Abstract
Retinal abnormality and visual disturbances occur in thiamine-responsive megaloblastic anaemia (TRMA), an autosomal recessive disorder caused by mutations in the human thiamine transporter-1 (hTHTR-1). Human retinal pigment epithelial cells play a pivotal role in supplying thiamine to the highly metabolically active retina but nothing is known about the mechanism, regulation or biological processes involved in thiamine transport in these cells. To address these issues, we used human-derived retinal pigment epithelial ARPE-19 cells to characterize the thiamine uptake process. Thiamine uptake is energy- and temperature-dependent, pH-sensitive, Na+-independent, saturable at both the nanomolar (apparent Km, 30 ± 5 nm) and the micromolar (apparent Km, 1.72 ± 0.3 μm) concentration ranges, specific for thiamine and sensitive to sulfhydryl group inhibition. The diuretic amiloride caused a concentration-dependent inhibition in thiamine uptake, whereas the anti-trypanosomal drug, melarsoprol, failed to affect the uptake process. Both hTHTR-1 and hTHTR-2 are expressed in ARPE-19 cells as well as in native human retinal tissue with expression of the former being significantly higher than that of the latter. Uptake of thiamine was adaptively regulated by extracellular substrate level via transcriptionally mediated mechanisms that involve both hTHTR-1 and hTHTR-2; it was also regulated by an intracellular Ca2+–calmodulin-mediated pathway. Confocal imaging of living ARPE-19 cells expressing TRMA-associated hTHTR-1 mutants (D93H, S143F and G172D) showed various expression phenotypes. These results demonstrate for the first time the existence of a specialized and regulated uptake process for thiamine in a cellular model of human retinal pigment epithelia that involves hTHTR-1 and hTHTR-2. Further, clinically relevant mutations in hTHTR-1 lead to impaired cell surface expression or function of the transporter in retinal epithelial ARPE-19 cells.
The retinal pigment epithelium is part of the blood–retinal barrier and plays an important role in the delivery of nutrients to the highly differentiated and metabolically active retina (Pow, 2001; Philp et al. 2003). Cells of this epithelium form a single layer that is located between the choriocapilliaris and the retina, and thus, impairment in the nutrient transport function of these cells may adversely affect the retina. Like all other cells of the body, cells of the retina cannot synthesize the water-soluble vitamin B1 (thiamine), and must obtain the micronutrient from exogenous sources. The importance of thiamine (in its pyrophosphate form) for cellular functions stems from the role it plays as a cofactor for transketolase, α-ketoglutarate dehydrogenease and pyruvate dehydrogenase, enzymes important in carbohydrate metabolism. Further, because thiamine bridges the glycolytic and the pentose phosphate metabolic pathway that is critical for creating chemical reducing power in cells, the vitamin is also considered as having a role in reducing oxidative stress (Calingasan et al. 1996, 1997; Frederikse et al. 1999). Thus, low intracellular levels of thiamine will lead to impairment in energy metabolism and a propensity for oxidative injury. In addition, a reduction in intracellular thiamine has been shown to lead to apoptotic cell death (Stagg et al. 1999). Clinically, thiamine deficiency (which occurs in disease conditions such as alcoholism, diabetes mellitus and coeliac disease; Leevy & Baker, 1968; Tomasulo et al. 1968; Saito et al. 1987; Victor et al. 1989; Tallaksen et al. 1992) leads to a plethora of abnormalities that include cardiovascular and neurological disorders (Victor et al. 1989; Tanphaichirt, 1994; Berdanier, 1998). Impaired cellular thiamine accumulation that occurs in the autosomal recessive disorder thiamine-responsive megaloblastic anaemia (TRMA), leads to (among other things) abnormalities in the retina and visual disturbances (Raz et al. 2000; Meire et al. 2000; Scharfe et al. 2000). TRMA is caused by mutational defects in the human thiamine transporter-1 (hTHTR-1) (see below). In contrast to the negative effects that thiamine deficiency causes in occular (and other) tissues, thiamine supplementation appears to be of potential benefit in preventing diabetic retinopathy (Hammes et al. 2003).
How cells obtain thiamine from their surrounding environment has been the subject of great interest over the past two decades. Two specific thiamine uptake systems have been identified, namely the human thiamine transporter-1 and -2 (hTHTR-1 and hTHTR-2, the products of the SLC19A2 and SLC19A3 genes, respectively), and are believed to play important roles in thiamine import to and across cells (Diaz et al. 1999; Dutta et al. 1999; Fleming et al. 1999; Labay et al. 1999; Eudy et al. 2000; Rajgopal et al. 2001; Said et al. 2004). The pattern of expression of these two thiamine transporters, their regulation, and the degree to which they contribute to cellular thiamine uptake, show some level of tissue specificity (Diaz et al. 1999; Fleming et al. 1999; Labay et al. 1999; Eudy et al. 2000; Reidling et al. 2002; Said et al. 2004). However, the mechanism by which retinal pigment epithelial cells transport thiamine and whether the process is regulated by intracellular and extracellular factors is not known. Addressing these issues is important from a physiological and nutritional prospective, and informative regarding the understanding of pathological disorders encountered in TRMA. Therefore, we used the human-derived retinal pigment epithelial (ARPE-19) cell line, as well as native human retina in our investigations towards this goal. Our choice of this cell line was based on the proven similarities between this and cell lines of the native human retinal pigment epithelial (hRPE), and its demonstrated suitability for physiological investigations that resulted in similar findings to those occurring in vivo (Dunn et al. 1996; Aukunuru et al. 2001; Busik et al. 2002). Results of our investigations showed for the first time the existence of a specialized uptake process for thiamine that is pH-dependent, Na+-independent and involves the activity of both hTHTR-1 and hTHTR-2. The results also showed the process to be adaptively regulated by the prevailing substrate level (via what appears to be a transcriptionally mediated mechanism that involves both hTHTR-1 and hTHTR-2), and by an intracellular Ca2+–calmodulin (CaM)-mediated pathway. Finally, our investigations also resolved the cellular targeting in ARPE-19 cells of three hTHTR-1 mutations found in TRMA patients.
Methods
Materials
Green fluorescent protein vector (GFP-N3) and human retinal RNA were obtained from Clontech (Palo Alto, CA, USA). ARPE-19 cell line was obtained from ATCC (Manassas, VA, USA). DNA oligonucleotide primers (Table 1) were from Sigma Genosys (Woodlands, TX, USA). [3H]-Thiamine (specific activity, 10 Ci mmol−1; radiochemical purity, > 98%) was obtained from American Radiolabeled Company (ARC) (St Louis, MO, USA). Unlabelled thiamine, and all other biochemicals and molecular biology reagents were purchased from commercial sources and were of analytical grade. Melarsoprol (Mel B) and melarsenoxide (Mel Ox) were kindly provided by Dr Reta Brun of the Swiss Tropical Institute, Basel, Switzerland. Mel B (90 mmol l−1) was dissolved in propylene glycol and Mel Ox (25 mmol l−1) in dimethyl sulfoxide (DMSO) as stock solutions.
Table 1.
Gene-specific primers used for generating mutations in SLC19A2
| Amino acid | Forward and reverse primers 5′–3′ |
|---|---|
| hTHTR-1 | CCGCTCGAGATGGATGTGCCCGGCC |
| CGGGATCCTGAAGTGGTTACTTGAGAACT | |
| D93H | GTTTCCTGTGTTCCTTGCCACACACTACCTCCGTTATAAAC |
| GTTTATAACGGAGGTAGTGTGTGGCAAGGAACACAGGAAAC | |
| S143F | ATTGCCTATTACTTTTATATCTACAGTGTGGTGGACCTGGGC |
| GCCCAGGTCCACCACACTGTAGATATAAAAGTAATAGGCAAT | |
| G172D | GGTGGGCTTTACAGTGGACTCTGTCCTAGGGCAAATCC |
| GGATTTGCCCTAGGACAGAGTCCACTGTAAAGCCCACC |
The table shows the required nucleotide changes in forward and reverse primers (bold face) used for generation of point mutations. Restriction sites for XhoI (italics) and BamHI (underlined) were incorporate into the SLC19A2 primer to aid subcloning into the GFP–N3 vector.
