Abstract
This study examined the influence of oestrogen on cardiovascular responses to hypotension produced by administration of isoproterenol (Isop) and on neural activation in hindbrain nuclei mediating these responses. We first measured mean arterial pressure (MAP) and heart rate (HR) after administration of isoproterenol, a β-adrenergic agonist that increases circulating levels of AngII, in ovariectomized (OVX) rats treated with oestradiol benzoate (EB). We then evaluated EB effects on Isop-induced Fos immunoreactivity (Fos-IR) in the hindbrain baroreflex circuit. To control for weight loss associated with oestrogen replacement in OVX rats, we food restricted a separate group of OVX rats and evaluated Isop-induced changes in MAP, HR and Fos-IR. The depressor response to Isop was significantly attenuated by EB, which also produced a disproportionate increase in HR. These effects were not secondary to loss of body weight after EB treatment, because cardiovascular responses to Isop in food restricted rats were similar to those in OVX rats treated with the oil vehicle. Isop significantly increased Fos-IR in the nucleus of the solitary tract (NTS), area postrema (AP), rostral ventrolateral medulla (RVLM), and lateral parabrachial nucleus (lPBN); however, EB significantly attenuated the increase in the AP and in the lPBN. Again, these effects were not secondary to body weight loss, because food restricted rats had the same pattern of Fos-IR as did rats treated with the oil vehicle. These results suggest that EB modifies cardiovascular responses to Isop, possibly by decreasing activation of the AP and lPBN.
Cardiovascular diseases are the leading cause of death in women and the risk is exacerbated after menopause (Kannel & Wilson, 1995). Postmenopausal women are four times more likely to suffer from heart disease and hypertension than are premenopausal women of the same age (Kannel & Wilson, 1995; Tremollieres et al. 1995; Harrison-Bernard & Raij, 2000). In postmenopausal women, hormone replacement decreases blood pressure and increases arterial compliance (Da Costa et al. 2004), suggesting a cardio-protective role for oestrogen. Similarly, studies using animal models show that elimination of endogenous oestrogen by ovariectomy results in hypertension (Harrison-Bernard et al. 2003; Hinojosa-Laborde et al. 2004) which can be reversed by subsequent oestrogen replacement (Chappell et al. 2003). Much of the animal research examining the effect of oestrogen on cardiovascular function has focused on hormone effects on the periphery (Mendelsohn & Karas, 2005). Oestrogen receptors are located in the heart and peripheral blood vessels (Brown et al. 2000), so it is not surprising that a role for oestrogen in cardiovascular physiology has been shown to involve oestrogen actions on cardiac and vascular tissue. For example, oestrogen has rapid non-genomic and genomic effects on nitric oxide production (Chen et al. 1999), which causes dilatation of blood vessels. Moreover, oestrogen down-regulates β-adrenergic receptors on the heart, thereby decreasing the action of β-adrenergic agonists on heart rate and contractility (Li et al. 2000; Thawornkaiwong et al. 2003).
In addition to direct peripheral effects on the heart and vasculature, oestrogen treatment increases baroreflex sensitivity and modulates autonomic tone (Saleh & Connell, 2000; Pamidimukkala & Hay, 2003b; Pamidimukkala et al. 2005). Clearly, changes in vascular distensibility might be expected to alter the sensitivity of the baroreceptors, thereby affecting afferent activity; however, electrical stimulation of cut baroreceptor afferent nerves enhanced the bradycardic response in OVX rats given EB compared to those that were not (Mohamed et al. 1999). Importantly, the efferent limb of the reflex was unaffected by oestrogen, as the bradycardia elicited by electrical stimulation of the efferent vagus was not changed by oestrogen (Mohamed et al. 1999). These observations are part of increasing evidence that has led to growing acceptance of the idea that oestrogen has central actions that are important in cardiovascular regulation and may, in fact, alter activity in the central baroreflex pathway that adjusts autonomic tone to buffer perturbations in blood pressure. Consistent with this idea, oestrogen receptors have been localized to the hindbrain nuclei that comprise the baroreflex circuit (Shughrue et al. 1997), including the nucleus of the solitary tract (NTS), ventrolateral medulla (VLM), and the hindbrain circumventricular organ, area postrema (AP). Moreover, site specific injection of oestrogen into many of these nuclei alters cardiovascular parameters and/or baroreflex sensitivity (Saleh et al. 2000a,b). An additional possibility is that oestrogen influences the detection of peripheral signals by the brain. Pamidimukkala & Hay (2003a) recently showed that bath application of oestrogen affects the responsiveness of cultured AP neurons to angiotensin II (AngII), a hormone associated with cardiovascular regulation, in vitro. This finding suggests that oestrogen also may modify the detection of and/or responses to AngII in vivo.
