Abstract
We investigated if acute endurance-type exercise interacts with insulin-stimulated activation of atypical protein kinase C (aPKC) and insulin signalling to peptide chain elongation in human skeletal muscle. Four hours after acute one-legged exercise, insulin-induced glucose uptake was ∼80% higher (N = 12, P < 0.05) in previously exercised muscle, measured during a euglycaemic–hyperinsulinaemic clamp (100 μU ml−1). Insulin increased (P < 0.05) both insulin receptor substrate (IRS)-1 and IRS-2 associated phosphatidylinositol (PI)-3 kinase activity and led to increased (P < 0.001) phosphorylation of Akt on Ser473 and Thr308 in skeletal muscle. Interestingly, in response to prior exercise IRS-2-associated PI-3 kinase activity was higher (P < 0.05) both at basal and during insulin stimulation. This coincided with correspondingly altered phosphorylation of the extracellular-regulated protein kinase 1/2 (ERK 1/2), p70S6 kinase (P70S6K), eukaryotic elongation factor 2 (eEF2) kinase and eEF2. aPKC was similarly activated by insulin in rested and exercised muscle, without detectable changes in aPKC Thr410 phosphorylation. However, when adding phosphatidylinositol-3,4,5-triphosphate (PIP3), the signalling product of PI-3 kinase, to basal muscle homogenates, aPKC was more potently activated (P = 0.01) in previously exercised muscle. Collectively, this study shows that endurance-type exercise interacts with insulin signalling to peptide chain elongation. Although protein turnover was not evaluated, this suggests that capacity for protein synthesis after acute endurance-type exercise may be improved. Furthermore, endurance exercise increased the responsiveness of aPKC to PIP3 providing a possible link to improved insulin-stimulated glucose uptake after exercise.
Insulin exerts a variety of actions favouring uptake and deposition of nutrients in many tissues, including skeletal muscle. In skeletal muscle, several of these actions are improved when insulin stimulation is preceded by muscle contraction (Zorzano et al. 1985; Cartee et al. 1989; Wasserman et al. 1991; Wojtaszewski et al. 1997, 2000; Biolo et al. 1999). This phenomenon is often referred to as improved insulin sensitivity, and may depend on intensity and type of exercise, time of evaluation and availability of nutrients. Correspondingly, exercise is generally endorsed as an important element in prevention and treatment of impaired insulin action in skeletal muscle, a hallmark feature of type 2 diabetes.
Immediately after endurance exercise, increased muscle glucose uptake is partly due to a residual effect of exercise whereas at later stages (> 3 h) increased insulin sensitivity to stimulate muscle glucose uptake is the primary mechanism (Richter et al. 1982, 1989; Garetto et al. 1984; Mikines et al. 1988; Cartee et al. 1989). At the cellular level this beneficial effect of prior exercise is manifested in an increased insulin-induced membrane abundance of GLUT4 (Hansen et al. 1998) and an increased activity of glycogen synthase (GS) (Richter et al. 1982; Wojtaszewski et al. 2000), the rate-limiting enzyme in glycogen synthesis. However, insulin signalling measured in whole muscle lysates through insulin receptor tyrosine kinase (IRTK), insulin receptor substrate (IRS)-1-associated phosphatidylinositol-3 (PI-3) kinase, Akt as well as glycogen synthase kinase-3 (GSK-3) does not appear to be enhanced (Treadway et al. 1989; Goodyear et al. 1995; Wojtaszewski et al. 1997, 2000). This suggests that improved overall delivery of insulin to muscle fibres cannot explain why insulin sensitivity of muscle glucose uptake is increased more than 3 h after endurance exercise. Rather, any molecular interaction between exercise and subsequent insulin action on glucose uptake is probably occurring at levels of the insulin signalling cascade not yet investigated and/or in processes directly related to GLUT4 translocation.
Downstream of IRS-1-associated PI-3 kinase, atypical PKC (aPKC) has emerged as an important signalling component to stimulate GLUT4 translocation (Standaert et al. 1997; Kotani et al. 1998; Bandyopadhyay et al. 2000; Imamura et al. 2003; Sajan et al. 2006). In human skeletal muscle, aPKC is activated in response to both insulin and exercise (Nielsen et al. 2003; Beeson et al. 2003; Rose et al. 2004; Richter et al. 2004; Perrini et al. 2004). Furthermore, dysregulation of aPKC is observed in a variety of situations of impaired insulin action on glucose uptake in humans including obesity, impaired glucose tolerance (IGT) and type 2 diabetes mellitus (T2DM) (Kim et al. 1999; Vollenweider et al. 2002; Beeson et al. 2003; Kim et al. 2003). On the other hand, restoration of insulin action through weight loss or rosiglitazone treatment normalizes aPKC function (Kim et al. 2003; Beeson et al. 2003), lending support to the idea of a causal relation between aPKC and insulin action on glucose uptake in human skeletal muscle. Activation of aPKC in response to insulin is thought to involve both allosteric binding of phosphatidylinositol-3,4,5-triphosphate (PIP3) in the membrane vicinity as well as covalent modifications including autophosphorylation on Thr560 and phosphorylation of Thr410 by phosphatidylinositol-dependent kinase-1 (PDK1) (Good et al. 1998; Standaert et al. 1999; Le Standaert et al. 2001). However, the mechanism of activation of aPKC in vivo in response to insulin in human skeletal muscle is not well documented.
One aim of this study was to investigate if in human skeletal muscle, insulin-stimulated aPKC activity, similar to glucose uptake, is enhanced in response to prior endurance exercise. Furthermore, we investigated if responsiveness of aPKC towards PIP3, the signalling product of PI-3 kinase, is altered by prior exercise.
Compared with insulin-stimulated glucose uptake, only few studies have evaluated the effect of endurance exercise on insulin regulation of muscle protein kinetics. Insulin is a potent anabolic stimulus in skeletal muscle, increasing uptake of amino acids and protein synthesis (Biolo et al. 1995). Both endurance and resistance exercise improves insulin-stimulated uptake of selected amino acids in skeletal muscle (Zorzano et al. 1985; Biolo et al. 1999) and furthermore, the anabolic efficacy of amino acids and insulin is increased after resistance exercise (Biolo et al. 1997, 1999). This has not been investigated in response to endurance exercise in humans in vivo, and results from two rodent studies in vitro are conflicting showing either increased or unaltered insulin-stimulated protein synthesis (Davis & Karl, 1986; Balon et al. 1990).
