Abstract
It has been proposed that different forms of rhythmic human limb movement have a common central neural control (‘common core hypothesis’), just as in other animals. We compared the modulation patterns of background EMG and cutaneous reflexes during walking, arm and leg cycling, and arm-assisted recumbent stepping. We hypothesized that patterns of EMG and reflex modulation during cycling and stepping (deduced from mathematical principal components analysis) would be comparable to those during walking because they rely on similar neural substrates. Differences between the tasks were assessed by evoking cutaneous reflexes via stimulation of nerves in the foot and hand in separate trials. The EMG was recorded from flexor and extensor muscles of the arms and legs. Angular positions of the hip, knee and elbow joints were also recorded. Factor analysis revealed that across the three tasks, four principal components explained more than 93% of the variance in the background EMG and middle-latency reflex amplitude. Phase modulation of reflex amplitude was observed in most muscles across all tasks, suggesting activity in similar control networks. Significant correlations between EMG level and reflex amplitude were frequently observed only during static voluntary muscle activation and not during rhythmic movement. Results from a control experiment showed that strong correlation between EMG and reflex amplitudes was observed during discrete, voluntary leg extension but not during walking. There were task-dependent differences in reflex modulation between the three tasks which probably arise owing to specific constraints during each task. Overall, the results show strong correlation across tasks and support common neural patterning as the regulator of arm and leg movement during various rhythmic human movements.
The innate capacity for generation of rhythmic movement patterns is found across the animal kingdom (Orlovsky et al. 1999). Humans produce a variety of rhythmic motor patterns during all forms of terrestrial and aquatic locomotion by way of walking, running, cycling, crawling, creeping and swimming. Considerable overlap with shared neurons and reorganization of synaptic activity to produce different rhythmic motor patterns with similar neuronal ensembles is well documented in invertebrate preparations such as the crayfish (Hooper & DiCaprio, 2004). A contribution to rhythmic motor outputs by spinal central pattern-generating elements (CPGs) has been suggested in many species, including humans (see for review Rossignol, 1996; Dietz, 2003; Zehr & Duysens, 2004; Yang et al. 2004; Rossignol et al. 2006). Using the research model of the infant walking paradigm, Yang and colleagues have shown that multiple locomotor tasks, including forward and backward walking and side-stepping, are probably controlled by the same reconfigured CPGs (Lamb & Yang, 2000).
We previously suggested that an estimate of the probable contributions of CPG activity can be evaluated by the phase-dependent modulation of reflex amplitudes evoked during rhythmic movement (Zehr & Duysens, 2004; Zehr et al. 2004a; Zehr & Hundza, 2005). This is essentially an extension of the proposal by Burke related to task-dependent regulation of reflexes as indicators of spinal processing (Burke, 1999). In this way, phase-dependent modulation of background EMG and reflex amplitudes have been suggested as hallmark ‘symptoms’ of CPG activity during rhythmic movement (for reviews see Duysens & Tax, 1994; Duysens, 1998; Duysens & Van de Crommert, 1998; Zehr & Duysens, 2004; Zehr et al. 2004a; Zehr, 2005). Additionally, independent modulation of reflex amplitude and background EMG level has been observed during rhythmic movements such as walking (Van Wezel et al. 1997; Komiyama et al. 2000; Zehr & Haridas, 2003; Haridas & Zehr, 2003; Lamont & Zehr, 2006) and cycling (Zehr & Hundza, 2005; Hundza & Zehr, 2006). Since this contrasts with the strong relation seen between EMG level and reflex amplitude during tonic contractions with no movement, this has been taken as indirect evidence for CPG involvement in the regulation of reflex amplitude during rhythmic activity. Therefore, the existence of shared circuitry and CPGs for different rhythmic locomotor movements within the human would be characterized by phase-modulation of reflex amplitudes and background locomotor activity which are relatively uncorrelated with each other. We previously argued that shared circuitry does exist in humans and should be seen as a ‘common core’ of CPG elements activated regardless of the specific locomotor task (Zehr, 2005). Recently, this hypothesis was tested by investigating the extent to which background muscle activity and cutaneous reflex modulation were conserved across three locomotor tasks that were all rhythmic but differed in task mechanics: level walking, incline walking and stair stepping (Lamont & Zehr, 2006). Considerable similarities amongst the tasks were identified, suggesting that the underlying neural mechanisms involved in co-ordinating level walking could be modified to also co-ordinate other locomotor tasks such as stair climbing. However, there were also task-specific differences between some tasks (e.g. in tibialis anterior (TA) activity during swing while stair climbing versus level walking) that may be indicative of a specific adaptation to the mechanical and stability constraints unique to each task.
Mathematical analyses of electromyographic recordings taken during gait have also revealed common neural factors involved in the control of locomotion (Davis & Vaughan, 1993; Olree & Vaughan, 1995). Recently, it was shown that muscle activity occurring during walking can be accounted for by five basic temporal activation patterns (Ivanenko et al. 2004). In this study, extensive EMG recordings were sampled while walking was performed at 1–5 km h−1 and under a range of body-weight-supported conditions. The stability of the principal components across these various conditions supported the suggestion of a contribution to the movement pattern by oscillatory neural circuits (e.g. CPGs). Common factors operational during walking with bent and erect postures (Grasso et al. 2000) suggest that broadly applicable principles of neural control are evident during human locomotion. Furthermore, recent comparison of muscle activation during walking and recumbent stepping has shown a high correspondence between these two locomotor tasks (Stoloff et al. 2007). Taken together, these studies support the concept of common neural regulation of locomotion arising from activity in shared CPG elements.
Whether common CPG control properties are maintained across multiple types of rhythmic arm and leg movement is uncertain. We examined three tasks involving movement of all four limbs in locomotor-like co-ordination patterns: treadmill walking, arm and leg cycling, and arm-assisted recumbent stepping. We tested the hypothesis that a common core for rhythmic movement control could be indirectly identified in humans using mathematical principal components analysis such as we recently performed for electromyographic patterns during recumbent stepping and walking (Stoloff et al. 2007). In this way, evidence for similar operational control would come from two main observations. Firstly, within each task the presence of phase-modulated background EMG and reflex amplitudes was determined. Secondly, across tasks, factor analysis was used to evaluate whether a simple set of factors common to the different locomotor tasks could be identified. Related to this analysis, the overall correlation between tasks was also examined. Additionally, we examined the specifics of functionally relevant task dependence in neural control which would be manifest in any task and phase-related differences in overt patterns of background muscle and cutaneous reflex activity (e.g. see Lamont & Zehr, 2006).
Methods
Much of the general methodology for nerve stimulation and muscle recording is similar to that previously described (Zehr et al. 1997, 2001; Zehr & Kido, 2001; Haridas & Zehr, 2003). Thus, methods will only be described briefly here.
Subjects and protocol
Ten subjects between the ages of 18 and 43 years participated with informed, written consent in a protocol approved by the Human Research Ethics Board at the University of Victoria and conforming with the Declaration of Helsinki. All subjects were free of documented neurological or metabolic impairment. Subjects were asked to perform three rhythmic arm and leg tasks: (1) level walking on a motorized treadmill (walking; Woodway Desmo M, Waukesha, WI, USA); (2) recumbent stepping assisted with the arms (stepping; using a coupled arm and leg stepping ergometer; NuStep, TRS 4000, Ann Arbor, Michigan, USA); and (3) seated arm and leg cycling (cycling; using a coupled arm and leg cycle ergometer; SciFit Pro II, Tulsa, Oklahoma, USA). Movement frequency was maintained at ∼1 Hz across tasks. That is, the period from ipsilateral heel contact to subsequent ipsilateral heel contact for walking, and for each complete movement cycle for cycling and stepping, was ∼1 s. This was achieved by having subjects walk at ∼3 mph and step/cycle at ∼1 rotation per second. Refer to the images at the far left of Fig. 1 for apparatus used for each task. For each task, two different cutaneous nerves innervating the hand and foot were stimulated in separate trials, thus yielding a total of six movement trials. Additionally, to obtain a control for the possible effects of automatic gain compensation, data for static voluntary activation were obtained during stationary postures with the arms and legs mimicking positions occurring during rhythmic movement, as in numerous recent studies from our laboratory (Zehr & Haridas, 2003; Haridas & Zehr, 2003; Lamont & Zehr, 2006; Balter & Zehr, 2007). For example, data were obtained for swing and stance for walking, and knee flexion and extension for cycling and stepping.
