Abstract
The effects of exercise on skeletal muscle are mediated by a coupling between muscle electrical activity and gene expression. Several activity correlates, such as intracellular Ca2+, hypoxia and metabolites like free fatty acids (FFAs), might initiate signalling pathways regulating fibre-type-specific genes. FFAs can be sensed by lipid-dependent transcription factors of the peroxisome proliferator-activated receptor (PPAR) family. We found that the mRNA for the predominant muscle isoform, PPARδ, was three-fold higher in the slow/oxidative soleus compared to the fast/glycolytic extensor digitorum longus (EDL) muscle. In histological sections of the soleus, the most oxidative fibres display the highest levels of PPARδ protein. When the soleus muscle was stimulated electrically by a pattern mimicking fast/glycolytic IIb motor units, the mRNA level of PPARδ was reduced to less than half within 24 h. In the EDL, a three-fold increase was observed after slow type I-like electrical stimulation. When a constitutively active form of PPARδ was overexpressed for 14 days in normally active adult fibres after somatic gene transfer, the number of I/IIa hybrids in the EDL more than tripled, IIa fibres increased from 14% to 25%, and IIb fibres decreased from 55% to 45%. The level of succinate dehydrogenase activity increased and size decreased, also when compared to normal fibres of the same type. Thus PPARδ can change myosin heavy chain, oxidative enzymes and size locally in muscle cells in the absence of general exercise. Previous studies on PPARδ in muscle have been performed in transgenic animals where the transgene has been present during muscle development. Our data suggest that PPARδ can mediate activity effects acutely in pre-existing adult fibres, and thus is an important link in excitation–transcription coupling.
Patients with metabolic syndrome are characterized by physical inactivity, obesity, cardiovascular disease and type 2 diabetes (Grimaldi, 2005). Such individuals have muscles with reduced oxidative capacity, increased glycolytic capacity and a high percentage of fast muscle fibres (Tanner et al. 2002). Animal experiments suggest that individuals gaining the most weight on high-fat diets are the ones possessing the highest percentage of fast fibres (Abou Mrad et al. 1992).
Muscle fibres can be classified into distinct types based on the myosin heavy chain (MyHC) isoenzyme expressed. MyHC largely determines the fibre's shortening velocity, and the expression patterns of these and other isoenzymes are correlated with the metabolic properties of the fibres. Typically, phenotypic characteristics range from slow/oxidative/small type I fibres to fast/glycolytic/large type IIb fibres. In rodents, IIa and IIx fibres are intermediate forms. Thus, in most muscles, the four types constitute a functional spectrum: I ↔ IIa ↔ IIx ↔ IIb. There are, however, exceptions: for example in rat soleus the IIa fibres are more oxidative than type I fibres (Nakatani et al. 1999).
Fully differentiated post-mitotic muscle fibres can undergo dramatic phenotypic change without degeneration/regeneration when subjected to an altered pattern of electrical activity. Normally, slow/oxidative motor units are active a large fraction of the time, and receive a large number of action potentials delivered in long, low-frequency trains. Fast/glycolytic units are much less active and the activity consists of brief high-frequency bursts of action potentials (Hennig & Lømo, 1985). These patterns maintain a normal phenotype; however, if the activity pattern changes, the gene expression is altered; in particular switching between different MyHC isoforms and between glycolytic and oxidative metabolism.
Several pathways have been suggested to mediate the effects of different patterns of nerve-evoked activity on fibre phenotype, such as during endurance exercise, or in a more controlled way during electrical nerve or muscle stimulation. Such pathways would have to be initiated by an activity correlate such as changes in intracellular free Ca2+, O2 (Mason et al. 2004; Pisani and Dechesne, 2005; Lundby et al. 2006) or metabolites such as free fatty acids (FFAs).
For Ca2+, it has been hypothesized that the long trains of impulses evoked in slow motor units lead to sustained moderate levels of free intracellular Ca2+, activating calcium sensors such as calmodulin, which in turn activates calmodulin-dependent protein kinases (CaMKs). The CaMKs can activate the Ras/Raf/MEK/ERK (Ras activated factor/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase) pathway (Agell et al. 2002), which seems to be involved in fast-to-slow transformation of adult fibres (Murgia et al. 2000). The calmodulin-dependent phosphatase calcineurin can activate transcription factors such as nuclear factor of activated T cells (NFAT) and mouse embryonic fibroblast myocyte enhancer factor 2 (mef-2), both of which have been reported to turn on slow muscle genes (Bassel-Duby & Olson, 2006). Calcineurin might also activate the myogenin promoter (Friday et al. 2003), and myogenin has been shown to increase the oxidative enzyme activity in adult fast muscles (Ekmark et al. 2003). Moreover, calcineurin seems to interact with the peroxisome proliferator-activated receptor (PPAR) transcription factor system. Expression of PPARα, PPARδ and the PPAR cofactor PGC-1α were promoted by expression of a constitutively active form of calcineurin in both fast and slow muscles in transgenic mice (Long et al. 2007).
