Abstract
We examined the controversial notion of whether lactate is directly oxidized by subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria obtained from red and white rat skeletal muscle. Respiratory control ratios were normal in SS and IMF mitochondria. At all concentrations (0.18–10 mm), and in all mitochondria, pyruvate oxidation greatly exceeded lactate oxidation, by 31- to 186-fold. Pyruvate and lactate oxidation were inhibited by α-cyano-4-hydroxycinnamate, while lactate oxidation was inhibited by oxamate. Excess pyruvate (10 mm) inhibited the oxidation of palmitate (1.8 mm) as well as lactate (1.8 mm). In contrast, excess lactate (10 mm) failed to inhibit the oxidation of either palmitate (1.8 mm) or pyruvate (1.8 mm). The cell-permeant adenosine analogue, AICAR, increased pyruvate oxidation; in contrast, lactate oxidation was not altered. The monocarboxylate transporters MCT1 and 4 were present on SS mitochondria, but not on IMF mitochondria, whereas, MCT2, a high-affinity pyruvate transporter, was present in both SS and IMF mitochondria. The lactate dehydrogenase (LDH) activity associated with SS and IMF mitochondria was 200- to 240-fold lower than in whole muscle. Addition of LDH increased the rate of lactate oxidation, but not pyruvate oxidation, in a dose-dependent manner, such that lactate oxidation approached the rates of pyruvate oxidation. Collectively, these studies indicate that direct mitochondrial oxidation of lactate (i.e. an intracellular lactate shuttle) does not occur within the matrix in either IMF or SS mitochondria obtained from red or white rat skeletal muscle, because of the very limited quantity of LDH within mitochondria.
At rest l-lactate is present in blood, skeletal muscle and other tissues. During prolonged exercise, when lactate is produced by skeletal muscle, a considerable portion of this substrate is transported to the liver and converted to glucose via hepatic gluconeogenesis. In addition, lactate is also oxidized by heart and oxidative types of skeletal muscle (Baldwin et al. 1978). Uptake of lactate into skeletal muscle and other tissues is facilitated by a family of monocarboxylate transporters (MCTs), of which 14 have been identified (Halestrap & Meredith, 2004). Distinct kinetic properties for lactate and pyruvate have been shown for MCT1, 2 and 4 (Broer et al. 1998, 1999; Dimmer et al. 2000), all of which are expressed in skeletal muscle (Bonen et al. 2000b, 2006; Benton et al. 2004). MCT4 is highly expressed in glycolytic muscles (Bonen et al. 1979, 2000a,c), while MCT1 content is greater in oxidative muscles (Bonen et al. 1979, 2000a,c) and is correlated with rates of lactate uptake into this tissue (McCullagh et al. 1996, 1997). MCT2 has a great affinity for pyruvate (Lin et al. 1998; Broer et al. 1999) and is associated with both subsarcolemmal (SS) and intramyofibrillar (IMF) mitochondria (Benton et al. 2004). In contrast, MCT1 and 4 are associated only with SS mitochondria (Benton et al. 2004). Since it appears that SS and IMF mitochondria have different capacities for substrate oxidation (Krieger et al. 1980; Cogswell et al. 1993; Chilibeck et al. 2002; Koves et al. 2005), the MCT differences in SS and IMF mitochondria (Benton et al. 2004) suggest that these mitochondria may have different capacities for the oxidation of pyruvate.
In recent years there has been a suggestion that direct oxidation of lactate to pyruvate can also occur within mitochondria (Brooks et al. 1999b), rather than in the cytosol, as has been accepted for many years. This process has been termed the intracellular lactate shuttle, in which it is postulated that lactate is transported into mitochondria via MCT1, after which LDH within mitochondria converts lactate to pyruvate to allow its oxidation (Brooks et al. 1999b). The notion that lactate might be converted to pyruvate in mitochondria is not new, as more than 30 years ago it was reported that LDH is located near or perhaps within mitochondria (Baba & Sharma, 1971). Recently, with more modern techniques, such proximity of LDH near mitochondria has been reconfirmed (Brooks et al. 1999b; Hashimoto et al. 2006). However, while LDH may be detectable near and/or in mitochondria, there is considerable consensus that in skeletal muscle and heart, LDH content and/or activity are only 0.5–2% of that found in the cytosol (Kline et al. 1986; Brandt et al. 1987; Rasmussen et al. 2002; Sahlin et al. 2002). This would suggest that lactate conversion to pyruvate within mitochondria is probably negligible. Nevertheless, evidence has been presented that rates of pyruvate and lactate oxidation are identical in isolated skeletal muscle mitochondria (Brooks et al. 1999b). However, all other experimental studies to date have been unable to replicate these findings (Popinigis et al. 1991; Rasmussen et al. 2002; Sahlin et al. 2002; Willis et al. 2003; Ponsot et al. 2005). In addition, the concept of direct oxidation of lactate within mitochondria has been challenged on theoretical grounds (Sahlin et al. 2002).
Despite theoretical concerns and the considerable lack of experimental support for direct oxidation of lactate within mitochondria, recent studies have shown an apparent co-localization of proteins (MCT1, CD147, LDH) that may be involved in an intracellular lactate shuttle in L6 muscle cells (Hashimoto et al. 2006), while in rodent skeletal muscle MCT1 and its chaperone CD147 may (Hashimoto et al. 2005) co-localize at or near mitochondria. Yet previously, this group had observed co-localization of MCT1 at or near mitochondria, but without its chaperone CD147 (Butz et al. 2004). Nevertheless, despite some discordant observations, these data have been interpreted as providing evidence for an intracellular lactate shuttle (Butz et al. 2004; Hashimoto et al. 2005, 2006). However, these descriptive studies have not been supported by evidence of direct oxidation of lactate by mitochondria. Indeed, the lactate shuttle hypothesis remains controversial.
We examined (a) whether the controversy concerning direct oxidation of lactate within mitochondria was attributable to a difference in the metabolic capacities of SS and IMF mitochondria obtained from either highly oxidative (red) or highly glycolytic (white) rat skeletal muscles. In addition (b) we also compared the rates of pyruvate and lactate oxidation in SS and IMF mitochondria obtained from red and white muscle. If lactate and pyruvate are equivalently metabolized by skeletal muscle mitochondria, as has been proposed elsewhere (Brooks et al. 1999b), then the following observations would be expected: (i) at given concentrations of lactate and pyruvate, CO2 production from each substrate would be similar, (ii) excess lactate could displace pyruvate oxidation, (iii) the AMPK-induced activation of pyruvate dehydrogenase (PDH) (Smith et al. 2005) would enhance lactate as well as pyruvate oxidation, and (iv) exogenous provision of LDH would not alter lactate or pyruvate oxidation rates. However, in the present study none of these suppositions (i–iv), derived from the putative intracellular lactate shuttle hypothesis (Brooks et al. 1999b), were supported by the experimental data. Indeed, our studies, which are the first to compare pyruvate and lactate oxidation in isolated SS and IMF mitochondria from red and white muscles, demonstrate that pyruvate oxidation in SS and IMF mitochondria is greatly in excess of lactate oxidation, due to the absence of LDH in mitochondria.
Methods
Mature, male Sprague–Dawley rats (250–350 g) were used in these studies. They were bred on site and maintained at 22.5 ± 0.5°C with a 12 h light (19.00–07.00) and 12 h dark (07.00–19.00) cycle in animal holding facilities. Food and water were provided ad libitum. After animals had been anaesthetized (Somnotol, 60 mg kg−1, i.p.) red (RTA) and white tibialis anterior (WTA), and red (RG) and white gastrocnemius (WG) were removed. Since the muscle fibre composition of the RG and RTA are similar (McCullagh et al. 1996), and those of the WG and WTA are also similar (McCullagh et al. 1996), these respective red and white muscles were pooled to ensure that sufficient quantities of mitochondria were isolated for the experiments. Immediately after harvesting the muscles, the animals were killed with an overdose of Somnotol. The procedures for harvesting the muscle tissue and killing of the animals were approved by the Animal Care Committee at the University of Guelph.
Materials
Antibodies against MCT1 and 4 were custom made (Qiagen, Tokyo, Japan). Antibodies against MCT2 were obtained from Chemicon International (Temecula, CA, USA). All of these MCT antibodies have been used in our previous work (Bonen et al. 2000b, 2006; Yoshida et al. 2004). The monoclonal antibody MO25 (Matsuno et al. 1996) was used to detect fatty acid translocase (FAT)/CD36. Commercially available antibodies were used to detect cytochrome c oxidase IV (COX IV) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), LDH (Abcam Inc., Cambridge, MA, USA), and Na+–K+-ATPase (Upstate Biotechnology, Lake Placid, NY, USA). A kit for determining LDH isozymes was also purchased (Paragon LD gel, Beckman, Fullerton, CA, USA). Radiolabelled [U-14C]-lactate, [l-14C]-pyruvate and [l-14C]-palmitate, were obtained from Amersham Biosciences (Buckinghamshire, UK). 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) was obtained from Toronto Research Chemicals Inc. (North York, ON, Canada). All other reagents were obtained from Sigma-Aldrich (St Louis, MO, USA).
Isolation of SS and IMF mitochondria
All studies were performed in freshly obtained subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria isolated separately from pooled RG and RTA muscles, and from pooled WG and WTA muscles. SS and IMF mitochondria were prepared on ice as we have recently described (Benton et al. 2004; Campbell et al. 2004), using modifications of the procedures published by Cogswell et al. (1993). Briefly, extracted muscles were placed in buffer 1 (100 mm KCl, 50 mm Tris HCl, 5 mm MgSO4 and 5 mm EDTA, pH 7.4). Then, the muscles were minced and diluted 10-fold in buffer 2 (buffer 1, supplemented with 0.84 mm ATP). Thereafter, muscle samples were homogenized only briefly using a polytron at 7500 r.p.m. (Polytron PT3100, Kinematica, Litttau-Lucerne, Switzerland) for 2 × 15 s. Subsequently the mitochondria were isolated by centrifugation at 800 g for 10 min. The resultant supernanant and pellet fractions were used to obtain SS and IMF mitochondria, respectively.
