Abstract
Acute exercise induces collagen synthesis in both tendon and muscle, indicating an adaptive response in the connective tissue of the muscle–tendon unit. However, the mechanisms of this adaptation, potentially involving collagen-inducing growth factors (such as transforming growth factor-β-1 (TGF-β-1)), as well as enzymes related to collagen processing, are not clear. Furthermore, possible differential effects of specific contraction types on collagen regulation have not been investigated. Female Sprague–Dawley rats were subjected to 4 days of concentric, eccentric or isometric training (n = 7–9 per group) of the medial gastrocnemius, by stimulation of the sciatic nerve. RNA was extracted from medial gastrocnemius and Achilles tendon tissue 24 h after the last training bout, and mRNA levels for collagens I and III, TGF-β-1, connective tissue growth factor (CTGF), lysyl oxidase (LOX), metalloproteinases (MMP-2 and -9) and their inhibitors (TIMP-1 and 2) were measured by Northern blotting and/or real-time PCR. In tendon, expression of TGF-β-1 and collagens I and III (but not CTGF) increased in response to all types of training. Similarly, enzymes/factors involved in collagen processing were induced in tendon, especially LOX (up to 37-fold), which could indicate a loading-induced increase in cross-linking of tendon collagen. In skeletal muscle, a similar regulation of gene expression was observed, but in contrast to the tendon response, the effect of eccentric training was significantly greater than the effect of concentric training on the expression of several transcripts. In conclusion, the study supports an involvement of TGF-β-1 in loading-induced collagen synthesis in the muscle–tendon unit and importantly, it indicates that muscle tissue is more sensitive than tendon to the specific mechanical stimulus.
Tendon and the extracellular matrix (ECM) of skeletal muscle are collagen-rich tissues that are important for muscle function, especially in relation to force transmission. The fibrillar collagens, type I and III, are the major components of tendon and muscle ECM. In tendon, type I collagen is predominant, while both types are expressed at equal levels in muscle ECM (Light & Champion, 1984). In cases of training leading to muscle hypertrophy and increased strength, it seems imperative that these collagen-rich tissues adapt in order to sustain the integrity of the muscle–tendon unit (Kjaer et al. 2006). In vivo human studies indicate that collagen synthesis increases in both muscle and tendon in response to acute exercise (Langberg et al. 1999; Heinemeier et al. 2003; Moore et al. 2005; Miller et al. 2005), and, similarly, prolonged training appears to elevate type I collagen synthesis in human tendon (Langberg et al. 2001). These findings indicate that collagen synthesis, and possibly collagen mRNA expression, in tendon and muscle is regulated by exercise/training, and a few studies have in fact shown elevated expression levels of type I and III collagen mRNA in both muscle and tendon in response to loading in animals (Han et al. 1999; Archambault et al. 2001; Koskinen et al. 2001; Olesen et al. 2006b). Meanwhile, the knowledge on how specific contraction types (e.g. concentric versus eccentric) may influence collagen expression and synthesis in a differential manner is very limited. In the present study we have measured changes in tendon and muscle expression of type I and III collagen in response to short-term training involving either pure, concentric, eccentric or isometric contractions in rats.
It is acknowledged that loading induces collagen synthesis in muscle and tendon, but the mechanisms responsible for mediating this effect are still unclear. Several observations point to transforming growth factor-β-1 (TGF-β-1) as an essential mediator of mechanically induced collagen synthesis in a variety of cell types (Gutierrez & Perr, 1999; Nakatani et al. 2002; Kim et al. 2002; Lindahl et al. 2002), including patella tendon fibroblasts (Yang et al. 2004), and this growth factor may well act as a link between loading and collagen synthesis in the muscle–tendon unit. In addition to TGF-β-1, connective tissue growth factor (CTGF), a member of the CCN (CTGF, cyr61 and Nov) family, could be implicated in this process, as it is involved in mechanically induced collagen synthesis (Hishikawa et al. 2001; Schild & Trueb, 2002), possibly acting down-stream of TGF-β-1 (Grotendorst et al. 1996; Duncan et al. 1999).
Besides induction of collagen synthesis, mechanical loading of tendon and muscle tissue has been suggested to induce certain changes in the collagen structure, possibly involving increased collagen cross-linking (Kovanen et al. 1984; Kubo et al. 2002) but also degradation (Langberg et al. 2001; Olesen et al. 2006a). Therefore, training may change the expression of enzymes involved in collagen processing, such as lysyl oxidase (LOX), which facilitates cross-linking of collagen (Kagan & Li, 2003), and matrix metalloproteinases (MMPs) and their inhibitors, which regulate degradation of collagen molecules (Visse & Nagase, 2003).
The aim of the present study was to investigate the specific effects of concentric, eccentric and isometric short-term training in rats, with regard to the expression of type I and III collagen mRNA, in tendon and muscle tissue. Furthermore, to identify possible mediators of loading-induced collagen regulation in these tissues, we studied the changes in expression of collagen-inducing growth factors as well as enzymes related to collagen processing and turnover. Our earlier findings indicate that an anabolic response is induced in tendon tissue by short-term training and that this response is independent of the contraction type performed by the muscle (Heinemeier et al. 2007). Based on this, we hypothesize that a loading-induced expression of collagen and collagen-related growth factors and enzymes in tendon will not depend on contraction type. In muscle tissue, however, there are several indications that the anabolic effect is more pronounced after eccentric loading compared with other loading types (Hather et al. 1991; Hortobagyi et al. 2000; Farthing & Chilibeck, 2003; Heinemeier et al. 2007) and the regulation of muscle connective-tissue-related genes may well follow a similar pattern.
Methods
Training of rats
The model used for training of rats in the present study has been described earlier (Adams et al. 2004; Heinemeier et al. 2007).
Animals
Young adult female Sprague–Dawley rats, weighing 238 ± 2 g (mean ±s.e.m.), were assigned randomly to three groups (minimum of 7 in each group). Each group was subjected to involuntary concentric training (CON), eccentric training (ECC), or isometric training (ISO). Rats were housed in groups in standard vivarium cages on a 12: 12 h light–dark cycle and had ad libitum access to standard rat chow and water. The study was conducted according to the American Physiological Society Guiding Principles in the Care and Use of Animals, and the protocol was approved by the University of California Institutional Animal Care and Use Committee.
Electrical muscle stimulation
Prior to each training bout, the rats were lightly anaesthetized with an intraperitoneal injection of ketamine (30 mg kg−1), xylazine (4 mg kg−1) and acepromazine (1 mg kg−1). To confirm a satisfactory level of anaesthesia, the animals were carefully monitored during the training sessions with pinch tests on the feet for reflex behaviour. Stainless steel wire electrodes, coated with Teflon, were used for stimulation. The electrodes were introduced subcutaneously in the region adjacent to the poplateal fossa, via 22-gauge hypodermic needles (which were withdrawn leaving the electrode in place). Before insertion, a section of the Teflon was removed, leaving the wire exposed in the area lateral and medial to the sciatic nerve and allowing for field stimulation of the nerve. The electrodes were attached to the output poles of a Grass stimulus isolation unit interfaced with a Grass S8 stimulator. This allowed for delivery of current to the sciatic nerve and thus muscle activation.
