Abstract
Despite its very low concentration in the plasma membrane, PIP2 is the precursor for the important second messenger InsP3 and, independently, is a key modulator of membrane signalling molecules such as ion channels. However, it has been difficult to determine the spatial and temporal characteristics of PIP2 and InsP3 during a cell signalling event. Our laboratory used bradykinin stimulation of N1E-115 neuroblastoma cells to infer the InsP3 dynamics from calcium imaging studies, biochemical analysis and InsP3 uncaging. We have used computational modelling with Virtual Cell to help analyse and interpret experimental data on the details of the calcium release process as well as to build a comprehensive image-based model of agonist-induced calcium release in a neuronal cell. These data provided a constraint for the further investigation of how low levels of cellular PIP2 could provide sufficient InsP3 for calcium release. Using biochemical assays, quantitative imaging of GFP-based probe translocation and computational analysis, it was shown that PIP2 synthesis is stimulated concomitant with its hydrolysis. This mechanism should be important not just for consideration of PIP2 as a precursor of InsP3, but for any pathway that can be directly or indirectly modulated by PIP2.
The hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) to produce inositol-1,4,5-trisphosphate (InsP3), which in turn triggers release of calcium from the endo-plasmic reticulum, is a ubiquitous signalling pathway that is evoked by many external stimuli (Berridge & Irvine, 1989; Berridge, 1993, 1998; Putney & Bird, 1993). Figure 1 shows that the neurotransmitter glutamate can bind to a G-protein coupled receptor leading to activation of phospholipase C-β (PLC-β), which catalyses the key hydrolysis reaction. Similar pathways, mediated by receptor tyrosine kinases, activate PLC-γ.
Figure 1. Cytosolic calcium handling following InsP3-dependent release from the endoplasmic reticulum.
The cascade of events is triggered when an agonist (here glutamate, Glu) binds to its receptor (mGluR) in the plasma membrane. This sets off a G-protein cascade (the G-protein complex is shown as αGq,βγ) that activates phospholipase C (PLC), which in turn hydrolyses the glycerolphsophate bond in phosphatidylinositolbisphosphate (PIP2). One product of this hydrolysis goes on to activate protein kinase C (PKC), but more importantly for our models it releases InsP3 from the membrane. The InsP3 is free to diffuse through the cytosol, be degraded by phosphatases and kinases, and bind to its receptor (InsP3R) in the endoplasmic reticulum (ER) membrane. The InsP3R is a calcium channel that is triggered to open when InsP3 is bound and when calcium itself binds to an activation site; a slower inactivation process also pertains to this channel. The calcium that is thus released binds to calcium buffers (B) in the cytosol including the fluorescent calcium indicator that is used experimentally to visualize the [Ca2+]c. Calcium influx through plasma membrane channels (CaCh) and release from the ER through the ryanodine receptor calcium channels (RyR) may also contribute to the cytosolic calcium changes. Finally, calcium is pumped back into the ER via a calcium ATPase (SERCA) and can also be extruded from the cell by a number of exchange and pump.
Fluorescent indicator dyes have allowed the calcium release following InsP3 generation to be studied in great detail. However, it has proven more difficult to determine the spatial and temporal characteristics of PIP2 and InsP3 during a cell signalling event. Our laboratory used N1E-115 neuroblastoma cells to infer the InsP3 dynamics from calcium imaging studies, biochemical analysis and InsP3 uncaging (Fink et al. 1999a,b, 2000). We have used the Virtual Cell, a computational modelling and simulation problem solving environment for cell biology (Schaff et al. 1997; Slepchenko et al. 2003), to help analyse and interpret experimental data on the details of the calcium release process as well as to build a comprehensive image-based model of agonist-induced calcium release in a neuronal cell. This led to the question of how a very limited supply of cellular PIP2 could provide sufficient InsP3 for calcium release. Quantitative imaging and analysis with Virtual Cell were able to provide some suggestions as to how the cell might solve this problem (Xu et al. 2003). This analysis of the translocation of GFP-based probes for PIP2 provides a live cell assay for PIP2 kinetics that accounts for binding to InsP3. Clearly, these issues are important not just for consideration of PIP2 as a precursor of InsP3, but for any pathway that can be directly or indirectly modulated by PIP2 levels, such as the modulation of ion channels.