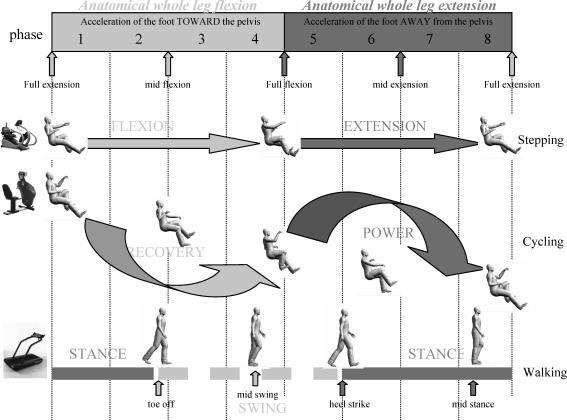
Figure 1. Overall schematic diagram relating the phases of movement for recumbent stepping, cycling and walking.
Functional phases related to movement phases are shown from top to bottom and are further discussed in the text. The descriptors flexion–extension for stepping, recovery–power for cycling, and stance–swing for walking refer to the ipsilateral (right) leg and correspond to the cartoons shown in the figure. The topmost terms of biomechanical flexion and extension refer to the overall motion of the leg as regards position of the foot moving towards or away from the pelvis, respectively. Numbers inside the top rectangle represent the phases used for averaging. For reference, at far left of the description for each task are images of the apparatus used for stepping (NuStep TRS 4000), cycling (SciFit Pro II) and walking (Woodway Desmo M treadmill).
Nerve stimulation
Cutaneous reflexes were evoked with trains (5 × 1.0 ms pulses at 300 Hz) of isolated constant current stimulation applied pseudorandomly across the movement phases using a Grass S88 stimulator with SIU5 and CCU1 isolation and constant current units (AstroMed-Grass Inc., Longueuil, Quebec, Canada). In separate trials, stimulation was applied to the superficial peroneal (SP) or to the superficial radial (SR) nerves on the right side using flexible 1 cm UNI-GEL single-use electrodes (Thought Technologies Ltd, Montreal, Quebec, Canada). Stimulation was set as a multiple of the threshold at which a clear radiating paresthesia (radiating threshold, RT) into the innervation area of the nerve was reported, i.e. into the dorsolateral portion of the hand and the dorsal surface of the foot for SR and SP nerves, respectively. Stimulation intensities were set to evoke a strong cutaneous sensation (during standing) which was not deemed painful by the subjects. This level ranged across subjects from 1.5 to 3 × RT but was typically set at ∼2 × RT.
Electromyography (EMG)
After abrasion and cleaning of the skin with alcohol, disposable 1 cm surface EMG electrodes (Thought Technologies Ltd) were applied in a bipolar configuration and using a 2 cm interelectrode distance over 10 muscles in the right arm and leg. The muscles were: tibialis anterior (TA); medial gastrocnemius (MG); soleus (SOL); biceps femoris (BF); vastus lateralis (VL); anterior deltoid (AD); posterior deltoid (PD); long head of biceps brachii (BB); long head of triceps brachii (TB); and flexor carpi radialis (FCR). Earth electrodes were placed over electrically neutral tissue. The EMG signals were preamplified and bandpass filtered at 100–300 Hz (P511 Grass Instruments, AstroMed, Inc.).
Kinematics and step-cycle detection
Movements at the hip, knee and elbow joints were recorded using bi-axial goniometers (Biometrics Ltd, Cwenfellinfach, UK) using methods previously described (Zehr et al. 1997; Zehr & Haridas, 2003). Step-cycle parameters (e.g. heel contact, toe-off) for walking were obtained with the use of custom-made force sensors, located in the insole of the subject's right shoe. Knee kinematics were used for establishing cycle timing and to anchor the movement cycle for off-line averaging.
Data acquisition and analysis
Neurophysiological and biomechanical analyses
Data were sampled at 1000 Hz with a 12 bit A/D converter connected to a microcomputer running custom-written (Dr Timothy Carroll, University of New South Wales, Australia) LabView software (National Instruments, Austin, TX, USA). Off-line, custom-written software programs (Matlab, The Mathworks, Inc., Natick, MA, USA) were used to separate the step cycles into eight equal parts or phases, aligned to begin with maximal knee extension. This corresponded to the onset of stance phase during walking trials. During the off-line analysis, EMG signals were full-wave rectified and filtered prior to averaging. At each phase of movement, the average trace from the non-stimulated steps was subtracted from the corresponding stimulated average trace to produce a subtracted EMG ‘reflex’ trace for each subject. Reflex traces that occurred within the same phase were averaged together (between 10 and 20 reflexes occurred in each phase for each subject).
The stimulus artifact was removed from the subtracted reflex trace and it was then filtered at 40 Hz using a dual-pass fourth order Butterworth low-pass filter. Cutaneous reflexes were examined at the middle (80–120 ms to peak) latency and reflexes for a given muscle were only analysed if at least one response at any latency exceeded a 2 s.d. band (centred about the mean prestimulus EMG level) for any task. Reflexes were quantified as the peak amplitude (from a 10 ms average window centred about the peak latency) within the middle-latency window. We concentrated on middle-latency reflexes, which tend to be largest and occur most frequently (Baken et al. 2005). Within this experiment, we were interested mainly in examining the pattern of reflex activation and locomotor muscle activity. Accordingly, all EMG and kinematic variables for each subject in each task were normalized to the averaged peak control (non-stimulated) EMG amplitude value and the range of motion, respectively, that occurred during each task and expressed as percentages. This procedure allows for the best representation of pattern but it may obscure differences that exist in the absolute values of the responses across tasks.
A schematic diagram relating the phase of arm and leg movements for the three tasks is shown in Fig. 1. Shown at the top of the figure are the eight numerical phases. Next are the relative movements for stepping and cycling. The bottom diagram indicates the phasing relative to walking. The relationship between the three movements and the biomechanical schema are addressed in the discussion.
Control experiments
To ensure that modulation of reflex amplitudes was not simply related to alterations in EMG levels occurring during the movements, we evaluated their relationship by performing correlation analysis between reflex amplitude and background EMG. This allowed for the discrimination of effects of automatic gain compensation between static voluntary tasks and rhythmic movement tasks. To further discriminate the degree of relations during rhythmic and static activity, Cohen's q statistic was calculated. A significant q statistic indicates a significant difference in the strength of correlation between rhythmic and static tasks. Lastly, four subjects participated in a further control experiment consisting of walking and discrete knee flexion or extension. The data from walking were obtained as described above. For the discrete actions, subjects were seated for knee extension and stood for knee flexion. The task was to extend the knee from ∼90 deg to full extension while seated or to flex the knee from full extension to ∼90 deg while standing. In both cases, subjects had to voluntarily stop the discrete movement to just touch a stationary reference marker with heel (flexion) or toe (extension) to complete the movement cycle. Reflexes were evoked at five equidistant portions of the flexion–extension cycle and averaged together (13 sweeps taken at each position). Reflex data were processed and analysed as described above.
Mathematical analysis
To examine basic patterns in neural control, we performed a principal components analysis (PCA) on reflex and background EMG data from the arm and leg muscles recorded during treadmill walking, recumbent stepping, and cycling (Olree & Vaughan, 1995). In an extension of procedures used by us recently (Stoloff et al. 2007), we analysed the middle-latency reflex amplitudes and background EMG data separately after dividing each task into eight discrete bins in the movement cycle. The PCA was performed using the princomp function in MATLAB 7.0 (The Mathworks, Inc.). The first step in the PCA was the creation of a 10 × 10 correlation matrix showing the linear dependence between muscles. Factor scores were determined using an orthogonal varimax rotation of the eigenvectors of this matrix, grouping variables with similar activity together. Using the eigenvalues and eigenvectors of this matrix, we calculated the variance of each factor. We determined the percentage of total variability described by each factor by dividing its associated variance by the sum of the variances. Calculation of the total variability described by each factor revealed that in all three tasks, the first four factors accounted for at least 93% of the variability. We therefore only considered scores for the first four factors for all further analysis. Much of our approach for the PCA analysis is similar to that used by Ivanenko et al. (2005), with the exception that our procedure was adapted to analyse EMG and reflex data that were binned into eight phases (instead of continuous points across all phases of movement).