Cell culture, uptake assays and transient transfection
ARPE-19 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 1 μm thiamine. Medium was supplemented with 10% fetal bovine serum (FBS), glutamine (0.29 g l−1), sodium bicarbonate (3.7 g l−1), penicillin (100 000 units l−1) and streptomycin (10 mg l−1). Routine [3H]-thiamine uptake assay was performed on confluent monolayers (3–4 days after confluence) of ARPE-19 cells grown on solid support according to a previously established procedure (Aukunuru et al. 2001; Busik et al. 2002; Said et al. 2005). To determine the functionality of hTHTR-1 clinical mutants, ARPE-19 cells were transiently transfected with hTHTR-1–GFP, hTHTR-1[D93H]–GFP, hTHTR-1[S143F]–GFP, hTHTR-1[G172D]–GFP and GFP vector alone. Uptake assay was performed after 24 h. Protein concentrations were estimated in parallel wells using a protein assay kit (Bio-Rad, CA, USA). For imaging transient transfection, cells were grown on sterile glass-bottomed Petri dishes (MatTek, MA, USA) and transfected at 90% confluency with 2 μg plasmid DNA using Lipofectamine 2000 (Invitrogen, CA, USA). After 24 h, cells were analysed by confocal microscopy.
In the studies to investigate the effect of growing the ARPE-19 cells in the presence of different exogenous thiamine levels on [3H]-thiamine uptake and related parameters, the cells were grown for 5 days in custom-made thiamine-deficient DMEM (Life Technologies Inc., MD, USA) supplemented with 2.5% dialysed FBS (Hyclone). Various levels of thiamine were then added to the culture medium as specified. To determine the degree of thiamine metabolism after uptake by ARPE-19 cells, a thin-layer chromatography (TLC) procedure employing cellulose gel-precoated plates and a solvent system of isopropanol/0.5 m acetate buffer (pH 4.5)/water (65/15/20, v/v/v) was used as previously described (Said et al. 2001).
Semiquantitative and real-time PCR analysis
Total RNA (5 μg) was isolated from ARPE-19 cells and primed with oligo-dT primers to synthesize first strand cDNA (Superscript; first strand synthesis RT-PCR kit, Invitrogen). To amplify the coding region of hTHTR-1, hTHTR-2 and β-actin, we used the following gene specific primers for semiquantitative PCR primers: hTHTR-1: forward, 5′-GCGTCGACGCCAGT-GGCCTTGGATTA-3′; reverse, 5′-CGGGATCCTGAA-GTGGTTACTTGAGAACT-3′; hTHTR-2: forward, 5′-GCGTCGACCAGAGAGGGCTCAACT-3′; reverse, 5′-CGGGATCCGAGTTTTGTTGACATGATGATATTAC-3′; β-actin: forward, 5′-TTGTAACCAACTGGGACGATAT-GG-3′; reverse, 5′-GATCTTGATCTTCATGGTGCTAGG-3′, and real-time PCR primers: hTHTR-1: forward, 5′-AGCCAGACCGTCTCCTTGTA-3′; reverse, 5′-TAGA-GAGGGCCCACCACAC-3′; hTHTR-2: forward, 5′-TTC-CTGGATTTACCCCACTG-3′; reverse, 5′-GTATGTCC-AAACGGGGAAGA-3′; β-actin: forward, 5′-CATCCT-GCGTCTGGACCT-3′, reverse, 5′-TAATGTCACG-CACGATTTCC-3′. Real-time PCR conditions were as previously described (Said et al. 2004; Reidling et al. 2006) and data were normalized to β-actin, and then quantified using a relative relationship method supplied by the iCycles manufacture (Bio-Rad). The sample with the lowest expression level is assigned an arbitrary value of one and the levels of the rest of the samples are expressed relative to the expression level above that sample. Each unit a given gene is expressed above the basal sample indicates a doubling of the expression level (Livak & Schmittgen, 2001; Reidling et al. 2006).
Analysis of hTHTR-1 and hTHTR-2 expression in ARPE-19 cells by Western blotting
Membranous fractions from ARPE-19 cells were isolated by mechanical homogenization of cells in a buffer containing (mm): mannitol 300, EGTA 5 and Tris-HCl 12, and a cocktail of protease inhibitors (Boehringer Manheim, NJ, USA). Western blot analysis was performed as we previously described using specific anti-hTHTR-1 and anti-hTHTR-2 polyclonal antibodies (Said et al. 2001, 2004). Blots were striped and reprobed with anti β-actin antibodies (Santa Cruz, CA, USA) to normalize data for sample loading.
Determination of hTHTR-1 and hTHTR-2 promoter activity: transient transfection and luciferase assay
We have previously described the full-length hTHTR-1 and hTHTR-2 promoters fused to luciferase (Reidling & Said, 2003; Nabokina & Said, 2004). ARPE-19 cells were cotransfected with Lipofectamine 2000 at ∼75% confluency with 2 μg of either full-length hTHTR-1 or hTHTR-2 reporter gene construct along with 100 ng of the pRL-TK (Renilla luciferase-thymidine kinase) (Promega) to normalize for transfection efficiency. Cell lysates were then prepared from cells 48 h after transfection, and luciferase activity was determined as previously described (Reidling & Said, 2003; Subramanian et al. 2003a; Nabokina & Said, 2004). Data are presented as fold increase in expression over pGL3 basic expression set arbitrarily at 1 as previously described (Reidling & Said, 2003; Subramanian et al. 2003a; Nabokina & Said, 2004).
Generation of hTHTR-1–GFP mutants and confocal imaging of live ARPE-19 cells
The QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) was used to introduce insertions or deletions of nucleotides into the open reading frame of hTHTR-1 (the product of SLC19A2 gene). The hTHTR-1–GFP fusion protein was constructed by performing PCR using gene-specific primers (Table 1). PCR products and the GFP–N3 vector were digested with XhoI and BamHI restriction enzymes and the products were isolated from the gel and ligated to generate an in-frame fusion protein (hTHTR-1–GFP), with GFP fused to the C-terminus of hTHTR-1, as previously described (Subramanian et al. 2003b). Paired sense- and anti-sense primer oligonucleotides encompassing the specified mutation sites (Table 1), as well as a plasmid containing hTHTR-1 fused to GFP–N3 were used as a template for PCR mutagenesis. Nucleotide changes in all constructs were confirmed by sequencing (Laragen, Los Angeles, CA, USA).
For confocal imaging of clinically relevant hTHTR-1 mutants in ARPE-19 cells, cell monolayers grown on coverslip-fixed Petri dishes were imaged for construct expression using a Nikon C-1 confocal scanner head attached to a Nikon inverted phase-contrast microscope. Fluorophores were excited using the 488 nm line from an argon ion laser, and emitted fluorescence was monitored with a 530 ± 20 nm band pass (GFP).
Data presentation and statistical analysis
All uptake determinations are the results of at least three separate determinations and are expressed as means ±s.e.m. in pmol or fmol per mg protein per unit time. Comparison was made relative to simultaneously performed controls using ANOVA or Student's t test (unpaired), with a significant P value set at < 0.05. Kinetic parameters of the saturable component of thiamine uptake were determined by subtracting the diffusing component (calculated from the slope of the line between uptake at by a pharmacological concentration of 500 μm and the point of origin, i.e. multiplication of the slope by individual concentration) from total uptake; data were then applied to a computerized model of the Michaelis–Menten equation as previously described (Wilkinson, 1961). Confocal imaging, real-time PCR, semiquantitative RT-PCR and Western blot analyses were performed and promoter activities were determined on at least three separate occasions.