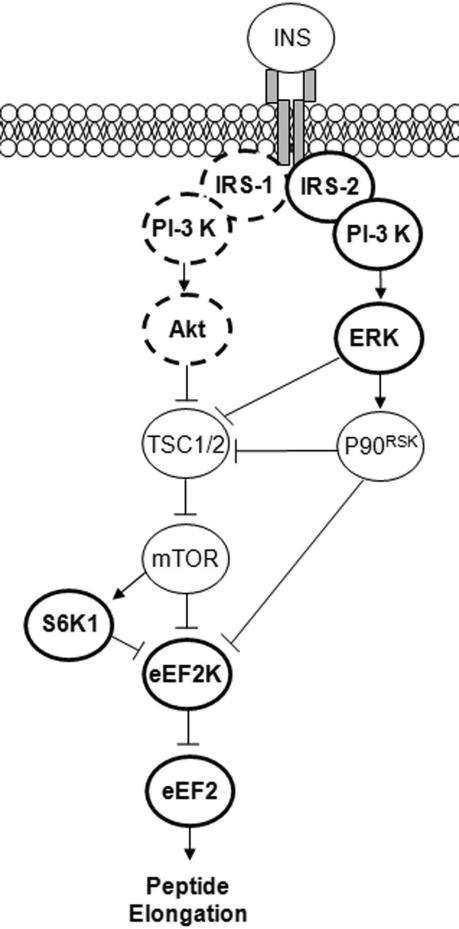
Acute insulin-stimulated protein synthesis (mRNA translation) primarily results from rapid activation of existing components of the translational apparatus. In particular, peptide elongation is stimulated by insulin through a signalling sequence involving PI-3 kinase, Akt, mammalian target of rapamycin (mTOR), eukaryotic elongation factor-2 (eEF2) kinase and ultimately eEF2 as illustrated in Fig. 1. Interestingly, interaction with this pathway has been observed at the level of eEF2 kinase in response to activation of the extracellular-regulated protein kinase 1/2 (ERK 1/2) (Wang et al. 1998, 2001; Wang & Proud, 2002b) and furthermore, ERK activation seems to be required for improved insulin-stimulated muscle protein synthesis 16 h after resistance exercise in rodents (Fluckey et al. 2006). In human skeletal muscle, insulin-stimulated ERK signalling is improved 24 h after endurance exercise, although this has only been investigated in the obese and subjects with T2DM (Cusi et al. 2000). In human skeletal muscle myotubes, ERK is a downstream target of IRS-2-associated PI-3 kinase (Bouzakri et al. 2006). Furthermore, in both rodent (Howlett et al. 2002) and human (Howlett et al. 2006) muscle, IRS-2-associated PI-3 kinase activity is markedly increased in response to insulin stimulation immediately after endurance exercise. Collectively, this suggests a possible signalling interaction between endurance exercise and insulin signalling to mRNA translation.
Figure 1. Diagram showing insulin signalling through IRS-1 and IRS-2 to stimulate peptide chain elongation.
Signalling through IRS-2 is suggested as an alternative pathway to signalling through IRS-1, allowing for exercise to interact with insulin signalling. Bold full circles show signalling improved by prior endurance exercise. Bold dashed circles show signalling not regulated by prior endurance exercise. Regular full circles show signalling components not investigated.
The second aim of the present study was to evaluate if the interaction between exercise and IRS-2-associated PI-3 kinase activity is present in human skeletal muscle 4 h after acute endurance-type exercise and furthermore if this coincides with altered downstream signalling to ERK and eEF2.
Methods
Subjects
Twelve young, healthy male subjects (age, 24 ± 1.3 years; weight, 80 ± 2.7 kg; height, 1.88 ± 0.02 m; BMI, 23 ± 0.5 kg m−2; V˙O2,peak, 4.6 ± 0.1 l min−1) gave their informed consent to participate in this study, which was approved by the local ethics committee (no. 01-180/01) and in accordance with the Declaration of Helsinki. The subjects had no family history of diabetes and fulfilled the criterion of a maximal difference of < 5% between the estimated mass of the quadriceps femoris muscle of the thighs.
Experimental design
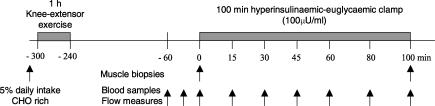
On the morning following 4 days of standardized diet (65% energy from carbohydrates, 20% from fat and 15% from protein) the subjects arrived at the laboratory in the fasted state. After a light, carbohydrate rich breakfast (5% daily energy intake) the subjects performed 60 min of dynamic one-legged knee extensor exercise (1 kick s−1) at a work intensity averaging 80% of the peak work load (PWL) of the knee extensors (see Fig. 2). Included in the exercise bout were 2 × 5 min intervals of work at 100% of PWL in order to ensure activation of the majority of muscle fibres in the thigh region. Care was taken to keep the contra-lateral leg at rest. After exercise, the subjects rested in the supine position and Teflon catheters were inserted below the inguinal ligament in one femoral artery and both femoral veins. A thermistor (Edslab probe 94-030-2.5F, Baxter, Alleroed, Denmark) was inserted through both femoral catheters and advanced 8 cm proximally for blood flow determination. Four hours after exercise the subjects underwent a 100 min euglycaemic–hyperinsulinaemic clamp (1.5 mU (min kg−1)−1) initiated with a bolus injection of insulin (9 mU kg−1) (Actrapid, Novo Nordisk, Bagsvaerd, Denmark) given over 1 min. Blood samples were drawn simultaneously from the femoral catheters before (−60, −30 and 0 min) and during (15, 30, 60, 80 and 100 min) insulin infusion. Each blood sampling was followed by venous blood flow measurements in the thighs, using the constant infusion thermo-dilution technique (Richter et al. 1989) after occlusion of the lower leg using an inflated cuff (> 220 mmHg). This allowed for calculation of thigh glucose uptake using the Fick's principle. At 5 min intervals a 1 ml arterial blood sample was drawn and analysed for glucose content in order to adjust the glucose infusion rate. Needle biopsies were obtained from the vastus lateralis muscle immediately before initiation and at termination of the clamp in both the rested and the previously exercised leg. Biopsies in the same leg were obtained from individual incisions. Incisions were performed under subcutaneous anaesthesia (∼2–3 ml Xylocain (10 mg ml−1 Lidocain), Astra, Sweden) and separated by 5–6 cm within each leg. Biopsies were quickly (< 20 s) frozen in liquid nitrogen and stored at −80°C for later analyses.
Figure 2. Schematic illustration of the experimental day.
Grey areas indicate periods of either exercise (1 h) or insulin infusion (100 min). Arrows indicate time point of either muscle biopsies or blood sampling/venous blood flow measures.
Blood analyses
Glucose and lactate concentrations in whole blood were measured with an ABL 615 (Radiometer Medical A/S, Copenhagen, Denmark) and plasma insulin concentrations were measured using a radioimmunoassay (RIA DSL-1600, Oxon, UK).
Glycogen
For measurements of muscle glycogen content, muscle specimens were freeze dried and dissected free of visible blood, fat and connective tissue. After acid hydrolysis, detection of glycosyl units was performed using fluorometry (Lowry & Passonneau, 1972).
Muscle processing
Lysate preparation
Freeze-dried and dissected muscle tissue was homogenized (1: 80, weight/volume) in ice-cold buffer (50 mm Hepes, 150 mm NaCl, 20 mm Na4P2O7, 20 mmβ-glycerophosphate, 10 mm NaF, 2 mm Na3VO4, 2 mm EDTA, 1% Nonidet P-40, 10% glycerol, 2 mm phenylmethylsulphonyl fluoride (PMSF), 1 mm MgCl2, 1 mm CaCl2, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin and 3 mm benzamidine) and the resultant homogenate was rotated end over end at 4°C for 1 h before being centrifuged for 30 min (17 500 g, 4°C). The supernatant was harvested and total protein content in the lysate was determined using the bicinchoninic acid method (Pierce, Rockford, IL, USA). Muscle lysate protein was used for Western blotting procedures as well as for measurements of PI-3 kinase activity.
Homogenate preparation
A 30 mg sample of wet weight muscle was homogenized (1: 20 weight/volume) in ice-cold buffer (250 mm sucrose, 20 mm Tris-HCl (pH 7.5), 1.2 mm EGTA, 20 mmβ-mercaptoethanol, 1 mm PMSF, 10 μg ml−1 aprotinin, 20 μg ml−1 leupeptin, 3 mm NaF, 3 mm Na4P2O7, and 1 μm LR-microcystin) using a sonicator. The homogenates were centrifuged for 10 min at 700 g to remove nuclei, cellular debris and floating fat. The supernatants were harvested and protein concentrations were determined using the Coomassie PLUS protein assay (Pierce, Rockford, IL, USA). Muscle homogenate protein was used for measurement of aPKC activity.