When stimulation electrodes were in place, the rats were positioned in a specially designed training platform previously described (Caiozzo et al. 1992). The left leg was positioned in a footplate, which was attached to the shaft of a Cambridge model H ergometer. The voltage and stimulation frequency (∼50 Hz) were adjusted to produce maximal isometric tension.
Training
During all training bouts the sciatic nerve of the left leg was stimulated for 2 s with 18 s between stimulations. Sets consisted of 10 stimulations and were separated by 5 min rest. Rats were trained for four consecutive days with two sets on day 1, three sets on day 2, and four sets on day 3 and 4. After each training session the electrodes were removed. The right leg served as control.
The training protocols were controlled by computer via a digital-to-analog board (model DDA-06, Keithley Instruments) used to control footplate movement and to trigger the stimulus. A separate analog-to-digital board (DAS-16) was used to acquire force measurements (100 Hz acquisition). Data acquisition, control of stimulus triggering and footplate movements were programmed by using LabTech Note-book (Laboratory Technologies). Data analysis was conducted by using Acqknowledge software (Biopac Systems). Force output was monitored in real time on the computer screen during each contraction.
Rats of the ISO group had their right foot placed in the footplate at an angle of ∼44 deg relative to the tibia. The footplate angle was fixed during muscle stimulation. For the CON group, the ergometer allowed the footplate to move from 44 deg to 64 deg after the development of maximal isometric tension in the beginning of muscle contraction. For the ECC group, the footplate was moved from 44 deg to 24 deg after the development of maximal isometric tension. The movement rate was limited to 29 deg s−1 in the CON and ECC groups in order to maintain force development. It is important to note that muscles trained under these conditions generate forces that are significantly different among the three training modes, i.e. eccentric trained muscles generated the most force, while the concentric trained muscle generated the least amount of force (see Results).
Tissue sampling and storage
Twenty-four hours after the last training bout the rats were killed via an intraperitoneal injection of Pentosol euthanasia solution (Medical-Pharmex) at a dose of 0.4 ml kg−1. The medial gastrocnemius including the Achilles tendon, from both legs, was dissected free and the mid-section of the muscle belly and the Achilles tendon were cut out and stored at −80°C for later use.
RNA extraction
Total RNA from all tissue types was extracted according to the method described by Chomczynski & Sacchi (1987). Muscle tissue was homogenized in TRI-reagent (MRC) with a Polytron homogeniser (Ultra-Turrax T8, Ika labourtechnik, Staufen, Germany), while tendon tissue was homogenized in TRI-reagent using a bead-mixer (Retsch, MM300) with the aid of 3 mm steel beads. The tubes containing tendon tissue, TRI-reagent and beads, were shaken at 24 Hz for 60 s and then cooled on ice. This was repeated seven times.
Following homogenization, BCP (1-bromo-3-chloropropane) (MRC) was added (100 μl per 1000 μl TRI-reagent) in order to separate the samples into an aqueous and an organic phase. In tendon samples, glycogen was added (120 μg per 1000 μl TRI-reagent) to improve RNA precipitation. Following isolation of the aqueous phase, RNA was precipitated using isopropanol. The RNA pellet was then washed in ethanol and subsequently dissolved in RNase-free water. All samples were weighed prior to RNA extraction. RNA concentrations were determined by spectroscopy at 260 nm. To account for absorbance of the glycogen added to the tendon samples, mock extractions were made in which no tissue was present, and the mean absorbance of these negative controls (n = 3) was subtracted from all test values. A good RNA quality was ensured by gel electrophoresis.
Real time PCR
Total RNA (500 ng from muscle and 200 ng from tendon) was converted into cDNA in 20 μl using the OmniScript reverse transcriptase (Qiagen, CA, USA) according to the manufacture's protocol. For each target mRNA, 0.25 μl cDNA was amplified in a 25 μl SYBR Green PCR reaction containing 1 × Quantitect SYBR Green Master Mix (Qiagen, CA, USA) and 100 nm of each primer (Table 1).
Table 1.
Primers
| MRNA | Sense primer | Anti-sense primer |
|---|---|---|
| COL1A1a | ATCAGCCCAAACCCCAAGGAGA | CGCAGGAAGGTCAGCTGGATAG |
| COL3A1a | TGATGGGATCCAATGAGGGAGA | GAGTCTCATGGCCTTGCGTGTTT |
| TGF-β-1a | CCCCTGGAAAGGGCTCAACAC | TCCAACCCAGGTCCTTCCTAAAGTC |
| TGF-β-1b | GCTGCTGACCCCCACTGATA | CCAACCCAGGTCCTTCCTAA |
| CTGFa | CAGGCTGGAGAAGCAGAGTCGT | CTGGTGCAGCCAGAAAGCTCAA |
| CTGFb | GGCGAGTCCTTCCAAAGCAGTT | GGTCTTAGAACAGGCGCTCCAC |
| LOXa | CAGGCACCGACCTGGATATGG | CGTACGTGGATGCCTGGATGTAGT |
| MMP-2a | CTGGGTTTACCCCCTGATGTCC | AACCGGGGTCCATTTTCTTCTTT |
| MMP-9a | GGATGTTTTTGATGCCATTGCTG | CCACGTGCGGGCAATAAGAAAG |
| TIMP-1a | ATAGTGCTGGCTGTGGGGTGTG | TGATCGCTCTGGTAGCCCTTCTC |
| TIMP-2a | GGACACGCTTAGCATCACCCAGA | GTCCATCCAGAGGCACTCATCC |
| GAPDHa | CCATTCTTCCACCTTTGATGCT | TGTTGCTGTAGCCATATTCATTGT |
| RPLP0a | AGGGTCCTGGCTTTGTCTGTGG | AGCTGCAGGAGCAGCAGTGG |
Primers for real-time RT-PCR.
Primers for Northern probe generation.
The amplification was monitored real-time using the MX3000P real-time PCR machine (Stratagene, CA, USA). The threshold cycle (Ct) values were related to a standard curve made with the cloned PCR products and specificity was confirmed by melting curve analysis after amplification. The general range of Ct values was 15–30. The large ribosomal protein P0 (RPLP0) had been chosen as internal control, assuming RPLP0 mRNA to be constitutively expressed (Dheda et al. 2004). To validate this assumption another unrelated ‘constitutive’ RNA, GAPDH mRNA, was measured and RPLP0 was normalized to GAPDH. However, the RPLP0/GAPDH ratio was not stable and we chose to normalize mRNA data to the weight of the tissue needed for extracting the amount of RNA used for cDNA synthesis (500 ng RNA for muscle and 200 ng RNA for tendon). This choice of normalization has been discussed in our earlier publication where other data from the present study were presented (Heinemeier et al. 2007).
