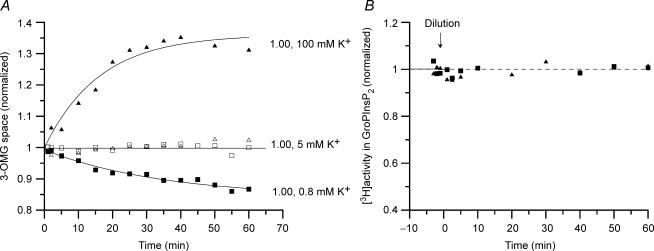
Figure 3. Cell water (3-OMG space) and PtdInsP2 content in Ehrlich ascites cells during incubation in isosmotic media with increased or decreased K+concentration.
At zero time (arrow) the cell suspensions were diluted 3- or 6-fold with isosmotic incubation medium with either high or low K+ concentration (obtained by replacing NaCl and KCl mole by mole), to give a final K+ concentration at 100 mm and 0.8 mm, respectively (marked with ▴ and ▪). Parallel groups (control, marked with open symbols) were diluted with the same volumes of standard incubation medium (5 mm K+). Protocol otherwise as in Fig. 1. A, typical experiment. The calculated 3-OMG spaces of the cells are normalized to the mean for the control group in isosmotic medium at 5 mm K+. One other experiment gave similar results. B, typical experiment. Other experiments with similar protocol gave consistent results. In 2, 3, and 3 further experiments at 5 mm, 0.8 mm, and 100 mm K+, respectively, the PtdInsP2 content was followed with 5 min intervals for 35 min, and normalized to the mean value within the group. The normalized data were pooled at each K+ concentration, and were at all K+ concentrations adequately fitted by a horizontal line. s.d. was calculated at s.d.= 0.045 (n = 14), 0.027 (n = 23), and 0.027 (n = 18), respectively, n being the number of pooled data points at 5, 0.8, and 100 mm K+, respectively.
From suspensions of myo-[3H]inositol labelled cells, samples were taken for extraction of phosphoinositide lipids and for analysis of cellular inositol phosphates (see below).
Extraction of phosphoinositide lipids
Samples (1000 μl) of the cell suspension were mixed with 2.5 ml ice-cold methanol containing butylated hydroxytoluene (BHT; 1.25 mg) and trans-1,2-diaminocyclohexano-N,N,N′,N′-tetraacetic acid (CDTA; 5 μmol), and extracted for 30 min at 0°C. After addition of 1.25 ml chloroform the samples were extracted for 2 h at room temperature, and finally stored at −20°C until lipid extraction. The phosphoinositide lipids were extracted as previously described (Christensen et al. 2003). Briefly, a two-phase system was established by addition of 1.25 ml chloroform, 2.50 ml ‘neutral equilibrated chloroform phase’, and 1.25 ml 2.4 m HCl. After vigorous mixing, immediately after addition of HCl, and centrifugation, the acidic aqueous phase was re-extracted with 5 ml chloroform. Both chloroform phases were re-extracted with 4 ml methanol/0.1 m HCl (1: 1, v/v). The combined chloroform phases were finally evaporated by vacuum concentration for 2 h in preparation for deacylation (see below).
Deacylation of phosphoinositides (PtdInsPn) and separation of deacylation products by anion-exchange chromatography
The extracted lipids were deacylated with methylamine as previously described (Christensen et al. 2003). Briefly, the lipids were dissolved in 300 μl n-butanol and mixed with 2.7 ml monomethylamine reagent (monomethylamine/methanol (5: 4, v/v), containing CDTA (0.75 μmol), mannitol (5.5 μmol), and InsP6-hydrolysate (600 nmol total P). The mixture was incubated for 60 min at room temperature, and subsequently evaporated by vacuum concentration overnight. The dry, deacylated product was dissolved in l ml 1 mm ammonium formate, and the fatty acids removed by extraction with 1.2 ml n-butanol/light petroleum/ethyl formate (20: 4: 1, v/v), followed by re-extraction with 0.75 ml of the same solvent mixture. The resulting water-soluble deacylation products of the [3H]phosphoinositides were finally separated by small-column, ambient pressure, anion-exchange chromatography, using glass columns packed with Bio-Rad AG 1-X8 formate resin, and step-wise elution with increasing concentrations of ammonium formate in formic acid (see Christensen et al. 2003). 3H radioactivity was recorded by liquid-scintillation counting with Hionic Fluor (Packard, BioScience) as scintillation cocktail in a Packard Tri-Carb 2300TR analyser with quench correction (tSIE/AEC). The 3H radioactivity eluted in the individual chromatographic peaks is expressed as a percentage of the total 3H radioactivity eluted from the column. The PtdInsP2 isomers have not been resolved, but PtdInsP2 is presumed to predominantly represent PtdIns(4,5)P2.