SDS-PAGE and Western blotting
Muscle lysate proteins were separated using 7.5% or 15% Bis-Tris gels (Invitrogen, Taastrup, Denmark), and transferred (semi-dry) to PVDF membranes (Immobilon Transfer Membrane, Millipore, Glostrup, Denmark). After blocking (10 mm Tris-base (pH 7.4), 0.9% NaCl, 1% Tween 20 (TBST) + 2% skimmed milk), the membranes were incubated with primary antibodies (anti-phospho-PKC-zeta(ζ)/iota(ι) Thr410/403, anti-phospho AKT Ser473, anti-phospho ERK1 (Thr202/Tyr204)/ERK2 (Thr185/Tyr187), anti-phospho P70S6K (Ser389), anti-phospho eEF2K (Ser366) and anti-phospho eEF2 (Thr56); Cell signalling Technology, Beverly, MA, USA; anti-PKC-ζ/ι, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; anti-phospho Akt Thr308, Upstate Biotechnology, Lake Placid, NY, USA) in TBST + 2% skimmed milk followed by incubation in horseradish peroxidase-conjugated secondary antibody (TBST + 2% skimmed milk; Amersham Pharmacia Biotech, Buckinghamshire, UK). After detection and quantification (Kodak Image Station 2000MM, Kodak, Ballerup, Denmark), the protein content was finally expressed in arbitrary units relative to an internal standard. Both antibodies targeting aPKC produced a band at the expected molecular weight (∼75 kDa) and furthermore, both antibodies were positively validated using recombinant full-length human aPKC. As previously reported, phospho-specificity of the phospho-PKC-ζ/ι Thr410/403 antibody has been validated (Richter et al. 2004). In brief, PVDF membranes including human lysate protein were pre-treated with or without λ-phosphatase. This treatment completely abolished the signal otherwise expected when using the anti-phospho-PKC-ζ/ι Thr410/403 antibody, whereas the signal was intact when incubating with the anti-PKC-ζ/ι antibody.
PI-3 kinase activity
Activity of PI-3 kinase was determined in muscle lysates as described previously (Wojtaszewski et al. 1997). In brief, immuno-precipitated PI-3 kinase associated with IRS proteins was assayed for 30 min allowing for 32P incorporation into phosphatidylinositol (PI) (Sigma Aldrich, Munich, Germany). Subsequently, PI was isolated using thin layer chromatography and the amount of radioactivity incorporated was detected by phosphor imaging (Storm 840, Molecular Dynamics, Amersham Pharmacia Biotech, Buckinghamshire, UK) and expressed in arbitrary units relative to a standard.
In the present study, sequential isoform-specific immuno-precipitation of IRS was performed. One milligram of lysate protein was immuno-precipitated overnight using an IRS-2-specific antibody (Upstate Biotechnology). Subsequently, 400 μg of supernatant protein was immuno-precipitated overnight with an IRS-1-specific antibody (raised against bacterially expressed glutathione S-transferase fusion protein using the 236 amino acids of the carboxy-terminal part of rat IRS-1) kindly donated by Dr Ken Siddle (Cambridge University, UK). During optimization of this 2-step procedure it was validated that the antibodies did not cross-react and furthermore that IRS-1-associated PI-3 kinase activity was not influenced by the procedure of previous IRS-2 immuno-precipitation. Assuming that immuno-precipitation and assay conditions are comparable, it can be crudely estimated that bulk PI-3 kinase activity associated with IRS-2 is approximately 1: 8 of IRS-1-associated PI-3 kinase activity in human muscle lysates. This crude estimate is obtained when comparing net activity (radioactivity incorporated) per sample weight used for immuno-precipitation.
aPKC activity
PKC-ζ/ι activity was measured in muscle homogenates as previously described (Beeson et al. 2003). In brief, PKC-ζ/ι in 500 μg of homogenate protein was immuno-precipitated with a rabbit polyclonal antiserum (anti-PKC-ζ/ι, Santa Cruz Biotechnologies). The pellet was incubated for 8 min at 30°C in 100 μl buffer containing 50 mm Tris-HCl (pH 7.5), 100 μm Na3VO4, 100 μm Na4P2O7, 1 mm NaF, 100 μm PMSF, 4 μg phosphatidylserine (Sigma Aldrich, St Louis, MO, USA), 50 μm (λ-32P)ATP (NEN/Life Science Products, Boston, MA, USA), 5 mm MgCl2, and, as substrate, 40 μm serine analogue of the PKC-ɛ pseudosubstrate (BioSource International, Camarillo, CA, USA). After incubation, 32P-labelled substrate was trapped using P-81 filter paper and counted in a liquid scintillation counter. Since recovery of aPKC during the immuno-precipitation procedure is only partial (∼60%) (Standaert et al. 1997; Chen et al. 2002), in theory the limiting amount of antibody should immuno-precipitate the same absolute number of aPKC molecules. Therefore, measured aPKC activity is expected to primarily reflect aPKC enzyme-specific activity.
In all basal samples, aPKC activity was also measured with addition of 10 μm PIP3 (Matreya, Pleasant Gap, PA, USA), which directly and maximally activates aPKC (Standaert et al. 1997, 1999, 2002). The difference in aPKC activity between basal and PIP3 addition was taken to reflect PIP3 responsiveness of aPKC.
Statistics
Values before and after insulin stimulation in both legs were compared using two-way analysis of variance with repeated measures for both time and leg. When a significant main effect was observed, a Student–Newman–Keuls test was used as a post hoc test. A significance level of 0.05 was chosen. When reporting relative changes, these are calculated from each individual and expressed as means ±s.e.m. All absolute data are expressed as means ±s.e.m.
Results
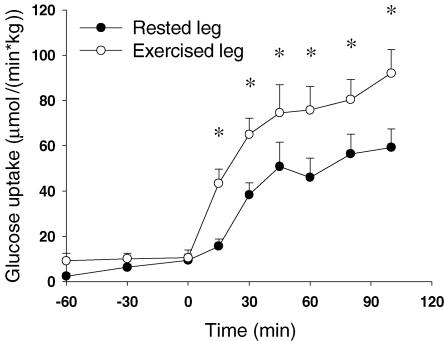
Four hours after termination of exercise (80% PWL; 46 ± 3 W) muscle glycogen content in the exercised muscle was 397 ± 61 μmol g−1 dry wt compared with 618 ± 48 μmol g−1 dry wt in the rested muscle (P < 0.001). Insulin stimulation did not change glycogen content (N.S., data not shown). In response to insulin infusion, arterial plasma insulin levels reached a plateau of 113 ± 8 μU ml−1 after 15 min and the arterial glucose concentration was clamped at 5.0 ± 0.1 mm. Femoral venous blood flow did not differ in the two thighs at rest or during the clamp. However, blood flow increased significantly during the clamp from 403 ± 28 ml min−1 (0 min) to 553 ± 38 ml min−1 (100 min) (P = 0.004). Glucose arterial–venous (A–V) difference likewise increased in response to insulin, and this to a significantly greater extent (P < 0.001) in the previously exercised thigh (rested leg: 0.2 ± 0.1 mm (0 min) to 1.0 ± 0.1 mm (100 min); exercised leg: 0.2 ± 0.1 mm (0 min) to 1.4 ± 0.2 mm (100 min). As shown in Fig. 3, muscle glucose uptake was similar (N.S.) before insulin infusion but increased to a greater extent (P < 0.001) with insulin infusion in the previously exercised muscle (area under curve: rested leg: 3.9 ± 0.6 mmol kg−1versus exercised leg: 6.7 ± 0.6 mmol kg−1. Lactate release was similar in the two thighs (N.S., data not shown).