Our recent studies show that oestrogen reduced water intake in response to systemic administration of isoproterenol (Isop), a β-adrenergic agonist that decreases blood pressure and stimulates AngII release (Krause et al. 2006). Importantly, Isop-induced neural activation in the subfornical organ (SFO), a forebrain circumventricular organ, also was attenuated by oestrogen (Krause et al. 2006). The effects were not attributable to differences in circulating levels of AngII, but rather to down-regulation of AngII receptors in the SFO (Krause et al. 2006). Together, these findings suggest that the observed behavioural effect is attributable to differences in the detection of AngII at the SFO. At present, however, it is not known whether oestrogen modulation of cardiovascular function may involve responses to circulating AngII by neurons in the baroreflex circuit and, in particular, in the AP. Accordingly, we examined the effect of oestrogen on cardiovascular responses and on neuronal activation in hindbrain nuclei mediating these responses after administration of Isop, a potent activator of the renin–angiotensin system (Stocker et al. 2000). We first measured blood pressure and heart rate in ovariectomized rats treated with oestrogen after systemic administration of Isop. We then evaluated oestrogen effects on Isop-induced neuronal activation, as indicated by fos immunoreactivity (Fos-IR), in the hindbrain baroreflex circuit. Body weight loss is associated with oestrogen replacement in ovariectomized rats (Krause et al. 2003; Krause et al. 2006) and weight loss is known to affect cardiovascular function (Emdin et al. 2001). Therefore, to control for the effects of weight loss, we also evaluated Isop-induced changes in blood pressure, heart rate and Fos-IR in ovariectomized rats that were food restricted to produce weight loss comparable to that which occurs after oestrogen treatment.
Methods
Animals
Adult female Sprague–Dawley rats weighing between 200 and 300 g were used in these studies. Animals were individually housed in plastic cages and given ad libitum access to Purina rodent chow (no. 5001) and water except where noted. Rats were kept in a temperature-controlled room (22–24°C) on a 12: 12 h light–dark cycle with lights on at 07.00 h. All procedures were approved by the Florida State University Animal Care and Use Committee.
Surgical procedures
Ovariectomy and telemetry device implantation were performed as previously described (Contreras et al. 2000; Krause et al. 2003). Briefly, under sodium pentobarbital anaesthesia (50 mg (kg body weight)−1; Abbott Laboratories, Chicago, IL, USA), rats were bilaterally ovariectomized (OVX) using a ventral approach and given 1 week to recover. Animals used to record mean arterial pressure (MAP) and heart rate (HR) were OVX and, after 7–10 days, again were anaesthetized with pentobarbital and implanted with a radiotelemetry transmitter (TA11PA-C40, Data Sciences International (DSI), St Paul, MN, USA). The descending aorta was exposed via an abdominal incision, and a catheter extending from the transmitter capsule was placed into the descending aorta and secured with tissue adhesive (Vetbond; St Paul, MN, USA) and a cellulose patch. The capsule was sutured to the abdominal musculature, the abdominal musculature was sutured, and wound clips were applied to the skin.
Oestrogen replacement
Ten to seven days after surgery, rats were given the oil vehicle (Oil; 0.1 ml, s.c.) or 17β-oestradiol-3-benzoate (EB; 10 μg/0.1 ml sesame oil, s.c.; Fisher Scientific, Fair Lawn, NJ, USA) on a schedule that mimics the pattern of oestrogen fluctuation during the estrous cycle. Specifically, rats were given subcutaneous injections of EB or Oil daily for two consecutive days, and tested 48 h following the second injection (i.e. Day 4). Oestrogen replacement using this schedule reliably elicits lordosis on Day 4 when progesterone also is given (McCarthy et al. 1991; Schumacher et al. 1991). Our previous studies show that this EB replacement schedule causes a 5–7% decrease in body weight (Krause et al. 2006); therefore, additional rats (WT Loss) were treated with Oil as described and food restricted on Day 3 to produce a 5–7% loss of body weight on Day 4.
Blood pressure and heart rate recording
MAP and HR responses to Isop were examined in 10 OVX rats. We used a within-subjects design so that each animal served as its own control. Rats were treated with EB or Oil as described and on Day 4 were injected with Isop (30 μg kg−1; s.c.) and with 0.15 m NaCl. Seven to ten days later, the same rats were treated with Oil or EB (i.e. rats previously treated with EB were treated with Oil and vice versa) and injected with Isop and with 0.15 m NaCl on Day 4. Seven to ten days later, a subset of these rats (n= 6) was tested in the WT Loss condition on Day 4. The WT Loss condition always was tested last to minimize differences in the number of days after OVX before rats were treated with EB.
