Abstract
Airway submucosal glands are sites of high expression of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel and contribute to fluid homeostasis in the lung. However, the molecular mechanisms of gland ion and fluid transport are poorly defined. Here, submucosal gland serous acinar cells were isolated from murine airway, identified by immunofluorescence and gene expression profiling, and used in physiological studies. Stimulation of isolated acinar cells with carbachol (CCh), histamine or ATP was associated with marked decreases in cell volume (20 ± 2% within 62 ± 5 s) that were tightly correlated with increases in cytoplasmic Ca2+ concentration ([Ca2+]i) as revealed by simultaneous DIC and fluorescent indicator dye microscopy. Simultaneous imaging of cell volume and the Cl−-sensitive fluorophore SPQ indicated that the 20% shrinkage was associated with a fall of [Cl−]i from 65 mm to 28 mm, reflecting loss of 67% of cell Cl− content, accompanied by parallel efflux of K+. Upon agonist removal, [Ca2+]i relaxed and the cells swelled back to resting volume via a bumetanide-sensitive Cl− influx pathway, likely to be NKCC1. Accordingly, agonist-induced serous acinar cell shrinkage and swelling are caused by activation of solute efflux and influx pathways, respectively, and cell volume reflects the secretory state of these cells. In contrast, elevation of cAMP failed to elicit detectible volume responses, or enhance those induced by submaximal [CCh], because the magnitude of the changes were likely to be below the threshold of detection using optical imaging. Finally, when stimulated with cholinergic or cAMP agonists, cells from mice that lacked CFTR, as well as wild-type cells treated with a CFTR inhibitor, exhibited identical rates and magnitudes of shrinkage and Cl− efflux compared with control cells. These results provide insights into the molecular mechanisms of salt and water secretion by lung submucosal glands, and they suggest that while murine submucosal gland fluid secretion in response to cholinergic stimulation can originate from CFTR-expressing serous acinar cells, it is not dependent upon CFTR function.
The submucosal glands found in cartilaginous airways are important in human lung physiology because they contribute to airway surface liquid (ASL) secretion, composition and homeostasis. Furthermore, submucosal glands synthesize and secrete a variety of macromolecules including mucins, lysozyme and lactoferrin that are important for airway defence against inspired pathogens (reviewed in Ballard & Inglis, 2004; Inglis & Wilson, 2005). Submucosal gland secretion may play important roles in airway diseases such as cystic fibrosis (CF; reviewed in Ballard & Inglis, 2004; Inglis & Wilson, 2005), asthma (reviewed in Jeffery, 1994; Rogers, 2004), and chronic obstructive pulmonary disease (COPD; reviewed in Jeffery, 1994; Rogers, 2001b). Submucosal glands are composed of several different cell types, defined mainly by visible histology and morphology in electron microscopy (Meyrick et al. 1969; Meyrick & Reid, 1970; Berger et al. 1999). These cell types include (i) serous cells arranged in acini at the distal ends of the glands, which are thought to be responsible for secretion of fluid and antimicrobial peptides; (ii) mucous cells that secrete mucin macromolecules; and (iii) both ciliated and non-ciliated collecting duct cells.
A fundamental model of submucosal gland fluid secretion (reviewed in Ballard & Inglis, 2004) proposes that a primary watery secretion emanates from the serous acini, which then washes over the mucous cells and hydrates released mucin granules, creating a mixture of ion-rich fluid and mucus that accumulates in the collecting duct. The fluid and mucus are propelled to the airway surface epithelium by combined actions of hydrostatic pressure, cilial beating and contraction of surrounding myoepithelial cells. The complex morphology and small gland size have limited experimental studies of the functions of the various cell types that comprise the glands. Current data have been derived from studies of intact glands in vivo or in excised pieces of mucosa. As a result, the functions and relative contributions of the various individual cell types to fluid and mucus secretion are not well understood.
Submucosal gland serous cells may be the major site of expression in the lung of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) Cl− channel (Engelhardt et al. 1992; Jacquot et al. 1993), and thus may be crucial to CF (reviewed in Ballard & Inglis, 2004; Inglis & Wilson, 2005), the most common lethal genetic recessive disease in the United States. In CF, the major lethal pathology involves the lung, where morbidity is manifested as mucus plugging of airways and chronic inflammation and infection (Boucher, 2004). It has been proposed that defective salt, fluid and mucus homeostasis is the underlying physiological consequence of impaired CFTR function in CF. Because the relative contributions of surface epithelial cells and submucosal glands to overall lung fluid homeostasis are unknown, it has been hypothesized that defective fluid homeostasis in CF may be partly due to defects in the amount or composition of submucosal gland fluid secretions (reviewed in Ballard & Inglis, 2004; Inglis & Wilson, 2005). The importance of CFTR in the secretory functions of submucosal glands is supported by observations of occluded mucus-filled gland ducts, as well as gland hypertrophy, hyperplasia and infection in lungs of CF patients (Ornoy et al. 1987; Jeffery & Brain, 1988). Pharmacological inhibition of glandular fluid secretion in porcine airways mimics some of the manifestations of CF pathology, including disrupted mucociliary clearance without reduction in ciliary beat frequency (reviewed in Ballard & Inglis, 2004). These results suggest that inhibition of glandular fluid secretion may be sufficient to cause CF-like pathology, independent of possible hyper-absorption of fluid by surface epithelial cells in the absence of CFTR function. Since glands contribute significantly to fluid secretion in large airways, it has been hypothesized that the absence of a functional CFTR Cl− conductance in CF glands may lead to defective salt and water secretion and contribute to CF lung pathology (reviewed in Ballard & Inglis, 2004; Inglis & Wilson, 2005).
Submucosal glands in airway tissue from individuals with CF appear to be defective in their ability to secrete fluid in response to agonists such as vasoactive intestinal peptide (VIP) that elevate intracellular concentrations of cyclic-AMP ([cAMP]i) (Joo et al. 2002a; Salinas et al. 2005; Song et al. 2005). However, freshly isolated CF glands still secrete significant volumes of fluid in response to cholinergic agonists that increase intracellular Ca2+ concentration ([Ca2+]i) (Joo et al. 2002a). In these studies, the cAMP-stimulated pathway appears to be the minor pathway of the two, as VIP induced sustained glandular secretion at a maximal rate that was only ∼30–40% of the secretion rate elicited by cholinergic stimulation (Trout et al. 2001; Joo et al. 2001b, 2002a,b). Thus, it is not obvious how diminished cAMP-dependent secretion contributes to CF pathology. The implications of observations of intact glands are not clear, largely because there are few data regarding the cellular and molecular mechanisms that underlie gland secretion. This knowledge is not only critical for our understanding of the general airway pathology of CF and other lung diseases, but would also be very important for the development of therapeutic agents that may target submucosal glands.
The experiments described below represent a significant step toward filling this important gap by helping to elucidate the molecular mechanisms of submucosal gland serous cell ion and fluid transport. Submucosal serous cells from the mouse airway were isolated, identified and studied using simultaneous bright-field differential interference contrast (DIC) imaging of cell volume and quantitative fluorescence microscopy of ion indicator dyes, to examine the molecular mechanisms of serous cell ion transport and its regulation in single cells. These results provide insights into the molecular mechanisms of regulated fluid secretion in murine submucosal gland serous cells, and they suggest that secretion can be mediated by Cl− efflux through a non-CFTR anion conductance in murine serous acinar cells that express CFTR.
Methods
Reagents
AlexaFluor (AF)-labelled anti-mouse and anti-rabbit IgG secondary antibodies, 6-methoxy-N-(3-sulfopropyl)quinolinium (SPQ), ionomycin, A23187, and acetoxymethylester (AM)-derivatives of fura-2, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), and 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF), and calcein were purchased from Molecular Probes (Eugene, OR, USA). RNasin RNase inhibitor was purchased from Promega (Madison, WI, USA). All other molecular biology reagents were from Invitrogen (Carlsbad, CA, USA). CFTRinh172 was a gift from Dr A. S. Verkman and also purchased from Calbiochem (San Diego, CA, USA). Anti-CFTR 24–1 monoclonal antibody was obtained from R & D Systems (Minneapolis, MN, USA). Anti-lysozyme polyclonal antibody was purchased from Dakocytomation (Carpinteria, CA, USA). Recombinant CFTR C-terminal peptide was a kind gift from Dr V. Raghuram (NIH/NHLBI; Bethesda, MD, USA). Microscope filters were obtained from Chroma Technologies, Inc. (Rockingham, VT, USA). All other reagents were obtained from Sigma (St Louis, MO, USA).
Submucosal gland serous acinar cell isolation
All animal handling procedures were performed in accordance with regulations of the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Nasal turbinate and septum were obtained from wild-type (WT), cftrtm1Unc−/− knockout, or heterozygote mice on a congenic C57BL/6 strain background housed in a pathogen-free facility after killing the animals by CO2 asphyxiation and cervical dislocation. Isolated tissue was first placed in a physiological saline buffer containing (mm): 125 NaCl, 5 KCl, 1.2 MgCl2, 1.2 CaCl2, 1.2 NaH2PO4, 11 glucose, 15 Hepes, pH 7.4. The tissue was mechanically minced with scissors and then incubated for 25 min at room temperature in medium containing (mm): 125 NaCl, 5 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 11 glucose, and 25 NaHCO3, gassed with 95% O2–5% CO2, supplemented with 0.8 mg ml−1 collagenase (Worthington Type II), 2 mm l-glutamine, 1 × MEM-vitamins, 1 × MEM-amino acids, and 1% BSA. After washing via gentle centrifugation, cells were plated on glass coverslips and allowed to adhere for 10–20 min. The isolation protocol yielded acini, single cells and strings of cells. Cells were identified based on visible morphology (size, polarized secretory granules, acinar structures) under DIC optics using a 40× oil-immersion objective lens (Nikon S Fluor 1.3 NA) on an inverted Nikon microscope. WT and cftrtm1Unc−/− mice used in these experiments ranged in age from 3 to 12 months and 3 to 7 months, respectively. No age-dependent differences were observed in cell viability (assessed by visible morphology and responses to agonists), yield, or responses to agonist (volume, [Ca2+]i, or [Cl−]i) between WT and cftrtm1Unc−/− mice.
Confocal immunofluorescence microscopy
After isolation, cells were plated for 30 min on poly l-lysine (poly K) coated coverslips (1–2 mg ml−1) and fixed in 4% formaldehyde for 20 min at 4°C. Blocking/primary antibody incubation was performed overnight at 4°C in Dulbecco's phosphate buffered saline (DPBS; with Ca2+ and Mg2+) containing 1% BSA, 2% goat serum, and 0.1% saponin. Following washing (3 × 5 min in DPBS), cells were incubated with AlexaFluor (AF)-conjugated secondary antibodies for 2 h at 4°C. After washing, cells were mounted in DPBS containing 40% glycerol and 1 mg ml−1p-phenylenediamine. Imaging and excitation of fluorescently labelled secondary antibodies was performed using the 488 and 568 nm lines of an Ar/Kr-ion laser (50% intensity) attached to a Perkin-Elmer spinning-disc confocal system coupled to an inverted Nikon microscope equipped with a 60× oil-immersion objective lens (Nikon Plan Apo 1.4 NA). Imaging and image overlays were performed using Perkin-Elmer Ultraview software.
Cell harvesting/aRNA amplification
Cells were plated on poly K-coated coverslips for ∼15 min and then gently washed to remove free-floating debris. Cells were harvested via gentle suction with a patch-clamp pipette with a ∼5 μm diameter opening. Care was taken to aspirate three- to four-cell structures of morphologically distinct acinar cells and to avoid contamination from surrounding cells and/or cellular debris. The cells were ejected into a solution of 15 mm DTT and 5–6 U μl−1 RNasin RNAse inhibitor and frozen overnight at −80°C. Three- to five-cell sheets of ciliated epithelial cells were isolated using identical procedures. Two rounds of antisense RNA (aRNA) amplification were carried out with a poly dT-oligo containing a T7 RNA polymerase promoter (Van Gelder et al. 1990). Control RNA was isolated from nasal turbinate, brain and kidney using TRIzol reagent according to the manufacturer's protocol. Reverse transcription PCR (RT-PCR) was carried out for 30 cycles, using denaturation, annealing, and extension temperatures of 94°C, 57°C and 72°C, respectively. Primers and expected product sizes are listed in online supplemental material, Supplemental Table 1.
Cell volume determinations
Cells were imaged under continuous perfusion with the HCO3− buffer described above (except lacking collagenase, vitamins, amino acids, glutamine and BSA but containing 1.2 mm CaCl2) at 37°C (chamber volume ∼300 μl, flow rate ∼2 ml min−1) with continuous gassing. Experiments performed in solutions lacking extracellular Ca2+ (0-Ca2+ solution) were done in the same buffer with the 1.2 mm CaCl2 omitted and 1 mm EGTA added. To avoid CO2 loss, the perfusion solutions were kept in heated overhead glass reservoirs connected by glass tubing to glass heating coils near the perfusion chamber. Changes in the extracellular solution were controlled by an electronic pinch-valve system (Warner Instruments; Hamden, CT, USA). No differences in the magnitude of cell volume changes were observed between single acinar cells and small acini composed of two to four cells. Large acinar clumps exhibited qualitatively similar volume responses but their complex structure prevented quantitative volume determinations. Since the isolated cells and small acini were approximately spherical and volume changes occurred in the same proportion in all directions (described below and in Supplemental Fig. 1), the cell outline (traced using ImageJ software; W. Rasband, NIH/NIMH, Bethesda, MD, USA) was used to determine volume by taking the cross-sectional area of the centre of the imaged cell raised to the power 3/2. Volume at time 0 (Vo) was normalized to 1, and relative volume expressed as V/Vo. All data are reported as means ± standard error of the mean (s.e.m.) unless otherwise noted. All graphing and linear regression fits were performed with Igor Pro software (Wavemetrics, Inc., Lake Oswego, OR, USA). P-values were determined in Excel using Student's two-tailed t test.
Confocal 3D reconstruction of isolated serous acinar cells
Acinar cells were loaded with calcein by incubation in 2 μm calcein-AM for 10–15 min, followed by incubation in calcein-free medium for 5–10 min to allow cells to recover/de-esterify the loaded dye. Cells were illuminated with the 488 nm line of an Ar/Kr laser attached to a Perkin-Elmer spinning disc confocal Nikon microscope equipped with a 40×, 1.3 NA objective lens and Prior Optiscan z-stepper focal motor controlled by Ultraview software. Fluorescence was collected by a CCD camera after passing through a 525/50 nm band-pass (bp) filter. Images were taken with a 0.5 μm z-step size. Images were taken in 305 mosmol l−1 extracellular solution and after the medium was supplemented with 100 mm sucrose (407 mosmol l−1 by vapour pressure osmometry), since it was observed that this concentration induced a cell shrinkage similar to the maximal shrinkage observed during exposure to 100 μm CCh (as measured by DIC; see Supplemental Fig. 2A). Image stacks were rendered into 3-D volumes and measurements were made of cell volume and x-, y-, and z-axis diameter using Volocity software (Improvision, Lexington, MA, USA).
