Abstract
TRPM7 (transient-receptor-potential melastatin 7) is an ion channel with α-kinase function. TRPM7 is divalent-selective and regulated by a range of receptor-stimulated second messenger pathways, intracellular Mg-nucleotides, divalent and polyvalent cations and pH. TRPM7 is ubiquitously found in mammalian cells, including kidney, the responsible organ for osmolyte regulation, posing the question whether the channel is osmosensitive. Recent reports investigated the sensitivity of native TRPM7-like currents to cell swelling with contradictory results. Here, we assess the sensitivity of TRPM7 to both hypo- and hyperosmotic conditions and explored the involvement of the channel's kinase domain. We find that hypotonicity facilitates TRPM7 at elevated intracellular magnesium and Mg·ATP (3–4 mm), but has no effect in the absence of these solutes. Hypertonic conditions, in contrast, inhibit TRPM7 with an IC50 of 430 mosmol l−1. This inhibitory effect is maintained in the complete absence of intra- and extracellular divalent ions, although shifted to higher osmolarities (IC50= 510 mosmol l−1). TRPM7 senses osmotic gradients rather than ionic strength and this is independent of cAMP or not affected by cytochalasin D treatment. Furthermore, the kinase-domain deletion mutant of TRPM7 shows a similar behaviour to osmolarity as the wild-type protein, both in the presence and absence of divalent ions. This indicates that at least part of the osmosensitivity resides in the channel domain. Physiologically, TRPM7 channels do not seem to play an active role in regulatory volume changes, but rather those volume changes modulate TRPM7 activity through changes in the cytosolic concentrations of free Mg, Mg-nucleotides and a further unidentified factor. We conclude that TRPM7 senses osmotically induced changes primarily through molecular crowding of solutes that affect channel activity.
TRPM7, a member of the melastatin-related transient-receptor-potential ion channel TRPM subfamily, is a ubiquitously expressed bifunctional plasma-membrane protein with both ion channel and α-kinase domains (Penner & Fleig, 2007). TRPM7 has a unique selectivity profile (Monteilh-Zoller et al. 2003) allowing cellular divalent ion influx, including the permeation of the physiologically two most abundant ions Ca2+ and Mg2+. Channel activity is regulated by a variety of second messengers (Runnels et al. 2002; Takezawa et al. 2004; Langeslag et al. 2007), intracellular Mg-nucleotides (Nadler et al. 2001; Hermosura et al. 2002; Demeuse et al. 2006), divalent and polyvalent ions and pH (Nadler et al. 2001; Kerschbaum et al. 2003; Jiang et al. 2005; Kozak et al. 2005; Demeuse et al. 2006). TRPM7 plays a central role in cellular Mg2+ homeostasis (Schmitz et al. 2003, 2005) and has been implicated in anoxic calcium overload and cell death (Aarts et al. 2003). Recent reports implicate TRPM7 function in synaptic transmission of sympathetic neurons (Krapivinsky et al. 2006), cell adhesion (Clark et al. 2006; Su et al. 2006) and cell growth (Hanano et al. 2004). Known downstream targets of TRPM7's kinase domain include annexin I, a protein involved in many of the cellular morphological changes induced by glucocorticoids (Dorovkov & Ryazanov, 2004), and myosin IIA heavy chain, linking TRPM7 to cell shape maintenance and formation (Clark et al. 2006). Maintenance of cellular morphology is essential for cell function, but is influenced by many physiological processes such as cell division, exocytosis and ionic exchange. Osmotic gradients also cause changes in cell morphology, which ultimately lead to changes in cell volume due to cellular water loss or uptake. Cell swelling is counteracted by an efflux of osmolytes followed by water and resulting in restoration of cell volume (regulatory volume decrease, RVD). Conversely, cell shrinkage activates osmolyte influx followed by water influx and compensatory swelling (regulatory volume increase, RVI). Several signalling pathways have been proposed to underlie RVD and RVI, including the involvement of ionic strength, membrane stretch-activated ion channels and transporters, changes in intracellular Ca2+ and Mg2+ concentration, phosphorylation of transporters and changes in macromolecular crowding (Lang et al. 1998; Pasantes-Morales et al. 2000; Okada et al. 2001, 2004; Sardini et al. 2003; Wehner et al. 2003).
Some members of the TRP superfamily are regulated or modulated by osmotic pressure (Harteneck & Reiter, 2007). TRPV4 and TRPM3 have been reported to increase Ca2+ conductance in response to hypotonic stress (Strotmann et al. 2000; Grimm et al. 2003), while TRPC1 responds to direct mechanical stress with increased Ca2+ conductance (Maroto et al. 2005). Endogenous TRPM7-like currents have been measured in many cell types and were originally called magnesium-nucleotide-regulated metal currents (MagNuM) due to their sensitivity to not only intracellular magnesium ions but also strong dependence on Mg-nucleotides (Nadler et al. 2001; Hermosura et al. 2002; Demeuse et al. 2006). Recent publications investigated the sensitivity of MagNuM to hypertonic environments reaching divergent conclusions. Jiang et al. (2003) probed the response of MagNuM to hypertonic extracellular challenges in rat brain microglia without any obvious effect on current activity. In contrast, Numata and colleagues observed stretch-induced activation of a 23 pS cationic conductance in human epithelial cells that was not observed after treatment with small interfering RNA (siRNA) targeted against TRPM7 (Numata et al. 2007b). They observed a similar 26 pS conductance in HEK293 cells overexpressing TRPM7 (Numata et al. 2007a).
We here report dose-dependent inhibition of native MagNuM and heterologously expressed TRPM7 channels. Our results demonstrate that channel regulation by osmolarity largely resides in the channel domain, is partially linked to intracellular Mg2+ and Mg-nucleotide concentration and is not altered by exposure of cells to cytochalasin D, a toxin of the actin cytoskeleton. We show that TRPM7 is not involved in RVD or RVI, but rather mediates osmolarity-induced changes in intracellular Ca2+ concentration, and possibly mediating cell detachment or adherence following volume changes.
Methods
Cell culture
HEK293 cells were stably expressing tetracycline-inducible Flag-murine TRPM7/pCDNA4-TO (Nadler et al. 2001), Flag-human TRPM7 or Flag-TRPM7-Δ-kinase (Schmitz et al. 2003). Cells were cultured in DME medium supplemented with 10% fetal bovine serum, blasticidin (5 μg ml−1) and zeocin (0.4 mg ml−1) in a 5% CO2-humidified atmosphere at 37°C. Protein expression was induced by 1 μg ml−1 tetracycline added to the cell culture 18–26 h before experiments. Wild-type HEK293 cells were cultured in DME medium supplemented with 10% fetal bovine serum.
Electrophysiology
Patch-clamp experiments were performed in the whole-cell configuration at ∼25°C, 18–26 h after tetracycline induction. High-resolution currents were filtered at 2.3 kHz and digitized at 100 μs intervals using a computer-based patch-clamp amplifier system (EPC-9, HEKA, Lambrecht, Germany) and the PULSE acquisition program (HEKA). Liquid junction potential was 10 mV and capacitance was compensated for using the automatic cancellation feature of the EPC-9. Voltage ramps of −100 to +100 mV were applied over 50 ms every other second for 600–700 s from a holding potential of 0 mV. In certain experiments, the holding the potential was kept at −80 mV for 5 s every 60 s. Current amplitudes were extracted at −80 or +80 mV using the PULSEFIT analysis program (HEKA). Statistical analysis and graphical display was done using IGOR PRO (Wavemetrics, USA). Statistical errors are standard error of the mean (s.e.m.) unless indicated otherwise.
