Abstract
This study addresses whether there is excitation from human hand muscles to flexor carpi radialis (FCR) motoneurones mediated through propriospinal circuits and, if so, whether it is used in specific motor tasks. Electrical stimuli to the ulnar nerve at wrist level produced an excitation in FCR motoneurones with characteristics typical of a propriospinally mediated effect: low threshold (0.6 × motor threshold (MT)), a group I effect that was not reproduced by purely cutaneous stimuli, long central delay (4.1 ± 0.4 ms in single units), suppression when the stimulus intensity was increased, and facilitation of the corticospinal excitation at the premotoneuronal level. Ulnar-induced propriospinally mediated excitation was compared during selective voluntary contractions of the FCR and, at equivalent level of FCR EMG, during tasks in which the FCR was activated automatically in postural contractions rather than voluntarily (grip, pinching and pointing). The excitation was significantly greater during grip (and pinching) than during voluntary FCR contractions and pointing, whether measured in single motor units or tonic EMG activity, or whether the response to motor cortex stimulation was assessed as the compound motor-evoked potential or the corticospinal peak in single units. The discrepancy between the tasks appeared with ulnar intensities above 0.8 × MT and was then present across a wide range of stimulus intensities. This suggests a reduction in the corticospinal control of ‘feedback inhibitory interneurones’ mediating peripheral inhibition to propriospinal neurones during grip and pinching. The resulting more effective background excitation of propriospinal neurones by the peripheral input from hand muscles could contribute to stabilizing the wrist during grip.
The present study addresses a controversial issue in human motor control (Lemon & Griffiths, 2005; Pierrot-Deseilligny & Burke, 2005): (i) whether the evolutionary changes associated with development of manual skill in primates has been associated with suppression of non-monosynaptic corticospinal projections through a propriospinal system located at C3–C4 level to upper limb motoneurones and (ii) if such a system does exist in human subjects, whether it is involved in a wider range of upper limb tasks than has been established for the propriospinal system of the cat. We confirm a connection from intrinsic muscles of the hand, probably transmitted through the putative propriospinal relay to motoneurones innervating flexor carpi radialis (FCR), and demonstrate that this circuitry is differentially controlled in different tasks involving voluntary and postural contractions of FCR.
In the cat, the descending command for visually guided target-reaching of the forelimb is mediated through a system of propriospinal neurones located at the C3–C4 level (for review, see Alstermark & Lundberg, 1992; Lundberg, 1999). The extensive convergence onto these neurones of descending excitation, descending inhibition and of peripheral inputs from the moving limb allows the descending command to be updated at the premotoneuronal level en route to the motoneurones. The presence of a significant contribution of the cervical propriospinal system to the control of upper limb movement in higher primates has been debated (see Nakajima et al. 2000; Lemon & Griffiths, 2005). Recent primate experiments confirm that, as postulated by Nakajima et al. (2000), there are species differences, but suggest that the major species difference is stronger corticospinal inhibitory control of the C3–C4 propriospinal neurones in the macaque monkey than in the cat (see Sasaki et al. 2004; Isa et al. 2006). In humans, there is mounting evidence that a substantial part of the cortical command for movement is transmitted to motoneurones through a relay located rostral to motoneurones (for review, see Pierrot-Deseilligny & Burke, 2005; see also Stinear & Byblow, 2004a), and that this may be important during recovery from stroke (Mazevet et al. 2003; Stinear & Byblow, 2004b) and during fatigue (Martin et al. 2007). An analogy has been drawn between the propriospinal system of the cat and macaque monkey and this system in humans, which is referred to as ‘propriospinal’ in the following.
Propriospinally mediated excitation (both homonymous and heteronymous) is widespread in all human proximal muscles (including extrinsic finger muscles), but attempts to demonstrate such propriospinal projections to intrinsic hand muscles have proved negative (Pierrot-Deseilligny, 1996). Due of this absence of projections to hand muscles, inputs to the propriospinal system from hand muscle afferents were not sought. Thus, when recently investigating spinal group II and transcortical group I pathways from hand muscles to FCR motoneurones, it was not expected that these long-latency responses would be preceded by significant non-monosynaptic group I excitation. This excitation was mentioned only briefly in the report which focused on group II and transcortical group I excitations (Lourenço et al. 2006), and one motive for the present study was to determine whether this non-monosynaptic excitation has the characteristics expected for a propriospinally mediated effect and to document its corticospinal control.
An additional and, indeed, the main aim of the study was to examine how the circuitry is used in different motor tasks. It has been suggested that the putative propriospinal system in humans could be used in a more expanded repertoire of upper limb tasks than in the cat (Burke, 2001). During selective tonic contractions of FCR, the early non-monosynaptic group I excitation from hand muscles to FCR motoneurones appeared to be quite potent, more so than usually observed from proximal muscles to forearm motoneurones (see Discussion). If substantiated, this might imply that the corresponding propriospinal pathway could play an important functional role by, for example, providing a firm support to the hand during contractions involving intrinsic hand muscles. The present investigation therefore seeks to compare the extent of involvement of this pathway during various motor tasks in which the involvement of forearm muscles differed: selective tonic voluntary contractions of the FCR without functional significance, grip, pinching and pointing. Other pathways in the human central nervous system have been shown to be used differently in different motor tasks. For example, in the human upper and lower limbs, cutaneomuscular responses of any given muscle vary significantly with the task that the subject is performing (Evans et al. 1989; Burke et al. 1991; Gibbs et al. 1995; see Pierrot-Deseilligny & Burke, 2005).
Methods
The experiments were carried out on 17 healthy subjects (10 females), aged 22–71 years, all of whom had given informed written consent to the experimental procedures, which had been approved by the appropriate institutional ethics committee and which conformed to the guidelines in the Declaration of Helsinki.
General experimental procedure
The subjects were seated comfortably in an armchair. The shoulder was in slight abduction (60 deg), the elbow semiflexed (110 deg) with the ulnar edge of the forearm and the hand resting on a table.
Motor tasks
The EMG was recorded from the FCR while subjects performed four different tasks, with the wrist in 90 deg pronation and 0 deg flexion-extension: (i) selective tonic voluntary contractions of FCR against resistance; (ii) gripping a plastic egg (5 or 6 cm diameter, depending on hand size) in the palm of the hand between the thumb and the last three fingers; (iii) pinching a spring clip between the thumb and the 4th and 5th fingers, carefully maintaining a constant distance between the two edges of the clip, there being no contact with the palm of the hand; (iv) pointing with the index finger extended and with the last three fingers flexed against the palm of the hand and the thumb in flexion-adduction. In tasks (ii)–(iv), the subject was instructed not to activate FCR voluntarily and had to perform the task sufficiently strongly to produce an equivalent level of FCR EMG through a postural contraction. The rectified and integrated background EMG activity was displayed on an oscilloscope. In experiments dealing with the modulation of the ongoing EMG or of the MEP, the subjects were instructed to adjust their effort to maintain an equivalent FCR EMG activity in the different tasks. In each subject, this level was chosen to be weak enough not to produce fatigue during grip sequences lasting 100 s, the task requiring the strongest effort. Depending on the subject, this corresponded to an EMG level of 5–15% of that recorded during maximal tonic voluntary contraction (MVC) lasting 5 s.
Recordings
EMG activity was recorded by surface electrodes (silver plates, 0.8 cm diameter, 1.5 cm apart) secured to the skin over the FCR muscle belly, in the upper part of the forearm, at a site where selective wrist flexion produced much more activity than selective flexion of the fingers, and over the abductor digiti minimi (ADM). Surface EMG recordings are never selective, but the placement of the recording electrodes was such that FCR activity dominated, and only low-level distant activity was recorded during selective contractions of other forearm muscles. Accordingly, the forearm flexor EMG is referred to as FCR EMG in the following.
Conditioning stimuli
Stimuli to mixed nerves were square electrical pulses of 1 ms duration delivered through bipolar surface electrodes 1 cm apart, cathode proximal, applied to the median nerve at elbow level and the ulnar nerve at wrist level. The intensity of stimulation was expressed as multiples of the threshold of the motor response (× MT) evoked in the target muscle (ADM for ulnar stimulation at wrist level, FCR for median stimulation at elbow level). The cutaneous paraesthesiae evoked by the ulnar nerve stimulation were reproduced by purely cutaneous stimuli applied through plate electrodes over the nerve projection to the 5th finger. The stimulus intensity was adjusted to reproduce the sensation evoked by ulnar nerve stimulation; it was often impossible for the subject to make the distinction between the sensation elicited by the two stimulations. In some experiments purely cutaneous stimuli were also applied over the palmar side of the distal phalanx of the fingers 3–5 (with allowance made for the extra peripheral conduction time, see legend of Fig. 6F and G).
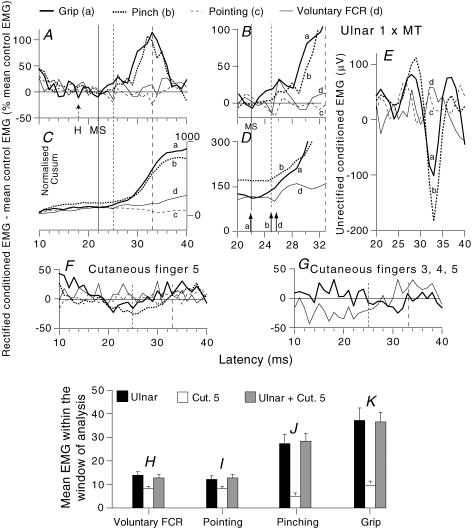
Figure 6. Modulation of the ongoing rectified and unrectified EMG by different stimuli in various motor tasks.