Northern blotting
Probe preparation
Human cDNA was amplified by PCR, using primers specific for the target genes (Table 1). The purified PCR products were ligated into the SmaI site of the pBlueScript II SK(+) vector and competent DH5αE. coli cells (Invitrogen, Carlsbad, CA, USA) were transformed with the plasmids. Single positive colonies were isolated, and cell cultures were grown and then purified with the High Pure Plasmid Isolation kit (Roche). Size and orientation of the inserted PCR products were tested by asymmetric restriction digest. Using the purified plasmids as templates, the inserts were amplified by PCR with 5′ biotinlylated M13 primers and non-biotinylated M13 primers complementary to the sequences flanking the inserts. The resulting PCR products were separated into single strands and the biotinylated strands were isolated with the use of streptavidin dynabeads. Radioactive probes complementary to the biotinylated DNA strands, were generated with the aid of Exonuklease-free Klenow DNA polymerase (StripEZ, Ambion) and isolated for use in the hybridization procedure.
Blotting and hybridization
Samples containing 460 ng of RNA for muscle, and 200 ng for tendon, were mixed with formaldehyde loading buffer (Ambion) and run on a formaldehyde agarose gel. The gel was dyed in SYBR Green II (Cambrex) and scanned on a fluorescence scanner (Imager FX, Biorad) in order to verify the integrity of the RNA. Gels were then blotted onto Appligene nylon membrane (Qbiogene, Irvine, CA, USA) by alkaline capillary transfer. Membranes were hybridized overnight at 50°C with 10 million counts min−1 per blot of probe in 6 ml hybridization buffer. After removal of excess probe, the blots were exposed on a phosphorscreen (Biorad).
Quantification
Phosphoscreen images were visualized using the phosphoimager (Imager FX, Biorad) and band intensity was measured with the use of Quantity One software (Biorad). All values were normalized to a reference mRNA (pooled samples) that was run on the gels at intervals alongside the test samples. 28S ribosomal RNA was intended for further normalization. However, as tissue weight was used for normalization of real-time RT-PCR data (discussed above), the same procedure was followed for the Northern data to allow comparison between the two methods.
Number of samples in each group
For muscle, all samples were successfully analysed (with both real-time RT-PCR and Northern blotting) and all groups included seven to nine samples: for tendon, four, six and eight samples for control, CON, ECC and ISO groups, respectively, and six, seven and nine samples for trained CON, ECC and ISO groups, respectively, were successfully analysed with real-time RT-PCR. Northern analysis was performed successfully on six to eight samples for all tendon groups.
Statistics
All mRNA data (except MMP-9 mRNA values) were log-transformed before statistical analyses and are presented as geometric means ± back-transformed s.e.m.
A two-way ANOVA on contraction × training was performed using the SAS (Statistical Analysis System) procedure mixed with autoregressive modelling. If the two-way ANOVA was significant, individual differences between trained and untrained leg within each contraction type, and between contraction types, were tested with a post hoc test (Bonferroni). Otherwise, training effect was tested against control on all contraction types combined. For MMP-9 mRNA, which was expressed at very low levels, a χ2 test was performed to test if the number of samples containing detectable levels of MMP-9 mRNA was altered as a result of the training intervention.
Differences were considered significant when P < 0.05.
Results
Force–time integral
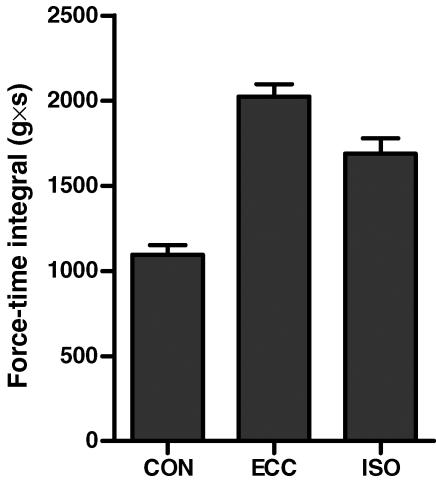
The force–time integral was significantly different between all three contraction modes (P < 0.01), with the greatest force–time integral being obtained during eccentric loading and the lowest during concentric loading (Fig. 1).
Figure 1. Mean force–time integral over the 2 s stimulation period for CON, ECC and ISO contractions.
There was a significant difference in force–time integral between all 3 contraction types (P < 0.01).
Type I collagen
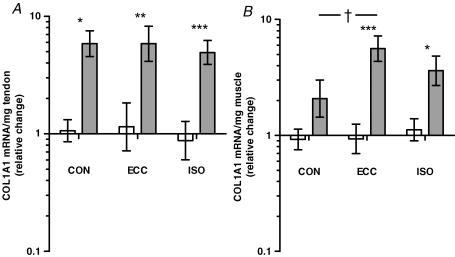
Achilles tendon levels of collagen type I, α1 (COL1A1) mRNA increased 5- to 6-fold in response to all types of training and no difference was seen between training types (P > 0.05) (Fig. 2A). In gastrocnemius muscle, only eccentric and isometric training increased COL1A1 mRNA expression (P < 0.05), and eccentric training tended to have a greater effect than concentric (P = 0.1) (Fig. 2B).
Figure 2. COL1A1 mRNA.
COL1A1 mRNA normalized to tissue weight, presented as fold changes relative to the mean of all control values, in Achilles tendon (A) and gastrocnemius muscle (B) subjected to concentric (CON), eccentric (ECC) and isometric (ISO) training (grey bars) versus contralateral controls (white bars). Values are geometric means ±s.e.m.A, COL1A1 increased in tendon in response to all training types (*P < 0.05, **P < 0.01, ***P < 0.001). B, muscle COL1A1 mRNA increased in response to ECC and ISO training (*P < 0.05, ***P < 0.001), and ECC training tended to have greater effect than CON (†P = 0.1).
Type III collagen
In tendon, collagen type III, α1 (COL3A1) mRNA increased markedly (15- to 20-fold) in response to all types of training (P < 0.001) (Fig. 3A) and in muscle, concentric training led to an ∼7-fold increase (P < 0.001), while eccentric and isometric training induced increases of approximately ∼13-fold (P < 0.001). However, no significant differences were seen between training types (P > 0.05) (Fig. 3B).
Figure 3. COL3A1 mRNA.
COL3A1 mRNA normalized to tissue weight, presented as fold changes relative to the mean of all control values, in Achilles tendon (A) and gastrocnemius muscle (B) subjected to concentric (CON), eccentric (ECC) and isometric (ISO) training (grey bars) versus contralateral controls (white bars). Values are geometric means ±s.e.m.A and B, COL3A1 increased in both tendon and muscle in response to all training types (***P < 0.001), and no difference was seen between training types (P > 0.05).