Results
Overall characteristics of the thiamine uptake process by ARPE-19 cells: time course, effect of incubation temperature, pH and Na+
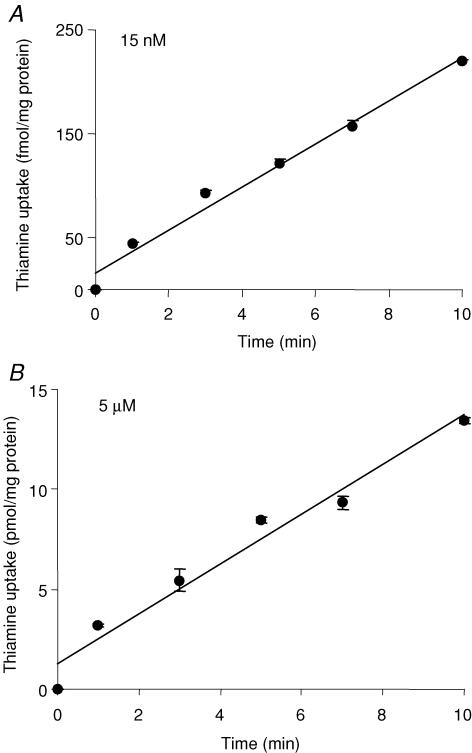
Thiamine uptake by ARPE-19 cells was linear with time during 10 min incubation (rate, 220 fmol (mg protein)−1 and 13.5 pmol (mg protein)−1 for 15 nm and 5 μm, respectively; r= 0.99 for both; Fig. 1). The metabolic form of the radioactivity taken up by the confluent ARPE-19 cells following incubation for 10 min with [3H]-thiamine (40 nm) was found (by cellulose-precoated TLC plates; see Methods) to be (95%) in the form of intact thiamine.
Figure 1. Time-dependent uptake of thiamine by confluent monolayers of ARPE-19 cells.
ARPE-19 cells were incubated at 37°C in Krebs–Ringer solution (pH 7.4) for 0–10 min in the presence of 15 nm (A) and 5 μm (B) thiamine. Values are means ±s.e.m. of n= 3–4 separate uptake determinations. Note, in all figures when not apparent, the error bars are smaller than the symbol.
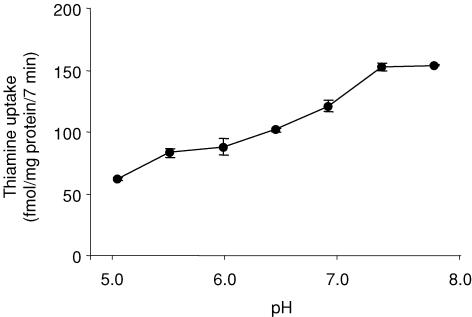
The initial rate of uptake of thiamine (15 nm) varied with pH (Fig. 2). The highest uptake was observed over a pH range of 7.5–8. Isosmotic replacement of Na+ by Li+ or mannitol did not affect the initial rate of thiamine (15 nm) uptake (153 ± 3.0, 149 ± 0.5 and 148 ± 1.0 fmol (mg protein)−1 (7 min)−1 in the presence of Na+, Li+ and mannitol, respectively). Pre-treating ARPE-19 monolayers (for 30 min at 37°C) with 1 mm ouabain (a Na+–K+-ATPase inhibitor) did not significantly affect the initial rate of thiamine (15 nm) uptake (157 ± 6.1 and 145 ± 3.3 fmol (mg protein)−1 (7 min)−1 in the presence and absence of ouabain, respectively). Finally, uptake of thiamine (15 nm) was temperature dependent (153 ± 4.0, 73 ± 5.0 and 28 ± 1.7 fmol (mg protein)−1 (7 min)−1 at 37°C, 22°C and 4°C, respectively).
Figure 2. Effect of incubation buffer pH on thiamine uptake by confluent monolayers of ARPE-19.
cells ARPE-19 cells were incubated at 37°C for 7 min in Krebs–Ringer buffer at pH 5.0–8.0. [3H]-Thiamine (15 nm) was added at the onset of incubation. Data are means ±s.e.m. of n= 3–4 separate uptake determinations.
Involvement of saturable processes in thiamine uptake by ARPE-19 monolayers
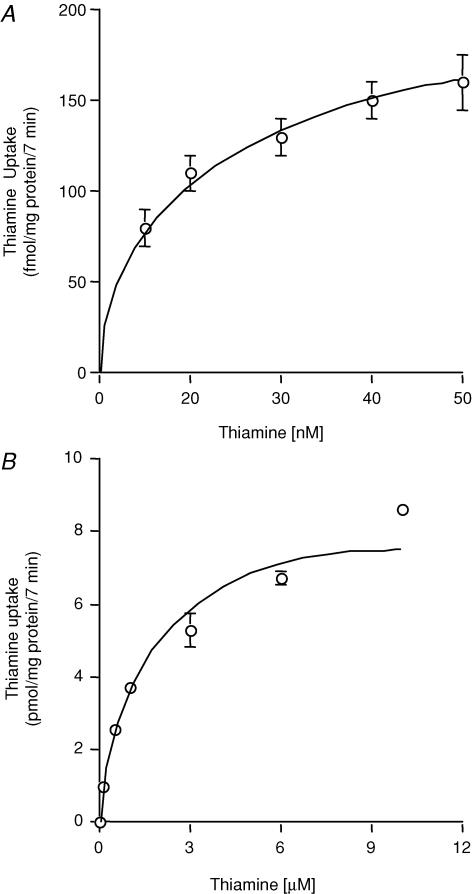
The initial rate of thiamine uptake as a function of concentration was examined over nanomolar (15–50 nm) and micromolar (0.1–10 μm) concentration ranges. Evidence for saturable uptake was observed for both concentration ranges (Fig. 3A and B). Kinetic parameters of both saturable components were determined as stated in the Methods. The apparent Km and Vmax of the saturable process in the nanomolar range were 30 ± 5 nm and 260 ± 21 fmol (mg protein)−1 (7 min)−1, respectively, and in the micromolar range were 1.72 ± 0.3 μm and 9.3 ± 0.8 pmol (mg protein)−1 (7 min)−1, respectively. This suggested that the thiamine uptake process of ARPE-19 cells is carrier-mediated. To confirm this, we also examined the effect of unlabelled thiamine and that of its structural analogues amprolium, benfotiamine and oxythiamine (all at 10 μm) on the initial rate of [3H]-thiamine (2 μm) uptake. Unlabelled thiamine caused significant (P < 0.01) inhibition of [3H]-thiamine uptake (18.3 ± 1 and 6.4 ± 0.03 pmol (mg protein)−1 (7 min)−1 in controls and in the presence of unlabelled thiamine, respectively); similarly, the thiamine analogues caused significant (P < 0.01 for all) inhibition of [3H]-thiamine uptake (19.2 ± 1, 7.5 ± 0.2, 8.7 ± 0.1 and 8.6 ± 0.3 pmol (mg protein)−1 (7 min)−1 in controls and in the presence of amprolium, benfotiamine and oxythiamine, respectively). No effect of biotin (1 mm) and the organic cations tetraethylammonium (TEA) and N-methylnicotinamide (NMN) (both at 100 μm) were observed on the uptake of [3H]-thiamine (15 nm) (155 ± 7.0, 156 ± 2.0, 152 ± 2.3 and 154 ± 2.0 fmol (mg protein)−1 (7 min)−1 in controls and in the presence of biotin, TEA and NMA, respectively).