InsP3 mediated calcium release in cultured smooth muscle and neuronal cells
The cytosolic concentration of InsP3 ([InsP3]c) required for calcium release in A7r5 cells, a smooth muscle cell line, and N1E-115 neuroblastoma cells, a neuronal cell line, were determined by a set of experimental procedures, employing quantitative confocal microscopy, for measurement of released InsP3 from cells microinjected with caged-InsP3 and mathematical modelling with the Virtual Cell to help analyse the data (Fink et al. 1999a). ‘Caged InsP3’ contains a photo-labile covalent link between one of the phosphates and an inactivating nitroaromatic moiety; microinjection of the caged InsP3 into a cell and release with pulses of UV light allows one to directly deliver active InsP3 to the cell's calcium release system. Using a specially developed quantitative uncaging procedure, we were able to titrate the [Ca2+]c signal with controlled doses of InsP3. For the A7r5 cells, the [InsP3]c required to evoke a half-maximal calcium response is 100 nm. Experiments with caged-GPIP2, a slowly metabolized analogue of InsP3, gave a much slower recovery and the concentration for half-maximal response was an order of magnitude greater than InsP3. Experimental data and highly constrained variables were used to construct a mathematical model of the InsP3-dependent [Ca2+]c changes; the resultant simulations show high fidelity to experiment. Among the elements considered in constructing this model are the mechanisms of the InsP3-receptor, InsP3 degradation, calcium buffering in the cytosol, and refilling of the ER stores via SERCA pumps. The model predicts a time constant of 0.8 s for InsP3 degradation, and 13 s for GPIP2. InsP3 degradation was found to be a prerequisite for [Ca2+]c recovery to baseline levels and is therefore critical to the pattern of the overall [Ca2+]c signal. Analyses of the features of this model provide insights into the individual factors controlling the amplitude and shape of the InsP3-mediated calcium signal. This study is an excellent example of the use of modelling not as an end in itself but as a means to extract more information from experiments.
In differentiated neuroblastoma cells, bradykinin (BK) triggers InsP3 dependent calcium waves that consistently start in the neurite proximal to the soma and rapidly propagate in both directions. The signalling network is generally similar to that depicted in Fig. 1, except that BK binding to the BK receptor triggers the system rather than glutamate. Using calcium imaging, quantitative uncaging of microinjected InsP3, biochemical analysis of InsP3 levels in cell suspensions, and simulations from the Virtual Cell, we found that BK triggers a build-up of InsP3 in the neurite at a rate and to an extent much greater than in the soma (Fink et al. 1999b, 2000). Simulations and experiments using focal applications of BK confirmed that the proximal segment of the neurite is the critical region for a response to a BK stimulus and is necessary and sufficient to initiate and propagate the calcium signal to other regions of the cell. The reciprocal relationship of a high density of calcium stores in the soma and a rapid rise of [InsP3]c in the neurite can explain these results. The latter is attributed to a high surface-to-volume ratio in the neurite, and may reflect a general principle whereby the neuronal morphology amplifies signals from diffusible second messengers in axons and dendrites.
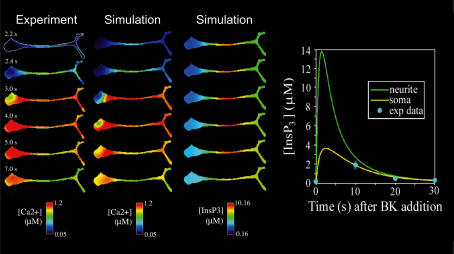
This study represents a good example of the interplay of experiment and modelling as fostered by the Virtual Cell system. The first column of Fig. 2 shows the results of an experiment for the response of intracellular Ca2+ to BK. These data were collected on a fast digital imaging microscope using the fluorescence from the indicator fura-2 to record the spatiotemporal changes in [Ca2+]c. The initial calcium increase is observed in the middle of the neurite after 2.2 s, spreading bi-directionally to the soma and growth cone, and reaching a peak [Ca2+]c everywhere of ∼1 μm. This pattern was observed in all the cells studied as long as they had this characteristic neuronal morphology. A quantitative model of the process shown in Fig. 2 was constructed with the Virtual Cell software to determine how all these individual components could interact to produce the observed calcium wave. We obtained the data for the model both from the literature and from experiments in our own lab. Indeed, we were forced in several instances to repeat previously reported experiments when we found that a model based on the literature data could not predict the observed calcium dynamics. One of the most interesting findings that came out of this iterative modelling ↔ experiment process was the prediction that the observed calcium wave could only be reproduced if the calcium store density in the soma was approximately twice as high as in the neurite. Subsequently, the results of immunofluorescence studies showed that the endoplasmic reticulum had precisely this predicted non-uniform distribution. The simulation for [Ca2+]c resulting from the completed model can be directly compared to the experiment in the first two columns of Fig. 2. This illustrates how simulations can be mapped to the same geometry as the experiment; thus, the investigator may analyse simulation results with the same familiar tools that are employed to analyse and reduce experimental image data.