Isotonic (310–330 mosmol l−1) intracellular pipette solutions contained (mm): caesium glutamate 140, NaCl 8, caesium BAPTA 10, Hepes-CsOH 10; pH 7.2. Hypo-osmotic intracellular solutions (200 mosmol l−1) contained 80 mm caesium glutamate instead of 140 mm. Hyper-osmotic intracellular pipette-filling solutions were adjusted with additional CsCl. Free [Mg2+] for each condition was adjusted with appropriate quantities of MgCl2 and calculated with MaxChelator software (http://www.stanford.edu/~cpatton/webmaxcS.htm). Standard free [Mg2+] was 0.9 mm in wild-type TRPM7 cells and 0.4 mm in TRPM7-Δ-kinase-expressing cells unless indicated otherwise. For Fura-2 experiments the intracellular pipette-filling solutions was supplemented with 200 μm K-Fura-2 (Molecular Probes, Eugene, OR, USA) instead of BAPTA. Hypotonic solutions for extracellular applications were composed of modified Ringer solution with low NaCl concentration (mm): NaCl 80, KCl 2.8, CaCl2 1, MgCl2 2, glucose 10, Hepes-NaOH 10; pH 7.2. Hypertonic application solutions were composed of the bath solution with osmolarity adjusted with appropriate amounts of sucrose, glucose or NaCl at pH 7.2. In certain experiments, cells were incubated in 5 μm cytochalasin D (Sigma, St Louis, MO, USA) overnight.
For balloon-patching, the intracellular pressure was increased by application of 30 mmH2O pressure via the patch-pipette connected to an air pressure controller (MPCU, Lorenz Messgeraetebau).
Fluorescence measurements
Combined patch-clamp and Fura-2 measurements were performed using a photomultiplier-based system with a monochromatic light source (TILL Photonics). Excitation was at 360 and 390 nm for 20 ms length each. Data acquisition was performed using X-Chart standalone (HEKA) at a sampling rate of 5 Hz. Analog/digital conversion was done using the ITC-16 (Instrutech, USA). In intact HEK293 cells, intracellular Ca2+ concentration changes and cell volume changes were monitored in cells pre-loaded with 5 μm Fura-2 ester dissolved in DMSO (Fura-2 AM, Molecular Probes) for 40–60 min at 37°C in external solution containing (mm): 140 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 5 glucose and 10 Hepes-NaOH; pH 7.2; 310–330 mosmol l−1. Real-time average of the pixels in the region of interest were analysed for cells superfused with hypertonic, hypotonic or isotonic saline and excited at 360 nm to determine volume changes. The ratio of 360 nm/390 nm excitation determined intracellular Ca2+ changes.
Results
TRPM7 is inhibited by hyperosmolarity
While the actual volume sensor remains elusive, several signalling pathways seem to be involved in volume regulation of cells, including its sensitivity to intracellular Mg2+ and the involvement of phosphorylation events via protein kinase mechanisms (Lang et al. 1998; Jakab et al. 2002; Wehner et al. 2003). Since TRPM7 is regulated by intracellular Mg2+ and Mg·ATP and contains an active α-kinase domain, we wondered whether TRPM7 channel activity would be sensitive to changes in the osmotic environment of the cell. We performed whole-cell patch-clamp experiments in HEK293 cells overexpressing mouse TRPM7 channels under control of a tetracycline-inducible promotor.
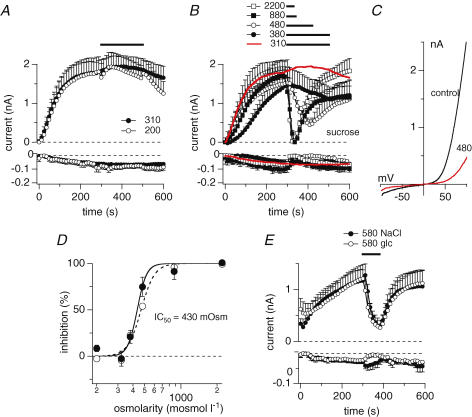
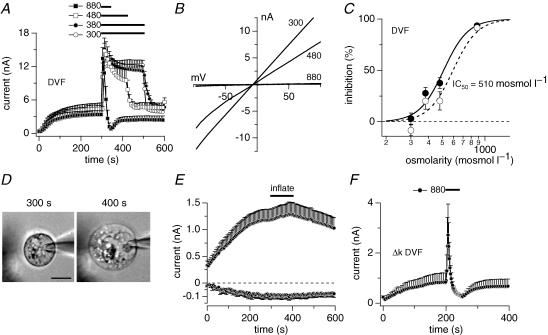
In a first set of experiments, we kept HEK293 cells overexpressing TRPM7 in our standard extracellular solution (see Methods) with osmolarity adjusted to 310–330 mosmol l−1. TRPM7 current development after whole-cell establishment was observed by perfusing cells with standard intracellular caesium glutamate-based solution where Mg2+ was adjusted to 0.9 mm and 0 Mg·ATP. At 300 s into the experiment, a time at which TRPM7 currents have reached their maximum, isotonic bath solution was applied via a wide-mouth pressure pipette (5–10 μm) to ascertain that pressure application alone had no effect on TRPM7 currents (Fig. 1A). Furthermore, applying hypotonic extracellular solution with reduced NaCl concentrations (80 mm) also had no significant effects on TRPM7 currents (Fig. 1A). However, when systematically increasing the osmolarity of the extracellular solution by adding the metabolically inactive sugar sucrose to our standard extracellular solution, elevated osmolarities of 380, 480, 880 or 2200 mosmol l−1, inhibited both inward and outward currents through TRPM7 in a dose-dependent manner (Fig. 1B and C). A dose–response curve fitted to the inhibitory effect on inward and outward currents as assessed at −80 mV and +80 mV, respectively, yielded an IC50 value of 430 mosmol l−1 with Hill coefficients of 8 (−80 mV) and 11 (+80 mV) (Fig. 1D). This effect was directly linked to hyperosmolarity rather than sucrose itself, since replacing sucrose with either glucose or NaCl as active osmolytes to achieve an osmolarity of 580 mosmol l−1 produced a comparable inhibitory effect as sucrose-based solutions (Fig. 1E).
Figure 1. Hyper-osmotic conditions inhibit TRPM7.
A, average current development of murine TRPM7 inward and outward currents measured in overexpressing HEK293 cells. Cells were superfused with either isotonic bath solution (•, n = 5) or hypo-osmotic bath solution (200 mosmol l−1, n = 8) as indicated by the black bar. Hypo-osmotic conditions were achieved by reducing NaCl to 70 mm. Currents were elicited by a voltage ramp from −100 mV to +100 mV over 50 ms at 0.5 Hz intervals. Holding potential was 0 mV throughout. Inward current amplitudes were extracted at −80 mV and outward currents at +80 mV, averaged and plotted versus time with error bars representing s.e.m. Cells were perfused with the standard intracellular caesium-based solution in the absence of Mg·ATP, with 0.9 mm free Mg2+ and 10 mm Cs-BAPTA. Error bars indicate s.e.m.B, average time-course of TRPM7 inward and outward currents where cells were superfused with increasing concentrations of hyperosmotic solutions as indicated by the black bars (red trace isotonic (same trace as in D, n = 9); •, 380 mosmol l−1, n = 8; ^, 480 mosmol l−1, n = 5; ▪, 880 mosmol l−1, n = 3; □, 2200 mosmol l−1, n = 4). Data were analysed as in A. Hyperosmotic conditions were established by using sucrose. C, current–voltage (I–V) relationship taken from example cells just before application of 480 mosmol l−1 (black trace) and at the end of application (red trace). D, dose–response curve of current inhibition caused by application of increasing hyperosmotic extracellular solutions. Closed circles represent current increase at +80 mV, open circles at −80 mV (n = 3–9). Currents were measured before application (300 s) and at the end of application (330 – 500 s) and normalized to 300 s as I/I300s. Lines indicate a dose–response curve to the data with an IC50 of 430 mosmol l−1 and Hill coefficient of 8 (−80 mV) and 11 (+80 mV). E, average time-course development of TRPM7 inward and outward currents overexpressed in HEK293 cells and superfused with a hypertonic extracellular solution (580 mosmol l−1) as indicated by the black bar. Hyperosmolarity was achieved by adding appropriate amounts of either NaCl (•, n = 5) or glucose (^, n = 5). Currents were analysed as in A. Error bars indicate s.e.m.