Figure 3. Uptake of thiamine by confluent monolayers of ARPE-19 cells as a function of nanomolar and micromolar concentrations.
ARPE-19 cells were incubated at 37°C for 7 min in Krebs–Ringer solution at pH 7.4 in the presence of nanomolar (15–50 nm; A) and micromolar (1–10 μm; B) concentrations of thiamine. Uptake plots shown are those of the saturable components calculated as described in the text. Values are means ±s.e.m. of n= 3–4 separate uptake determinations.
We also tested possible trans-stimulation of [3H]-thiamine transport across the ARPE-19 cell membrane by unlabelled thiamine. Here we first preloaded the cells with 15 nm[3H]-thiamine (for 10 min at 37°C) then incubated the cells (for 10 min) in the presence and absence of 100 μm unlabelled thiamine in the incubation buffer. The results showed the cellular content of 3H radioactivity to be significantly (P < 0.01) lower in ARPE-19 cells incubated in the presence of unlabelled thiamine compared to those incubated in its absence (cell content of 3H radioactivity was 78.7 ± 1.6 and 112 ± 2.0 fmol (mg protein)−1, respectively).
Effect of pharmacological agents and sulfhydryl group inhibitor on thiamine uptake by ARPE-19 cells
The diuretic amiloride, and the anti-trypanosomal drug melarsoprol and its metabolite melarsenoxide have been reported to inhibit thiamine transport in certain human cell types as well as in yeast and bacteria (Said et al. 1999, 2001; Schweingruber, 2004; Szyniarowski et al. 2006). For this reason we examined the effect of these compounds on initial rate of thiamine uptake by ARPE-19 cells. Amiloride was found to cause a concentration-dependent inhibition of thiamine uptake (157 ± 7.1, 90.6 ± 3.4, 55.8 ± 1.7 and 38.9 ± 1.2 fmol (mg protein)−1 (7 min)−1 for control and in the presence of 0.1, 0.5 and 1 mm amiloride, respectively). On the other hand, neither melarsoprol nor melarsenoxide (each at 150 μm) showed an effect (119 ± 3.8, 115 ± 6.7 and 120 ± 4 fmol (mg protein)−1 (7 min)−1 for control and in the presence of melarsoprol and melarsenoxide, respectively) on thiamine uptake by ARPE-19 cells.
The effect of pretreating (for 30 min) the ARPE-19 cells with the sulfhydryl group inhibitor p-chloromercuriphenyl sulphonate (p-CMPS, 0.05 mm) on the initial rate of thiamine (15 nm) uptake was also tested. The results showed significant (P < 0.01) inhibition of thiamine uptake by p-CMPS (143 ± 2.3 and 75.3 ± 0.7 fmol (mg protein)−1 (7 min)−1 for control and p-CMPS pretreated cells, respectively). Further treating the p-CMPS-treated ARPE-19 cells with 10 mm of the reducing agent dithiothreitol (DTT) led to a significant (P < 0.05) reversal of the inhibitory effect of pCMPS on thiamine uptake (143 ± 2.3, 75.3 ± 0.7 and 102.2 ± 1.7 fmol (mg protein)−1 (7 min)−1 for control, p-CMPS and p-CMPS plus DTT-treated cells, respectively).
Expression of the hTHTR-1 and hTHTR-2 in ARPE-19 cells and in native human retina
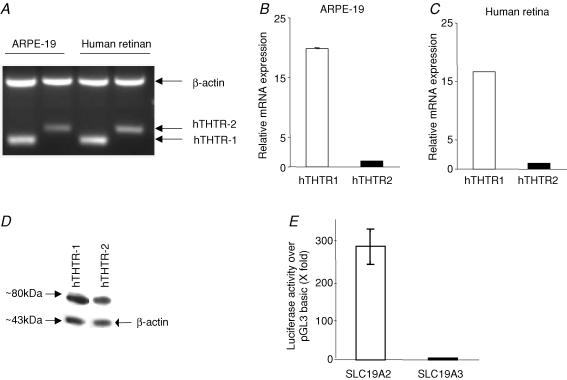
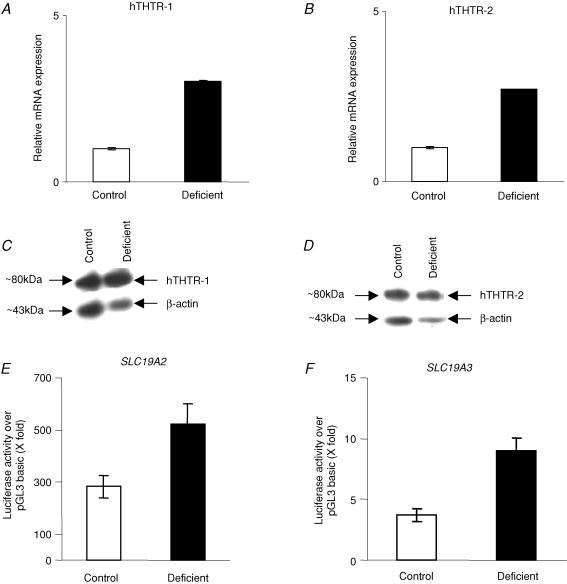
Expression of the hTHTR-1 and hTHTR-2 at the RNA level was examined by means of semiquantitative and real-time PCR techniques. The semiquantitative RT-PCR (described in the Methods) showed clear expression of hTHTR-1 and hTHTR-2 in ARPE-19 cells (Fig. 4A). The two transporters are also expressed in native human retinal tissue (Fig. 4A). We also more accurately quantified the relative level of mRNA expression of hTHTR-1 and hTHTR-2 in ARPE-19 cells (Fig. 4B) and native human retina (Fig. 4C) using real-time PCR (see Methods) and found the level of expression of the hTHTR-1 to be significantly (P < 0.01) higher than the level of expression of hTHTR-2 (Fig. 4B and C). Expression of the hTHTR-1 and hTHTR-2 at the protein level in ARPE-19 cells was also determined by means of Western blot analysis (see Methods) with the results showing expression of both transporters. The level of hTHTR-1 protein, however, was significantly (P < 0.05) higher than that of hTHTR-2 (Fig. 4D). We also tested the relative activity of the SLC19A2 and SLC19A3 promoters in ARPE-19 cells (see Methods). Activity of both promoters was found to be significantly higher than that of pGL3 basic (∼280- and ∼4-fold, for SLC19A2 and SLC19A3 promoters, respectively). Relative activity of the SLC19A2 promoter was found to be significantly (P < 0.01) higher than that of the SLC19A3 promoter (Fig. 4E).
Figure 4. Expression of hTHTR-1 and hTHTR-2 in ARPE-19 cells at the mRNA and protein levels and on activity of SLC19A2 and SLC19A3 promoters.
Semiquantitative RT-PCR products from ARPE-19 cells and native human retina for hTHTR-1 and hTHTR-2 mRNA levels were analysed on an agarose gel as described in the Methods. Data shown are representative of three separate sets of experiments (A). Real-time quantitative PCR of hTHTR-1 and hTHTR-2 mRNA levels in ARPE-19 cells (B) and native human retina (C). Data are from at least three independent sets of experiments and normalized against β-actin and then quantified using a relative relationship method supplied by the iCycles manufacturer (Bio-Rad, CA, USA) and as described in the Methods. Western blot analysis was performed using 150 μg membranous fractions of ARPE-19 cells and specific polyclonal antibodies against hTHTR-1 and hTHTR-2 (D). SLC19A2 and SLC19A3 promoter-luciferase activity was determined in ARPE-19 cells as described in the Methods. Values presented are means ±s.e.m. of three separate determinations performed on different occasions and are expressed as luciferase acivity of each construct over the promoter-less vector pGL3 basic following transient transfection into ARPE-19 cells. To account for transfection efficiency, Firefly luciferase activity was normalized relative to the activity of simultaneously transfected Renilla luciferase (D).