Measurement of cellular inositol phosphates (InsPn)
Samples were taken from the suspension of myo-[3H]inositol labelled cells and processed for chromatographic separation of inositol phosphates as previously described (Christensen et al. 1994). Briefly, 500 μl samples were rapidly mixed with 500 μl ice-cold 1 m perchloric acid (PCA) with 0.2% Triton X-100, dipped into an alcoholic mix of frozen carbon dioxide for a few seconds to ensure complete stoppage of enzymic activity, and finally extracted for 20 min at 0°C. After centrifugation the supernatant, still kept at 0°C, received ethylene-diamino-N,N,N′,N′-tetraacetic acid (EDTA; final concentration 4 mm) and was finally neutralized with tri-n-octylamine/freon. After centrifugation the neutralized extract (pH 6.2 approx.) was stored at −20°C until analysis. The inositol phosphates were analysed by HPLC or usually by small-column, ambient-pressure anion-exchange chromatography as previously described (Christensen et al. 1994). The cell content of [3H]InsPn was calculated with correction for the content in the extracellular medium, as measured in duplicate samples of the supernatant taken before and after the hypotonic dilution (see Jørgensen et al. 1997).
Statistical analysis
The data are expressed as mean values ±s.d. or s.e.m. as appropriate, with n indicating the number of independent experiments. The figures show typical experiments, with the number of replicate experiments given in the legends. The curves were drawn by eye. In Fig. 3B two data points at 0.8 mm K+ have been omitted as outliers, since the deviation from the mean was 4.6 and 7.0 times s.d., respectively.
Results
In unstimulated Ehrlich ascites cells labelled in vivo with myo-[2-3H]- or l-myo-[1-3H]inositol and suspended in isotonic medium the labelling pattern of cellular phosphoinositides was quite reproducible between experiments (see Table 1), suggesting a dynamic balance between the phosphoinositide species. The labelling pattern was identical to that found in a previous study, demonstrating reduced aberrant 3H-labelling of nucleotides with the use of 3H-myo-inositol labelled at L-C1 rather than at the C2 position (Christensen et al. 2003). The dominating phosphoinositide species was PtdIns, with PtdInsP2 contributing about 7% and PtdInsP only about 3% of total PtdInsPn. No PtdInsP3 could be detected.
Table 1.
Distribution of phosphoinositides in Ehrlich ascites cells (unstimulated cells)
| 3H radioactivity (% of total) | ||
|---|---|---|
| Series 1 | Series 2 | |
| PtdIns | 88.5 ± 1.22 (5) | 89.9 ± 0.72 (7) |
| PtdInsP | 2.8 ± 0.69 (5) | 3.4 ± 0.66 (7) |
| PtdInsP2 | 8.7 ± 0.86 (5) | 6.7 ± 1.09 (7) |
The data are means ± s.d. and the values in parentheses are the number of independent experiments. The cells in two series of experiments were labelled in vivo with myo-[2-3H]inositol (series 1) or l-myo-[1-3H]inositol (series 2). 3H-labelled PtdInsPn (PtdIns, PtdInsP, and PtdInsP2) were measured as the water-soluble glycerophosphoinositol (GroPInsPn) head groups resulting from methylamine-deacylation of a methanol/chloroform/HCl lipid extract of the cell suspension, and separated by small-column, ambient pressure anion-exchange chromatography. The PtdInsPn content is given as percent of the total 3H radioactivity eluted from the column.