In all conditions, rats were injected with both 0.15 m NaCl and Isop on Day 4. The order of the injections was randomized so that half the rats in each condition were injected with Isop first and the other half with 0.15 m NaCl. These injections were separated by 2.5 h, during which time MAP and HR were recorded continuously to ensure MAP and HR had returned to resting levels. Water was not available during recording. After Isop injection, MAP and HR were averaged over the 15 min period corresponding to the largest decrease in MAP, which typically occurred after 30 min. MAP and HR after 0.15 m NaCl were averaged during the same relative period. Changes in MAP and HR for each rat in each condition were calculated as the difference between the levels after 0.15 m NaCl versus those after Isop. The ratio between change in HR and change in MAP for each rat in each condition also was calculated.
c-Fos immunohistochemistry
Separate groups of OVX rats were treated with EB or Oil and were injected with Isop (Oil n= 10, EB n= 10) or 0.15 m NaCl (Oil n= 6, EB n= 6) on Day 4 as described. Two additional groups were tested in the WT Loss condition (Isop n= 5, 0.15 m NaCl n= 5) on Day 4. Ninety minutes after injection, rats were deeply anaesthetized with sodium pentobarbital and then perfused with 0.15 m NaCl, followed by 4% paraformaldehyde in 0.1 m phosphate buffer. Brains were removed, placed into 30% sucrose for 48 h, and then cut into 40 μm coronal sections using a cryostat.
c-Fos immunocytochemistry was performed as previously described (Curtis et al. 2002). Briefly, free-floating sections were washed in 0.05 m Tris-NaCl, soaked in 0.05 m Tris-NaCl containing 0.5% Triton X-100 and 10% normal goat serum (NGS) for 1 h, then incubated for 20 h with a rabbit polyclonal anti-c-Fos peptide antisera (Santa Cruz Biotechnology, Inc.) diluted 1: 30 000 in 2% NGS. Sections were washed in 2% NGS and incubated for 2 h at room temperature with a biotinylated goat anti-rabbit antibody (Vector Laboratories) diluted 1: 300 in NGS. Bound secondary antibody was amplified during a 1.5 h incubation in an avidin–biotin complex (ABC Elite Kit, Vector Laboratories). Antibody complexes were visualized using nickel intensified diaminobenzidine (Kirkegaard and Perry Laboratories, Gaithersberg, MD, USA) and this reaction was terminated by washing the sections in 0.05 m Tris-NaCl. Sections were mounted on microscope slides and coverslipped.
Fos-IR was examined in the hindbrain baroreflex circuit, including the AP, NTS, rostral ventrolateral medulla (RVLM), and caudal ventrolateral medulla (CVLM). Sections through the lateral parabrachial nucleus (lPBN) also were examined. Serial sections were taken from the pyramidal decussation to the rostral extent of the parabrachial nucleus (−14.60 to −8.72 mm from Bregma). Every third section was processed for Fos-IR to ensure that neurons were not counted twice. Using NIH Image software, the numbers of Fos-positive nuclei were counted in representative sections from each of these areas, which were matched between animals based on anatomical landmarks as described by Paxinos & Watson (1997). Specifically, AP sections were chosen at caudal, middle, and rostral levels (3 representative sections, −14.08 to −13.68 mm from Bregma). In the NTS, four representative sections were chosen (−14.50 to −13.68 from Bregma), one from the portion caudal to the AP (cNTS) and three from the middle portion (mNTS) corresponding to caudal, middle and rostral AP. For the ventral lateral medulla, Fos-IR was evaluated in the RVLM (3 sections, −12.80 to −11.80 mm from Bregma) and in the CVLM (3 representative sections, −14.60 to −14.20 mm from Bregma). Fos-IR was evaluated in the lPBN in two representative sections (−9.80 to −8.72 mm from Bregma). In the cases of bilateral structures, counts were taken from one side. The average number of Fos-positive nuclei in each area from each animal was calculated and group means for each area in each experimental condition were determined.
Statistics
All data are expressed as means ±s.e.m. Data were analysed using Statistica (StatSoft, Tulsa, OK, USA). Differences in absolute MAP, HR and Fos-IR were assessed with a 2-factor analysis of variance (ANOVA) with group (EB, Oil, WT Loss) and drug (0.15 m NaCl and Isop) as the factors. Differences in change in MAP and in HR and in the ratio between change in HR and change in MAP were assessed with a 1-factor ANOVA with group (EB, Oil, WT Loss) as the factor. Significant main effects or interactions (P < 0.05) were evaluated with Student–Newman–Keuls tests; additional specific planned comparisons were made using Bonferroni corrections.
Results
Cardiovascular responses to Isop
Table 1 shows the absolute MAP and HR after 0.15 m NaCl or Isop in Oil, EB and WT Loss conditions. As expected, there was a significant effect of drug on MAP (F1,46= 38.5, P < 0.001). Regardless of group, MAP was significantly less after Isop than after 0.15 m NaCl. There was no effect of group and no interaction between group and drug. Similarly, there was a significant effect of drug on HR (F1,46= 631.9, P < 0.001) and, regardless of group, HR was significantly greater after Isop than after 0.15 m NaCl (Table 1). There also was a significant effect of group on HR (F2,10= 5.39, P < 0.05). Overall, HR was lower in the EB and WT Loss conditions than in the Oil condition (P < 0.05); however, there was no interaction between group and drug.
