Abstract
The TRP superfamily of cation channels encompasses 28 mammalian members related to the product of the Drosophila trp (transient receptor potential) gene. TRP channels have a widespread distribution in many cell types and organs and gate in response to a broad variety of physical and chemical stimuli; as such, they can be considered as ubiquitous cellular sensors. Several recent studies reported modulation of different TRP channels by phosphoinositides, in particular by phosphatidylinositol 4,5-bisphosphate (PIP2). In most cases, PIP2 promotes TRP channel activation. Here we provide a brief overview of current insights and controversies about the mechanisms and structural determinants of PIP2–TRP channel interactions, and zoom in on the regulation of the Ca2+- and voltage-gated TRPM4 by phosphoinositides.
In 1969, Cosens & Manning performed a screening for Drosophila mutants with impaired vision and identified a mutant that was rapidly blinded by bright light (Cosens & Manning, 1969). Subsequent electrophysiological analysis of the photoreceptor cells of the mutant fly strain revealed that sustained light induced a transient rather than the normal sustained receptor potential, and the mutant was therefore named trp, for transient receptor potential (Minke et al. 1975). The trp gene was cloned in 1989 (Montell & Rubin, 1989), and soon thereafter it became clear that the trp gene product, TRP, forms a Ca2+-permeable cation channel (Hardie & Minke, 1992; Phillips et al. 1992). In the years following the cloning of the trp gene, it became clear that TRP is the founding member of a large family of cation channels. Published genomes reveal the presence of multiple trp-related genes in yeast, worms, insects, fish and mammals (Vriens et al. 2004; Montell, 2005b), and there are 27 trp-related genes in humans. Based on sequence homology, the mammalian TRP channels can be subdivided into the TRPC, TRPV, TRPM, TRPP, TRPML and TRPA subfamilies (Montell, 2005b; Nilius & Voets, 2005). A further subfamily, the TRPN channels, does not contain any mammalian member.
TRP channels are homo- or heterotetramers of subunits containing six transmembrane segments (S1–S6) and cytoplasmic N- and C-terminal tails. S5, S6 and the connecting pore loop form the central cation-conducting pore, whereas S1–S4 and the cytoplasmic N- and C-terminal parts are involved in the regulation of channel gating and the interaction with ligands or proteins (Voets et al. 2005; Owsianik et al. 2006). Overall, the transmembrane configuration of TRP channels is highly similar to that of other six-transmembrane (6TM) channels such as voltage-gated and Ca2+-activated K+ channels, cyclic nucleotide-gated (CNG) cation channels, hyperpolarization-activated cyclic-nucleotide-gated (HCN) channels and to the four repeats in voltage-gated Ca2+ and Na+ channels (Swartz, 2004; Voets et al. 2005). Given that several of these related 6TM channels are regulated by the plasma membrane PIP2 levels (Hilgemann et al. 2001), it came as no surprise that the gating of at least some of the TRP channels is PIP2 dependent.
TRP channels and phosphoinositides: consensus and controversies
Already at the time of its cloning, it was realized that the gating of TRP in the Drosophila photoreceptor cells is tightly coupled to PIP2 metabolism. In these cells, TRP and the closely related TRPL (TRP-like) (Phillips et al. 1992) function as receptor-operated channels that are activated downstream of the G-protein-coupled receptor rhodopsin. Light-induced activation of rhodopsin leads to the Gαq-dependent activation of phospholipase C (PLC), which mediates hydrolysis of PIP2 into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) (Hardie & Raghu, 2001). Although the exact gating mechanism of TRP/TRPL in the photoreceptor cells remains unsettled, there is good evidence that DAG or polyunsaturated fatty acids derived from DAG can directly gate these channels (Chyb et al. 1999). PIP2 by itself may also have an inhibitory effect on heterologously expressed TRPL (Estacion et al. 2001), but it is unlikely that PIP2 depletion plays a crucial role in the activation of TRP/TRPL in vivo. In fact, Ca2+ influx through TRP is required for maintaining PIP2 levels in the photoreceptors. The exact mechanisms whereby Ca2+ influx sustains the cellular PIP2 levels are not fully known, and may involve both an inhibition of Drosophila PLC by the high micromolar intracellular Ca2+, and a Ca2+-dependent facilitation of PIP2 recycling (Hardie et al. 2001).