We performed a cross-correlation analysis on factor scores to assess the relative similarity between treadmill walking, recumbent stepping, and cycling (MATLAB 7.0, xcorr function). We calculated the maximal correlation coefficient and corresponding lag of each factor score between each pair of tasks (walking–stepping, walking–cycling and stepping–cycling). We calculated a summarizing correlation coefficient using a variance-weighted average of the four factors. The summarizing correlation coefficient was the mathematically derived total correlation between two tasks, taking into account similarity in each of the four factor scores. Since the main point of the study was to compare both recumbent stepping and cycling with walking, we used the treadmill walking data as our reference point for comparing walking with stepping and walking with cycling. For these two comparisons, we determined the summarizing correlation coefficient by summing the products of percentage variance explained by each factor for walking and the correlation between walking and stepping, or walking and cycling, respectively. For the comparison between stepping and cycling, we determined the summarizing correlation coefficient by summing the product of mean percentage variance explained by each factor for stepping and cycling and the correlation between stepping and cycling. Previous research using similar methods (Ivanenko et al. 2005) used a criterion with greater than 0.4 indicating a good correlation between factors. We have adopted that criterion here as well.
We did not use principal component analysis on joint kinematic data because it did not seem theoretically valid. During recumbent stepping and arm and leg cycling, the motions of the joints are essentially dictated by the kinematic linkages of the devices. As such, derivation of principal factors contributing to joint motion would seem to have little functional basis.
Statistics
In all cases, analysis was performed using the averaged normalized values for each subject from each phase of the step cycle. Repeated-measures analysis of variance (RM ANOVA) was used to determine significant differences in the background EMG level, cutaneous reflex amplitudes, and kinematics. Tukey's HSD test was used for post hoc analysis of any significant main effects observed. Using these statistical procedures, large differences in the pattern of responses would be detected as task–phase interactions, whereas general amplitude differences (e.g. scaling effects) would be seen as significant main effects for task. Linear regression analysis using Pearson's correlation coefficient (r) was used to determine relationships between reflex amplitudes and background EMG levels for each muscle across rhythmic and static tasks. This was also used for comparison between walking and discrete knee flexion or extension in the control experiments. Additionally, to contrast differences between the tasks, Cohen's q statistic (Cohen, 1992) was calculated from the differences in the z-score transformed correlation coefficients for each task. In all cases, we used a conservative critical value of r of 0.62 (2-tailed test at P < 0.05) which accounted for repeated sampling from the same subjects. To determine whether differences in the frequency of significant correlations between tasks was significant, a χ2 square test of association was implemented (Glass & Hopkins, 1984).
Descriptive statistics included means ±s.e.m., and statistical significance was set at P < 0.05.
Results
Background EMG
Arm muscles
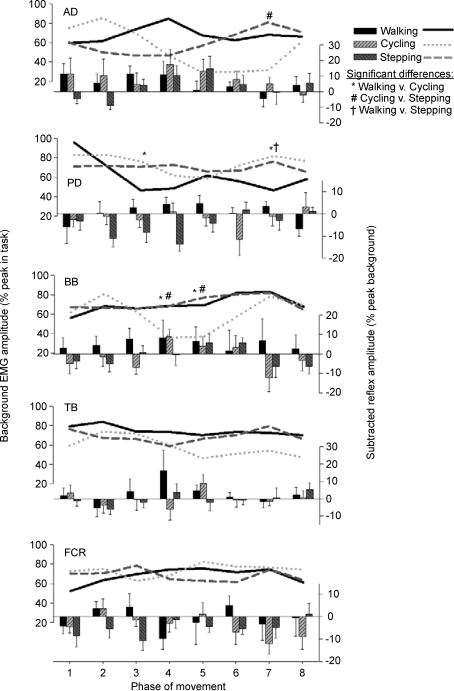
Background EMG patterns for all five arm muscles during SR nerve and SP nerve movement trials are shown as line plots in Figs 2 and 3, respectively. Values for walking (continuous black line), cycling (dotted grey line) and stepping (dashed grey line) are all expressed as percentages of the peak amplitudes in each task. There was significant phase-dependent modulation of background EMG in AD and BB muscles for all three tasks. For walking and cycling, background EMG amplitude in PD was phase-modulated, while FCR and TB were phase-modulated only during walking and cycling, respectively.
Figure 2. Background EMG (line plots) and middle-latency reflexes (bar plots) for muscles of the arms averaged across all subjects for cutaneous stimulation at the wrist (SR nerve).
The EMG amplitudes are means (±s.e.m.) from all subjects and are normalized to the peak control (i.e. background) EMG recorded in each task. Statistical differences from post hoc testing are as indicated by the three symbols in the figure. Specific contrasts are shown above or below each plot. Abbreviations are: AD, anterior deltoid; PD, posterior deltoid; BB, biceps brachii; TB, triceps brachii; and FCR, flexor carpi radialis.
Figure 3. Background EMG (line plots) and middle-latency reflexes (bar plots) for muscles of the arms averaged across all subjects for stimulation at the ankle (SP nerve).
The EMG amplitudes are normalized to the peak control (i.e. background) EMG recorded in each task and are means (±s.e.m.) taken across all subjects. Statistical differences from post hoc testing are as indicated by the three symbols in the figure. Abbreviations are as given in legend to Fig. 2.
Generally, the extent of background EMG amplitude modulation was similar across the three tasks (i.e. there were few statistically significant differences between tasks). For SR nerve trials (Fig. 2), there were no significant differences for TB or FCR muscles and there were few significant differences between cycling and walking (2 each for AD and PD, and 3 phases for BB; see * in Fig. 2). Three phases were different for cycling compared with stepping (1 each for AD, PD and BB; see # in Fig. 2) and one phase was different for walking compared with stepping for PD (see † in Fig. 2). These observations were similar for the SP nerve trials (Fig. 3), where there were also no differences for TB or FCR muscles and some differences for AD (1 phase for cycling–stepping), PD (2 phases for walking–cycling and 1 phase for walking–stepping) and BB (2 phases each for walking–cycling and for cycling–stepping). The small differences can be better appreciated by considering the number of phases in which significant differences could have been observed, which is 120 [equal to the number of phases (8) × number of muscles recorded (5) × number of tasks (3)]. In this context, there were 11 differences out of 120 for SR and 8 out of 120 for SP trials.
Leg muscles
Background EMG patterns recorded in the leg muscles for SR nerve and SP nerve trials are indicated as line plots at the top of each panel in Figs 4 and 5, respectively. As with the arm muscles, all values are expressed as percentages of the peak amplitudes recorded in each task. There was significant phase-dependent modulation of background EMG in four of the five leg muscles (VL, TA, MG and SOL) for all three tasks. The BF was phase-modulated only during walking and stepping.
Figure 4. Background EMG (line plots) and middle-latency reflexes (bar plots) for muscles of the legs averaged across all subjects for stimulation applied at the wrist (SR nerve).
The EMG amplitudes are normalized to the peak control (i.e. background) EMG recorded in each task. Statistical differences from post hoc testing are as indicated by the three symbols in the figure. Values are means (±s.e.m.) across all subjects. Abbreviations are: VL, vastus lateralis; BF, biceps femoris; TA, tibialis anterior; MG, medial gastrocnemius; SOL, soleus.
Figure 5. Background EMG (line plots) and middle-latency reflexes (bar plots) for muscles of the legs averaged across all subjects for stimulation applied at the ankle (SP nerve).
The EMG amplitudes are normalized to the peak control (i.e. background) EMG recorded in each task. Statistical differences from post hoc testing are as indicated by the three symbols in the figure. Mean values (±s.e.m.) from all subjects are plotted. Abbreviations are as given in legend to Fig. 4.
The patterns of background EMG amplitude modulation for each muscle have similarity across the three tasks as evidenced by few statistically significant differences between tasks. Data in Fig. 4 are for trials with SR nerve stimulation. There were few significant differences between cycling and walking (3 phases for BF, and 1 each for TA, MG; and SOL; see * in Fig. 4) and only one difference for cycling–stepping (in BF; see # in Fig. 4). The largest number of differences was seen for walking–stepping (1 phase for VL and MG, 2 for BF and SOL, and 4 for TA; see † in Fig. 4). The observations from SP nerve (Fig. 5) were generally consistent with the SR results. However, there were no differences for VL or MG background EMGs. For BF there were three differences for cycling–stepping and one for walking–cycling. The TA had one difference for walking–cycling and cycling–stepping and three for walking–stepping. There were two walking–stepping differences identified for SOL. When the number of phases with significant differences is considered, as described for the arm muscles above, there were 17 differences out of 120 for SR and 11 out of 120 for SP trials.
Kinematics
The movements at the elbow, hip and knee across all phases of movement for each task are plotted in Fig. 6. There are clear differences for the pattern of motion at the elbow when comparing walking with either cycling or stepping. While the general pattern appears to be closer for hip and knee movements, there were still many significant differences (see Fig. 6). Summarized as for the background EMG, there were 39 differences out of a total of 72 possible. As seen in Fig. 6, there were more differences when comparing walking with stepping (n= 14) and walking with cycling (n= 16) than when comparing stepping with cycling (n= 9). It must be noted further that there are large differences in both the total range of motion and the anatomical excursions (given as maximal and minimal values in the Table 1) reached for the elbow, knee, and hip when values in degrees are examined (see Table 1).