A, F and G, time course of the modulation of rectified EMG recorded during different motor tasks (force ∼10% of MVC): grip, thick continuous lines (a); pinching, thick dotted lines (b); pointing, thin dashed lines (c); selective voluntary contractions of FCR, thin continuous lines (d). A–E, ulnar-induced (1 × MT) effects. B, same traces as in A zooming in on the window of analysis. C and D, Cusums corresponding to A and B. E, unrectified responses recorded in parallel with the rectified EMG in A. Each trace is the average of 200 trials. (Calculations for heteronymous monosynaptic latency: homonymous monosynaptic response, 18 ms in the ongoing EMG (H reflex, dotted arrow ‘H’ in A); distance wrist to elbow, 0.28 m; supplementary afferent conduction time for the ulnar Ia volley, 4 (0.28/69) ms; ulnar-induced heteronymous Ia excitation expected at 22 (18 + 4) ms as indicated by the thin continuous vertical line in A–D (MS); note the existence of a peak of monosynaptic facilitation during grip (a) but not during pinching (b).) Vertical dotted and dashed lines, limits of the window of analysis (25–33 ms). D, single-headed vertical arrows as in Fig. 2. F and G, effects of purely cutaneous stimulation reproducing the sensation elicited by ulnar stimulation (F) or applied to the palmar side of fingers 3–5 (G, another subject, but with the same expected latency for heteronymous monosynaptic latency), with allowance for the supplementary peripheral conduction time. H–K, mean EMG within the window of analysis (see Methods) for 9 subjects. Data following separate ulnar stimulation (1 × MT, black columns), separate cutaneous stimulation of the 5th finger (white columns) and combined cutaneous and ulnar stimulation (grey columns) are compared during selective voluntary contractions (H), pointing (I), pinching (J) and grip (K).
Modulation of the ongoing EMG activity
Ongoing FCR EMG activity was filtered (100 Hz–1 kHz), amplified (× 10 000), recorded, using a sampling rate of 1 kHz, unrectified (Fig. 6E) and full-wave rectified (Figs 2A–D and 6A, B, F and G) and averaged for 60 ms against the conditioning stimulus. Trials conditioned by different stimuli (ulnar and cutaneous) and unconditioned were randomly alternated (0.5 s) during short sequences of 100 s to avoid muscular fatigue. Data from several sequences were averaged to produce a single run containing 200 conditioned (for each stimulus) and 200 unconditioned responses. The background EMG was measured in the corresponding unconditioned trials and then integrated over 60 ms to provide a fixed measure of baseline EMG over the sequence.
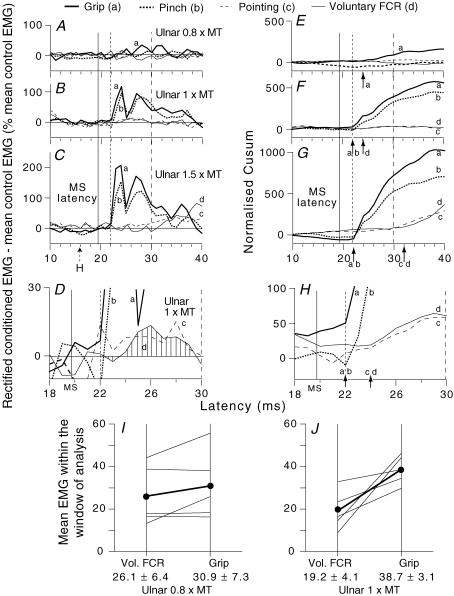
Figure 2. Ulnar-induced modulation of the ongoing rectified EMG of FCR in various motor tasks.
Time course of the rectified ongoing EMG activity of FCR during different tasks at a contraction level ∼15% of MVC: grip, thick continuous lines (a); pinching, thick dotted lines (b); pointing, thin dashed lines (c); selective voluntary contractions, thin continuous lines (d). Each trace is the average of 200 trials. Intensity of ulnar stimulation at wrist level: 0.8 (A and E), 1 (B, F, D and H), 1.5 (C and G) × MT. A–D, rectified EMG, difference: conditioned EMG – mean unconditioned EMG as a percentage of the mean unconditioned EMG. E–H, corresponding Cusums (the excursion of the Cusum was normalized by dividing against the baseline mean EMG). D and H, rectified EMG (D) and Cusum (H) at 1 × MT zooming in on the window of analysis (22–30 ms). Single-headed vertical arrows in E–H indicate the onset of the slope in the Cusum. (Calculations for heteronymous monosynaptic latency: homonymous monosynaptic response, 16 ms in the ongoing EMG (i.e. FCR H reflex, dotted arrow ‘H’ in C); distance wrist to elbow, 0.26 m; supplementary afferent conduction time for the ulnar Ia volley, 3.7 (0.26/69) ms; ulnar-induced heteronymous Ia excitation expected at 19.7 (16 + 3.7) ms, as indicated by the thin continuous vertical line in A–H; note the absence of peak at this latency in E–H). Vertical dotted and dashed lines, limits of the window of analysis (see Methods). I and J, group data: 5 subjects (averaged data for 2 experiments for 2 subjects). Each thin line represents one subject and the thick lines (and •) the mean values for the group. Ulnar-induced facilitation of the rectified EMG (area below the curve divided against the number of bins (1 ms width) within the window of analysis) is compared with stimulation at 0.8 (I) and 1 (J) × MT, during selective voluntary contractions (left vertical bar) and grip (right vertical bar). Mean values (± 1 s.e.m.) for the group are shown beneath the corresponding vertical bars.
Onset of excitation and window of analysis
Given the variability of the baseline, a cumulative sum (Cusum) technique was used to help identify the onset in excitation in the rectified ongoing EMG activity (King et al. 2006). The Cusum was calculated by adding the difference in the EMG from the mean background EMG (instead of the pre-stimulus mean used by Ellaway, 1978) to the preceding value of the Cusum. In order to avoid contamination by the conditioning stimulus artefact the first bin of the Cusum was 10 ms after ulnar stimulation. The single-headed vertical arrows in Figs 2E–G (and zooming in in H), and 6D indicate the onset of the slope in the Cusum (i.e. the latency of the excitation) in each task. The earliest such onset in the Cusums (generally grip and/or pinching) was chosen as the onset of the window of analysis, provided that it was within the intervals corresponding to propriospinal excitation (see Results). This window onset (vertical dotted line in Figs 2A–H and 6A–D, F and G) was then used for all tasks. The offset of the window was fixed 8 ms later (vertical dashed line in Figs 2A–H and 6A–D, F and G) to avoid contamination by later group II excitation at ulnar intensities above 1 × MT (see Results and Lourenço et al. 2006).
Statistical analysis
The difference between the grand average of rectified conditioned values in a single subject and the baseline EMG was expressed as a percentage of this baseline (Figs 2A–D and 6A, B, F and G). The mean EMG within the window of analysis was calculated (area below the curve divided by the number of bins (1 ms width) within the window) to compare data within the group (Figs 2I and J, and 6H–K). The excursion of the Cusum was also assessed and normalized by dividing by the baseline mean EMG. ANOVA and a post hoc test (Scheffé's F test) were used to determine whether the differences between conditioned and unconditioned EMG were significant for each subject. In nine subjects, the modulation of ongoing EMG activity (by ulnar and cutaneous stimuli) during the four different tasks was alternated in the same experiment. The significance of variations with the task in the group was examined with Kruskal–Wallis k sample test, and comparison between the results obtained in two different tasks by a Wilcoxon matched-pairs signed-rank test.
Study of single motor units
Post-stimulus time histograms (PSTHs) for a voluntarily activated motor unit isolated from surface EMG recordings were constructed for the period following a conditioning stimulus. Five subjects were able to keep the same motor unit firing during grip and during selective voluntary contractions of FCR. Thus, the effects of ulnar stimulation could be compared in the two tasks for 20 units. An additional 8 units (four additional subjects) were investigated only during selective FCR voluntary contractions.
Methods for PSTHs
EMG potentials were converted into standard pulses by a window discriminator with variable trigger levels. These pulses were fed to a computer, which subsequently triggered the stimulators about once every 0.5 s. PSTHs for single units were constructed for the 15–80 ms following the conditioning stimulus using a 0.5 ms bin width. Stimuli were triggered at a fixed delay after the preceding motor unit action potential. The details of the technique used are given elsewhere (see Pierrot-Deseilligny & Burke, 2005). The delay was chosen so that the afterhyperpolarization (AHP) following the previous motoneurone discharge reduced the firing probability due to the monosynaptic Ia EPSP, but not the effects of the late synaptic events, which occurred later during the recovery from AHP. This explains why heteronymous monosynaptic Ia excitation was rarely seen in the present investigation, in contrast to a previous study (Marchand-Pauvert et al. 2000), in which the stimulation was triggered at a delay favouring early Ia effects. Trials with and without stimulation were randomly alternated, and histograms of the firing probability were constructed without stimulation (control situation, filled columns in Fig. 5A and C) and after the conditioning stimulus (open columns in Fig. 5A and C). The control count was subtracted from the conditioned count for each bin in the PSTH. This accounts for the negative values in Figs 1, 5B and D, and 7. Changes in discharge probability were normalized as a percentage of the number of triggers. Sequences in which irregularities in the control sequence contributed significantly to the difference between the two situations were not retained for further analysis.