Endogneous MagNuM currents are inhibited by hyperosmolarity
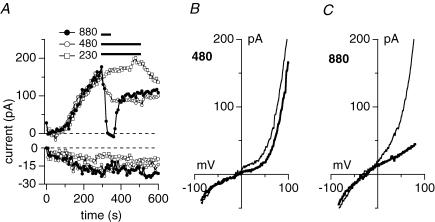
We next tested whether osmolarity changes in the extracellular milieu of a cell would also affect endogenous TRPM7-like MagNuM currents. We perfused wild-type HEK293 cells with our standard pipette solution that contained free Mg2+ levels of 0.9 mm and 0 Mg·ATP. Application of a hypo-osmotic solution (230 mosmol l−1) did not affect MagNuM currents, but hypertonic conditions suppressed MagNuM in a dose-dependent manner (Fig. 2A, B and C). It should be pointed out that under these experimental conditions the store-operated calcium influx pathway ICRAC is activated in addition to MagNuM currents, indicating that the smaller effect of hyperosmotic conditions seen on inward currents is most probably due to the insensitivity of ICRAC to osmotic changes in the extracellular milieu.
Figure 2. Hyperosmotic conditions inhibit endogenous TRPM7-like MagNuM currents.
A, representative current development of MagNuM inward and outward currents measured in wild-type HEK293 cells. Cells were superfused with hypo-osmotic extracellular solution (□, n = 8) or hypertonic extracellular solutions at 480 mosmol l−1 (^, n = 5) or 880 mosmol l−1 (•, n = 4) as indicated by the black bar. Hypotonic conditions were achieved by removal of NaCl (total of 80 mm NaCl) and hypertonic conditions were established using appropriate amounts of sucrose. Currents were elicited by a voltage ramp from −100 mV to +100 mV over 50 ms at 0.5 Hz intervals. Inward current amplitudes were extracted at −80 mV and outward currents at +80 mV and plotted versus time. Cells were perfused with the standard intracellular Cs-based solution in the absence of Mg·ATP, with 0.9 mm free Mg2+ and 10 mm Cs-BAPTA. Baseline correction was done by measuring basal leak currents between 6 s and 22 s from each curve and subtracted from the overall current. B, I–V relationship taken from the same cells as in A just before application of 480 mosmol l−1 (thin trace) and at the end of application (thick trace). Note that I–V data are not basal leak subtracted. C, I–V relationship taken from the same cells as in A just before application of 880 mosmol l−1 (thin trace) and at the end of application (thick trace). Data are not basal leak subtracted. Note that the slightly more negative reversal potential could be due to somewhat more active background channels in this particular cell that are not completely suppressed by intracellular Cs2+.
Osmosensitivity is not affected by ionic strength, cAMP or cytochalasin D
How does TRPM7 sense osmolarity? Several of the mechanisms that are thought to be involved in cellular volume regulation are also linked to TRPM7 activity. These include the sensing of ionic strength, intracellular Mg2+ levels, remodelling of the cytoskeleton and regulation through cAMP via protein kinase A (PKA) (Wehner et al. 2003). In addition, TRPM7 has been linked to cell adhesion (Clark et al. 2006; Su et al. 2006). We therefore wondered whether any of these mechanisms would be involved in the osmoregulation of TRPM7.
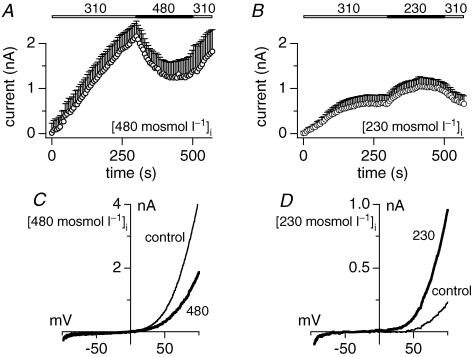
First, we wanted to find out whether the osmotic effects on TRPM7 were due to the osmotic gradient or rather the intracellular ionic strength. To this end, HEK293 cells were kept in standard extracellular solution of 310 mosmol l−1 and perfused with standard intracellular solution whose osmolarity was adjusted to higher or lower levels than the bath solution, thus creating an osmotic gradient across the plasma membrane. After TRPM7 currents had fully developed under these conditions, an extracellular solution was applied that restored isotonic conditions across the plasma membrane. When perfusing cells with intracellular solutions of 480 mosmol l−1 with 310 mosmol l−1 outside, TRPM7 developed to normal current amplitudes (around 2 nA at +80 mV), albeit with somewhat slower kinetics (Fig. 3A). These conditions would establish relative hypotonicity across the membrane similar to a hypotonic solution application, which has no effect on TRPM7 activity (see Fig. 1A). However, when restoring isotonicity by application of an extracellular solution at 480 mosmol l−1 to match intracellular tonicity, TRPM7 currents reversibly returned to smaller current amplitudes (Fig. 3A and C). Conversely, when perfusing cells with intracellular solutions of 230 mosmol l−1 with 310 mosmol l−1 outside, effectively mimicking the osmotic gradient of a hypertonic solution application, the restoration of isotonicity slightly enhanced TRPM7 currents (Fig. 3B and D). These data indicate that osmotic gradients, rather than the overall ionic strength, may be the primary mechanism that regulates TRPM7 activity. However, changes in intracellular osmolarity have a slightly different effect on TRPM7 from those seen with extracellular changes in osmolarity. While relative decreases in intracellular osmolarity or relative increases in extracellular osmolarity suppress TRPM7 currents, a relative increase in intracellular osmolarity can facilitate TRPM7 currents while a corresponding decrease in extracellular osmolarity fails to do so (Fig. 1A).
Figure 3. Osmosensitivity is independent of the direction of the osmotic gradient.
A, averaged outward current development of overexpressed murine TRPM7 in HEK293 cells. Cells were perfused with standard intracellular solution adjusted to 480 mosmol l−1 and allowed to develop in normal (310 mosmol l−1) extracellular solution. Superfusion of isotonic (480 mosmol l−1) extracellular solution is indicated by the black bar (n = 4). Currents were elicited by a voltage ramp from −100 mV to +100 mV over 50 ms at 0.5 Hz intervals. Currents were extracted at +80 mV and plotted versus time. Cells were perfused with the standard intracellular Cs-based solution in the absence of Mg·ATP, with 0.9 mm free Mg2+ and 10 mm Cs-BAPTA. Intracellular osmolarity was adjusted adding CsCl. Error bars indicate s.e.m.B, averaged outward current development of TRPM7 in HEK293. Cells were perfused with standard intracellular solution adjusted to 230 mosmol l−1 and allowed to develop in normal (310 mosmol l−1) extracellular solution. Superfusion of isotonic (230 mosmol l−1) extracellular solution is indicated by the black bar (n = 7). Intracellular osmolarity was adjusted omitting caesium glutamate. Error bars indicate s.e.m.C, I–V relationship of an example cell perfused with 480 mosmol l−1 and before (300 s, thin line) and at the end of application of 480 mosmol l−1 extracellular solution (500 s, thick line). D, I–V relationship of an example cell perfused with 230 mosmol l−1 and before (300 s, thin line) and at the end of application of 230 mosmol l−1 extracellular solution (500 s, thick line).