Regulation of the thiamine uptake process of ARPE-19 cells by extracellular thiamine level and by intracellular regulatory pathways
Effect of extracellular thiamine levels
The effect of maintaining the ARPE-19 cells in growth medium (for 5 days) containing specific levels of thiamine (1 nm and 1, 12 and 100 μm) on the initial rate of uptake of [3H]-thiamine (15 nm) was examined. The results showed [3H]-thiamine uptake to be inversely correlated with the level of thiamine in the growth medium (181 ± 6, 125 ± 2, 113 ± 3 and 94 ± 2 fmol (mg protein)−1 (7 min)−1 in the presence of 1 nm and 1, 12 and 100 μm thiamine, respectively). Uptake of the unrelated biotin, however, was similar in cells grown in the presence of 1 nm and 12 μm thiamine (161 ± 8 and 170 ± 1 fmol (mg protein)−1 (7 min)−1, respectively). To determine whether extracellular thiamine concentrations affect the level of expression of the hTHTR-1 and hTHTR-2 at the mRNA and protein levels, we performed real-time PCR and Western blotting, respectively, on samples obtained from cells grown in the presence of 1 nm and 12 μm thiamine. The results showed a significant (P < 0.05) increase in the steady-state mRNA (Fig. 5A and B) and protein levels (Fig. 5C and D) of both the hTHTR-1 and hTHTR-2 in ARPE-19 cells grown in the presence of 1 nm thiamine compared to those grown in the presence of 12 μm thiamine. We also tested the effect of growing the ARPE-19 cells in the presence of 1 nm and 12 μm thiamine on activity of the full-length SLC19A2 and SLC19A3 promoters. The results showed significantly (P < 0.05) higher SLC19A2 (Fig. 5E) and SLC19A3 (Fig. 5F) promoter activities in cells grown in the presence of 1 nm compared to those grown in the presence of 12 μm thiamine.
Figure 5. Effect of thiamine deficiency on hTHTR-1 and hTHTR-2 mRNA and protein levels and on activity of SLC19A2 and SLC19A3 promoters in ARPE-19 cells.
Real-time PCR analysis of hTHTR-1 (A) and hTHTR-2 (B) mRNA levels in ARPE-19 cells grown under control (12 μm) and thiamine deficient (1 nm) conditions. Data are from at least three independent sets of experiments and normalized against β-actin and then quantified as described in the Methods. Western blot analysis of hTHTR-1 (C) (relative density of the bands were determined and are as follows: for hTHTR-1 the intensity was 946385 and 1100047 and for β-actin the intensity was 707596 and 32997 for control and thiamine-deficient cells, respectively) and hTHTR-2 (D) proteins in membranous fractions of ARPE-19 cells grown under control (12 μm) and thiamine-deficient (1 nm) conditions. Data shown are representative of three separate sets of experiments. Activity of full-length SLC19A2 (E) and SLC19A3 (F) promoters in ARPE-19 cells grown in control (12 μm) and thiamine-deficient (1 nm) conditions. Data are means ±s.e.m. of three separate determinations performed on different occasions.
Role of intracellular signalling pathways
We examined the possible involvement of a number of intracellular signalling regulatory mechanisms (Ca2+–CaM, protein kinase C (PKC), protein kinase A (PKA), protein tyrosine kinase (PTK) and nitric oxide (NO)) in regulating thiamine uptake by ARPE-19 cells. The role of the Ca2+–CaM-mediated pathway in the regulation of thiamine uptake by ARPE-19 cells was tested by examining the effect of pretreating confluent ARPE-19 cells (for 1 h) with three different modulators of the Ca2+–CaM- mediated pathways, namely calmidazolium, trifluoperazine (TFP) and 1-[N, O-bis (5-isoquinolinesulphonyl)-N-methyl-l-tyrosyl]-4-phenyl piperazine (KN-62) on the initial rate of [3H]-thiamine (15 nm) uptake. All inhibitors tested were found to cause significant (P < 0.01) inhibition of thiamine uptake (124 ± 3, 64.6 ± 2, 75 ± 3.8 and 48 ± 2 fmol (mg protein)−1 (7 min)−1 for control and in the presence 10 μm calmidazolium, 25 μm TFP and 10 μm KN-62, respectively).
No role for the PKC-mediated pathway in the regulation of thiamine uptake by ARPE-19 cells was evident as uptake of thiamine (15 nm) was not affected by pretreatment with modulators of this pathway (116 ± 3.8, 106 ± 0.34, 112 ± 3.9 and 114 ± 10.2 fmol (mg protein)−1 (7 min)−1 for control and in cells pretreated with (all 10 μm) phorbol 12-myristate 13-acetate, staurosporin and chelerythrine, respectively). Similarly no role for the PKA-mediated pathway was evident as modulators of this pathway also failed to significantly affect thiamine uptake by ARPE-19 cells (123 ± 2.6, 118 ± 3.7 and 120 ± 3.2 fmol (mg protein)−1 (7 min)−1 for control and following pretreatment with 0.5 mm dibutyryl cAMP or 8-bromo-cAMP, respectively). Similarly, no role for the PTK-mediated pathway (as indicated by lack of effect of modulators of this pathway –genistein and tyrophospin A-25) or for the NO-mediated pathway (as indicated by lack of effect of modulators of this pathway –S-nitrose-N-acetylpenicillamine, sodium nitroprusside and 8-bromo cGMP) in the regulation of thiamine uptake by ARPE-19 cells was observed (data not shown).
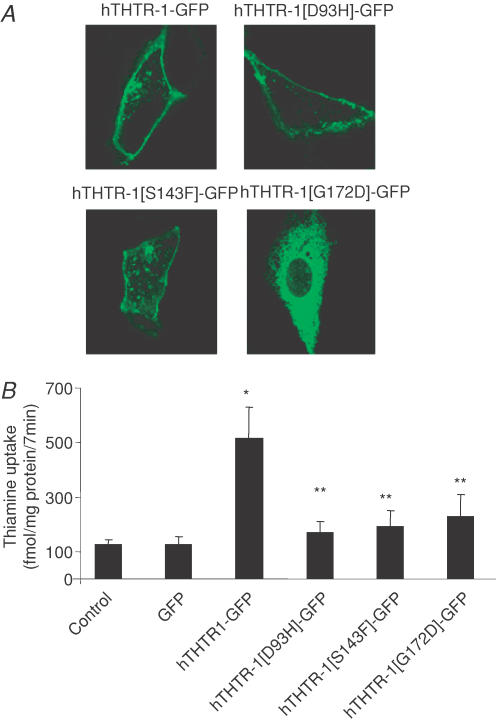
Imaging of hTHTR-1 mutants found in TRMA in ARPE-19 cells
TRMA is caused by mutations in hTHTR-1 and is associated with visual disturbances and retinal abnormality (Fleming et al. 1999; Diaz et al. 1999; Labay et al. 1999; Raz et al. 2000; Meire et al. 2000; Scharfe et al. 2000). In the present study we resolved the cellular localization of three clinically identified TRMA mutants in hTHTR-1 (D93H, S143F and G172D) by generating these mutants by means of site-directed mutagenesis followed by their transfection individually into ARPE-19 cells and by performing live cell imaging of their cellular distribution by means of confocal microscopy. The results showed wild-type hTHTR-1–GFP protein to be targeted predominantly to the plasma membrane; it was also evident within a variety of intracellular vesicular structures (Fig. 6A). The individual mutants, however, showed different patterns of cellular expression (Fig. 6A). The D93H and S143F mutants were also expressed at the cell surface with a lower level of expression efficiency than that of the wild-type (hTHTR-1). By contrast, the G172D mutant was found to be completely retained within the endoplasmic reticulum (Fig. 6A). Further, all mutants showed significantly (P < 0.01) impaired functionality when compared to wild-type hTHTR-1 following transfection into ARPE-19 cells (Fig. 6B).