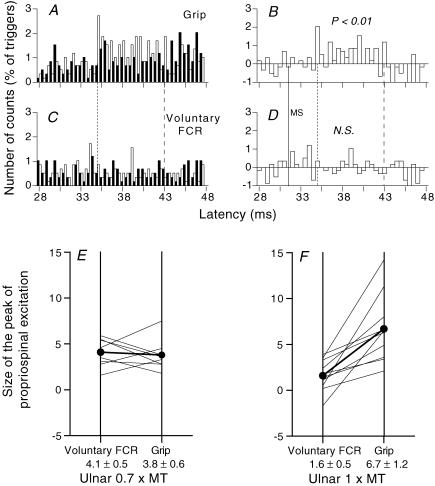
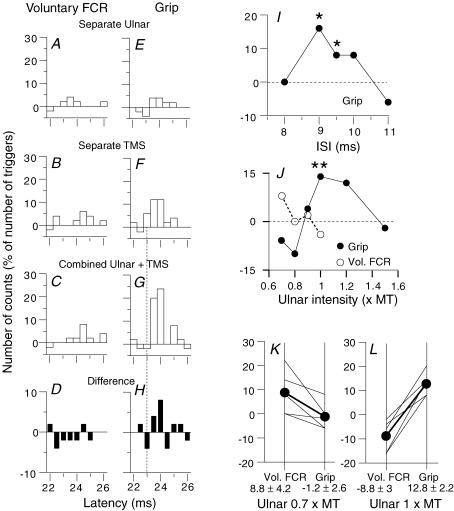
Figure 5. PSTHs for FCR units after ulnar stimulation.
A–D, PSTHs for the same FCR unit during grip (A and B) and selective FCR voluntary contractions (C and D). A and C, histograms without (filled bars) and with (open bars) stimulation of the ulnar nerve at wrist level (1 × MT). B and D, the difference between the histograms with and without stimulation. (Calculations for heteronymous monosynaptic latency: homonymous monosynaptic response (not corrected for trigger delay), 27.5 ms; distance wrist to elbow, 0.28 m; supplementary afferent conduction time for the ulnar Ia volley, 4 (0.28/69) ms; ulnar-induced heteronymous Ia excitation expected at 31.5 (27.5 + 4) ms indicated by the thin vertical continuous line (MS) in B and D). Vertical dotted and dashed lines, limits of the window (35–43 ms) in which the facilitation was assessed. E and F, group data. Each thin line represents one unit and the thick lines (and •) the mean values for the group. The amount of facilitation (difference (conditioned – unconditioned counts, expressed as a percentage of the number of triggers)) within the window of analysis (mean central delay 4.2 ± 0.5 ms, mean duration 3.6 ± 0.4 ms) is compared with ulnar stimulation at 0.7 × MT (E, 8 units) and 1 × MT (F, 10 units), during selective voluntary contractions (left vertical bar) and grip (right vertical bar). Mean values (± 1 s.e.m.) for the group are shown beneath the corresponding vertical bars.
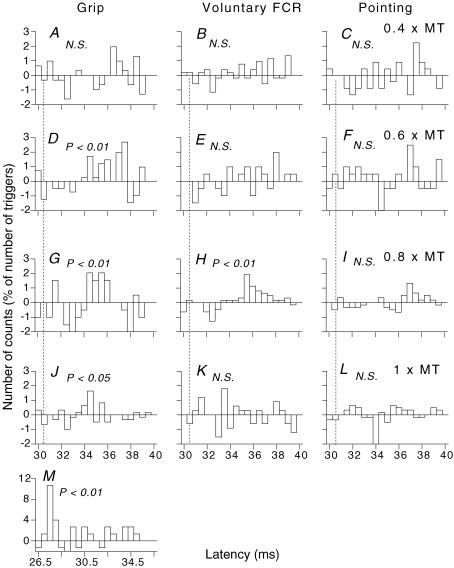
Figure 1. PSTHs to ulnar stimulation for a single FCR unit discharging in various motor tasks.
In this and Figs 5 and 7, the number of counts in each 0.5 ms bin is expressed as a percentage of the number of triggers, and plotted against the latency after stimulation. Each histogram shows the difference between conditioned and control histograms (see Methods). Intensity of ulnar stimulation at wrist level: 0.4 (A–C), 0.6 (D–F), 0.8 (G–I), 1 (J–L) × MT. A, D, G and J, grip. B, E, H and K, selective voluntary contractions of FCR. C, F, I and L, pointing with the index finger while clenching the last three fingers. M, monosynaptic peak evoked by median nerve stimulation (0.8 × MT) at elbow level. The significance of the peak is indicated above each histogram (N.S., not significant). (Calculations for heteronymous monosynaptic latency: homonymous monosynaptic response (not corrected for trigger delay), 27 ms (M); distance wrist to elbow, 0.26 m; supplementary afferent conduction time for the ulnar Ia volley, 3.7 (0.26/69) ms; ulnar-induced heteronymous Ia excitation therefore expected at 30.7 (27 + 3.7) ms, indicated by the vertical dotted line in A–L).
Figure 7. Ulnar modulation of the corticospinal peak in the PSTHs for single motor units in FCR.
PSTHs for the same unit are shown after subtraction of the background firing during selective voluntary contractions of FCR (A–D) and during grip (E–H). A and E, effects of separate ulnar stimulation (1 × MT). B and F, effects of separate TMS (44%). C and G, effects of combined stimulation (9.5 ms ISI). D and H, extra effect on combined stimulation, i.e. effect on combined stimulation minus the sum of effects of separate stimuli. The dotted vertical line in F–H indicate the latency of the first bin of the corticospinal peak produced by separate TMS. A–H, latencies not corrected for trigger delay. I, time course of the extra effect on combined stimulation (ulnar 1 × MT) during grip for the unit illustrated in A–H. J, the extra effect on combined stimulation for another unit (from a different subject) is plotted against the intensity of ulnar stimulation during selective voluntary contractions of FCR (^) and grip (•). I and J, the asterisks indicate P < 0.05 (*) or 0.01 (**). K and L, group data (5 units). Each thin line represents one unit and the thick lines (and •) the mean values for the group. The extra effect on combined stimulation is compared with ulnar stimulation at 0.7 (K) and 1 (L) × MT, during selective voluntary contractions (left vertical bar) and grip (right vertical bar). Mean values (± 1 s.e.m.) for the group are shown beneath the corresponding vertical bars.
Statistical analysis
The statistical analysis of changes in firing probability was confined to visually identified peaks of excitation within the window covering the intervals over which propriospinal excitation would have occurred. Consecutive bins with an increase in firing probability above the background discharge were grouped together and assessed with a χ2 test to determine the extent to which the distribution of firing probability after conditioning stimulation within this group differed from that in the control situation. For each ulnar stimulus intensity, a peak of excitation was accepted if the firing probability was significantly increased in a group of consecutive bins (at a probability of at least P < 0.01). The conclusion about the ulnar stimulus threshold for evoking propriospinal facilitation was therefore at least P < 0.05 (progressive Bonferroni correction). The onset of each peak (i.e. its latency) was taken as the initial bin (e.g. 34 ms in Fig. 1D) of the first group of two or three consecutive bins which was statistically significant (see Pierrot-Deseilligny & Burke, 2005). Although the relation between the amplitude of a peak in the PSTH and that of the underlying EPSP is complex (Gustafsson & McCrea, 1984), the larger the EPSP, the higher the peak. Thus, the size of the peak was estimated as the sum of the difference (conditioned – unconditioned counts, expressed as a percentage of the number of triggers) in the consecutive bins with increased firing probability (e.g. the peak in Fig. 5B reached 11.2% between 35 and 43 ms (66 counts/588 triggers)). The results obtained with different units during grip and voluntary contractions of FCR were compared using a Wilcoxon matched-pairs signed-rank test.
Modulation of responses evoked by TMS
A comparison was made between the effects of ulnar volleys at wrist level on corticospinal responses evoked in the FCR during voluntary contractions of FCR and grip. Transcranial magnetic stimulation (TMS) was applied over the motor cortex using a Magstim 200 (Magstim, Whitland, Dyfed, UK) through a 9 cm coil held at the vertex with the optimal inclination for the FCR. Ulnar-induced modulation was studied on the full-wave rectified motor-evoked potential (MEP) of FCR and on TMS-evoked peaks in PSTHs for single units. Four combinations of stimuli were randomly alternated in the same sequence: background activity with no stimulation (tonic EMG activity or tonic firing of single units), separate ulnar stimulation, separate TMS, and combined stimulation. The background activity was subtracted from the conditioned responses and the extra effect on combined stimulation was the difference between the response to combined stimulation minus the sum of the effects of separate stimuli. The intensity of TMS was 25–50% of the maximal stimulator output, dependent on the subject. The results obtained in different subjects during grip and voluntary contractions of FCR were compared using a Wilcoxon matched-pairs signed-rank test.
MEPs
The surface rectified control and conditioned responses were assessed within a window corresponding to the full MEP (e.g. 15–30 ms in Fig. 3A). Results in Figs 3D and 4A, D and E represent the extra effect on combined stimulation (see above), expressed as a percentage of the control MEP. ANOVA and a post hoc test (Scheffé's F test) were used to test the significance of this result in individual subjects. The effects of ulnar stimulation on the MEP during voluntary contractions of FCR were investigated in 10 subjects. In three of them, the time course of the ulnar modulation of the MEP was compared to that of the H reflex (elicited by 1 ms stimuli to the median nerve in the cubital fossa). When the latency of the MEP was shorter than that of the H reflex, the latency difference was subtracted from the latency of the ulnar modulation of the H reflex so that the appropriate interstimulus intervals (ISIs) were aligned (Fig. 3D). Ulnar modulation of the MEP was compared during grip and voluntary contractions of FCR in five subjects (10 experiments) and during grip and pointing in three subjects.
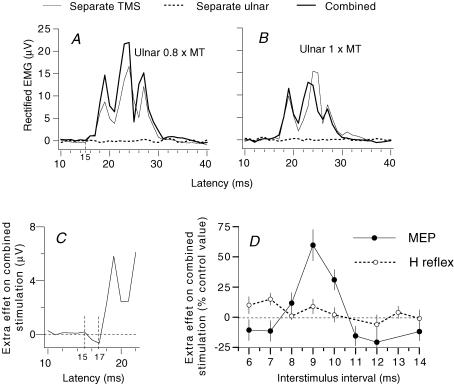
Figure 3. Time course of effects of ulnar stimulation at wrist level on the MEP and H reflex of FCR.