Simultaneous single cell volume and [Ca2+]i determinations
Cells were loaded with the Ca2+-sensitive dye fura-2 by incubation in a medium containing 0.5–1 μm fura-2-AM for 10–15 min, followed by 5 min incubation in fura-2-free solution to allow cells to recover and to de-esterify the loaded dye. Simultaneous DIC and fura-2 fluorescence imaging was performed as previously described (Foskett, 1988, 1990b; Foskett & Melvin, 1989) except the analyser was housed in the emission filter wheel and moved out of the light path during measurements of fluorescence. To minimize toxicity, fluorescence was sampled as infrequently as possible while still accurately tracking changes in [Ca2+]i. The ratio of emitted light upon excitation with 340 nm and 380 nm light was used to determine [Ca2+]i, as described (Foskett & Melvin, 1989).
Simultaneous single cell volume and [Cl−]i determinations
Cells were loaded with SPQ by incubation in medium containing 10 mm SPQ for 30–40 min. SPQ fluorescence was excited with a 340/10 nm filter, with emission collected with a 450/50 nm filter. Simultaneous DIC and SPQ fluorescence imaging was performed as described (Foskett, 1990a) except that DIC illumination was filtered at 530/30 nm. SPQ properties were calibrated in vivo in a separate group of calibration cells as previously described (Foskett, 1990a). Osmotic shrinkage of serous acinar cells was accomplished using Hepes-buffered solutions containing (mm) 150 K+, 65 Cl− (determined to be the resting [Cl−]i, described below), 85 gluconate, supplemented with nigericin and tributyltin (to clamp [Cl−]i at 65 mm), and containing varying sucrose concentrations (0, 50, 100, or 150 mm). Acinar cells were exposed to the 0-sucrose nigericin–tributyltin solution (308 mosmol l−1 by osmometry) until a stable SPQ fluorescence level was achieved, followed by exposure to one of three hyperosmotic solutions (356, 407 and 453 mosmol l−1) to induce cell shrinkage while keeping [Cl−]i clamped at 65 mm. Due to possible effects of volume regulatory mechanisms, each experiment was limited to one hyperosmotic exposure. The cells shrank rapidly (within 10–20 s) upon exposure to hyperosmotic medium and remained shrunken until re-exposure to 308 mosmol l−1 solution, upon which they swelled back to resting volume.
Results
Isolation and identification of murine submucosal gland serous acinar cells
Serous and mucous cells isolated from the lung are difficult to culture, and they rapidly de-differentiate and begin to express markers of the opposite cell type (Marin & Culp, 1986; Sommerhoff & Finkbeiner, 1990; Yamaya et al. 1991; Chopra et al. 1994; Emery et al. 1995). Consequently, data obtained from cultured airway gland cells must be interpreted with caution. The Calu-3 cell line has been frequently used as a surrogate model for serous cell function, because it is a lung-derived secretory cell that expresses high levels of CFTR (Shen et al. 1994), and Calu-3 cells have proven valuable for understanding CFTR-mediated fluid secretion. However, this carcinoma-derived cell line is anneuploid and expresses some markers of goblet cells while lacking some serous cell markers (Duszyk, 2001; Dubin et al. 2004), indicating that extrapolation of data obtained from Calu-3 cells to submucosal gland serous cell function must also be done cautiously. To examine fluid transport properties of lung submucosal gland acinar cells directly, we developed a protocol to isolate living primary submucosal gland cells from murine nasal turbinate and septum. The mouse was chosen because it is currently the only animal available with CFTR mutant and knockout models (reviewed in Grubb & Boucher, 1999), which was felt to be valuable for deciphering the role of CFTR in submucosal gland function.
In mice, submucosal glands are present only in the nasal cavity and the upper cartilaginous rings of the proximal trachea (Yamaya et al. 1991; Song & Verkman, 2001; Widdicombe et al. 2001). Preliminary experiments determined that acinar cells were more efficiently isolated from nasal tissue than from tracheal tissue, and therefore nasal turbinate and septum were used as a source of submucosal gland cells. Previous immunohistochemical characterization (Groneberg et al. 2003; Martinez-Anton et al. 2006), as well as functional measurements of intact human nasal gland secretion (Salinas et al. 2005; Song et al. 2005) have indicated no significant differences in the properties of upper airway and tracheal or bronchial glands.
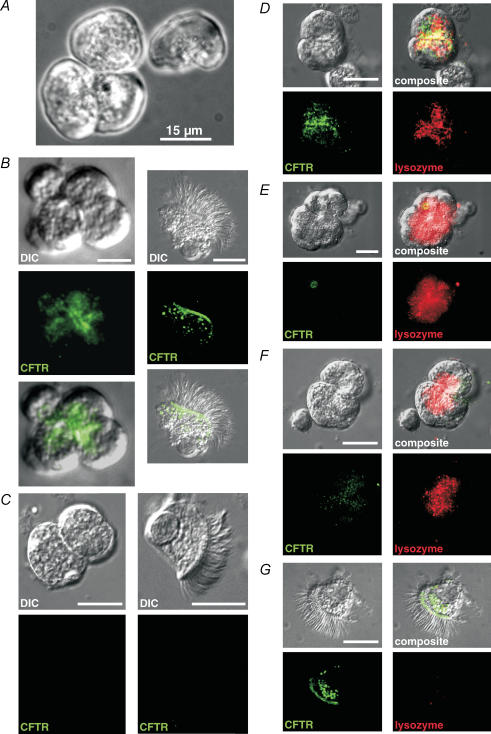
Serous cells were first identified by the appearance of small apically localized secretory granules, their ‘smooth’ basolateral side, and the acinar morphology of isolated cell clumps (Fig. 1A). To confirm the morphological identification, confocal microscopy and immunocytochemistry were employed using antibodies directed against CFTR and serous cell-specific markers (Klockars & Reitamo, 1975). Fixed acinar cells displayed bright immunofluorescence at the apical region using anti-CFTR monoclonal antibody 24–1 (Fig. 1B), which was substantially reduced when purified recombinant C-terminal CFTR peptide (peptide sequence N-(LC-Biotin)-QIAALKEETEEEVQDTRL-C, containing the 24–1 epitope) was added to the primary antibody incubation solution (Fig. 1E). In contrast, the CFTR peptide did not affect lysozyme immunofluorescence (below), indicating that the reduced CFTR staining was caused by competition of the peptide for specific antibody binding. CFTR immunofluorescence was absent in cells isolated from cftrtm1Unc−/− mice (Fig. 1C), confirming that the antibody detected CFTR specifically. The morphologically identified isolated acinar cells also displayed lysozyme immunofluorescence (Fig. 1D) that consistently appeared to be localized to the secretory granules, and was reduced to near-background levels when 0.2 mg ml−1 recombinant human lysozyme was added to the primary antibody incubation solution (not shown). Ciliated epithelial cells from WT mice displayed CFTR immunofluorescence at the apical membranes (Fig. 1B and G) but did not display lysozyme immunofluorescence (Fig. 1G), supporting specific acinar localization.
Figure 1. Isolated murine nasal acinar cells stain for CFTR and lysozyme using confocal immunofluorescence.
A, isolated murine acinus and single acinar cell after collagenase digestion and before fixation. Cells were identified by the presence of small (< 1 μm) polarized secretory granules and an acinar morphology. B, fixed acinar cells (first column) stained for CFTR using monoclonal 24–1 antibody (recognizing a C-terminal epitope) and AF488 anti-mouse secondary antibody exhibited strong fluorescence at the apical region of the cells. The immunofluorescence appeared strongest at the apical membrane where cells were joined together, but also extended somewhat into the apical pole of the cells, consistent with CFTR present in secretory vesicles and/or CFTR being trafficked to the plasma membrane. Fixed ciliated epithelial cells (second column) exposed to the same antibody conditions showed bright apical membrane fluorescence. C, CFTR immunofluorescence was absent in cells isolated from cftrtm1Unc−/− knockout mice. Fluorescence recorded with exposure/camera settings identical to those in B. Intensity was equivalent to background levels observed in reactions containing secondary antibody alone (not shown). D, cells isolated from WT mice costained for CFTR and lysozyme (using polyclonal anti-lysozyme antibody and AF568 anti-rabbit secondary antibody). Lysozyme immunofluorescence consistently appeared localized to the secretory granules. E, cells isolated from the cftrtm1Unc−/− knockout mouse lacked CFTR immunofluorescence, but stained brightly for lysozyme. F, cells isolated from WT mice incubated with CFTR antibody in the presence of C-terminal CFTR peptide (0.01 mg ml −1, ∼10-fold excess by weight) did not display strong CFTR fluorescence but exhibited normal lysozyme immunofluorescence. G, ciliated epithelial cells display CFTR immunofluoresence, but did not exhibit lysozyme immunofluorescence. All scale bars represent 15 μm.
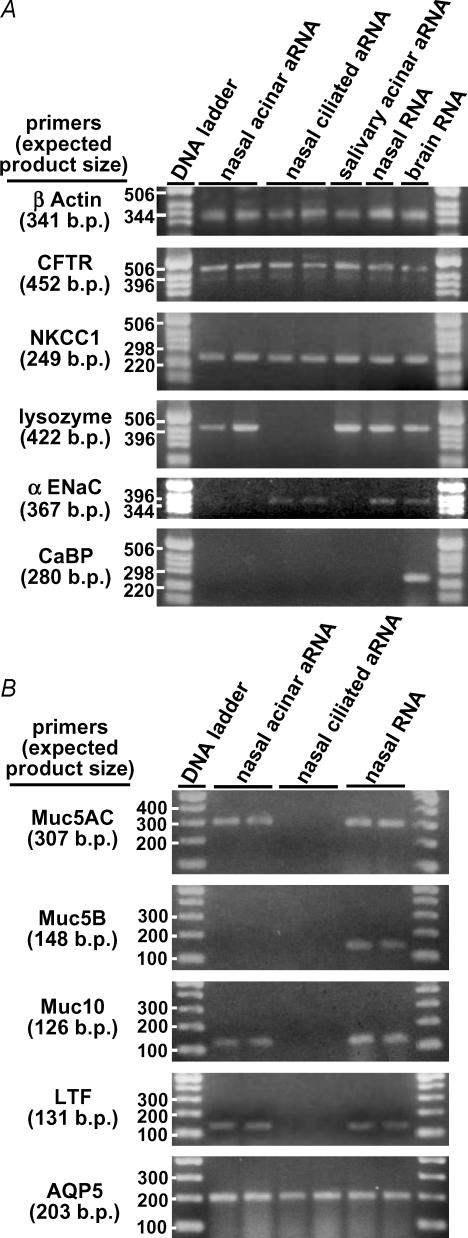
To further confirm the identity of the cells that we believed were CFTR-expressing serous acinar cells, three- to four-cell acini were harvested for mRNA amplification via the Eberwine antisense RNA (aRNA) method (Van Gelder et al. 1990; reviewed in Eberwine, 2001). Because this method uses the mRNA poly A tail for amplification (via an oligo dT primer), it selectively amplifies expressed mRNA and not genomic DNA. This sensitive method allows amplification and detection of as few as two to three copies of a single mRNA species. After two rounds of amplification (∼106-fold), reverse transcription (RT)-PCR revealed expression of mRNA sequences for CFTR, NKCC1 and lysozyme in the cells identified as serous acinar cells (n = 4 reactions from three WT mice; Fig. 2A). Neither the α-subunit of the epithelial sodium channel (αENaC) nor CaBP1 (a neuronal Ca2+ binding protein) was detected. RNA amplification performed on two to three isolated ciliated cells revealed expression of mRNA transcripts for CFTR and NKCC1 as well as αENaC, but not lysozyme (n = 4 reactions). As a control, aRNA amplified from salivary gland acinar cells revealed expression of CFTR, NKCC1 and lysozyme mRNA, but not αENaC mRNA, as expected. To validate the integrity/function of all primers, RT-PCR was performed on RNA extracted from murine nasal turbinate tissue. CFTR, NKCC1, lysozyme, lactoferrin and αENaC transcripts were detected, but not mRNA for the neuronal CaBP1. RNA extracted from mouse brain revealed expression of CFTR, NKCC1, CaBP1, lysozyme and αENaC transcripts, as expected. No PCR products were detected in control reactions run for all primer sets and RNA samples with the reverse transcription step omitted (data not shown), suggesting that the products detected were not amplified contaminating genomic DNA.
Figure 2. Isolated mouse acinar cells express mRNA for serous acinar cell-specific markers.
A, Eberwine aRNA amplification reactions were performed on 3–4 nasal acinar cells, ciliated nasal epithelial cells, or salivary gland acinar cells as indicated. B, isolated mouse nasal acinar cells express Muc5AC and Muc10, but not the mucous cell marker Muc5B. Acinar cells also expressed the serous cell proteins LTF and AQP5.
Isolated acinar cells expressed mRNA encoding Muc5AC as well as Muc10 (Fig. 2B). Muc10 is considered a mouse homologue of human Muc7 (Rose & Voynow, 2006), which is thought to be restricted in human airway to serous cells of the submucosal gland (Sharma et al. 1998). Muc5AC is believed to be expressed primarily in airway goblet cells (reviewed in Rogers, 2003), although it is expressed in Calu-3 cells (Berger et al. 1999) and has been detected in submucosal gland cells (Martinez-Anton et al. 2006). Neither Muc5AC nor Muc10 was detected in RNA amplified from ciliated cells. Muc5B was not detected in either amplified acinar cell or ciliated cell aRNA, although its expression was detected in RNA isolated from total nasal turbinate, consistent with the suggestion that Muc5B expression is restricted mainly to airway gland mucous cells (Sharma et al. 1998; reviewed in Rose & Voynow, 2006) and possibly goblet cells (Groneberg et al. 2003). Acinar cells (but not ciliated cells) also expressed the serous cell proteins lactoferrin (LTF; Raphael et al. 1989) and aquaporin 5 (AQP5; Fig. 2B), previously shown to be important for glandular fluid secretion in the murine trachea (Song & Verkman, 2001). Taken together, these expression data indicate that the cells from the isolation that were visually identified as submucosal gland serous acinar cells express serous-cell specific markers, including CFTR.