Figure 6. Distribution of individual clinically relevant hTHTR-1–GFP mutations in ARPE-19 cells.
A, representative of confocal lateral (xy) images showing localization of individual mutant constructs in ARPE-19 cells, 24–48 h after transient transfection. B, uptake of [3H]-thiamine in control, GFP, hTHTR-1–GFP, hTHTR-1[D93H]–GFP, hTHTR-1[S143F]–GFP and hTHTR-1[G172D]–GFP transiently expressing ARPE-19 cells. *Significantly different from control (P < 0.01); **no significant difference between mutants and control. Results represents means ±s.e.m. from three separate determinants performed on three different occasions.
Discussion
In this study we investigated the thiamine uptake process of hRPE using the ARPE-19 cell line as an in vitro model system. This cell line possesses many of the structural and physiological characteristics of hRPE in vivo and has been extensively used in physiological investigations (Dunn et al. 1996; Aukunuru et al. 2001; Busik et al. 2002; Philp et al. 2003). Our aims in this study were: (i) to investigate the physiological characteristics/mechanism of thiamine uptake; (ii) to assess the expression of the hTHTR-1 and hTHTR-2 in ARPE-19 and in native human retina; (iii) to examine possible regulation of the thiamine uptake process by extracellular and intracellular factors; and (iv) to study the effect of specific clinically relevant hTHTR-1 mutations on the expression of the hTHTR-1 transporter in live cells. All these issues are discussed in turn below.
The physiological characteristics/mechanism of the thiamine uptake process
Thiamine uptake by the ARPE-19 cells occurs without metabolic alterations in the transported substrate, and the initial rate of thiamine uptake is both temperature- and energy-dependent in nature. Thiamine uptake was found to be Na+-independent as replacing Na+ in the incubation buffer or pretreating the cells with the Na+–K+-ATPase inhibitor ouabain failed to affect thiamine uptake. Uptake of thiamine, however, was pH-dependent and decreased with a decrease in the incubation buffer pH from 7.4 to 5.0. These findings are similar to those reported previously for thiamine uptake in epithelial cell types where the thiamine uptake process was suggested to involve a thiamine–H+-exchange mechanism (Rindi & Laforenza, 2000; Said et al. 2001).
The thiamine uptake process of the ARPE-19 cells appears to involve two saturable systems, one being functional in the nanomolar range with high affinity but low capacity, and the other being functional in the micromolar range with lower affinity but higher capacity. This may be a reflection of the functionality of the hTHTR-2 and hTHTR-1 (both of which are expressed in ARPE-19 cells), respectively. This is because the apparent Km values of these thiamine transport systems fall within the nanomolar and the micromolar concentration ranges, respectively (Dutta et al. 1999; Said et al. 2004). The ability of unlabelled thiamine and its structural analogues amprolium, benfotiamine and oxythiamine to inhibit the uptake of [3H]-thiamine further confirms the carrier-mediated nature of the thiamine uptake process of the ARPE-19 cells. Because uptake of the cationic thiamine was not affected by the presence of the unrelated organic cations TEA and NMN in the incubation buffer, we conclude that the thiamine uptake process in these cells is specific in nature. Finally the ability of unlabelled thiamine in the incubation buffer to stimulate [3H]-thiamine efflux from preloaded cells further confirmed the carrier-mediated nature of the uptake process.
We also examined the effect of the diuretic amiloride (which inhibits thiamine uptake in other human epithelial cells; Said et al. 1999, 2001; Ashokkumar et al. 2006) and the anti-trypanosomal drug melarsoprol and its metabolite melarsenoxide (which inhibit thiamine uptake by neuroblastoma cells as well as by yeast and bacteria; Schweingruber, 2004; Szyniarowski et al. 2006) on the initial rate of thiamine (15 nm) uptake. The results showed that whereas melarsoprol and melarsenoxide do not affect thiamine uptake, amiloride causes a concentration-dependent inhibition of thiamine uptake. The former findings raise the possibility that the thiamine uptake process exhibits different pharmacological porperties in different cell types; the latter findings, on the other hand, reveal another way in which amiloride appears to interfere with thiamine transport and points to the need for careful investigation of the potential adverse effect of this pharmacological agent on body/cellular thiamine homeostasis in vivo.
The uptake process of thiamine by ARPE-19 cells was found for the first time to be sensitive to the inhibitory effect of the SH group inhibitor p-CMPS. The ability of the reducing agent DTT to significantly reverse the inhibitory effect of p-CMPS on thiamine uptake by ARPE-19 cells suggests possible involvement of such groups in the uptake of thiamine.
Expression of hTHTR-1 and hTHTR-2 in ARPE-19 cells and in native human retina
Previous studies have shown that the hTHTR-1 and hTHTR-2 are differentially expressed in variety of tissues, but their level of expression in the hRPE has not been tested previously. This issue was addressed in this investigation by means of semiquantitative, real-time PCR and Western blotting with the results showing expression of both carriers at both the mRNA and protein levels. Similarly, the two thiamine transporters were found for the first time to be expressed in native human retina. In both of the above cases, expression of the hTHTR-1 was found to be significantly higher than that of the hTHTR-2, a scenario that has been seen previously in other human epithelial cells (Said et al. 2004; Ashokkumar et al. 2006). It is interesting that when activity of the full-length SLC19A2 and SLC19A3 promoters were examined in ARPE-19 cells, activity of the former promoter was also found to be significantly higher than that of the latter promoter, a finding that is in line with the level of mRNA expression of the two carriers.
Regulation of thiamine uptake
Possible regulation of the thiamine uptake process of the ARPE-19 cells by extracellular and an intracellular factors was also investigated in these studies. Performing such investigations is physiologically important as such factors affect nutrient/substrate transport in a cell-specific manner (Traebert et al. 1999; Reidling & Said, 2005). Studies on the effect of extracellular thiamine level showed an inverse correlation between substrate uptake and its prevailing level in the growth medium. Maintaining ARPE-19 cells in a thiamine-deficient growth medium was found to lead to a specific and significant up-regulation of [3H]-thiamine uptake compared to cells grown in the presence of high concentrations of thiamine. This induction of thiamine uptake was found to be associated with an increase in the level of expression of the hTHTR-1 and hTHTR-2 at the mRNA and protein levels as well as in the level of activity of the SLC19A2 and SLC19A3 promoters in cells maintained in the thiamine-deficient growth medium compared to those supplemented with a high dose of thiamine. The latter observation suggests the possible involvement of transcriptional regulatory mechanism(s) in this adaptive regulation of the thiamine uptake process in ARPE-19 cells. Further studies are needed to delineate the cis- and trans-regulatory elements that are responsible for mediating this adaptive response.
Investigating the possible role of specific intracellular regulatory pathways in the regulation of the thiamine uptake process of ARPE-19 cells has revealed that the PKC-, PKA-, PTK- and NO-mediated pathways appear to have no impact on thiamine uptake in ARPE-19 cells. However, a possible role for a Ca2+–CaM-mediated intracellular pathway appeared to exist. The latter conclusion is based on the observations that modulators of this pathway cause significant inhibition of thiamine uptake. It is interesting that thiamine uptake by other epithelial cells also appears to be regulated by a Ca2+–CaM-mediated pathway (Said et al. 1999; Ashokkumar et al. 2006), thus suggesting the possible existence of a common intracellular mechanism for regulating thiamine uptake in different cell types. Further studies are needed to elucidate the molecular mechanism(s) through which the Ca2+–CaM-mediated intracellular pathway exerts its effect on thiamine uptake.