Figure 6. Summary of the kinematic recordings for movement at the elbow, hip and knee across walking, stepping and cycling.
Data are the means of all 10 subjects, and values are normalized to the peak joint excursion for each task. Statistical differences from post hoc testing are as indicated by the three symbols in the figure. Refer to Table 1 for peak values and range of motion in degrees.
Table 1.
Average joint excursions and range of motion (ROM) for all kinematic measures recorded during all tasks
| Elbow | Knee | Hip | ||||
|---|---|---|---|---|---|---|
| SP | SR | SP | SR | SP | SR | |
| Walking | ||||||
| Minimum | 130.05 | 132.07 | 104.27 | 104.09 | 83.82 | 84.80 |
| Maximum | 150.56 | 153.26 | 150.00 | 148.86 | 101.40 | 102.07 |
| ROM | 20.51 | 21.18 | 45.74 | 44.78 | 17.58 | 17.27 |
| Cycling | ||||||
| Minimum | 55.62 | 59.88 | 59.54 | 59.49 | 46.90 | 46.92 |
| Maximum | 130.10 | 130.60 | 130.50 | 129.07 | 71.18 | 70.74 |
| ROM | 74.49 | 70.72 | 70.97 | 69.58 | 24.28 | 23.82 |
| Stepping | ||||||
| Minimum | 62.77 | 63.08 | 84.43 | 88.43 | 53.71 | 54.43 |
| Maximum | 128.07 | 124.07 | 138.01 | 137.68 | 70.67 | 70.86 |
| ROM | 65.30 | 60.98 | 53.58 | 49.25 | 16.96 | 16.44 |
Values are in degrees.
Reflex modulation
Superficial radial nerve
Middle-latency reflexes evoked in arm and leg muscles by SR nerve stimulation are plotted as bars in Figs 2 and 4, respectively. As can be seen in the figures, there are differences in response amplitude across tasks. When comparisons were made between the tasks, few significant differences were found for arm and leg muscles. Reflex amplitude was significantly larger during cycling compared with walking at phase 1 for AD (see * in the AD panel of Fig. 2). There was some difference in the pattern for interlimb SR reflexes evoked in TA, as indicated by a task–phase interaction. However, no significant post hoc differences were identified. Lastly, SR-evoked interlimb middle-latency reflexes in MG consisted of larger suppressions during cycling and stepping than during walking (significant main effect for task; not marked in Fig. 4).
Superficial peroneal nerve
Reflexes evoked at middle latency by SP nerve stimulation are plotted as bars in Figs 3 and 5 for arm and leg muscles, respectively. As with the SR reflexes described above, there were differences in the amplitude of SP nerve reflexes in various muscles across the three tasks. When examining the pattern of responses and contrasting the tasks directly, there were few statistically significant differences between the tasks. In this context, VL had the largest differences. One phase (phase 4; * and † in Fig. 5) showed significant differences between walking and both stepping and cycling. Indeed, this phase showed a complete reflex reversal between walking (facilitation) and the other two tasks (suppression). The MG (Fig. 5) did have one phase (phase 8) in which reflex amplitude during walking was significantly larger than that observed during stepping. There were also task–phase interactions for PD (Fig. 3) and TA (Fig. 5) but these differences were not large enough to be detected in post hoc testing.
The general conservation in the pattern of reflex responses can be seen in the grand average reflex traces plotted in Fig. 7. Note that the pattern is generally similar (e.g. sign of the responses) but that there are some differences in relative amplitude.
Figure 7. Ensemble reflex grand average traces (from all phases) for all subjects for SP and SR nerves for all three tasks in a muscle of the leg.
There is a similar pattern of cutaneous reflex modulation regardless of motor task. Note that while there is some change in amplitude, the general pattern is similar. This lack of extensive task dependency suggests similar neural control across the walking, stepping and cycling tasks.
Mathematical PCA
The summary for the principal components analysis for SR and SP nerve trials is shown in Fig. 8 (lines for background EMG; bars for reflexes). Across all three tasks, four common factors explained more than 98% of the variance for background EMG for both SR and SP trials. Similarly, for middle-latency reflexes, more than 92% of the variance for SR and SP trials was accounted for by four factors. There was a substantial difference between walking and the other two tasks in the magnitude of variance accounted for by the first principal component of reflex modulation. The first factor explained 77–85% of the variance in middle-latency reflexes during cycling and stepping but only 46–53% during walking. This suggests that reflex modulation during cycling and stepping had a less complex pattern compared with walking.
Figure 8. Summary of principal component analysis for background EMG (bEMG; line plots) and middle-latency reflex amplitude (bar plots).
Data represent the variance accounted for (VAF) by each factor. Note that both cycling and stepping could be well explained by only two factors but that walking required additional factors to achieve similar values for VAF.
Correlations in EMG and reflex factors indicated that the three tasks had similar neural patterns. Coefficients between walking–stepping, walking–cycling and cycling–stepping for background EMG and middle-latency reflexes ranged from 0.43 to 0.65 (see Table 2).
Table 2.
Overall summarizing correlations between tasks from PCA analysis
| SR Nerve | SP Nerve | |||
|---|---|---|---|---|
| Background EMG | Middle-latency reflex amplitude | Background EMG | Middle-latency reflex amplitude | |
| Walking–cycling | 0.6 | 0.48 | 0.57 | 0.43 |
| Walking–stepping | 0.63 | 0.44 | 0.6 | 0.65 |
| Cycling–stepping | 0.48 | 0.65 | 0.48 | 0.49 |
Values show correlations amongst the tasks of walking, cycling and stepping. Note that previous research using similar methods (Ivanenko et al. 2005) used a criterion with greater than 0.4 indicating a good correlation. Thus, our correlations show good correspondence between tasks.
Control experiments
The results of the regression analysis for background EMG and middle-latency cutaneous reflex amplitudes are shown in Table 3 for SR nerve and Table 4 for SP nerve. Examination of Tables 3 and 4 reveals that significant correlation was commonly seen during static contraction but rarely seen during rhythmic movement. Note also that for the few muscles for which correlations were found to be significant during static contraction and rhythmic movement, the sign of the relation was reversed between the two conditions. Out of a total possible of 60, there were 24 significant correlations during static contraction and only 7 during rhythmic movement. The frequency of significant correlations was significantly lower in the rhythmic compared with the static tasks. When considering SP nerve cutaneous reflexes in leg muscles only, there were 8 of 15 significant correlations during static contraction compared with 2 of 15 during rhythmic movement (χ2 squared, P < 0.02). Similarly, for SR nerve reflexes in arm muscles, during static contraction 7 of 15 correlations were significant, whereas during rhythmic movement only 1 of 15 were significant (χ2 squared, P < 0.02).
Table 3.
Summary of linear regression analysis between background EMG and SR nerve middle-latency reflex amplitudes for all muscles across all rhythmic and static tasks
| Walking | Cycling | Stepping | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rhythmic | Static | q | Rhythmic | Static | q | Rhythmic | Static | q | |
| AD | 0.52 | −0.11 | 0.69* | 0.38 | 0.95* | 1.43* | 0.36 | 0.93* | 1.28* |
| PD | 0.24 | −0.43 | 0.71* | −0.06 | −0.09 | 0.03 | 0.35 | −0.89* | 1.79* |
| BB | −0.26 | −0.25 | 0.01 | 0.31 | −0.74* | 1.27* | −0.13 | −0.58 | 0.53 |
| TB | 0.59 | −0.21 | 0.89* | 0.46 | 0.90* | 0.98* | 0.02 | 0.97* | 2.07* |
| FCR | −0.18 | −0.92* | 1.41* | −0.89* | −0.44 | 0.95* | −0.37 | −0.49 | 0.15 |
| VL | −0.71* | 0.61 | 1.60* | −0.28 | −0.49 | 0.25 | −0.75* | −0.76* | 0.02 |
| BF | 0.18 | −0.88* | 1.19* | 0.03 | −0.42 | 0.48 | −0.01 | 0.22 | 0.23 |
| TA | −0.37 | −0.97* | 1.70* | −0.48 | −0.86* | 0.77* | −0.53 | −0.58 | 0.07 |
| MG | 0.55 | −0.85* | 1.87* | −0.60 | −0.89* | 0.73* | −0.26 | −0.90* | 1.74* |
| SOL | −0.27 | −0.19 | 0.09 | 0.03 | 0.27 | 0.25 | −0.13 | −0.50 | 0.42 |
Significant Pearson r coefficients and significant values of Cohen's q statistic (indicating significant difference between correlation from rhythmic task compared with static task) are indicated by an asterisk. The critical value for this 2-tailed comparison (P < 0.05) was 0.62. Abbreviations: AD, anterior deltoid; PD, posterior deltoid; BB, biceps brachii; TB, triceps brachii; FCR, flexor carpi radialis; VL, vastus lateralis; BF, biceps femoris; TA, tibialis anterior; MG, medial gastrocnemius; and SOL, soleus.