SS mitochondria
The supernatant fraction derived from the initial centrifugation step was recentrifuged at 10 000 g for 10 min. Thereafter, the pellet was washed twice in buffer 2 (buffer 1 supplemented with 0.84 mm ATP) and centrifuged at 10 000 g for 10 min. The final pellet was resuspended in a final volume of 700 μl buffer (220 mm sucrose, 70 mm d-mannitol, 10 mm Tris-HCl, and 1 mm EDTA, pH 7.4)
IMF mitochondria
The pellet fraction derived from the initial centrifugation step was rehomogenized using the polytron and centrifuged again at 800 g for 10 min. The supernatant fraction was discarded and the pellet was diluted 10-fold in buffer 2 and treated with subtilisin A (Sigma-Aldrich) for exactly 5 min. Addition of 5 ml of ice-cold buffer 2 arrested the protease activity. The samples were then centrifuged at 5000 g for 5 min. The pellet was resuspended in a 10-fold dilution of buffer 2 and centrifuged at 800 g for 10 min. The resultant supernatant fraction was centrifuged at 10 000 g for 10 min. The resultant pellet was washed twice in buffer 2, and was centrifuged at 10 000 g for 10 min. The final pellet was resuspended in a final volume of 700 μl buffer (220 mm sucrose, 70 mm d-mannitol, 10 mm Tris-HCl, and 1 mm EDTA, pH 7.4)
Mitochondrial purification for Western blotting
To detect proteins associated specifically with mitochondria, it was necessary to remove contaminating subcellular materials, as we have done in our previous work (Benton et al. 2004; Campbell et al. 2004). For these purposes, the SS and IMF mitochondria were purified using a 60% Percoll gradient. The SS and IMF mitochondria were centrifuged at 20 000 g for 1 h after which the mitochondrial layer was removed from the interface between the Percoll and the aqueous phase. The Percoll was removed from the samples by further centrifugation at 20 000 g for 5 h. Thereafter, the purified mitochondria were frozen at −80°C until the samples were analysed.
Subcellular fractionation of skeletal muscle
We used pooled rat hindlimb muscles (1.5 g) to examine the subcellular distribution of MCTs. For these purposes we used a procedure designed to retain all muscle constituents, except debris and mitochondria. We have described this fractionation procedure in detail previously (Bonen et al. 2000a). After the last centrifugation step, plasma membrane and low-density membrane fractions were removed and frozen (−80°C) until analysed by Western blotting.
Giant sarcolemmal vesicles
Giant sarcolemmal vesicles, which consist of plasma membrane proteins (10%) and cytosolic protein (90%), but contain no mitochondria, were prepared from red and white skeletal muscle, as we have previously described in detail (Bonen et al. 1998, 2000a; Tonouchi et al. 2002; Enoki et al. 2006). Once prepared, the vesicles were frozen (−80°C) until analysed for LDH and COX IV with Western blotting.
Mitochondrial respiratory capacity
To determine the respiratory capacities of the red and white muscle SS and IMF mitochondria we have used the procedures outlined elsewhere (Ljubicic et al. 2004). Briefly, samples of isolated SS and IMF mitochondrial subfractions were incubated with VO2 buffer (250 mm sucrose, 50 mm KCl, 25 mm Tris-HCl, 10 mm K2HPO4 and 0.2% BSA, pH 7.4) at 30°C in a water-jacketed respiratory chamber with continuous stirring. Oxygen consumption (n atoms O2 (mg protein)−1 min−1) was assessed in the presence of 11 mm glutamate (state 4 respiration), or glutamate plus 0.4 mm ADP (state 3 respiration) using a Clark oxygen electrode (YSI, Yellow Springs, OH, USA), as we have reported previously (Ljubicic et al. 2004). The degree of coupling was described by the respiratory control ratio (RCR), which expresses how strongly ADP is capable of stimulating oxygen consumption compared with the basal (or state 4) rate. The RCR was calculated by dividing the state 3 rate by the state 4 rate of each isolated mitochondrial preparation.
Lactate and pyruvate oxidation in SS and IMF mitochondria
Oxidation of lactate and pyruvate were determined using radiolabelled [U-14C]-lactate and [l-14C]-pyruvate in a sealed system as we have previously described (Campbell et al. 2004; Bezaire et al. 2006; Holloway et al. 2006). Briefly, oxidation experiments were performed in sealed 20 ml scintillation vials. Mitochondria (100 μl, protein concentration > 2 mg ml−1) were added to a 900 μl aliquot of pre-gassed (5% CO2 and 95% O2) modified Krebs–Ringer buffer (MKR: 115 mm NaCl, 2.6 mm KCl, 1.2 mm KH2PO4, 10 mm NaHCO3, 10 mm Hepes, pH 7.4), which was supplemented with ATP (5 mm), NAD+ (1 mm), l-carnitine (0.5 mm), l-malate (0.5 mm), coenzyme A (0.1 mm) and cytochrome C (25 μm) for the oxidation studies, as we have reported previously (Campbell et al. 2004; Bezaire et al. 2006; Holloway et al. 2006). Oxidation (30 min, 37°C) was initiated by the addition of 0.18, 1.8 or 10 mm l-lactate and l-[U-14C]-lactate (0.4 μCi vial−1) or 0.18, 1.8 and 10 mm l-pyruvate and [1-14C]-pyruvate (0.4 μCi vial−1) (Amersham Biosciences, Buckinghamshire, UK). Oxidation was terminated with the addition of 70% perchloric acid (Sigma-Aldrich). During the incubation the 14CO2 produced was trapped in 1 m benzethonium hydroxide (500 μl) contained in a microcentrifuge tube suspended within the scintillation vial. Acidifying the reaction mixture with 1.0 ml of 1 m H2SO4, liberated 14CO2 from the incubating buffer and captured it in the benzethonium hydroxide trap (60 min at room temperature). Thereafter, the microcentrifuge tube containing the 14CO2 was placed in a scintillation vial, and radioactivity was counted.
Inhibition studies
Inhibition of lactate (1.8 mm) and pyruvate (0.18 mm) oxidation was examined with 5 mmα-cyano-4-hydroxycinnamate (CINN) and with 50 mm sodium oxamate. For these purposes mitochondria were preincubated with each inhibitor for 30 min. The same volumes of ethanol (for CINN) or H2O (for oxamate) used to dissolve the inhibitors were added to control vials. After the 30 min preincubation, mitochondria were centrifuged at 10 000 g for 10 min and washed twice. Thereafter, lactate and pyruvate oxidation were determined as above.
Effects of LDH on mitochondrial lactate and pyruvate oxidation
To examine the effects of exogenously provided LDH on lactate (1.8 mm) and pyruvate (0.18 mm) oxidation, red and white muscle SS and IMF mitochondria were incubated with progressively increased quantities of LDH-1 (0–10 units). Lactate and pyruvate oxidation were determined as described above.
Acute AICAR stimulation
To examine the stimulatory effects of AMPK on mitochondrial lactate and pyruvate oxidation, the cell-permeant adenosine analogue AICAR (1 mg (g body weight)−1 in 0.45% saline, 3 ml (300 g body weight)−1) was injected subcutaneously (s.c.) into the back neck of rats. Control rats were injected with an equivalent volume of 0.45% saline. After 1 h, RTA, RG, WTA and WG muscles were isolated and rates of lactate (1.8 mm) and pyruvate (0.18 mm) oxidation were also determined in SS and IMF mitochondria from red and white muscles as described above.
Substrate competition studies
To determine whether mitochondria preferred oxidation of pyruvate or lactate, competition studies were performed. For these purposes we determined whether excess quantities of lactate (10 mm) inhibited the oxidation of pyruvate (1.8 mm), and whether excess pyruvate (10 mm) inhibited oxidation of lactate (1.8 mm). In addition, we also examined whether lactate (10 mm) and pyruvate (10 mm) inhibited palmitate (1.8 mm) oxidation in red and white muscle SS and IMF mitochondria. Palmitate oxidation (1.8 mm, [1-14C]-palmitate, 0.5 μCi vial−1) was determined in a similar manner as was done for lactate and pyruvate, and as we have previously described (Campbell et al. 2004).
Western blotting
MCT1, 2, 4 as well as other proteins (LDH, Na+–K+-ATPase, FAT/CD36), were detected on mitochondria, and on plasma membranes in some instances, using Western blotting as we have previously reported (Benton et al. 2004; Campbell et al. 2004). Proteins were visualized by chemiluminescence detection, according to the manufacturer's instructions (Hyperfilm-ECL; Amersham). Blots were quantified using the ChemiGenius 2 Bioimaging system (SynGene, Cambridge, UK).
LDH activity and LDH isoenzymes detection
LDH activity and isoenzymes were determined as previously described (Chi et al. 1983; McCullagh et al. 1996). Briefly, mitochondrial fractions and whole muscle homogenates were diluted with 10 mm Tris-buffer (pH 7.5). The LDH activity was determined in the pyruvate-to-lactate direction using a fluorometric assay (Chi et al. 1983; McCullagh et al. 1996). LDH isoenzymes in mitochondrial fractions and whole muscle homogenates were separated by adding 20 μg or 1 μg protein, respectively, to agarose gels followed by electrophoresis for 40 min at 100 V according to the manufacture's instructions (Paragon LD isoenzyme electrophoresis kit, Beckman, Fullerton, CA, USA). Thereafter, gels were covered with a blotter saturated in substrate (208 mm lithium l-lactate, 5.6 mm NAD, 2.4 mmp-nitro blue tetrazolium chloride and 0.33 mm phenazine methosulphate (PMS)) and incubated for 20 min at 45°C. Gels were fixed in 5% acetic acid and dried. LDH isoenzyme bands were scanned and quantified using the ChemiGenius 2 Bioimaging system (SynGene).