PPARs are ligand-activated transcription factors and members of the nuclear receptor superfamily. They form dimers with retinoid X receptors (RXRs) and have FFAs and other lipids as ligands; hence they work as lipid sensors. The level of FFAs is elevated in active muscles (Nawrocki & Gorski, 2004), thus PPARs might mediate activity-dependent signalling independently of other signal systems. Three mammalian subtypes of PPARs, α, γ, and δ (also called β), have been identified (Dreyer et al. 1992; Kliewer et al. 1994). PPARα and PPARγ are predominantly expressed in liver and adipose tissue, respectively, while PPARδ is the predominant isoform present in skeletal and heart muscle (Kliewer et al. 1994; Braissant et al. 1996; Escher et al. 2001; Muoio et al. 2002).
Transgenic mice overexpressing wild-type PPARδ selectively in skeletal muscle develop muscles with a higher oxidative capacity, but show no alterations in MyHC fibre type (Luquet et al. 2003). Wang et al. (2004) further explored PPARδ's function in muscle development by expressing a constitutively active form of the protein. They found that such transgenic animals developed muscles with more type I fibres, and they were capable of continuous running of up to twice the distance that wild-type littermates could manage. Similarly, transgenic mice overexpressing the PPAR cofactors PGC-1α or PGC-1β develop muscles with slower/more oxidative muscle fibres (Lin et al. 2002; Arany et al. 2007).
In contrast to previous authors who used transgenic mice in which the transgene is expressed during development, we have used an in vivo transfection technique allowing for acute expression of active PPARδ in pre-existing muscle fibres of adult animals. Under these conditions, transformation of fast fibres towards a slower and more oxidative phenotype was observed. Moreover, we demonstrate that PPARδ expression is increased by a slow electrical activity pattern, while it is decreased by a fast pattern.
Methods
Animal experiments
All surgical procedures were performed on male Wistar rats weighing 200–300 g, under deep anaesthesia after i.p. injections of 5 μl g−1 Equithesin (Sykehusapoteket Rikshospitalet, Norway). Animals were killed by neck dislocation while still deeply anaesthetized. The animal procedures were reviewed and approved by the Norwegian Animal Research Authority and were conducted in accordance with the Norwegian Animal Welfare Act of 20 December 1974, no. 37, Chapter VI, Sections 20–22, and the Regulation of Animal Experimentation of 15 January 1996.
Electrical stimulation
Stimulations of soleus and extensor digitorum longus (EDL) muscles were performed by placing Teflon-coated steel electrodes (AS632, Cooner Sales, Chatsworth) parallel to the sciatic nerve and sutured to the surrounding tissue. Their proximal ends were placed under the skin, exiting through the head via a 6 mm silicon tube fixed to the scalp with steel screws (Frederick Haer and Co, Brunswick, Maine, USA) and dental cement (Swebond, SVEDIA). The electrodes were then connected to a rotating contact approximately 0.5 m above the animal's head to allow free movement in the holding cage, and finally to electrical stimulators.
Stimulation commenced 3 days after implantation. Two different stimulation patterns were used. For EDL, a ‘slow’ pattern mimicking slow type I motor units (Hennig & Lømo, 1985) was used, consisting of 20 Hz pulse trains each lasting 10 s and repeated every 30 s. For soleus, the pattern was designed based on the motoneuronal discharges observed in type IIb motor units (Hennig & Lømo, 1985), and consisted of trains of 25 pulses at 150 Hz every 15 min. For both patterns, the sciatic nerve was stimulated for 3, 6, 12 or 24 h. Sciatic nerves stimulated with the slow electrical pattern were kept intact, while sciatic nerves stimulated with the ‘fast’ electrical pattern and its contralateral control were denervated by removing a 3–5 mm-long segment of the sciatic nerve high up in the thigh, in order to abolish the frequent endogenous slow activity. Denervation was done just prior to the start of stimulation. Sham electrodes were implanted in the contralateral leg. Soleus and EDL muscles of both legs were harvested at the end of stimulation, snap frozen in liquid nitrogen, and stored at −80°C for subsequent RNA analysis.
RNA analysis
RNA was extracted from soleus and EDL muscles with RNAwiz. RNA isolation Reagent (Ambion). Total RNA was then purified on a CsCl gradient. The Roche Lightcycler 1.2 instrument with version 5.32 Lightcycler software and the LightCycler FastStart DNA Master SYBR Green I kit (Roche Applied Science) were used for all reactions. β-actin was used for normalization. The primers were as follows: PPARδ forward, 5′-GCA GGC TCT AGA ATT CCA TC-3′ and PPARδ reverse, 5′-GTG CAG CCT TAG TAC ATG TC-3′; β-actin forward, 5′-ACC AAC TGG GAC GAT ATG GAG AAG A-3′ and β-actin reverse, 5′-CGC ACG ATT TCC CTC TCA GC-3′. Cycling with PPARδ consisted of an initial denaturation step at 95°C for 10 min, 57°C for 10 s and 72°C for 15 s. For β-actin the annealing temperature was optimized to 55°C. A final [Mg2+] of 3 mm was used for all reactions.
Plasmids and plasmid products
The pCMX-VP16-PPARδ plasmid encodes an intrinsically active VP16-PPARδ fusion protein driven by the cytomegalovirus promoter, with VP16-PPARδ inserted into the HindIII/BamHI sites (Umesono et al. 1991; Wang et al. 2004). pAP-lacZ (Kisselev et al. 1995) is a reporter plasmid encoding the Escherichia coliβ-galactosidase sequence driven by the Rouse sarcoma virus promoter.