Following anisotonic perturbation, however, the cell content of PtdInsP2 and PtdInsP was markedly changed. In hypotonic medium there was a marked decrease in the cellular PtdInsP2 content that reached a minimum after about 2 min and partially recovered after 5 min (see Fig. 1B). Concurrently, there was a small, transient increase in PtdInsP (see Fig. 1C). A similar rapid, transient decrease in PtdInsP2 content was observed in a much earlier study using 32P-labelled cells (Christensen et al. 1988).
The sum of the 3H radioactivity eluted from the columns remained constant (not illustrated), and no evidence of the presence of PtdInsP3 could be detected in any experiment, not even at the time of maximal decrease in PtdInsP2 (no 3H radioactivity eluted at higher eluent concentrations than InsP3, see column labelled ‘Rest’ in Fig. 1C). In parallel control groups with isotonic dilution, as expected no changes in PtdInsP2 and PtdInsP could be detected (not illustrated). Of note, following the hypotonic dilution (relative osmolarity 0.50) no changes could be detected in cytosolic inositol phosphates (InsPn), when analysed by small-column chromatographic separation of neutralized PCA extracts of serial aliquots of the cell suspension, and with due correction for the content in the extracellular medium (single experiment, data not shown). In particular, no increase in cellular InsP3, InsP4, or InsP2 could be demonstrated. In separate experiments with HPLC analysis of the PCA extracts, the cell content of both Ins(1,4,5)P3 and Ins(1,3,4)P3) remained constant, consistent with previous results (see Jørgensen et al. 1997).
The time-dependent changes in phosphoinositide pattern were concurrent with the changes in cell water (3-OMG space, see Fig. 1A). Cells showed the well-known RVD response, studied in detail in this cell type among many others (see, e.g. Hoffmann et al. 1993). After the initial, nearly osmometric cell swelling the cells partially recover their initial, isotonic cell volume by KCl loss during the RVD response. The volume recovery is only partial, because the ‘set-point’ for the RVD response is elevated in hypotonic media (see Harbak & Simonsen, 1999).
The changes in cellular PtdInsP2 and PtdInsP content increased with the degree of hypotonicity, as summarized in Table 2 (see also Fig. 1C). Concurrently, the steady-state 3-OMG space attained after completed RVD response and reflecting the elevation of the volume regulatory ‘set-point’ also increased with the degree of hypotonicity (see Table 2).
Table 2.
3-OMG space, PtdInsP2 and PtdInsP in Ehrlich ascites cells following anisotonic exposure
| Rel. osmolarity | 3-OMG spacea | PtdInsP2a | PtdInsPb |
|---|---|---|---|
| 1.50 | 0.752 ± 0.018 (2) | 1.237 ± 0.002 (2) | 0.802 ± 0.008 (2) |
| 1.00c | 1.056 ± 0.011 (5) | 0.986 ± 0.003 (3) | 1.030 ± 0.017 (4) |
| 0.90 | 1.087 | 0.899 | 1.042 |
| 0.80 | 1.181 ± 0.005 (2) | 0.848 ± 0.015 (3) | 1.104 ± 0.048 (3) |
| 0.70 | 1.244 ± 0.007 (4) | 0.840 ± 0.012 (4) | 1.120 ± 0.033 (3) |
| 0.50 | 1.283 ± 0.014 (3) | 0.787 ± 0.038 (3) | 1.180 ± 0.053 (2) |
The data are normalized means ± s.e.m., with the number of independent experiments given in parentheses.
Mean of data points 3, 4 and 5 min after anisotonic exposure, normalized to mean of data points taken before anisotonic perturbation.
‘Peak’ value 1 min after anisotonic exposure, normalized to mean of data points taken before anisotonic perturbation.