Among mammalian TRP channels, at least seven have been reported to be regulated by the cellular PIP2 levels. Julius and colleagues presented data suggesting an inhibitory effect of PIP2 on the heat- and capsaicin-sensitive TRPV1, and proposed that pro-algesic agents such as nerve growth factor (NGF) or bradykinin sensitize TRPV1 by reducing plasma membrane PIP2 levels following PLC activation (Chuang et al. 2001). A C-terminal region of TRPV1 rich in basic residues was found to be required for NGF-induced sensitization of the channel, suggesting that it may function as a PIP2 binding site (Prescott & Julius, 2003). Although this mechanism represents an appealing modus operandi of pro-algesic compounds, recent studies have strongly questioned the inhibitory effect of PIP2 on TRPV1. First, it was found that PIP2 levels are strongly reduced upon TRPV1 activation, and that the recovery of TRPV1 activity after desensitization requires and coincides with recovery of cellular PIP2 levels (Liu et al. 2005). Notably, current recovery was independent of the putative C-terminal PIP2 binding site (Liu et al. 2005). In addition, another study showed that direct application of PIP2 to the inner leaflet of TRPV1-expressing membranes leads to channel activation rather than inhibition, whereas sequestering PIP2 using polylysine inhibits channel function (Stein et al. 2006). The authors of this latter study suggested an alternative mechanism for NGF-induced TRPV1 potentiation, whereby NGF leads to phosphoinositide 3-kinase-dependent trafficking of TRPV1 to the plasma membrane (Stein et al. 2006). Although these studies seem strongly at odds with the initially proposed inhibitory effect of PIP2 on TRPV1, unpublished data suggest that PIP2 may have a dual effect on TRPV1: inhibitory at low agonist concentrations and activatory in the presence of stronger TRPV1-activating stimuli (Lukacs et al. 2007).
A second mammalian TRP reported to be modulated by PIP2 is the Mg2+ and Mg2+-nucleotide-regulated cation channel TRPM7, although this modulation has also been subsequently challenged. One study reported that receptor-mediated inhibition of TRPM7 is mediated by PLC-induced PIP2 breakdown (Runnels et al. 2002). However, a later study found that overexpression of TRPM7, which was originally identified as a PLC-interacting protein, leads to a full suppression of PLC activity, and presented evidence that receptor stimulation modulates TRPM7 via cAMP signalling (Takezawa et al. 2004). Further research will be required to establish whether both mechanisms of TRPM7 are operational in vivo, and which of them is most predominant.
Less controversial (at least to this point) are the stimulatory effects of PIP2 on TRPV5, TRPM4, TRPM5 and TRPM8. TRPV5 is a highly Ca2+-selective epithelial channel, mainly involved in Ca2+ transport processes in epithelia (Hoenderop et al. 2005). Intracellular Mg2+ ions inhibit TRPV5, both through a direct and fully reversible voltage-dependent block of the pore and by inducing a slower, pore-independent, and irreversible channel rundown (Voets et al. 2003). This rundown can be reversed by stimulation of the resynthesis of PIP2 or by direct application of PIP2 to the inner leaflet of the plasma membrane. Moreover, PIP2 reduces the channel's sensitivity to slow Mg2+ inhibition (Lee et al. 2005). TRPM8 is a voltage-dependent channel activated by cold and by cooling agents such as menthol (McKemy et al. 2002; Peier et al. 2002; Voets et al. 2004). Activation of the channel leads to desensitization, which depends on Ca2+ influx (McKemy et al. 2002). Ca2+ influx through TRPM8 leads to activation of Ca2+-dependent phospholipase C (e.g. PLCδ1) (Rohacs et al. 2005), which causes depletion of cellular PIP2 levels resulting in channel desensitization (Liu & Qin, 2005; Rohacs et al. 2005), mainly by shifting the voltage dependence of channel activation to more positive potentials. Positively charged residues in the TRP domain, which is located C-terminal of S6, appear to play a role in the interaction of the channel with membrane-bound PIP2, suggesting that this conserved region may play a more general role as a PIP2-interacting site (Rohacs et al. 2005).