Table 4.
Summary of linear regression analysis between background EMG and SP nerve middle-latency reflex amplitudes for all muscles across all rhythmic and static tasks
| Walking | Cycling | Stepping | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rhythmic | Static | q | Rhythmic | Static | q | Rhythmic | Static | q | |
| AD | −0.08 | 0.23 | 0.31 | −0.16 | −0.68* | 0.67* | 0.03 | 0.74* | 0.92* |
| PD | −0.54 | −0.13 | 0.47 | −0.46 | −0.28 | 0.21 | −0.31 | 0.13 | 0.45 |
| BB | −0.18 | −0.33 | 0.16 | −0.70* | −0.50 | 0.32 | 0.02 | −0.52 | 0.60 |
| TB | −0.61 | −0.04 | 0.67* | −0.56 | 0.49 | 1.17 | −0.22 | −0.13 | 0.10 |
| FCR | −0.40 | 0.24 | 0.67* | −0.77* | −0.37 | 0.63* | −0.52 | −0.22 | 0.35 |
| VL | −0.31 | −0.89* | 1.10* | −0.91* | −0.90* | 0.06 | −0.58 | −0.89* | 0.76* |
| BF | 0.02 | 0.37 | 0.37 | 0.18 | 0.11 | 0.07 | −0.20 | 0.86* | 1.50* |
| TA | −0.18 | −0.36 | 0.20 | −0.37 | −0.99* | 2.26* | −0.54 | −0.96* | 1.34* |
| MG | −0.61 | 0.03 | 0.74* | −0.67* | −0.11 | 0.70* | −0.37 | 0.19 | 0.58 |
| SOL | −0.27 | 0.78* | 1.32* | −0.13 | 0.30 | 0.44 | 0.17 | 0.91* | 1.70* |
Significant Pearson r coefficients and significant values of Cohen's q statistic (indicating significant difference between correlation from rhythmic task compared with static task) are indicated by the asterisk. The critical value for this 2-tailed comparison (P < 0.05) was 0.62. Abbreviations as in Table 3.
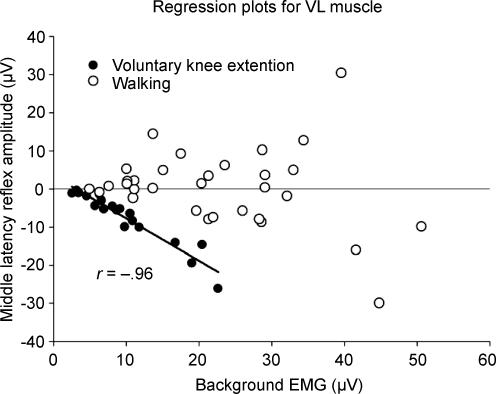
Typical data from the control experiment contrasting voluntary discrete knee extension and flexion with rhythmic activity during walking are plotted in Fig. 9 for VL muscle and SP nerve reflexes. The graph in Fig. 9 shows a significant correlation between reflex amplitude and background EMG during discrete knee extension (•) and the lack of correlation during walking (○). Further, although not plotted, reflexes were phase-dependently modulated (including reflex reversals) during walking but not during voluntary knee extension (when responses were not phase-modulated and were always suppressive). The BF muscle (not shown) was also phase-modulated, with reflex reversals during walking while the voluntary knee flexion data remained facilitatory across the movement cycle. Therefore, the independence between reflex amplitude and background EMG and the phase-dependent nature of the cutaneous reflexes were observed typically only during rhythmic motor tasks.
Figure 9. Strong correlation between reflexes evoked by SP nerve stimulation and background EMG during isolated discrete action is absent during walking.
Reflexes in the knee extensor muscle vastus lateralis (VL) during walking (○) are uncorrelated with background EMG, whereas those taken during discrete voluntary knee extension are highly correlated with EMG amplitude (•; continuous regression line). Values are data taken from 4 subjects.
Discussion
Background EMG and reflex amplitudes of most arm and leg muscles were phase-dependently modulated during all three rhythmic motor tasks of walking, cycling and stepping. The modulation patterns of reflex amplitudes were typically uncoupled from the patterns of background EMG during the rhythmic tasks but coupled during static voluntary activation or discrete actions. The mathematical analysis revealed a dependence on common factors and high correlation amongst the tasks. We interpret this as evidence for common neural control of rhythmic arm and leg movement across walking, coupled cycling and seated stepping. This commonality appears to result from neural acitivity specifically expressed during rhythmic movement and is suggestive of CPG regulation, as seen in other animals. The findings support the proposal of a common core regulating the basic pattern of arm and leg movement during rhythmic motor tasks in humans. This basic pattern is finely tuned and sculpted to the movement task constraints, as evidenced by the task-dependent reflex control observed at some phases across tasks.
Limitations of comparison across locomotor tasks using neurophysiological and mathematical analyses
There are several issues which must be addressed when contrasting and comparing EMG and kinematic recordings across walking, stepping and cycling. By the very nature of the apparati used, there is mechanical coupling between and within the arms and legs during recumbent stepping and arm and leg cycling that is not present during walking. Because of this, discrepancies across tasks are to be anticipated. Indeed, as shown in this study, there are some differences across all three tasks. However, there are relatively few overt and significant differences, suggesting that the nature of the mechanical coupling may not substantially alter the neural control. Also, there is a relatively greater activation of the arm muscles during the stepping and cycling tasks (see ‘Translational implications’ subsection for more discussion). We attempted to minimize the effect this could have on our analysis by performing amplitude normalization within each task.
Overall, we evaluated the data taking the widest perspective possible in an attempt to examine overall patterns across walking, stepping and cycling and to search for evidence of common neural control across those locomotor tasks. A hallmark of rhythmic locomotor activity (regulated by presumed CPG activity) is phase-dependent modulation of background EMG and reflex amplitudes (Rossignol, 1996; Zehr & Duysens, 2004; Zehr et al. 2004a; Zehr, 2005). Therefore, it was necessary to evaluate the individual patterns of background muscle activity and reflex responses within each task as a gauge of operational control in each task. It should be noted that, while not present in every muscle or nerve condition across all tasks (see Figs 2–5), phase-dependent modulation of background EMG and cutaneous reflex amplitudes was observed in numerous muscles for each task and nerve stimulation condition. Thus, the essential criteria indicative of similar control across tasks were met using the ‘conventional’ neurophysiological approach. Note that within this definition, it is not a requirement that the amplitudes and patterns match exactly at each phase of movement across tasks. The mathematical principal components analysis can extract common control factors across all tasks, as applied previously by others (Grasso et al. 2000; Ivanenko et al. 2004; Stoloff et al. 2007).
Implications for neural control: cycling and stepping as ‘reduced’ walking
Rhythmic motor activity in animals is produced in large part by the activity of CPGs in the spinal cord which can produce a variety of locomotor rhythms and patterns (Grillner, 1981; Orlovsky et al. 1999). If a similar organization also operates in humans, common mechanisms of neural control should be active across many different rhythmic limb movements. Here we show correspondence of rhythmic muscle activity and reflex control across three locomotor tasks, suggesting shared operational control. On the basis of other work, the most likely explanation is the activity of CPGs contributing to this muscle activity across tasks (Dietz et al. 2001; Dietz, 2002a, 2003; Zehr & Duysens, 2004; Zehr, 2005; Carroll et al. 2005). The robust nature of rhythmic movement control in humans was recently examined by superimposing voluntary movement (such as kicking a ball) onto an on-going rhythmic walking task (Ivanenko et al. 2005). The results suggested a simple integration of the voluntary action with the automatic pattern of gait, since invariance in the main components accounting for walking were maintained. In the study of Ivanenko et al. (2004), unimpeded walking was compared with walking with a kick task and a walk over an obstacle. These tasks showed correlations of ∼0.65 and were described as similar. In contrast, correlations for walking with a ‘stoop’ to pick up an object showed r values less than 0.2. Thus our r values of 0.43–0.63 for walking, cycling and stepping (see Table 2) correspond well to this previous work.