Disruption of the cytoskeleton with colchicine or cytochalasin D can enhance the mechanosensitivity of the K+ channel TRAAK (Maingret et al. 1999). TRPM7 itself has been linked to annexin I phosphorylation and control of cell adhesion (Dorovkov & Ryazanov, 2004; Clark et al. 2006; Su et al. 2006). We therefore examined the possibility that the osmotic effects on TRPM7 might be due to strain on the membrane-cortical actin cytoskeleton. Overnight treatment of cells with 5 μm of the f-actin toxin cytochalasin D resulted in cell swelling and rounding, but cells still responded with TRPM7 current inhibition by about 50% when challenged with 480 mosmol l−1 (n = 3, data not shown).
Cellular volume changes have been reported to result in changed cAMP levels in some cells but not in others. Since TRPM7 channel activity is facilitated by cAMP (Takezawa et al. 2004), we perfused TRPM7-overexpressing HEK293 cells with standard intracellular solution supplemented with 100 μm cAMP. However, this neither prevented nor enhanced the inhibitory effect of 480 mosmol l−1 applied extracellularly (n = 3, data not shown). Together, these results indicate that TRPM7 is responsive to osmotic gradients rather than ionic strength and is independent of changes in cAMP or the actin cytoskeleton.
Hypertonic sensitivity of TRPM7 is partially conveyed by intracellular Mg2+
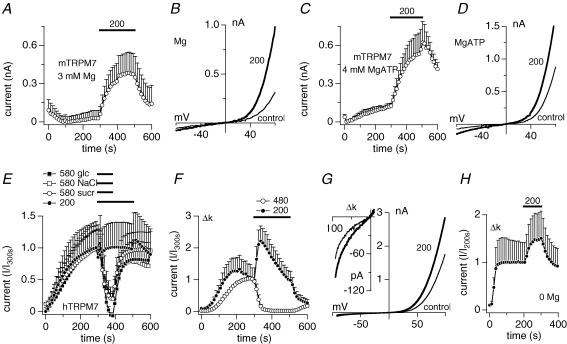
Volume changes occurring during osmotic stress will lead to a dilution or concentration of intracellular molecules, including the TRPM7 suppressors Mg2+ and Mg·ATP. Our data show that TRPM7 currents recorded with standard intracellular conditions containing 0.9 mm Mg2+ were not facilitated when cells were challenged with 200 mosmol l−1. However, when increasing intracellular Mg2+ to 3 mm or Mg·ATP to 4 mm (at 0.9 free Mg2+), hypotonic conditions did cause a significant current increase (Fig. 4A–D). We conclude that the osmosensitivity of TRPM7 is connected to osmolarity-induced volume and hence concentration changes of intracellular regulators of TRPM7.
Figure 4. Sensitivity to hyperosmolarity partially resides in the channel domain.
A, average time-course of overexpressed murine TRPM7 currents where cells were superfused with solutions adjusted to 200 mosmol l−1 but intracellular standard solution was supplemented with 3 mm free Mg2+ in the absence of Mg·ATP (n = 6). Error bars represent s.e.m.B, I–V relationship taken from an example cell from D before application of 200 mosmol l−1 (300 s, thin trace) and at the end of application (500 s, thick trace). C, average time-course of overexpressed murine TRPM7 currents where cells were superfused with solutions adjusted to 200 mosmol l−1 but intracellular standard solution was supplemented with 4 mm Mg·ATP with free Mg2+ adjusted to 0.9 mm (n = 5). Error bars represent s.e.m.D, I–V relationship taken from an example cell from F before application of 200 mosmol l−1 (300 s, thin trace) and at the end of application (500 s, thick trace). E, average time-course of overexpressed human TRPM7 outward currents where cells were superfused with solutions adjusted to either 200 mosmol l−1 or 580 mosmol l−1 as indicated by the black bars. Hyperosmolarity was achieved by adding appropriate amounts of either NaCl (□, n = 6), sucrose (^, n = 5) or glucose (▪, n = 4). Hypo-osmolarity was achieved by omission of NaCl (•, n = 4). Currents were elicited by a voltage ramp from −100 mV to +100 mV over 50 ms at 0.5 Hz intervals. Current amplitudes were extracted at +80 mV, averaged and plotted versus time with error bars representing s.e.m. Cells were perfused with the standard intracellular Cs-based solution in the absence of Mg·ATP, with 0.9 mm free Mg2+ and 10 mm Cs-BAPTA. Error bars represent s.e.m.F, average time-course of overexpressed human Δ-kinase TRPM7 outward currents where cells were superfused with a solution adjusted to 480 mosmol l−1 using sucrose (^, n = 3) or with a solution adjusted to 200 mosmol l−1 by omission of NaCl (•, n = 4). Application as indicated by the black bar. Cells were perfused with the standard intracellular Cs-based solution in the absence of Mg·ATP, with 0.4 mm free Mg2+ and 10 mm Cs-BAPTA. Error bars represent s.e.m.G, I–V relationship taken from an example cell before application of 200 mosmol l−1 (300 s, thin trace) and at the end of application (500 s, thick trace). The inset magnifies the inward currents. H, average time-course of overexpressed human Δ-kinase TRPM7 outward currents in the absence of intracellular magnesium, where cells were superfused with a solution adjusted to 200 mosmol l−1 (•, n = 5). Data were normalized to the current measured before application as I/I200s. Application as indicated by the black bar.
Based on previous work, the TRPM7 channel may possess two distinct binding sites for Mg2+ and Mg·NTP, the latter being located within the channel's endogenous kinase domain and the former upstream, possibly close to the inner mouth of the channel (Schmitz et al. 2003; Demeuse et al. 2006). We therefore wanted to know whether the channel would retain sensitivity to osmotic gradients in the absence of intracellular and extracellular divalents and Mg-nucleotides. Indeed, when perfusing mouse TRPM7 overexpressing HEK293 cells with internal solution devoid of the latter molecules and supplemented with EDTA, application of divalent-free extracellular solutions with increasing osmolarities caused inhibition of TRPM7 currents in a dose-dependent manner (Fig. 5A and B). Plotting the percentage inhibition of currents versus osmolarity and fitting the resulting data points revealed an IC50 of 590 mosmol l−1 for inward (−80 mV) and 510 mosmol l−1 for outward currents (+80 mV) in these divalent-free conditions (Fig. 5C, Hill coefficient = 5). Thus, the IC50 for divalent-free conditions is shifted by about 100 mosmol l−1 to the right compared with divalent ions (see Fig. 1), suggesting that Mg2+ and Mg-nucleotides indeed contribute to the osmosensitivity of TRPM7. However, since reduced osmosensitivity was retained in the absence of Mg2+, additional factors may be involved in shaping the osmosensitivity of TRPM7.
Figure 5. The TRPM7 channel domain retains osmo-sensitivity in the absence of Mg2+ or Mg-nucleotides.