Serous acinar cells respond to cholinergic stimulation with a rapid decrease in cell volume
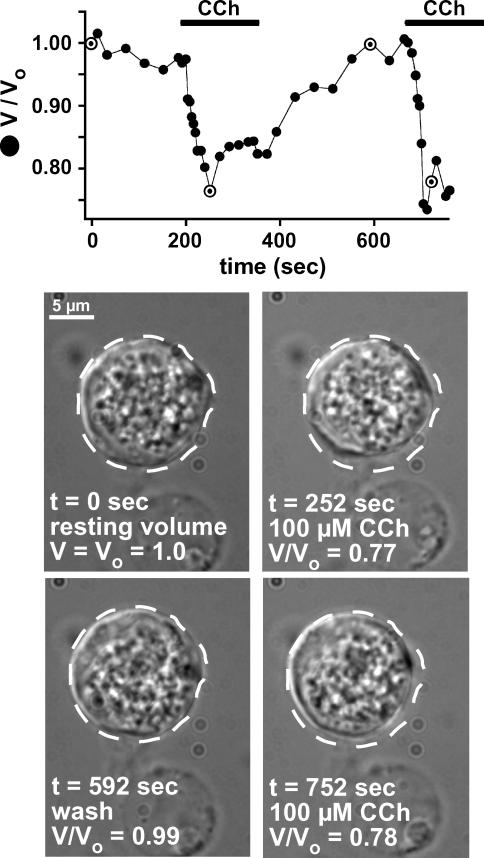
Submucosal glands are cholinergically innervated (Yu et al. 1989; Hejal et al. 1993; reviewed in Rogers, 2001a), and the greatest glandular response to fluid-secreting agonists is seen upon cholinergic stimulation. The responses of isolated acinar cells to the acetylcholine (ACh) agonist carbachol (CCh) were examined using DIC imaging to track changes in cell volume. The rationale for imaging cell volume is based on the fact that water is almost always at equilibrium across membranes, so changes in cell solute content as a consequence of alterations of ion transport rates result in parallel changes of cell volume. Real time measurements of cell volume, combined with appropriate pharmacology and ion substitutions, can provide insights into the molecular mechanisms of ion transport. Furthermore, when combined with simultaneous measurements of ion-indicator dyes, this method affords an approach to correlate concentrations of transported solutes and signalling molecules with secretion in intact cells. Optical studies of cell volume combined with simultaneous quantitative low-light-level fluorescence microscopy using ion indicator dyes has been successfully employed in primary exocrine cells to characterize the molecular mechanisms involved in cholinergic-induced salivary gland ion and fluid secretion (Foskett, 1988, 1990a; Foskett & Melvin, 1989). Confocal three-dimensional reconstruction of isolated calcein-loaded serous acinar cells indicated that the cells behaved as spheres that changed size approximately equally in all dimensions (Supplemental Fig. 1) validating the use of changes in the area of a single optical section to track changes in cell volume. Stimulation with CCh caused cell volume to decrease by 20 ± 2% (volume/original volume (V/Vo) = 0.80 ± 0.02) within 62 ± 5 s (n = 11; Fig. 3). Upon agonist removal, the cells swelled back to the resting volume. We hypothesized that these changes in cell volume were reflective of changes in ionic content in the isolated serous cells as a consequence of changes in their secretory state, and thus set out to determine the utility of this technique for monitoring serous cell fluid secretion.
Figure 3. Serous acinar cells shrink in response to CCh.
Time course of serous acinar cell volume in response to repetitive stimulation with 100 μm CCh. Images below represent the nasal acinar cell at specific time points from the trace shown, which are highlighted on the graph. Dotted line representing the outline of the cell at time 0 is superimposed on the subsequent next four images to illustrate cell volume compared to cell volume at t = 0. Results typical of 11 similar experiments.
CCh-stimulated cell shrinkage occurs in parallel with increases in [Ca2+]i
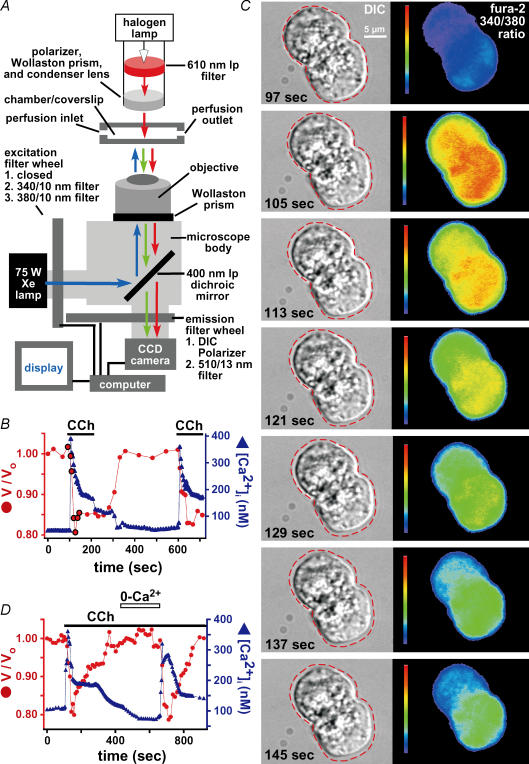
Submucosal gland fluid secretion in response to cholinergic stimulation is believed to result from agonist-induced increases in [Ca2+]i (reviewed in Ballard & Inglis, 2004). To examine the role of [Ca2+]i in the observed CCh-induced cell volume responses, an inverted microscope was modified to carry out simultaneous DIC and fluorescence microscopy of the ratiometric Ca2+ indicator dye fura-2 (Fig. 4A). Resting [Ca2+]i was 89 ± 2 nm (n = 19). Stimulation with 100 μm CCh caused [Ca2+]i to rapidly increase to 447 ± 15 nm within 25 ± 2 s (n = 19; Fig. 4B), typically followed by a plateau phase of increased [Ca2+]i (240 ± 10 nm; n = 15). Upon washout of agonist, [Ca2+]i relaxed to resting levels (Fig. 4B). Reapplication of CCh caused a similar spike (468 ± 15 nm within 25 ± 2 s) and plateau (230 ± 6 nm) of increased [Ca2+]i (n = 15). These agonist-induced changes in [Ca2+]i were associated with profound changes in cell volume observed simultaneously by DIC microscopy (Fig. 4C). The initial CCh-induced peak of increased [Ca2+]i was accompanied by a maximal decrease in cell volume of 21 ± 1% within 62 ± 3 s (n = 19 fura-2-loaded cells; Fig. 4B). Initiation of cell shrinkage occurred after the onset of the rapid rise of [Ca2+]i. Although the sustained [Ca2+]i response to CCh was somewhat variable between cells, cell volume was nevertheless tightly correlated with [Ca2+]i. Thus, an elevated plateau phase of [Ca2+]i was associated with sustained cell shrinkage, whereas more substantial relaxation of [Ca2+]i toward resting levels was accompanied by swelling of cell volume toward resting levels. In ∼20% of cells and acini, stimulation with 100 μm CCh caused only a transient increase of [Ca2+]i (Fig. 4D). The transient rise of [Ca2+]i was accompanied by a transient cell shrinkage (Fig. 4D). In this event, wash out and reintroduction of extracellular Ca2+ in the continued presence of CCh caused another transient spike of [Ca2+]i that induced another transient cell shrinkage.
Figure 4. CCh-induced serous acinar cell shrinkage is accompanied by an increase in [Ca2+]i.
A, microscope set-up for simultaneous DIC and quantitative low-light fluorescence microscopy to measure cell volume and [Ca2+]i using fura-2. See Methods for complete description. B, simultaneous cell volume and [Ca2+]i responses in single serous acinar cells stimulated with 100 μm CCh as indicated. Cell volume is plotted as volume/volume at time 0 (V/Vo)). A representative response is shown (n = 19). Highlighted points on the graph represent time points shown in C. C, time-lapse images of the volume and [Ca2+]i responses to CCh in the two acinar cells imaged in the experiment shown in B. The background-subtracted fura-2 340/380 ratio images illustrate the change in [Ca2+]i. The dotted line represents the outline of the cell at t = 97 s (immediately before CCh exposure), which was then superimposed upon subsequent images to illustrate cell volume compared to volume at t = 97 s. Note that the peak [Ca2+]i increase (t = 105 s) precedes the peak shrinkage (t = 129 s). D, in 10% of cells, stimulation with CCh raised [Ca2+]i only transiently. This was accompanied by cell shrinkage followed by swelling back to near-resting volume. In the continued presence of agonist, removal (∼100 s) and re-introduction of extracellular Ca2+ induced a second, similar response.
The magnitude of the maximum shrinkage observed as well as the time to maximum shrinkage were both dependent on the concentration of CCh (a representative experiment is shown in Fig. 5A). The lowest concentration of CCh used (100 nm) most often elicited a volume response that was indistinguishable from background fluctuations in cell volume (maximal shrinkage 4 ± 1%; n = 10; Fig. 5B, top panel). A greater cell shrinkage of 16 ± 1% within 114 ± 8 s (n = 22) was observed in cells stimulated with 1 μm CCh (Fig. 5B; P < 0.001 compared to 100 nm CCh). A higher concentration of CCh (10 μm; n = 19; Fig. 5B) elicited a volume decrease of 20 ± 1% (P = 0.003 compared to 1 μm CCh) within 62 ± 3 s (P < 0.001 compared to 1 μm CCh), which was nearly identical to the results obtained with 100 μm CCh (maximal shrinkage 21 ± 1% within 62 ± 3 s; P = 0.7 and 0.3, respectively, compared to 10 μm CCh), suggesting that this is a saturating agonist-induced volume response. The magnitude of the peak [Ca2+]i response, the time to the peak [Ca2+]i response after application of CCh, and the plateau level of increased [Ca2+]i (if observed) under conditions of sustained CCh exposure were also dose dependent (Fig. 5C). Thus, CCh elicits increases in [Ca2+]i and associated cell volume decreases in a dose-dependent manner. To determine the role of [Ca2+]i in the observed volume responses, the mechanisms involved in CCh-induced [Ca2+]i dynamics were examined in greater detail.
Figure 5. CCh induces cell shrinkage and increased [Ca2+]i in a dose-dependent manner.
A, representative experiment in which a serous acinar cell was stimulated successively with 100 nm and 1 μm CCh (representative of 3 experiments). B, mean maximal shrinkage and time to maximal shrinkage are plotted for different concentrations of CCh (n = 10, 22, 19 and 28 for 100 nM, 1 μM, 10 μM and 100 μM CCh, respectively). C, mean peak and plateau [Ca2+]i responses and time to peak [Ca2+]i response plotted for 100 nM (n = 10), 1 μM (n = 20), 10 μM (n = 9) and 100 μM CCh (n = 19). After stimulation with 100 nM CCh (n = 10), the peak [Ca2+]i increase was 145 ± 12 nM within 53 ± 2 s followed by a plateau [Ca2+]i of 132 ± 10 nM. After stimulation with 1 μM CCh (n = 20), [Ca2+]i peaked at 321 ± 16 nM within 39 ± 4 s (P < 0.001 for peak [Ca2+]i and P = 0.07 for time to peak compared to 100 nM CCh) followed by a plateau [Ca2+]i of 163 ± 10 nM (P = 0.3 compared to 100 nM CCh). Cells exposed to 10 μM CCh (n = 9) exhibited a similar peak [Ca2+]i of 355 ± 17 nM (P = 0.2 compared to 1 μM CCh) within 29 ± 2 s (P = 0.1 compared to 1 μM CCh), followed by a plateau [Ca2+]i of 214 ± 14 nM (P = 0.01 compared to 1 μM CCh). The maximal [CCh] used (100 μM as reported above) induced a peak [Ca2+]i= 447 ± 15 nM within 25 ± 2 s (P = 0.1 and 0.4, respectively, compared to 10 μM CCh) along with a plateau of 240 ± 10 nM [Ca2+]i (P = 0.3 compared to 10 μM CCh). Significance P-values (compared to results from 10-fold lower [CCh]) are indicated by asterisks (*P = 0.1; **P = 0.01).
Changes in cell volume are tightly coupled to changes in [Ca2+]i
To examine the role of extracellular Ca2+, serous acinar cells were stimulated in a Ca2+-free solution containing no added Ca2+ and 1 mm EGTA (0-Ca2+ solution). Stimulation with 100 μm CCh in 0-Ca2+ solution caused a transient peak increase in [Ca2+]i (283 ± 6 nm within 31 ± 3 s; n = 10) that returned to resting levels (below 100 nm) within 190 ± 25 s (Fig. 6A). This was accompanied by a transient cell shrinkage (21 ± 1% within 62 ± 5 s) followed by swelling back to resting volume within 200 ± 11 s. In the continued presence of CCh, reintroduction of extracellular Ca2+ caused a sustained increase in [Ca2+]i and associated sustained cell shrinkage (Fig. 6A). In a second protocol (Fig. 6B), cells were similarly stimulated in 0-Ca2+ solution, causing a transient rise of [Ca2+]i and cell shrinkage. Washout and re-application of CCh was without effect on [Ca2+]i, indicating that intracellular Ca2+ stores had been depleted by the first exposure to CCh in the absence of extracellular Ca2+. This lack of CCh-induced [Ca2+]i signal was associated with no change of cell volume (Fig. 6B). In contrast, reintroduction of Ca2+ during a third exposure to CCh induced a rapid increase in [Ca2+]i accompanied by cell shrinkage (Fig. 6B). These data suggest that influx of extracellular Ca2+ is required for both the sustained increase of [Ca2+]i and the sustained cell shrinkage. After depletion of [Ca2+]i by prolonged CCh stimulation (> 300 s) in 0-Ca2+ solution and subsequent washout of CCh, reintroduction of [Ca2+]o alone was sufficient to cause a small transient increase in [Ca2+]i that caused a transient decrease in cell volume (Fig. 6C). This suggests that the increased [Ca2+]i caused by Ca2+-influx across the plasma membrane is sufficient to induce cell shrinkage independently of the presence of an agonist.
Figure 6. Changes in serous cell volume are tightly coupled to changes in [Ca2+]i.
A, responses of cell volume and [Ca2+]i to stimulation with 100 μm CCh in 0-Ca2+–1 mm EGTA solution, with extracellular Ca2+ reintroduced in the presence of CCh (representative of n = 10 cells). B, after stimulation with 100 μm CCh in 0-Ca2+ solution, neither cell volume nor [Ca2+]i responded upon washout and reintroduction of CCh in the continued absence of extracellular Ca2+ (representative of n = 5 cells). C, after depletion of intracellular Ca2+ stores by stimulation in 0-Ca2+–1 mm EGTA solution, reintroduction of extracellular Ca2+ in absence of agonist induced a small transient spike in [Ca2+]i that was sufficient to induce a marked volume change (n = 4). D, response of cell volume to 100 μm CCh of cells incubated for 30 min in 5 mm BAPTA-AM. E, cells (obtained from the same preparation used in D) incubated for 30 min in 5 mm BCECF-AM shrank normally when stimulated with 100 μm CCh. F, reversal of CCh (10 μm)-induced changes in [Ca2+]i and cell volume by atropine (100 μm). G, ionomycin (5 μm) was sufficient to induce an agonist independent rise in [Ca2+]i and decrease in cell volume (n = 3 cells). Identical results were obtained with 5 μm A23187 (data not shown, n = 3 cells).