To confirm the actual transcription and translation of the VP16-PPARδ transgene into a functional protein, human embryonic kidney cells (HEK-293) were transfected with the expression plasmid pCMX-VP16-PPARδ, using a Lipofectamine 2000 kit from Invitrogen. A sham control group was transfected only with the reporter plasmid, pAP-lacZ. Proteins were extracted from the cells as described by Andrews and Faller (1991) and 30 μg of protein from the two groups was run on SDS-PAGE according to Laemmli (1970), followed by Western blotting (BIO-RAD protocol) (Burnette, 1981). The VP16-PPARδ protein was visualized by application of a specific rabbit anti-VP16 IgG primary antibody diluted 1: 1000 (V4388, Sigma) and a goat horse radish peroxidase (HRP) conjugated antirabbit IgG secondary antibody diluted 1: 1000 (ab6721, Abcam), followed by the use of an ECL Western Blotting Detection kit (Amersham).
Somatic gene transfer
In vivo electroporation of muscle fibres was performed essentially as previously described by Mathiesen (1999). Following surgical exposure of the EDL muscle, 100 μl of DNA solution was injected into the interstitium in the centre of the muscle from the distal end, using a U-100 insulin BD Micro-Fine syringe. Subsequently, five trains of 1000 symmetrical bipolar pulses (200 μs in each direction) with a peak-to-peak voltage of 50 V were run across the muscle by two 1 mm-thick cylindrical silver electrodes placed 3–4 mm apart on each side of the muscle.
pCMX-VP16-PPARδ was cotransfected with the pAP-lacZ plasmid into the muscle cells, with pAP-lacZ transfection alone serving as sham control in the contralateral EDL. The total DNA concentration was always 1 g l−1 in 0.9% NaCl, and in the cotransfection studies, the DNA concentration for each of the plasmids was 0.5 g l−1.
DNA used for electroporation was purified using a JETstar Plasmid Purification Maxi kit (Genomed), using the recommended protocol, followed by 1–2 phenol extractions to eliminate proteins.
Histology and quantitative histochemistry
The histological analysis of electroporated muscles was done essentially as we have described before (for method see Ekmark et al. 2003). EDL muscles were surgically excised 5 or 14 days after electroporation. The muscles were slightly stretched before being frozen in melting isopentane and liquid nitrogen, and stored at −80°C. The frozen muscles were cryosectioned at 10 μm in the cryotome (HM560M Microme). Transverse serial sections were mounted on SuperFrost Plus slides (Menzel-Gläser) before being analysed histochemically to determine β-galactosidase expression, MyHC isoform, succinate dehydrogenase (SDH) activity and fibre cross-sectional area (CSA). β-Galactosidase expression was visualized on sections as previously described (Sanes et al. 1991). VP16 expression was determined by applying the same antibody as used on the Western blot, diluted 1: 300 in 1% BSA in PBS and detected with an antirabbit fluorescein IgG diluted 1: 500 in 0.5% BSA in PBS (FI-1000, Vector Laboratories Inc.). EDL and soleus muscles from non-transfected rats were analysed to determine PPARδ protein by applying a PPARδ antibody to the sections (Sc-7197, Santa Cruz Biotechnology), diluted 1: 100 in 1% BSA in PBS and detected with the same secondary antibody as for the VP16 staining. MyHC type was determined using the following panel of monoclonal antibodies (Schiaffino et al. 1989): BA-D5 (I), SC-71 (IIa), BF-F3 (IIb) and BF-35 (non-IIx). The SDH activity was visualized histochemically as described by Bancroft (1975), and quantified by measurement of the grey tone (scale 0–255) of manually encircled muscle fibres in ImageJ (NIH). The light and camera settings were standardized, and the scale was standardized individually for all sections as the mean grey value of normal IIb fibres was subtracted from all grey values and divided by the average IIa grey value; in effect average IIb values were set to 0, while that of normal IIa fibres was set to 1. Thus, the SDH activity of experimental fibres was presented relative to that of normal IIb and IIa fibres from the same section. The CSA of muscle fibres was determined by manually encircling individual fibres in ImageJ.
Imaging
Bright-field images were taken using a CCD video camera (C2400, Hamamatsu) connected to a microscope (BX50W1, Olympus), while fluorescence images were taken using a SIT video camera (C2400-08, Hamamatsu). The images were digitalized through an image-processing unit (Argus-20, Hamamatsu) prior to transferral to a Macintosh computer and further analyses in Photoshop (Adobe) or ImageJ (NIH).
Statistics
Differences in mRNA levels between soleus and EDL were tested with a Mann–Whitney t test. Differences in mRNA levels after stimulation, CSA and SDH activity levels were tested using one-way ANOVA and post testing with Bonferroni correction. Differences in fibre-type distribution were tested using Fisher's exact test.