Statistical analysis
The data were analysed with analyses of variance. Fisher's least-squares difference test was used for post hoc analyses, when appropriate. The significance level was set at P≤ 0.05 and all data are reported as means ±s.e.m.
Results
Mitochondrial respiratory capacity was established from oxygen consumption rates under state 4 and 3 conditions. State 4 respiratory rates did not differ among mitochondria, except for the lower rate in white muscle SS mitochondria (P < 0.05, Table 1). State 3 respiratory rates were lower in SS than in IMF mitochondria in both red and white muscle (P < 0.05, Table 1), and in white SS mitochondria than in red muscle SS mitochondria (P < 0.05, Table 1). These results are comparable to those we (Cogswell et al. 1993) and others (Rasmussen et al. 2002; Sahlin et al. 2002) have reported previously.
Table 1.
Respiratory rate in IMF and SS mitochondria obtained from red and white skeletal muscle
| Respiratory rate (n atoms O2 mg protein−1 min−1) | ||||
|---|---|---|---|---|
| Muscle | Mitochondria | State 4 | State 3 | RCR |
| Red | IMF | 13.9 ± 1.9 | 111.2 ± 13.8b | 8.5 ± 1.1 |
| SS | 11.0 ± 2.3 | 79.3 ± 9.5a,b | 8.2 ± 1.1 | |
| White | IMF | 14.8 ± 2.8 | 126.6 ± 15.9b | 9.4 ± 1.3 |
| SS | 6.7 ± 0.8a,c | 52.6 ± 5.1a,b,c | 7.2 ± 1.0a | |
Values are mean ± s.e.m. RCR, respiratory control ratio. N = 6–7 independent preparations.
P < 0.05, SS versus IMF
P < 0.05, state 3 versus state 4
P < 0.05, white SS versus red SS.
Pyruvate and lactate oxidation in IMF and SS mitochondria
The rates of pyruvate and lactate oxidation were determined over a wide range of concentrations (i.e. 0.18, 1.8 and 10 mm), in SS and IMF mitochondria, obtained from both red and white skeletal muscle (Fig. 1).
Figure 1. Lactate and pyruvate oxidation at different concentrations (0.18, 1.8 and 10 mm) in red and white muscle IMF (A) and SS (C) mitochondria. Lactate oxidation is also shown on a 30-fold more sensitive scale in red and white muscle IMF (B) and SS (D) mitochondria (mean ±s.e.m.).
N = 5–20 independent mitochondrial preparations per point. With increasing concentrations both lactate and pyruvate oxidation rates increased (P < 0.05), with those of pyruvate greatly exceeding those of lactate (P < 0.05).
Pyruvate oxidation
White muscle
With increasing concentrations of pyruvate (0.18–10 mm) there was a marked increase in pyruvate oxidation in white muscle IMF and SS mitochondria (Fig. 1A and C). Pyruvate oxidation did not differ in SS and IMF mitochondria, either at 0.18 mm or 1.8 mm pyruvate (P > 0.05). However, at 10 mm pyruvate, the oxidation rates were greater in IMF (+44%) compared with SS mitochondria (P < 0.05, Fig. 1A and C).
Red muscle
There was also a marked increase in pyruvate oxidation in red muscle IMF and SS mitochondria (Fig. 1) with increasing concentrations of pyruvate (0.18–10 mm). The pyruvate oxidation in red muscle SS mitochondria was greater than in the IMF mitochondria, at concentrations of 0.18 mm (+145%, P < 0.05) and 1.8 mm (+65%, P < 0.05, Fig. 1A and C). However, at 10 mm pyruvate this was reversed, with the oxidation rates in IMF being greater (+20%) than in SS mitochondria (P < 0.05, Fig. 1A and C).
Red versus white muscle
Comparisons between red and white muscle revealed that the oxidation rate of pyruvate was higher in white muscle IMF compared with red muscle IMF mitochondria at 0.18 mm (+90%) and 1.8 mm (+60%) (P < 0.05, Fig. 1A), but not at 10 mm. In contrast, no difference in pyruvate oxidation was observed between red and white muscle SS mitochondria at any of the concentrations (Fig. 1C).
Lactate oxidation
White muscle
With increasing lactate concentrations (0.18–10 mm), the rates of lactate oxidation increased linearly in white muscle IMF and SS mitochondria. There were no differences in the rates of lactate oxidation in SS and IMF mitochondria (Fig. 1A–D).
Red muscle
The rate of lactate oxidation in white muscle IMF and SS mitochondria also increased linearly, with increasing lactate concentrations (0.18–10 mm) (P < 0.05, Fig. 1A–D). These rates did not differ in SS and IMF mitochondria, except at 10 mm lactate (IMF > SS, P < 0.05).
Red versus white muscle
Except for a somewhat higher rate of lactate oxidation in red muscle IMF mitochondria at 10 mm lactate (+62%, P < 0.05), no other differences in lactate oxidation were observed among red and white muscle SS and IMF mitochondria.
Comparison between pyruvate and lactate oxidation
The absolute rate of lactate oxidation was far less than that of pyruvate at all concentrations, in both IMF and SS mitochondria (Fig. 1). At comparable concentrations (0.18, 1.8 and 10 mm) the pyruvate oxidation rates were, respectively, 31-, 52- and 29-fold greater in red muscle IMF mitochondria, 76-, 124- and 36-fold greater in red muscle SS mitochondria, 110-, 143- and 56-fold greater in white muscle IMF mitochondria, and 125-, 186- and 38-fold greater than in white muscle mitochondria (P < 0.05).
Direct comparison of the pyruvate and lactate concentrations required to achieve equivalent rates of oxidation (Table 2) were derived from the formulas obtained when a curve was fitted to the pyruvate and lactate data (Fig. 1). At 0.18 mm and 1.8 mm pyruvate, the lactate concentrations needed to be 53- to 139-fold greater to produce equivalent oxidation rates (Table 2).
Table 2.
Comparison of pyruvate and lactate required to obtain equivalent rates of oxidation
| Muscle Mitochondria | Pyruvate concentration (mm) | Pyruvate oxidation nmol mg prot−1 h−1 | Lactate required to attain equivalent oxidation rate (mm) | Ratio of La to Pyr to attain equivalent rates of oxidation |
|---|---|---|---|---|
| Red IMF | 0.18 | 30 | 9.5 | 53: 1 |
| Red SS | 0.18 | 49 | 23 | 127: 1 |
| White IMF | 0.18 | 47 | 24 | 133: 1 |
| White SS | 0.18 | 50 | 25 | 139: 1 |
| Red IMF | 1.8 | 319 | 102 | 57: 1 |
| Red SS | 1.8 | 462 | 210 | 117: 1 |
| White IMF | 1.8 | 430 | 235 | 131: 1 |
| White SS | 1.8 | 446 | 226 | 126: 1 |
Oxidation rates were determined from the equations derived from the data shown in Fig. 1. Data were not calculated for 10 mm pyruvate, as this is physiologically unrealistic.
Effects of selected inhibitors on pyruvate and lactate oxidation
The MCT inhibitor α-hydroxy-4-cinnamate (5 mm) inhibited the oxidation of lactate in all mitochondrial fractions (Fig. 2A). Similarly, the rates of pyruvate oxidation were also inhibited by α-hydroxy-4-cinnamate in all mitochondrial fractions (Fig. 2B).
Figure 2. Effects of α-cyano-4-hydroxycinnamate (5 mm) on lactate (1.8 mm) (A) and pyruvate (0.18 mm) (B) oxidation in red and white muscle IMF and SS mitochondria (±s.e.m.).
N = 4 independent mitochondrial preparations for each treatment in A and B. Note the 10-fold difference in the scaling used for pyruvate and lactate oxidation. *P < 0.05, respective control mitochondria versus mitochondria treated with α-cyano-4-hydroxycinnamate. **P < 0.05, in control mitochondria: red SS versus red IMF, or white SS versus white IMF. ***P < 0.05, in control mitochondria: red IMF versus white IMF, or red SS versus white SS.
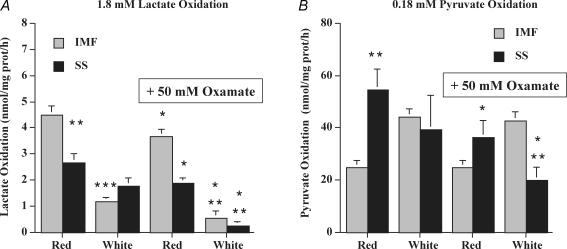
The LDH inhibitor oxamate (50 mm) inhibited the oxidation of lactate in all mitochondrial fractions (Fig. 3A). The reductions were more pronounced in white muscle IMF (−53%) and SS mitochondria (−85%), than in red muscle IMF (−18%) and SS mitochondria (−30%) (Fig. 3A). Pyruvate oxidation was not inhibited by oxamate in red and white muscle IMF mitochondria (Fig. 3B), but unexpectedly and for unknown reasons, oxamate did inhibit pyruvate oxidation in red and white muscle SS mitochondria (Fig. 3B).
Figure 3. Effects of oxamate (50 mm) on lactate (1.8 mm) (A) and pyruvate (0.18 mm) (B) oxidation in red and white muscle IMF and SS mitochondria (mean ±s.e.m.).
N = 4 independent mitochondrial preparations for each treatment in A and B. Note the 10-fold difference in the scaling used for pyruvate and lactate oxidation. *P < 0.05, respective control mitochondria versus mitochondria treated with oxamate. **P < 0.05, in control mitochondria: red SS versus red IMF, or white SS versus white IMF. ***P < 0.05, in control mitochondria: red IMF versus white IMF, or red SS versus white SS.