Table 1.
MAP and HR after injection with 0.15 m NaCl or Isop
| 0.15 m NaCl | Isop | |||
|---|---|---|---|---|
| MAP (mmHg) | HR (BPM) | MAP (mmHg) | HR (BPM) | |
| Oil | 93.2 ± 4.8 | 355.8 ± 9.2 | 71.8 ± 4.2* | 507.7 ± 4.8α |
| EB | 84.8 ± 2.9 | 324.4 ± 9.1 | 72.6 ± 2.8* | 493.2 ± 4.6α |
| WT Loss | 88.6 ± 2.2 | 325.4 ± 10 | 66.0 ± 4.5* | 489.7 ± 7.8α |
Significantly different from MAP after injection of 0.15 m NaCl (P < 0.001). α= significantly different from HR after injection of 0.15 m NaCl (P < 0.001).
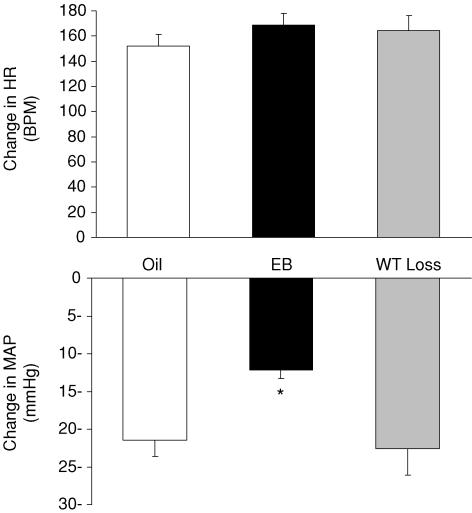
Figure 1 shows the change in MAP and in HR in the three conditions. There was a significant effect of group on the depressor response to Isop (F2,23= 6.35, P < 0.01). Post hoc analyses showed that the decrease in MAP was of smaller magnitude in rats treated with EB (−12.2 ± 1.1 mmHg) compared to those in the WT Loss (−22.6 ± 3.5 mmHg; P < 0.01) or Oil (−21.4 ± 3.1 mmHg; P < 0.01) conditions. Isop also elicited a robust tachycardia (approximately a 40–50% increase from baseline HR) that was similar in all three conditions. Examination of the ratio between change in HR and change in MAP revealed a significant effect of group (F2,23= 7.745, P < 0.01). Post hoc analyses showed that the ratio in rats treated with EB (14.0 ± 1.7) was significantly greater than that in Oil-treated (7.8 ± 0.9) and WT Loss (8.6 ± 1.8) conditions (both P < 0.01).
Figure 1. The Isop-induced change in HR (top) and MAP (bottom) in Oil, EB and WT Loss rats.
*Significantly different from Oil and WT Loss (P < 0.01).
Fos-IR after Isop
Nucleus of the solitary tract
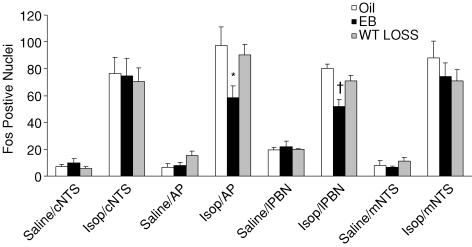
As shown in Fig. 2, there was a significant effect of drug on Fos-IR in cNTS (F1,36= 64.6, P < 0.01) and mNTS (F1,35= 70.7, P < 0.01), with approximately a sixfold increase after Isop compared to that after 0.15 m NaCl. There was no effect of group and no interaction between group and drug. Figure 3 shows digital photomicrographs of representative coronal sections from the cNTS.
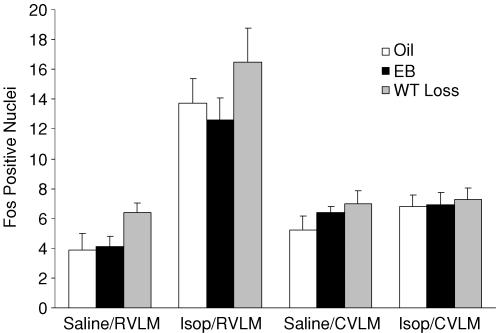
Figure 2. Fos positive nuclei after injection of 0.15 m NaCl or Isop in rats treated with Oil, EB, or WT Loss.
Abbreviations: caudal NTS (cNTS), area postrema (AP), lateral parabrachial nucleus (lPBN), and middle NTS (mNTS). *Significantly different from Oil/Isop (P < 0.01).†Significantly different from Oil/Isop and WT Loss/Isop (P < 0.01).