Results
PPARδ is found in highest concentration in the most oxidative fibres
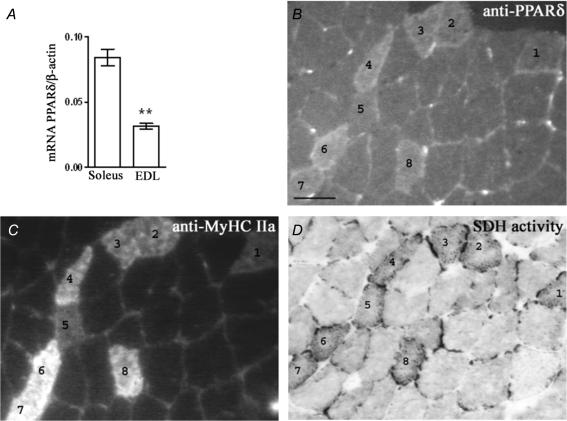
Homogenates from muscles with a high content of slow/oxidative fibres have been shown to have higher levels of PPARδ protein than typical fast/glycolytic muscles (Wang et al. 2004; Long et al. 2007) and we found that the level of PPARδ mRNA is almost three times higher in the slow/oxidative soleus than in the fast/glycolytic EDL (Fig. 1A). PPARδ expression has, to our knowledge, previously only been investigated in muscle homogenates, and in order to present a more detailed picture, we also stained normal muscles with a PPARδ antibody and antibodies against MyHC and for SDH activity (Fig. 1B–D). The rat soleus contains essentially type I and some IIa fibres, and the IIa fibres are the most oxidative (Nakatani et al. 1999). All IIa fibres displayed stronger PPARδ staining than type I fibres. Furthermore, within the IIa group, the fibres that stained weakly for PPARδ also had lower oxidative activity (for example no. 5 in Fig. 1). In the EDL we were unable to discern differences in PPARδ staining between the different fast fibre types (data not shown). We conclude that the PPARδ level seems to correlate with MyHC expression and high oxidative capacity, at least in the soleus muscle.
Figure 1. PPARδ is mainly expressed in oxidative muscle fibres.
A, PPARδ mRNA level relative to β-actin as mean ±s.e.m. in six normal slow/oxidative solei and six fast glycolytic EDL muscles. B–D, serial cross sections of soleus stained for PPARδ (B), MyHC IIa (C) and SDH activity (D). The type IIa fibres are marked 1–8, the rest are type I fibres. In rats, soleus type IIa fibres have higher oxidative capacity than type I fibres. Scale bar: 50 μm **P < 0.01; statistically different from soleus muscle mRNA level.
PPARδ expression is regulated by electrical activity
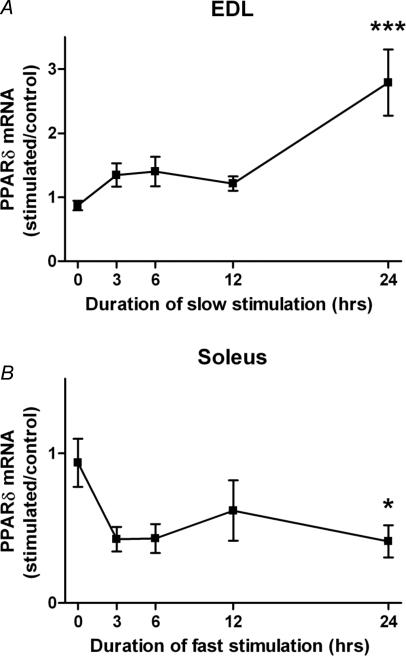
In order to investigate if activity per se affects the level of PPARδ expression, we subjected the fast EDL to a slow pattern (see Methods) of action potentials that over time will induce a slower/more oxidative fibre phenotype. After 24 h of stimulation, this pattern increased the mRNA level of PPARδ three-fold, while only small effects were observed after 3–12 h of stimulation (Fig. 2A).
Figure 2. PPARδ mRNA levels change in response to different patterns of electrical activity.
A, a slow pattern of electrical stimulation increases the mRNA level of PPARδ in the EDL relative to the contralateral non-stimulated leg, and B, a fast pattern had the opposite effect on the soleus. For animals where the soleus was studied, the sciatic nerve was cut at the onset of stimulation in order to avoid the large amounts of activity from the CNS delivered to this muscle (Hennig & Lømo, 1985). Each data point represents the mean ±s.e.m. of six observations. *P < 0.05; ***P < 0.001; statistically different from normal muscles.
We also stimulated the soleus muscle with a fast pattern of action potentials. In this case, the muscles had to be denervated in order to prevent the high levels of background activity in slow motor units; however, the transmission was intact within the 24 h period of stimulation. Denervation itself had little effect on the PPARδ mRNA level (data not shown), and the stimulated muscles were compared to the denervated contralateral muscles. The fast activity pattern reduced the levels of PPARδ mRNA to less than half of the control values (Fig. 2B). The response seemed faster than for EDL, but the effect was only statistically significant after 24 h of stimulation.
We conclude that PPARδ expression is increased by an activity pattern that induces slow/oxidative properties, and reduced by a pattern inducing fast/glycolytic properties. The effects are probably not coupled to systemic effects of training, since only a small group of muscles in one leg was stimulated, and since similar effects were not observed in the contralateral control muscles.