TRPM4 and TRPM5 are voltage-dependent Ca2+-activated but Ca2+-impermeable cation channels (Launay et al. 2002; Hofmann et al. 2003; Nilius et al. 2003). TRPM5 is highly expressed in taste cells, where its activation downstream of taste receptor stimulation leads to membrane depolarization, which appears crucial in the transduction of sweet, bitter and umami taste (Zhang et al. 2003). TRPM4 has a more widespread expression pattern and appears to function as a brake on Ca2+ influx in non-excitable cells, by reducing the driving force for Ca2+ influx through non-voltage-gated Ca2+-permeable channels (Launay et al. 2002). This was recently nicely illustrated in TRPM4−/– mice, which exhibit increased anaphylactic responses due to increased IgE-induced Ca2+ influx and histamine release in mast cells that lack the TRPM4 brake (Vennekens et al. 2007). Analogous to what has been described for TRPM8, a rise in the intracellular Ca2+ concentration, which is an absolute prerequisite for TRPM4/5 activation, leads to desensitization of both channels, and this desensitization process can be reversed by application of PIP2 to the inner leaflet of the plasma membrane (Liu & Liman, 2003; Nilius et al. 2006). Below, we will discuss in more detail the current insight on the functional interaction between phosphoinositides and TRPM4.
Modulation of TRPM4 by PIP2
To analyse the effect of phosphoinositides on TRPM4, we have used several protocols expected to affect the plasma membrane PIP2 content and/or its breakdown (Nilius et al. 2006). Application of the synthetic steroid U73122, an inhibitor of PLC activity, fully prevented the decay of TRPM4 current that normally follows Ca2+-dependent activation in whole-cell and inside-out patches, indicative of an involvement of Ca2+-dependent PLC activity and PIP2 depletion in this process. Consistent with this hypothesis, we found that direct application of PIP2 to the inner leaflet of the plasma membrane fully restores the initial TRPM4 current after current decay. Similarly, we observed that application of Mg-ATP to inside-out patches led to full restoration of TRPM4 currents, which can be attributed to Mg-ATP-dependent activation of the different PI(P) kinases that lead to replenishment of the PIP2 pool. In contrast, a number of experimental approaches that promote depletion of the plasma PIP2 pool all led to a reduction of TRPM4 currents. These include direct application of the PIP2 scavenger polylysine to inside-out patches, inhibition of PIP2 synthesis by the PI(P) kinase inhibitor wortmannin, and overexpression of 5-phosphatase IV, which catalyses the dephosphorylation of PIP2 to phosphatidyl 4-phosphate. Interestingly, our results indicate that several enzymes involved in PIP2 synthesis and breakdown remain active in inside-out patch-clamp experiments for several minutes, suggestive of a tight coupling between TRPM4 and these enzymes (Nilius et al. 2006). This raises the possibility of a configuration similar to the so-called ‘signalplex’ that couples TRP and TRPL to the different components of the rhodopsin-induced signal transduction pathway in the Drosophila photoreceptor cells (Montell, 2005a).
Previous studies in TRP and other 6TM channels as well as in inwardly rectifying K+ channels had found that C-terminal regions rich in basic residues may act as PIP2 binding sites. It is thought that these positively charged side chains interact with the negatively charged headgroup of PIP2. We analysed the role of two such regions in the C-terminal tail of TRPM4, namely the proximal TRP domain and a more distal region that contains twice the consensus sequence for a putative pleckstrin homology (PH) domain, [R/K]-X3-11-[R/K]-X-[R/K]-[R/K]. Whereas neutralization of basic residues in the TRP domain did not affect the effects of PIP2 on TRPM4, charge neutralizing mutations to distal domain, particularly those that abolish the first PH consensus sequence, led to a significant decrease in PIP2 sensitivity (Nilius et al. 2006). Interestingly, this region, in contrast to the TRP domain, is not conserved in TRPM5, which may explain the lower PIP2 sensitivity of this channel compared to TRPM4 (Liu & Liman, 2003; Nilius et al. 2006).
When analysing the potentiating effects of PIP2 on TRPM4 channels in more detail, two distinct phenomena can be distinguished. First, application of PIP2 results in a ∼100-fold increase in apparent Ca2+ affinity. The difference in affinity is mainly due to a slower rate of Ca2+ unbinding, as can be deduced from the ∼100-fold slower rate of current decay upon removal of activating Ca2+ in inside-out patches perfused with PIP2. One possible explanation could be that the phosphate groups of PIP2 somehow contribute to the Ca2+-binding site on the channel. Second, PIP2 causes a strong leftward shift of the voltage-dependent activation curve, leading to an increase in channel open probability at physiological voltages. This can be attributed to a decrease in the rate of voltage-dependent channel deactivation, suggesting that PIP2 stabilizes the open state rather than destabilizing the closed state (Nilius et al. 2006). This effect is therefore similar to known effects of ligands on other TRP channels, such as for example the activation of TRPM8 by menthol (Voets et al. 2004, 2007).