Parallel control groups receiving isotonic dilution. The small increase in 3-OMG space probably represents a small systematic error (see Legend to Fig. 1).
In hypertonic medium the cell content of PtdInsP2 and PtdInsP was also shifted, and in a direction opposite of that observed in hypotonic medium (see Fig. 2B and Table 2). Again, the sum of the 3H radioactivities eluted from the columns remained constant (not illustrated).
The changes in phosphoinositide pattern were, as in hypotonic medium, concurrent with changes in 3-OMG space (see Fig. 2A). After the initial, nearly osmometric cell shrinkage, the cells showed a small RVI response that was only just detectable, reflecting the marked depression of the cell volume ‘set-point’ in the hypertonic medium (see Harbak & Simonsen, 1999).
Addition of glucose to a suspension of Ehrlich ascites cells leads to a marked transient fall in cytosolic ATP concentration, due to the ‘Crabtree effect’ as described by Overgaard-Hansen (1965). Addition of glucose (10 mm, at 4–5% cytocrit), with a nearly 50% reduction in ATP concentration, was, however, without any effect on the cellular PtdInsP2 and PtdInsP content (two experiments, not illustrated).
In isosmotic medium cells swelled slowly at high K+ concentration (100 mm) and conversely shrank slowly at low K+ concentration (0.8 mm), reflecting net uptake and loss, respectively, of KCl and osmotically obliged cell water (see Fig. 3A). Interestingly, the cell PtdInsP2 content remained constant during cell swelling and cell shrinkage in isosmotic media with high and low K+ concentration, respectively (see Fig. 3B).
Discussion
Like nearly all other animal cells, Ehrlich ascites cells regulate their volume in anisotonic media, as studied in detail in this cell model before (see, e.g. Hoffmann et al. 1993). In hypotonic media the initial osmometric cell swelling is followed by an RVD (regulatory volume decrease) response with KCl loss via swelling-activated K+ and Cl− channels (see Niemeyer et al. 2000; Pedersen et al. 1998), and in hypertonic media the initial cell shrinkage is followed by a small and barely detectable RVI (regulatory volume increase) response with activation of the Na+,K+,2Cl−-cotransporter, as illustrated in Figs. 1A and 2A. In both cases the volume recovery in Ehrlich cells is clearly incomplete, because the ‘set-point’ for volume regulation, as discussed further below, is shifted in anisotonic media, being elevated in hypotonic media and depressed in hypertonic media (see Harbak & Simonsen, 1999). In the anisotonic media the cell volume changes are concurrent with changes in the cell PtdInsP2 and PtdInsP content. In hypotonic media PtdInsP2 decreases and PtdInsP increases, with changes in the opposite direction in hypertonic media (see Figs. 1B and 2B and Table 2). The decrease in the cell PtdInsP2 content in hypotonic medium cannot be caused by phosphoinositide kinase-mediated phosphorylation of PtdInsP2, since no PtdInsP3 was detectable, not even at the time of maximal decrease in PtdInsP2 (see Fig. 1C). Also, the decrease in PtdInsP2 cannot be due to a swelling-induced activation of phosphoinositidase C with hydrolysis of PtdInsP2, since no increase in cytosolic inositol phosphates, including both Ins(1,4,5)P3 and Ins(1,3,4)P3, could be detected (see Results).
The reciprocal changes in cellular PtdInsP2 and PtdInsP content both in hypotonic and in hypertonic medium would suggest a shift in the ‘PtdInsPn shuttle’:
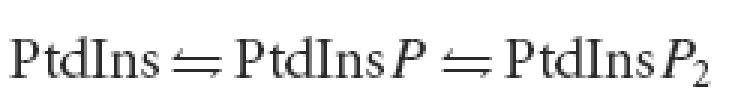 |
In hypotonic medium the shift would be to the left and in hypertonic medium to the right, perhaps being caused by activation/inhibition of phosphoinositide-specific phosphatases/kinases. As a simple interpretation the shifts could conceivably be due to the changes in cytosolic ATP concentration concomitant to the cell volume changes. This interpretation seems unlikely, however, since no changes in PtdInsP2 and PtdInsP content could be detected during a marked transient fall of nearly 50% in cytosolic ATP concentration induced by addition of glucose (see Results). In support of the proposed shift in the ‘PtdInsPn shuttle’ in anisotonic media, hypertonicity has recently been shown to induce a rapid increase in PtdInsP2 in HeLa cells, due to membrane recruitment and activation of PtdIns(4)5-kinase by a volume-dependent, calyculin A-sensitive protein phosphatase-mediated dephosphorylation of the 5-kinase (Yamamoto et al. 2006).