TGF-β-1 and CTGF
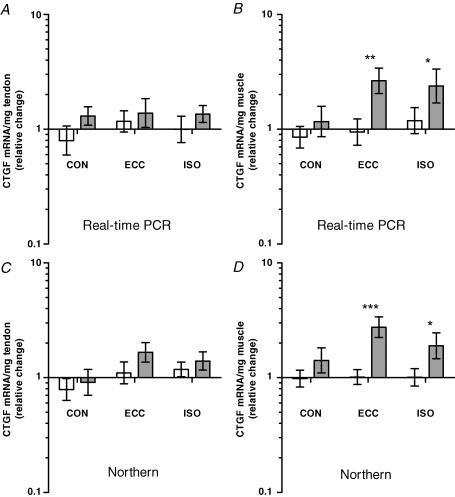
TGF-β-1 and CTGF mRNA were measured with both real-time RT-PCR and Northern blotting, and though fold changes appeared slightly lower with Northern blotting, the observed changes in mRNA levels for TGF-β-1 and CTGF were comparable (Figs 4 and 5). All types of training induced an increase in tendon levels of TGF-β-1 mRNA in a range of 2- to 5-fold (P < 0.001), with no difference between training types (Fig. 4A and C). In muscle, an increase was also seen in TGF-β-1 mRNA in response to all training types (P < 0.01); however, eccentric training induced a greater increase than concentric training (P < 0.05) (Fig. 4B and D). For CTGF, no differences were seen between control and trained tendon (P > 0.05) (Fig. 5A and C). In muscle, a moderate increase (1- to 2-fold) in CTGF mRNA was observed in response to eccentric and isometric training (P > 0.05), while no change was found after concentric training (P > 0.05) (Fig. 5B and D).
Figure 4. TGF-β-1 mRNA.
TGF-β-1 mRNA normalized to tissue weight, presented as fold changes relative to the mean of all control values, in Achilles tendon (A and C) and gastrocnemius muscle (B and D) subjected to concentric (CON), eccentric (ECC) and isometric (ISO) training (grey bars) versus contralateral controls (white bars). Levels of mRNA were measured with real-time RT-PCR (A and B) and Northern blotting (C and D). Values are geometric means ±s.e.m.A and C, in tendon, TGF-β-1 increased in response to all training types (**P < 0.01, ***P < 0.001). B and D, in muscle, TGF-β-1 increased in response to all training types (**P < 0.01, ***P < 0.001), but ECC training had greater effect than CON (#P < 0.05, ##P < 0.01).
Figure 5. CTGF mRNA.
CTGF mRNA normalized to tissue weight, presented as fold changes relative to the mean of all control values, in Achilles tendon (A and C) and gastrocnemius muscle (B and D) subjected to concentric (CON), eccentric (ECC) and isometric (ISO) training (grey bars) versus contralateral controls (white bars). Levels of mRNA were measured with real-time RT-PCR (A and B) and Northern blotting (C and D). Values are geometric means ±s.e.m.A and C, in tendon, no changes were seen in CTGF mRNA levels (P > 0.05). B and D, in muscle, CTGF increased in response to ECC and ISO (*P < 0.05, **P < 0.01, ***P < 0.001), while no effect was seen of CON (P > 0.05).
Lysyl oxidase
A marked increased was seen in tendon tissue expression of LOX mRNA in response to all training types (up to 37-fold increase) (P < 0.001) (Fig. 6A). In muscle, LOX expression increased more moderately (3- to 10-fold) and the effect of concentric training was just below significance (P = 0.06) (P < 0.001 for ECC and ISO) (Fig. 6B). No specific effect was seen of contraction type in either tissue type (P > 0.05).
Figure 6. Lysyl oxidase, MMP-2, TIMP-1 and TIMP-2 mRNA.
Lysyl oxidase, MMP-2, TIMP-1 and TIMP-2 mRNA normalized to tissue weight, presented as fold changes relative to the mean of all control values, in Achilles tendon (A, C, E and G) and gastrocnemius muscle (B, D, F and H) subjected to concentric (CON), eccentric (ECC) and isometric (ISO) training (grey bars) versus contralateral controls (white bars). Values are geometric means ±s.e.m.A and B, LOX mRNA levels increased in tendon and muscle in response to all training types, though this only tended to be significant for CON trained muscle (†P = 0.06, ***P < 0.001). C, tendon MMP-2 mRNA increased after CON and ISO and tended to do so after ECC (†P = 0.1, *P < 0.05 **P < 0.01). D, ECC and ISO (and possibly CON) loading-induced MMP-2 mRNA expression in muscle (†P = 0.09, **P < 0.01, ***P < 0.001). E–H, TIMP-1 and TIMP-2 mRNA levels were increased in both tendon and muscle in response to all training types (P < 0.05) and in muscle tissue the effect of eccentric training was greater than concentric (P < 0.05).
MMPs
MMP-2 mRNA expression appeared to increase moderately in both tendon and muscle in response to all types of training (1- to 3-fold), though not reaching significance for ECC tendon (P = 0.1) and CON muscle (P = 0.09). No specific effect was seen of contraction mode on MMP-2 expression (P > 0.05) (Fig. 6C and D).
MMP-9 was below detection level in nearly all control samples of both muscle and tendon. In response to training, the number of samples containing detectable levels of MMP-9 mRNA increased significantly (P < 0.001) (data not shown). However, even in loaded tissue the measured level of MMP-9 mRNA was still negligible compared with MMP-2 mRNA in both tissue types (data not shown).
TIMPs
Both TIMP-1 and -2 mRNA increased in response to all training types in tendon and muscle tissue (P < 0.05) (Fig. 6E–H). The induction of TIMP-2 was moderate (1- to 4-fold), whereas TIMP-1 was highly induced, especially in muscle tissue (up to 60-fold) (Fig. 6F). The effect of eccentric training was greater than concentric on both TIMP-1 and 2 expression in muscle (P < 0.05), while in tendon no specific effect was seen of contraction type (P > 0.05).
Discussion
The presented findings support the view that tendon and muscle connective tissue adapt to training by increased collagen synthesis and are in agreement with an involvement of transforming growth factor-β-1 (TGF-β-1) in this adaptive response in vivo. Furthermore, the results suggest that muscle tissue is more sensitive to differences in contraction type and/or force production than tendon tissue.
In humans, exercise/training has been shown to induce collagen synthesis in both tendon and muscle tissue, as measured by changes in the interstitial pro-collagen I C-terminal peptide (PICP) levels for tendon tissue (Langberg et al. 1999, 2001; Hishikawa et al. 2001; Heinemeier et al. 2003; Crameri et al. 2004; Olesen et al. 2006a), and by increases in incorporation of amino acid tracers for both tendon and muscle (Moore et al. 2005; Miller et al. 2005). Likewise, earlier animal studies show increased collagen synthesis rates during stretch-induced muscle hypertrophy (Laurent et al. 1985). These observations indicate that the supporting connective tissues of the skeletal muscle undergo an adaptive response when subjected to increased loading. In line with this, we find a noticeably increased expression of type I and III collagen in both tendon and muscle in response to short-term strength training in rats. Very few in vivo results have been published with regard to tendon expression of collagen mRNA in response to loading. Archambault et al. showed increased type III collagen expression in rabbit Achilles tendon in response to 11 weeks of high-frequency loading at low stress/strain levels, but did not detect any change in type I collagen mRNA levels (Archambault et al. 2001). The longer loading duration in that study, compared with the present one, could explain why no change was observed for type I collagen expression, as an increase may have occurred at an earlier time-point. In accordance with the present observations, we have recently shown that functional loading of the plantaris tendon in rats leads to increased expression levels of both type I collagen (after 8 days of loading) and type III collagen (after 2 days of loading) (Olesen et al. 2006b). Regarding the regulation of collagen expression in skeletal muscle, our findings are in line with two earlier studies showing increases in collagen type I and III mRNA in response to acute strenuous eccentric exercise in both damaged and undamaged rat muscle (Han et al. 1999; Koskinen et al. 2001), and with findings of increased type I collagen expression in rabbit muscle after long-term jump training (Ducomps et al. 2004).