Recombinant VP16-PPARδ is expressed in muscle fibres after electroporation
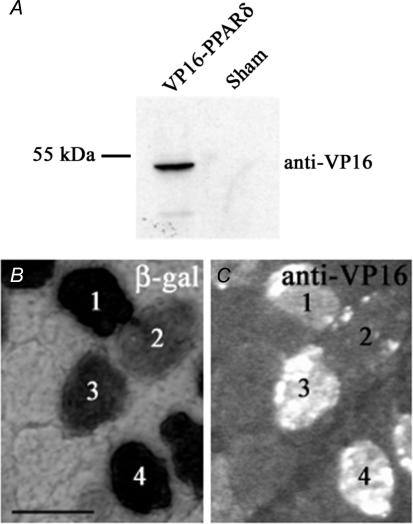
We first checked for expression of VP16-PPARδ after transfection of pCMX-VP16-PPARδ into HEK-293 cells. Nuclear protein extracts were visualized on a Western blot using a VP16 antibody, demonstrating that pCMX-VP16-PPARδ-transfected cells displayed a fusion protein of the expected size: 53 kDa (Fig. 3A).
Figure 3. Transfection with pCMX-VP16-PPARδ led to expression of VP-16-PPARδ fusion protein.
A, nuclear protein extracts from HEK-293 cells transfected with pCMX-VP16-PPARδ, but not from pAP-lacZ sham transfected cells, yielded a 53 kDa band, the expected size of the fusion protein, on a Western blot probed with a VP16 antibody. B and C, on serial cross sections of EDL muscles cotransfected with pCMX-VP16-PPARδ and pAP-lacZ, β-galactosidase-positive fibres also displayed VP16 staining (numbered fibres). Fibres staining weakly for β-galactosidase also stained weakly for VP16 (e.g. fibre 2). Scale bar: 50 μm.
We also confirmed that the fibres in the cotransfected muscles that expressed lacZ, also expressed VP16-PPARδ (Fig. 3B and C). The sensitivity of the antibody staining is probably lower than for the β-galactosidase enzymatic staining, and in some β-galactosidase-stained fibres, VP16-PPARδ was not detectable. We did not detect VP16 in any β-galactosidase-negative fibres. As discussed previously, essentially all fibres transfected with two plasmids probably express both after electroporation (Rana et al. 2004). We cannot, however, exclude that some of the negative control fibres in the present study expressed recombinant protein at levels that were not detectable; however, the control fibres in muscles transfected with VP16-PPARδ were no different from controls in sham transfected muscles (see below).
Active PPARδ induces smaller and more oxidative fibres
Since PPARδ protein level showed a correlation to an oxidative phenotype and responded to activity patterns that would alter the muscle phenotype, we investigated if elevated levels of an active PPARδ could induce slow/oxidative properties in a typical fast muscle of adult rats. To this end, we investigated muscle fibres cotransfected with a constitutively active VP16-PPARδ fusion protein and a β-galactosidase reporter protein in adult EDL muscles. In addition, an equivalent number of randomly selected normal, non-transfected fibres from the same muscles served as internal controls. pAP-lacZ-transfected fibres in the contralateral EDL expressing β-galactosidase alone served as sham control. Normal control fibres were pooled into one group as there were no statistical differences between them in any of the tested parameters, and statistical analyses, with or without pooling the data, yielded the same result. The same applied to sham fibres.
In some muscles, minor areas appeared damaged by the electroporation, and such areas were excluded from the analysis. No embryonic myosin indicative of regenerating fibres was detected when staining with the BF-G6 antibody (Schiaffino et al. 1986) (data not shown).
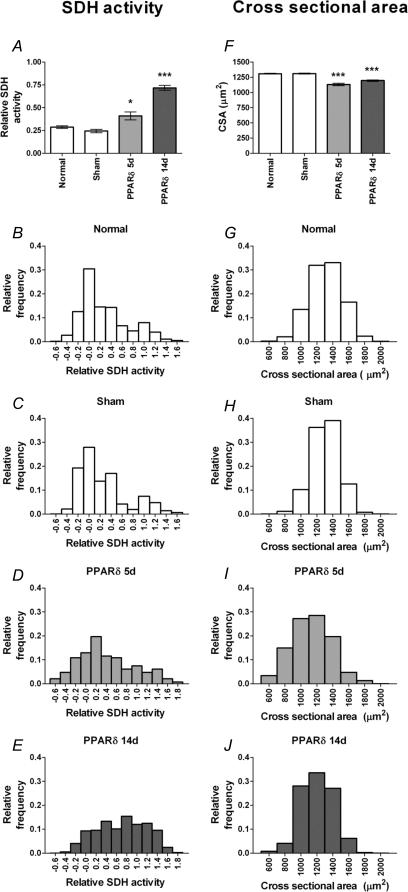
After 5 days, the average SDH value (see Methods) of PPARδ-transfected fibres was increased by 43%, and after 14 days the SDH value was 2.5 times higher than that of normal fibres. Sham transfected fibres were similar to normal fibres both at 5 and 14 days (Fig. 4A–E).
Figure 4. Forced VP16-PPARδ expression induces an oxidative phenotype.
Succinate dehydrogenase (SDH) activity level (A–E) and cross-sectional area (F–J) after 5 and 14 days of treatment with PPARδ, sham transfection, and in non-transfected (normal) fibres given as mean ±s.e.m. (A and F) and as distributions of the 2124 and 2096 fibres from 18 different EDL muscles, respectively (B–E and G–J). For SDH scale see Methods. *P < 0.05; ***P < 0.001; statistically different from normal non-transfected fibres.
Consistent with these results, we found that the average CSA of PPARδ-transfected fibres was reduced after 5 days by 14%, from 1311 μm2 to 1131 μm2, and on average somewhat less reduced after 14 days (Fig. 4F–J).