Figure 3. Glucose uptake in the thigh measured using the Fick's principle.
•, values in the rested leg. ○, values in the previously exercised leg. Insulin infusion is initiated at 0 min *P < 0.05 versus values in the rested leg. Values are means ±s.e.m., N = 12.
PI-3 kinase activity
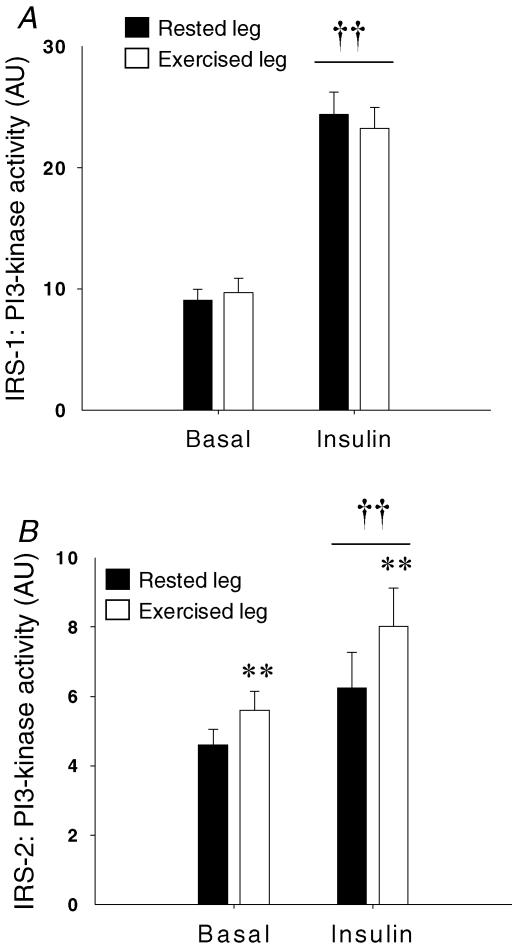
IRS-1-associated PI-3 kinase activity increased in response to insulin stimulation (P < 0.001) (rested muscle 187 ± 26% of basal values; exercised muscle 160 ± 20%) and was not influenced by prior exercise (N.S.) (Fig. 4A). A more modest increase (rested muscle 33 ± 14%; exercised muscle 41 ± 11%) was observed in IRS-2-associated PI-3 kinase activity in response to insulin stimulation (P = 0.008) (Fig. 4B) and the response was similar in the two thighs (N.S.). However, a main effect of exercise was observed (P = 0.005) (Fig. 4B) demonstrating that 4 h after acute exercise, IRS-2-associated PI-3 kinase activity was higher in both basal and insulin-stimulated muscle (basal 26 ± 12%; insulin-stimulated 39 ± 15%) when compared with rested muscle.
Figure 4. PI-3 kinase activity measured in muscle lysates.
A, IRS-1-associated PI-3 kinase activity. B, IRS-2-associated PI-3 kinase activity. Filled bars are values in the rested leg. Open bars are values in the previously exercised leg. **P < 0.005 versus values in the rested leg, ††P < 0.005 versus basal values. Values are means ±s.e.m., N = 12.
Signalling to peptide elongation
Considering the proposed link between IRS-2, ERK and stimulation of peptide elongation, we next measured phosphorylation levels of ERK 1/2, P70S6K, eEF2K and eEF2 in order to provide support for a signalling sequence stimulating the process of peptide elongation.
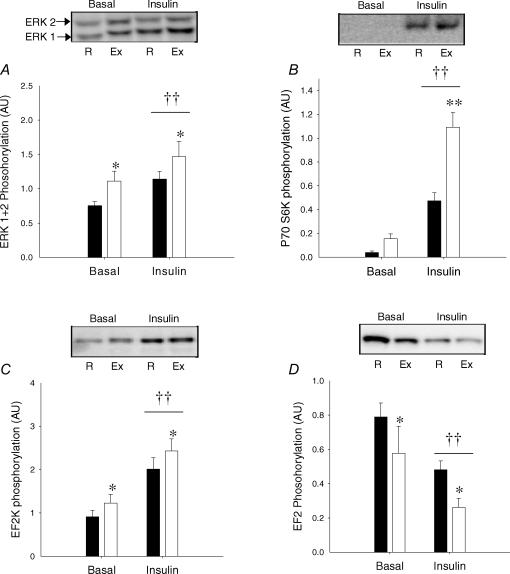
Corresponding to increased IRS-2-associated PI-3 kinase activity, insulin stimulation increased phosphorylation of ERK 1/2 (Fig. 5A, P = 0.005), P70S6K (Fig. 5B, P < 0.005) and eEF2K (Fig. 5C, P < 0.001) and subsequently decreased phosphorylation of eEF2 (Fig. 5D, P = 0.002) in both rested and exercised muscle. Furthermore, prior exercise resulted in further increased phosphorylation of ERK (P = 0.02) and eEF2K (P < 0.001) in both basal and insulin-stimulated muscle corresponding to further decreased phosphorylation of eEF2 (P = 0.05) in exercised muscle. Finally, the phosphorylation level of P70S6K increased significantly more (P < 0.005) in response to insulin in previously exercised muscle when compared with rested muscle.
Figure 5. Signalling to peptide chain elongation measured in muscle lysates using Western blotting.
A, ERK1 (Thr202/Tyr204)/ERK2 (Thr185/Tyr187) phosphorylation. B, p70S6K (Thr389) phosphorylation. C, eEF2K (Ser366) phosphorylation. D, EF2 Thr56 phosphorylation. Filled bars are values in the rested (R) leg. Open bars are values in the previously exercised (Ex) leg. ††P < 0.005 versus basal values. **P < 0.005 versus values in the rested leg. *P < 0.05 versus values in the rested leg. Values are means ±s.e.m., N = 12.
aPKC phosphorylation
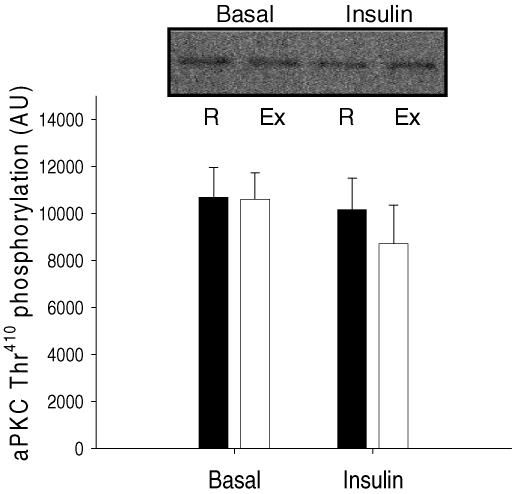
As illustrated in Fig. 6, no effect of prior exercise or insulin stimulation was observed on phosphorylation of aPKC on Thr410, when measured by Western blotting in muscle lysates (N.S.).
Figure 6. aPKC Thr410 phosphorylation measured in muscle lysates using Western blotting.