Figure 2. The outputs of the Virtual Cell allow direct comparison of experiment with simulation.
The left column shows the experimental calcium changes following addition of BK at time 0 s in a differentiated N1E-115 neuroblastoma cell as determined by the fluorescent calcium indicator fura-2. The centre column displays the corresponding output of the Virtual Cell simulation. The right column displays the output of the simulation for [IP3], illustrating that the simulation permits estimation of the spatiotemporal distribution of molecules that are not accessible experimentally. The scale at the bottom of each column defines the range of values encoded by the colours in the images. The graph at the right shows the model results for the change in [InsP3] in the centre of the neurite and the centre of the soma; the experimentally derived concentrations for 4 time points are shown as well. (For additional details, see Fink et al. 2000.)
It is difficult to experimentally map the intracellular pattern of [InsP3] during signalling, so the model provides a unique view of the spatial and temporal distribution of this key metabolite. The spatiotemporal pattern of [InsP3]c predicted by the model is shown in the third column of Fig. 2. The calculated [InsP3]c dynamics show a rapid build-up in the neurite to a peak of ∼10 μm, while [InsP3]c in the soma increases more slowly and to lower peak concentrations (∼3 μm). The production of InsP3 is much faster than its diffusion throughout the intracellular volume and also outpaces the rate of degradation through putative cytosolic kinase and phosphatase based pathways. Therefore, because InsP3 is produced from the plasma membrane, the cytosolic concentrations of InsP3 will rise faster and with greater maximum amplitude in the neurite than in the soma. This is primarily because of the high surface-to-volume ratio of the neurite compared to the soma. The graph on the right of Fig. 2 shows the calculated time course from regions of interest in the centre of the neurite and soma; the latter, representing the bulk of the cellular volume, nicely fits the experimentally derived points from a biochemical analysis of InsP3 from a cell suspension following BK treatment. Interestingly, despite the higher level of InsP3 in the neurite, the maximum calcium amplitudes are similar in all parts of the cell. This is because the non-uniform distribution of calcium stores, discussed above, balances the non-uniform levels of InsP3. Additional experiments that were suggested by predictions of the model can be found in Fink et al. (1999b, 2000). The model itself is available as part of the Virtual Cell database, which can be accessed through http://vcell.org.
PIP2 dynamics in neuroblastoma cells – stimulated production concomitant with hydrolysis
Having thus established a quantitative picture of InsP3 production and degradation in these cells, the rate and extent of PIP2 hydrolysis was effectively constrained. We set out to determine if the loss of PIP2 matched the production of InsP3 via biochemical experiments measuring the kinetics of the bradykinin-induced changes in PIP2 mass in suspensions of N1E-115 neuroblastoma cells (Xu et al. 2003). The biochemical results are summarized in Fig. 3 shown in relation to a fit of these data to a model that also accounts for the InsP3 production. We found that the depletion of the initial steady state concentration of PIP2 would be insufficient to account for the large and rapid production of InsP3. The time course of PIP2 and the production of InsP3 could only be reconciled with each other by invoking a rapid stimulated synthesis of PIP2 that would keep up with its hydrolysis. The modelling predicted a remarkable initial stimulated increase in PIP2 concomitant with activation of PLC-mediated PIP2 hydrolysis. Interestingly, the sensitivity of the InsP3R in these cells is an order of magnitude lower than in non-neuronal cells (Watras et al. 2005). Thus, the ability of these cells to replenish PIP2 as it is being hydrolysed makes it possible for the cell to produce sufficient InsP3 to produce a robust calcium release.
Figure 3. Experimental (points) and simulated (curves) time courses of bradykinin-induced changes of PIP2 (black) and PIP (purple) in N1E-115 cells.
[3H]Inositol-prelabelled N1E-115 cells were incubated in the presence of bradykinin (1 μm) for the indicated times. These data along with our prior study of InsP3 dynamics (inset, Fink et al. 2000) in this cell were used to constrain a model that produced the respective black and purple solid curves. The prediction of the model that there was an initial increase in [PIP2], led us to determine the change in PIP2 at 5 s, shown as a red diamond. From Xu et al. (2003); reproduced from The Journal of Cell Biology, 2003, 161: 779–791. Copyright 2003 The Rockefeller University Press.