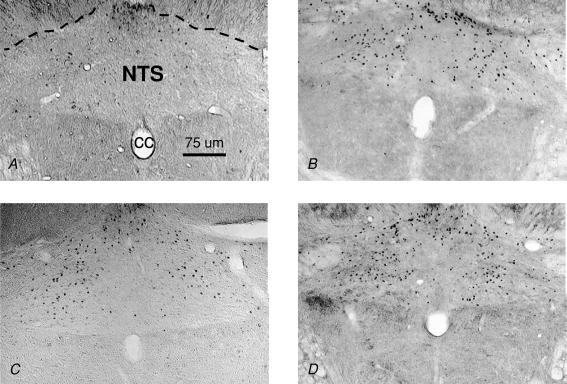
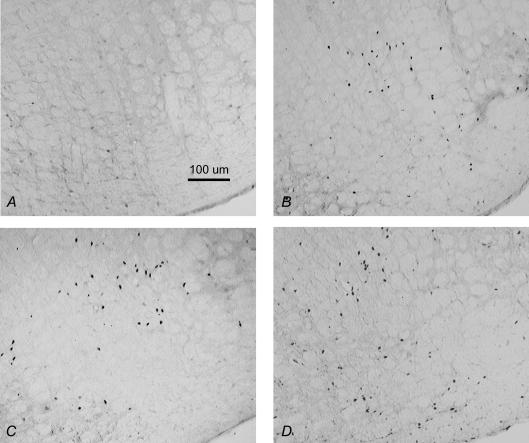
Figure 3. Coronal sections (40 μm) showing Fos IR in the cNTS of rats treated with 0.15 m NaCl (A), Oil and Isop (B), EB and Isop (C), and WT Loss and Isop (D).
cc; central canal.
Area postrema
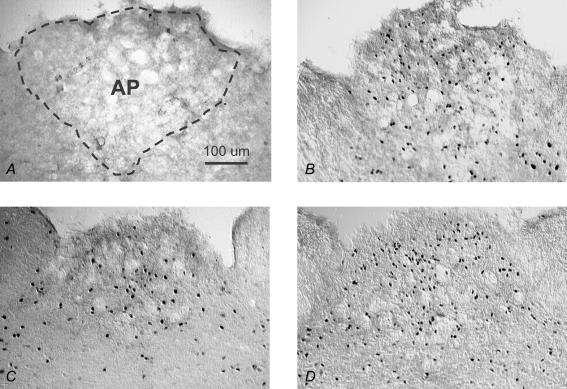
There also was a significant effect of drug on Fos-IR in the AP (F1,35= 70.4, P < 0.01), with a 10-fold increase after Isop compared to that after 0.15 m NaCl (Fig. 2). There was no effect of group and no interaction between group and drug; however, examination of Fos-IR in the AP (Fig. 4) and of the group means (Fig. 2) suggested an attenuation of Fos-IR after Isop in EB-treated rats. This possibility was further analysed using specific planned comparisons with Bonferonni corrections, which revealed significantly less Fos-IR after Isop in the AP of rats treated with EB compared to those treated with Oil (P < 0.01). Fos-IR in the AP of EB-treated rats did not differ from that of rats in the WT Loss condition (P= 0.12), which, in turn, did not differ from that in Oil-treated rats (P= 0.60). Figure 4 shows digital photomicrographs of representative sections from the AP.
Figure 4. Coronal sections (40 μm) showing Fos IR in the AP of rats treated with: 0.15 m NaCl (A), Oil and Isop (B), EB and Isop (C), and WT Loss and Isop (D).
Ventrolateral Medullá
There was no effect of drug or group on Fos-IR in the CVLM and no interaction between drug and group (Fig. 5). In contrast, there was a significant effect of drug on Fos-IR in the RVLM, as shown in Fig. 5 (F1,35= 52.7, P < 0.01), with nearly a fourfold increase after Isop compared to that after 0.15 m NaCl. There was no effect of group and no interaction between drug and group. Figure 6 shows digital photomicrographs of representative sections from the RVLM.
Figure 5. Fos positive nuclei after injection of 0.15 m NaCl or Isop in rats treated with Oil, EB, or WT Loss.
Abbreviations: caudal ventrolateral medulla (CVLM), rostral ventrolateral medulla (RVLM).
Figure 6. Coronal sections (40 μm) showing Fos IR in the RVLM of rats treated with 0.15 m NaCl (A), Oil and Isop (B), EB and Isop (C), and WT Loss and Isop (D).