The observed transient increase in [Ca2+]i upon stimulation in 0-Ca2+ solution suggests that release of Ca2+ from intracellular stores alone is sufficient to induce transient cell shrinkage. To determine whether intracellular Ca2+ release is required for cell shrinkage in response to CCh in 0-Ca2+solution, cells were incubated in 5 mm BAPTA-AM (the cell permeant acetoxymethyl (AM) ester-derivative of the Ca2+-chelator BAPTA) for 30 min to achieve a sufficiently high concentration to buffer intracellular Ca2+ release. BAPTA-loaded cells failed to shrink when stimulated with 100 μm CCh in 0-Ca2+ solution (n = 5) (Fig. 6E). As a control for AM-ester loading, cells from the same preparation were incubated for 30 min in 5 mm BCECF-AM, an AM-ester derivative of a pH-sensitive fluorophore that does not chelate Ca2+. BCECF-loaded cells shrank normally in response to CCh (maximal shrinkage was 20 ± 3%, n = 4 cells; Fig. 6D). Thus, the lack of response observed in BAPTA-loaded cells was not due to toxicity arising from a high amount of AM-ester hydrolysis. These data suggest that a [Ca2+]i rise as a result of release from intracellular stores is necessary for cell shrinkage during the response to CCh in 0-Ca2+ solution.
We hypothesized that CCh increases [Ca2+]i via binding to a muscarinic receptor, based on previous evidence of M1 and M3 receptor expression in submucosal glands (Ishihara et al. 1990; reviewed in Rogers, 2001a). Muscarinic activation of receptor-coupled Gq proteins leads to production of InsP3 and release of Ca2+ from the endoplasmic reticulum via the InsP3 receptor. In agreement, atropine (100 μm), a competitive muscarinic receptor antagonist, blocked the normally sustained increase in [Ca2+]i stimulated during exposure to 10 μm CCh, causing both [Ca2+]i and cell volume to return to resting levels (Fig. 6F).
Together, the above data suggest that an increased [Ca2+]i is necessary to induce cell shrinkage. To determine whether elevated [Ca2+]i was sufficient, cells were treated with the calcium ionophores ionomycin or A23187. Both ionophores raised [Ca2+]i and induced similar cell shrinkage (Fig. 6G; maximum shrinkage was 25 ± 3% with ionomycin and 24 ± 2% with A23187; n = 3 experiments with each ionophore). After application of either ionophore, the extent of cell shrinkage achieved a plateau despite the fact that [Ca2+]i continued to rise, suggesting that a saturating concentration of [Ca2+]i was reached and that a ∼20–25% volume decrease is a maximal response.
Histamine and ATP induce similar [Ca2+]i-dependent volume responses in murine serous acinar cells
To determine if other receptors similarly mobilized Ca2+ and induced cell-volume responses, acinar cells were perfused with solutions containing either histamine or ATP. Histamine is a local mediator of inflammatory responses; its release during inflammation in CF lungs could possibly stimulate gland secretion via serous cell H1 receptors. Histamine (100 μm) increased [Ca2+]i (peak 350 ± 16 nm within 25 ± 2 s; plateau 253 ± 5 nm; n = 9) that was accompanied by cell shrinkage (maximal shrinkage was 21 ± 1% within 62 ± 4 s) comparable to that elicited by CCh (Fig. 7A). ATP is a coneurotransmitter in adrenergic and cholinergic neurons and is also released from airway epithelial cells, where it may play a role in ASL homeostasis (Donaldson et al. 2000; Lazarowski et al. 2004). Exposure to 100 μm ATP elicited similar changes in [Ca2+]i and cell volume to that observed with CCh (peak [Ca2+]i= 348 ± 14 nm within 26 ± 3 s; plateau [Ca2+]i= 249 ± 3 nm; maximal shrinkage was 21 ± 1% within 57 ± 3 s; n = 8 (Fig. 7B). While the peak [Ca2+]i increase with both agonists was somewhat less than that observed with 100 μm CCh, both agonists increased [Ca2+]i sufficiently to result in a significant sustained cell shrinkage. We conclude that cell shrinkage is a generalized response of submucosal gland serous acinar cells in response to a variety of secretagogues that signal through changes in [Ca2+]i.
Figure 7. Histamine and ATP elevate [Ca2+]i and induce cell volume changes similar to those observed with CCh.
A, stimulation with 100 μm histamine, as indicated. Representative of n = 9 cells. B, stimulation with 100 μm ATP as indicated. Representative of n = 8 cells.
Ca2+-dependent cell volume changes reflect changes in cell solute content
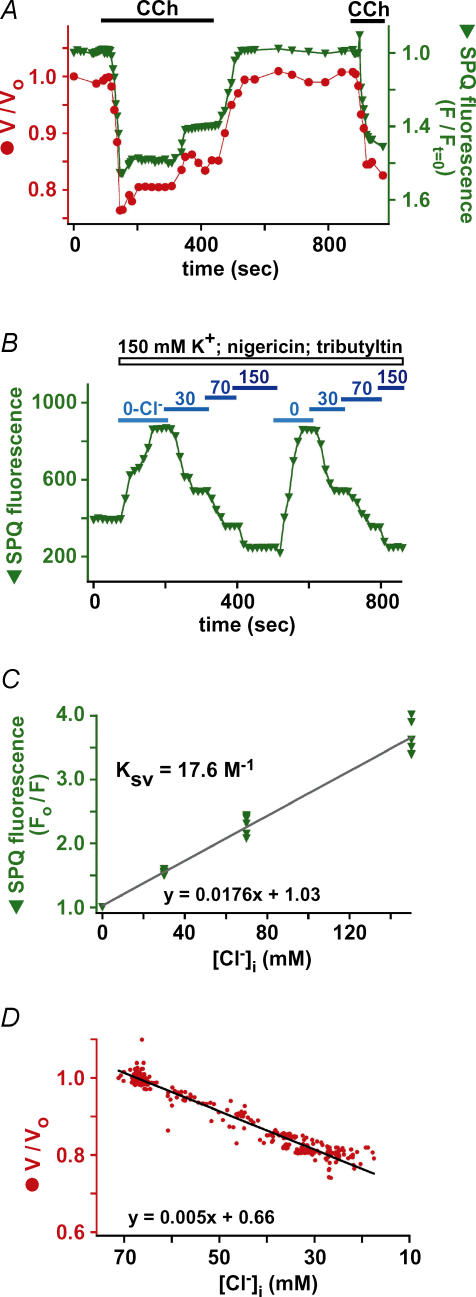
We hypothesized that the observed changes in cell volume upon CCh-stimulation were due to changes in intracellular solute content caused by [Ca2+]i-dependent alterations of ion permeabilities, as previously shown in salivary acinar cells (Foskett, 1990a). However, other possible explanations exist, including loss of cell content during exocytosis of secretory granules, internalization of the plasma membrane, or cell shrinkage via contractile mechanisms. To determine if cell volume changes were caused by changes in cell solute content, we combined optical measurements of acinar cell volume with a fluorescent indicator to track the solute content of the cell. As discussed in Foskett (1990b), because Cl− is not the overwhelmingly predominant anion in cells (typically > 50% of cellular anionic content is made up of organic membrane-impermeant molecules), changes in Cl− content will be reflected in changes in [Cl−]i. Accordingly, changes in cell volume should be associated with changes in cell Cl− content that would be reflected in corresponding changes in [Cl−]i. To test this hypothesis, cell volume and [Cl−]i were monitored by simultaneous DIC imaging and fluorescence imaging of the halide-sensitive fluorophore SPQ. SPQ fluorescence is quenched by Cl− via a collisional mechanism, leading to a linear reciprocal relationship between its fluorescence and [Cl−].
CCh-induced cell shrinkage was paralleled quantitatively by increases in SPQ fluorescence (plotted inversely as fluorescence normalized to fluorescence at time 0 (F/Ft=0; Fig. 8A). In SPQ-loaded cells, 100 μm CCh caused a 20 ± 2% decrease in cell volume that was accompanied by a 55 ± 2% increase in SPQ fluorescence (n = 9). Under all conditions, cell volume was correlated tightly with the magnitude of SPQ fluorescence. Thus, partial recovery of cell volume towards prestimulation levels was associated with parallel decrease in SPQ fluorescence, and full recovery of cell volume following removal of CCh was associated with a decrease of SPQ fluorescence to prestimulation levels.
Figure 8. Changes in [Cl−]i parallel observed cell volume changes.
A, simultaneous determination of cell volume and SPQ fluorescence intensity (normalized to the intensity at t = 0 (F/Ft = 0) and plotted inversely) changes in response to 100 μm CCh. B, representative SPQ calibration experiment in acinar cells. C, composite Stern–Volmer plot of 6 calibration experiments. All values are represented as fluorescence in 0-Cl−/fluorescence (F0/F). Average F0/F-values were 1.55 ± 0.02 for 30 mm Cl−, 2.29 ± 0.06 for 70 mm Cl−, and 3.64 ± 0.1 for 150 mm Cl−. F0/F was 2.16 ± 0.06 under resting conditions before calibration, indicating resting [Cl−]i= 64.5 ± 4 mm. The quenching constant, KSV, determined from the slope of the least squares regression fit, was 17.6 m−1 (standard deviation (s.d.) = 0.5 m−1). D, composite plot of simultaneous [Cl−]i and cell volume determinations from 9 experiments during CCh stimulation that demonstrates tight correlation between the two parameters. Data were fitted with least squares linear regression, with slope equal to 5.56 m−1 (s.d. of slope = 0.210−3m−1).
Use of a non-ratiometric dye in cells undergoing changes in volume is not ideal. However, as previously discussed (Foskett, 1990a), changes in cell volume do not strongly influence the recorded SPQ fluorescence intensity. Because the fluorescence was imaged using wide-field optics with its considerable depth of field, emitted fluorescence was collected along the entire depth of the cells. The focal plane could be moved along the z-axis from the top to the bottom of the cell without a noticeable change in the raw SPQ fluorescence intensity recorded. Thus it appeared that the wide-field optics employed enable collection of fluorescence emitted during an experiment from a constant number of dye molecules. Nevertheless, to determine if changes in cell volume per se affect the measured changes in SPQ fluorescence, and to rule out SPQ quenching by intracellular organic anions such as proteins, cells were osmotically shrunk by exposing them to hypertonic solutions containing 150 mm K+ with [Cl−]i kept constant (see Methods). Exposure to 356 (n = 5), 407 (n = 19), and 453 (n = 7) mosmol l−1 solutions induced shrinkages of 12 ± 1.1%, 19 ± 0.7% and 28 ± 1.3%, respectively (Supplemental Fig. 2A). Shrinkage decreased SPQ fluorescence by 1 ± 1%. These results confirm that changes in serous acinar cell volume per se have negligible effects on the measured SPQ fluorescence. A van't Hoff plot of normalized cell volumes against the inverse of the measured osmolarities (1/osmolarity) was linear with the y-axis intercept indicating that the osmotically inactive volume is ∼20% (Supplemental Fig. 2B), a value that agrees with observations in other mammalian cells (discussed in Foskett, 1990b).
The observed CCh-induced changes in SPQ fluorescence and their tight correlation with cell volume strongly suggested that agonist-induced changes in cell volume are caused by changes in cell solute content, reflected by quantitative changes in [Cl−]i. To quantify [Cl−]i, SPQ fluorescence was calibrated using the tributyltin and nigericin along with solutions of known [Cl−] to clamp [Cl−]i=[Cl−]o and [pH]i=[pH]o in a separate group of calibration cells (as described in Foskett, 1990a). Six calibration experiments were performed (an example is shown in Fig. 8B), with each consisting of two exposures of a cell or acinus to a range of [Cl−]. Only cells in which similar fluorescence intensities were observed during the first and second exposures were used, since these had no dye leakage during the course of the calibrations. The Stern–Volmer plot (Fig. 8C) indicated that the quenching constant (Ksv; the slope of the linear fit) was 17.6 m−1, nearly identical to the quenching constant of 17 m−1 previously measured in salivary gland acinar cells (Foskett, 1990a) and similar to the reported values of 13 m−1 found for both canine tracheal epithelial cells (Chao et al. 1990) and fibroblasts (Chao et al. 1989). Extrapolation of the average SPQ fluorescence intensity in the cells used in the calibration experiments before their exposure to the nigericin–tributyltin solutions indicated that resting [Cl−]i in isolated murine serous acinar cells is 64.5 ± 4 mm. Using this value and the Stern–Volmer plot, average changes in SPQ fluorescence during agonist stimulation were converted to quantitative changes in [Cl−]i. The average increase in SPQ fluorescence of 55% upon stimulation with CCh corresponds to a decrease of [Cl−]i from 64.5 in unstimulated cells to 28 mm during the peak cell shrinkage. The relationship between [Cl−]i and cell volume was plotted in aggregate for nine cells during CCh-induced stimulation (Fig. 8D). The linear relationship demonstrates that cell volume quantitatively tracks [Cl−]i. Thus, cell volume is a quantitative measure of cell solute content as well as [Cl−]i. Importantly, these results establish that cell volume changes are caused by changes in cell solute content.