A, average time-course of overexpressed murine TRPM7 outward currents where cells were superfused with isotonic divalent-free solution (DVF; ^, n = 4) or DVF solutions adjusted to either 380 mosmol l−1 (•, n = 5), 480 mosmol l−1 (□, n = 5) or 880 mosmol l−1 (▪, n = 5). Application time as indicated by the black bars. Hyper-osmolarity was achieved by adding sucrose. Currents were elicited by a voltage ramp from −100 mV to +100 mV over 50 ms at 0.5 Hz intervals. Current amplitudes were extracted at +80 mV, averaged and plotted versus time with error bars representing s.e.m. Cells were perfused with the standard intracellular Cs-based solution in the absence of Mg·ATP and Mg2+ and in the presence of 10 mm Cs-BAPTA and 5 mm EDTA. The extracellular solution contained no divalents and 5 mm EDTA. B, I–V relationships extracted from an example cells taken from A at the end of application of either 300, 480 or 880 mosmol l−1 application. C, dose–response curve of current inhibition caused by increased hypertonicity in the absence of intracellular and extracelluar divalents. • inhibition at +80 mV ^ inhibition at −80 mV (n = 4–5). Currents were measured before application (300 s) and at the end of application time (40–200 s) and normalized to 300 s as I/I300s. Lines indicate a dose–response curve to the data with a IC50 of 600 mosmol l−1 (−80 mV) and 510 mosmol l−1 (+80 mV) and Hill coefficient of 5 each. Same data set as in A. D, example of a TRPM7-overexpressing HEK293 cell before (300 s) and after inflation of the cell by application of 30 mmH2O pressure via the patch pipette (400 s). Scale bar represents 5 μm. E, average development of TRPM7 currents at +80 mV and −80 mV in standard isotonic solutions with intracellular Mg2+ at 0.9 mm and absence of ATP (n = 6). Intracellular pressure of 30 mmH2O was applied through the patch pipette as indicated by the black bar. F, average time-course of overexpressed human Δ-kinase TRPM7 outward currents where cells were superfused with isotonic divalent-free solution (n = 5). Application time as indicated by the black bars. Hyperosmolarity was achieved by adding sucrose. Cells were perfused with the standard intracellular Cs-based solution in the absence of Mg·ATP and Mg2+ and in the presence of 10 mm Cs-BAPTA and 5 mm EDTA. The extracellular solution contained no divalents and 5 mm EDTA.
Mechano-sensitive cation channels have been studied in the whole-cell configuration by applying positive pressure through the patch pipette, which causes the plasma membrane to separate from other cellular structures (Hamill & McBride, 1997). Such manipulations inflate the cells like a balloon, thereby altering the cell volume and increasing membrane stretch in much the same way as hypotonic solutions. The significant difference to hypotonic swelling, however, is that pressure-induced inflation occurs without altering the cytosolic concentrations of pipette-infused ions. Using this approach, we assessed whether part of the sensitivity of TRPM7 to hypotonic challenge in the presence of high Mg2+ or nucleotide concentrations could be due to mechano-sensitivity. To isolate stretch-sensitivity, we kept cells in standard external solution and perfused them with standard intracellular solution with 0.9 mm free Mg2+ and no nucleotides. At 300 s into the experiment, we applied 30 mmH2O pressure to the patch pipette via a manometer for 100 s. This caused 6 out of 8 cells to balloon. The average increase in cell diameter of these 6 cells was 19 ± 3% (n = 6). A representative balloon-patched cell is shown in Fig. 5D, before (300 s) and at the end (400 s) of pressure application. Cells remained ballooned after pressure was removed. The membrane stretch caused by balloon patching did not significantly increase TRPM7 currents (Fig. 5E). This further indicates that intracellular factors rather than membrane stretch account for the osmosensitvity of TRPM7.
Osmosensitivity does not require a functional kinase domain
The TRPM7 protein contains a cytosolic α-kinase domain in addition to the transmembrane channel domain (Nadler et al. 2001; Runnels et al. 2001; Ryazanova et al. 2001). The kinase domain mediates the Mg-nucleotide-dependent regulation of channel activity (Demeuse et al. 2006). We wondered whether the observed osmosensitivity of channel activity requires the presence of the kinase domain. We tested this with a human TRPM7 truncation mutant with the complete deletion of the kinase domain (Δ-kinase-TRPM7) (Schmitz et al. 2003), after confirming that human TRPM7 exhibited similar osmosensitivity as mouse TRPM7. Indeed, similar to mouse, currents measured in tetracycline-induced HEK293 cells overexpressing human TRPM7 were not significantly affected when exposed to 200 mosmol l−1 extracellularly (Fig. 4E), whereas hypertonic conditions (580 mosmol l−1) inhibited fully developed TRPM7 to a similar extent as seen with the mouse construct, independent of the means used to achieve hyperosmolarity (sucrose, glucose or NaCl, Fig. 4E). We next proceeded probing the sensitivity of Δ-kinase-TRPM7 to osmotic changes. Hypotonic conditions (200 mosmol l−1) strongly facilitated Δ-kinase-TRPM7 currents (Fig. 4F and G), whereas a hypertonic solution of 480 mosmol l−1 was sufficient to completely inhibit the currents compared with the 580 mosmol l−1 required for wild-type TRPM7 (Fig. 4F), suggesting that the mutant channels are more sensitive to osmotic changes that WT channels. Our previous work demonstrated that Δ-kinase-TRPM7 is significantly more sensitive to intracellular Mg2+ and Mg·ATP concentrations (Schmitz et al. 2003). Since intracellular free Mg2+ partially contributes to TRPM7's osmosensitivity, this may explain the higher sensitivity of the Δ-kinase-TRPM7 channels. To assess the contribution of intracellular Mg2+, we repeated the hypotonic experiment in the absence of any intracellular Mg2+ (and presence of 5 mm EDTA) and then superfused Δ-kinase-TRPM7-expressing cells with 200 mosmol l−1 external solution (Fig. 4H). This resulted in reduced current facilitation compared with experiments in which Mg2+ was present, but not complete suppression of osmosensitivity. Furthermore, the absence of the kinase domain did not interfere with 880 mosmol l−1 inhibiting the current even in divalent-free (DVF) conditions (Fig. 5F). As already concluded for the WT channel, it appears that intracellular free Mg2+ is partially responsible for the osmosensitivity, with an as yet unknown further factor contributing to it.
TRPM7 overexpression does not alter cell volume responses to osmolarity
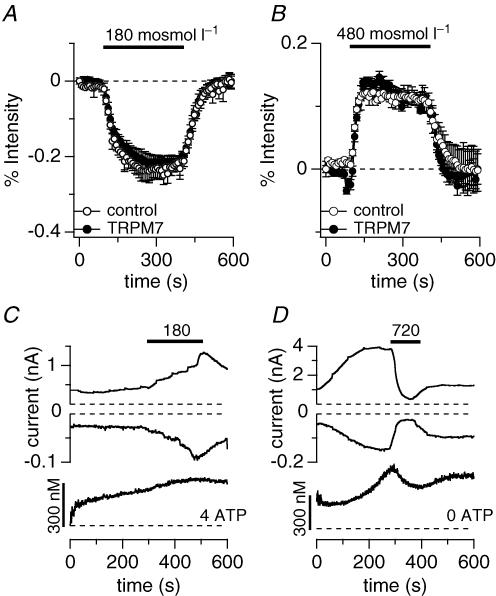
In order to see whether overexpression of TRPM7 alters the cell's ability to react to osmotic challenges, HEK293 cells were incubated in 5 μm Fura-2 AM for 30 min. Changes in fluorescent emission to osmotic challenges directly reflect cell expansion or shrinkage by diluting or concentrating the Fura-2 dye. As can be seen in Fig. 6A, the decrease of fluorescence intensity was identical in both wild-type HEK293 cells and cells overexpressing TRPM7 for 24 h when challenged with 180 mosmol l−1. Conversely, superfusion of a hyper-osmotic extracellular solution (480 mosmol l−1) also did not produce any difference in cell volume regulation when comparing wild-type and TRPM7-expressing HEK293 (Fig. 6B). This indicates that TRPM7 is not actively involved in regulatory volume increase (RVI) or regulatory volume decrease (RVD), but rather is regulated as a consequence of such volume changes.