In isosmotic medium increasing or reducing the external K+ concentration results in slow changes in the cell volume (see Fig. 3A). Under these conditions, however, the cell PtdInsP2 content is unaffected by the cell volume changes (see Fig. 3B), in contrast to the findings during cell volume changes in anisotonic media. The cell swelling and shrinkage attained after 30 min in high and low K+ medium correspond roughly to those that are attained in anisotonic media at 0.70 and 1.50 relative osmolarity, respectively, and that showed marked concurrent changes in PtdInsP2 content. The absence of a PtdInsP2 response during the cell volume changes in isosmotic media shows that PtdInsP2 is not volume sensitive per se and is not simply acting as a volume ‘sensor’ or indicator. The absence of a PtdInsP2 response in isosmotic media shows, moreover, that the response observed in anisotonic media cannot be caused by volume-dependent changes in cytosolic enzyme concentrations or in ‘macromolecular crowding’ (Minton et al. 1992), or be dependent on membrane tension per se or on mechanical transduction by the actin cytoskeleton (see Czech, 2000).
PtdInsP2 has been implicated in both direct and indirect modulation of the function of ion channels and transporters (see recent review by Hilgemann et al. 2001; Zeng et al. 2003). However, it seems in the present case unlikely that the depletion of cell membrane PtdInsP2 is directly involved in the activation of the K+ and Cl− channels mediating the RVD response in hypotonic medium. The K+ and Cl− channels are activated also during the isosmotic cell swelling, since a rapid KCl efflux can be induced by restoring a gradient for K+ and Cl− efflux by a sudden reduction of the external K+ concentration (Harbak, Jensen & Simonsen, 2001), and this channel activation is seen in the absence of changes in cell membrane PtdInsP2.
An important difference between the cell volume perturbations in isosmotic and in anisotonic media is that the cytoplasmic ionic strength is largely unchanged during cell volume changes in isosmotic media, but is markedly reduced and increased, respectively, during cell swelling and shrinkage in anisotonic media. In parallel with the ionic strength, the cell PtdInsP2 content remains constant during isosmotic cell volume perturbations, but markedly changes, and in opposite directions, during hypotonic and hypertonic cell swelling and shrinkage.
There has been considerable interest in the intracellular Cl− concentration as a regulated parameter, and the existence of a Cl− sensor has been suggested (see, e.g. O'Neill, 1999; Lytle & McManus, 2002; and references therein). In our experimental set-up with changes in the external K+ concentration in isosmotic media, the cells swell or shrink by taking up or releasing isosmotic KCl, that is, KCl at a concentration of about 160 mm. This is about the same as the normal K+ concentration in unperturbed cells in isosmotic media, so the intracellular K+ concentration will not change much during these isosmotic cell volume changes. The intracellular Cl− concentration, on the other hand, is about 50 mm in unperturbed Ehrlich ascites cells. This means that when the cells swell by taking up isosmotic KCl, the intracellular Cl− concentration will increase. Conversely, when the cells shrink by releasing isosmotic KCl, the intracellular Cl− concentration will decrease. (For a discussion of the changes in intracellular Cl− concentration due to isosmotic KCl fluxes, see Fig. 2 in O'Neill, 1999.) Despite these changes in the intracellular Cl− concentration, the PtdInsPn content is unchanged during isosmotic volume perturbations.