The mechanisms that link mechanical loading during exercise/training to increased expression of collagen in the muscle–tendon unit are still unclear. There are several indications, however, that mechanically induced collagen synthesis in general relies on an increased expression of collagen inducing stress/strain-responsive growth factors, including transforming growth factor-β-1 (TGF-β-1) and connective tissue growth factor (CTGF) (Chiquet et al. 2003). A positive correlation between mechanical loading of cells and TGF-β-1 expression has been shown in both in vitro and in vivo studies, on a large variety of human and animal cell and tissue types (Villarreal & Dillmann, 1992; Robbins et al. 1997; Cucina et al. 1998; Li et al. 1998; Gutierrez & Perr, 1999; Lee et al. 1999; Cillo et al. 2000; Joki et al. 2000; O'Callaghan & Williams, 2000; Ruwhof et al. 2000; Zheng et al. 2001; Skutek et al. 2001; Lindahl et al. 2002). Importantly, loading-induced type I and/or type III collagen expression appears to depend directly on TGF-β-1 activity in human ligament (Nakatani et al. 2002; Kim et al. 2002) and patella tendon fibroblast (Yang et al. 2004). Thus, the in vitro evidence that suggests TGF-β-1 as an important mediator of mechanically induced collagen synthesis in fibroblasts is relatively strong, and the recent findings by Yang et al. support such a role for TGF-β-1 in tendon (Yang et al. 2004). However, only few in vivo data have been published with regard to the role of TGF-β-1 in loaded tendon tissue and while one study supports the idea that TGF-β-1 could be important for Achilles tendon adaptation to uphill running in humans (Heinemeier et al. 2003), recent work by Legerlotz et al. showed no change in TGF-β-1 expression in response to different types of long-term training in rats (Legerlotz et al. 2007). However, in that study mRNA levels were only measured after 12 weeks of training and an increase in TGF-β-1 expression may well have been present at earlier stages. The present in vivo observations of increased TGF-β-1 mRNA levels in the early phase of resistance training, combined with an elevated type I and III collagen expression, provide significant support for the suggestion of TGF-β-1 as a link between exercise/training and collagen expression in tendon tissue.
In skeletal muscle, TGF-β-1 has been suggested to be involved in both exercise-induced angiogenesis (Breen et al. 1996; Gavin & Wagner, 2001) and pathological muscle fibrosis (Li et al. 2004), and increases in TGF-β-1 mRNA have been observed in response to muscle damaging eccentric exercise in humans (Hamada et al. 2005) and to acute endurance exercise and short-term endurance training in rats (Breen et al. 1996; Gavin & Wagner, 2001). In the present study we find an elevated expression of TGF-β-1 in skeletal muscle, along with elevated collagen mRNA levels, in response to short-term training. These findings, combined with the well-known collagen-inducing effect of TGF-β-1, support a role for this growth factor in relation to training-induced non-pathological muscle ECM production. The present and earlier studies do not specify the source of TGF-β-1 mRNA, and though myotubes do not appear to increase TGF-β-1 expression in response to strain (Tsivitse et al. 2005), indicating that other cell types present in skeletal muscle (e.g. fibroblasts, vascular cells) are a likely source, further investigation is needed in order to determine which cell types are responsible for the production of TGF-β-1 mRNA. This could help to pinpoint the functions of TGF-β-1 in skeletal muscle. Importantly, however, it should be considered that a general increase in TGF-β-1 protein concentration, regardless of the source, is likely to affect gene expression in a number of cell types, including collagen expression in muscle fibroblasts.
Connective tissue growth factor (CTGF) – a potent stimulator of collagen synthesis – is induced by mechanical stimuli (Schild & Trueb, 2002) and appears to be involved in mechanically induced collagen synthesis in certain cell types (Hishikawa et al. 2001). In fibroblasts, CTGF is induced by TGF-β-1 and, by acting as a down-stream mediator, CTGF appears to be at least partly responsible for the collagen-inducing actions of TGF-β-1 (Grotendorst et al. 1996; Duncan et al. 1999). The role of CTGF in tendon and muscle is largely unknown. However, one recent study by Nakama et al. has shown an increase in the amount of CTGF-positive (immuno-stained) cells in rabbit tendon after 80 accumulated hours of low force repetitive loading (Nakama et al. 2006). In contrast, we did not observe any significant change in tendon CTGF mRNA levels in response to training. However, the results of the present study are not easily compared with the findings of Nakama et al. as the amount and duration of stress/strain applied to tendons in the two studies are fundamentally different. Furthermore, the loading regime applied by Nakama et al. led to microtears in the tendon tissue and initiation of a wound-healing response might explain the observed increase in CTGF staining (Nakama et al. 2005). Our findings do not indicate an involvement of CTGF in the adaptation of tendon tissue to training; however, an increase in CTGF expression may have occurred at an earlier time-point than the one chosen for measurement, and further experiments are needed to clarify the role of CTGF in tendon.
The function of CTGF in skeletal muscle is unknown; however, recent observations show that rat myoblasts and myotubes express CTGF in vitro, and that its expression is increased by TGF-β-1 (Obreo et al. 2004; Maeda et al. 2005). Furthermore, several types of fibroblast increase their expression of CTGF in response to both mechanical loading (Schild & Trueb, 2002) and exogenous TGF-β-1 (Grotendorst et al. 1996; Duncan et al. 1999; Blalock et al. 2003) and a similar response in muscle ECM fibroblasts seems likely. Thus, an increased TGF-β-1 expression, along with the mechanical stimulus during training, are possible explanations for the observed increases in muscle CTGF expression in response to eccentric and isometric training. A possible connection between TGF-β-1 and CTGF expression is supported by the fact that concentric training had a relatively low effect on TGF-β-1 expression and no effect on CTGF expression.
In addition to increased collagen synthesis, the adaptation of tendon and muscle connective tissue to training is thought to involve changes in the quality of the tissue structure, such as increased levels of cross-linking between collagen molecules (Kovanen et al. 1984; Buchanan & Marsh, 2002; Kubo et al. 2002). The enzyme lysyl oxidase (LOX) could be important for this type of adaptation as it facilitates the formation of lysine-derived covalent cross-links between and within collagen molecules, leading to stabilization and strengthening of fibrillar collagen structures (Kagan & Li, 2003). Though one recent study indicates that habitual tendon loading may be necessary to maintain normal LOX expression (Arruda et al. 2007), the present study is apparently the first to show that LOX mRNA expression in tendon is highly responsive to mechanical stimuli. A similar trend was seen in muscle, which is in accordance with earlier work by Han et al. (1999), and although these observations should be supplemented with more mechanistic experiments (and verified on the protein level) our findings definitely support the idea of an increased collagen cross-linking as part of tendon and muscle connective tissue adaptation to loading.