Figure 6. Overexpression of TRPM7 does not alter cell volume regulation in response to osmotic challenges.
A, fluorescent indicator dye imaging of cell volume change of wild-type (^, n = 5) or TRPM7 overexpressing HEK293 cells (•, n = 5) pre-loaded with Fura-2 ester and evalulated at 360 nm excitation. Intact Fura-2-loaded cells were superfused with 180 mosmol l−1 external solution as indicated by the black bar. The concomitant cell swelling was assessed as emission intensity decrease in per cent. Data were normalized to the intensity before application. Error bars indicate s.e.m.B, fluorescent indicator dye imaging of cell volume change of wild-type (^, n = 4) or TRPM7 overexpressing HEK293 cells (•, n = 3) pre-loaded with Fura-2 ester and evaluated at 360 nm. Intact Fura-2-loaded cells were superfused with 480 mosmol l−1 external solution as indicated by the black bar. The concomitant cell shrinkage was assessed as emission intensity increase in per cent. Data were normalized to the intensity before application. Error bars indicate s.e.m.C, combined Fura-2 and patch-clamp experiments. Upper panel shows development of inward and outward currents of an example cell where currents were assessed at −80 mV and +80 mV, respectively. Application of 180 mosmol l−1 as indicated by the bar. Cells were perfused with standard internal caesium glutamate-based solution supplemented with 4 mm Mg·ATP, with 0.9 mm free Mg2+ and 200 μm K5Fura-2. Lower panel shows concomitant intracellular calcium concentration of the same cell. Note that currents are caused by a Cl− conductance, not TRPM7 (n = 4). D, combined Fura-2 and patch-clamp experiments. Upper panel shows development of inward and outward currents of an example cell where currents were assessed at −80 mV and +80 mV, respectively. Application of 720 mosmol l−1 as indicated by the bar (n = 5). Cells were perfused with standard internal caesium glutamate-based solution in the absence of Mg·ATP, with 0.9 mm free Mg2+ and 200 μm K5Fura-2. Lower panel shows concomitant intracellular calcium changes of the same cell.
Inhibition of TRPM7 by hypertonic solutions affects intracellular calcium concentrations
Osmotic changes seem to cause alterations in intracellular calcium signalling (Wehner et al. 2003). Since TRPM7 is an important divalent ion influx pathway, we wondered whether hyper- or hypo-osmotic changes directly would affect intracellular calcium concentrations. To this end we performed combined patch-clamp and Fura-2 experiments perfusing HEK293 cells overexpressing the murine TRPM7 protein with 5 mm Mg·ATP and superfusing the cells with a hypotonic solution of 180 mosmol l−1. Under these hypo-osmotic experimental conditions, we were unable to assess TRPM7 currents, since there was a concomitant reversible increase in volume-sensitive Cl− conductance (Fig. 6C). This is probably due to the presence of intracellular calcium compared with the conditions above, where 10 mm BAPTA maintained intracellular calcium levels at near zero (Fig. 4C). Additionally, intracellular calcium concentrations did not increase significantly in these conditions, although all cells showed a steady, slow increase in overall calcium over the time of the experiment (Fig. 6C, lower panel). However, when superfusing TRPM7-expressing HEK293 cells with hyperosmotic solution (720 mosmol l−1) while concurrently measuring currents and intracellular calcium, we observed that both TRPM7 currents and intracellular calcium concentration were significantly reduced (Fig. 6D).
Discussion
TRPM7 is a homeostatic divalent cation influx pathway, whose basal activity is regulated in synergy by intracellular Mg-nucleotides and Mg2+ (Nadler et al. 2001; Monteilh-Zoller et al. 2003; Demeuse et al. 2006). Consequently, dilution or concentration of these membrane-impermeant intracellular solutes will affect channel activity, implicating a role for TRPM7 in cell volume physiology. We set forth to test this hypothesis and find that increasing cell volume by an extracellular hypertonic challenge facilitates TRPM7 currents but only in the presence of either physiological intracellular Mg·ATP or high Mg2+ concentration. In the absence of Mg·ATP the facilitatory effect is lost.
On the other hand, hypertonicity inhibits TRPM7 in a dose-dependent manner. This is only partially dependent on the presence of divalent ions and is linked to the protein's channel domain, since the TRPM7 kinase-deletion mutant is similarly sensitive to hyperosmotic conditions as wild-type channels, even in the complete absence of divalent ions. Inhibition by hypertonic conditions seems independent of the solute used to increase the osmolarity (NaCl, glucose or sucrose) and neither perturbing the f-actin cytoskeleton by cytochalasin-D nor interfering with the PKA signalling pathway using cAMP alters TRPM7's inhibitory response to hyperosmolarity.
TRPM7 does not seem to be directly involved in either RVD or RVI, because overexpression of the protein does not alter the onset or recovery from osmosis-induced cell volume changes when compared with wild-type cells expressing native TRPM7 channels. Rather, it seems that cell-volume-induced changes in TRPM7 activity lead to changes of intracellular calcium concentrations, since suppressing TRPM7 during hypertonic solution applications concomitantly reduces cytosolic calcium concentrations.
A recent study reported shear stress-induced recruitment of TRPM7 ion channels to the plasma membrane via an exocytotic event (Oancea et al. 2006). While this does not implicate TRPM7 as a mechano-sensitive ion channel, it has to be considered whether the current facilitation observed in our study is due to shear stress rather than osmolarity. Several observations argue against this mechanism in our cells. Superfusion of control (isotonic) extracellular solution at a constant application pipette pressure of 12 cmH2O did not alter the size of fully developed TRPM7 currents nor did it change the cell size as assessed by capacitance (23 ± 2 pF, n = 5), which one would expect to see upon integration of additional membrane by vesicle fusion events (Fernandez et al. 1984). Furthermore, although our wide-mouthed application pipettes had similar diameters (5–10 μm) and hydrostatic pressure was identical for all application conditions used in this study, differential effects on current behaviour could be seen when either using hypertonic or hypotonic solutions or when removing intracellular Mg·ATP. This would not be expected if current changes were caused by vesicular insertion.