To confirm this finding, we used fluorescence imaging of a probe that translocates from the plasma membrane to the cytosol as PIP2 is hydrolysed. This technique, nearly simultaneous introduced by the labs of Balla and Meyer (Stauffer et al. 1998; Varnai & Balla, 1998), uses a construct in which the pleckstrin homology domain from PLCδ1 (abbreviated, PHδ1) is fused to green fluorescent protein (GFP). This probe was originally designed as an indicator of PIP2, because the PHδ1 domain is known to have a high affinity for this lipid. Indeed, in our experiments, activation of PLC-mediated PIP2 hydrolysis causes a dramatic translocation of the fluorescence from the plasma membrane to the cytosol (Fig. 4). However, the initial increase in PIP2 from Fig. 3 was not reflected in an initial increase in membrane fluorescence. Since it was known that PHδ1-GFP has an affinity for InsP3 as well as for PIP2, it was not clear how to interpret this result. We used Virtual Cell to analyse the data and develop a quantitative mechanistic understanding of the system based on the known initial concentrations and the measured affinities of the probe for PIP2 and InsP3. The modelling results (top 2 rows of Fig. 5) produced excellent agreement with the experiment of Fig. 4 and at the same time (bottom row of Fig. 5) was fully consistent with the biochemical studies of Fig. 3 indicating an initial increase in PIP2. The model reveals that translocation is sensitive to both InsP3 and PIP2 and that the presence of the indicator distorts the amplitude and time course of changes in both of these molecules through a buffering effect.
Figure 4. Left:Time series of confocal images with the time indicated on each frame in seconds.
Bradykinin was added at time 0. Right: relative change in GFP fluorescence in the cytosol and membrane averaged over 15 experiments. From Xu et al. (2003); reproduced from The Journal of Cell Biology, 2003, 161: 779–791. Copyright 2003 The Rockefeller University Press.
Figure 5. Virtual Cell simulation of the experiment in Fig. 4 using the geometry of central cell in the image.
The percentage change in fluorescence of the GFP probe in the cytosol (top) and the membrane (PIP2-PH-GFP) are consistent with Fig. 4, while the triphasic behaviour of PIP2 (bottom series with a scale showing molecules μm−2) is consistent with Fig. 3. From Xu et al. (2003); reproduced from The Journal of Cell Biology, 2003, 161: 779–791. Copyright 2003 The Rockefeller University Press.
Conclusion
The ability of the cell to use stimulation of a paradigmatic G-protein coupled receptor to effect PIP2 hydrolysis is a familiar and well established mechanism for controlling PIP2 levels. Our study uses several lines of evidence to establish that PIP2 synthesis is also stimulated either directly or indirectly by the same receptor. The speed of the initial PIP2 build-up suggests that stimulation of synthesis may arise from a relatively upstream branch off the G-protein pathway, presumably via the sequential phosphorylation of phosphatidylinositol (PI) at the 4′ position to produce phosphatidylinositol 4-phosphate (PIP) and then at the 5′ position to produce PIP2 (Tolias & Cantley, 1999). Indeed, in our work we showed that wortmannin, an inhibitor of PI kinases, abrogates the initial increase in PIP2. Several other studies have shown that the build-up of InsP3 is too rapid compared to the decrease and subsequent recovery in membrane PIP2 (Kaya et al. 1989; Willars et al. 1998). Additionally, there have been other reports that both InsP3 and PIP2 increase at the time of fertilization in sea urchin, Xenopus and mouse eggs (Turner et al. 1984; Ciapa et al. 1992; Stith et al. 1993; Stith et al. 1994; Snow et al. 1996; Halet et al. 2002) and during the stimulation of platelets (Lassing & Lindberg, 1990). We suggest that hydrolysis and synthesis of plasma membrane PIP2 may be tightly coupled such that PIP2 synthesis rapidly compensates for, or is independently stimulated by, its hydrolysis.
Acknowledgments
I am pleased to acknowledge Charles Fink, Chang Xu, James Watras, Jim Schaff, Ion Moraru and Boris Slepchenko who contributed to various aspects of the research reviewed herein. I am grateful for the support of the National Institute of Biomedical Imaging and Bioengineering through grant no. EB001963 and the National Center for Research Resource through grant no. RR13186.