Thus, the active PPARδ induces two traits typical of oxidative fibres: (1) higher SDH activity shifting the metabolism towards a more oxidative phenotype, and (2) smaller size, facilitating oxygen diffusion.
Active PPARδ shifts the MyHC fibre type distribution to slower isoforms
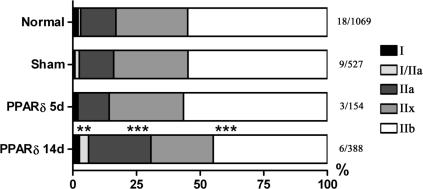
As the most oxidative fibres in rat muscles express MyHC type I and IIa, we next investigated if active PPARδ had an effect on MyHC fibre-type distribution. Among the fibres transfected with PPARδ, the number of I/IIa hybrids was more than tripled, the number of IIa fibres increased from 14% to 25% and the number of IIb fibres was reduced from 55% to 45%, when compared to normal controls 14 days after transfection (Fig. 5). Thus, the main effect was a significant increase in the number of IIa fibres and a decrease in the number of IIb fibres. Five days after transfection, active PPARδ had no effect on MyHC fibre-type distribution. No significant increase in pure type I fibres was observed in this study; however, the increased number of type I hybrids suggests that, given more time, active PPARδ might have induced type II to I conversion in EDL.
Figure 5. VP16-PPARδ induces MyHC fibre-type transformation in the slow direction in rat EDL.
Fibre types were defined according to MyHC expression on transverse serial sections (see Methods) and the distribution is given in percentages. Data are given for a total of 2138 fibres from 18 different EDL muscles 5 or 14 days after transfection and in sham and normal control groups. Muscles (n/number of fibres) for each group as indicated. **P < 0.01; ***P < 0.001; statistically different from the normal distribution.
PPARδ induces smaller and more oxidative fibres within the same MyHC fibre type
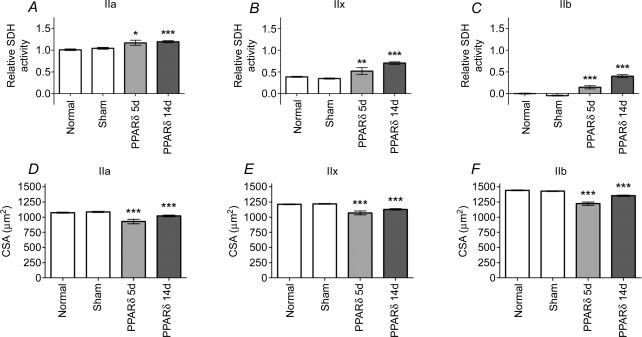
The finding that the active PPARδ influenced MyHC expression could imply that the observed changes in size and SDH activity reflected a type conversion and not a regulation of oxidative capacity per se. We therefore investigated whether SDH activity and size were different in PPARδ transfected and normal fibres of the same MyHC type.
For type IIa, IIx and IIb fibres, a significant increase in SDH activity (Fig. 6A–C) and a decrease in size (Fig. 6D–F) were observed in PPARδ-tranfected fibres compared to sham transfected and normal controls of the same type. After 14 days, IIb fibres, which normally have very low levels of SDH activity, reached levels similar to those found in normal IIx fibres. In PPARδ-transfected IIx fibres, the oxidative capacity was increased by 82% and the size reduced by 7%, and in IIa fibres the oxidative capacity was increased by 20%, and the size decreased by 5%, compared to normal fibres of the same type. Thus, PPARδ had clear effects on oxidative traits even in fibres of the same MyHC type. In terms of relative changes, the effects were most dramatic in types that are normally relatively glycolytic, such as IIb and IIx fibres; however, at 14 days these types still remained much less oxidative than normal type I and IIa fibres in spite of overexpressing the active PPARδ.
Figure 6. VP16-PPARδ expression fibres of the same MyHC type have a more oxidative phenotype and a smaller cross-sectional area.
A–C, relative SDH activity levels and D–F, cross-sectional areas of PPARδ fibres 5 and 14 days after transfection, and in sham transfected and non-transfected (normal) controls, given as mean ±s.e.m. for individual fibre types of 2025 and 2052 fibres from 18 different EDL muscles, respectively. For SDH scale see Methods. *P < 0.05; **P < 0.01; ***P < 0.001; statistically different from normal levels of the same fibre type.
Discussion
The present data suggest that PPARδ is linking slow electrical muscle activity to a slow/oxidative conversion of fibre type in adult muscle. This conclusion is based on three findings: (1) PPARδ protein was found in high levels in the slow/oxidative soleus muscle, in particular in type IIa fibres which have the highest oxidative capacity; (2) fast/glycolytic EDL muscles subjected to a slow activity pattern rapidly started expressing more PPARδ, while slow soleus muscles subjected to a fast activity pattern expressed less; and (3) forced expression of active PPARδ in adult fast muscles fibres induced fibre-type transformation in the slow/oxidative direction, and also increased oxidative capacity irrespective of fibre type.
More specifically, the same activity patterns that we found to be efficient in elevating PPARδ expression in the present study, elicit changes similar to the effects of forced expression of active PPARδ in normally active fibres reported here, such as more IIa fibres and higher oxidative capacity (Leberer et al. 1987; Windisch et al. 1998).