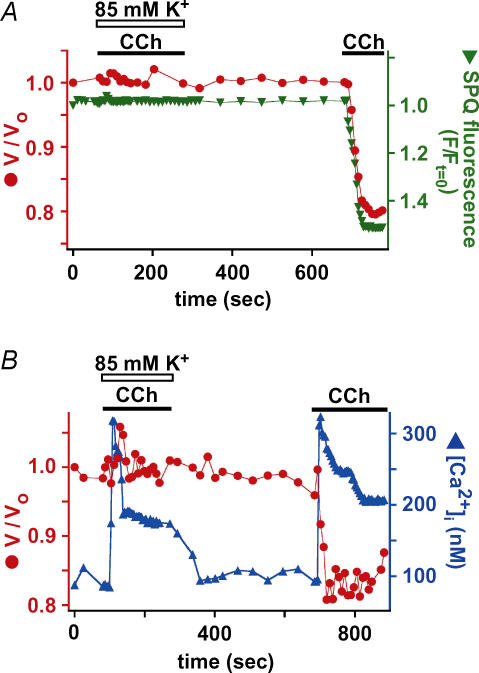
K+ efflux is required for cell shrinkage and [Cl−]i decrease
Because the results suggested that changes in cell volume and Cl− content are tightly coupled, it is expected that a similar quantity of cation must accompany net Cl− fluxes to preserve electroneutrality. Because K+ is the predominant cation inside cells, we hypothesized that cell shrinkage predominately reflected loss of KCl. We therefore examined whether K+ efflux contributed to agonist-evoked cell shrinkage. As previously discussed (Foskett, 1990a), isosmotic loss of KCl will be expected to result in only negligible changes in [K+]i, and thus measurements of [K+]i would not be informative. Instead, we examined the effects of changing the driving forces for K+ efflux. When [K+]o in the medium was increased from 5 mm to 85 mm (by isosmotic replacement of Na+), normal CCh-induced cell shrinkage was prevented and no change in SPQ fluorescence was observed (Fig. 9A). This result is consistent with a requirement of K+ efflux during agonist-induced cell shrinkage. However, because exposure of the cell to 85 mm K+ is likely to have depolarized the plasma membrane potential, it was possible that the cells failed to shrink because the high [K+]o solution reduced the magnitude of the CCh-induced [Ca2+]i signal. However, the initial [Ca2+]i increase, which is primarily contributed to by release from intracellular stores, was substantially unaltered in the high K+ solution (Fig. 9B). The sustained plateau phase of increased [Ca2+]i was somewhat lower under these conditions (Fig. 9B), likely to be due to reduction of the driving force for Ca2+ entry across the plasma membrane during the sustained phase. Nevertheless, our other observations suggest that the magnitude of the [Ca2+]i during the sustained phase was still sufficient to induce cell shrinkage under normal conditions. Therefore, we conclude that the effects of high K+ to block normal cell shrinkage and efflux of intracellular Cl− were unrelated to effects on [Ca2+]i signals. Rather, the results are consistent with the hypothesis that K+ efflux from the cell is required for agonist-induced serous acinar cell shrinkage. Together with the SPQ data, our results suggest that the observed shrinkage is due to loss of cellular K+ and Cl− content in response to agonist-induced increased [Ca2+]i.
Figure 9. K+ efflux is required for CCh-induced cell shrinkage and Cl− loss.
Simultaneous determinations of cell volume and SPQ fluorescence (A; n = 4) or [Ca2+]i (B; n = 10) during stimulation by 100 μm CCh in bathing solution containing either 85 mm K+ (as indicated) or normal extracellular K+ (5 mm).
Cell swelling reflects NKCC1-mediated solute uptake
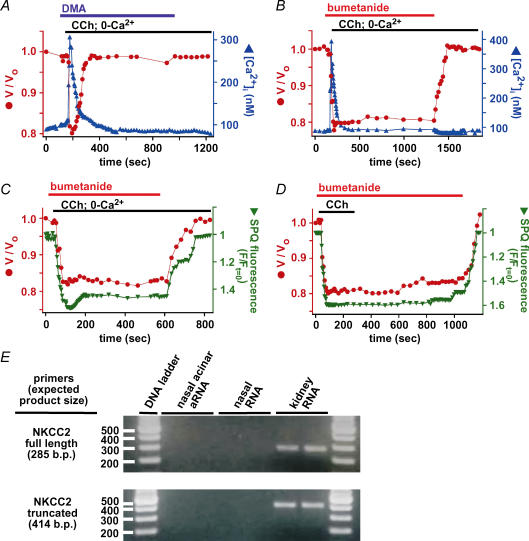
If cell volume decrease is due to loss of solute, cell swelling towards resting levels upon lowering of [Ca2+]i should reflect solute uptake. In a paradigm of fluid secretion by epithelial cells, solute uptake mechanisms in the basolateral membrane accumulate [Cl−]i above its electrochemical equilibrium value, enabling it to exit the cell across the apical membrane through Cl− channels there. The best-characterized Cl− uptake pathways involve the Na+/H+ exchanger (NHE) coupled in parallel to a Cl−/OH− exchanger (AE), as well as the Na+–K+–2Cl− cotransporter (NKCC). We hypothesized that the activities of either or both of these pathways were responsible for solute accumulation that accounted for cell swelling. To determine the relative contributions of these pathways to the observed cell volume increases, cells were exposed to dimethylamiloride (DMA) or bumetanide, specific inhibitors of NHE and NKCC, respectively. Application of either reagent was without effect on the steady-state volume of unstimulated cells for up to 5 min (not shown). This result suggests that steady-state maintenance of cell solute content (and therefore [Cl−]i) is not dependent on these pathways, indicating either that other transport mechanisms are involved, or that solute efflux and influx pathways are both inactive under resting conditions. Maximal shrinkage upon 100 μm CCh stimulation was 20 ± 1% within 64 ± 4 s (n = 17) in the presence of 30 μm DMA (Fig. 10A) and 22 ± 2% within 62 ± 4 s (n = 25) in the presence of 100 μm bumetanide (Fig. 10B). Thus, neither inhibitor affected the rate or extent of cell shrinkage during CCh stimulation. The agonist-stimulated changes in [Ca2+]i were also unaffected by either inhibitor. In DMA-treated cells stimulated with 100 μm CCh in 0-Ca2+ solution, the mean [Ca2+]i peak was 300 ± 7 nm within 23 ± 2 s, and [Ca2+]i returned to resting levels within 165 ± 18 s (n = 17). Bumetanide-treated cells stimulated with 100 μm CCh in 0-Ca2+ solution exhibited a rise in [Ca2+]i to 310 ± 8 nm within 27 ± 3 s, and [Ca2+]i returned to resting levels within 211 ± 15 s (n = 15).
Figure 10. Cell volume recovery is mediated by solute uptake via a NKCC-dependent pathway.
Simultaneous determinations of cell volume and [Ca2+]i (A and B) or SPQ fluorescence (C and D) during stimulation by 100 μm CCh in the absence (A–C) or presence (D) of extracellular [Ca2+]i in the presence of the Na+/H+ exchanger inhibitor DMA (30 μm; A) or the NKCC cotransporter inhibitor bumetanide (100 μm; B–D). Experiments shown in A–D are representative of 17, 15, 5 and 4 identical experiments, respectively. E, expression of NKCC2 was not detected in RNA from either isolated nasal serous acinar cells or from intact nasal tissue, whereas it was detected using both primer sets and RNA extracted from murine kidney.
Under conditions of CCh/0-Ca2+ stimulation, DMA-treated cells transiently shrank and swelled back to resting volume within 213 ± 23 s (Fig. 10A), a rate that was similar to that of untreated cells, suggesting that DMA had little effect on solute uptake during stimulated conditions. In marked contrast, bumetanide nearly completely blocked both the normal volume increase (Fig. 10B–D; maximal volume recovery was 20 ± 5% of control cells (V/Vo= 0.84 ± 0.01 after 600 s; n = 23) and recovery of [Cl−]i after stimulation in 0-Ca2+ solution (Fig. 10C; n = 5) or wash-out of the agonist (Fig. 10D; n = 4).
These results suggest that cell swelling following Ca2+-induced shrinkage is due to solute uptake through a bumetanide-sensitive pathway, most likely a NKCC transporter. The cotransporter involved is most likely NKCC1, as NKCC2 expression is believed to be restricted to the kidney (reviewed in Mount et al. 1998). Mouse nasal acinar cells express detectable levels of NKCC1 mRNA (Fig. 2). RNA amplification and RT-PCR of NKCC2 in mouse nasal acinar cells as well as mouse nasal tissue was employed to determine whether NKCC2 might play a role in serous cell solute uptake. Although several splice variants of NKCC2 exist in the kidney, most of them differ only in the exons encoding the second transmembrane domain, with the various transcripts having only two different C-terminal ends (one full length and one truncated; reviewed in Mount et al. 1998). Therefore, all NKCC2 isoforms should be detectable with only two 3′-biased primer sets (listed in Supplemental Table 1). Both primers sets detected expression of NKCC2 using RNA isolated from murine kidney (Fig. 10E). However, NKCC2 transcripts could not be detected in either isolated acinar cell aRNA or RNA extracted from mouse nasal tissue. Thus, NKCC1 appears to be the major NKCC isoform expressed in airway gland serous acinar cells. Taken together, our results indicate that solute uptake after Ca2+-induced acinar cell shrinkage is mediated by NKCC1.
Ca2+-evoked fluid secretion does not require CFTR
Since previous data (Engelhardt et al. 1992; Jacquot et al. 1993) as well as our own immunocytochemistry suggest that serous acinar cells are sites of CFTR expression, we explored the possible role of CFTR in the observed cell shrinkage and Cl− loss in response to CCh stimulation. To this end, we used the cftrtm1Unc−/− mouse, which lacks functional CFTR. The maximal cell volume decrease in acinar cells from heterozygote WT/cftrtm1Unc mice was 22 ± 2.5% within 61 ± 3 s (n = 7; data not shown) in response to 10 μm CCh and 21 ± 2% within 61 ± 4 s (n = 8; data not shown) in response to 100 μm CCh. Serous cells isolated from homozygous cftrtm1Unc−/− mice (cftr−/− cells) shrank robustly in response to both 10 μm CCh (19 ± 2% maximal shrinkage within 64 ± 4 s; n = 8, data not shown) and 100 μm CCh (20 ± 2% maximal shrinkage within 63 ± 4 s; n = 19, Fig. 11A). Cells from cftr−/− mice also shrank robustly in response to 100 μm histamine (maximal shrinkage 20 ± 1% within 65 ± 4 s; n = 7, data not shown) and 100 μm ATP (maximal shrinkage 20 ± 1% within 64 ± 3 s; n = 7, data not shown). Thus, no differences in either magnitude or rate of cell shrinkage were observed among cftr−/−, WT, or heterozygote WT/cftrtm1Unc cells. CCh-induced [Ca2+]i signals were nearly identical in cftr−/− and WT cells. Upon stimulation with 100 μm CCh, [Ca2+]i rose to 400 ± 19 nm within 30 ± 2 s and then typically remained at an elevated level of 248 ± 5 nm (n = 14; Fig. 11A). These data indicate that CCh-induced [Ca2+]i increases are capable of inducing cell volume changes in cells lacking CFTR in a manner quantitatively similar to CFTR-expressing WT acinar cells.
Figure 11. CFTR is not required for the agonist-stimulated Cl− loss.
Simultaneous determinations of cell volume and [Ca2+]i (A) or SPQ fluorescence (B) in serous acinar cells from cftrtm1unc−/− mice in response to 100 μm CCh. Representative of n = 15 (A) and 6 (B) experiments. C, representative in vivo calibration experiment of SPQ fluorescence in serous acinar cells from cftrtm1unc−/− mice. D, composite Stern–Volmer plot of 3 calibration experiments. Average F0/F values obtained were 1.55 ± 0.03 for 30 mm Cl−, 2.32 ± 0.01 for 70 mm Cl−, and 3.60 ± 0.06 for 150 mm Cl−. The Stern–Volmer plot yielded KSV= 17.3 m−1 (standard deviation of the slope of the fitted line = 0.4 m−1), with resting [Cl−]i= 66 ± 2.3 mm (F0/F = 2.17 ± 0.04). E, composite data from 6 experiments during stimulation of serous acinar cells from cftrtm1unc−/− mice with 100 μm CCh showing correlation of [Cl−]i, determined from SPQ fluorescence, and cell volume. The slope of the fit was 5.32 m−1 (s.d.= 0.0710−3m−1). F, lack of effect of the CFTR inhibitor CFTRinh172 (10 μm) on cell volume (n = 15) or [Cl−]i (SPQ fluorescence; n = 6) responses of serous acinar cells from WT mice.
To further explore a possible role of CFTR, isolated cftr−/− acinar cells were loaded with SPQ and stimulated with CCh. In response to 100 μm CCh, the peak increase in SPQ fluorescence was 56 ± 2% with maximal shrinkage of 20 ± 2% (n = 6; Fig. 11B), similar to those observed in cells from the WT animals. Calibration of SPQ fluorescence in cells from cftrtm1Unc−/− mice (Fig. 11C and D) revealed that both the resting [Cl−]i (65.9 ± 2.3 mm) and KSV (17.3 m−1) were nearly identical to the values measured in WT cells (64.5 ± 4 mm and 17.5 m−1, respectively). Quantitative analysis of the increase in SPQ fluorescence indicated that 100 μm CCh caused a reduction in [Cl−]i to 30 ± 2 mm, also similar to WT cells. Cell volume varied linearly with [Cl−]i in a manner nearly identical to WT cells (Fig. 11E).
A potential caveat in using cells from the cftrtm1Unc−/− mouse is that they may possess compensatory mechanisms in the absence of CFTR, for example the up-regulation of one or more alternative Cl− conductance(s). To address this possibility, the specific inhibitor CFTRinh172 was used to inhibit CFTR in WT cells. CFTRinh172 has been shown to block glandular fluid secretion in response to both forskolin and pilocarpine (Thiagarajah et al. 2004). WT serous acinar cells that had been pretreated for 2–3 min with 10 μm CFTRinh172 shrank normally in response to both 100 μm CCh (20 ± 2% maximal shrinkage within 65 ± 5 s; n = 15 cells; Fig. 11F) and 100 μm histamine (20 ± 2% maximal shrinkage within 60 ± 4 s; n = 8 cells, data not shown). In agreement, no difference was observed in the maximal increase in SPQ fluorescence in response to 100 μm CCh (55 ± 3%; n = 6) compared with control cells (Fig. 11F). These data suggest that CFTR does not play an essential role in CCh- or histamine-stimulated fluid secretion under these conditions, and it appears that serous acinar cells lacking functional CFTR are still capable of secreting Cl− in response to stimulation with Ca2+-mobilizing agonists.