References
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling (Review) Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Ciapa B, Borg B, Whitaker M. Polyphosphoinositide metabolism during the fertilization wave in sea urchin eggs. Development. 1992;115:187–195. doi: 10.1242/dev.115.1.187. [DOI] [PubMed] [Google Scholar]
- Fink CC, Slepchenko B, Loew LM. Determination of time-dependent inositol-1,4,5-trisphosphate concentrations during calcium release in a smooth muscle cell. Biophys J. 1999a;77:617–628. doi: 10.1016/S0006-3495(99)76918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Slepchenko B, Moraru II, Schaff J, Watras J, Loew LM. Morphological control of inositol-1,4,5-trisphosphate-dependent signals. J Cell Biol. 1999b;147:929–935. doi: 10.1083/jcb.147.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Slepchenko B, Moraru II, Watras J, Schaff J, Loew LM. An image-based model of calcium waves in differentiated neuroblastoma cells. Biophys J. 2000;79:163–183. doi: 10.1016/S0006-3495(00)76281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halet G, Tunwell R, Balla T, Swann K, Carroll J. The dynamics of plasma membrane PtdIns(4,5)P2 at fertilization of mouse eggs. J Cell Sci. 2002;115:2139–2149. doi: 10.1242/jcs.115.10.2139. [DOI] [PubMed] [Google Scholar]
- Kaya H, Patton GM, Hong SL. Bradykinin-induced activation of phospholipase A2 is independent of the activation of polyphosphoinositide-hydrolyzing phospholipase C. J Biol Chem. 1989;264:4972–4977. [PubMed] [Google Scholar]
- Lassing I, Lindberg U. Polyphosphoinositide synthesis in platelets stimulated with low concentrations of thrombin is enhanced before the activation of phospholipase C. FEBS Lett. 1990;262:231–233. doi: 10.1016/0014-5793(90)80197-q. [DOI] [PubMed] [Google Scholar]
- Putney JW, Jr, Bird GS. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993;14:610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Schaff J, Fink CC, Slepchenko B, Carson JH, Loew LM. A general computational framework for modeling cellular structure and function. Biophys J. 1997;73:1135–1146. doi: 10.1016/S0006-3495(97)78146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepchenko BM, Schaff JC, Macara IG, Loew LM. Quantitative cell biology with the virtual cell. Trends Cell Biol. 2003;13:570–576. doi: 10.1016/j.tcb.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Snow P, Yim DL, Leibow JD, Saini S, Nuccitelli R. Fertilization stimulates an increase in inositol trisphosphate and inositol lipid levels in Xenopus eggs. Dev Biol. 1996;180:108–118. doi: 10.1006/dbio.1996.0288. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Current Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- Stith BJ, Espinoza R, Roberts D, Smart T. Sperm increase inositol 1,4,5-trisphosphate mass in Xenopus laevis eggs preinjected with calcium buffers or heparin. Dev Biol. 1994;165:206–215. doi: 10.1006/dbio.1994.1247. [DOI] [PubMed] [Google Scholar]
- Stith BJ, Goalstone M, Silva S, Jaynes C. Inositol 1,4,5-trisphosphate mass changes from fertilization through first cleavage in Xenopus laevis. Mol Biol Cell. 1993;4:435–443. doi: 10.1091/mbc.4.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Cantley LC. Pathways for phosphoinositide synthesis. Chem Phys Lipids. 1999;98:69–77. doi: 10.1016/s0009-3084(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Turner PR, Sheetz MP, Jaffe LA. Fertilization increases the polyphosphoinositide content of sea urchin eggs. Nature. 1984;310:414–415. doi: 10.1038/310414a0. [DOI] [PubMed] [Google Scholar]
- Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras J, Fink CC, Loew LM. Endogenous inhibitors of InsP3-induced Ca2+ release in neuroblastoma cells. Brain Res. 2005;1055:60–72. doi: 10.1016/j.brainres.2005.06.091. [DOI] [PubMed] [Google Scholar]
- Willars GB, Nahorski SR, Challiss RAJ. Differential regulation of muscarinic acetylcholine receptor-sensitive polyphosphoinositide pools and consequences for signaling in human neuroblastoma cells. J Biol Chem. 1998;273:5037–5046. doi: 10.1074/jbc.273.9.5037. [DOI] [PubMed] [Google Scholar]
- Xu C, Watras J, Loew LM. Kinetic analysis of receptor-activated phosphoinositide turnover. J Cell Biol. 2003;161:779–791. doi: 10.1083/jcb.200301070. [DOI] [PMC free article] [PubMed] [Google Scholar]