Swimming exercise increased muscle PPARδ mRNA in rats on a high-fat diet, but had no effect in lean rats (Kannisto et al. 2006). In normally fed mice, PPARδ protein increased significantly after three weeks of moderate treadmill exercise, but short-term effects were not investigated in this study (Luquet et al. 2003). In a microarray study on man, the PPARδ signal was found to be elevated during recovery after a single bout of cycling exercise to exhaustion (Mahoney et al. 2005). We observed increased expression after 24 h, and since only a limited group of muscles below the knee in one leg was stimulated in otherwise normally active rats, and since the effects were compared to contralateral muscles, these experiments link PPARδ expression to muscle activity per se. Systemic factors accompanying full-body exercise triggered from the CNS seem not to be required, and muscle electrical activity might affect PPARδ in a muscle autonomous fashion.
While a slow pattern induced higher PPARδ expression, a fast pattern had the opposite effect. The latter seemed to be a specific effect of fast-patterned activity, and not to a reduced level of activity, since muscles in the contralateral leg that were denervated did not show similar changes. It is possible that, for example, the high instantaneous frequency of action potentials in the fast pattern (see Methods) actively regulates the PPARδ gene similar to what we have discussed previously for the isoforms of troponin I (Rana et al. 2005). The enhancers for fast and slow isoforms of troponin I were activated by matching fast and slow activity patterns, respectively, while removal of nerve-evoked activity by denervation reduced transcriptional activity of both enhancers. These results strongly suggested that distinct signalling pathways can couple both fast and slow patterns of activity to different enhancers.
How the pattern of muscle action potential activity influences PPARδ expression is still unclear. PPARα, PPARδ and PGC-1α is promoted by expression of a constitutively active form of calcineurin in transgenic mice (Long et al. 2007). This could be an indirect effect, or an effect on development, but calcineurin is currently one of the major candidates for a link between slow activity patterns and activation of slow genes (Bassel-Duby & Olson, 2006) involving other transcription factors such as NFAT, Mef-2 (Bassel-Duby & Olson, 2006) and myogenin (Ekmark et al. 2003; Friday et al. 2003). PPARδ might be similarly regulated by activity.
Systemic treatment with the PPARδ agonist GW501516 has been shown to alter muscle metabolic properties (Dressel et al. 2003; Tanaka et al. 2003; Wang et al. 2004). Our study indicates that the effects of PPARδ on muscle phenotype are intrinsic to the muscle fibres; systemic effects accompanying drug administration, physical exercise, diet, or alterations in systemic availability of lipids are probably not necessary. We used, however, a constitutively active VP16 fusion protein in the overexpression experiments. In vivo, wild-type PPARδ might regulate muscle fibre type by integrating information about the cells' activity pattern, leading to altered levels of the transcription factor (this paper), and ligand availability, that is also activity dependent. In other words, our data suggest that activity regulates the lipid sensitivity of the PPAR gene-regulatory system in muscle fibres.
Conclusions
In contrast to previous reports from transgenic mice where the transgene is expressed during development, we have studied the acute expression of active PPARδ in individual adult, pre-existing muscle fibres, and demonstrate that PPARδ transforms fast fibres towards a slower and more oxidative phenotype. Thus, elevated PPARδ activity is a sufficient condition for transforming adult fibres in the slow direction. We also demonstrate that PPARδ expression is regulated by muscle electrical activity in a pattern-dependent fashion, suggesting that PPARδ is an important local mediator of the changes in phenotype that can be observed upon altered muscle activity, such as after physical exercise.
Acknowledgments
We thank Dr Ronald M. Evans, San Diego for donating the pCMX-VP16-PPARδ plasmid, Dr Stefano Schiaffino, Padova, for donating MyHC antibodies and Tove Klungervik Larsen for technical assistance. We are grateful for comments on the manuscript from Dr Jo C. Bruusgaard, Ida B. Johansen and Professor Arild Rustan. This work was supported by grant 170473 from The Research Council of Norway.