Two reports have tested the response of native TRPM7-like MagNuM to hypotonic gradients. Jiang and colleagues found that reduction of extracellular osmolarity to 75% of normal values did not alter MagNuM in rat brain microglia cells (Jiang et al. 2003), an observation that our results confirm in wild-type HEK293 cells. Numata and colleagues conducted a detailed analysis of a cationic conductance in HeLa cells that could be activated by either suction-induced membrane stretch or exposure of cells to hypotonic solutions (Numata et al. 2007b). Single channel activity of the cationic conductance was absent in HeLa cells transfected with siRNA targeted against TRPM7 and in HEK293 cells transiently overexpressing TRPM7, they described a similar conductance (Numata et al. 2007a). The authors concluded that the stretch-induced channels represent TRPM7. However, several biophysical properties of these channels are incompatible with this conclusion. A number of reports confirm a single channel conductance of 40 pS for MagNuM and TRPM7 (Kerschbaum & Cahalan, 1999; Nadler et al. 2001; Kerschbaum et al. 2003), which is significantly different from the 23 pS reported for the stretch-induced single channels in HeLa cells (Numata et al. 2007b) and the 26 pS conductance seen in HEK-TRPM7 cells (Numata et al. 2007a). Furthermore, the suction-induced conductance has a low open probability at negative potentials (−50 mV and below), whereas many studies confirm high open probabilities independent of voltage (Kerschbaum & Cahalan, 1999; Fomina et al. 2000; Nadler et al. 2001; Hermosura et al. 2002; Kozak et al. 2002, 2005; Prakriya & Lewis, 2002; Kerschbaum et al. 2003; Monteilh-Zoller et al. 2003; Schmitz et al. 2003). The current is blocked by Gd3+ in a voltage-independent manner, while in our studies we did not see any effects of Gd3+ on MagNuM at +80 mV (Hermosura et al. 2002). Finally, the standard experiments in both HeLa and HEK293 cells were conducted under intracellular and extracellular divalent-free conditions. Using these conditions, the current–voltage relationship of TRPM7 currents would be almost linear (Schmitz et al. 2003 and Fig. 5), irrespective of whether cells were kept in isotonic or hypotonic extracellular conditions. However, this is clearly not the case for the conductance reported in HeLa cells, whose appearance is strongly outwardly rectifying under control conditions and assumes a linear shape only upon induction of cell swelling. Taken together, it seems unlikely that the proposed stretch-activated conductance is mediated by TRPM7, although MagNuM seems to play a role in hypotonicity-induced volume regulation due to the significant delay of volume decrease in intact cells treated with TRPM7-siRNA (Numata et al. 2007b). It should be noted, however, that TRPM7 siRNA experiments may also down-regulate other ion channels, e.g. TRPM2 (Aarts et al. 2003).
We show here that under physiological divalent conditions hypotonic gradients facilitated TRPM7 only when using high osmotic intracellular conditions, or in the presence of intracellular Mg·ATP when decreasing extracellular osmolarity. Hypotonic solutions had no effect on TRPM7 activity in the absence of Mg·ATP even when the intracellular solution was supplemented with 0.9 mm Mg2+. These deviating results argue against a stretch-activated mechanism, as this would be expected to be independent of intracellular solutes and direction of osmotic gradients. On the other hand, effects of hyperosmotic conditions have not yet been studied in TRPM7. Our results show a dose-dependent inhibition of the current induced by hypertonicity. The inhibitory effectiveness is enhanced in the presence of Ca2+ and Mg2+ ions, left-shifting the IC50 from 510 mosmol l−1 to 430 mosmol l−1. Thus our data indicate that TRPM7 senses a wide range of osmotic gradients.
The osmosensitivity of TRPM7 could be due to several mechanisms. Several TRP channels, including TRPA1, TRPV1 and TRPC1, have been reported to be mechanosensitive (Mutai & Heller, 2003; Barritt & Rychkov, 2005); however, membrane stress-induced activation seems an unlikely major mechanism for TRPM7 since the mere absence of intracellular Mg·ATP at physiological free Mg2+ (0.9 mm) abolishes hypotonicity-induced current facilitation. Furthermore, application of extracellular pressure via the application pipette (12 cmH2O) had no effect on current size despite increasing cell volume to a similar extent as hypotonic solutions. Mechano-sensitive cation channels have been studied using the balloon-patch technique (Hamill & McBride, 1997), but TRPM7 currents were not significantly affected by inflating the cell through application of intracellular pressure. Interference with the f-actin cytoskeleton has been reported to increase activity of stretch-activated BK and Cl channels (Sakai et al. 1999; Piao et al. 2003). However, in our hands overnight treatment of TRPM7-overexpressing cells with cytochalasin-D did not enhance current sizes nor was hypertonicity-induced inhibition alleviated.
The activity of many transporters that are involved in cell volume regulation depend on phosphorylation events using cAMP and cAMP-independent protein kinases during RVI, whereas dephosphorylation events cause RVD (Wehner et al. 2003). TRPM7 is regulated through Gs/Gi receptor stimulation involving PKA-dependent cAMP production (Takezawa et al. 2004). However, perfusion of TRPM7-overexpressing cells with maximal cAMP concentrations (100 μm) had no effect on the inhibitory action of hypertonic stress, indicating that these two regulatory pathways may act on different parts of the protein. This is further strengthened by the observation that cAMP-induced current enhancement requires a functional kinase domain (Takezawa et al. 2004), whereas the effect of hypertonicity does not.
Volume-activated Cl− channels (VRACs) respond to changes in ionic strength caused by altering osmolarity (Nilius et al. 2000). However, our data show that relative osmotic pressure or osmolyte concentration, rather than ionic strength, seems to cause TRPM7 inhibition, as the inhibitory effect of hypertonic solutions is similar whether NaCl, glucose or sucrose are used to increase osmolarity. Another argument against TRPM7's osmosensitivity being due to sensing ionic strength can be made from our results that TRPM7 is facilitated or inhibited by osmotic changes irrespective of the direction of the osmotic gradient. One interesting observation here is that creating relative hypo-osmotic conditions by increasing intracellular osmolarity facilitates TRPM7 currents even in the absence of intracellular Mg·ATP, unlike the result when intracellular tonicity remains constant and the cell is superfused with a 200 mosmol l−1 solution.
The most straightforward interpretation of how TRPM7 increases or decreases channel activity in response to changes in osmolarity is through volume-induced concentration changes of solutes that interfere with channel activity, such as Mg-nucleotides, Mg2+ or polyvalent cations (Nadler et al. 2001; Hermosura et al. 2002; Kozak et al. 2005; Demeuse et al. 2006). Most of our data support this conclusion. Nevertheless, TRPM7's sensitivity to hyperosmotic conditions is retained to some extent even in the complete absence of divalent ions or the kinase domain. This could be due to concentration changes of a hitherto unidentified cellular factor or process that resists washout imposed by cell perfusion. Indeed, Kozak et al. (2002) have previously noted that MagNuM develops slower than anticipated for the time it would take to washout relatively small solutes such as divalents or Mg-nucleotides (Pusch & Neher, 1988).
Numata and colleagues observed delayed volume regulation upon hypotonic challenge in intact cells treated with siRNA against TRPM7 (Numata et al. 2007b), which was dependent on the presence of extracellular Ca2+. In our hands, overexpression of TRPM7, which causes enhanced TRPM7 activity in intact cells (Monteilh-Zoller et al. 2003), did not alter the cells' ability to perform RVD or RVI compared with control. On the other hand, we could observe a marked decrease in cytosolic calcium levels when exposing TRPM7-expressing HEK293 cells to hypertonic conditions, consistent with an inhibition of Ca2+ influx through TRPM7 when the channels close during cell shrinkage. When exposing cells to hypotonic solutions, we observed only small calcium increases, although TRPM7 activity was probably enhanced. While TRPM7 currents could not be resolved due to the concomitant development of Cl− currents, the TRPM7-mediated influx of Ca2+ resulted in a relatively small increase in global free Ca2+, presumably due to the presence of high ATP levels, which may help remove cytosolic Ca2+ through enhanced pump activity. Since many studies have reported the importance of extracellular Ca2+ in TRPM7's physiology, it could be that relative Ca2+ increases through hypotonic TRPM7 facilitation are concentrated locally around the channel pore and are too small to be detected globally.