Lateral parabrachial nucleus
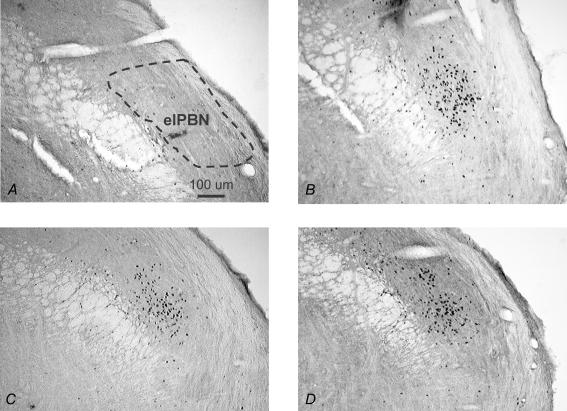
As shown in Fig. 2, there was a significant effect of drug on Fos-IR in the lPBN (F1,20= 220, P < 0.001), with a threefold increase after Isop compared to that after 0.15 m NaCl. There also was a significant effect of group (F2,20= 6, P < 0.01), with the EB-treated group having the least Fos-IR overall. Finally, there was a significant interaction between drug and group (F2,20= 8.3, P < 0.01). Post hoc analyses revealed that EB-treated rats had significantly less Fos-IR after Isop than did rats in the WT Loss (P < 0.01) and Oil (P < 0.01) conditions, whereas Fos-IR in the lPBN after Isop was similar in WT Loss and Oil-treated rats (P= 0.10). Figure 7 shows digital photomicrographs of representative sections from the lPBN illustrating the prevalence of Fos-IR after Isop in the external lateral portion.
Figure 7. Coronal sections (40 μm) showing Fos IR in the lPBN of rats treated with 0.15 m NaCl (A), Oil and Isop (B), EB and Isop (C), and WT Loss and Isop (D).
elPBN; external portion of the lateral parabrachial nucleus
Discussion
This study examined the effect of circulating oestrogen on cardiovascular parameters and on neuronal activation in the hindbrain nuclei that comprise the baroreflex circuit in response to systemic administration of Isop. The results show that EB attenuated the depressor response to Isop and the smaller decrease in MAP was accompanied by a disproportionate increase in HR. We also found that Isop increased Fos-IR, an indication of neuronal activation, in the NTS, AP, RVLM and lPBN and show for the first time that EB treatment attenuated Fos-IR in specific medullary (AP) and supramedullary (lPBN) nuclei implicated in blood pressure regulation. EB modulation of the cardiovascular and central responses to Isop was not secondary to hormonal effects on body weight because Isop-induced changes in MAP, HR, and Fos-IR in WT Loss rats were similar to those of rats treated with Oil.
Cardiovascular effects of EB
Despite the reduced depressor response to Isop in rats treated with EB, the accompanying increase in HR was of the same magnitude as that in WT Loss and Oil-treated rats. In fact, the compensatory tachycardia in EB-treated rats was similar to that in rats in which MAP decreased nearly two-fold more. This observation, in conjunction with the exaggerated ratio between change in HR and change in MAP in EB-treated rats, suggests that EB augments the HR response to Isop and may, in fact, account for the attenuated depressor response. Consistent with this idea, Ciric & Susic (1980) found that, when hypotensive responses to Isop were similar, OVX rats treated with EB had a greater increase in HR than did OVX rats without EB. Because Isop is a β-adrenergic agonist that increases heart rate and contractility, Ciric & Susic (1980) attributed the enhanced tachycardia to oestrogen modulation of β-adrenergic receptors on the heart. However, more recent studies have shown that EB blunts the stimulatory actions of Isop on heart rate and cardiac contractility in isolated rat hearts (Li et al. 2000) and prevents the up-regulation of β-adrenergic receptors on the heart that typically occurs after OVX (Thawornkaiwong et al. 2003). Thus, our finding of enhanced tachycardia in response to Isop in EB-treated rats is unlikely to be due to EB effects on β-adrenergic receptors on the heart.
Another possibility is that the weight loss associated with oestrogen replacement influenced the HR response to Isop. In conjunction with reports of enhanced parasympathetic tone after systemic administration of oestrogen (Saleh et al. 2000a,b), our observation of a mild basal bradycardia in EB-treated rats (Table 1) is consistent with increased vagal influence such as may occur as a result of weight loss (Rissanen et al. 2001). Thus, hypotension may have a greater effect on HR in EB-treated rats due to the withdrawal of elevated vagal efferent activity that is secondary to weight loss. Accordingly, we food restricted rats to produce body weight loss similar to that which occurs in EB-treated rats. Moreover, rats in the WT Loss condition exhibited basal bradycardia indicative of elevated parasympathetic tone (Rissanen et al. 2001) and comparable to that of EB-treated rats (Table 1). Nonetheless, enhanced compensatory tachycardic responses to Isop did not occur in WT Loss rats, even though the hypotension after Isop was the greatest in this condition (Table 1). Hence, weight loss alone does not affect reflex HR responses to Isop-induced hypotension, nor do slight basal bradycardia or hypotension (Table 1) related to body weight loss. Rather, given that EB augments the increase in vagal nerve activity (and thereby results in greater bradycardia) in response to hypertension (Saleh et al. 2000a,b), it is feasible that EB may contribute directly to the augmented HR response to Isop by altering baroreflex-mediated parasympathetic withdrawal.