Filled bars are values in the rested (R) leg. Open bars are values in the previously exercised (Ex) leg. Values are means ±s.e.m., N = 12.
aPKC activity
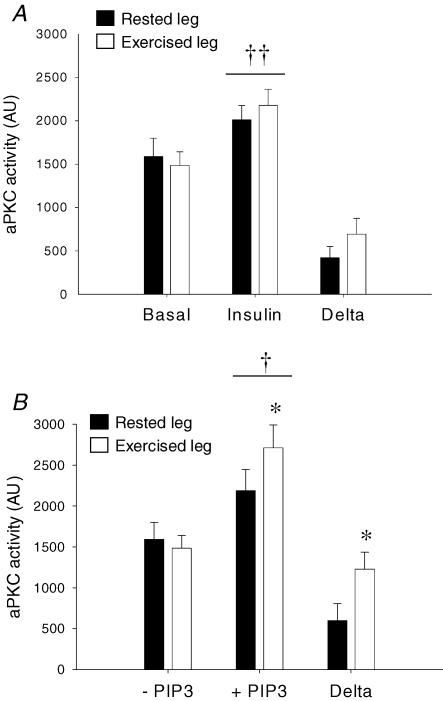
As seen in Fig. 7A, a significant increase in in vitro aPKC activity was observed in response to insulin stimulation in both exercised and rested muscle (P < 0.001). The increase in the exercised leg was 58 ± 15% compared with basal values. When compared with an increase of 35 ± 8% in the rested leg, no significant interaction by prior exercise was observed (P = 0.17). Addition of PIP3 to basal samples (Fig. 7B) increased in vitro aPKC activity in both rested and exercised muscle (P < 0.001). However, the response to PIP3 was significantly greater in previously exercised muscle (P = 0.01), producing an increase of 89 ± 14% of basal values compared with 52 ± 21% in the previously rested muscle.
Figure 7. aPKC activity measured in vitro.
A, aPKC activity in basal and insulin-stimulated samples. B, aPKC activity in basal samples, with or without addition of 10 μm PIP3. Filled bars are values in the rested leg. Open bars are values in the previously exercised leg. ††P < 0.005 versus basal values. †P < 0.05 versus basal values. *P < 0.05 versus response to PIP3 in the rested leg. Values are means ±s.e.m., N = 12.
Akt phosphorylation
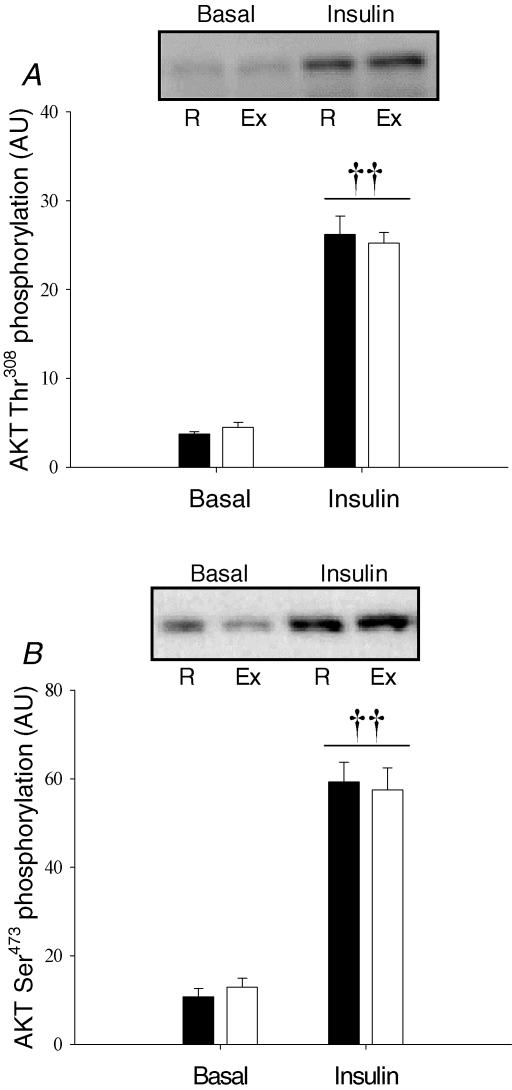
Insulin stimulation led to a marked increase (P < 0.001) in Thr308 and Ser473 phosphorylation of Akt (Fig. 8A and B). This effect of insulin stimulation was not affected by prior exercise (N.S.).
Figure 8. Akt phosphorylation measured in muscle lysates using Western blotting.
A, Akt Thr308 phosphorylation. B, Akt Ser473 phosphorylation. Filled bars are values in the rested (R) leg. Open bars are values in the previously exercised (Ex) leg. ††P < 0.005 versus basal values. Values are means ±s.e.m., N = 12.
Discussion
The main findings of the present study are that improved insulin-stimulated glucose uptake in human skeletal muscle after exercise coincides with improved responsiveness of aPKC towards PIP3, the endogenous signalling product of PI-3 kinase activity. However, insulin-stimulated aPKC activity measured in vitro was not significantly greater after exercise. In response to prior exercise, IRS-2 (but not IRS-1)-associated PI-3 kinase activity was increased in basal and insulin-stimulated muscle. This coincided with corresponding altered phosphorylation levels of ERK 1/2, P70S6K, eEF2K and eEF2, suggesting a signalling mechanism that may allow for an additive effect of prior endurance exercise on subsequent insulin-stimulated protein synthesis.
Exercise is a useful intervention in order to increase insulin-stimulated glucose uptake in human skeletal muscle. However, the underlying mechanisms are still poorly understood (Wojtaszewski et al. 2002). A working hypothesis is that prior exercise interacts with downstream elements of the insulin-signalling pathway allowing for a more potent response upon subsequent insulin stimulation. This is supported by findings that metabolic endpoint events of insulin action in skeletal muscle (i.e. GS activity, GLUT4 translocation and glucose uptake) are increased more by insulin in previously exercised compared with rested muscle (Richter et al. 1982; Hansen et al. 1998; Thorell et al. 1999; Wojtaszewski et al. 2000). However, work from our group has previously shown that in human skeletal muscle insulin signalling at the level of IRTK, IRS-1 tyrosine phosphorylation, IRS-1-associated PI-3 kinase, Akt and GSK-3 is not enhanced by prior exercise (Wojtaszewski et al. 1997, 2000). Data from the present study confirm that prior exercise does not influence insulin-stimulated activation of IRS-1-associated PI-3 kinase (Fig. 4A) and Akt Ser473 phosphorylation (Fig. 8A). Furthermore, this study shows for the first time that Akt Thr308 phosphorylation (Fig. 8B) is likewise not affected by prior exercise.
A primary aim of the present study was to investigate if improved insulin action on glucose uptake coincided with altered regulation of aPKC in human skeletal muscle. In response to insulin stimulation, in vitro aPKC activity increased 1.3- to 1.5-fold. This somewhat lesser response compared with previous findings in healthy humans (Kim et al. 2003; Beeson et al. 2003) should probably be ascribed to the lower dose of insulin used in the present study (∼100 μU ml−1versus 700–900 μU ml−1). The increased aPKC activity was observed in the absence of any detectable changes in aPKC Thr410 phosphorylation. This seems inconsistent with the proposed model of activation based on studies in rat adipocytes (Bandyopadhyay et al. 1999; Kanoh et al. 2001) and the finding of increased aPKC Thr410 phosphorylation (Kanoh et al. 2003) in response to supra-physiological insulin stimulation in rat skeletal muscle. However, it should be emphasized that this has not previously been investigated in response to physiological insulin stimulation in humans. In this context, two previous studies have failed to show increased Thr410 phosphorylation in response to exercise in human muscle, despite increased in vitro activity of aPKC (Rose et al. 2004; Richter et al. 2004). This shows that activation of the enzyme does not always involve changes in Thr410 phosphorylation. Clearly more research is needed in order to fully understand the pattern of activation of aPKC in human skeletal muscle. However, based on the above, the proposed model of activation of aPKC based on studies in cells and rodent muscle should be cautiously adapted to intact human muscle.