In our first report on TRPM7 (Nadler et al. 2001), we had noted that overexpression of TRPM7 causes HEK293 cells to swell and detach from the substrate. This was recently confirmed and reported to be due to TRPM7 regulating cell adhesion through controlling the calcium-dependent protease calpain (Su et al. 2006), where Ca2+ influx through TRPM7 promotes activation of m-calpain leading to decreases in peripheral adhesion complexes. In contrast to this, another study observed an increase in cell adhesion and cell spreading in response to ‘mild’ overexpression of TRPM7 in N1E-115 neuroblastoma (Clark et al. 2006). These differing results may be due to the two different cell types investigated or related to the relative expression levels of TRPM7. Nevertheless, in the light of our results, it is tempting to speculate that regulation of TRPM7 channel activity by osmolarity will lead to enhanced or reduced Ca2+ influx, ultimately affecting the integrity of the cytoskeleton. Thus, TRPM7 would promote regulated substrate detachment under hypotonic stress and reduced or ablated m-calpain activity under hypertonic stress. Hence, we can hypothesize that in physiological settings, osmotic stress will cause cellular volume changes, which secondarily regulate TRPM7 activity by either increasing or decreasing the cytosolic concentrations of free Mg2+, Mg-nucleotides and a further unidentified factor. The resulting changes in the influx rates of divalent cations such as Mg2+ and Ca2+ may then regulate processes that control cytoskeletal functions. Deregulation of TRPM7 activity in either direction will cause the deleterious effects observed following TRPM7 overexpression or knockdown.
Acknowledgments
We thank K.L. Dang and M.K. Monteilh-Zoller for excellent technical support. This work was supported by NIH grant RO1-GM65360 (A.F.).
References
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Barritt G, Rychkov G. TRPs as mechanosensitive channels. Nat Cell Biol. 2005;7:105–107. doi: 10.1038/ncb0205-105. [DOI] [PubMed] [Google Scholar]
- Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeuse P, Penner R, Fleig A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol. 2006;127:421–434. doi: 10.1085/jgp.200509410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorovkov MV, Ryazanov AG. Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem. 2004;279:50643–50646. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- Fernandez JM, Neher E, Gomperts BD. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature. 1984;312:453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fomina AF, Fanger CM, Kozak JA, Cahalan MD. Single channel properties and regulated expression of Ca2+ release-activated Ca2+ (CRAC) channels in human T cells. J Cell Biol. 2000;150:1435–1444. doi: 10.1083/jcb.150.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem. 2003;278:21493–21501. doi: 10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr Induced membrane hypo/hyper-mechanosensitivity: a limitation of patch-clamp recording. Annu Rev Physiol. 1997;59:621–631. doi: 10.1146/annurev.physiol.59.1.621. [DOI] [PubMed] [Google Scholar]
- Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, Sumimoto H, Ito Y, Mori Y, Inoue R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci. 2004;95:403–419. doi: 10.1254/jphs.fp0040273. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Reiter B. TRP channels activated by extracellular hypo-osmoticity in epithelia. Biochem Soc Trans. 2007;35:91–95. doi: 10.1042/BST0350091. [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A. Dissociation of the store-operated calcium current ICRAC and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol. 2002;539:445–458. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab M, Furst J, Gschwentner M, Botta G, Garavaglia ML, Bazzini C, Rodighiero S, Meyer G, Eichmueller S, Woll E, Chwatal S, Ritter M, Paulmichl M. Mechanisms sensing and modulating signals arising from cell swelling. Cell Physiol Biochem. 2002;12:235–258. doi: 10.1159/000067895. [DOI] [PubMed] [Google Scholar]
- Jiang J, Li M, Yue L. Potentiation of TRPM7 inward currents by protons. J Gen Physiol. 2005;126:137–150. doi: 10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Newell EW, Schlichter LC. Regulation of a TRPM7-like current in rat brain microglia. J Biol Chem. 2003;278:42867–42876. doi: 10.1074/jbc.M304487200. [DOI] [PubMed] [Google Scholar]
- Kerschbaum HH, Cahalan MD. Single-channel recording of a store-operated Ca2+ channel in Jurkat T lymphocytes. Science. 1999;283:836–839. doi: 10.1126/science.283.5403.836. [DOI] [PubMed] [Google Scholar]
- Kerschbaum HH, Kozak JA, Cahalan MD. Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys J. 2003;84:2293–2305. doi: 10.1016/S0006-3495(03)75035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak JA, Kerschbaum HH, Cahalan MD. Distinct properties of CRAC and MIC channels in RBL cells. J Gen Physiol. 2002;120:221–235. doi: 10.1085/jgp.20028601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak JA, Matsushita M, Nairn AC, Cahalan MD. Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J Gen Physiol. 2005;126:499–514. doi: 10.1085/jgp.200509324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–496. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Langeslag M, Clark K, Moolenaar WH, van Leeuwen FN, Jalink K. Activation of TRPM7 channels by phospholipase C-coupled receptor agonists. J Biol Chem. 2007;282:232–239. doi: 10.1074/jbc.M605300200. [DOI] [PubMed] [Google Scholar]
- Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem. 1999;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutai H, Heller S. Vertebrate and invertebrate TRPV-like mechanoreceptors. Cell Calcium. 2003;33:471–478. doi: 10.1016/s0143-4160(03)00062-9. [DOI] [PubMed] [Google Scholar]
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Droogmans G. The endothelial volume-regulated anion channel, VRAC. Cell Physiol Biochem. 2000;10:313–320. doi: 10.1159/000016364. [DOI] [PubMed] [Google Scholar]
- Numata T, Shimizu T, Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell Physiol Biochem. 2007a;19:1–8. doi: 10.1159/000099187. [DOI] [PubMed] [Google Scholar]
- Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol. 2007b;292:C460–C467. doi: 10.1152/ajpcell.00367.2006. [DOI] [PubMed] [Google Scholar]
- Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- Okada Y. Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem Biophys. 2004;41:233–258. doi: 10.1385/CBB:41:2:233. [DOI] [PubMed] [Google Scholar]
- Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) J Physiol. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasantes-Morales H, Cardin V, Tuz K. Signaling events during swelling and regulatory volume decrease. Neurochem Res. 2000;25:1301–1314. doi: 10.1023/a:1007652330703. [DOI] [PubMed] [Google Scholar]
- Penner R, Fleig A. The Mg2+ and Mg2+-nucleotide-regulated channel-kinase TRPM7. Handb Exp Pharmacol. 2007;179:313–328. doi: 10.1007/978-3-540-34891-7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao L, Ho WK, Earm YE. Actin filaments regulate the stretch sensitivity of large-conductance, Ca2+-activated K+ channels in coronary artery smooth muscle cells. Pflugers Arch. 2003;446:523–528. doi: 10.1007/s00424-003-1079-y. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J Gen Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- Ryazanova LV, Pavur KS, Petrov AN, Dorovkov MV, Ryazanov AG. Novel type of signaling molecules: protein kinases covalently linked with ion channels. Mol Biol. 2001;35:271–283. [PubMed] [Google Scholar]
- Sakai H, Nakamura F, Kuno M. Synergetic activation of outwardly rectifying Cl- currents by hypotonic stress and external Ca2+ in murine osteoclasts. J Physiol. 1999;515:157–168. doi: 10.1111/j.1469-7793.1999.157ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardini A, Amey JS, Weylandt KH, Nobles M, Valverde MA, Higgins CF. Cell volume regulation and swelling-activated chloride channels. Biochim Biophys Acta. 2003;1618:153–162. doi: 10.1016/j.bbamem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem. 2005;280:37763–37771. doi: 10.1074/jbc.M509175200. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, Yue L, Runnels LW. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci U S A. 2004;101:6009–6014. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RK. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol. 2003;148:1–80. doi: 10.1007/s10254-003-0009-x. [DOI] [PubMed] [Google Scholar]