A transient increase in collagen degradation appears to be an additional aspect of the tendon-tissue response to mechanical loading (Langberg et al. 2001; Olesen et al. 2006a), and matrix metalloproteinases, MMP-2 and -9, which can degrade intact fibrillar collagen, may be involved in this process (Visse & Nagase, 2003). We have previously shown a pronounced increase in MMP-9 gelatinolytic activity in dialysate obtained from human peritendinous tissue immediately after acute exercise (Koskinen et al. 2004), and recent data suggest increased levels of both MMP-9 protein and mRNA in skeletal muscle shortly after a single exercise bout in men (Rullman et al. 2007). In the present study, however, MMP-9 mRNA was below the detection level in non-loaded muscle/tendon tissue and barely detectable in loaded tissue. In support of this, Koskinen et al. could not detect MMP-9 mRNA in control or eccentrically loaded rat muscle (Koskinen et al. 2001), and similar observations have recently been made in rat muscle subjected to long-term high-intensity exercise (Carmeli et al. 2005). Additionally, studies on human cadaver tissue suggest very low MMP-9 expression in tendon (Riley et al. 2002). This discrepancy could have several explanations, one being that the two studies, which show a pronounced loading-induced increase in MMP-9 activity, both included traumatic interventions prior to exercise (i.e. insertion of microdialysis probes or multiple muscle biopsy procedures) (Koskinen et al. 2004; Rullman et al. 2007), which may have contributed to the post-exercise increase in MMP-9 activity/expression (Madlener, 1998). However, this is speculative, and evidently further studies are needed to clarify the presence and importance of MMP-9 in tendon and skeletal muscle.
Though MMP-2 is known to be expressed in both human and rat tendon tissue (Riley, 2005; Jones et al. 2006; Legerlotz et al. 2007), the relationship between mechanical loading and MMP-2 expression in tendon is unclear. In the present study we found a moderate increase in MMP-2 expression in loaded tendon, and previously we have observed a transient decrease followed by an increase in MMP-2 gelatinolytic activity in human Achilles peritendon tissue after acute exercise (Koskinen et al. 2004). Meanwhile, a recent study by Legerlotz et al. did not indicate any regulation of tendon MMP-2 mRNA expression in response to long-term strength training in rats (Legerlotz et al. 2007). Our results on skeletal muscle are in line with earlier studies showing elevated MMP-2 expression in rat muscle after acute downhill running (Koskinen et al. 2001) and after long-term endurance training (Carmeli et al. 2005). Considering that MMP-2 alone can mediate degradation of mature collagen molecules (Visse & Nagase, 2003), our findings could support a loading-induced collagen degradation. However, along with the increased MMP-2 expression, we observed a significant induction of the tissue inhibitors of metalloproteinases (TIMPs), especially TIMP-1 (Fig. 6), and given their inhibitory effect on MMP activity it is not possible to predict the net effect on collagen turnover.
With regard to the specific effects of different contraction types, we saw a similar increase in tendon expression of collagens and regulatory factors in response to the three different loading types. In muscle, however, eccentric training had a greater effect than concentric training on expression on TGF-β-1 and TIMPs. Furthermore, concentric training did not change muscle CTGF expression, while an effect was seen of both eccentric and isometric training. Taking into account the well-known effect of both TGF-β-1 and CTGF on collagen expression, it could be expected that eccentric training would have a greater effect on muscle collagen expression than concentric. In support of this, collagen I expression was not significantly induced by concentric training, while a 4-fold increase was seen after eccentric loading, and although the difference between eccentric and concentric loading did not reach significance (P = 0.1), we cannot conclude that collagen expression in muscle is unrelated to specific training types. The differences seen in the muscle response to specific contraction types could possibly be linked to differences in generation of shear stress between muscle fibres and ECM, as this could lead to a differential mechanical loading on both muscle fibres and connective tissue cells. However, the differences in force production between the training modes are also a likely explanation. The adaptive response of muscle was generally lowest after concentric training and highest in response to eccentric training, which corresponds well with forces generated during the different contraction types (Fig. 1). The possibility that the muscle response is primarily related to the force production is supported by recent data showing an equal increase in the expression of several anabolic genes, as well as type III collagen, in rat skeletal muscle in response to concentric, eccentric and isometric training with equal force–time integrals (Garma et al. 2007). Although the differential muscle response may simply relate to force production, it is still interesting that skeletal muscle tissue appears sensitive to the specific force development (and/or contraction type), while no differential response is seen in tendon, even though the stress/strain experienced by this tissue is directly related to the force generated by muscle. These observations, combined with our corresponding results on anabolic growth factors (Heinemeier et al. 2007), indicate a more specific adaptive response in muscle, compared with tendon, and potentially, certain training modes may lead to an unbalanced adaptation of tendon versus muscle.
Eccentric training might have been expected to induce a specific tendon response, as several studies in humans show a superior effect of eccentric loading in treatment of achilles tendinosis compared with other loading types (e.g. Mafi et al. 2001; Silbernagel et al. 2001). Thus, based on the present observations, it could be speculated that the beneficial effect of eccentric training in the treatment of tendon pathology is unrelated to collagen and growth factor expression. However, when considering the differences in species, duration of training intervention and range of movement during contraction, this is by no means certain.
In conclusion, we have demonstrated that short-term training induces collagen expression in both skeletal muscle and tendon tissue, and our findings support the hypothesis that TGF-β-1 acts as a mediator of training-induced collagen expression in these tissues. Furthermore, eccentric training appears to have a larger potential than concentric training for increasing the expression of collagen-inducing growth factors in muscle. This may be connected to a greater force production during eccentric contraction, or perhaps to a greater local shear stress in the muscle tissue. The changes seen in tendon were not dependent on stress/strain levels or contraction type, which indicates that this tissue is less sensitive than skeletal muscle to differences in mechanical stimulus.
Acknowledgments
The authors address a special thanks to Ann-Christina Reimann, Christina Bøg Sørensen, Lisbeth Tranholm Andersen and Satu Koskinen for excellent technical assistance. This study was supported by grants from the Danish Rheumatism Association, The Danish Ministry of Culture Committee for Sports Research, Danish Medical Research Counsel (22-04-0191), Lundbeck Foundation (128/03), Novo Nordic Foundation, and Medical Faculty, University of Copenhagen, NSBRI-NC9–58 to K.M.B.