Taken together, these observations suggest that the cardiovascular effects we observed after Isop reflect selective EB enhancement of baroreflex responses. Several studies have shown that OVX rats treated with EB had greater decreases in HR in response to phenylephrine-induced increases in blood pressure (el-Mas & Abdel-Rahman, 1998, Mohamed et al. 1999; Saleh & Connell, 2000). Although oestrogen effects on peripheral vasculature may contribute to EB-mediated changes in such compensatory HR responses, the enhancement of reflex bradycardia is likely to also involve EB modulation of central baroreflex pathways: EB treatment enhanced HR responses elicited by electrical stimulation of the cut aortic depressor nerve, but did not affect HR responses to electrical stimulation of the efferent vagus nerve (Mohamed et al. 1999). Therefore, the present findings that EB-treated rats exhibit compensatory increases in HR disproportionate to the decreased blood pressure after Isop also may be attributable to EB actions within the central baroreflex pathway.
Central effects of EB
During decreased blood pressure, the central baroreflex pathway is activated by unloading of arterial baroreceptors in the aortic arch and carotid sinus. Afferent fibres from these baroreceptors terminate on cell bodies of neurons in the NTS which integrate input from baroreceptors and, in turn, influence neural activity in the ventrolateral medulla. Hypotension decreases activity in GABAergic neurons in the CVLM that normally inhibit RVLM neurons, thereby increasing sympathetic outflow via disinhibition of excitatory projections from RVLM to sympathetic preganglionic neurons (Dampney & Horiuchi, 2003; Babic & Ciriello, 2004). Similar to other studies that examined Fos-IR after hypotension, we found that Isop increased Fos-IR in cNTS, mNTS and RVLM (Chan & Sawchenko, 1994; Dun et al. 1995; Graham et al. 1995; Curtis et al. 1999). We did not see an effect of Isop on Fos-IR in the CVLM, whereas others report increased Fos-IR in CVLM after hypotension (Graham et al. 1995; Curtis et al. 1999). Methodological differences including type of drug, route of administration, or duration of hypotension may account for the discrepancy; nonetheless, our observation is consistent with reports that hypotension reduces activity in CVLM neurons that send inhibitory projections to RVLM (Dampney & Horiuchi, 2003) and that Fos expression is not associated with decreased neuronal activity (Dragunow & Faull, 1989).
Increased Fos-IR in cNTS, mNTS, CVLM and RVLM after Isop did not differ among the groups, indicating no differences in afferent activation of the NTS or activation in CVLM and RVLM. In other words, neither EB treatment nor weight loss altered activity in the afferent terminal field or in the ‘preautonomic’ area of the hindbrain in response to Isop-induced hypotension. Although we did not record sympathetic nerve activity in this study, similar levels of Fos-IR in the RVLM after Isop suggest that sympathetic outflow also was comparable among the groups. Ultimately, then, activation in the efferent limb of the reflex pathway produced comparable reflex tachycardia in the three groups. However, the idea that typical patterns of activation in the afferent terminal field and in the sympathoexcitatory area lead to predictable and comparable changes in HR rests on the assumption that there were no differences in depressor responses (i.e. changes from baseline MAP) among the groups, which was not the case.
It is well known that hypotension increases Fos-IR in the NTS (Chan & Sawchenko, 1994; Curtis et al. 1999; Potts et al. 1999) and the effect requires intact arterial baroreceptors (Potts et al. 1999). Thus, the Fos-IR we observed in the NTS following Isop is likely to be attributable to unloading of arterial baroreceptors. We would therefore have expected that differences in depressor responses after Isop (Fig. 1) would produce different levels of Fos-IR in the NTS, as suggested from studies of hypotensive and non-hypotensive haemorrhage (Badoer et al. 1993; Buller et al. 1999). Clearly, however, there were no differences among EB, Oil, or WT Loss rats in the number of Fos-IR neurons in the NTS after Isop (Fig. 2). One possible explanation for this observation is that EB increases baroreceptor sensitivity such that afferent signals arising from peripheral baroreceptors are comparable among the groups and lead to similar activation in the NTS. An alternative possibility is that oestrogen increases the responsiveness of neurons in the NTS to the reduced baroreceptor input by classic steroid actions that lead to changes in protein expression, especially of neurotransmitter receptors (e.g. serotonin, noradrenaline). Consistent with this possibility, oestrogen receptors α (ERα) and β (ERβ) have been localized to the NTS (Schlenker & Hasen, 2006) and Fos-IR in the NTS produced by other stimuli (Flanagan-Cato et al. 1998) also is modulated by oestrogen. However, it is important to note that, without phenotypic labelling and neuroanatomical tract-tracing, we can say only that the number (Fig. 2) and general distribution (Fig. 4) of Fos-IR neurons in the NTS are comparable, not whether these are the same population of cells. It is possible that EB differentially affects subpopulations of neurons within the NTS or alters input to the NTS from other brain areas involved in cardiovascular regulation. Clearly, additional studies will be necessary to assess these possibilities, but the latter is an especially intriguing explanation for the comparable Fos-IR in the NTS given the broad distribution of oestrogen receptors throughout the central nervous system (McEwen, 2002) and the convergence of ascending and descending neural pathways at the NTS (Spyer, 1994).