Despite the overall correspondence between the rhythmic arm and leg movement tasks studied here, clearly there are variables which have been removed (or which have had their effective contribution reduced) during the recumbent stepping and cycling. Postural stabilization control, visual flow information and vestibular input are likely to be dramatically different for the two seated tasks compared with treadmill walking (Kennedy et al. 2003). Further, lower limb loading (owing to reduced body weight support while seated) is less during the recumbent tasks. Load-related feedback is important for sculpting the locomotor output in many species, including the bipedal human (Duysens et al. 2000; Dietz & Duysens, 2000). Despite the fact that the sensory feedback from multiple sources was probably different across the three tasks, strong correspondence in neural control was observed. This overall result agrees with the prediction that neural elements comprising CPGs are fundamentally concerned with the overall locomotor rhythm and are secondarily affected by afferent feedback to appropriately sculpt motor output to local conditions. The reflex data obtained in this study point to this latter issue of task modulation. That is, we have previously suggested that alterations in environmental (i.e. unstable versus stable walking; Haridas et al. 2005) or task contraints (i.e. stair stepping versus level walking; Lamont & Zehr, 2006) can lead to dynamic regulation of cutaneous reflex amplitudes. It is probable that the task-related differences in reflex amplitudes noted here are also indicative of this. This may also be reflected in the principal components analysis. As seen in Fig. 8, during walking the initial factor accounts for ∼50% of the variance, whereas it accounts for ∼80% for cycling and stepping. In contrast, the percentage variance accounted for by the initial factor in background EMG is between 60 and 70% for all three tasks. In sum, we suggest that our data can be taken as evidence for common and shared regulation of rhythmic arm and leg movement across a variety of human locomotor tasks.
Common control variables for rhythmic movement?
While there was correspondence between background EMG and cutaneous reflex modulation patterns across all three tasks, there were many instances where both the pattern and the amplitude of movement at the elbow, knee and hip differed. Thus, a similar pattern of neural output gave rise to a different movement pattern. This corresponds to the situation of backwards walking, in which kinematics and EMG patterns are similar to those of forwards walking except that they are essentially reversed in time and the amplitudes are usually higher (Thorstensson, 1986; Winter et al. 1989; Duysens et al. 1996; Grasso et al. 1998). Grasso et al. (1998) suggested that activity of similar CNS mechanisms regulates forwards and backwards walking. Indeed, the similarities between forwards and backwards locomotion also extend to the modulation of reflexes and could reflect the activity of CPG networks ‘running in reverse’ (Duysens et al. 1996). Thus several patterns of rhythmic movement could be regulated by similar circuits. This argument could also explain the observations seen during ‘crouched’ or bent walking (Grasso et al. 2000). On the basis of observations during forwards and backwards walking and side-stepping, it has also been suggested that the same CPG mechanisms may regulate various patterns of locomotion in the human infant (Lamb & Yang, 2000). The present data, taken together with these observations, suggests a pattern of control that is broadly applicable to all forms of rhythmic arm and leg movement. We suggest that it is the local feedback associated with the specific kinematics for each movement task that yield the small differences in muscle activation that are seen. We previously addressed this issue in the context of the ‘common core hypothesis’ (Zehr, 2005) and provided a model for interaction between common CPG timing element for rhythm generation (the ‘common core’), interneuronal reflex networks and afferent feedback. That model is revised here in Fig. 10 to encompass walking, stepping and cycling. The data are consistent with the idea that afferent feedback modulates background EMG level independently of reflex amplitude. That is, there were more differences in background EMG than there were for the cutaneous reflexes. Previously, we suggested that this represents the differential regulation of EMG and reflex amplitudes effected by rhythmic pattern-generating elements (Zehr & Hundza, 2005; Hundza & Zehr, 2006).
Figure 10. Schematic conceptual overview for the regulation of rhythmic human movement.
Note that the effect of feedback projects to the motoneuronal pools, interneuronal pathways and the CPG itself and is subsumed in the output of the shared common oscillator. The effect of supraspinal input is an important trigger and regulatory input but is not shown for simplicity. Adapted from Zehr (2005).
Lacquaniti et al. (1999) proposed that organizational principles underlying CPG regulation of walking are related to common principles of kinematic control. These are related to mechanical and neural constraints required to maintain balance and produce a more efficient locomotor pattern. Thus, normal walking should be considered according to the inverted pendulum model (Kuo et al. 2005). An interesting outcome from this work is that kinematic control is structured to produce muscle patterns that generally relate to the pattern of motion of the centre of mass (COM) as it vaults over the legs. This yields a ‘planar law of intersegmental co-ordination’ for elevation angles of thigh, shank and foot during walking. There is planar covariation such that the CPG output can reflect the movement of the end-point environmental interface for locomotion, namely the foot (Ivanenko et al. 2003). Thus, CPGs regulate limb segment movements based upon a net integration of the segmental elevation angles. This concept has direct relevance to our work here. In cycling and stepping, the nervous system essentially has a comparable but inverse problem to that experienced during walking. That is, the relation between the foot and the COM (or pelvis) is still the global control variable but restricted now to the net movement of the entire limb in the sagittal plane (derived from motion at the hip, knee and ankle). During walking, whole limb movement may be controlled to allow for the vaulting of the COM over the foot. During recumbent stepping and cycling, the COM is fixed but the end-point trajectory of the foot relative to the pelvis may become the variable of importance. In this context, it is helpful to refer back to Fig. 1. Positions of the limbs for each task are relative to the biomechanical movement of the whole leg (using the terminology of Ting et al. 1998, 1999, 2000). We use whole limb flexion and extension of the leg in the strictest anatomical terms, meaning movement of the foot towards and away from the body, respectively. In this way, the overall correspondence between the tasks of walking, cycling and stepping studied here could be regulated by the same neural substrate.
Evidence for CPG contributions to the common control of rhythmic tasks?
In contrast to the use of reduced lower animal preparations, such as the lamprey, in which direct cellular measurements can be made, indirect evidence and inference are used to estimate CPG contributions to human locomotor movements. Evidence from studies of clinical populations, such as patients after spinal cord injury, suggests that CPG mechanisms contribute to the locomotor pattern for human walking (Dietz et al. 1994; Barbeau & Rossignol, 1994; Harkema et al. 1997; Dietz, 1997; MacKay-Lyons, 2002; Steldt & Schmit, 2004; Ferris et al. 2004). Importantly, afferent feedback contributes strongly to the modulation of the putative CPG output during human walking (Duysens & Van de Crommert, 1998; Duysens, 1998; Van de Crommert et al. 1998). Accordingly, reflex modulation during rhythmic movement can be used to infer the activity of CPG circuits (Burke, 1999; Burke et al. 2001; Zehr & Duysens, 2004). For example, modulation of afferent feedback via premotoneuronal gating driven by CPG output could explain observations of phase dependency and task dependency of reflex amplitudes during rhythmic movement (Duysens & Tax, 1994; Duysens & Van de Crommert, 1998; MacKay-Lyons, 2002; Dietz, 2002a,b). Further, during rhythmic locomotor movements of the arms or legs, the general observation is that reflex amplitude is typically uncoupled from the locomotor EMG and is instead related to the phase of the movement cycle during which the reflex is evoked (‘phase-dependent modulation’; see Komiyama et al. 2000; for review see Duysens & Tax, 1994; Brookes et al. 1997). This modulation of reflex amplitude (including reversal of sign) suggests the premotoneuronal gating of afferent inputs to motoneurons by the activity of neural circuits which are active during rhythmic movement. These circuits could be supraspinal and/or be related to spinal CPG networks. A required distinction to infer CPG activity is to show that the patterns of reflex control are differentially modulated in motor tasks where voluntary control of static contractions can be separated from more automatic control of rhythmic muscle activity. This uncoupling stands in stark contrast to the close association between reflex amplitude and background EMG level seen during a static voluntary contraction (i.e. ‘automatic gain compensation’; Matthews, 1986), as shown in many other lower animal (Forssberg, 1979; Duysens & Loeb, 1980; Drew & Rossignol, 1987; Pratt & Loeb, 1991; LaBella et al. 1992) and human studies (and comprehensively discussed recently by Hundza & Zehr, 2006; Sakamoto et al. 2006; Balter & Zehr, 2007). Here we provide evidence that while there is a strong direct relation between reflex and background EMG amplitude during static contraction or voluntary discrete action, there is a weak or completely absent relation between these two parameters during walking, cycling or stepping. Interestingly, recent neuroimaging results also suggest the contribution of subcortical, presumed spinal, CPG networks operating to regulate muscle activity during human locomotion. Using functional magnetic resonance imaging data acquired during imagined standing, lying, walking or running, Jahn et al. (2004) showed distinct shifts of activity for the locomotor tasks. This was observed as deactivation of higher cortical areas and a shift to spinal and cerebellar regulation (Jahn et al. 2004). We speculate that the neurophysiological data obtained in the present experiments can be taken as additional confirmation of this concept.