The simplest interpretation of the present results would be an effect of ionic strength on the cellular level of PtdInsP2. On the other hand it cannot be excluded that the cells could perhaps have both a volume and a Cl− sensor, with the effect of increasing cell volume and decreasing Cl− concentration augmenting each other in hypotonic media, whereas in isosmotic high K+ medium the effect of increasing cell volume and increasing Cl− concentration following the isosmotic KCl uptake would counteract each other.
During cell volume regulation opposing ion channels and transporters are activated during the RVD and RVI response, respectively. In Ehrlich ascites cells the activities of these opposing transporters are controlled with tight negative coordination by a common cell volume ‘set-point’ (Harbak & Simonsen, 1999). Thus, a cell volume above the ‘set point’ elicits an RVD response with silent RVI transporters, and conversely a cell volume below the ‘set-point’ elicits an RVI response with silent RVD transporters. It is intriguing that this volume-regulatory ‘set-point’ is elevated in hypotonic medium and depressed in hypertonic medium (Harbak & Simonsen, 1999), but unchanged during cell swelling in isosmotic high K+ medium (Harbak et al. 2001). It would appear that in anisotonic media there is a connection between the changes in ionic strength, the changes in PtdInsP2, and the shift in cell volume ‘set-point. The intracellular signalling mechanism involved remains, however, to be identified.
Cell volume plays an essential role in the regulation of a multitude of cellular functions (see, e.g. Lang et al. 1998; and references therein), and the signal transduction involved in cell volume regulation has been studied intensively (see, e.g. review by Wehner et al. 2003). The ‘sensing’ mechanism(s) for the regulation of volume regulatory transporters remain(s), however, enigmatic. The present study implies a putative role of phosphoinositides in intracellular signalling in cell volume regulation. PtdInsP2 is found to change concurrent with the ‘set-point’ shift during cell volume regulation in anisotonic media, but to remain unchanged during isosmotic cell volume regulation where the cell volume ‘set-point’ is also unchanged. The results show that PtdInsP2 is not volume sensitive per se, and moreover, that the regulatory volume adjustments in Ehrlich ascites cells are not mediated by PtdInsP2 hydrolysis and its subsequent production of the second messengers DAG and InsP3, corroborating previous findings demonstrating the absence of Ca2+ signalling during RVD in Ehrlich ascites cells (Jørgensen et al. 1997). The simplest interpretation of the observed effects is that PtdInsP2 is controlled by the changes in ionic strength in anisotonic media, probably via activation/inhibition of phosphoinositide-specific phosphatases/kinases in the ‘PtdInsPn shuttle’. PtdInsP2 itself is a multifunctional regulator that is known to modulate a number of ion channels and transporters. We hypothesize, however, that PtdInsP2, at least in Ehrlich ascites cells, does not interact directly with the volume regulatory transporters, but rather acts ‘upstream’ to control the volume-regulatory ‘set-point’ setting. It would be interesting to study if a similar mechanism might operate in other cell types.
The activity of volume regulatory transporters is often affected by protein phosphatase/kinase activators/inhibitors, indicating a role for phosphorylation in the regulation of transporter activity. The hypothesis proposed above that changes in PtdInsP2 under anisotonic conditions are controlled by the activity of phosphatases/kinases in the ‘PtdInsPn shuttle’, and itself controls the volume-regulatory ‘set-point’ setting, implies that an effect of a phosphatase/kinase activator/inhibitor on the activity of a volume regulatory transporter does not necessarily reflect a direct effect on the specific transporter, but might perhaps rather reflect a ‘set-point’ shift. In support of this notion it may be noted that the protein phosphatase 1 (PP1) inhibitor calyculin A raises the volume regulatory ‘set-point’ in Ehrlich ascites cells in isotonic medium and activates the Na+,K+,2Cl−-cotransporter (Harbak, 1999). Clearly, in this case the elevation of the ‘set-point’ would account for the cotransporter activation. The effect of calyculin A on PtdInsP2 was not recorded in the study by Harbak (1999), but the PtdIns(4)5-kinase is, at least in HeLa cells, activated by a calyculin A-sensitive protein phosphatase-mediated dephosphorylation (Yamamoto et al. 2006). Hence, PtdInsP2 would be expected to decrease in the presence of calyculin A, with an elevated ‘set-point’ as the predicted result, by analogy with the effects of hypotonicity.