Altering substrate oxidation in response to metabolic stimuli
If lactate and pyruvate are oxidized within mitochondria, then it may be expected that these monocarboxylates would inhibit each other's oxidation as well as inhibiting fatty acid oxidation. Moreover, they should also exhibit increased rates of oxidation after muscle AMPK has been activated by AICAR, as AICAR stimulates PDH activity (Smith et al. 2005).
Displacing lactate, pyruvate and fatty acid oxidation
Excess pyruvate (10 mm) inhibited mitochondrial lactate (1.8 mm) oxidation in red muscle IMF (−75%) and SS (−62%) mitochondria (P < 0.05, Fig. 4A), and in white muscle IMF (−40%) and SS (−90%) mitochondria (P < 0.05, Fig. 4A). In contrast, excess lactate (10 mm) did not inhibit mitochondrial pyruvate (1.8 mm) oxidation in red and white muscle IMF and SS mitochondria. (P > 0.05, Fig. 4B)
Figure 4. Effects of excess pyruvate (10 mm) on lactate (1.8 mm) oxidation (A), and effects of excess lactate (10 mm) on pyruvate (1.8 mm) oxidation (B) in red and white muscle IMF and SS mitochondria (±s.e.m.).
N = 4 independent mitochondrial preparations for each treatment in A and B. Note the 100-fold difference in the scaling used for the pyruvate and lactate oxidation. *P < 0.05, respective control mitochondria versus mitochondria treated with pyruvate (A) or lactate (B). **P < 0.05, in control mitochondria: red SS versus red IMF, or white SS versus white IMF. ***P < 0.05, in control mitochondria: red IMF versus white IMF, or red SS versus white SS.
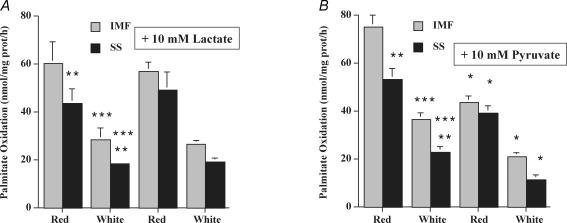
Lactate (10 mm) did not inhibit mitochondrial palmitate (1.8 mm) oxidation, in either SS or IMF mitochondria from red and white muscle (P > 0.05, Fig. 5A). In contrast, pyruvate (10 mm) did inhibit mitochondrial palmitate (1.8 mm) oxidation by 40–50% in IMF and SS mitochondria obtained from red and white muscle (P < 0.05, Fig. 5B).
Figure 5. Effects of lactate (10 mm) (A) and pyruvate (10 mm) (B) on palmitate (1.8 mm) oxidation in red and white muscle IMF and SS mitochondria (±s.e.m.).
N = 3 independent mitochondrial preparations for each treatment in A and B. *P < 0.05, respective control mitochondria versus mitochondria treated with lactate (A) or pyruvate (B). **P < 0.05, in control mitochondria: red SS versus red IMF, or white SS versus white IMF. ***P < 0.05, in control mitochondria: red IMF versus white IMF, or red SS versus white SS.
Effects of AICAR on mitochondrial oxidation of pyruvate and lactate
Lactate oxidation was not altered after the administration (s.c.) of AICAR, either in SS or IMF mitochondria (Fig. 6A). In contrast, AICAR administration increased pyruvate oxidation in SS mitochondria (+25%, P < 0.05, Fig. 6B) but not in IMF mitochondria. AICAR induced a similar selective increase in palmitate oxidation in SS mitochondria (+26%) but not in IMF mitochondria (data not shown).
Figure 6. Effects of AICAR (1 mg (g body weight)−1s.c., 60 min) on the oxidation of lactate (1.8 mm) (A) and pyruvate (0.18 mm) (B) in mixed muscle IMF and SS mitochondria (±s.e.m.).
N = 4 independent mitochondrial preparations for each treatment in A and B. Note the 10-fold difference in the scaling used for lactate and pyruvate oxidation. *P < 0.05, control versus AICAR in SS mitochondria.
Subcellular distribution of MCTs
MCT1 and 4, but not MCT2, were abundantly present in muscle homogenates (Fig. 7A). MCT1 and 4 were present on the plasma membrane and in intracellular membranes obtained from mixed muscles (Fig. 7B). The lack of Na+–K+-ATPase in purified mitochondria confirmed that this preparation was not contaminated with plasma membrane fragments (Fig. 7C). MCT1 and 4 were present in SS but not IMF mitochondria (Fig. 7C), while MCT2 was present in both SS and IMF mitochondria (Fig. 7C). Quantification of the MCTs revealed that in white muscle SS mitochondria, MCT1 was lower (Fig. 7D) and MCT4 was greater (Fig. 7E) than in red muscle SS mitochondria. There were no differences in MCT2 in red and white muscle mitochondria, except that somewhat less MCT2 was present in white muscle IMF mitochondria (−23%, Fig. 7F).
Figure 7. Subcellular distribution of MCT1, 2 and 4.
A, Western blots of MCT1, 2, and 4 in red (R) and white (W) muscle homogenates (the same quantity of protein was loaded in each lane for red and white muscle). B, total MCT1 and 4 content (arbitrary units per fraction) on fractions of plasma membrane (PM) and low density microsomal (LDM) membranes of mixed muscles (mean ±s.e.m., from 3 independent experiments). In this preparation all the muscle is fractionated and none is discarded, except for mitochondria. The different fractions contain different concentrations of proteins, and therefore, to make direct comparisons between fractions the data are expressed as total MCT content per fraction. The mitochondrial marker COX IV is not detected in the PM or LDM compartments. Due to the low expression of MCT2 in muscle homogenates, it was not examined in the PM and LDM compartments. In each preparation the most abundant lane was set to 100 and all other lanes were expressed relative to that lane. Therefore, the MCT quantities within each of the PM and LDM fractions are quantitatively comparable, but the MCT quantities between the PM and LDM fractions are not directly quantitatively comparable. C, purified mitochondria show that MCT1 and 4 are present in SS but not IMF mitochondria, while MCT2 is present in both SS and IMF mitochondria. Lack of an Na+–K+-ATPase signal indicates that mitochondria are not contaminated with plasma membrane fragments, while COX IV and FAT/C36 are known SS and IMF mitochondrial proteins. D–F, quantification of mitochondrial MCT1, 4 and 2 (±s.e.m., from 4 independent preparations). ND = not detected. *P < 0.05, white versus red muscle SS or IMF mitochondria. **P < 0.05, white muscle IMF versus red muscle IMF mitochondria.
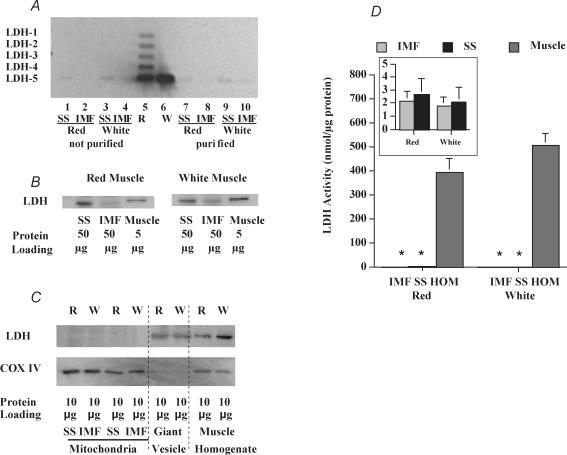
LDH protein and activity in mitochondria and in muscle
The presence of LDH and its activity were compared in SS and IMF mitochondria, and in muscle homogenates. Separation of LDH via agarose gel electrophoresis revealed that in red muscle the expected LDH1–5 pattern was observed, and that in white muscle the expected prevalence of LDH-5 was found (Fig. 8A). With the same procedure, an LDH signal was barely detectable when the mitochondrial protein content was 20-fold greater than in muscle homogenates (Fig. 8A). Similarly, with Western blots we could only detect signal for LDH in association with mitochondria, but this required a 10-fold greater quantity of protein than that used for the muscle homogenate (Fig. 8B). At equivalent protein concentrations in each lane (10 μg), LDH was not detected in mitochondria but was observed in muscle homogenates and in giant sarcolemmal vesicles (Fig. 8C), which are largely (90%) comprised of cytosolic proteins. Mitochondria are not present in this cytosolic preparation as can be seen from the absence of COX IV. Quantitative determinations of LDH activity showed that the muscle homogenate LDH activity was 200- to 240-fold greater than the LDH activity observed in SS and IMF mitochondria (Fig. 8D, and inset).
Figure 8. Comparison of LDH presence in muscle homogenate, isolated mitochondria and cytosol (giant vesicles) (A–C), and LDH activity (D) in red and white muscle homogenates and in red and white muscle IMF and SS mitochondria.
A, agarose gel electrophoresis showing LDH isozyme pattern in red and white muscle homogenates (lanes 5 and 6; 1 μg protein lane−1) and in non-purified mitochondria (lanes 1–4, 20 μg protein lane−1) and in purified mitochondria (lanes 7–10, 20 μg protein lane−1) (sample blot of 3 preparations). B, Western blot of LDH in red and white muscle (5 μg protein lane−1) and in SS and IMF mitochondria (50 μg protein lane−1) (sample blot of 4 independent preparations). C, Western blot of LDH and COX IV in red (R) and white (W) muscle homogenates, and their SS and IMF mitochondria, and giant vesicles (primarily of cytosolic proteins, 90%) which lack mitochondria (sample blot of 3 independent preparations). D, LDH activity in IMF and SS mitochondria, and in muscle homogenates (±s.e.m.). N = 4 independent mitochondrial preparations for SS and IMF mitochondria and muscle homogenates. Due to the extremely low LDH activity in SS and IMF mitochondria relative to muscle homogenates, the LDH activity in IMF and SS mitochondria is also shown in the inset (scale of inset is 160-fold more sensitive). *P < 0.05, IMF or SS mitochondria versus muscle homogenate.