A and B, mean control and conditioned FCR MEPs (TMS, intensity 42%; 20 sweeps, thin and thick lines, respectively) at the 9 ms ISI after ulnar stimulation at 0.8 (A) and 1 (B) × MT. Rectified EMG responses are shown after subtraction of the mean background EMG (integrated over 60 ms) and expressed in μV. Dotted line, effect of ulnar stimulation by itself (the negligible effect is due to the small number of stimuli (n = 20)). C, initial part of the extra effect on combined stimulation (see Methods) of the traces shown in A to illustrate that the facilitation spares the initial bins (16–17 ms). D, comparison of the time courses of the effects of ulnar volleys (0.8 × MT) on the MEP (•, TMS intensity 42%) and the H reflex of FCR (^) (same experiment as in A–C) during selective FCR voluntary contractions. The extra effect of combined stimulation on the MEP, i.e. difference (conditioned MEP – (control MEP + effect of separate ulnar stimulation) as a percentage of control MEP) and the size of the H reflex (expressed as a percentage of its control value) are plotted against the ISI (2 ms has been subtracted from the ISI between conditioning and test H reflex volleys so that the appropriate intervals are aligned, see Methods). (Calculations for the central delay: MEP and H reflex had latencies of 15 and 17 ms, respectively, so that simultaneous arrival of median-induced Ia and corticospinal volleys at the FCR motoneurone pool should occur when median stimulation at elbow level was delivered 2 ms before TMS. Since the ulnar volley had a supplementary peripheral afferent conduction time of ∼4 ms (see above), its simultaneous arrival at motoneurone level with the corticospinal volley requires 6 (2 + 4) ms, and the ISI of 9 ms for the peak of extra facilitation could be accounted for by these 6 ms added to the central delay, which may therefore be estimated at 3 (9–6) ms.)
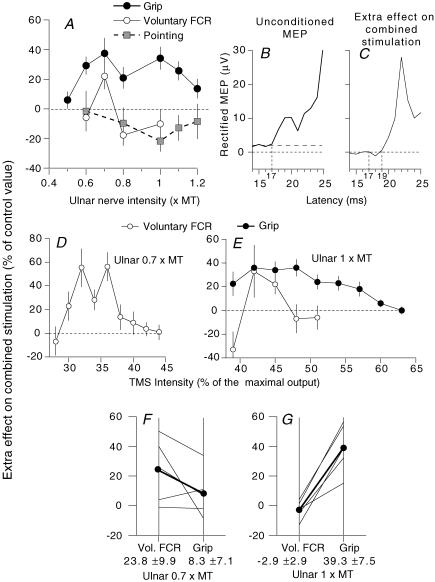
Figure 4. Effects of changing the intensity of ulnar stimulation or TMS on the MEP.
A, D and E, extra effect on combined stimulation, i.e. difference (conditioned MEP – (control MEP + effect of separate ulnar stimulation) as a percentage of control MEP), same subject as in Fig. 3. A, ulnar intensity was varied (TMS 40%, ISI 7 ms) during selective voluntary contractions of FCR (^), grip (•) and pointing (grey squares). B, initial part of the unconditioned MEP (20 sweeps, expressed in μV), which, although recorded from the same subject as in Fig. 3, had a longer latency (17 ms, in the different experiments of A, D and E), presumably because it required the input from a later I wave. C, initial part of the extra effect on combined stimulation during grip (ISI 7 ms, ulnar 1 × MT) showing that the facilitation spares the initial bins (18–19 ms), and thus confirming that both the MEP and the excitation of propriospinal neurones are produced by a later I wave than in Fig. 3. (Calculations for the central delay: the ISI at which the ulnar-induced peak of extra facilitation occurred was shorter (7 ms). However, the central delay of the extra facilitation, estimated as in Fig. 3 (ISI at the peak of extra facilitation minus (difference between latencies of the H reflex and the MEP + supplementary peripheral afferent conduction time)), remained the same: 7 – (0 + 4) = 3 ms.) D and E, TMS intensity was varied (ISI 7 ms; ulnar stimulation 0.7 (D), 1 (E) × MT) during selective voluntary contractions (D and E, ^) and grip (E, •). (Data in A–C, D and E come from three different experiments. This explains that the MEP was obtained with a lower intensity in D than in E and that the peak of extra facilitation was with TMS ∼40% in A and E, but ∼30% in D.) F and G, group data: 5 subjects (averaged data for three experiments for one subject). Each thin line represents one subject and the thick lines (and •) the mean values for the group. The extra effect on the MEP is compared with ulnar stimulation at 0.7 (F) and 1 (G) × MT, during selective voluntary contractions (left vertical bar) and grip (right vertical bar). Mean values (± 1 s.e.m.) for the group are shown beneath the corresponding vertical bar.
Corticospinal peaks in PSTHs
With five units (5 subjects) it was possible to compare the ulnar modulation of the corticospinal peak during grip and during voluntary contractions of FCR. The TMS intensity was set so that, during voluntary activation of the unit, cortical stimulation only affected the firing probability of the recorded unit, i.e. it did not cause any other unit to fire. A χ2 test was used to assess the difference between the response to combined stimulation and the sum of effects of separate stimuli. This was done using a window of analysis (i) starting with the first bin with facilitation (or inhibition) on combined stimulation, since the first bin(s) of the peak of corticospinal excitation were not affected by peripheral afferent volleys (see Pierrot-Deseilligny & Burke, 2005 and Discussion), and (ii) lasting 1–2 ms so as to include the whole first wave of the corticospinal peak when there were several successive waves (see Rothwell, 1997).
Results
Several approaches were used to document a possible ulnar-induced propriospinally mediated excitation of FCR motoneurones and to investigate task-related changes in it. This included how, during different motor tasks, ulnar volleys of different intensity modulated tonic firing of single units, tonic EMG activity or the responses to motor cortex stimulation.
Ulnar-induced non-monosynaptic group I excitation of FCR motoneurones
The first step was to confirm that the ulnar-induced non-monosynaptic group I excitation of FCR motoneurone mentioned in a previous paper (Lourenço et al. 2006) could be attributed to a propriospinally mediated excitation with a reasonable certainty.
Timing of the effects
Heteronymous monosynaptic Ia excitation from hand muscles to FCR motoneurones has been previously described (Marchand-Pauvert et al. 2000). However, it was found in only 5/28 units (for reasons mentioned in Methods), and a peak of monosynaptic excitation in the ongoing EMG was found in only 2/9 subjects (during grip, see Fig. 6A–D). This is because, in contrast to a previous study (Lourenço et al. 2006), most experiments involved ulnar stimuli of intensity equal to or less than 1 × MT, and such stimuli recruit only a small proportion of Ia afferents (see Pierrot-Deseilligny & Burke, 2005). The expected latency of the ulnar-induced heteronymous monosynaptic excitation was then estimated by adding the peripheral afferent conduction time between wrist and elbow to the latency of the median-induced homonymous monosynaptic excitation (monosynaptic peak in PSTHs for single units, H reflex in the ongoing EMG). The former (∼4 ms) was calculated from the conduction velocity of the fastest Ia afferents from hand muscles (69 m s−1; Marchand-Pauvert et al. 2000) and the distance (0.26–0.28 m) between the stimulation sites for the median nerve at the elbow and the ulnar nerve at the wrist.
Non-monosynaptic group I excitation in the PSTHs for single units
For the FCR unit illustrated in Fig. 1, the middle column of histograms (Fig. 1B, E, H and K) shows the effects of increasing ulnar stimulus intensity on the PSTH for this unit recorded during selective FCR contractions. At 0.4 × MT (Fig. 1B), there was no effect. At 0.6 × MT (Fig. 1E), the increase in firing probability within the window 34–38 ms (4.4%) was not statistically significant (though at the same intensity, there was a significant facilitation during grip, see Fig. 1D). At 0.8 × MT (Fig. 1H), there was a significant increase in firing probability (P < 0.01) between 35 and 37.5 ms (i.e. at a latency exceeding that of the calculated monosynaptic Ia latency by 4.3 ms, see legend to Fig. 1). At 1 × MT (Fig. 1K) no significant peak was detected. A similar significant excitation following stimuli at 0.7–0.8 × MT was observed in 16/28 units (P < 0.01; 8/9 subjects). The threshold of the excitation was low (0.6 × MT), and it could not be reproduced by purely cutaneous stimulation (see Discussion), suggesting a group I effect. It occurred on average 4.1 ± 0.4 ms longer than monosynaptic Ia excitation, presumably the result of a long central delay, and had a mean size of 5.7 ± 1.4%. In no unit was a significant peak detected at 1–1.2 × MT.
Modulation of the FCR EMG
The thin continuous line in Fig. 2A–D shows the effects of increasing ulnar stimulus intensity on the ongoing rectified FCR EMG recorded during selective FCR tonic contractions. At 0.8 × MT (Fig. 2A), there was no effect, although there was a significant facilitation during grip (curve a). At 1 × MT, facilitation was small during voluntary contractions (at least on the scale of Fig. 2B and F), but there was clear facilitation during grip and pinch (a and b) starting at 22 ms (i.e. 2.3 ms longer than the calculated monosynaptic latency, see legend to Fig. 2). However, zooming in on the window of analysis (22–30 ms, see Methods) reveals a small facilitation that was significant during voluntary contractions of FCR both in the rectified EMG (Fig. 2D, dashed area under the curve d) and the Cusum (Fig. 2H, d). The latency (24 ms, Fig. 2H) was 2 ms longer (d) than during grip (a) or pinch (b). In 8/9 subjects, there was similar facilitation at 1 × MT during voluntary contractions, occurring on average 4.6 ± 0.1 ms longer (as calculated from the Cusum) than the calculated monosynaptic latency.