Murine serous acinar cells stimulated with cAMP-increasing agonists exhibit no change in cell volume
These data suggest that CFTR Cl− conductance is not necessary for Ca2+-evoked fluid secretion in serous acinar cells, but submucosal glands also secrete fluid in response to agonists that stimulate production of cAMP. It is possible that CFTR is involved specifically in cAMP-mediated fluid secretion. Whereas [Ca2+]i-mediated secretory responses were normal in intact glands isolated from CF patients, cAMP-mediated responses were absent (reviewed in Ballard & Inglis, 2004). Therefore, we hypothesized that CFTR, while not required for [Ca2+]i-evoked fluid secretion, may instead play an essential role in cAMP-evoked fluid secretion in serous acinar cells. To understand the role of CFTR in this pathway, similar optical methods were used to track cell volume in acinar cells stimulated with the adenylyl cyclase-activating compound forskolin. In acinar cells isolated from WT mice treated with either 10 μm (n = 6; Supplemental Fig. 3A) or 25 μm (n = 4; data not shown) forskolin for up to 600 s, no cell volume changes were observed. Because PKA phosphorylation has also been implicated in up-regulation of NKCC1 activity (reviewed in Hebert et al. 2004), a possible reason for the observed lack of shrinkage could be that forskolin activated both ion efflux (though CFTR) and ion influx (through NKCC1 or another solute transporter) pathways in parallel. Because observation of a change of cell volume requires a perturbation of the steady state balance between solute influx and efflux, simultaneous activation of both pathways could result in no or only small changes in cell volume. To address this, we treated cells with forskolin in the presence of 100 μm bumetanide to inhibit NKCC1-mediated solute influx. Under these conditions, 10 μm forskolin treatment still had no effect on cell volume (n = 4; Supplemental Fig. 3B). Serous acinar cells were also stimulated with forskolin in the presence of acetazolamide, an inhibitor of carbonic anhydrase (CA), to minimize Cl−/HCO3− exchange (Sterling & Casey, 2002; McMurtrie et al. 2004; Nguyen et al. 2004). Acetazolamide was shown to block 50% of the forskolin-stimulated short circuit current in Calu-3 monolayers (Krouse et al. 2004) and inhibit fluid secretion in intact glands (Inglis et al. 1997a; Inglis et al. 1998). However, 10 μm forskolin had no effect on the volume of serous acinar cells simultaneously treated with 20 μm acetazolamide (Supplemental Fig. 3C). Taken together, these data suggest that the inability to observe cell shrinkage in response to forskolin is unlikely to be caused by concomitant activation of ion influx and efflux pathways.
We also considered that the lack of effect of forskolin could be caused by an inability to activate adenylyl cyclase in these cells, or to its inability to generate cAMP in the proper subcellular compartment required to activate CFTR. It is possible that PKA-dependent activation of CFTR could be coupled to specific localized pools of cAMP and/or specific receptors. To address this possibility, we stimulated serous cells with a cocktail of cAMP-elevating agents that included 10 μm forskolin, 1 mm IBMX (to inhibit phosphodiesterase), 200 μm adenosine (which could activate adenylyl cyclase via purinergic A2 receptors), 200 μm isoproterenol (a β-adrenergic agonist), and 5 μm vasoactive intestinal peptide (VIP). However, no volume changes were noted under these conditions even during prolonged stimulation (n = 7; Supplemental Fig. 3D). Addition of 250 μm 8-Br-cAMP (a cell permeant cAMP analogue) to this cocktail also failed to elicit any effects on cell volume (n = 3; not shown). To again address the possibility of simultaneous stimulation of solute uptake mechanisms, this cocktail was supplemented with 100 μm bumetanide, 50 μm DMA, and 50 μm acetazolamide to inhibit NKCC1, NHE, and AE, respectively. Surprisingly, no volume changes were observed under these conditions (n = 5; Supplemental Fig. 3D).
Ca2+-stimulated cell shrinkage is not enhanced by cAMP
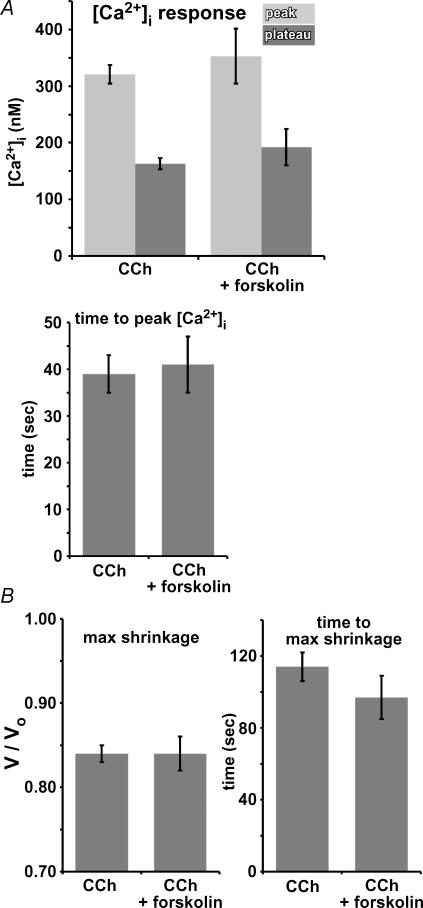
There are several possible explanations for the lack of an observed volume response to cAMP stimulation. First, the magnitude of volume change could be below the threshold of detection of our assay. This would not be surprising since the maximal glandular fluid secretion rate observed upon stimulation with VIP is only ∼3% of that induced by cholinergic stimulation in murine glands (Ianowski et al. 2007). Another possibility is that K+ permeability is low under conditions of cAMP stimulation, so whereas CFTR Cl− permeability may become activated, K+ conductance could be rate limiting for solute efflux and associated cell shrinkage. We therefore considered that cAMP activation of CFTR Cl− conductance might only become manifest as observable cell shrinkage if K+ permeability was simultaneously enhanced. We hypothesized that low concentrations of CCh that normally elicit submaximal volume responses might raise [Ca2+]i sufficiently to stimulate the basolateral K+ permeability to an extent that would make cAMP-stimulated CFTR-mediated Cl− permeability rate-limiting for salt efflux, enabling visualization of cell shrinkage during cAMP stimulation. One potential complication would be the possibility of cross talk between the [Ca2+]i and cAMP-signalling pathways that could result in altered [Ca2+]i dynamics under conditions of cAMP stimulation. For example, it has been reported that cAMP potentiates [Ca2+]i responses to purinergic stimulation in parotid gland cells (Brown et al. 2004).
To address this, serous acinar cells were loaded with fura-2 and stimulated with 15 μm forskolin for ∼250–300 s followed by stimulation with 1 μm CCh in the continued presence of forskolin. Forskolin was without effect on resting [Ca2+]i. CCh (1 μm) caused [Ca2+]i to rise to 353 ± 50 nm within 41 ± 6 s, typically followed by a plateau of increased [Ca2+]i of 192 ± 32 nm (n = 9; Fig. 12A). As described, control cells stimulated with 1 μm CCh without forskolin treatment exhibited a [Ca2+]i peak of 320 ± 16 nm (P = 0.41 compared to forskolin-treated cells) within 39 ± 4 s (P = 0.85 compared to forskolin-treated cells), followed by a plateau of 163 ± 10 nm (P = 0.25 compared to forskolin-treated cells; n = 20; Fig. 12A), suggesting that forskolin did not effect the observed [Ca2+]i responses. In cells pretreated with 15 μm forskolin, 1 μm CCh induced cell shrinkage of 16 ± 2% within 97 ± 12 s (n = 9; Fig. 12B). Control cells without forskolin treatment shrank by 16 ± 1% within 115 ± 8 s (n = 20; Fig. 12B; P = 0.8 and 0.3 for max shrinkage and time to max shrinkage, respectively, for forskolin treated versus untreated cells). Thus, forskolin treatment induced no significant differences in either the observed [Ca2+]i signals or cell shrinkage elicited by 1 μm CCh.
Figure 12. Stimulation of serous acinar cells with 1 μm CCh in the presence of increased [cAMP]i does not increase the rate or magnitude of cell volume changes or [Ca2+]i dynamics compared to control cells.
A, peak [Ca2+]i (top panel, lighter grey bars), plateau [Ca2+]i (top panel, darker grey bars), and time to peak [Ca2+]i (bottom panel) responses to 1 μm CCh with or without 15 μm forskolin pretreatment. B, maximal shrinkage (first panel) and time to maximal shrinkage (second panel) upon stimulation with 1 μm CCh ± pretreatment with 15 μm forskolin.
Discussion
Fluid secretion by airway submucosal glands is believed to play important roles in airway surface liquid volume and composition homeostasis and in lung innate immunity. In previous studies of submucosal gland secretion employing intact glands, the ability of glands to secrete a mucus fluid was established (Yang et al. 1988; Ballard et al. 1995; Inglis et al. 1997a,b; Trout et al. 1998, 2001), and the volume and composition of the secreted fluid in normal and cystic fibrosis tissues was determined (Inglis et al. 1998; Jayaraman et al. 2001; Joo et al. 2001a,b, 2002b, 2006; Song & Verkman, 2001; Thiagarajah et al. 2004; Salinas et al. 2005; Song et al. 2005; Ianowski et al. 2007). Nevertheless, the complex geometry of the glands and the presence of numerous cell types have limited insights into the molecular mechanisms in specific cell types that contribute to the generation and composition of the mucus secretion that emerges from the gland onto the airway surface. To address these issues, in the present study, we employed cell isolation protocols and immunocytochemical and gene-expression techniques to specifically identify murine submucosal gland acinar cells for use in physiological studies. We used optical imaging of single cells and acini to determine the mechanisms involved in agonist-stimulated fluid secretion in serous acinar cells. Murine acinar cells that express CFTR secrete fluid in response to agonists that raise [Ca2+]i, including ATP, histamine and CCh, by a process that involves Cl− and K+ conductances and NKCC1 cotransport, but that does not appear to require CFTR. The implications of these finding are discussed in more detail below.
Identification of submucosal gland serous acinar cells
Collagenase digestion of murine nasal submucosal glands yielded primarily two cell types, ciliated cells and cells that had an acinar appearance with small (∼1 μm) apical secretory granules. Cells with larger secretory granules filling their cytoplasm, presumably representing goblet and/or mucous cells, were also observed infrequently. The reasons for the relative abundance of only ciliated and serous-like cells are unclear, but may include specific cell loss during the isolation protocol, and/or a more simple cellular composition of murine nasal glands by comparison with human airway submucosal glands. Serous cells may outnumber mucous cells by at least 50% in human airway submucosal glands (reviewed in Ballard & Inglis, 2004), so a higher yield of serous cells during cell digestion may be expected. Both major identified isolated cell types (serous and ciliated) expressed CFTR specifically in their apical poles. The specificity of the immunostaining was confirmed by examination of cells from mice lacking CFTR, as well as three- to four-cell RT-PCR to examine expression of CFTR mRNA. Immunostaining and RT-PCR confirmed that the cells with an acinar appearance were serous acinar cells, since they expressed CFTR, lactoferrin, and lysozyme but not αENaC. Although we could not visually differentiate between surface epithelial- and collecting-duct ciliated cells, all isolated ciliated cells in the preparation from WT mice stained apically for CFTR.
Simultaneous optical imaging of cell volume and [Ca2+]i during agonist stimulation
We employed optical imaging approaches to dissect the ion transport pathways that mediate acinar cell fluid secretion. By simultaneous DIC and quantitative low-light fluorescence microscopy, we have been able to simultaneously record single cell or acinus volume and the concentrations of intracellular ions involved in either mediating fluid secretion (Cl−) or regulating it (Ca2+). Although these approaches do not provide insights into the biophysical properties of specific ion conductances, they enable cellular responses to be observed in cells with an intact cytoplasm and intact signal transduction mechanisms, making these techniques less invasive than conventional whole-cell patch clamping. Furthermore, they are useful in examining the activities of electroneutral transport pathways.
Because lung submucosal glands are mainly under cholinergic control, we first examined the responses of isolated serous acinar cells to the cholinergic agonist CCh. We observed that muscarinic-induced elevation of [Ca2+]i was accompanied by profound cell shrinkage. Recovery of [Ca2+]i towards or to resting levels was accompanied by cell swelling towards or to prestimulation levels, respectively. Thus, there was a tight correlation between changes in [Ca2+]i and changes in cell volume during agonist stimulation. This observed coupling of cell volume and [Ca2+]i is very similar to previous observations of rat parotid exocrine acinar cells (Foskett & Melvin, 1989). Increased [Ca2+]i appears to be both necessary and sufficient to cause serous acinar cell shrinkage since the cell volume responses could be similarly elicited with Ca2+ ionophores, and intracellular buffering of the Ca2+ transient prevented the shrinkage. Similar [Ca2+]i responses and associated cell volume changes were also elicited by histamine and ATP, suggesting that cell shrinkage is a general response of submucosal gland acinar cells to agonists that elevate [Ca2+]i. Furthermore, these responses provide functional evidence for the expression of H1 and P2Y receptors in submucosal gland serous cells. Expression of these receptors is likely to have physiological importance. For example, histamine released during lung inflammation may result in gland fluid secretion, and release of ATP from nearby cells may stimulate acinar cell fluid secretion in vivo.
Agonist-induced cell volume changes are due to changes in cell solute content
That the observed changes in cell volume reflect changes in the solute content of the cell was confirmed by quantitative measurements of [Cl−]i using SPQ during simultaneous measurements of cell volume. Changes in [Cl−]i were observed that quantitatively paralleled changes in cell volume. Stimulation with 100 μm CCh caused a rapid increase in SPQ fluorescence that was calibrated to indicate a peak reduction of [Cl−]i from a resting value of ∼65 mm to ∼28 mm. With ∼20% of the cell comprised of osmotically inactive solids (Supplemental Fig. 2) and assuming that observed agonist-induced cell shrinkage of 20% reflects loss of osmotically active volume (new total volume was 0.8; new osmotically active volume was 0.6; new osmotic volume/original osmotic volume was 0.8/0.6 = 0.75), then the observed post-CCh-stimulation [Cl−]i of 28.0 mm is equivalent to a cell Cl− content of 21.0 meq Cl− l−1 (0.75 × 28.0 mm). Thus, agonist stimulation caused the cells to lose 43.5 meq Cl− l−1 (64.5 – 21.0), or 67% of their Cl− content. These results are very similar to previous measurements of Cl− secretion in rat parotid gland acinar cells, where CCh induced a loss of 62% of cellular Cl− content associated with a ∼17% cell shrinkage (Foskett, 1990a).
To account for the observed 20% cell shrinkage by isosmotic loss of cell solute content requires ∼75 meq l−1 solute in a solution of ∼300 mosmol l−1 (volume loss/total osmotic volume × the extracellular osmolarity = 0.2/0.8 × 300 mosmol l−1= 75 meq l−1). The measured loss of Cl− content (∼44 meq l−1) therefore accounts for approximately half of the total solute loss associated with the observed cell shrinkage. The other half, the cationic component, is most likely accounted for by loss of cell K+, the predominant cation in the cell and the only one that could account for the necessary ionic equivalents. Abolition of the driving force for KCl efflux, by raising [K+] in the bathing medium, completely abrogated Ca2+-induced cell shrinkage and loss of Cl−. Accordingly, agonist-induced elevation of [Ca2+]i in serous acinar cells leads to cell shrinkage as a result of a reduction of K+ and Cl− content and osmotically obliged cell water by stimulation of KCl efflux. Thus, cell shrinkage is caused by and is associated with activation of Cl− and/or K+ permeabilities.
Relationship of cell volume to the secretory state of the cell
The observation that agonist-induced [Ca2+]i signals cause profound changes in acinar cell volume raises several issues, the most important of which is what they mean. We propose that cell volume can provide information regarding the secretory state of the acinar cell, with a stimulated shrunken cell in an actively secreting state and a swelling or resting cell reflecting a less active or inactive state. In a model of epithelial cell fluid secretion driven by secondarily active Cl− secretion, a regulated Cl− conductance is present in the apical membrane, and a (regulated) K+ conductance is present in the basolateral membrane. Thus, we suggest that the observed Ca2+-activated loss of KCl is mediated by an apical membrane Cl− conductance and basolateral membrane K+ conductance.