Effects of exogenous LDH on mitochondrial lactate and pyruvate oxidation
Addition of LDH-1 (H type) did not alter the rate of pyruvate (0.18 mm) oxidation in IMF and SS mitochondria (Fig. 9A and B). In contrast, addition of LDH to SS and IMF mitochondria markedly increased, in a dose-dependent manner, the rate of lactate (1.8 mm) oxidation in red (42-fold) and white (127-fold) muscle IMF mitochondria, and in red (89-fold) and white (107-fold) muscle SS mitochondria (Fig. 9A and B). At maximal levels of added LDH, the oxidation of 1.8 mm lactate approached that of 1.8 mm pyruvate (Fig. 1) in red (82%) and white (88%) muscle IMF mitochondria (Fig. 1), but not quite in red (72%) nor white (54%) muscle SS mitochondria.
Figure 9. Effects of providing LDH (0–10 units) on pyruvate (0.18 mm) and lactate (1.8 mm) oxidation in red and white muscle IMF (A) and SS mitochondria (B) (mean ±s.e.m.).
When LDH is provided to red and white muscle IMF and SS mitochondria, the rate of lactate oxidation is increased, P < 0.05. N = 3–4 independent mitochondrial preparations at each concentration in A and B.
Discussion
We have examined the controversial notion that lactate is directly oxidized within mitochondria (Brooks et al. 1999b). We used SS and IMF mitochondria obtained from red and white rat skeletal muscle and found that: (i) at all concentrations (0.18–10 mm), lactate oxidation was negligible, being only 0.5–3.2% of pyruvate oxidation (i.e. 31- to 186-fold lower) in SS and IMF mitochondria; (ii) excess pyruvate was able to inhibit the oxidation of both palmitate and lactate; whereas (iii) excess lactate failed to inhibit the oxidation of either palmitate or pyruvate; (iv) AICAR increased pyruvate oxidation but not lactate oxidation; (v) MCT1 and 4 were present on SS but not IMF mitochondria, while MCT2 was present in both SS and IMF mitochondria; (vi) the LDH activity associated with mitochondria was 200- to 240-fold lower than in muscle homogenates; and (vii) exogenous LDH-1 provision increased lactate oxidation, but not pyruvate oxidation, in a dose-dependent manner, such that lactate approached the rates of pyruvate oxidation. Collectively, these studies indicate that there is no mitochondrial matrix component of an intracellular lactate shuttle in either IMF or SS mitochondria obtained from either red or white rat skeletal muscle, because of the severely limited quantity of LDH within mitochondria (i.e. ≤ 0.5% of that present in muscle homogenates).
Lactate oxidation and pyruvate in IMF and SS mitochondria
We used a range of lactate and pyruvate concentrations (0.18–10 mm) that were comparable to those used in other experiments with isolated mitochondria (5–10 mm; (Popinigis et al. 1991; Brooks et al. 1999b; Rasmussen et al. 2002; Sahlin et al. 2002). In the present studies, the rates of pyruvate oxidation (0.18–10 mm), which were comparable to a previous report (Bookelman et al. 1978), increased rapidly and reached a plateau between 2 and 10 mm in both SS and IMF mitochondria. In contrast, lactate oxidation rates increased linearly and at a much slower rate than pyruvate in all mitochondria. There is one report in which rates of mitochondrial lactate and pyruvate oxidation were found to be identical (Brooks et al. 1999b). However, all other studies concur that the rates of pyruvate oxidation by mitochondria are many-fold greater than those for lactate (Popinigis et al. 1991; Rasmussen et al. 2002; Sahlin et al. 2002; Willis et al. 2003; Ponsot et al. 2005). Indeed, our results and others (Willis et al. 2003) have shown that pyruvate oxidation rates are 31- to 186-fold greater than for lactate. This is clearly shown in Table 2, in which we have determined the quantities of lactate required to obtain oxidation rates comparable to that of pyruvate.
Effects of selected inhibitors on pyruvate and lactate oxidation
In the current study, reductions in pyruvate and lactate oxidation in the presence of α-cyano-4-hydroxycinnamate, concur with previous reports (Halestrap & Denton, 1974; Mole et al. 1978; Brooks et al. 1999b). This indicates that an MCT contributes to the transport of these monocarboxylates into SS and IMF mitochondria.
The oxamate-mediated reductions in lactate oxidation in mixed mitochondria (Brooks et al. 1999b) and in SS and IMF mitochondria (present study) indicated that there is some LDH associated with mitochondria. However, whether this LDH was contained within mitochondria or represented a low level of contamination with cytosolic LDH has not been determined. Why oxamate reduced pyruvate oxidation in IMF mitochondria only is unclear, and may reflect some non-specific effects of this inhibitor. Unusual effects of oxamate on pyruvate oxidation have also been reported elsewhere; namely, this LDH inhibitor stimulated pyruvate oxidation in mitochondria derived from liver, heart and skeletal muscle (Brooks et al. 1999b). These anomalies with oxamate (present study and Brooks et al. 1999b) point to the need for some caution in interpreting results obtained with this inhibitor.
Altering substrate oxidation in response to selected metabolic perturbations
We tested the metabolic responsiveness of isolated mitochondria to lactate, pyruvate and palmitate by (a) conducting substrate inhibition studies, in which excess quantities of one substrate can be expected to inhibit the oxidation of another substrate, and (b) by increasing mitochondrial substrate oxidation rate via AICAR-mediated activation of AMPK. Our studies revealed that direct mitochondrial lactate oxidation within SS and IMF mitochondria appears to be negligible as (i) this substrate failed to inhibit pyruvate and palmitate oxidation, as has been observed elsewhere (Rasmussen et al. 2002), and (ii) lactate oxidation, unlike pyruvate and palmitate oxidation, did not respond to AICAR, a known stimulator of acetyl-CoA carboxylase and PDH (Smith et al. 2005).
Examination of putative mechanism of the intracellular lactate shuttle
All investigators agree that several mechanisms must be present to allow for the operation of an intracellular lactate shuttle, including MCT proteins to take lactate into the mitochondria, and most importantly, a substantial pool of LDH within mitochondria to allow lactate to be reconverted to pyruvate.
Mitochondrial MCT1, MCT2, MCT4
Experiments with α-hydroxy-4-cinnamate demonstrate that monocarboxylate transporters are present in skeletal muscle mitochondria (present study and Brooks et al. 1999b). These include MCT1, 2 and 4 (present study and Brooks et al. 1999b; Benton et al. 2004). However, MCT4 is probably not a pyruvate transporter (Km 18–25 mm), and is considered to be a low-affinity, high-capacity transporter for lactate (MCT4: Km 28–34 mm) (Dimmer et al. 2000; Manning Fox et al. 2000). Hence, the functional significance of the MCT4 association with SS mitochondria (present study and Benton et al. 2004) is unclear.
Brooks and colleagues (Brooks et al. 1999a; Butz et al. 2004; Hashimoto et al. 2005; Hashimoto et al. 2006) believe that MCT1 is critical for the intracellular lactate shuttle. However, this argument is based solely on the detection of some MCT1 associated with mitochondria, while other MCTs, known to be present in muscle (Bonen et al. 2006), are not considered to be important (Brooks et al. 1999a; Butz et al. 2004; Hashimoto et al. 2005; Hashimoto et al. 2006). Yet, neither the quantity of mitochondrial MCT1 relative to that of whole muscle, nor the functional capacities of lactate or pyruvate transport by MCT1 in mitochondria have been examined. Moreover, in the past decade others have shown that (a) in the heart MCT1 is located near but not within mitochondria (Johannsson et al. 1997), (b) MCT1 has a 2.4- to 4.7-fold higher affinity for pyruvate than for lactate (Garcia et al. 1995; Broer et al. 1998; Lin et al. 1998), and (c) MCT1 is present only in SS mitochondria, while MCT2 is associated with both SS and IMF mitochondria (present study and Benton et al. 2004). While immunohistochemical studies in L6 cells (Hashimoto et al. 2006) appear to show that some MCT1 is co-localized with mitochondria located some distance from the cell surface, this appears to be far less than some of the co-localization with mitochondria near the cell surface. Taken altogether, these observations suggest that the limited presence or absence of MCT1 in IMF mitochondria undermines its putative, critical role in an intracellular lactate shuttle in these mitochondria. By extension, this may also question the role of MCT1 in such a process in SS mitochondria. Indeed, MCT1 may not be the key mitochondrial lactate transporter.
The presence of MCT2 in both SS and IMF mitochondria also challenges the idea of an MCT1-facilitated intracellular lactate shuttle. MCT2 appears to be a pyruvate transporter, since it has 100- to 260-fold greater affinity for pyruvate (Km 25–80 μm) than for lactate (Km 6.5–8.1 mm) (Garcia et al. 1995; Lin et al. 1998) and the MCT2 Km for pyruvate is around the range of pyruvate concentrations measured in skeletal muscle homogenates (50–200 μm) (Linnarsson et al. 1974; Aragon & Lowenstein, 1980; Spencer et al. 1991; Gibala et al. 1998), although intracellular pyruvate concentrations may be higher at rest and during exercise (heart: 320–650 μm; Goodwin et al. 1998). This very high affinity of MCT2 for pyruvate, and its presence in both SS and IMF mitochondria (present study and Benton et al. 2004) may well mean that this transporter is key for importing pyruvate rather than lactate into the mitochondria. In addition, the relatively easy detection of MCT2 in mitochondria but not whole muscle (present study) may indicate that MCT2 is largely confined to mitochondria. Taken altogether there is substantial evidence to indicate that pyruvate entry into the mitochondria, whether via MCT2 and/or the ‘traditional’ pyruvate carrier (Halestrap & Denton, 1974; Halestrap, 1976; Hildyard & Halestrap, 2003; Hildyard et al. 2005), is most probably greatly in excess of lactate. Indeed, our studies in which pyruvate inhibited lactate oxidation, while lactate completely failed to inhibit pyruvate oxidation, also provides support for this conclusion.