Modulation of the MEP
Figure 3A illustrates the averaged rectified EMG activity observed in one subject in response to separate and combined stimuli (ulnar and TMS) during a tonic voluntary contraction of FCR. By itself ulnar stimulation at 0.8 × MT had a negligible effect (dotted line), but the MEP produced by separate TMS (42%, thin continuous line), was clearly facilitated on combined stimulation (9 ms ISI, thick continuous line, from 16 to ∼22 μV). However, the facilitation spared the first 2 ms of the MEP (see the extra effect on combined stimulation in Figs 3C (16–17 ms) and 4C (18–19 ms)). Figure 3D (filled circles) shows the time course of the extra effect on combined stimulation (see Methods) measured within the window 15–30 ms. There was significant extra facilitation (59%) of the MEP peaking at the 9 ms ISI (P < 0.05). The amplitude of the FCR H reflex (open circles) was minimally facilitated at the corresponding ISI (see Methods). Similar results were obtained in the three subjects so tested (mean values for the facilitation: 45 ± 7%, MEP; 7 ± 7%, H reflex). During selective FCR voluntary contractions, weak ulnar volleys at 0.7 × MT (facilitation had often a lower threshold at 0.6 × MT) consistently enhanced the MEPs elicited by low TMS intensities (10/10 subjects, mean value of the extra facilitation 46 ± 7.4%), a facilitation which was significant (Scheffé's F test) in 9/10 subjects. The central delay of the extra facilitation was ∼3 ms (see legend of Figs 3 and 4).
In 10/10 subjects, increasing the ulnar stimulus intensity to or above 0.8 × MT caused the facilitation to disappear and/or to be replaced by inhibition: e.g. compare Fig. 3A (0.8 × MT) and 3B (1 × MT), and note that in Fig. 4A (open circles) the facilitation present at 0.7 × MT was replaced by inhibition at 0.8 × MT. Finally, as previously described (Nicolas et al. 2001), increasing TMS intensity also caused the extra facilitation of the MEP to disappear, less abruptly, however, when ulnar stimulation was at 0.7 than at 1 × MT (Fig. 4D and E, open circles and see Discussion).
Task-related changes in ulnar-induced nonmonosynaptic group I excitation
Task-related changes in ulnar modulation of PSTHs for single units
Three subjects were able to isolate the same single FCR unit during three tasks (grip, selective voluntary contractions of FCR and pointing) and to maintain its discharge long enough to explore the effects of ulnar stimulation at different intensities. Figure 1 illustrates one of these units. During grip, significant facilitation was present at 0.6 × MT (Fig. 1D, 8.3%; P < 0.01) and 0.8 × MT (Fig. 1G, 7.1%; P < 0.01). Although reduced, the facilitation was still significant at 1 × MT (Fig. 1J, 4%; P < 0.05). Whatever the stimulus intensity, there was no significant facilitation during pointing (Fig. 1C, F, I and L). During selective voluntary contractions of FCR, the facilitation was significant only at 0.8 × MT (Fig. 1H, P < 0.01), and, at 5.3%, it was smaller than during grip. There was a similar facilitation, greater during grip than during pointing, whatever the stimulus intensity, with the other two units tested. In these two units, the facilitation during grip at high intensities (0.8–1 × MT) was also greater than during selective voluntary contractions of FCR.
Figure 5A–D shows for another subject that ulnar-induced facilitation at 1 × MT was considerably greater during grip (Fig. 5A and B) than during selective voluntary contractions of FCR (Fig. 5C and D): 11.2 versus 0.5% within the window 35–43 ms (central delay 3.5 ms, see legend of Fig. 5). Figure 5E and F shows the pooled data for five subjects who were able to keep the same unit during selective voluntary contractions of FCR (left vertical bar) and grip (right vertical bar). Ulnar stimulation at 0.6–0.7 × MT (Fig. 5E, 8 units) and 1 × MT (Fig. 5F, 10 units) produced excitation at a mean central delay of 4.2 ± 0.5 ms. At 0.6–0.7 × MT, there was no difference in the peak of group I non-monosynaptic excitation in the two tasks (Fig. 5E; mean values 4.1 ± 0.5% during voluntary contractions of FCR versus 3.8 ± 0.6% during grip; P = 0.57, Wilcoxon). However, in all 10 units explored at 1 × MT, the facilitation was greater during grip (mean values 1.6 ± 0.5%, voluntary contractions; 6.7 ± 1.2%, grip; P < 0.01, Wilcoxon).
Task-related changes in modulation of the ongoing EMG
Figure 2A–H compares the ulnar-induced modulation of the rectified (Fig. 2A–D) ongoing FCR EMG activity and the corresponding Cusums (Fig. 2E–H) during different tasks. Whatever the stimulus intensity (0.8, Fig. 2A and E; 1, Fig. 2B and F, zoom in, Fig. 2D and H; 1.5 × MT, Fig. 2C and G), the non-monosynaptic ulnar-induced group I facilitation of the rectified EMG was much greater during grip (a) and pinch (b) than during selective voluntary contractions of FCR (d) or pointing (c). Unrectified averages recorded in parallel confirmed the greater facilitation during grip and pinch (compare Fig. 6E and A). In Fig. 2I and J, the changes produced by ulnar stimulation at 0.8 and 1 × MT in the mean EMG within the window of analysis (see Methods) are compared in five subjects during selective voluntary contractions of FCR (left vertical bar) and grip (right vertical bar). At 0.8 × MT (Fig. 2I), there was little difference in the group between the facilitation of ongoing EMG activity in the two situations (26.1 ± 6.4%, selective FCR contractions; 30.9 ± 7.3%, grip; P = 0.34, Wilcoxon). However, in all five subjects, ulnar stimulation at 1 × MT (Fig. 2J) enhanced the ongoing EMG activity more during grip than during selective FCR contractions (38.7 ± 3.1%versus 19.2 ± 4.1%, respectively; P < 0.05, Wilcoxon). In 3 of the 5 subjects, increasing the ulnar stimulation to 1.4–1.5 × MT (Fig. 2C and G) greatly attenuated the facilitation at propriospinal latency during selective voluntary contractions of FCR (mean EMG amplitude within the window 22–30 ms: 0.9% in Fig. 2C (1.5 × MT) versus 4.6% in Fig. 2B (1 × MT)) or pointing. In parallel, a later excitation appeared during voluntary contractions and pointing (Fig. 2C and G (c and d)), presumably due to high-threshold long-latency group II excitation (Lourenço et al. 2006), which was not observed during grip or pinch (Fig. 2C and G (a and b)).
Changes in the rectified ongoing EMG were used to compare the effects of ulnar stimulation and those of cutaneous stimuli (Fig. 6). Panels A–D show, in another subject at 1 × MT, the much greater ulnar-induced non-monosynaptic group I facilitation of the ongoing EMG during grip (a) and pinching (b) than during pointing (c) and selective voluntary contractions of FCR (d). (Note that this peak was preceded by a peak of monosynaptic facilitation during grip but not during pinching.) Figure 6F and G shows that purely cutaneous stimulation, evoking the sensation produced by ulnar stimulation (Fig. 6F) or applied to the palmar side of fingers 3–5 (Fig. 6G), did not reproduce ulnar-induced facilitation in the same tasks during the window of analysis (see figure legend). The mean values for the nine subjects are shown in Fig. 6H–K within the window beginning (see Methods) at a mean central delay of 3 ± 0.1 ms (range 2.2–4.2 ms). Ulnar-induced facilitation (black columns) varied significantly with the motor task (P < 0.01, Kruskal–Wallis). It was significantly greater (P < 0.05, Wilcoxon) during grip (Fig. 6K, 38.6 ± 7%) and pinching (Fig. 6J, 28.6 ± 4%) than during selective voluntary contractions of FCR (Fig. 6H, 14.6 ± 3%) or pointing (Fig. 6I, 11.8 ± 4%). (It is noted that the monosynaptic excitation observed in two subjects during grip could have contributed to the increased ulnar-induced facilitation within the window of analysis during grip, but not during pinching.) The task-related differences in ulnar-induced facilitation were not accompanied by differences in the effects of cutaneous afferents in the ulnar nerve: indeed, purely cutaneous stimulation reproducing the sensation evoked by ulnar nerve stimulation produced the same small facilitation in the different tasks (white columns in Fig. 6H–K), and the addition of cutaneous stimulation did not modify the effects of ulnar stimulation by itself (compare black and grey columns in Fig. 6H–K). Finally, in the four subjects so tested, cutaneous stimulation applied to the palmar side of fingers 3–5 had no effect during voluntary contractions of FCR or grip.
Task-related changes in ulnar modulation of the MEP
Figure 4A shows the effects obtained when conditioning the MEP (ISI 7 ms, see figure legend and Discussion) by ulnar stimuli of various intensity, during grip (filled circles) and selective voluntary contractions of FCR (open circles). During selective voluntary contractions of FCR, extra facilitation of the MEP was present only at 0.7 × MT, whereas, during grip, it was present across a wider range of stimulus intensities, up to 1.2 × MT. Similar results were obtained in the five subjects tested (Fig. 4F and G). In panels F and G, the results obtained at 0.7 and 1 × MT are compared during selective voluntary contractions of FCR (left vertical bar) and grip (right vertical bar). At 0.7 × MT, the extra facilitation of the MEP tended to be stronger during selective voluntary contractions than during grip in 4/5 subjects (Fig. 4F, mean values 23.8 ± 9.9%, voluntary contractions; 8.3 ± 7.1%, grip; P = 0.14, Wilcoxon). Conversely, at 1 × MT, in all five subjects, the extra facilitation of the MEP was not present during selective voluntary contractions, but was significant during grip (Fig. 4G, mean values –2.9 ± 2.9%, voluntary contractions; 39.3 ± 7.5%, grip; P < 0.05, Wilcoxon). Figure 4A also shows that ulnar stimulation, whatever its intensity, did not produce any extra facilitation of the MEP during pointing (grey squares), a consistent finding in the three subjects so tested. Finally, Fig. 4E illustrates that the suppression of the extra facilitation of the MEP (ulnar 1 × MT) induced by increasing TMS intensity was much less abrupt during grip (filled circles) than during voluntary contractions of FCR (open circles), a result that was confirmed in all five subjects.