Clinical TRMA mutants
At least 16 distinct mutations in the hTHTR-1 have been identified in TRMA patient, encompassing missense, nonsense and frame-shift mutations (Raz et al. 2000; Lagarde et al. 2004; Ricketts et al. 2006). In this study, we report for the first time the cellular effects of three missense mutations (D93H, S143F and G172D) on membrane expression and functionality of the protein in living ARPE-19 cells. The results showed different cellular targeting phenotypes with mutants D93H and S143F being expressed at the cell surface with a lower level of expression efficiency than wild-type, and G172D mutant exhibiting a completely impaired trafficking phenotype with the protein being retained in the endoplasmic reticulum in a manner analogous to that obtained with clinical point mutations occurring within other nutrient transporters (Martin et al. 1997; Stein et al. 2002). Furthermore, all mutants displayed impaired thiamine uptake.
From the discussion presented above, the question may arise as to how mutations in hTHTR-1 in TRMA patients lead to low thiamine supplies (and retinal abnormalities and visual disturbances) knowing that RPE cells can up-regulate hTHTR-2 when faced with thiamine deficiency. The most likely explanation to this is that the high metabolic rates of the retina and other ocular tissues require them to need large amounts of thiamine that could not be provided even by up-regulated hTHTR-2 in patients exhibiting impaired expression/activity of hTHTR-1 (which as shown here is the dominant thiamine transporter in retina).
In summary, our results characterized, for the first time, the thiamine uptake process in ARPE-19 cells and demonstrated the involvement of a specialized carrier-mediated process. Both the hTHTR-1 and hTHTR-2 were found to be expressed in ARPE-19 cells as well as in native human retina. The results also show that the hRPE thiamine uptake process is adaptively regulated by the extracellular thiamine level via a mechanism that involves both the hTHTR-1 and hTHTR-2; the uptake process also appears to be regulated by an intracellular Ca2+–CaM-mediated pathway. Further, clinically relevant mutations of the hTHTR-1 in TRMA were found to impair cell surface expression or function of the transporter in these cells, thus providing the connecting link between transport physiology and disease condition.
Acknowledgments
We would like to thank Mrs Shuling Wang for her excellent technical assistance in this work. This study was supported by grants from the National Institutes of Health (DK58057 and DK56061 (H.M.S.) and DK 71538 (V.S.S.)) and the Department of Veterans Affairs.
References
- Ashokkumar B, Vaziri ND, Said HM. Thiamin uptake by the human-derived renal epithelial (HEK 293) cells: cellular and molecular mechanisms. Am J Physiol Renal Physiol. 2006;291:F796–F805. doi: 10.1152/ajprenal.00078.2006. [DOI] [PubMed] [Google Scholar]
- Aukunuru JV, Sunkara G, Bandi N, Threson WB, Kompella UB. Expression of multi-drug resistance-associated protein (MRP) in human retinal pigment epithelial cells and its interaction with BAPSG, a noval aldose reductase inhibitor. Pharm Res. 2001;18:565–572. doi: 10.1023/a:1011060705599. [DOI] [PubMed] [Google Scholar]
- Berdanier CD. Advanced Nutrition-Micronutrients. New York: CRC Press; 1998. [Google Scholar]
- Busik JV, Olson LK, Grant MB, Henry DN. Glucose-induced activation of glucose uptake in cells from the inner and outer blood–retinal barrier. Invest Ophthalmol Vis Sci. 2002;43:2356–2363. [PubMed] [Google Scholar]
- Calingasan NY, Gandy SE, Baker H, Sheu KF, Smith JD, Lamb BT, Gearhart JD, Buxbaum JD, Harper C, Selkoe DJ, Price DL, Sisodia SS, Gibson GE. Noval neuritic clusters with accumulations of amyloid precursor protein and amyloid precursor-like protein 2 immunoreactivity in brain regions damaged by thiamine deficiency. Am J Pathol. 1996;149:1063–1071. [PMC free article] [PubMed] [Google Scholar]
- Calingasan NY, Gandy SE, Gibson GE. Thiamine deficiency alters APP but not presenilin-1 immunoreactivity in vulnerable brain regions. Neuroreport. 1997;8:2631–2634. doi: 10.1097/00001756-199707280-00041. [DOI] [PubMed] [Google Scholar]
- Diaz GA, Banikazemi M, Oishi K, Desnick RJ, Gelb BD. Mutations in a new gene encoding a thiamine transporter cause thiamine-responsive megaloblastic anaemia syndrome. Nat Genet. 1999;22:309–312. doi: 10.1038/10385. [DOI] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Dutta B, Huang W, Molero M, Kekuda R, Leibach FH, Devoe LD, Ganapathy V, Prasad PD. Cloning of the human thiamine transporter, a member of the folate transporter family. J Biol Chem. 1999;274:31925–31929. doi: 10.1074/jbc.274.45.31925. [DOI] [PubMed] [Google Scholar]
- Eudy JD, Spiegel O, Barber RC, Wlodarczyk BJ, Talbot J, Finnell RH. Identification and characterization of the human and mouse SLC19A3 gene: a novel member of the reduced folate family of micronutrient transporter genes. Mol Genet Metab. 2000;71:581–590. doi: 10.1006/mgme.2000.3112. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Tartaglini E, Steinkamp M, Schorderit DF, Cohen N, Neufeld EJ. The gene mutated in thiamine responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat Genet. 1999;22:305–308. doi: 10.1038/10379. [DOI] [PubMed] [Google Scholar]
- Frederikse PH, Farnsworth P, Zigler JS., Jr Thiamine deficiency in vivo produces fiber cell degeneration in mouse lenses. Biochem Biopys Res Commun. 1999;258:703–707. doi: 10.1006/bbrc.1999.0560. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- Labay V, Raz T, Baron D, Mandel H, Williams H, Barrett T, Szargel R, McDonald L, Shalata A, Nosaka K, Gregory S, Cohen N. Mutations in SLC19A2 cause thiamine-responsive megaloblastic anaemia associated with diabetes mellitus and deafness. Nat Genet. 1999;22:300–304. doi: 10.1038/10372. [DOI] [PubMed] [Google Scholar]
- Lagarde WH, Underwood LE, Moats-Staats BM, Calikoglu AS. Novel mutation in the SLC19A2 gene in an African-American female with thiamine-responsive megaloblastic anemia syndrome. Am J Med Genet A. 2004;123:299–305. doi: 10.1002/ajmg.a.20506. [DOI] [PubMed] [Google Scholar]
- Leevy CM, Baker H. Vitamins and alcoholism. Am J Clin Nutr. 1968;21:325–328. doi: 10.1093/ajcn/21.11.1325. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Martin MG, Lostao MP, Turk E, Lam J, Wright EM, Kreman M. Compound missense mutations in the sodium/d-glucose cotransporter result in trafficking defects. Gastroenterology. 1997;112:1206–1212. doi: 10.1016/s0016-5085(97)70132-x. [DOI] [PubMed] [Google Scholar]
- Meire FM, Van Genderen MM, Lemmens K, Ens-Dokkum MH. Thiamine-responsive megaloblastic anemia syndrome (TRMA) with cone-rod dystrophy. Ophthalmic Genet. 2000;21:243–250. [PubMed] [Google Scholar]
- Nabokina SM, Said HM. Characterization of the 5′-regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. J Cell Physiol. 2004;287:G822–G829. doi: 10.1152/ajpgi.00234.2004. [DOI] [PubMed] [Google Scholar]