Translational implications for rehabilitation
While recumbent stepping and cycling are similar to walking, an important difference is the extent of arm muscle activation and the direct mechanical coupling between the arms and legs. That is, the devices have handles and pedals that are mechanically coupled, allowing the arm movement to assist leg movement. This has been suggested to be of potential value in facilitating locomotor recovery after neurotrauma (Huang & Ferris, 2004; Kao & Ferris, 2005; Ferris et al. 2006). Recently, we also determined that rhythmic arm cycling could affect reflexes in leg muscles (Frigon et al. 2004; Zehr et al. 2004b). Circuits active only during rhythmic movement (e.g. CPG activity) were suggested to cause this reflex attenuation and were speculated to represent a portion of the co-ordinated linkage between the arms and legs during locomotion (Dietz, 2002a; Zehr & Duysens, 2004). This suggests that normal co-ordination between the legs during walking is affected by activity in the arms (Balter & Zehr, 2007). This has implications for recovery of walking after neurotrauma, since the recovery of arm muscle co-ordination during rhythmic movement could assist with recovery of leg muscle activity. It is currently unknown whether this pattern persists as well after stroke and spinal cord injury. However, the extension of the results from this project would help to provide a neurophysiological basis for recommending or opposing the use of recumbent stepping and arm and leg cycling as complementary therapy for gait rehabilitation after spinal cord injury and stroke (for review see Ferris et al. 2006).
Acknowledgments
This work was supported by grants to E.P.Z. from the Natural Sciences and Engineering Council of Canada (NSERC), the Heart and Stroke Foundation of Canada (BC & Yukon), and the Michael Smith Foundation for Health Research. D.P.F. and R.H.S. were supported by the Christopher Reeve Paralysis Foundation and the Paralysed Veterans of America Spinal Cord Research Foundation. S.R.H. was supported by fellowships from the Heart and Stroke Foundation of Canada (BC & Yukon) and the Michael Smith Foundation for Health Research.
References
- Baken BC, Dietz V, Duysens J. Phase-dependent modulation of short latency cutaneous reflexes during walking in man. Brain Res. 2005;1031:268–275. doi: 10.1016/j.brainres.2004.10.058. [DOI] [PubMed] [Google Scholar]
- Balter JE, Zehr EP. Neural coupling between the arms and legs during rhythmic locomotor-like cycling movement. J Neurophysiol. 2007;97:1809–1818. doi: 10.1152/jn.01038.2006. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Enhancement of locomotor recovery following spinal cord injury. Curr Opin Neurol. 1994;7:517–524. doi: 10.1097/00019052-199412000-00008. [DOI] [PubMed] [Google Scholar]
- Brooke JD, Cheng J, Collins DF, McIlroy WE, Misiaszek JE, Staines WR. Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog Neurobiol. 1997;51:393–421. doi: 10.1016/s0301-0082(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Exp Brain Res. 1999;128:263–277. doi: 10.1007/s002210050847. [DOI] [PubMed] [Google Scholar]
- Burke RE, Degtyarenko AM, Simon ES. Patterns of locomotor drive to motoneurons and last-order interneurons: clues to the structure of the CPG. J Neurophysiol. 2001;86:447–462. doi: 10.1152/jn.2001.86.1.447. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Zehr EP, Collins DF. Modulation of cutaneous reflexes in human upper limb muscles during arm cycling is independent of activity in the contralateral arm. Exp Brain Res. 2005;161:133–144. doi: 10.1007/s00221-004-2050-7. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psyc Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Davis BL, Vaughan CL. Signals during gait: use of multivariate statistics. J Electromyogr Kinesiol. 1993;3:51–60. doi: 10.1016/1050-6411(93)90023-P. [DOI] [PubMed] [Google Scholar]
- Dietz V. Locomotor recovery after spinal cord injury. Trends Neurosci. 1997;20:346–347. doi: 10.1016/s0166-2236(97)89934-1. [DOI] [PubMed] [Google Scholar]
- Dietz V. Do human bipeds use quadrupedal coordination? Trends Neurosci. 2002a;25:462–467. doi: 10.1016/s0166-2236(02)02229-4. [DOI] [PubMed] [Google Scholar]
- Dietz V. Proprioception and locomotor disorders. Nat Rev Neurosci. 2002b;3:781–790. doi: 10.1038/nrn939. [DOI] [PubMed] [Google Scholar]
- Dietz V. Spinal cord pattern generators for locomotion. Clin Neurophysiol. 2003;114:1379–1389. doi: 10.1016/s1388-2457(03)00120-2. [DOI] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Jensen L. Locomotor activity in spinal man. Lancet. 1994;344:1260–1263. doi: 10.1016/s0140-6736(94)90751-x. [DOI] [PubMed] [Google Scholar]
- Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture. 2000;11:102–110. doi: 10.1016/s0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Dietz V, Fouad K, Bastiaanse CM. Neuronal coordination of arm and leg movements during human locomotion. Eur J Neurosci. 2001;14:1906–1914. doi: 10.1046/j.0953-816x.2001.01813.x. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. A kinematic and electromyographic study of cutaneous reflexes evoked from the forelimb of unrestrained walking cats. J Neurophysiol. 1987;57:1160–1184. doi: 10.1152/jn.1987.57.4.1160. [DOI] [PubMed] [Google Scholar]
- Duysens J. From cat to man: basic aspects of locomotion relevant to motor rehabilitation of SCI. Neurorehabilitation. 1998;10:107–118. doi: 10.3233/NRE-1998-10203. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Duysens J, Loeb GE. Modulation of ipsi- and contralateral reflex responses in unrestrained walking cats. J Neurophysiol. 1980;44:1024–1037. doi: 10.1152/jn.1980.44.5.1024. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax T. Interlimb reflexes during gait in cat and human. In: Swinnen SP, Heuer H, Massion J, Casaer P, editors. Interlimb Coordination: Neural, Dynamical, and Cognitive Constraints. San Diego, Calif, USA: Academic Press, Inc.; 1994. pp. 97–126. [Google Scholar]
- Duysens J, Tax AA, Murrer L, Dietz V. Backward and forward walking use different patterns of phase-dependent modulation of cutaneous reflexes in humans. J Neurophysiol. 1996;76:301–310. doi: 10.1152/jn.1996.76.1.301. [DOI] [PubMed] [Google Scholar]
- Duysens J, Van de Crommert HW. Neural control of locomotion; The central pattern generator from cats to humans. Gait Posture. 1998;7:131–141. doi: 10.1016/s0966-6362(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Ferris DP, Gordon KE, Beres-Jones JA, Harkema SJ. Muscle activation during unilateral stepping occurs in the nonstepping limb of humans with clinically complete spinal cord injury. Spinal Cord. 2004;42:14–23. doi: 10.1038/sj.sc.3101542. [DOI] [PubMed] [Google Scholar]
- Ferris DP, Huang HJ, Kao PC. Moving the arms to activate the legs. Exerc Sport Sci Rev. 2006;34:133–120. doi: 10.1249/00003677-200607000-00005. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phasedependent compensatory reaction during locomotion. J Neurophysiol. 1979;42:936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Frigon A, Collins DF, Zehr EP. Effect of rhythmic arm movement on reflexes in the legs: modulation of soleus H-reflexes and somatosensory conditioning. J Neurophysiol. 2004;91:1516–1523. doi: 10.1152/jn.00695.2003. [DOI] [PubMed] [Google Scholar]
- Glass GV, Hopkins KD. Statistical methods in Education and Psychology. Needham Heights, MA, USA: Allyn & Bacon; 1984. Inferences among correlation coefficients; pp. 300–323. [Google Scholar]
- Grasso R, Bianchi L, Lacquaniti F. Motor patterns for human gait: backward versus forward locomotion. J Neurophysiol. 1998;80:1868–1885. doi: 10.1152/jn.1998.80.4.1868. [DOI] [PubMed] [Google Scholar]
- Grasso R, Zago M, Lacquaniti F. Interactions between posture and locomotion: motor patterns in humans walking with bent posture versus erect posture. J Neurophysiol. 2000;83:288–300. doi: 10.1152/jn.2000.83.1.288. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, editors. Handbook of Physiology, section 1, The Nervous System, Motor Control. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 1179–1236. [Google Scholar]
- Haridas C, Zehr EP. Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. J Neurophysiol. 2003;90:2850–2861. doi: 10.1152/jn.00531.2003. [DOI] [PubMed] [Google Scholar]