LDH protein and activity in mitochondria and in muscle
A key requirement for the intracellular lactate shuttle is the presence of LDH within the mitochondria, to convert lactate to pyruvate inside this organelle. However, LDH has long been considered a cytosolic protein, as only 0.5–2% of the total LDH in muscle is found with isolated mitochondria (present study and Kline et al. 1986; Brandt et al. 1987; Rasmussen et al. 2002; Sahlin et al. 2002). In contrast, qualitative data have been presented indicating that both muscle mitochondria and cytosol contained abundant quantities of LDH isozymes (Brooks et al. 1999b; McClelland & Brooks, 2002; Butz et al. 2004; Hashimoto et al. 2005, 2006). Yet, in these studies, direct quantitative comparisons of cytosolic and mitochondrial LDH appear not to have been performed. However, in one of these reports it appeared that mitochondrial LDH was far less abundant than cytosolic LDH, as 6- to 7-fold more mitochondrial protein than cytosolic protein was required to detect LDH qualitatively (McClelland & Brooks, 2002). Loading of apparently equal quantities of muscle homogenate protein and mitochondrial protein showed very weak LDH signals in non-purified, mitochondrial preparations compared with muscle homogenates (McClelland & Brooks, 2002). Our quantitative analysis established that the LDH activity per milligram of protein was 200- to 240-fold greater in the cytosol than in mitochondria. These differences would be even greater if we take the small volume density of mitochondria into account (< 10% of muscle) (Hoppeler et al. 1987). Moreover, replenishment of LDH rapidly increased lactate oxidation in a dose-dependent manner, whereas pyruvate oxidation, as expected, was not altered (present study and Popinigis et al. 1991; Rasmussen et al. 2002; Sahlin et al. 2002). Thus, (a) extramitochondrial LDH converts lactate to pyruvate without requiring an intramitochondrial pool of LDH, and (b) under normal circumstances the rate of lactate oxidation in mitochondria isolated from skeletal muscle is severely limited due the virtual absence of LDH within mitochondria.
These suggestions are not necessarily at odds with recent immunofluorescent studies showing the co-localization of LDH and COX in L6 muscle cells (Hashimoto et al. 2006). Such co-localization probably indicates the close proximity of LDH near mitochondria, as we have shown for MCT1 (Johannsson et al. 1997), rather than the sharing of the same subcellular compartment (i.e. mitochondria). This is also suggested by the fact that a considerable quantity of LDH also does not co-localize with COX (Hashimoto et al. 2006), and there is some non-specific labelling of both proteins (Hashimoto et al. 2006). Finally, there is also increasing recognition that fluorescent microscopy is fraught with dangers, as it has been estimated that as many as half of all experiments that report the co-localization of two proteins have not been performed properly (Pearson, 2007). In particular, the phenomenon known as ‘bleed-through,’ in which conditions used to excite one tag also partially excites the second tag, may be particularly problematical, as this can erroneously indicate that two proteins are co-localized (Pearson, 2007).
Does use of nagarse obviate direct lactate oxidation by mitochondria?
It is perplexing that we (present study) and many others (Popinigis et al. 1991; Rasmussen et al. 2002; Sahlin et al. 2002; Willis et al. 2003; Ponsot et al. 2005) have been unable to replicate the work of Brooks et al. (1999b) with respect to (a) the equivalent rates of lactate and pyruvate oxidation in isolated mitochondria, and (b) the presence of large quantities of LDH in isolated mitochondria. To buttress the lack of support for an intracellular lactate shuttle, it has been speculated (Brooks, 2002; Hashimoto et al. 2005) that the protease treatment (nagarse) used to isolate mitochondria by other groups (Popinigis et al. 1991; Rasmussen et al. 2002; Sahlin et al. 2002; Willis et al. 2003; Benton et al. 2004) leads to the loss of intramitochondrial LDH and mitochondrial membrane-bound MCTs. However, considerable evidence suggests that this is probably not the case. For example, why the loss of other mitochondrial membrane-bound proteins such as MCT2, FABPpm and FAT/CD36 (present study and Benton et al. 2004) does not occur, when nagarse has been used, is not obvious. Moreover, there is also substantial evidence that the concern about nagarse treatment may not be warranted, as (i) no protease is used to isolate SS mitochondria, in which minimal direct lactate oxidation and mitochondrial LDH were observed (present study); (ii) there were no differences in either LDH or MCT content, nor in direct lactate oxidation in IMF mitochondria, when these were prepared with or without nagarse (present study, data not shown); (iii) state 3 respiratory capacities are similar or better in the nagarse-treated mitochondria than in mechanically isolated mitochondria (Palmer et al. 1977; Matlib et al. 1978; Cogswell et al. 1993; Philippi & Sillau, 1994); (iv) neither mitochondrial membrane-bound proteins nor intramitochondrial proteins appear to be lost with nagarse treatment, as fatty acid oxidation is not compromised in either SS (no nagarse) or IMF mitochondria (+ nagarse, present study and Campbell et al. 2004); (v) in permeabilized muscle, not exposed to nagarse, cytosolic LDH is absent and direct mitochondrial lactate oxidation is negligible (Ponsot et al. 2005). Collectively, these studies indicate that the widely used procedures for mitochondrial isolation probably do not account for the failure to observe direct mitochondrial lactate oxidation in many studies (present study and Popinigis et al. 1991; Rasmussen et al. 2002; Sahlin et al. 2002; Willis et al. 2003; Benton et al. 2004; Ponsot et al. 2005).
Is direct mitochondrial lactate oxidation due to mitochondrial contamination?
There is, however, concern about mitochondrial contamination in some studies. The very early studies, in which direct mitochondrial lactate oxidation had been observed (Szczesna-Kaczmarek, 1990), have been retracted, because of mitochondrial contamination with cytosolic LDH (Popinigis et al. 1991). To avoid this problem, when analysing mitochondria for selected proteins, we (present study and Benton et al. 2004; Campbell et al. 2004) employ (i) a purification protocol to minimize the presence of other, non-mitochondrial constituents, although 100% purification is probably impossible, and (ii) we examine thoroughly the mitochondrial purification with abundantly expressed proteins of selected subcellular components (present study and Benton et al. 2004; Campbell et al. 2004). Use of very low-abundant sarcolemmal proteins such GLUT1 to establish mitochondrial purification (Brooks et al. 1999a; Dubouchaud et al. 2000), will predictably underestimate the extent of contamination that may be present. In some other studies, in which LDH has been reported in mitochondria, a thorough purification step appears not to have taken place (Brooks et al. 1999a; Dubouchaud et al. 2000; McClelland & Brooks, 2002), and indeed, mitochondrial contamination with plasmalemmal GLUT1 and 4 is observed (Brooks et al. 1999a; Dubouchaud et al. 2000). Additionally, contamination of skeletal muscle plasma membrane with mitochondrial fragments has also been observed by this group (Hashimoto et al. 2006). Thus, in view of these mitochondrial (Brooks et al. 1999a; Dubouchaud et al. 2000) and sarcolemmal (Hashimoto et al. 2006) contamination problems, the apparent detection of LDH within mitochondria in some of these studies (Brooks et al. 1999a; Dubouchaud et al. 2000; McClelland & Brooks, 2002) may well reflect some degree of cytosolic LDH contamination, as has been found by others (Popinigis et al. 1991). Interestingly, given that LDH content in mitochondria is typically observed to be only 0.5–2% relative to cytosolic LDH (present study and Kline et al. 1986; Brandt et al. 1987; Rasmussen et al. 2002; Sahlin et al. 2002), this may well represent the ‘normal’ contamination of isolated mitochondria with the very abundant cytosolic LDH. This small contamination is, however, not without substantial metabolic consequences when attempting to examine direct lactate oxidation. We can calculate from our studies with LDH addition (Fig. 9), that a 1% LDH contamination would increase the rate of lactate oxidation by 450–1330%, and a 10% LDH contamination would increase these rates by 1900–6300% in SS and IMF mitochondria obtained from red and white muscle. Taken altogether, we cannot ignore the possibility that even a small amount of LDH contamination in mitochondria, possibly 0.5–2% (Kline et al. 1986; Brandt et al. 1987; Rasmussen et al. 2002; Sahlin et al. 2002) may explain the apparent direct oxidation of lactate by mitochondria. Moreover, even in the present studies we cannot discount the possibility that some level of LDH contamination of the mitochondria accounted for the very low rates of lactate oxidation. With less purified mitochondria this LDH contamination problem would be significantly worse, and could lead to misinterpretations.
Reconciling different views
In view of the fact that there is a considerable lack of support for the intracellular lactate shuttle in virtually all studies (present study and Popinigis et al. 1991; Rasmussen et al. 2002; Sahlin et al. 2002; Willis et al. 2003; Ponsot et al. 2005), but yet, LDH and COX appear to co-localize (Hashimoto et al. 2006), it seems that another explanation for lactate oxidation within muscle is needed. We favour the proposal outlined by Gladden (2004) based on older schemes (Peuhkurinen et al. 1983; Stainsby & Brooks, 1990). Specifically, there may well be subcompartmental metabolism of lactate and pyruvate, such that pyruvate and NADH concentrations are highest in cytosolic sites farthest removed from mitochondria, while very low pyruvate and NADH concentrations would occur near mitochondria where pyruvate carrier(s) and NADH shuttles would be taking these products into the mitochondria (Gladden, 2004). In this scheme, lactate and NAD+ production would occur in remote cytosolic locations away from mitochondria, from where lactate would diffuse within the cytosol to areas near the mitochondria, where lactate is then converted back to pyruvate and NADH to be taken up into mitochondria (Fig. 10). Indeed, recent work has shown that glycogen metabolism is subcompartmentalized (Marchand et al. 2007) and others have speculated about the existence of subcompartmentation of pyruvate metabolism in the heart (Peuhkurinen et al. 1983; Goodwin et al. 1998). In addition, studies in the heart showing that glycolytically derived lactate production and oxidation of exogenous lactate operate as functionally separate pathways (Chatham et al. 2001) would be fully consistent with this scheme (Fig. 10). The model (Fig. 10) allows for the observed close association of LDH near mitochondria (Hashimoto et al. 2006), while still not requiring an intracellular shuttle such as has been proposed (Brooks et al. 1999b) and for which there is virtually no experimental support (present study and Popinigis et al. 1991; Rasmussen et al. 2002; Sahlin et al. 2002; Willis et al. 2003; Ponsot et al. 2005).