References
- Abou Mrad J, Yakubu F, Lin D, Peters JC, Atkinson JB. Skeletal muscle composition in dietary obesity-susceptible and dietary obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 1992;262:R684–R688. doi: 10.1152/ajpregu.1992.262.4.R684. [DOI] [PubMed] [Google Scholar]
- Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and calmodulin. Cell Signal. 2002;14:649–654. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIx fibers in skeletal muscle. Cell Metab. 2007;5:35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Bancroft JD. Histochemical Techniques. London: Butterworths; 1975. [Google Scholar]
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Burnette WN. Western Blotting-electrophoretic transfer of proteins from sodium Dodecyl Sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein-A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17:2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- Ekmark M, Gronevik E, Schjerling P, Gundersen K. Myogenin induces higher oxidative capacity in pre-existing mouse muscle fibres after somatic DNA transfer. J Physiol. 2003;548:259–269. doi: 10.1113/jphysiol.2002.036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142:4195–4202. doi: 10.1210/endo.142.10.8458. [DOI] [PubMed] [Google Scholar]
- Friday BB, Mitchell PO, Kegley KM, Pavlath GK. Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation. 2003;71:217–227. doi: 10.1046/j.1432-0436.2003.710303.x. [DOI] [PubMed] [Google Scholar]
- Grimaldi PA. Regulatory role of PPAR delta in muscle metabolism. A new target for metabolic syndrome treatment? Biochimie. 2005;87:5–8. doi: 10.1016/j.biochi.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Kannisto K, Chibalin A, Glinghammar B, Zierath JR, Hamsten A, Ehrenborg E. Differential expression of peroxisomal proliferator activated receptors alpha and delta in skeletal muscle in response to changes in diet and exercise. Int J Mol Med. 2006;17:45–52. [PubMed] [Google Scholar]
- Kisselev O, Pronin A, Ermolaeva M, Gautam N. Receptor–G protein coupling is established by a conformational switch in the beta gamma complex. Proc Natl Acad Sci U S A. 1995;92:9102–9106. doi: 10.1073/pnas.92.20.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leberer E, Staron RS, Gundersen K, Lømo T, Pette D. Control of parvalbumin expression in rat skeletal muscle by motor-unit specific activity patterns. In: Norman AW, Vanaman TC, Means AR, editors. Calcium-binding Proteins in Health and Disease. New York: Academic Press; 1987. pp. 287–289. [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Long YC, Glund S, Garcia-Roves PM, Zierath JR. Calcineurin regulates skeletal muscle metabolism via coordinated changes in gene expression. J Biol Chem. 2007;282:1607–1614. doi: 10.1074/jbc.M609208200. [DOI] [PubMed] [Google Scholar]
- Lundby C, Gassmann M, Pilegaard H. Regular endurance training reduces the exercise induced HIF-1alpha and HIF-2alpha mRNA expression in human skeletal muscle in normoxic conditions. Eur J Appl Physiol. 2006;96:363–369. doi: 10.1007/s00421-005-0085-5. [DOI] [PubMed] [Google Scholar]
- Luquet S, Lopez-Soriano J, Holst D, Gaudel C, Jehl-Pietri C, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17:2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- Mahoney DJ, Parise G, Melov S, Safdar A, Tarnapolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005;19:1498–1500. doi: 10.1096/fj.04-3149fje. [DOI] [PubMed] [Google Scholar]
- Mason SD, Howlett RA, Kim MJ, Olfert IM, Hogan MC, McNulty W, Hickey RP, Wagner PD, Kahn CR, Giordano FJ, Johnson RS. Loss of skeletal muscle HIF-1alpha results in altered exercise endurance. PLoS Biol. 2004;2:e288. doi: 10.1371/journal.pbio.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 1999;6:508–514. doi: 10.1038/sj.gt.3300847. [DOI] [PubMed] [Google Scholar]
- Muoio DM, MacLean PS, Lang DB, Li S, Houmard JA, Way JM, Winegar DA, Corton JC, Dohm GL, Kraus WE. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor alpha knock-out mice. J Biol Chem. 2002;277:26089–26097. doi: 10.1074/jbc.M203997200. [DOI] [PubMed] [Google Scholar]
- Murgia M, Serrano L, Calabria E, Pallafacchina G, Lømo T, Schiaffino S. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nature Cell Biol. 2000;2:142–147. doi: 10.1038/35004013. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Nakashima T, Kita T, Hirofuji C, Itoh K, Itoh M, Ishihara A. Succinate dehydrogenase activities of fibers in the rat extensor digitorum longus, soleus, and cardiac muscles. Arch Histol Cytol. 1999;62:393–399. doi: 10.1679/aohc.62.393. [DOI] [PubMed] [Google Scholar]
- Nawrocki A, Gorski J. Effect of plasma free fatty acid concentration on the content and composition of the free fatty acid fraction in rat skeletal muscles. Hormone Metab Res. 2004;36:601–606. doi: 10.1055/s-2004-825922. [DOI] [PubMed] [Google Scholar]
- Pisani DF, Dechesne CA. Skeletal muscle HIF-1alpha expression is dependent on muscle fiber type. J Gen Physiol. 2005;126:173–178. doi: 10.1085/jgp.200509265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana ZA, Ekmark M, Gundersen K. Coexpression after electroporation of plasmid mixtures into muscle in vivo. Acta Physiol Scand. 2004;181:233–238. doi: 10.1111/j.1365-201X.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- Rana ZA, Gundersen K, Buonanno A, Vullhorst D. Imaging transcription in vivo: distinct regulatory effects of fast and slow activity patterns on promoter elements from vertebrate troponin I isoform genes. J Physiol 562. 2004;3:815–828. doi: 10.1113/jphysiol.2004.075333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Johnson YR, Kotzbauer PT, Mudd J, Hanley T, Martinou JC, Merlie JP. Selective expression of an acetylcholine receptor-lacZ transgene in synaptic nuclei of adult muscle fibers. Development. 1991;113:1181–1191. doi: 10.1242/dev.113.4.1181. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Carli M. Embryonic myosin heavy chain as a differentiation marker of developing human skeletal muscle and rhabdomyosarcoma. A monoclonal antibody study. Exp Cell Res. 1986;163:211–220. doi: 10.1016/0014-4827(86)90574-4. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282:E1191–E1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Zhang C-LYuRT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPAR delta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch A, Gundersen K, Szabolcs MJ, Gruber H, Lomo T. Fast to slow transformation of denervated and electrically stimulated rat muscle. J Physiol. 1998;510:623–632. doi: 10.1111/j.1469-7793.1998.623bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]