Some mechanistic thoughts about PIP2 action on TRP channels
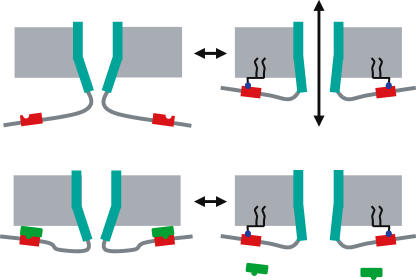
An important and still fully open question is how binding of PIP2 and/or other phosphatidylinositides regulate opening and closing of the TRP channel pores. In general, two main and not necessarily mutually exclusive mechanisms have been proposed to explain potentiation of channel activity by PIP2 (Fig. 1). In the first mechanism, PIP2 acts as a molecular ‘glue’ that pulls the intracellular PIP2 binding site to the plasma membrane, thereby exerting force on the inner gate of the pore leading to stabilization of the open state (Fig. 1, upper panels). Although appealing, direct functional evidence for such a mechanism has not yet been presented for TRPs or other channels. In the second mechanism, PIP2 disrupts the interaction between channel and an inhibitory partner, by competing for the same or overlapping binding sites on the channel (Fig. 1, lower panels). A recent study has shown that phosphoinositides, particularly PIP3 and PIP2, disrupt the interaction between TRPC6 and calmodulin, as a consequence of the strong overlap between the PIP2- and calmodulin-binding sites (Kwon et al. 2007). Given that calmodulin has an inhibitory effect on channel function, relief of the interaction leads to channel activation. The same study also provides evidence that a similar mechanism may be operational in several other (TRP) channels (Kwon et al. 2007). Interestingly, these authors also find that channel–calmodulin interaction can be disrupted, albeit less efficiently, by inositol 6-phosphate (IP6), which is water soluble and not normally bound to the plasma membrane (Kwon et al. 2007).
Figure 1. Possible modes of PIP2–TRP channel interactions.
Two possible mechanisms for PIP2-dependent activation of TRP channels. See text for more details.
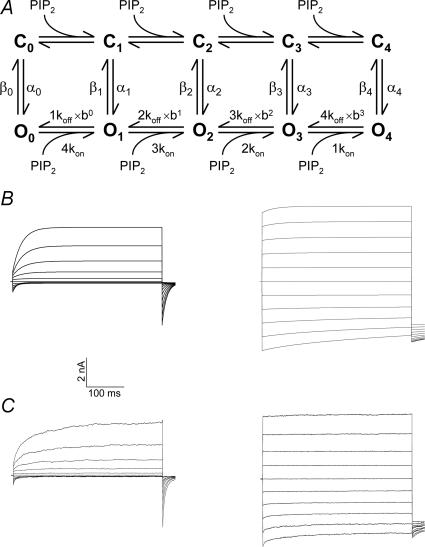
It should also be noted that at present we have no information concerning the stoichiometry of the PIP2–TRP channel interactions. Given that these channels are tetramers (Hoenderop et al. 2003), it is expected that each functional channel contains four equivalent PIP2 interaction sites (or a multiple of four, in case a subunit contains more than one PIP2 site). This immediately raises a number of important mechanistic questions, for example: Does the effect of PIP2 on the channels require PIP2 binding to all four subunits, or does each individual binding step influence the stability of the open channel in an equal manner? Does binding of PIP2 to one subunit influence the affinity of the other binding sites, i.e. is there any cooperativity in PIP2 binding? From a more theoretical point of view, the effects of PIP2 on a homotetrameric TRP channel may be described by a scheme as shown in Fig. 2A. In this scheme, it is assumed that the four subunits undergo a concerted transition between the closed (C) and open (O) states, in a manner similar to the Monod-Wyman-Changeux model for oxygen binding to haemoglobin (Voets et al. 2007). In the absence of ligand, the channel shuttles between the unbound closed (C0) and open (O0) states, a transition that is described by the forward and backward rate constants α0 and β0, which depends on temperature, transmembrane voltage and other energies imparted on the channel according to Eyring rate theory. Each subunit can bind one PIP2 molecule with an affinity given by Kd=koff/kon. A cooperativity factor (b) is introduced to account for possible cooperativity in PIP2 binding (P > 1 for positive cooperativity). In most cases, PIP2 leads to a stabilization (i.e. a decrease in the Gibbs free energy) of the open state, which mainly leads to a slowing of the closing of the channel (i.e. β1–4 < β0). Even with a few assumptions (no cooperativity of PIP2 binding (P = 1), PIP2-independent opening rates (αi=α0) and each individual binding step influencing the stability of the open channel in an equal manner), this model can yield a quite satisfactory simulation of the effects of PIP2 on TRPM4 (Fig. 2B and C). Clearly, further research is needed to establish the mechanism and stoichiometry of the modulation of TRP channels by PIP2, for example through the use of channel concatemers consisting of wild-type and PIP2-insensitive subunits. Detailed crystal structures of PIP2 binding sites or even of entire TRP channels may also provide novel insights into the modulatory interaction between phosphoinositides and TRP channels.