Task-related changes in ulnar modulation of the corticospinal peak in the PSTHs
Figure 7A–H shows data (after subtraction of the background firing) for 1 of the 5 units (5 subjects) in which it was possible to compare the effects of ulnar stimulation at wrist level on the corticospinal peak produced by TMS during selective voluntary contractions of FCR (Fig. 7A–D) and grip (Fig. 7E–H). Ulnar stimulation (1 × MT) by itself barely altered the firing probability of the unit in the two tasks (Fig. 7A and E). The corticospinal peak evoked by separate TMS (Fig. 7B and F) was not enhanced on combined stimulation (9.5 ms ISI) during selective voluntary contractions of FCR (Fig. 7C), but was significantly increased during grip (Fig. 7G; P < 0.05), a facilitation which did not involve the first bin of the peak (at 23 ms, see the vertical dotted line). Figure 7D and H shows the subtraction histograms, which illustrate the extra effect on combined stimulation over and above that expected from the sum of the two separate responses. Figure 7I shows the time course of the extra effect of the corticospinal peak on combined stimulation during grip. As previously described with stimulation of other peripheral nerves, extra facilitation on combined stimulation was brief, appearing at the 9 ms ISI and disappearing at the 11 ms ISI (see Pauvert et al. 1998 and Discussion). The plots in Fig. 7J compare the effects of varying ulnar stimulus intensity on the extra effect on combined stimulation during the two tasks for another unit: during selective voluntary flexion (open circles), extra facilitation was only present at low stimulus intensity (0.7 × MT) whereas, during grip (filled circles), it appeared with a higher threshold (0.9 × MT) and was still present at 1.2 × MT. Similar results were obtained in the five subjects tested, as shown in Fig. 7K and L, in which the results obtained at 0.7 and 1 × MT are compared during selective voluntary contractions of FCR (left vertical bar) and grip (right vertical bar). At 0.7 × MT, in the group data, the extra facilitation of the corticospinal peak during selective voluntary contractions, though small, was significantly greater than during grip (Fig. 7K; 8.8 ± 4.2%, FCR contractions; −1.2 ± 2.6%, grip; P < 0.05, Wilcoxon). This difference was not statistically significant for any of the units tested by themselves. Conversely, at 1 × MT, in all five units, there was prominent extra facilitation of the corticospinal peak during grip but not during selective voluntary contractions (Fig. 7L; −8.8 ± 3% (inhibition not significant for individual units), voluntary contractions; 12.8 ± 2.2%, grip; P < 0.05, Wilcoxon).
Discussion
The major findings of this study are: (i) that there is probably a projection of group I afferents from the intrinsic muscles of the hand to FCR motoneurones through a propriospinal relay, (ii) that the extent of this ulnar-induced propriospinally mediated excitation of FCR motoneurones depends on the motor task in which FCR is involved, being greater during grip (and pinching) than during voluntary contractions of FCR (and pointing), and (iii) that this discrepancy between the tasks only appears with ulnar intensities above 0.8 × MT.
Evidence for ulnar-induced propriospinally mediated excitation of FCR motoneurones
The excitation produced by ulnar stimulation at wrist level has the characteristics previously described for the propriospinally mediated excitation produced by stimulation of other peripheral nerves (see Pierrot-Deseilligny & Burke, 2005).
(1) Afferent pathway
In the PSTHs of single units, the excitation had a low threshold corresponding to that of group I fibres in the upper limb (Malmgren & Pierrot-Deseilligny, 1988a). We have previously demonstrated that this excitation was not reproduced by purely cutaneous stimulation (Lourenço et al. 2006). In addition, Fig. 6F, and H–K shows that, during tonic FCR contraction (and other tasks), cutaneous stimulation reproducing the paraesthesiae evoked by ulnar stimulation did not reproduce ulnar-induced facilitation of the ongoing EMG during the window of analysis. The excitation is therefore probably group I in origin.
(2) Central delay
The latency of the excitation was longer than that of the heteronymous monosynaptic Ia excitation. This presumably reflects a longer central delay, since afferents with the same threshold (and thus the same afferent conduction time) were responsible for the two excitations. The central delay (4.1 ± 0.4 ms) corresponds closely to that previously reported for the propriospinally mediated excitation evoked by stimulation of other peripheral nerves (median, musculo-cutaneous) in FCR single units (4.24 ms, see Gracies et al. 1991). This central delay is shorter than that of the ulnar-induced excitation of flexor digitorum superficialis (FDS) motoneurones (5.3 ± 0.4 ms, see Lourenço et al. 2006; P < 0.05, Mann–Whitney U test). The different central delays can be explained by different rostro-caudal locations of the relevant motor nuclei in the spinal cord (FCR C6–C8; FDS C7–T1). As previously argued (see Pierrot-Deseilligny & Burke, 2005), this suggests that the candidate interneurones are located rostral to the motoneurone pools, as is the case for C3–C4 propriospinal neurones. The mean central delay for the facilitation of the ongoing EMG calculated from the Cusum was of the same order of magnitude (4.6 ms during voluntary contractions, 3 ms during grip and pinching). Finally, the central delay of the ulnar-induced facilitation of the MEP was also ∼3 ms (see legends of Figs 3 and 4).
(3) Feedback inhibition
During selective voluntary contractions of FCR, increasing the stimulus intensity caused the facilitation to disappear in the PSTHs for single units (Fig. 1K), the ongoing EMG (Fig. 2C and G, (d)), the MEP (Fig. 4A (open circles) and the corticospinal peak evoked in single units (Fig. 7J). This is consistent with previous findings, that when the afferent volleys from other muscles are strong, propriospinally mediated excitation to FCR motoneurones is suppressed. This is because inhibitory interneurones mediating feedback inhibition to propriospinal neurones are fed by the same afferents (see the sketch in Fig. 8), and their discharge then becomes sufficient to overwhelm the facilitation.
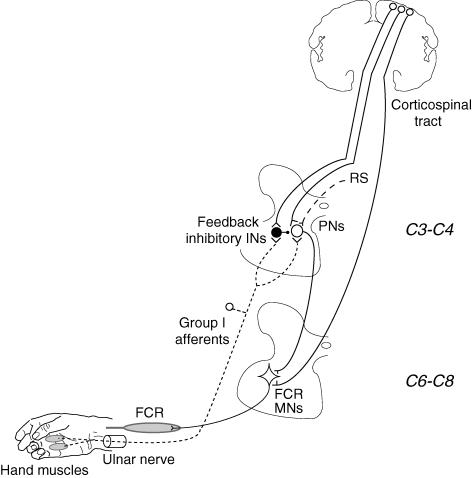
Figure 8. Sketch of the presumed propriospinal system from hand muscles to FCR.
Excitatory synapses are represented by Y-shaped bars and inhibitory synapses by small filled circles, excitatory interneurones by open circles and inhibitory interneurones by large filled circles. Group I afferents from hand muscles in the ulnar nerve (dotted line) have excitatory projections onto C3–C4 propriospinal neurones (PNs), which mediate excitation to FCR motoneurones (MNs) located at C6–C8. PNs are inhibited from feedback inhibitory interneurones (INs), which are excited by the same group I afferents. The corticospinal tract has monosynaptic projections onto MNs, and also excites both PNs and feedback inhibitory INs. PNs also receive excitation from other descending tracts, including the reticulospinal (RS) tract. Heteronymous monosynaptic Ia projections from hand muscles on FCR MNs have been omitted.
(4) Convergence of peripheral and corticospinal inputs onto propriospinal neurones
Evidence for this convergence is suggested by the ulnar facilitation of the MEP. The finding that the facilitation was greater than that of the H reflex (Fig. 3D) indicates that facilitation cannot be attributed to an interaction between the two volleys at motoneuronal level (by facilitation of the subliminal fringe of excitation created by the MEP, see Marchand-Pauvert et al. 1999b). Alternative explanations may be rejected: in a voluntarily activated motoneurone pool, the recruitment sequence is the same with Ia and corticospinal inputs (see Morita et al. 2000), and presynaptic inhibition of Ia afferents mediating the afferent volley of the H reflex may also be ruled out because ulnar volleys do not produce detectable inhibition of the FCR H reflex (Malmgren & Pierrot-Deseilligny, 1988a; Burke et al. 1992a). Finally, in agreement with previous studies, convergence of the two volleys on interneurones is further supported by the absence of facilitation in the initial bin(s) of the corticospinal peak in the PSTHs (Fig. 7F–H) and the initial part of the MEP (Figs 3C and 4C). This is what would be expected if the two volleys converged onto common interneurones rather than directly onto the motoneurone (see Pauvert et al. 1998; Nicolas et al. 2001).
There is therefore evidence for a propriospinally mediated excitation from intrinsic muscles of the hand to FCR motoneurones. This excitation is quite potent when compared with the propriospinal excitation of forearm motoneurones produced by stimulation at 0.8 × MT of afferents from proximal muscles (e.g. only 4% in extensor carpi radialis EMG after stimulation of the musculo-cutaneous nerve innervating elbow flexors (Marchand-Pauvert et al. 1999a) versus 26% for the mean ulnar-induced facilitation of the FCR EMG, Fig. 2I). The potency of this connection led to the following studies of the tasks in which it could be used.
Task-related changes in propriospinally meditated excitation from hand muscle afferents to FCR
Ulnar-induced propriospinally mediated excitation from the intrinsic muscles of the hand to FCR motoneurones was significantly greater during grip than during voluntary contractions of FCR, whether measured in FCR motoneurones as tonic firing in individual units, tonic EMG activity, or as the response to motor cortex stimulation (assessed in the compound MEP and in the corticospinal peak in individual units). Even though the selective voluntary contractions of FCR and the automatic postural activity required during grip were adjusted to produce an equivalent level of EMG activity in the FCR, several differences exist between the two tasks. The extent to which these differences could account for the different level of ulnar-induced propriospinal excitation is considered below:
(i) Different motoneurones?