Acknowledgments
The authors would like to thank Inga Somer for excellent and devoted technical assistance. The work was supported by The Velux Foundation of 1989 (SCC).
References
- Aharonovitz O, Zaun HC, Balla T, York JD, Orlowski J, Grinstein S. Intracellular pH regulation by Na+/H+ exchange requires phosphatidyl 4,5-bisphosphate. J Cell Biol. 2000;150:213–224. doi: 10.1083/jcb.150.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S, Harbak H, Simonsen LO. Aberrant 3H labelling of ATP during in vitro labelling of Ehrlich mouse ascites tumour cells with [2-3H]inositol is significant for the study of isomers of InsP3 and InsP4. Biochem J. 1994;300:859–863. doi: 10.1042/bj3000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S, Hoffmann EK, Saermark TS, Simonsen LO. Inositol trisphosphate may be a second messenger in regulatory volume decrease in Ehrlich mouse ascites-tumour cells. J Physiol. 1988;403:109P. [Google Scholar]
- Christensen SC, Jensen AK, Simonsen LO. Aberrant 3H in Ehrlich mouse ascites tumor cell nucleotides after in vivo labelling with myo-[2-3H]- and l-myo-[1-3H]inositol: implications for measuring inositol phosphate signaling. Anal Biochem. 2003;313:283–291. doi: 10.1016/s0003-2697(02)00592-4. [DOI] [PubMed] [Google Scholar]
- Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- Downes CP, Gray A, Lucocq JM. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005;15:259–268. doi: 10.1016/j.tcb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J Biol Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- Fujii M, Ohtsubo M, Ogawa T, Kamata H, Hirata H, Yagisawa H. Real-time visualization of PH domain-dependent translocation of phospholipase C-δ1 in renal epithelial cells (MDCK): response to hypo-osmotic stress. Biochem Biophys Res Commun. 1999;254:284–291. doi: 10.1006/bbrc.1998.9936. [DOI] [PubMed] [Google Scholar]
- Harbak H. Copenhagen, Denmark: August Krogh Institute, University of Copenhagen; 1999. Set point for cell volume regulation by K+ and Cl− transport in Ehrlich cells. PhD Thesis. [Google Scholar]
- Harbak H, Jensen AK, Simonsen LO. The swelling-induced chloride and taurine channels are separate and distinct in the Ehrlich mouse ascites tumour cell. J Physiol. 2001;531:125P. [Google Scholar]
- Harbak H, Nielsen DK, Christensen C, Simonsen LO. Cell volume-induced changes in phosphatidylinositol(4,5)bisphosphate in Ehrlich mouse ascites tumour cells. J Physiol. 1995;489:114P. doi: 10.1113/jphysiol.2007.132308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbak H, Simonsen LO. The set point for cell volume regulation is shifted in anisotonic media in Ehrlich mouse ascites tumour cells. J Physiol. 1999;517:22P–23P. [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Science STKE. 2001 doi: 10.1126/stke.2001.111.re19. 2001, RE19. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Simonsen LO, Lambert IH. Cell volume regulation: Intracellular transmission. Adv Comp Envir Physiol. 1993;14:187–248. [Google Scholar]
- Jørgensen NK, Christensen C, Harbak H, Brown AM, Hoffmann EK, Simonsen LO. On the role of calcium in the regulatory volume decrease (RVD) response in Ehrlich mouse ascites tumor cells. J Membr Biol. 1997;157:281–299. doi: 10.1007/s002329900236. [DOI] [PubMed] [Google Scholar]
- Kletzien RF, Pariza MW, Becker JE, Potter VR. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975;68:537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lytle C, McManus T. Coordinate modulation of Na-K-2Cl cotransport and K-Cl cotransport by cell volume and chloride. Am J Physiol Cell Physiol. 2002;283:C1422–C1431. doi: 10.1152/ajpcell.00130.2002. [DOI] [PubMed] [Google Scholar]