Figure 2. Modelling PIP2–TRP channel interactions.
A, general scheme describing the effect of PIP2 binding to a tetrameric channel analogous to the Monod–Wyman–Changeux model. B, simulation of the effect of PIP2 on TRPM4 currents in inside-out patches. The voltage protocol consisted of 400 ms voltage steps ranging from –100 to +100 mV from a holding potential of 0 mV; the tail current potential was –80 mV. C, experimental data obtained with the same voltage protocol showing the effect of addition of 10 μm PIP2 to inside-out patches from TRPM4-expressing HEK293 cells. For experimental details, see Nilius et al. (2006).
Acknowledgments
We thank all the members of our laboratory for discussion, suggestions and criticisms. Work in our laboratory is supported by grants from the Human Frontiers Science Program (RGP 32/2004), the Belgian Federal Government (IUAP P5/05), the Research Foundation-Flanders Flemish (G.0136.00, G.0172.03, and G.0565.07) and the Research Council of the KU Leuven (GOA 2004/07 and EF/95/010).
References
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5),P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Estacion M, Sinkins WG, Schilling WP. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J Physiol. 2001;530:1–19. doi: 10.1111/j.1469-7793.2001.0001m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P, Moore S, Juusola M, Baines RA, Sweeney ST. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron. 2001;30:149–159. doi: 10.1016/s0896-6273(01)00269-0. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- Hoenderop JGJ, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJM. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels, TRPV5 and TRPV6. EMBO J. 2003;22:776–785. doi: 10.1093/emboj/cdg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Lee J, Cha SK, Sun TJ, Huang CL. PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+ J Gen Physiol. 2005;126:439–451. doi: 10.1085/jgp.200509314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:1674–1681. doi: 10.1523/JNEUROSCI.3632-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs V, Thyagarajan B, Balla A, Varnai P, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphatidylinositol 4,5-bisphosphate. Biophysical Society Meeting Abstracts. Biophysical Journal, Suppl. 2007 290a, Abstract, 1366-Pos. [Google Scholar]
- McKemy DD, Neuhäusser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- Montell C. Drosophila TRP channels. Pflugers Arch. 2005a;451:19–28. doi: 10.1007/s00424-005-1426-2. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005b;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25:467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 2003;278:30813–30820. doi: 10.1074/jbc.M305127200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch. 2005;451:1–10. doi: 10.1007/s00424-005-1462-y. [DOI] [PubMed] [Google Scholar]
- Owsianik G, D'hoedt D, Voets T, Nilius B. Structure-function relationships of the TRP channel superfamily. Rev Physiol Biochem Pharmacol. 2006;156:61–90. [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P(2) regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz KJ. Towards a structural view of gating in potassium channels. Nat Rev Neurosci. 2004;5:905–916. doi: 10.1038/nrn1559. [DOI] [PubMed] [Google Scholar]
- Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci U S A. 2004;101:6009–6014. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. 2007;8:312–320. doi: 10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Voets T, Janssens A, Prenen J, Droogmans G, Nilius B. Mg2+-dependent gating and strong inward rectification of the cation channel TRPV6. J Gen Physiol. 2003;121:245–260. doi: 10.1085/jgp.20028752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol. 2007;3:174–182. doi: 10.1038/nchembio862. [DOI] [PubMed] [Google Scholar]
- Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol. 2005;1:85–92. doi: 10.1038/nchembio0705-85. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Voets T, Droogmans G, Nilius B. Invertebrate TRP proteins as functional models for mammalian channels. Pflugers Arch. 2004;449:213–226. doi: 10.1007/s00424-004-1314-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]