References
- Adams GR, Cheng DC, Haddad F, Baldwin KM. Skeletal muscle hypertrophy in response to isometric, lengthening, and shortening training bouts of equivalent duration. J Appl Physiol. 2004;96:1613–1618. doi: 10.1152/japplphysiol.01162.2003. [DOI] [PubMed] [Google Scholar]
- Archambault JM, Hart DA, Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect Tissue Res. 2001;42:13–23. doi: 10.3109/03008200109014245. [DOI] [PubMed] [Google Scholar]
- Arruda EM, Mundy K, Calve S, Baar K. Denervation does not change the ratio of collagen I and collagen III mRNA in the extracellular matrix of muscle. Am J Physiol Regul Integr Comp Physiol. 2007;292:R983–R987. doi: 10.1152/ajpregu.00483.2006. [DOI] [PubMed] [Google Scholar]
- Blalock TD, Duncan MR, Varela JC, Goldstein MH, Tuli SS, Grotendorst GR, Schultz GS. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003;44:1879–1887. doi: 10.1167/iovs.02-0860. [DOI] [PubMed] [Google Scholar]
- Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol. 1996;81:355–361. doi: 10.1152/jappl.1996.81.1.355. [DOI] [PubMed] [Google Scholar]
- Buchanan CI, Marsh RL. Effects of exercise on the biomechanical, biochemical and structural properties of tendons. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1101–1107. doi: 10.1016/s1095-6433(02)00139-3. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Ma E, McCue SA, Smith E, Herrick RE, Baldwin KM. A new animal model for modulating myosin isoform expression by altered mechanical activity. J Appl Physiol. 1992;73:1432–1440. doi: 10.1152/jappl.1992.73.4.1432. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Moas M, Lennon S, Powers SK. High intensity exercise increases expression of matrix metalloproteinases in fast skeletal muscle fibres. Exp Physiol. 2005;90:613–619. doi: 10.1113/expphysiol.2004.029462. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Renedo AS, Huber F, Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22:73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cillo JE, Jr, Gassner R, Koepsel RR, Buckley MJ. Growth factor and cytokine gene expression in mechanically strained human osteoblast-like cells: implications for distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:147–154. doi: 10.1067/moe.2000.107531. [DOI] [PubMed] [Google Scholar]
- Crameri RM, Langberg H, Teisner B, Magnusson P, Schroder HD, Olesen JL, Jensen CH, Koskinen S, Suetta C, Kjaer M. Enhanced procollagen processing in skeletal muscle after a single bout of eccentric loading in humans. Matrix Biol. 2004;23:259–264. doi: 10.1016/j.matbio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Cucina A, Sterpetti AV, Borrelli V, Pagliei S, Cavallaro A, D'Angelo LS. Shear stress induces transforming growth factor-β 1 release by arterial endothelial cells. Surgery. 1998;123:212–217. [PubMed] [Google Scholar]
- Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37:112–119. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- Ducomps C, Larrouy D, Mairal A, Doutreloux JP, Lebas F, Mauriege P. Effects of jump training on procollagen α1 (I) mRNA expression and its relationship with muscle collagen concentration. Can J Appl Physiol. 2004;29:157–171. doi: 10.1139/h04-012. [DOI] [PubMed] [Google Scholar]
- Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor b-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- Farthing JP, Chilibeck PD. The effects of eccentric and concentric training at different velocities on muscle hypertrophy. Eur J Appl Physiol. 2003;89:578–586. doi: 10.1007/s00421-003-0842-2. [DOI] [PubMed] [Google Scholar]
- Garma T, Kobayashi C, Haddad F, Adams GR, Bodell PW, Baldwin KM. Similar acute molecular responses to equivalent volumes of isometric, lengthening, or shortening mode resistance exercise. J Appl Physiol. 2007;102:135–143. doi: 10.1152/japplphysiol.00776.2006. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Wagner PD. Effect of short-term exercise training on angiogenic growth factor gene responses in rats. J Appl Physiol. 2001;90:1219–1226. doi: 10.1152/jappl.2001.90.4.1219. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor b response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]
- Gutierrez JA, Perr HA. Mechanical stretch modulates TGF-β1 and α1 (I) collagen expression in fetal human intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 1999;277:G1074–G1080. doi: 10.1152/ajpgi.1999.277.5.G1074. [DOI] [PubMed] [Google Scholar]
- Hamada K, Vannier E, Sacheck JM, Witsell AL, Roubenoff R. Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB J. 2005;19:264–266. doi: 10.1096/fj.03-1286fje. [DOI] [PubMed] [Google Scholar]
- Han XY, Wang W, Komulainen J, Koskinen SO, Kovanen V, Vihko V, Trackman PC, Takala TE. Increased mRNAs for procollagens and key regulating enzymes in rat skeletal muscle following downhill running. Pflugers Arch. 1999;437:857–864. doi: 10.1007/s004240050855. [DOI] [PubMed] [Google Scholar]
- Hather BM, Tesch PA, Buchanan P, Dudley GA. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand. 1991;143:177–185. doi: 10.1111/j.1748-1716.1991.tb09219.x. [DOI] [PubMed] [Google Scholar]
- Heinemeier K, Langberg H, Olesen JL, Kjaer M. Role of TGF-b1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol. 2003;95:2390–2397. doi: 10.1152/japplphysiol.00403.2003. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol. 2007;102:573–581. doi: 10.1152/japplphysiol.00866.2006. [DOI] [PubMed] [Google Scholar]
- Hishikawa K, Oemar BS, Nakaki T. Static pressure regulates connective tissue growth factor expression in human mesangial cells. J Biol Chem. 2001;276:16797–16803. doi: 10.1074/jbc.M010722200. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Dempsey L, Fraser D, Zheng D, Hamilton G, Lambert J, Dohm L. Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans. J Physiol. 2000;524:293–304. doi: 10.1111/j.1469-7793.2000.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joki N, Kaname S, Hirakata M, Hori Y, Yamaguchi T, Fujita T, Katoh T, Kurokawa K. Tyrosine-kinase dependent TGF-b and extracellular matrix expression by mechanical stretch in vascular smooth muscle cells. Hypertens Res. 2000;23:91–99. doi: 10.1291/hypres.23.91. [DOI] [PubMed] [Google Scholar]
- Jones GC, Corps AN, Pennington CJ, Clark IM, Edwards DR, Bradley MM, Hazleman BL, Riley GP. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006;54:832–842. doi: 10.1002/art.21672. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Kim SG, Akaike T, Sasagaw T, Atomi Y, Kurosawa H. Gene expression of type I and type III collagen by mechanical stretch in anterior cruciate ligament cells. Cell Struct Funct. 2002;27:139–144. doi: 10.1247/csf.27.139. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Magnusson P, Krogsgaard M, Moller JB, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat. 2006;208:445–450. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen SO, Heinemeier KM, Olesen JL, Langberg H, Kjaer M. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J Appl Physiol. 2004;96:861–864. doi: 10.1152/japplphysiol.00489.2003. [DOI] [PubMed] [Google Scholar]
- Koskinen SO, Wang W, Ahtikoski AM, Kjaer M, Han XY, Komulainen J, Kovanen V, Takala TE. Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1292–R1300. doi: 10.1152/ajpregu.2001.280.5.R1292. [DOI] [PubMed] [Google Scholar]