In a range of models where insulin action is impaired, in rodent (Kanoh et al. 2001; Hori et al. 2002; Standaert et al. 2004), monkey (Chen et al. 2002) and human skeletal muscle (Vollenweider et al. 2002; Beeson et al. 2003; Kim et al. 2003), both IRS-1-associated PI-3 kinase activity as well as in vitro aPKC activity is decreased. This indicates that production of PIP3 through IRS-1-associated PI-3 kinase action is probably a key upstream signalling event in regulation of aPKC activity and stimulation of glucose uptake. It has furthermore been demonstrated that responsiveness of aPKC to direct allosteric activation by PIP3 is a site of regulation as illustrated by impaired activation of aPKC in muscle samples from obese glucose-intolerant as well as type 2 diabetic subjects in response to PIP3 in vitro (Beeson et al. 2003). An interesting finding of the present study is that responsiveness of aPKC towards PIP3 is significantly greater in basal samples of previously exercised muscle. In contrast to PIP3 responsiveness, insulin-stimulated aPKC activation measured in vitro was not significantly greater in previously exercised muscle (P = 0.17). This apparent dissociation between PIP3 responsiveness and in vitro aPKC activity might seem a bit puzzling if the improved PIP3 responsiveness is of physiological relevance. To our knowledge, no absolute measures of PIP3 concentrations in skeletal muscle are available. However, it cannot be ruled out that sensitivity of the in vitro activity assay does not enable detection of improved PIP3 responsiveness within a lower physiological range of PIP3 concentrations. Nonetheless, the present study demonstrates a novel interaction between exercise and regulation of aPKC, warranting further investigation. This interaction is probably of a robust nature since it can be detected in vitro after the procedure of immuno-precipitation. This study supports previous correlative findings that the ability of aPKC to be activated in response to PIP3 may be relevant to insulin-stimulated glucose uptake (Beeson et al. 2003) and furthermore that measurement of PIP3 responsiveness in vitro may be a useful analytical tool in this regard. However, based on the existing data it can only be speculated if altered PIP3 responsiveness is directly involved in improving insulin action on glucose uptake after exercise.
Activation of PI-3 kinase is involved in many of the insulin actions in skeletal muscle (Yeh et al. 1995; Lund et al. 1995; Shepherd et al. 1997, 1998). PI3-kinase is activated during insulin stimulation through binding of the regulatory src homology 2 domain to a phosphorylated IRS protein. The present study shows that in response to physiological insulin stimulation in vivo both IRS-1- and IRS-2-associated PI-3 kinase activities are increased in human skeletal muscle. Furthermore, it is shown that prior exercise increases IRS-2-associated PI-3 kinase activity in basal and insulin-stimulated muscle. Previously, it has been shown that immediately after exercise, IRS-2-associated PI-3 kinase activity is increased (Howlett et al. 2002, 2006) in insulin-stimulated (but not basal) muscle. Together with the present findings, this suggests that in a prolonged period after exercise, enzymatic capacity for production of PIP3 is increased due to regulation at the level of IRS-2-associated PI-3 kinase.
During insulin stimulation, signalling to protein synthesis is believed to involve PI-3 kinase and Akt to increase both initiation and elongation in the process of mRNA translation (Proud, 2006). However, other stimuli of protein synthesis (i.e. phorbol ester in HEK 293 cells, and phenylephrine and endothelin-1 in cardiac myocytes) have been shown to act through ERK in a PI-3 kinase- and Akt-independent manner (Herbert et al. 2000; Wang & Proud, 2002a,b). Importantly, these stimuli result in dephosphorylation of eEF2, supposedly through phosphorylation at Ser366 and deactivation of the upstream eEF2 kinase (Wang et al. 2001; Wang & Proud, 2002b). This study shows that phosphorylation of eEF2 decreases with physiological insulin stimulation in human muscle and, interestingly, in basal and insulin-stimulated muscle, eEF2 phosphorylation is further reduced by prior exercise. This finding is supported by increased Ser366 phosphorylation (leading to deactivation) of the upstream eEF2K in exercised muscle. Furthermore, this site on eEF2K is a target of S6 kinases (Wang et al. 2001) and correspondingly we observe increased phosphorylation of P70S6K Thr389 (a marker of activity) in exercised muscle during insulin stimulation. Although correlative, this study suggests an interaction between prior exercise and insulin signalling to peptide elongation through IRS-2-associated PI3-K, ERK and P70S6K leading to deactivation of eEF2K thus relieving inhibitory phosphorylation of eEF2 (see Fig. 1). In this context it is interesting that ERK activation seems to be required for improved insulin-stimulated muscle protein synthesis 16 h after resistance exercise in rodents, supporting the proposed link (Fluckey et al. 2006). Furthermore, it is noteworthy that immediately after exercise the interaction at the level of IRS-2 is markedly greater compared with the present study (Wang et al. 2001; Howlett et al. 2006), suggesting that at earlier (< 4 h) time points after exercise perhaps capacity for protein synthesis is even greater. Whether insulin signalling to translation initiation is similarly influenced by prior exercise remains to be investigated. However, this seems questionable since signalling through Akt and glycogen synthase kinase 3 (GSK-3), required for insulin-stimulated initiation (Proud, 2007), is not regulated by prior exercise using the present protocol (this study and Wojtaszewski et al. 2000). In the present study, protein kinetics were not evaluated, and as described, results from previous rodent studies are unequivocal (Davis & Karl, 1986; Balon et al. 1990). Interestingly, in human muscle during 3 h recovery from endurance exercise of comparable duration and intensity to the present study, a positive fractional synthesis rate of protein is observed (Levenhagen et al. 2001; Bolster et al. 2005); however, a positive net protein balance is only observed when exogenous amino acids are supplemented during recovery (Levenhagen et al. 2001). This suggests that in the present investigation protein synthesis most probably occurs, particularly considering the anabolic effect of insulin whereas net protein balance may still be negative. However, future studies will be needed to test if improved capacity for peptide elongation as observed at the signalling level in this study results in improved insulin-stimulated protein synthesis.
Activity of IRS-associated PI-3 kinase is mainly regulated by tyrosine/serine phosphorylation of the IRS protein and in particular through activation of IRTK during insulin stimulation. Since IRTK activity is not altered in response to prior exercise (Wojtaszewski et al. 2000), a possible explanation for increased signalling through IRS-2 after exercise is that exercise regulates activity of other kinases/phosphatases able to interact with IRS proteins. A number of kinases including PKB, c-Jun NH2 kinase, AMPK and aPKC (Aronson et al. 1998; Fujii et al. 2000; Jakobsen et al. 2001; Sakamoto et al. 2002; Moeschel et al. 2004; Weigert et al. 2005) have these characteristics; however, most research so far has evaluated regulation in regard to IRS-1. Interestingly, impaired IRS-2 signalling seems to be part of the metabolic syndrome as demonstrated by the finding of impaired IRS-2-associated PI-3 kinase activity in insulin-stimulated muscle from obese subjects with IGT (Vollenweider et al. 2002) and type 2 diabetic subjects (Kim et al. 1999; Vollenweider et al. 2002). Data from the present study thus suggest acute exercise as a beneficial interaction in prevention or treatment of this defect in human skeletal muscle.