- Haridas C, Zehr EP, Misiaszek JE. Postural uncertainty leads to dynamic control of cutaneous reflexes from the foot during human walking. Brain Res. 2005;1062:48–62. doi: 10.1016/j.brainres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Hooper SL, DiCaprio RA. Crustacean motor pattern generator networks. Neurosignals. 2004;13:50–69. doi: 10.1159/000076158. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Ferris DP. Neural coupling between upper and lower limbs during recumbent stepping. J Appl Physiol. 2004;97:1299–1308. doi: 10.1152/japplphysiol.01350.2003. [DOI] [PubMed] [Google Scholar]
- Hundza S, Zehr E. Cutaneous reflexes during rhythmic arm cycling are insensitive to asymmetrical changes in crank length. Exp Brain Res. 2006;168:165–177. doi: 10.1007/s00221-005-0089-8. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F. Coordination of locomotion with voluntary movements in humans. J Neurosci. 2005;25:7238–7253. doi: 10.1523/JNEUROSCI.1327-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Zago M, Molinari M, Scivoletto G, Castellano V, Macellari V, Lacquaniti F. Temporal components of the motor patterns expressed by the human spinal cord reflect foot kinematics. J Neurophysiol. 2003;90:3555–3565. doi: 10.1152/jn.00223.2003. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol. 2004;556:267–282. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage. 2004;22:1722–1731. doi: 10.1016/j.neuroimage.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Kao PC, Ferris DP. The effect of movement frequency on interlimb coupling during recumbent stepping. Motor Control. 2005;9:144–163. doi: 10.1123/mcj.9.2.144. [DOI] [PubMed] [Google Scholar]
- Kennedy PM, Carlsen AN, Inglis JT, Chow R, Franks IM, Chua R. Relative contributions of visual and vestibular information on the trajectory of human gait. Exp Brain Res. 2003;153:113–117. doi: 10.1007/s00221-003-1633-z. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Zehr EP, Stein RB. Absence of nerve-specificity in human cutaneous reflexes during standing. Exp Brain Res. 2000;133:267–272. doi: 10.1007/s002210000411. [DOI] [PubMed] [Google Scholar]
- Kuo AD, Donelan JM, Ruina A. Energetic consequences of walking like an inverted pendulum: step-to-step transitions. Exerc Sport Sci Rev. 2005;33:88–97. doi: 10.1097/00003677-200504000-00006. [DOI] [PubMed] [Google Scholar]
- LaBella LA, Niechaj A, Rossignol S. Low-threshold, short-latency cutaneous reflexes during fictive locomotion in the ‘semi-chronic’ spinal cat. Exp Brain Res. 1992;91:236–248. doi: 10.1007/BF00231657. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Grasso R, Zago M. Motor patterns in walking. News Physiol Sci. 1999;14:168–174. doi: 10.1152/physiologyonline.1999.14.4.168. [DOI] [PubMed] [Google Scholar]
- Lamb T, Yang JF. Could different directions of infant stepping be controlled by the same locomotor central pattern generator? J Neurophysiol. 2000;83:2814–2824. doi: 10.1152/jn.2000.83.5.2814. [DOI] [PubMed] [Google Scholar]
- Lamont EV, Zehr EP. Task-specific modulation of cutaneous reflexes expressed at functionally relevant gait cycle phases during level and incline walking and stair climbing. Exp Brain Res. 2006;173:185–192. doi: 10.1007/s00221-006-0586-4. [DOI] [PubMed] [Google Scholar]
- MacKay-Lyons M. Central pattern generation of locomotion: a review of the evidence. Phys Ther. 2002;82:69–83. doi: 10.1093/ptj/82.1.69. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol. 1986;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olree KS, Vaughan CL. Fundamental patterns of bilateral muscle activity in human locomotion. Biol Cybern. 1995;73:409–414. doi: 10.1007/BF00201475. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion: from Mollusc to Man. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- Pratt CA, Loeb GE. Functionally complex muscles of the cat hindlimb. I. Patterns of activation across sartorius. Exp Brain Res. 1991;85:243–256. doi: 10.1007/BF00229404. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 173–216. [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Endoh T, Nakajima T, Tazoe T, Shiozawa S, Komiyama T. Modulations of interlimb and intralimb cutaneous reflexes during simultaneous arm and leg cycling in humans. Clin Neurophysiol. 2006;117:1301–1311. doi: 10.1016/j.clinph.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Steldt RE, Schmit BD. Modulation of coordinated muscle activity during imposed sinusoidal hip movements in human spinal cord injury. J Neurophysiol. 2004;92:673–685. doi: 10.1152/jn.00677.2003. [DOI] [PubMed] [Google Scholar]
- Stoloff R, Zehr EP, Ferris DP. Recumbent stepping has similar but simpler neural control compared to walking. Exp Brain Res. 2007;178:427–438. doi: 10.1007/s00221-006-0745-7. [DOI] [PubMed] [Google Scholar]
- Thorstensson A. How is the normal locomotor program modified to produce backward walking? Exp Brain Res. 1986;61:664–668. doi: 10.1007/BF00237595. [DOI] [PubMed] [Google Scholar]
- Ting LH, Kautz SA, Brown DA, Van der Loos HF, Zajac FE. Bilateral integration of sensorimotor signals during pedaling. Ann N Y Acad Sci. 1998;860:513–516. doi: 10.1111/j.1749-6632.1998.tb09091.x. [DOI] [PubMed] [Google Scholar]
- Ting LH, Kautz SA, Brown DA, Zajac FE. Phase reversal of biomechanical functions and muscle activity in backward pedaling. J Neurophysiol. 1999;81:544–551. doi: 10.1152/jn.1999.81.2.544. [DOI] [PubMed] [Google Scholar]
- Ting LH, Kautz SA, Brown DA, Zajac FE. Contralateral movement and extensor force generation alter flexion phase muscle coordination in pedaling. J Neurophysiol. 2000;83:3351–3365. doi: 10.1152/jn.2000.83.6.3351. [DOI] [PubMed] [Google Scholar]
- Van de Crommert HW, Mulder T, Duysens J. Neural control of locomotion: sensory control of the central pattern generator and its relation to treadmill training. Gait Posture. 1998;7:251–263. doi: 10.1016/s0966-6362(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Van Wezel BM, Ottenhoff FA, Duysens J. Dynamic control of location-specific information in tactile cutaneous reflexes from the foot during human walking. J Neurosci. 1997;17:3804–3814. doi: 10.1523/JNEUROSCI.17-10-03804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA, Pluck N, Yang JF. Backward walking: a simple reversal of forward walking? J Mot Behav. 1989;21:291–305. doi: 10.1080/00222895.1989.10735483. [DOI] [PubMed] [Google Scholar]
- Yang JF, Lam T, Pang MY, Lamont E, Musselman K, Seinen E. Infant stepping: a window to the behaviour of the human pattern generator for walking. Can J Physiol Pharmacol. 2004;82:662–674. doi: 10.1139/y04-070. [DOI] [PubMed] [Google Scholar]
- Zehr EP. Neural control of rhythmic human movement: the common core hypothesis. Exerc Sport Sci Rev. 2005;33:54–60. [PubMed] [Google Scholar]
- Zehr EP, Carroll TJ, Chua R, Collins DF, Frigon A, Haridas C, Hundza SR, Kido A. Possible contributions of spinal CPG activity to rhythmic human arm movement. Can J Physiol Pharmacol. 2004a;82:556–568. doi: 10.1139/y04-056. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Collins DF, Chua R. Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp Brain Res. 2001;140:495–504. doi: 10.1007/s002210100857. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Duysens J. Regulation of arm and leg movement during human locomotion. Neuroscientist. 2004;10:347–361. doi: 10.1177/1073858404264680. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Haridas C. Modulation of cutaneous reflexes in arm muscles during walking: further evidence of similar control mechanisms for rhythmic human arm and leg movements. Exp Brain Res. 2003;149:260–266. doi: 10.1007/s00221-003-1377-9. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Hoogenboom N, Frigon A, Collins DF. Facilitation of soleus H-reflex amplitude evoked by cutaneous nerve stimulation at the wrist is not suppressed by rhythmic arm movement. Exp Brain Res. 2004b;159:382–388. doi: 10.1007/s00221-004-2092-x. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Hundza SR. Forward and backward arm cycling are regulated by equivalent neural mechanisms. J Neurophysiol. 2005;93:633–640. doi: 10.1152/jn.00525.2004. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Kido A. Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. J Physiol. 2001;537:1033–1045. doi: 10.1111/j.1469-7793.2001.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Komiyama T, Stein RB. Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol. 1997;77:3311–3325. doi: 10.1152/jn.1997.77.6.3311. [DOI] [PubMed] [Google Scholar]