Figure 10. Schematic representation of compartmentalized lactate and pyruvate metabolism in skeletal muscle.
The scheme is based on a model presented by Gladden (2004) and on a recent observation of compartmentalized lactate metabolism (Chatham et al. 2001), co-localization of selected proteins with mitochondria (Hashimoto et al. 2006), as well as data from the present study indicating (a) minimal LDH in mitochondria, (b) the presence of MCT2 (a high-affinity pyruvate transporter) in SS and IMF mitochondria subcellular distribution, and (c) the preference for pyruvate rather than lactate oxidation by mitochondria. Note that this scheme shows LDH, MCT2 and mitochondria are in close proximity to each other, i.e. they are co-localized. Importantly, LDH is not contained within the mitochondrion.
Summary
Studies in Brooks' laboratory have raised the intriguing, but controversial, idea that direct oxidation of lactate can occur in skeletal muscle mitochondria (Brooks et al. 1999b). We examined whether the widespread lack of support for the intracellular lactate shuttle was attributable to a failure to consider that SS and IMF obtained from highly glycolytic and highly oxidative muscles can exhibit differences in substrate oxidations. However, our studies fail to provide any support for the idea that direct oxidation of lactate occurs in skeletal muscle mitochondria, as (a) rates of oxidation are far less than those of pyruvate and (b) lactate fails to displace pyruvate oxidation. The failure to observe direct oxidation of lactate by SS and IMF mitochondria fully supports all other studies, except one, on this topic to date. We find, in agreement with many other studies, that LDH is essentially absent in mitochondria, while replacing this enzyme rescues lactate oxidation, consistent with the well-known role of this enzyme in converting lactate to pyruvate in the cytosol, after which pyruvate enters the mitochondria to be oxidized. We suggest that low levels of contamination of isolated mitochondria, with cytosolic LDH, are probably ‘normal’. Unfortunately, this may give the appearance of direct oxidation of lactate within mitochondria. The severity of this problem was calculated to be dependent on the degree of LDH contamination. The proposal by Gladden (2004) that there could be different subcompartmental areas of lactate and pyruvate metabolism in skeletal muscle, is consistent with the many observations that direct oxidation of lactate in mitochondria is negligible, while LDH can exists in close association (co-localizes) with mitochondria, but need not be present within mitochondria.
Acknowledgments
These studies were supported by grants from the Natural Sciences and Engineering Research Council of Canada (D.A.H., L.L.S., A.B.), the Canadian Institutes of Health Research (A.B.), the Heart and Stroke Foundation of Ontario (A.B.), and the Canada Research Chair program (D.A.H. and A.B.). D.A.H. is the Canada Research Chair in Cell Physiology. A.B. is the Canada Research Chair in Metabolism and Health.
References
- Aragon JJ, Lowenstein JM. The purine-nucleotide cycle. Comparison of the levels of citric acid cycle intermediates with the operation of the purine nucleotide cycle in rat skeletal muscle during exercise and recovery from exercise. Eur J Biochem. 1980;110:371–377. doi: 10.1111/j.1432-1033.1980.tb04877.x. [DOI] [PubMed] [Google Scholar]
- Baba N, Sharma HM. Histochemistry of lactic dehydrogenase in heart and pectoralis muscles of rat. J Cell Biol. 1971;51:621–635. doi: 10.1083/jcb.51.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin KM, Hooker AM, Herrick RE. Lactate oxidative capacity in different types of muscle. Biochem Biophys Res Commun. 1978;83:151–157. doi: 10.1016/0006-291x(78)90410-2. [DOI] [PubMed] [Google Scholar]
- Benton CR, Campbell SE, Tonouchi M, Hatta H, Bonen A. Monocarboxylate transporters in subsarcolemmal and intermyofibrillar mitochondria. Biochem Biophys Res Comm. 2004;323:249–253. doi: 10.1016/j.bbrc.2004.08.084. [DOI] [PubMed] [Google Scholar]
- Bezaire V, Bruce CR, Heigenhauser GJ, Tandon NN, Glatz JF, Luiken JJ, Bonen A, Spriet LL. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: essential role in fatty acid oxidation. Am J Physiol Endocrinol Metab. 2006;290:E509–E515. doi: 10.1152/ajpendo.00312.2005. [DOI] [PubMed] [Google Scholar]
- Bonen A, Campbell CJ, Kirby RL, Belcastro AN. A multiple regression model for blood lactate removal in man. Pflug Arch. 1979;380:205–210. doi: 10.1007/BF00582897. [DOI] [PubMed] [Google Scholar]
- Bonen A, Heynen M, Hatta H. Distribution of monocarboxylate transporters MCT1-8 in rat tissue and human skeletal muscle. Appl Physiol Nutr Metab. 2006;31:31–39. doi: 10.1139/h05-002. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJFP, Arumugam Y, Glatz JFC, Tandon NN. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000a;275:14501–14508. doi: 10.1074/jbc.275.19.14501. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJFP, Lui S, Dyck DJ, Kiens B, Kristiansen S, Turcotte L, van der Vusse GJ, Glatz JFC. Palmitate transport and fatty acid transporters in red and white muscles. Am J Physiol Endocrinol Metab. 1998;275:E471–E478. doi: 10.1152/ajpendo.1998.275.3.E471. [DOI] [PubMed] [Google Scholar]
- Bonen A, Miskovic D, Tonouchi M, Lemieux K, Wilson MC, Marette A, Halestrap AP. Abundance and subcellular distribution of MCT1 and MCT4 in heart and fast-twitch skeletal muscles. Am J Physiol Endocrinol Metab. 2000b;278:E1067–E1077. doi: 10.1152/ajpendo.2000.278.6.E1067. [DOI] [PubMed] [Google Scholar]
- Bonen A, Tonuchi M, Miskovic D, Heddle C, Heikkila JJ, Halestrap AP. Isoform-specific regulation of the lactate transporters MCT1 and MCT4 by contractile activity. Am J Physiol Endocrinol Metab. 2000c;279:E1131–E1138. doi: 10.1152/ajpendo.2000.279.5.E1131. [DOI] [PubMed] [Google Scholar]
- Bookelman H, Trijbels JM, Sengers RC, Janssen AJ, Veerkamp JH, Stadhouders AM. Pyruvate oxidation in rat and human skeletal muscle mitochondria. Biochem Med. 1978;20:395–403. doi: 10.1016/0006-2944(78)90089-3. [DOI] [PubMed] [Google Scholar]
- Brandt RB, Laux J, Spainhour SE, Kline ES. Lactate dehydrogenase in rat mitochondria. Arch Biochem Biophys. 1987;259:412–422. doi: 10.1016/0003-9861(87)90507-8. [DOI] [PubMed] [Google Scholar]
- Broer S, Broer A, Schneider HP, Stegen C, Halestrap AP, Deitmer JW. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J. 1999;341:529–535. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer S, Schneider HP, Broer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J. 1998;333:167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA. Lactate shuttle – between but not within cells? J Physiol. 2002;541:333. doi: 10.1113/jphysiol.2002.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA, Brown MA, Butz CE, Sicurello JP, Dubouchaud H. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol. 1999a;87:1713–1718. doi: 10.1152/jappl.1999.87.5.1713. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci U S A. 1999b;96:1129–1134. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz CE, McClelland GB, Brooks GA. MCT1 confirmed in rat striated muscle mitochondria. J Appl Physiol. 2004;97:1059–1066. doi: 10.1152/japplphysiol.00009.2004. [DOI] [PubMed] [Google Scholar]
- Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJFP, Glatz JFC, Bonen A. A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem. 2004;279:36235–36241. doi: 10.1074/jbc.M400566200. [DOI] [PubMed] [Google Scholar]
- Chatham JC, Des Rosiers C, Forder JR. Evidence of separate pathways for lactate uptake and release by the perfused rat heart. Am J Physiol Endocrinol Metab. 2001;281:E794–E802. doi: 10.1152/ajpendo.2001.281.4.E794. [DOI] [PubMed] [Google Scholar]
- Chi MM-Y, Hintz CS, Coyle EF, Martin WH, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol Cell Physiol. 1983;244:C276–C287. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- Chilibeck PD, Syrotuik DG, Bell GJ. The effect of concurrent endurance and strength training on quantitative estimates of subsarcolemmal and intermyofibrillar mitochondria. Int J Sports Med. 2002;23:33–39. doi: 10.1055/s-2002-19269. [DOI] [PubMed] [Google Scholar]
- Cogswell AM, Stevens RJ, Hood DA. Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol Cell Physiol. 1993;264:C383–C389. doi: 10.1152/ajpcell.1993.264.2.C383. [DOI] [PubMed] [Google Scholar]
- Dimmer K-S, Friedrich B, Lang F, Deitmer JW, Broer S. The low affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350:219–227. [PMC free article] [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- Enoki T, Yoshida Y, Lally J, Hatta H, Bonen A. Testosterone increases lactate transport, monocarboxylate transporter (MCT) 1 and MCT4 in rat skeletal muscl. J Physiol. 2006;577:433–443. doi: 10.1113/jphysiol.2006.115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, MacLean DA, Graham TE, Saltin B. Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. Am J Physiol Endocrinol Metab. 1998;275:E235–E242. doi: 10.1152/ajpendo.1998.275.2.E235. [DOI] [PubMed] [Google Scholar]
- Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. Transport of lactate and pyruvate into human erythrocytes. Biochem J. 1976;156:193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Denton RM. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by α-cyano-4-hydroxycinnamate. Biochem J. 1974;138:313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D. The SLC16 gene family – from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Brooks GA. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidationn complex. Am J Physiol Endocrinol Metab. 2006;290:E1237–E1244. doi: 10.1152/ajpendo.00594.2005. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Masuda S, Taguchi S, Brooks GA. Immunohistochemical analysis of MCT1, MCT2 and MCT4 expression in rat plantaris muscle. J Physiol. 2005;567:121–129. doi: 10.1113/jphysiol.2005.087411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildyard JC, Ammala C, Dukes ID, Thomson SA, Halestrap AP. Identification and characterisation of a new class of highly specific and potent inhibitors of the mitochondrial pyruvate carrier. Biochim Biophys Acta. 2005;1707:221–230. doi: 10.1016/j.bbabio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Hildyard JCW, Halestrap AP. Identification of the mitochondrial pyruvate carrier in Saccharomyces cerevisae. Biochem J. 2003;374:607–611. doi: 10.1042/BJ20030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway GP, Bezaire V, Heigenhauser GJ, Tandon NN, Glatz JF, Luiken JJ, Bonen A, Spriet LL. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase 1 activity in human skeletal muscle during aerobic exercise. J Physiol. 2006;571:201–210. doi: 10.1113/jphysiol.2005.102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppeler H, Hudlicka O, Uhlmann E. Relationship between mitochondria and oxygen consumption in isolated cat muscles. J Physiol. 1987;385:661–675. doi: 10.1113/jphysiol.1987.sp016513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsson E, Nagelhus EA, McCullagh KJA, Sejersted OM, Blackstad TW, Bonen A, Ottersen O-P. Cellular and subcellular expression of the monocarboxylate transporter MCT1 in rat heart. A high resolution immunogold analysis. Circ Res. 1997;80:400–407. [PubMed] [Google Scholar]
- Kline ES, Brandt RB, Laux JE, Spainhour SE, Higgins ES, Rogers KS, Tinsley SB, Waters MG. Localization of L-lactate dehydrogenase in mitochondria. Arch Biochem Biophys. 1986;246:673–680. doi: 10.1016/0003-9861(86)90323-1. [DOI] [PubMed] [Google Scholar]
- Koves TR, Noland RC, Bates AL, Henes ST, Muoio DM, Cortright RN. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol. 2005;288:C1074–C1082. doi: 10.1152/ajpcell.00391.2004. [DOI] [PubMed] [Google Scholar]
- Krieger DA, Tate CA, McMillin-Wood J, Booth FW. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol. 1980;48:23–28. doi: 10.1152/jappl.1980.48.1.23. [DOI] [PubMed] [Google Scholar]
- Lin RY, Vera JC, Chaganti RS, Golde DW. Human monocarboxylate transporter 2 (MCT2) is a high affinity pyruvate transporter. J Biol Chem. 1998;273:28959–28965. doi: 10.1074/jbc.273.44.28959. [DOI] [PubMed] [Google Scholar]
- Linnarsson D, Karlsson J, Fagraeus L, Saltin B. Muscle metabolites and oxygen deficit with exercise in hypoxia and hyperoxia. J Appl Physiol. 1974;36:399–402. doi: 10.1152/jappl.1974.36.4.399. [DOI] [PubMed] [Google Scholar]
- Ljubicic V, Adhihetty PJ, Hood DA. Role of UCP3 in state 4 respiration during contractile activity-induced mitochondrial biogenesis. J Appl Physiol. 2004;97:976–983. doi: 10.1152/japplphysiol.00336.2004. [DOI] [PubMed] [Google Scholar]
- McClelland GB, Brooks GA. Changes in MCT 1, MCT 4, and LDH expression are tissue specific in rats after long-term hypobaric hypoxia. J Appl Physiol. 2002;92:1573–1584. doi: 10.1152/japplphysiol.01069.2001. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Poole RC, Halestrap AP, O'Brien M, Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. Am J Physiol Endocrinol Metab. 1996;271:E143–E150. doi: 10.1152/ajpendo.1996.271.1.E143. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Poole RC, Halestrap AP, O'Brien M, Bonen A. Chronic electrical stimulation increases MCT1 and lactate uptake in red and white skeletal muscle. Am J Physiol Endocrinol Metab. 1997;273:E239–E246. doi: 10.1152/ajpendo.1997.273.2.E239. [DOI] [PubMed] [Google Scholar]
- Manning Fox JE, Meredith D, Halestrap AP. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol. 2000;529:285–293. doi: 10.1111/j.1469-7793.2000.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand I, Tarnopolsky MA, Adamo KB, Bourgeois JM, Chorneyko K, Graham TE. Quantitative assessment of human muscle glycogen granules size and number in subcellular locations during recovery from prolonged exercise. J Physiol. 2007;580:617–628. doi: 10.1113/jphysiol.2006.122457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlib MA, Rouslin W, Kraft G, Berner P, Schwartz A. On the existence of two populations of mitochondria in a single organ. Respiration, calcium transport and enzyme activities. Biochem Biophys Res Commun. 1978;84:482–488. doi: 10.1016/0006-291x(78)90194-8. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Diaz-Ricard M, Montgomery RR, Aster T, Jamieson GA, Tandon NN. Inhibition of platelet adhesion to collagen by monoclonal anti CD36 antibodies. Br J Haematol. 1996;92:960–967. doi: 10.1046/j.1365-2141.1996.422962.x. [DOI] [PubMed] [Google Scholar]
- Mole PA, Van Handel PJ, Sandel WR. Extra O2 consumption attributable to NADH2 during maximum lactate oxidation in the heart. Biochem Biophys Res Commun. 1978;85:1143–1149. doi: 10.1016/0006-291x(78)90661-7. [DOI] [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- Pearson H. The good, the bad and the ugly. Nature. 2007;447:138–140. doi: 10.1038/447138a. [DOI] [PubMed] [Google Scholar]
- Peuhkurinen KJ, Hiltunen JK, Hassinen IE. Metabolic compartmentation of pyruvate in the isolated perfused rat heart. Biochem J. 1983;210:193–198. doi: 10.1042/bj2100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi M, Sillau AH. Oxidative capacity distribution in skeletal muscle fibers of the rat. J Exp Biol. 1994;189:1–11. doi: 10.1242/jeb.189.1.1. [DOI] [PubMed] [Google Scholar]
- Ponsot E, Zoll J, N'Guessan B, Ribera F, Lampert E, Richard R, Veksler V, Ventura-Clapier R, Mettauer B. Mitochondrial tissue specificity of substrates utilization in rat cardiac and skeletal muscles. J Cell Physiol. 2005;203:479–486. doi: 10.1002/jcp.20245. [DOI] [PubMed] [Google Scholar]
- Popinigis J, Antosiewicz J, Crimi M, Lenaz G, Wakabayashi T. Human skeletal muscle: participation of different metabolic activities in oxidation of 1-lactate. Acta Biochim Pol. 1991;38:169–175. [PubMed] [Google Scholar]
- Rasmussen HN, van Hall G, Rasmussen UF. Lacate dehydrogenase is not a mitochondrial enzyme in human and mouse vastus lateralis muscle. J Physiol. 2002;541:575–580. doi: 10.1113/jphysiol.2002.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K, Fernstrom M, Tonkonogi M. No evidence of an intracellular lactate shuttle in rat skeletal muscle. J Physiol. 2002;541:569–574. doi: 10.1113/jphysiol.2002.016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Bruce CR, Dyck DJ. AMP kinase activation with AICAR simultaneously increases fatty acid and glucose oxidation in resting rat soleus muscle. J Physiol. 2005;565:537–546. doi: 10.1113/jphysiol.2004.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MK, Yan Z, Katz A. Carbohydrate supplementation attenuates IMP accumulation in human muscle during prolonged exercise. Am J Physiol Cell Physiol. 1991;261:C71–C76. doi: 10.1152/ajpcell.1991.261.1.C71. [DOI] [PubMed] [Google Scholar]
- Stainsby WN, Brooks GA. Control of lactic acid metabolism in contracting muscles and during exercise. Exerc Sport Sci Rev. 1990;18:29–63. [PubMed] [Google Scholar]
- Szczesna-Kaczmarek A. L-Lactate oxidation by skeletal muscle mitochondria. Int J Biochem. 1990;22:617–620. doi: 10.1016/0020-711x(90)90038-5. [DOI] [PubMed] [Google Scholar]
- Tonouchi M, Hatta H, Bonen A. Muscle contraction increases lactate transport while reducing sarcolemmal MCT4, but not MCT1. Am J Physiol Endocrinol Metab. 2002;282:E1062–E1069. doi: 10.1152/ajpendo.00358.2001. [DOI] [PubMed] [Google Scholar]
- Willis WT, Thompson A, Messer JI, Thresher JS. Vmax of mitochondrial electron shuttles in rat skeletal muscle and liver. Med Sci Sports Exerc Suppl. 2003;35:S396. [Google Scholar]
- Yoshida Y, Hatta H, Kato M, Enoki T, Kato H, Bonen A. Relationship between skeletal muscle MCT1 and accumulated exercise during voluntary wheel running. J Appl Physiol. 2004;97:527–534. doi: 10.1152/japplphysiol.01347.2003. [DOI] [PubMed] [Google Scholar]