In the different tasks, it is possible that the FCR motoneurones responsible for the ongoing EMG activity or the MEP were different, and/or had a different sequence of recruitment, or that the recruitment gain in the motoneurone pool was different (see Kernell & Hultborn, 1990; Pierrot-Deseilligny & Burke, 2005). It is therefore important that, in the PSTHs for single units (including the corticospinal peak), the same results were obtained as in the compound ongoing EMG and MEP, i.e. a propriospinally mediated excitation produced by ulnar stimuli above 0.8 × MT which was much more potent during grip than during voluntary contractions of FCR.
(ii) Co-contractions
Another difference between the two tasks is that, during grip, FCR contraction was accompanied by a voluntary contraction of the flexors of the fingers and of intrinsic hand muscles. This could be of particular importance for propriospinal excitation, since, during voluntary contraction, descending excitation has been proposed to be focused on subsets of propriospinal neurones receiving the afferent feedback from the contracting muscle (see Pierrot-Deseilligny & Burke, 2005). However, during pointing with the index while clenching the other fingers, there was a contraction of many muscles activated during grip: flexors of the fingers and hand muscles (first dorsal interosseus, adductor of the thumb, other intrinsic muscles). Despite these contractions, ulnar-induced facilitation was not as great as during grip, whether assessed as tonic firing in individual units (Fig. 1) or tonic EMG activity (Figs 2A–C and 6A, B, I and K). If anything, it tended to be weaker than during selective voluntary contractions of FCR (Figs 1 and 6B (c)). Nor was there any extra facilitation of the MEP on combined stimulation during pointing (Fig. 4A, grey squares). Activation of hand muscles and finger flexors is therefore insufficient by itself to explain the potent propriospinal-induced excitation from hand muscle group I afferents to FCR motoneurones during grip.
(iii) Cutaneous stimulation
Cutaneous stimulation of the palmar side of the hand and fingers produced by gripping the object constituted another obvious difference from selective FCR voluntary contractions. However, electrical stimulation applied to the skin of the palmar side of the last three fingers to produce a tactile sensation did not facilitate the ongoing EMG of FCR during grip or voluntary contractions (Fig. 6G); nor did tactile stimuli applied to the palmar side of the 2nd and 3rd fingers (if anything, these stimuli can produce inhibition at propriospinal latency in the PSTHs of FCR units, Lourenço et al. 2007). Notwithstanding, it is conceded that brief stimulation of cutaneous afferents does not equate to natural tonic stimulation of the skin. However, during pointing, continuous cutaneous stimulation would result from clenching the tip of the last three fingers against the palm of the hand and the thumb against the 3rd finger, and the absence of facilitation during pointing is consistent with the lack of significant cutaneous facilitation during grip.
(iv)Difficulty of the task
Maintaining selective voluntary contractions of FCR at 5–15% of MVC during the experimental interventions was relatively easy. Producing the same level of automatic postural FCR EMG activity during the other tasks was less easy for the subjects, though the different tasks were not equally difficult. Thus, maintaining the same level of EMG activity during pointing was reported as rather easy, even though somewhat more difficult than during selective voluntary contractions of FCR. With pinching there was greater task difficulty, because it was necessary to maintain steady voluntary contractions of the finger flexors in the act of pinching and the postural contraction of FCR. All subjects agreed that greatest difficulty involved producing the same level of ongoing postural EMG activity during grip. In particular, to isolate a single FCR unit during grip without an associated voluntary contraction of FCR required concentration. This gradation within the difficulty of the task suggests that a switch in the drive to the propriospinal system could account for the higher ulnar-induced propriospinally mediated excitation observed during pinching and grip.
Which mechanism?
We can present no definitive experimental evidence on this issue, but some unanticipated findings justify speculation so that the results can be explained in a functionally meaningful context and serve as a stimulus to future experiments.
Change in the peripheral or descending excitatory input?
Despite the contraction of hand muscles and finger flexors during pointing, propriospinally mediated excitation was not higher during this task than during selective voluntary contractions of FCR. It is therefore unlikely that the increased excitation of the relevant propriospinal neurones by the group I afferent input related to the contraction of these muscles is sufficient by itself to account for the higher propriospinal excitation observed during pinching and grip. In addition, this would not explain why the greater facilitation appeared only with high ulnar stimulus intensities (above 0.8 × MT). Group I afferents projecting to propriospinal neurones are subjected to pre-synaptic inhibition and this increases during voluntary contraction of the target muscle (Burke et al. 1992b). Greater pre-synaptic gating during selective voluntary contractions of FCR than during grip would result in greater facilitation during grip. However, here again, this mechanism could not account for the finding that the discrepancy between the two tasks only appeared with ulnar intensities above 0.8 × MT, still less for the effects of low and high ulnar intensities on the responses evoked by cortical stimulation (where facilitation at low intensities is greater during selective contractions than during grip; see Figs 4F and G and 7K and L). A different descending control in the different tasks is therefore likely.
Interaction between excitatory and inhibitory inputs to propriospinal neurones
Much as with other muscle afferent volleys (Malmgren & Pierrot-Deseilligny, 1988b; Nicolas et al. 2001), ulnar volleys can have opposing effects on the propriospinal circuits projecting to FCR motoneurones: on the one hand, they directly excite propriospinal neurones and, on the other hand, they also excite inhibitory interneurones mediating feedback inhibition to propriospinal neurones (see the sketch in Fig. 8). The feedback inhibition was manifest in the present studies by the finding that, during selective voluntary contractions of FCR, increasing the ulnar stimulus intensity above 0.8 × MT caused the facilitation to disappear, whether assessed in the PSTHs for single units, the ongoing EMG, the compound MEP or the corticospinal peak in single units (see above). This has been interpreted as follows (see Pierrot-Deseilligny & Burke, 2005): because of the spatial facilitation between descending and peripheral inputs at the level of propriospinal neurones, and because excitation involves a pathway with one less interneurone than inhibition, excitation will dominate at low stimulus intensities. However, when the peripheral volley is strong, the discharge of feedback inhibitory interneurones becomes sufficient to overwhelm the facilitation in propriospinal neurones, probably because peripheral afferent excitation is stronger to neurones mediating feedback inhibition than to propriospinal neurones themselves, much as demonstrated for the propriospinal system of the cat (cf. Alstermark et al. 1984). Similarly, at higher TMS intensities, the facilitation is reversed to suppression (Fig. 4D and E (open circles)), because corticospinal facilitation of feedback inhibitory interneurones may then be sufficient to allow the peripheral volley to discharge the inhibitory interneurones, thereby producing large inhibitory postsynaptic potentials in propriospinal neurones and overwhelming the spatial facilitation of excitatory inputs. The finding that this suppression was less abrupt with ulnar stimulation at 0.7 than at 1 × MT (Fig. 4D and E (open circles)) supports the view of a convergence of peripheral and corticospinal volleys onto feedback inhibitory interneurones. Feedback inhibition also accounts for the finding that the extra facilitation of the corticospinal responses only occurs when the peripheral and the corticospinal volleys are timed to arrive simultaneously at propriospinal level (Pauvert et al. 1998). Indeed, summation in feedback inhibitory interneurones of the EPSPs due to the peripheral volley and early waves in a complex corticospinal volley can produce inhibition of propriospinal neurones. This would be manifest 1–2 ms later and could prevent later corticospinal I waves from evoking extra facilitation.
Descending facilitation or disinhibition of propriospinal neurones?
The question then arises whether the mechanism underlying the greater ulnar-induced facilitation during grip is a more potent descending excitation of the relevant propriospinal neurones or a less potent descending inhibition. The former should be expected to enhance the ulnar-induced facilitation during grip with respect to selective FCR voluntary contractions, whatever the stimulus intensity. This was not the case at low stimulus intensities: (i) ulnar-induced facilitation at 0.6–0.8 × MT had the same effects in both the ongoing EMG activity (Fig. 2I) and the PSTH of single units (Fig. 5E); and (ii) ulnar stimuli at 0.7 × MT produced the opposite effect on the cortical response, i.e. an extra facilitation which was greater during selective voluntary contractions than during grip, whether assessed as the compound MEP (Fig. 4F) or as the corticospinal peak in single units (Fig. 7K). This suggests that the descending excitation of propriospinal neurones to FCR motoneurones is not greater during grip, and also that the efficiency of the TMS-induced corticospinal volley in facilitating the relevant propriospinal neurones is less during grip than during selective voluntary contractions of FCR. Under these conditions, the much more potent facilitation observed during grip at 1 × MT (and with higher intensities) in all the responses (ongoing EMG, Fig. 2B, C and J; PSTHs for single units, Fig. 5F; MEP, Fig. 4G; corticospinal peak in single units, Fig. 7L) could indicate that the ulnar-induced feedback inhibition mediated to propriospinal neurones is reduced during grip with respect to selective voluntary contractions of FCR. In the cat, these inhibitory interneurones receive a facilitatory input from the corticospinal tract (Alstermark et al. 1984; see the sketch in Fig. 8), a convergence for which there is also evidence in humans (see above), and the most parsimonious explanation for the present results would be a reduction in corticospinal facilitation of these inhibitory interneurones during grip. During grip, convergence of the ulnar volley on inhibitory interneurones receiving less corticospinal excitation would explain why the ulnar-induced facilitation did not disappear at high peripheral stimulus intensities (Fig. 4A, filled circles) and at high TMS intensities (Fig. 4E, filled circles). During pointing, ulnar-induced excitation was, if anything, weaker than during selective FCR voluntary contractions. This could be explained if the corticospinal input to feedback inhibitory interneurones was not reduced in this task. Ulnar-induced feedback inhibition to propriospinal neurones could therefore be manifest fully: indeed the feedback inhibition could be even stronger than during selective voluntary contractions of FCR because of the peripheral input that inhibitory interneurones receive from contracting hand muscles.