- Kovanen V, Suominen H, Heikkinen E. Mechanical properties of fast and slow skeletal muscle with special reference to collagen and endurance training. J Biomech. 1984;17:725–735. doi: 10.1016/0021-9290(84)90103-9. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Fukunaga T. Effects of resistance and stretching training programmes on the viscoelastic properties of human tendon structures in vivo. J Physiol. 2002;538:219–226. doi: 10.1113/jphysiol.2001.012703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent GJ, McAnulty RJ, Gibson J. Changes in collagen synthesis and degradation during skeletal muscle growth. Am J Physiol Cell Physiol. 1985;249:C352–C355. doi: 10.1152/ajpcell.1985.249.3.C352. [DOI] [PubMed] [Google Scholar]
- Lee AA, Delhaas T, McCulloch AD, Villarreal FJ. Differential responses of adult cardiac fibroblasts to in vitro biaxial strain patterns. J Mol Cell Cardiol. 1999;31:1833–1843. doi: 10.1006/jmcc.1999.1017. [DOI] [PubMed] [Google Scholar]
- Legerlotz K, Schjerling P, Langberg H, Bruggemann GP, Niehoff A. The effect of running, strength, and vibration strength training on the mechanical, morphological, and biochemical properties of the Achilles tendon in rats. J Appl Physiol. 2007;102:564–572. doi: 10.1152/japplphysiol.00767.2006. [DOI] [PubMed] [Google Scholar]
- Li Q, Muragaki Y, Hatamura I, Ueno H, Ooshima A. Stretch-induced collagen synthesis in cultured smooth muscle cells from rabbit aortic media and a possible involvement of angiotensin II and transforming growth factor-β. J Vasc Res. 1998;35:93–103. doi: 10.1159/000025570. [DOI] [PubMed] [Google Scholar]
- Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-β1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light N, Champion AE. Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem J. 1984;219:1017–1026. doi: 10.1042/bj2191017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl GE, Chambers RC, Papakrivopoulou J, Dawson SJ, Jacobsen MC, Bishop JE, Laurent GJ. Activation of fibroblast procollagen α1 (I) transcription by mechanical strain is transforming growth factor-β-dependent and involves increased binding of CCAAT-binding factor (CBF/NF-Y) at the proximal promoter. J Biol Chem. 2002;277:6153–6161. doi: 10.1074/jbc.M108966200. [DOI] [PubMed] [Google Scholar]
- Madlener M. Differential expression of matrix metalloproteinases and their physiological inhibitors in acute murine skin wounds. Arch Dermatol Res. 1998;290:S24–S29. doi: 10.1007/pl00007450. [DOI] [PubMed] [Google Scholar]
- Maeda N, Kanda F, Okuda S, Ishihara H, Chihara K. Transforming growth factor-b enhances connective tissue growth factor expression in L6 rat skeletal myotubes. Neuromuscul Disord. 2005;15:790–793. doi: 10.1016/j.nmd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Mafi N, Lorentzon R, Alfredson H. Superior shortterm results with eccentric calf muscle training compared to concentric training in a randomized prospective multileft study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2001;9:42–47. doi: 10.1007/s001670000148. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–E1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- Nakama LH, King KB, Abrahamsson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Nakama LH, King KB, Abrahamsson S, Rempel DM. VEGF, VEGFR-1, and CTGF cell densities in tendon are increased with cyclical loading: An in vivo tendinopathy model. J Orthop Res. 2006;24:393–400. doi: 10.1002/jor.20053. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Marui T, Hitora T, Doita M, Nishida K, Kurosaka M. Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-β1. J Orthop Res. 2002;20:1380–1386. doi: 10.1016/S0736-0266(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Obreo J, Diez-Marques L, Lamas S, Duwell A, Eleno N, Bernabeu C, Pandiella A, Lopez-Novoa JM, Rodriguez-Barbero A. Endoglin expression regulates basal and TGF-b1-induced extracellular matrix synthesis in cultured L6E9 myoblasts. Cell Physiol Biochem. 2004;14:301–310. doi: 10.1159/000080340. [DOI] [PubMed] [Google Scholar]
- O'Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-β1. Hypertension. 2000;36:319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- Olesen JL, Heinemeier KM, Gemmer C, Kjaer M, Flyvbjerg A, Langberg H. Exercise dependent IGF-I, IGFBPs and type-I collagen changes in human peritendinous connective tissue determined by microdialysis. J Appl Physiol. 2006a;102:214–220. doi: 10.1152/japplphysiol.01205.2005. [DOI] [PubMed] [Google Scholar]
- Olesen JL, Heinemeier KM, Haddad F, Langberg H, Flyvbjerg A, Kjaer M, Baldwin KM. Expression of insulin-like growth factor I, insulin-like growth factor binding proteins, and collagen mRNA in mechanically loaded plantaris tendon. J Appl Physiol. 2006b;101:183–188. doi: 10.1152/japplphysiol.00636.2005. [DOI] [PubMed] [Google Scholar]
- Riley GP. Gene expression and matrix turnover in overused and damaged tendons. Scand J Med Sci Sports. 2005;15:241–251. doi: 10.1111/j.1600-0838.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- Riley GP, Curry V, DeGroot J, van El B, Verzijl N, Hazleman BL, Bank RA. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-b regulate proteoglycan synthesis in tendon. Arch Biochem Biophys. 1997;342:203–211. doi: 10.1006/abbi.1997.0102. [DOI] [PubMed] [Google Scholar]
- Rullman E, Rundqvist H, Wagsater D, Fischer H, Eriksson P, Sundberg CJ, Jansson E, Gustafsson T. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol. 2007;102:2346–2351. doi: 10.1152/japplphysiol.00822.2006. [DOI] [PubMed] [Google Scholar]
- Ruwhof C, van Wamel AE, van der Egas JM, van der Egas LA. Cyclic stretch induces the release of growth promoting factors from cultured neonatal cardiomyocytes and cardiac fibroblasts. Mol Cell Biochem. 2000;208:89–98. doi: 10.1023/a:1007046105745. [DOI] [PubMed] [Google Scholar]
- Schild C, Trueb B. Mechanical stress is required for high-level expression of connective tissue growth factor. Exp Cell Res. 2002;274:83–91. doi: 10.1006/excr.2001.5458. [DOI] [PubMed] [Google Scholar]
- Silbernagel KG, Thomee R, Thomee P, Karlsson J. Eccentric overload training for patients with chronic Achilles tendon pain – a randomised controlled study with reliability testing of the evaluation methods. Scand J Med Sci Sports. 2001;11:197–206. doi: 10.1034/j.1600-0838.2001.110402.x. [DOI] [PubMed] [Google Scholar]
- Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur J Appl Physiol. 2001;86:48–52. doi: 10.1007/s004210100502. [DOI] [PubMed] [Google Scholar]
- Tsivitse SK, Mylona E, Peterson JM, Gunning WT, Pizza FX. Mechanical loading and injury induce human myotubes to release neutrophil chemoattractants. Am J Physiol Cell Physiol. 2005;288:C721–C729. doi: 10.1152/ajpcell.00237.2004. [DOI] [PubMed] [Google Scholar]
- Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-β1, fibronectin, and collagen. Am J Physiol Heart Circ Physiol. 1992;262:H1861–H1866. doi: 10.1152/ajpheart.1992.262.6.H1861. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Yang G, Crawford RC, Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37:1543–1550. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Zheng W, Seftor EA, Meininger CJ, Hendrix MJ, Tomanek RJ. Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGF-β. Am J Physiol Heart Circ Physiol. 2001;280:H909–H917. doi: 10.1152/ajpheart.2001.280.2.H909. [DOI] [PubMed] [Google Scholar]