In conclusion, the present study shows several novel molecular sites of interaction between exercise and subsequent insulin signalling in human skeletal muscle. Four hours after acute exercise IRS-2-associated PI-3 kinase activity, ERK phosphorylation and subsequent signalling to activation of eEF2 is increased in both basal and insulin-stimulated muscle, establishing a molecular link to increased capacity for insulin-stimulated protein synthesis after exercise. Furthermore, responsiveness of aPKC towards PIP3 is improved in response to prior exercise. This should be expected to counteract the observed impairment in PIP3 responsiveness in states of insulin resistance and overt T2DM. However, in the present study no effect of prior exercise on in vitro aPKC activity was observed (P = 0.17) thus a link to improved insulin-stimulated glucose uptake remains to be established.
Acknowledgments
We kindly thank Dr Ken Siddle (Cambridge University, UK) for donating the antibody against IRS-1 and Dr Adam J Rose for helpful advice and discussions during this study. The study was supported by grants from the Danish Medical and Natural Science Research Councils, the Novo Nordisk Foundation, the Danish Diabetes Association, the Copenhagen Muscle Research Centre, the Lundbeck Foundation and an Integrated Project Funded by the European Union (LSHM-CT-2004–005272). J.F.P.W. was supported by a Hallas Møller fellowship from the Novo Nordisk Foundation. B.K. acknowledges the financial support of the Danish Ministry of Food, Agriculture and Fisheries, as well as the Danish Ministry of Family and Consumer Affairs. S.J.M. and N.B. were supported by the Novo scholarship programme, granted by the Novo Nordisk Foundation and Novozymes.
References
- Aronson D, Boppart MD, Dufresne SD, Fielding RA, Goodyear LJ. Exercise stimulates c-Jun NH2 kinase activity and c-Jun transcriptional activity in human skeletal muscle. Biochem Biophys Res Commun. 1998;251:106–110. doi: 10.1006/bbrc.1998.9435. [DOI] [PubMed] [Google Scholar]
- Balon TW, Zorzano A, Treadway JL, Goodman MN, Ruderman NB. Effect of insulin on protein synthesis and degradation in skeletal muscle after exercise. Am J Physiol Endocrinol Metab. 1990;258:E92–E97. doi: 10.1152/ajpendo.1990.258.1.E92. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Kanoh Y, Sajan MP, Standaert ML, Farese RV. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-λ on insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 2000;141:4120–4127. doi: 10.1210/endo.141.11.7766. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Standaert ML, Sajan MP, Karnitz LM, Cong L, Quon MJ, Farese RV. Dependence of insulin-stimulated glucose transporter 4 translocation on 3-phosphoinositide-dependent protein kinase-1 and its target threonine-410 in the activation loop of protein kinase C-ζ. Mol Endocrinol. 1999;13:1766–1772. doi: 10.1210/mend.13.10.0364. [DOI] [PubMed] [Google Scholar]
- Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95:811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Pikosky MA, Gaine PC, Martin W, Wolfe RR, Tipton KD, Maclean D, Maresh CM, Rodriguez NR. Dietary protein intake impacts human skeletal muscle protein fractional synthetic rates after endurance exercise. Am J Physiol Endocrinol Metab. 2005;289:E678–E683. doi: 10.1152/ajpendo.00060.2005. [DOI] [PubMed] [Google Scholar]
- Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, Zierath JR. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab. 2006;4:89–96. doi: 10.1016/j.cmet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocriol Metab. 1989;256:E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV, Jr, Farese RV. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and aminoimidazole-4-carboxamide-1-β-d-riboside (AICAR)-stimulated glucose transport. J Biol Chem. 2002;277:23554–23562. doi: 10.1074/jbc.M201152200. [DOI] [PubMed] [Google Scholar]
- Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TA, Karl IE. Response of muscle protein turnover to insulin after acute exercise and training. Biochem J. 1986;240:651–657. doi: 10.1042/bj2400651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckey JD, Knox M, Smith L, Dupont-Versteegden EE, Gaddy D, Tesch PA, Peterson CA. Insulin-facilitated increase of muscle protein synthesis after resistance exercise involves a MAP kinase pathway. Am J Physiol Endocrinol Metab. 2006;290:E1205–E1211. doi: 10.1152/ajpendo.00593.2005. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Garetto LP, Richter EA, Goodman MN, Ruderman NB. Enhanced muscle glucose metabolism after exercise in the rat: the two phases. Am J Physiol Endocrinol Metab. 1984;246:E471–E475. doi: 10.1152/ajpendo.1984.246.6.E471. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Giorgino F, Balon TW, Condorelli G, Smith RJ. Effects of contractile activity on tyrosine phosphoproteins and PI 3-kinase activity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 1995;268:E987–E995. doi: 10.1152/ajpendo.1995.268.5.E987. [DOI] [PubMed] [Google Scholar]
- Hansen PA, Nolte LA, Chen MM, Holloszy JO. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol. 1998;85:1218–1222. doi: 10.1152/jappl.1998.85.4.1218. [DOI] [PubMed] [Google Scholar]
- Herbert TP, Kilhams GR, Batty IH, Proud CG. Distinct signalling pathways mediate insulin and phorbol ester-stimulated eukaryotic initiation factor 4F assembly and protein synthesis in HEK 293 cells. J Biol Chem. 2000;275:11249–11256. doi: 10.1074/jbc.275.15.11249. [DOI] [PubMed] [Google Scholar]
- Hori H, Sasaoka T, Ishihara H, Wada T, Murakami S, Ishiki M, Kobayashi M. Association of SH2-containing inositol phosphatase 2 with the insulin resistance of diabetic db/db mice. Diabetes. 2002;51:2387–2394. doi: 10.2337/diabetes.51.8.2387. [DOI] [PubMed] [Google Scholar]
- Howlett KF, Sakamoto K, Yu H, Goodyear LJ, Hargreaves M. Insulin-stimulated insulin receptor substrate-2-associated phosphatidylinositol 3-kinase activity is enhanced in human skeletal muscle after exercise. Metabolism. 2006;55:1046–1052. doi: 10.1016/j.metabol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Howlett KF, Sakamoto K, Hirshman MF, Aschenbach WG, Dow M, White MF, Goodyear LJ. Insulin signaling after exercise in insulin receptor substrate-2-deficient mice. Diabetes. 2002;51:479–483. doi: 10.2337/diabetes.51.2.479. [DOI] [PubMed] [Google Scholar]
- Imamura T, Huang J, Usui I, Satoh H, Bever J, Olefsky JM. Insulin-induced GLUT4 translocation involves protein kinase C-λ-mediated functional coupling between Rab4 and the motor protein kinesin. Mol Cell Biol. 2003;23:4892–4900. doi: 10.1128/MCB.23.14.4892-4900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001;276:46912–46916. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- Kanoh Y, Bandyopadhyay G, Sajan MP, Standaert ML, Farese RV. Rosiglitazone, insulin treatment, and fasting correct defective activation of protein kinase C-ζ/λ by insulin in vastus lateralis muscles and adipocytes of diabetic rats. Endocrinology. 2001;142:1595–1605. doi: 10.1210/endo.142.4.8066. [DOI] [PubMed] [Google Scholar]
- Kanoh Y, Sajan MP, Bandyopadhyay G, Miura A, Standaert ML, Farese RV. Defective activation of atypical protein kinase C ζ and λ by insulin and phosphatidylinositol-3,4,5-(PO4)3 in skeletal muscle of rats following high-fat feeding and streptozotocin-induced diabetes. Endocrinology. 2003;144:947–954. doi: 10.1210/en.2002-221017. [DOI] [PubMed] [Google Scholar]
- Kim YB, Kotani K, Ciaraldi TP, Henry RR, Kahn BB. Insulin-stimulated protein kinase C λ/ζ activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes. 2003;52:1935–1942. doi: 10.2337/diabetes.52.8.1935. [DOI] [PubMed] [Google Scholar]
- Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest. 1999;104:733–741. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Requirement of atypical protein kinase cλ for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenhagen DK, Gresham JD, Carlson MG, Maron DJ, Borel MJ, Flakoll PJ. Postexercise nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis. Am J Physiol Endocrinol Metab. 2001;280:E982–E993. doi: 10.1152/ajpendo.2001.280.6.E982. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci U S A. 1995;92:5817–5821. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol Endocrinol Metab. 1988;254:E248–E259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- Moeschel K, Beck A, Weigert C, Lammers R, Kalbacher H, Voelter W, Schleicher ED, Haring HU, Lehmann R. Protein kinase C-ζ-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem. 2004;279:25157–25163. doi: 10.1074/jbc.M402477200. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Frosig C, Sajan MP, Miura A, Standaert ML, Graham DA, Wojtaszewski JF, Farese RV, Richter EA. Increased atypical PKC activity in endurance-trained human skeletal muscle. Biochem Biophys Res Commun. 2003;312:1147–1153. doi: 10.1016/j.bbrc.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Perrini S, Henriksson J, Zierath JR, Widegren U. Exercise-induced protein kinase C isoform-specific activation in human skeletal muscle. Diabetes. 2004;53:21–24. doi: 10.2337/diabetes.53.1.21. [DOI] [PubMed] [Google Scholar]
- Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans. 2006;34:213–216. doi: 10.1042/BST20060213. [DOI] [PubMed] [Google Scholar]
- Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982;69:785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol. 1989;66:876–885. doi: 10.1152/jappl.1989.66.2.876. [DOI] [PubMed] [Google Scholar]
- Richter EA, Vistisen B, Maarbjerg SJ, Sajan M, Farese RV, Kiens B. Differential effect of bicycling exercise intensity on activity and phosphorylation of atypical protein kinase C and extracellular signal-regulated protein kinase in skeletal muscle. J Physiol. 2004;560:909–918. doi: 10.1113/jphysiol.2004.071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Michell BJ, Kemp BE, Hargreaves M. Effect of exercise on protein kinase C activity and localization in human skeletal muscle. J Physiol. 2004;561:861–870. doi: 10.1113/jphysiol.2004.075549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajan MP, Rivas J, Li P, Standaert ML, Farese RV. Repletion of atypical protein kinase C following RNA interferance–mediated depletion restores insulin–stimulated glucose transport. J Biol Chem. 2006;281:17466–17473. doi: 10.1074/jbc.M510803200. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ. Contraction regulation of Akt in rat skeletal muscle. J Biol Chem. 2002;277:11910–11917. doi: 10.1074/jbc.M112410200. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Siddle K, Nave BT. Is stimulation of class-1 phosphatidylinositol 3-kinase activity by insulin sufficient to activate pathways involved in glucose metabolism. Biochem Soc Trans. 1997;25:978–981. doi: 10.1042/bst0250978. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Withers DJ, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert ML, Bandyopadhyay G, Kanoh Y, Sajan MP, Farese RV. Insulin and PIP3 activate PKC-ζ by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and autophosphorylation (T560) sites. Biochemistry. 2001;40:249–255. doi: 10.1021/bi0018234. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, Sajan MP, Cenni V, Sirri A, Moscat J, Toker A, Farese RV. Insulin activates protein kinases C-ζ and C-λ by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J Biol Chem. 1999;274:25308–25316. doi: 10.1074/jbc.274.36.25308. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-ζ as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Ortmeyer HK, Sajan MP, Kanoh Y, Bandyopadhyay G, Hansen BC, Farese RV. Skeletal muscle insulin resistance in obesity-associated type 2 diabetes in monkeys is linked to a defect in insulin activation of protein kinase C-ζ/λ/ι. Diabetes. 2002;51:2936–2943. doi: 10.2337/diabetes.51.10.2936. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Sajan MP, Miura A, Kanoh Y, Chen HC, Farese RV, Jr, Farese RV. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem. 2004;279:24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- Thorell A, Hirshman MF, Nygren J, Jorfeldt L, Wojtaszewski JF, Dufresne SD, Horton ES, Ljungqvist O, Goodyear LJ. Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am J Physiol Endocrinol Metab. 1999;277:E733–E741. doi: 10.1152/ajpendo.1999.277.4.E733. [DOI] [PubMed] [Google Scholar]
- Treadway JL, James DE, Burcel E, Ruderman NB. Effect of exercise on insulin receptor binding and kinase activity in skeletal muscle. Am J Physiol Endocrinol Metab. 1989;256:E138–E144. doi: 10.1152/ajpendo.1989.256.1.E138. [DOI] [PubMed] [Google Scholar]
- Vollenweider P, Menard B, Nicod P. Insulin resistance, defective insulin receptor substrate 2-associated phosphatidylinositol-3′ kinase activation, and impaired atypical protein kinase C (ζ/λ) activation in myotubes from obese patients with impaired glucose tolerance. Diabetes. 2002;51:1052–1059. doi: 10.2337/diabetes.51.4.1052. [DOI] [PubMed] [Google Scholar]
- Wang X, Flynn A, Waskiewicz AJ, Webb BL, Vries RG, Baines IA, Cooper JA, Proud CG. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J Biol Chem. 1998;273:9373–9377. doi: 10.1074/jbc.273.16.9373. [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90 (RSK1) and p70, S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Proud CG. Ras/Erk signaling is essential for activation of protein synthesis by Gq protein-coupled receptor agonists in adult cardiomyocytes. Circ Res. 2002a;91:821–829. doi: 10.1161/01.res.0000041029.97988.e9. [DOI] [PubMed] [Google Scholar]
- Wang L, Proud CG. Regulation of the phosphorylation of elongation factor 2 by MEK-dependent signalling in adult rat cardiomyocytes. FEBS Lett. 2002b;531:285–289. doi: 10.1016/s0014-5793(02)03536-6. [DOI] [PubMed] [Google Scholar]
- Wasserman DH, Geer RJ, Rice DE, Bracy D, Flakoll PJ, Brown LL, Hill JO, Abumrad NN. Interaction of exercise and insulin action in humans. Am J Physiol Endocrinol Metab. 1991;260:E37–E45. doi: 10.1152/ajpendo.1991.260.1.E37. [DOI] [PubMed] [Google Scholar]
- Weigert C, Hennige AM, Brischmann T, Beck A, Moeschel K, Schauble M, Brodbeck K, Haring HU, Schleicher ED, Lehmann R. The phosphorylation of SER318 of insulin receptor substrate 1 is not per se inhibitory in skeletal muscle cells, but is necessary to trigger the attenuation of the insulin-stimulated signal. J Biol Chem. 2005;280:37393–37399. doi: 10.1074/jbc.M506134200. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Hansen BF, Gade J, Kiens B, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes. 1997;46:1775–1781. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen JN, Richter EA. Invited review: effect of acute exercise on insulin signaling and action in humans. J Appl Physiol. 2002;93:384–392. doi: 10.1152/japplphysiol.00043.2002. [DOI] [PubMed] [Google Scholar]
- Yeh JI, Gulve EA, Rameh L, Birnbaum MJ. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem. 1995;270:2107–2111. doi: 10.1074/jbc.270.5.2107. [DOI] [PubMed] [Google Scholar]
- Zorzano A, Balon TW, Garetto LP, Goodman MN, Ruderman NB. Muscle α-aminoisobutyric acid transport after exercise: enhanced stimulation by insulin. Am J Physiol Endocrinol Metab. 1985;248:E546–E552. doi: 10.1152/ajpendo.1985.248.5.E546. [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]