- Philp NJ, Wang D, Yoon H, Hjelmeland LM. Polarized expression of monocarboxlate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci. 2003;44:1716–1721. doi: 10.1167/iovs.02-0287. [DOI] [PubMed] [Google Scholar]
- Pow DV. Amino acids and their transporters in the retina. Neurochem Int. 2001;38:463–484. doi: 10.1016/s0197-0186(00)00114-5. [DOI] [PubMed] [Google Scholar]
- Rajgopal A, Edmondson A, Goldman D, Zhao R. SLC19A3 encodes a second thiamine transporter ThTr2. Biochim Biopys Acta. 2001;1537:175–178. doi: 10.1016/s0925-4439(01)00073-4. [DOI] [PubMed] [Google Scholar]
- Raz T, Labay V, Baron D, Szargel R, Anbinder Y, Barrett T, Rabl W, Viana MB, Mandel H, Baruchel A, Cayuela JM, Cohen N. The spectrum of mutations, including four novel ones, in the thiamine-responsive megaloblastic anaemia gene SLC19A2 of eight families. Hum Mutat. 2000;13:37–42. doi: 10.1002/1098-1004(200007)16:1<37::AID-HUMU7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Reidling JC, Nabokina SM, Balamurugan K, Said HM. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and -2 promoters. J Cell Physiol. 2006;206:371–377. doi: 10.1002/jcp.20492. [DOI] [PubMed] [Google Scholar]
- Reidling JC, Said HM. In vitro and in vivo characterization of the minimal promoter region of the human thiamin transporter SLC19A2. Am J Physiol Cell Physiol. 2003;285:C633–C641. doi: 10.1152/ajpcell.00076.2003. [DOI] [PubMed] [Google Scholar]
- Reidling JC, Said HM. Adaptive regulation of intestinal thiamin uptake: molecular mechanism using wild-type and transgenic mice carrying hTHTR-1 and -2 promoters. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1127–G1134. doi: 10.1152/ajpgi.00539.2004. [DOI] [PubMed] [Google Scholar]
- Reidling JC, Subramanian VS, Dudeja PK, Said HM. Expression and promoter analysis of SLC19A2 in the human intestine. Biochim Biophys Acta. 2002;1561:180–187. doi: 10.1016/s0005-2736(02)00341-3. [DOI] [PubMed] [Google Scholar]
- Ricketts CJ, Minton JA, Ariyawansa I, Wales JK, Lo IF, Barrett TG. Thiamine-responsive megaloblastic anaemia syndrome: long-term follow-up and mutation analysis of seven families. Acta Paediatr. 2006;95:99–104. doi: 10.1080/08035250500323715. [DOI] [PubMed] [Google Scholar]
- Rindi G, Laforenza U. Thiamine intestinal transport and related issues: recent aspects. Proc Soc Exp Biol Med. 2000;224:246–255. doi: 10.1046/j.1525-1373.2000.22428.x. [DOI] [PubMed] [Google Scholar]
- Said HM, Balamurugan K, Subramanian VS, Marchant JS. Expression and functional contribution of the human thiamin transporter-2 (hTHTR-2) in thiamin absorption in the human intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G491–G498. doi: 10.1152/ajpgi.00361.2003. [DOI] [PubMed] [Google Scholar]
- Said HM, Ortiz A, Kumar C, Chatterjee N, Dudeja P, Rubin S. Transport of thiamine in human intestine: mechanism and regulation in intestinal epithelial cell model Caco-2. Am J Physiol Cell Physiol. 1999;277:C645–C651. doi: 10.1152/ajpcell.1999.277.4.C645. [DOI] [PubMed] [Google Scholar]
- Said HM, Ortiz A, Subramanian VS, Neufeld EJ, Moyer MP, Dudeja P. Mechanism of thiamine uptake by human colonocytes: studies with cultured colonic epithelial cell line NCM 460. Am J Physiol Cell Physiol. 2001;277:C645–C651. doi: 10.1152/ajpgi.2001.281.1.G144. [DOI] [PubMed] [Google Scholar]
- Said HM, Wang S, Ma TY. Mechanism of riboflavin uptake by cultured human retinal pigment epithelial ARPE-19 cells: possible regulation by an intracellular Ca2+–calmodulin-mediated pathway. J Physiol. 2005;566:369–377. doi: 10.1113/jphysiol.2005.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N, Kimura M, Kuchiba A, Itokawa Y. Blood thiamine levels in outpatients with diabetes mellitus. J Nutr Sci Vitaminol (Tokyo) 1987;33:421–430. doi: 10.3177/jnsv.33.421. [DOI] [PubMed] [Google Scholar]
- Scharfe C, Hauschild M, Klopstock T, Janssen AJM, Heidemann PH, Meitinger T, Jaksch M. A noval mutation in the thiamine responsive megaloblastic anaemia gene SLC19A2 in a patient with deficiency of respiratory chain complex I. J Med Genet. 2000;37:669–673. doi: 10.1136/jmg.37.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweingruber ME. The melaminophenyl arsenicals melarsoprol and melarsenoxide interfere with thiamin metabolism in the fission yeast Schizosaccharomyces pombe. Antimicrob Agents Chemother. 2004;48:3268–3271. doi: 10.1128/AAC.48.9.3268-3271.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg AR, Fleming JC, Baker MA, Sakamoto M, Cohen N, Neufeld EJ. Defective high-affinity thiamine transporter leads to cell death in thiamine-responsive megaloblastic anemia syndrome fibroblasts. J Clin Invest. 1999;103:723–729. doi: 10.1172/JCI3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M-P, Wandinger-Ness A, Roitbak T. Altered trafficking and epithelial cell polarity in disease. Trends Cell Biol. 2002;12:374–381. doi: 10.1016/s0962-8924(02)02331-0. [DOI] [PubMed] [Google Scholar]
- Subramanian VS, Chatterjee N, Said HM. Folate uptake in the human intestine: promoter activity and effect of folate deficiency. J Cell Physiol. 2003a;196:403–408. doi: 10.1002/jcp.10324. [DOI] [PubMed] [Google Scholar]
- Subramanian VS, Marchant JS, Parker I, Said HM. Cell biology of the human thiamine transporter-1 (hTHTR1): intracellular trafficking and membrane targeting mechanisms. J Biol Chem. 2003b;278:3976–3984. doi: 10.1074/jbc.M210717200. [DOI] [PubMed] [Google Scholar]
- Szyniarowski P, Bettendorff L, Schweingruber ME. The antitrypanosomal drug melarsoprol competitively inhibits thiamin uptake in mouse neuroblastoma cells. Cell Biol Toxicol. 2006;22:183–187. doi: 10.1007/s10565-006-0034-z. [DOI] [PubMed] [Google Scholar]
- Tallaksen CME, Bohmer T, Bell H. Blood and serum thiamin and thiamin phosphate esters concentrations in patients with alcohol dependence syndrome before and after thiamin treatment. Alcohol Clin Exp Res. 1992;16:320–325. doi: 10.1111/j.1530-0277.1992.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Tanphaichirt V. Thiamin. In: Shils ME, Olsen JA, Shike M, editors. Modern Nutrition in Health and Disease. New York: Lea & Febiger; 1994. pp. 359–375. [Google Scholar]
- Tomasulo PA, Kater RMH, Iber FL. Impairment of thiamine absorption in alcoholism in alcoholism. Am J Clin Nutr. 1968;21:1341–1344. doi: 10.1093/ajcn/21.11.1341. [DOI] [PubMed] [Google Scholar]
- Traebert M, Hattenhauer O, Murer H, Kaissling B, Biber J. Expression of type II Na-P(i) cotransporter in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 1999;277:L868–L873. doi: 10.1152/ajplung.1999.277.5.L868. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke–Korsakoff Syndrome and Related Neurological Disorders due to Alcoholism and Malnutrition. Philadelphia, PA: Davis; 1989. [Google Scholar]
- Wilkinson GN. Statistical estimation in enzyme kinetics. Biochem J. 1961;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]