Significance of the findings
It is postulated that, during grip, the automatic postural activation of the FCR is accompanied by a switch in the descending drives to the relevant propriospinal system. On the one hand, corticospinal excitation of feedback inhibitory interneurones may be reduced with respect to its level during selective voluntary contractions of the target muscle. This would allow the peripheral input from hand muscles to provide effective background excitation of propriospinal neurones, contributing to stabilization of the wrist during grip. On the other hand, the higher threshold of the ulnar-induced facilitation found for the modulation of the TMS-induced responses during grip suggests that TMS is less efficient in activating corticospinal neurones projecting to the relevant propriospinal neurones during grip than during voluntary contractions. There would be therefore a parallel decrease in the corticospinal control of propriospinal neurones and of their feedback inhibitory interneurones. However, the finding that the threshold of the modulation of the ongoing EMG (Fig. 2I) and the PSTHs in single units (Fig. 5E) was the same in the two tasks suggests that the overall excitatory drive to propriospinal neurones remains similar. This could be due to the more effective background excitation of propriospinal neurones by the peripheral input from hand muscles no longer counteracted by excitation of feedback inhibitory interneurones (see above). Alternatively, because of the extensive convergence of excitatory descending tracts onto propriospinal neurones (see Alstermark & Lundberg, 1992 and the sketch in Fig. 8), the reduction of the corticospinal excitation during a postural FCR contraction could be compensated for by an increased activity in other descending tracts projecting to propriospinal neurones (in particular the reticulospinal tract).
Human intrinsic hand muscles are characterized by the absence of propriospinally mediated excitation to the corresponding motoneurones co-existing with a particularly large monosynaptic corticospinal input. This indeed indicates a species differences (Lemon & Griffiths, 2005). However, whatever the mechanisms underlying the task-related changes in propriospinal excitation from hand muscle afferents described in the present investigation, these changes suggest that the propriospinal system in humans plays a functionally significant role. In this connection, the contrasted results observed at high ulnar intensities (increase in propriospinal excitation during grip and pinching, which disappears during voluntary contractions of FCR or pointing while a long-latency group II excitation then appears, Fig. 2C and G) suggest that different pathways from hand muscles could contribute to stabilizing the wrist during different movements: group II excitation mediated through a segmental pathway during voluntary contractions of FCR or pointing versus propriospinally mediated group I excitation (through rostrally located interneurones) during grip and pinching. Grip is the first meaningful task in which the propriospinal system has been investigated in humans. Given the potency of the propriospinally mediated excitation disclosed during this task, it would be of particular interest, when technically possible, to investigate transmission in the propriospinal system during reaching, the task in which it is specifically involved in the cat (see Lundberg, 1999).
Acknowledgments
This work was also supported by grants from Institut pour la Recherche sur la Moelle Épinière (IRME). C.I. was supported by a grant from Università degli Studi di Milano, G.L. by a grant from Institut Garches, and D.B. by the National Health & Medical Research Council of Australia.
References
- Alstermark B, Lundberg A. The C3-C4 propriospinal system: Target reaching and food taking. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferent and Spinal Control of Movement. Oxford-New York-Seoul-Tokyo: Pergamon Press; 1992. pp. 327–354. IBRO Series. [Google Scholar]
- Alstermark B, Lundberg A, Sazaki S. Integration in descending motor pathways controlling the forelimb in the cat. 11. Inhibitory pathways from higher motor center and forelimb afferents to C3–C4 propriospinal neurones. Exp Brain Res. 1984;56:293–307. doi: 10.1007/BF00236285. [DOI] [PubMed] [Google Scholar]
- Burke D. Clinical relevance of the putative C3–4 propriospinal system in humans. Muscle Nerve. 2001;24:1437–1439. doi: 10.1002/mus.1166. [DOI] [PubMed] [Google Scholar]
- Burke D, Dickson HG, Skuse NF. Task-dependent changes in the responses to low-threshold cutaneous afferent volleys in the human lower limb. J Physiol. 1991;432:445–458. doi: 10.1113/jphysiol.1991.sp018393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Convergence of descending and various peripheral inputs onto common propriospinal-like neurones in man. J Physiol. 1992a;449:655–671. doi: 10.1113/jphysiol.1992.sp019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of afferents to propriospinal-like neurones in man during voluntary contractions. J Physiol. 1992b;449:673–687. doi: 10.1113/jphysiol.1992.sp019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histogram. Electroencephalog Clin Neurophysiol. 1978;45:302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Evans AL, Harrison LM, Stephens JA. Task-dependent changes in cutaneous reflexes recorded from various muscles controlling finger movement in man. J Physiol. 1989;418:1–12. doi: 10.1113/jphysiol.1989.sp017825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Harrison LM, Stephens JA. Cutaneomuscular reflexes recorded from the lower limb in man during different tasks. J Physiol. 1995;487:237–242. doi: 10.1113/jphysiol.1995.sp020874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracies JM, Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of propriospinal-like excitation to different species of human upper limb motoneurones. J Physiol. 1991;434:151–167. doi: 10.1113/jphysiol.1991.sp018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol. 1984;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Ohki Y, Seki K, Alstermark B. Properties of propriospinal neurons in the C3–C4 segments mediating disynaptic pyramidal excitation to forelimb motoneurons in the macaque monkey. J Neurophysiol. 2006;95:3674–3685. doi: 10.1152/jn.00103.2005. [DOI] [PubMed] [Google Scholar]
- Kernell D, Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Res. 1990;507:176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- King NCC, Kuppuswamy A, Strutton PH, Davey NJ. Estimation of cortical silent period following transcranial magnetic stimulation using a computerised cumulative sum method. J Neurosci Methods. 2006;150:96–104. doi: 10.1016/j.jneumeth.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Lourenço G, Iglesias C, Cavallari P, Pierrot-Deseilligny E, Marchand-Pauvert V. Mediation of late excitation from human hand muscles via parallel group II spinal and group I transcortical pathways. J Physiol. 2006;572:585–603. doi: 10.1113/jphysiol.2005.102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço G, Iglesias C, Marchand-Pauvert V. Effects produced in human arm and forearm motoneurones after electrical stimulation of ulnar and median nerves at wrist level. Exp Brain Res. 2007;178:267–284. doi: 10.1007/s00221-006-0729-7. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Descending control of forelimb movements in the cat. Brain Res Bull. 1999;50:323–324. doi: 10.1016/s0361-9230(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Malmgren K, Pierrot-Deseilligny E. Evidence for non-monosynaptic Ia excitation of wrist flexor motoneurones, possibly via propriospinal neurones. J Physiol. 1988a;405:747–764. doi: 10.1113/jphysiol.1988.sp017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren K, Pierrot-Deseilligny E. Inhibition of neurones transmitting non-monosynaptic Ia excitation to human wrist flexor motoneurones. J Physiol. 1988b;405:765–783. doi: 10.1113/jphysiol.1988.sp017360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Mazevet D, Pierrot-Deseilligny E, Pol S, Pradat-Diehl P. Handedness-related asymmetry in transmission in a system of human cervical premotoneurones. Exp Brain Res. 1999a;125:323–334. doi: 10.1007/s002210050688. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nicolas G, Pierrot-Deseilligny E. Monosynaptic Ia projections from intrinsic hand muscles to forearm motoneurones in humans. J Physiol. 2000;525:241–252. doi: 10.1111/j.1469-7793.2000.t01-1-00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Simonetta-Moreau M, Pierrot-Deseilligny E. Cortical control of spinal pathways mediating group II excitation to thigh motoneurones. J Physiol. 1999b;517:301–313. doi: 10.1111/j.1469-7793.1999.0301z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Gandevia SC, Taylor JL. Muscle fatigue changes cutaneous suppression of propriospinal drive to human upper limb muscles. J Physiol. 2007;580:211–223. doi: 10.1113/jphysiol.2006.125997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain. 2003;126:988–1000. doi: 10.1093/brain/awg088. [DOI] [PubMed] [Google Scholar]
- Morita H, Olivier E, Baumgarten J, Petersen NT, Christensen LO, Nielsen JB. Differential changes in corticospinal and Ia input to tibialis anterior and soleus motor neurones during voluntary contraction in man. Acta Physiol Scand. 2000;170:65–76. doi: 10.1046/j.1365-201x.2000.00762.x. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Maier MA, Kirkwood PA, Lemon RN. Striking differences in transmission of corticospinal excitation to upper limb motoneurons in two primate species. J Neurophysiol. 2000;84:698–709. doi: 10.1152/jn.2000.84.2.698. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Marchand-Pauvert V, Burke D, Pierrot-Deseilligny E. Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans. J Physiol. 2001;533:903–919. doi: 10.1111/j.1469-7793.2001.t01-1-00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauvert V, Pierrot-Deseilligny E, Rothwell JC. Role of spinal premotoneurones in mediating corticospinal input to forearm motoneurones in man. J Physiol. 1998;508:301–312. doi: 10.1111/j.1469-7793.1998.301br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical premotoneurones. Prog Neurobiol. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord. Its Role in Motor Control and Movement Disorders. Cambridge, New York: Cambridge University Press; 2005. [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, Seki K, Ohki Y. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol. 2004;92:3142–3147. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Modulation of human cervical premotoneurons during bilateral voluntary contraction of upper-limb muscles. Muscle Nerve. 2004a;29:506–514. doi: 10.1002/mus.20003. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke pastients. J Clin Neurophysiol. 2004b;21:426–434. doi: 10.1097/00004691-200411000-00006. [DOI] [PubMed] [Google Scholar]