- Minton AP, Colclasure GC, Parker JC. Model for the role of macromolecular crowding in regulation of cellular volume. Proc Natl Acad Sci U S A. 1992;89:10504–10506. doi: 10.1073/pnas.89.21.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasuhoglu C, Feng S, Mao Y, Shammat I, Yamamato M, Earnest S, Lemmon M, Hilgemann DW. Modulation of cardiac PIP2 by cardioactive hormones and other physiologically relevant interventions. Am J Physiol Cell Physiol. 2002;283:C223–C234. doi: 10.1152/ajpcell.00486.2001. [DOI] [PubMed] [Google Scholar]
- Nielsen DK, Harbak H, Christensen S, Simonsen LO. Decrease in phosphatidylinositol(4,5)bisphosphate in Ehrlich mouse ascites tumor cells in response to hypotonic cell swelling. Role in second messenger signaling? Acta Physiol Scand. 1995;155:22A. [Google Scholar]
- Niemeyer MI, Hougaard C, Hoffmann EK, Jørgensen F, Stutzin A, Sepulveda F. Characterization of a cell swelling-activated K+-selective conductance of Ehrlich mouse ascites tumour cells. J Physiol. 2000;524:757–767. doi: 10.1111/j.1469-7793.2000.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill WC. Physiological significance of volume-regulatory transporters. Am J Physiol Cell Physiol. 1999;276:C995–C1011. doi: 10.1152/ajpcell.1999.276.5.C995. [DOI] [PubMed] [Google Scholar]
- Overgaard-Hansen K. Metabolic regulation of the adenine nucleotide pool. Studies on the transient exhaustion of the adenine nucleotides by glucose in Ehrlich ascites tumor cells. Biochem Biophys Acta. 1965;104:330–347. [PubMed] [Google Scholar]
- Pedersen SF, Prenen J, Droogmans G, Hoffmann EK, Nilius B. Separate swelling- and Ca2+-activated anion currents in Ehrlich ascites tumor cells. J Membr Biol. 1998;163:97–110. doi: 10.1007/s002329900374. [DOI] [PubMed] [Google Scholar]
- Santarius M, Lee CH, Anderson RA. Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J. 2006;398:1–13. doi: 10.1042/BJ20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Toker A. The synthesis and cellular roles of phosphatidyl 4,5-bisphosphate. Curr Opin Cell Biol. 1998;10:254–261. doi: 10.1016/s0955-0674(98)80148-8. [DOI] [PubMed] [Google Scholar]
- Van der Kaay J, Beck M, Gray A, Downes CP. Distinct phosphatidylinositol 3-kinase lipid products accumulate upon oxidative and osmotic stress and lead to different cellular responses. J Biol Chem. 1999;274:35963–35968. doi: 10.1074/jbc.274.50.35963. [DOI] [PubMed] [Google Scholar]
- Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RKH. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol. 2003;148:1–80. doi: 10.1007/s10254-003-0009-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Chen MZ, Wang Y-J, Sun H-Q, Wei Y, Martinez M, Yin HL. Hypertonic stress increases phosphatidylinositol 4,5-bisphosphate levels by activating PIP5KIβ. J Biol Chem. 2006;281:32630–32638. doi: 10.1074/jbc.M605928200. [DOI] [PubMed] [Google Scholar]
- Zeng W-Z, Li X-J, Hilgemann DW, Huang C-L. Protein kinase C inhibits ROMK1 channel activity via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. J Biol Chem. 2003;278:16852–16856. doi: 10.1074/jbc.M300619200. [DOI] [PubMed] [Google Scholar]
- Zonia L, Munnik T. Osmotically induced cell swelling versus cell shrinking elicits specific changes in phospholipid signals in tobacco pollen tubes. Plant Physiol. 2004;134:813–823. doi: 10.1104/pp.103.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]