While it is not possible to assign an apical versus basolateral polarity to the Cl− efflux pathway(s) we have observed in isolated serous acinar cells, it is highly likely that most of the Cl− efflux observed here is through the apical membrane. First, all existing models of epithelial exocrine fluid secretion depend upon the apical Cl− conductance being greater than the basal Cl− conductance to create vectoral Cl− movement during secretion (reviewed in Melvin et al. 2005; Ballard & Inglis, 2004). Second, the presence of a CCh-stimulated basolateral membrane-localized Cl− efflux in serous cells is not supported by studies of intact glands. Both WT and cftrtm1Unc−/− mouse glands secrete copious fluid in response to CCh stimulation (Ianowski et al. 2007), and CCh-induced fluid secretion emanates from serous acini in porcine and human glands (Wu et al. 2006). Assuming that fluid secreted by CCh-stimulated murine submucosal glands (Ianowski et al. 2007) emanates from serous acini as suggested here, the presence of a substantial CCh/Ca2+-activated basolateral Cl− conductance would inhibit rather than stimulate fluid secretion, by ‘short-circuiting’ vectoral movement of Cl−. Third, a significant regulated basolateral Cl− conductance has not been described in primary exocrine cells, and the presence of one here would make this cell unique among exocrine fluid-secreting cells. Thus, the simplest and most logical explanation for our data, as well as the explanation that agrees with observations of intact mouse, porcine and human glands (Inglis et al. 1997b, 1998; Trout et al. 1998, 2001; Ballard et al. 1999; Jayaraman et al. 2001; Joo et al. 2001a,b, 2002b, 2006; Song & Verkman, 2001; Thiagarajah et al. 2004; Salinas et al. 2005; Song et al. 2005; Wu et al. 2006; Ianowski et al. 2007), is that the CCh-stimulated Cl− efflux pathway we have observed is an apical membrane-localized pathway that contributes to cholinergic-induced fluid secretion.
To sustain secretion, transport mechanisms in the basolateral membrane facilitate Cl− entry into the cytoplasm from the serosal fluid. The known mechanisms, such as Na+–K+–2Cl− cotransporter and/or parallel operations of Cl−/HCO3− and Na+/H+ exchangers, utilize the energy of the Na+ gradient to mediate Cl− salt uptake. The activities of these Cl− transport mechanisms mediate solute uptake. To the extent that their activities exceed those of the KCl efflux pathways, the cell will accumulate solute and swell. Cell swelling was in fact observed when [Ca2+]i relaxed back towards resting levels. When Ca2+ declined completely back to resting levels, the cells continued to swell (accumulate solute) until resting cell volume (cell solute content) was restored. We interpret these observations to indicate that agonist stimulation is associated with activation of solute influx pathways, as well as KCl efflux pathways. When [Ca2+]i relaxes towards resting levels, the KCl permeability deactivates, enabling the activated solute influx pathways to cause net solute accumulation and consequent cell swelling. With [Ca2+]i remaining high during the plateau phase, both influx and efflux pathways are activated, and a steady-state shrunken volume is achieved. The simultaneous activation of influx and efflux pathways defines the actively secreting state. Thus, a shrunken cell during stimulation is actively secreting, whereas recovery of cell volume to less than maximum shrinkage reflects the cell in a submaximal secretory state.
Molecular identity of the basolateral Cl− influx pathway
The molecular identity of the basolateral solute influx pathway(s) was explored by examining the pharmacological sensitivity of cell swelling and [Cl−]i recovery following Ca2+-induced shrinkage. The strong inhibition by bumetanide of cell swelling and [Cl−]i recovery, together with the lack of effect of DMA, strongly suggests that the major Cl− influx pathway in murine serous acinar cells is the Na+–K+–2Cl− cotransporter. This conclusion is consistent with the observed expression of NKCC1 mRNA in serous acinar cells. It also complements previous observations that a substantial fraction of muscarinic agonist-induced glandular fluid secretion can be blocked by bumetanide (Trout et al. 1998; Joo et al. 2002a; Joo et al. 2002b). While the secretion response to CCh measured in intact murine glands is significantly blocked by bumetanide (Ianowski et al. 2007), it additionally shows a strong dependence on HCO3− (evidenced by inhibition of secretion upon replacement of extracellular HCO3− with Hepes) not observed here. Additionally, the lack of effects of DMA in our experiments is surprising since they were performed in a CO2 buffer and the NHE/AE coupled NaCl uptake pathway is often highly active in exocrine cells upon muscarinic stimulation (Robertson & Foskett, 1994; Robertson et al. 1997). In rat parotid acinar cells, DMA blocked ∼75% of CCh-stimulated Na+ influx compared to ∼24% inhibition by bumetanide (Robertson & Foskett, 1994). The requirement of NKCC1 for Cl− re-accumulation and consequent cell swelling also indicates that the Cl− efflux and influx pathways are distinct and that [Cl−]i is not solely determined by membrane potential in acinar cells.
The role of CFTR
The molecular identities of the pathways that mediate K+ and Cl− effluxes in serous acinar cells are unknown. CFTR mRNA is expressed in these cells, and immunocytochemistry revealed it to be present near the apical membrane. To examine the role of CFTR in Ca2+-evoked fluid secretion, we employed cells from the cftrtm1Unc−/− mouse and we pharmacologically inhibited CFTR in WT mouse cells. The results demonstrated that mouse nasal acinar cells from both WT and cftrtm1Unc−/− mice, as well as WT cells treated with CFTRinh172, all exhibited similar rates of Cl− efflux in response to muscarinic stimulation. Isolated intact submucosal glands from CF individuals that lack functional CFTR can secrete fluid in response to cholinergic agonists (Joo et al. 2002a), and it was recently shown that intact glands in WT and cftrtm1Unc−/− mice trachea secrete fluid in response to CCh at similar rates (Ianowski et al. 2007). It has remained unclear if cholinergic-induced fluid secretion in CF glands emanates from cells that normally express CFTR, or whether a different cell type is involved. The model of serous cell fluid secretion based upon Calu-3 cells (reviewed in Ballard & Inglis, 2004) suggests that CFTR is the primary functional apical membrane Cl− conductance that mediates Cl− secretion (Haws et al. 1994; Shen et al. 1994; Moon et al. 1997; Illek et al. 1999). The results here suggest that murine acinar cells that express CFTR can secrete fluid in a CFTR-independent manner in response to cholinergic stimulation. Whether this is also true in human serous acinar cells remains to be determined, but the approaches described here could be used to address this issue. Future electrophysiology and gene-expression studies may help to identify this alternative Cl− permeability in murine serous acinar cells.
Surprisingly, stimulation with a cocktail of reagents designed to raise [cAMP]i (including the physiological agonists VIP and adenosine) failed to show functional evidence of CFTR activity. One caveat to using cell volume to track solute and fluid flux is that cell volume changes depend upon a change in the steady-state balance of ion influx or efflux. If both pathways are up- or down-regulated simultaneously, a cell could have no change of total solute content yet vectorally transport solute. However, even in conditions designed to favour solute efflux by preventing compensatory solute influx (presence of bumetanide, DMA and acetazolamide) cAMP-induced cell shrinkage was not observed. Furthermore, cAMP stimulation did not potentiate the rate of KCl efflux stimulated by subsaturating [CCh]. These results suggest that the magnitude of the plasma membrane CFTR Cl− conductance is much lower than the Cl− conductance activated by elevated [Ca2+]i, and may be below the resolution of our optical techniques. Interestingly, serous acinar cells isolated from the cftrtm1Unc−/− mice had a similar resting [Cl−]i to that measured in the cells from WT mice. It might have been anticipated that a diminished plasma membrane Cl− conductance in cells lacking CFTR might lead to a higher resting [Cl−]i if the Cl− uptake mechanisms are unaltered in the cftr−/− cells (supported by our observations that bumetanide or DMA did not alter cell volume in unstimulated cells from either strain). The similar resting [Cl−]i in WT and CFTR-knockout cells suggests a similar resting Cl− permeability in these cells. Again, this result also suggests that the magnitude of the CFTR Cl− conductance is small in murine serous acinar cells. This hypothesis is supported by recent data from intact murine glands (Ianowski et al. 2007), in which the rate of VIP-stimulated fluid secretion was found to be only ∼2–3% of the maximal rate elicited by CCh. In contrast, the maximal rate of cAMP-evoked fluid secretion is much greater in porcine and human glands (∼30–40% of that evoked by CCh) (Trout et al. 2001; Joo et al. 2001b, 2002a,b). It is important to note that whereas CFTR mRNA and apically localized protein were detected here in WT serous acinar cells, quantitative inferences about absolute expression levels cannot be drawn from these studies. Future electrophysiological studies of isolated serous acinar cells will enable the magnitude of the CFTR and other Cl− conductances to be quantitatively compared, while more quantitative genetic approaches (i.e. real-time rtPCR) will enable measurement of CFTR mRNA expression levels. This smaller ratio of VIP- to CCh-induced secretion in murine submucosal glands (Ianowski et al. 2007) is a major caveat to using the mouse as a model system. Despite the advantage of the mouse as a unique source of relatively pathology-free cftr−/− serous cells, studies of ion transport in murine serous cells may not accurately reflect the behaviour of serous cells in humans or other species.
A major unanswered question regarding submucosal gland physiology is the mechanism of ACh/Ca2+-stimulated fluid secretion. It is not clear why this secretion pathway is still significantly intact in CF glands lacking an apical CFTR conductance. Furthermore, it has been unclear whether fluid secretion in response to ACh derives from serous cells or from a different glandular cell type. It was recently demonstrated that CCh-induced fluid secretion by intact porcine and human submucosal glands emanates from serous acini and not from gland ducts (Wu et al. 2006), in agreement with our results, but these studies were not carried out in glands lacking CFTR. While the presence of non-CFTR-mediated, niflumic acid-sensitive, CCh-evoked fluid secretion has been observed in intact mouse tracheal glands (Ianowski et al. 2007), it has not been directly demonstrated that serous acinar cells specifically can secrete fluid in the absence of CFTR. The data described here directly establish that murine serous acinar cells are capable of secreting salt and water in response to cholinergic stimulation, and that, at least in the mouse, this secretion is not dependent upon CFTR. Several non-exclusive models have been proposed to account for cholinergic activation of serous cell fluid secretion (reviewed in Ballard & Inglis, 2004). First, a Ca2+-dependent pathway might activate CFTR. However, such a mechanism has not yet been described in other experimental systems. An alternative hypothesis is that basolaterally localized Ca2+-sensitive K+ channels hyperpolarize the apical membrane upon increase of [Ca2+]i, enhancing the driving force for Cl− efflux through a constitutively activated CFTR. However, neither of these hypotheses accounts for the CFTR-independent secretion observed in cholinergically stimulated intact glands. A third possibility is that a non-CFTR apical membrane Cl− conductance(s), in either the same or different cells, is responsible for ACh-induced fluid secretion. The data obtained in the present study support this third model in murine submucosal glands, and suggest that the mechanism of cholinergic-induced murine serous cell fluid secretion involves CFTR-expressing cells but is not solely dependent on CFTR.
Acknowledgments
We thank Dr V. Rhaghuram for C-terminal CFTR peptide, Dr J. Eberwine, Ms L. Barrett, and Ms J. Jochems for assistance with the cell harvesting/aRNA amplification procedures, Dr A. S. Verkman for CFTRinh172, Drs D. Mak and C. White for helpful technical advice and discussion, Dr A. Stout for advice on confocal 3D reconstruction, Ms L. Mei for help with primer design and molecular biology protocols, and Ms R. Munden for excellent animal assistance. These studies were supported in part by an NSF graduate research fellowship to R.J.L., P01 HL-051746 to J.M.W., and a grant by the Cystic Fibrosis Foundation to J.K.F.
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.131995/DC1 and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.131995
References
- Ballard ST, Fountain JD, Inglis SK, Corboz MR, Taylor AE. Chloride secretion across distal airway epithelium: relationship to submucosal gland distribution. Am J Physiol Lung Cell Mol Physiol. 1995;268:L526–L531. doi: 10.1152/ajplung.1995.268.3.L526. [DOI] [PubMed] [Google Scholar]
- Ballard ST, Inglis SK. Liquid secretion properties of airway submucosal glands. J Physiol. 2004;556:1–10. doi: 10.1113/jphysiol.2003.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ST, Trout L, Bebok Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol Lung Cell Mol Physiol. 1999;277:L694–L699. doi: 10.1152/ajplung.1999.277.4.L694. [DOI] [PubMed] [Google Scholar]
- Berger JT, Voynow JA, Peters KW, Rose MC. Respiratory carcinoma cell lines. MUC genes and glycoconjugates. Am J Respir Cell Mol Biol. 1999;20:500–510. doi: 10.1165/ajrcmb.20.3.3383. [DOI] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Brown DA, Bruce JI, Straub SV, Yule DI. cAMP potentiates ATP-evoked calcium signaling in human parotid acinar cells. J Biol Chem. 2004;279:39485–39494. doi: 10.1074/jbc.M406201200. [DOI] [PubMed] [Google Scholar]
- Chao AC, Dix JA, Sellers MC, Verkman AS. Fluorescence measurement of chloride transport in monolayer cultured cells. Mechanisms of chloride transport in fibroblasts. Biophys J. 1989;56:1071–1081. doi: 10.1016/S0006-3495(89)82755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AC, Widdicombe JH, Verkman AS. Chloride conductive and cotransport mechanisms in cultures of canine tracheal epithelial cells measured by an entrapped fluorescent indicator. J Membr Biol. 1990;113:193–202. doi: 10.1007/BF01870071. [DOI] [PubMed] [Google Scholar]
- Chopra DP, Reddy L, Gupta SK, Wan L, Mathieu PA, Shoemaker RL, Rhim JS. Differentiation of immortalized epithelial cells derived from cystic fibrosis airway submucosal glands. In Vitro Cell Dev Biol Anim. 1994;30A:539–546. [PubMed] [Google Scholar]
- Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC. Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol Med. 2000;6:969–982. [PMC free article] [PubMed] [Google Scholar]
- Dubin RF, Robinson SK, Widdicombe JH. Secretion of lactoferrin and lysozyme by cultures of human airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2004;286:L750–L755. doi: 10.1152/ajplung.00326.2003. [DOI] [PubMed] [Google Scholar]
- Duszyk M. CFTR and lysozyme secretion in human airway epithelial cells. Pflugers Arch. 2001;443(Suppl 1):S45–S49. doi: 10.1007/s004240100643. [DOI] [PubMed] [Google Scholar]
- Eberwine J. Single-cell molecular biology. Nat Neurosci. 2001;4(Suppl):1155–1156. doi: 10.1038/nn1101-1155. [DOI] [PubMed] [Google Scholar]
- Emery N, Place GA, Dodd S, Lhermitte M, David G, Lamblin G, Perini JM, Page AM, Hall RL, Roussel P. Mucous and serous secretions of human bronchial epithelial cells in secondary culture. Am J Respir Cell Mol Biol. 1995;12:130–141. doi: 10.1165/ajrcmb.12.2.7865212. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Foskett JK. Simultaneous Nomarski and fluorescence imaging during video microscopy of cells. Am J Physiol Cell Physiol. 1988;255:C566–C571. doi: 10.1152/ajpcell.1988.255.4.C566. [DOI] [PubMed] [Google Scholar]
- Foskett JK. [Ca2+]i modulation of Cl− content controls cell volume in single salivary acinar cells during fluid secretion. Am J Physiol Cell Physiol. 1990a;259:C998–C1004. doi: 10.1152/ajpcell.1990.259.6.C998. [DOI] [PubMed] [Google Scholar]
- Foskett JK. Optical studies of ion and water transport in single living cells. In: Foskett JK, editor. Noninvasive Techniques in Cell Biology. New York: Wiley-Liss, Inc; 1990b. pp. 237–272. [Google Scholar]
- Foskett JK, Melvin JE. Activation of salivary secretion: coupling of cell volume and [Ca2+]i in single cells. Science. 1989;244:1582–1585. doi: 10.1126/science.2500708. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Peiser C, Dinh QT, Matthias J, Eynott PR, Heppt W, Carlstedt I, Witt C, Fischer A, Chung KF. Distribution of respiratory mucin proteins in human nasal mucosa. Laryngoscope. 2003;113:520–524. doi: 10.1097/00005537-200303000-00023. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Haws C, Finkbeiner WE, Widdicombe JH, Wine JJ. CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl− conductance. Am J Physiol Lung Cell Mol Physiol. 1994;266:L502–L512. doi: 10.1152/ajplung.1994.266.5.L502. [DOI] [PubMed] [Google Scholar]
- Hebert SC, Mount DB, Gamba G. Molecular physiology of cation-coupled Cl− cotransport: the SLC12 family. Pflugers Arch. 2004;447:580–593. doi: 10.1007/s00424-003-1066-3. [DOI] [PubMed] [Google Scholar]
- Hejal R, Strohl KP, Erokwu B, Cherniack NS, Haxhiu MA. Pathways and mechanisms involved in neural control of laryngeal submucosal gland secretion. J Appl Physiol. 1993;75:2347–2352. doi: 10.1152/jappl.1993.75.6.2347. [DOI] [PubMed] [Google Scholar]
- Ianowski JP, Choi JY, Wine JJ, Hanrahan JW. Mucus secretion by single tracheal submucosal glands from normal and CFTR knock-out mice. J Physiol. 2007;580:301–314. doi: 10.1113/jphysiol.2006.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illek B, Tam AW, Fischer H, Machen TE. Anion selectivity of apical membrane conductance of Calu 3 human airway epithelium. Pflugers Arch. 1999;437:812–822. doi: 10.1007/s004240050850. [DOI] [PubMed] [Google Scholar]
- Inglis SK, Corboz MR, Ballard ST. Effect of anion secretion inhibitors on mucin content of airway submucosal gland ducts. Am J Physiol Lung Cell Mol Physiol. 1998;274:L762–L766. doi: 10.1152/ajplung.1998.274.5.L762. [DOI] [PubMed] [Google Scholar]
- Inglis SK, Corboz MR, Taylor AE, Ballard ST. Effect of anion transport inhibition on mucus secretion by airway submucosal glands. Am J Physiol Lung Cell Mol Physiol. 1997a;272:L372–L377. doi: 10.1152/ajplung.1997.272.2.L372. [DOI] [PubMed] [Google Scholar]
- Inglis SK, Corboz MR, Taylor AE, Ballard ST. In situ visualization of bronchial submucosal glands and their secretory response to acetylcholine. Am J Physiol Lung Cell Mol Physiol. 1997b;272:L203–L210. doi: 10.1152/ajplung.1997.272.2.L203. [DOI] [PubMed] [Google Scholar]
- Inglis SK, Wilson SM. Cystic fibrosis and airway submucosal glands. Pediatr Pulmonol. 2005;40:279–284. doi: 10.1002/ppul.20183. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Shimura S, Sato M, Masuda T, Ishide N, Miura M, Sasaki T, Sasaki H, Takishima T. Intracellular calcium concentration of acinar cells in feline tracheal submucosal glands. Am J Physiol Lung Cell Mol Physiol. 1990;259:L345–L350. doi: 10.1152/ajplung.1990.259.6.L345. [DOI] [PubMed] [Google Scholar]
- Jacquot J, Puchelle E, Hinnrasky J, Fuchey C, Bettinger C, Spilmont C, Bonnet N, Dieterle A, Dreyer D, Pavirani A, et al. Localization of the cystic fibrosis transmembrane conductance regulator in airway secretory glands. Eur Respir J. 1993;6:169–176. [PubMed] [Google Scholar]
- Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. Proc Natl Acad Sci U S A. 2001;98:8119–8123. doi: 10.1073/pnas.131087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery PK. Comparative morphology of the airways in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:S6–S13. doi: 10.1164/ajrccm/150.5_Pt_2.S6. [DOI] [PubMed] [Google Scholar]
- Jeffery PK, Brain AP. Surface morphology of human airway mucosa: normal, carcinoma or cystic fibrosis. Scanning Microsc. 1988;2:553–560. [PubMed] [Google Scholar]
- Joo NS, Irokawa T, Robbins RC, Wine JJ. Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem. 2006;281:7392–7398. doi: 10.1074/jbc.M512766200. [DOI] [PubMed] [Google Scholar]
- Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J Biol Chem. 2002a;277:50710–50715. doi: 10.1074/jbc.M208826200. [DOI] [PubMed] [Google Scholar]
- Joo NS, Krouse ME, Wu JV, Saenz Y, Jayaraman S, Verkman AS, Wine JJ. HCO3− transport in relation to mucus secretion from submucosal glands. JOP. 2001a;2:280–284. [PubMed] [Google Scholar]
- Joo NS, Saenz Y, Krouse ME, Wine JJ. Mucus secretion from single submucosal glands of pig. Stimulation by carbachol and vasoactive intestinal peptide. J Biol Chem. 2002b;277:28167–28175. doi: 10.1074/jbc.M202712200. [DOI] [PubMed] [Google Scholar]
- Joo NS, Wu JV, Krouse ME, Saenz Y, Wine JJ. Optical method for quantifying rates of mucus secretion from single submucosal glands. Am J Physiol Lung Cell Mol Physiol. 2001b;281:L458–L468. doi: 10.1152/ajplung.2001.281.2.L458. [DOI] [PubMed] [Google Scholar]
- Klockars M, Reitamo S. Tissue distribution of lysozyme in man. J Histochem Cytochem. 1975;23:932–940. doi: 10.1177/23.12.1104708. [DOI] [PubMed] [Google Scholar]
- Krouse ME, Talbott JF, Lee MM, Joo NS, Wine JJ. Acid and base secretion in the Calu-3 model of human serous cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1274–L1283. doi: 10.1152/ajplung.00036.2004. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MG, Culp DJ. Isolation and culture of submucosal gland cells. Clin Chest Med. 1986;7:239–245. [PubMed] [Google Scholar]
- Martinez-Anton A, Debolos C, Garrido M, Roca-Ferrer J, Barranco C, Alobid I, Xaubet A, Picado C, Mullol J. Mucin genes have different expression patterns in healthy and diseased upper airway mucosa. Clin Exp Allergy. 2006;36:448–457. doi: 10.1111/j.1365-2222.2006.02451.x. [DOI] [PubMed] [Google Scholar]
- McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem. 2004;19:231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- Meyrick B, Reid L. Ultrastructure of cells in the human bronchial submucosal glands. J Anat. 1970;107:281–299. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B, Sturgess JM, Reid L. A reconstruction of the duct system and secretory tubules of the human bronchial submucosal gland. Thorax. 1969;24:729–736. doi: 10.1136/thx.24.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S, Singh M, Krouse ME, Wine JJ. Calcium-stimulated Cl− secretion in Calu-3 human airway cells requires CFTR. Am J Physiol Lung Cell Mol Physiol. 1997;273:L1208–L1219. doi: 10.1152/ajplung.1997.273.6.L1208. [DOI] [PubMed] [Google Scholar]
- Mount DB, Delpire E, Gamba G, Hall AE, Poch E, Hoover RS, Hebert SC. The electroneutral cation-chloride cotransporters. J Exp Biol. 1998;201:2091–2102. doi: 10.1242/jeb.201.14.2091. [DOI] [PubMed] [Google Scholar]
- Nguyen HV, Stuart-Tilley A, Alper SL, Melvin JE. Cl−/HCO3− exchange is acetazolamide sensitive and activated by a muscarinic receptor-induced [Ca2+]i increase in salivary acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G312–G320. doi: 10.1152/ajpgi.00158.2003. [DOI] [PubMed] [Google Scholar]
- Ornoy A, Arnon J, Katznelson D, Granat M, Caspi B, Chemke J. Pathological confirmation of cystic fibrosis in the fetus following prenatal diagnosis. Am J Med Genet. 1987;28:935–947. doi: 10.1002/ajmg.1320280420. [DOI] [PubMed] [Google Scholar]
- Raphael GD, Jeney EV, Baraniuk JN, Kim I, Meredith SD, Kaliner MA. Pathophysiology of rhinitis. Lactoferrin and lysozyme in nasal secretions. J Clin Invest. 1989;84:1528–1535. doi: 10.1172/JCI114329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MA, Foskett JK. Na+ transport pathways in secretory acinar cells: membrane cross talk mediated by [Cl−]i. Am J Physiol Cell Physiol. 1994;267:C146–C156. doi: 10.1152/ajpcell.1994.267.1.C146. [DOI] [PubMed] [Google Scholar]
- Robertson MA, Woodside M, Foskett JK, Orlowski J, Grinstein S. Muscarinic agonists induce phosphorylation-independent activation of the NHE-1 isoform of the Na+/H+ antiporter in salivary acinar cells. J Biol Chem. 1997;272:287–294. [PubMed] [Google Scholar]
- Rogers DF. Motor control of airway goblet cells and glands. Respir Physiol. 2001a;125:129–144. doi: 10.1016/s0034-5687(00)00209-7. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Mucus hypersecretion in chronic obstructive pulmonary disease. Novartis Found Symp. 2001b;234:65–77. doi: 10.1002/0470868678.ch5. discussion 77–83. [DOI] [PubMed] [Google Scholar]
- Rogers DF. The airway goblet cell. Int J Biochem Cell Biol. 2003;35:1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004;4:241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- Salinas D, Haggie PM, Thiagarajah JR, Song Y, Rosbe K, Finkbeiner WE, Nielson DW, Verkman AS. Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J. 2005;19:431–433. doi: 10.1096/fj.04-2879fje. [DOI] [PubMed] [Google Scholar]
- Yu J, Schultz HD, Goodman J, Coleridge JC, Coleridge HM, Davis B. Pulmonary rapidly adapting receptors reflexly increase airway secretion in dogs. J Appl Physiol. 1989;67:682–687. doi: 10.1152/jappl.1989.67.2.682. [DOI] [PubMed] [Google Scholar]
- Sharma P, Dudus L, Nielsen PA, Clausen H, Yankaskas JR, Hollingsworth MA, Engelhardt JF. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am J Respir Cell Mol Biol. 1998;19:30–37. doi: 10.1165/ajrcmb.19.1.3054. [DOI] [PubMed] [Google Scholar]
- Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am J Physiol Lung Cell Mol Physiol. 1994;266:L493–L501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- Sommerhoff CP, Finkbeiner WE. Human tracheobronchial submucosal gland cells in culture. Am J Respir Cell Mol Biol. 1990;2:41–50. doi: 10.1165/ajrcmb/2.1.41. [DOI] [PubMed] [Google Scholar]
- Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol. 2005;290:C741–C749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- Song Y, Verkman AS. Aquaporin-5 dependent fluid secretion in airway submucosal glands. J Biol Chem. 2001;276:41288–41292. doi: 10.1074/jbc.M107257200. [DOI] [PubMed] [Google Scholar]
- Sterling D, Casey JR. Bicarbonate transport proteins. Biochem Cell Biol. 2002;80:483–497. doi: 10.1139/o02-152. [DOI] [PubMed] [Google Scholar]
- Thiagarajah JR, Song Y, Haggie PM, Verkman AS. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J. 2004;18:875–877. doi: 10.1096/fj.03-1248fje. [DOI] [PubMed] [Google Scholar]
- Trout L, Corboz MR, Ballard ST. Mechanism of substance P-induced liquid secretion across bronchial epithelium. Am J Physiol Lung Cell Mol Physiol. 2001;281:L639–L645. doi: 10.1152/ajplung.2001.281.3.L639. [DOI] [PubMed] [Google Scholar]
- Trout L, Gatzy JT, Ballard ST. Acetylcholine-induced liquid secretion by bronchial epithelium: role of Cl− and HCO3− transport. Am J Physiol Lung Cell Mol Physiol. 1998;275:L1095–L1099. doi: 10.1152/ajplung.1998.275.6.L1095. [DOI] [PubMed] [Google Scholar]
- Van Gelder RN, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci U S A. 1990;87:1663–1667. doi: 10.1073/pnas.87.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JH, Chen LL, Sporer H, Choi HK, Pecson IS, Bastacky SJ. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J Anat. 2001;198:207–221. doi: 10.1046/j.1469-7580.2001.19820207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JV, Krouse M, Wine JJ. Acinar origin of Cftr-dependent airway submucosal gland fluid secretion. Am J Physiol Lung Cell Mol Physiol. 2006;292:L304–L311. doi: 10.1152/ajplung.00286.2006. [DOI] [PubMed] [Google Scholar]
- Yamaya M, Finkbeiner WE, Widdicombe JH. Ion transport by cultures of human tracheobronchial submucosal glands. Am J Physiol Lung Cell Mol Physiol. 1991;261:L485–L490. doi: 10.1152/ajplung.1991.261.6.L485. [DOI] [PubMed] [Google Scholar]
- Yang CM, Farley JM, Dwyer TM. Muscarinic stimulation of submucosal glands in swine trachea. J Appl Physiol. 1988;64:200–209. doi: 10.1152/jappl.1988.64.1.200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.131995/DC1 and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.131995