The AP is a hindbrain circumventricular organ that, by virtue of reciprocal connections with the NTS, is among the brain areas that may influence activity in the NTS. Importantly, the AP itself receives baroreceptor input (Papas & Ferguson, 1991) and, due to the incomplete blood–brain barrier that is characteristic of circumventricular organs, also receives humoral signals pertaining to blood pressure and blood volume from circulating hormones such as AngII. The AP expresses angiotensin type 1 (AT1) receptors (Lenkei et al. 1998), which bind circulating AngII, thereby activating neurons that modify baroreflex responses (Hasser et al. 2000; Xue et al. 2003). In addition to receptors for AngII, ERα and ERβ have been localized to the AP (Shughrue et al. 1997), raising the possibility of interactions between AngII and EB. In fact, Pamidimukkala & Hay (2003a) recently reported that EB inhibited AngII activation of cultured AP neurons, suggesting that EB also inhibits AngII activation of AP neurons in vivo. These observations are of particular importance because Isop is a potent activator of the renin–angiotensin system (RAS) and leads to large increases in levels of circulating AngII (Stocker et al. 2000). Although our overall ANOVA did not reveal an interaction between group and drug, our planned comparisons revealed an effect of EB treatment on Fos-IR in the AP after Isop. Thus, the attenuation of Isop-induced Fos-IR in the AP of EB-treated rats observed in the present study may be attributable to EB inhibition of AP neural responses to AngII.
The present finding of attenuated Fos-IR in a circumventricular organ is similar to that in our previous study (Krause et al. 2006), in which EB treatment attenuated Isop-induced Fos-IR in a forebrain circumventricular organ, the subfornical organ (SFO). The decreased neural activation in that study was associated with EB reduction of a behavioural response, water intake. The central neural pathways that mediate AngII-stimulated water intake have not been defined as well as the baroreflex pathway, so it has been difficult to elaborate on the previous observation. On the other hand, many years of research on the neurochemistry, electrophysiology and neuroanatomy of the baroreflex pathway (Spyer, 1994) allow speculation about the functional implications of attenuated Fos-IR in the AP. AngII binds to and activates AP neurons that modulate activity of barosensitive neurons in the NTS (Cai et al. 1994), in part, via inhibitory GABAergic projections from the AP to the NTS (Pickel et al. 1989). Thus, EB attenuation of AngII-induced activity in the AP may reduce GABAergic inhibiton of the NTS and thereby contribute to normal baroreceptor activation of NTS after Isop despite the reduced depressor response in EB-treated rats.
As an additional parallel between attenuated AngII-induced activation in forebrain and hindbrain circumventricular organs, it is interesting that Fos-IR in the SFO after Isop and Isop-induced water intake were reduced by both EB treatment and weight loss. In contrast, EB treatment, but not weight loss, reduced Fos-IR in the AP after Isop and increased baroreflex HR responses to Isop. These observations raise the possibility of regional differences in the effects of EB (or weight loss) on AngII-mediated activation of circumventricular organs and in the consequences of such activation. But is there also a hindbrain–forebrain difference in the mechanisms by which such effects occur? Our previous study showed that both EB treatment and weight loss reduced AT1 receptor mRNA in the SFO (Krause et al. 2006). Similar analyses of AngII receptors in the AP remain to be done; nonetheless, these findings suggest that EB treatment (but not weight loss) reduces AT1 receptor mRNA in the AP which underlies the attenuation of Isop-induced Fos-IR in the AP and the concomitant enhancement of baroreflex HR responses.
We have focused on the role of AngII in modulating baroreflex responses largely because of the ability of Isop to stimulate RAS and produce large increases in circulating AngII (Stocker et al. 2000). However, hypotension also increases circulating levels of other hormones, including vasopressin. Vasopressin promotes important compensatory responses to decreased blood pressure such as fluid retention and peripheral vasoconstriction. In the present context, it is equally important that vasopressin augments baroreflex responses (Hasser et al. 2000), particularly since oestrogen increases both circulating levels of vasopressin (Peysner & Forsling, 1990) and vasopressin release stimulated by haemorrhage (Hartley et al. 2004). Vasopressin receptors have been localized to the AP and application of vasopressin to the AP alters activity in AP neurons that, in turn, influence neurons in the NTS that also receive baroreceptor input (Nakayama et al. 1997). Thus, alternative explanations for the present findings are that the augmented baroreflex HR response to Isop in EB-treated rats is attributable to oestrogen enhancement of vasopressin release or of AP responsiveness to vasopressin. However, several observations argue against these explanations. First, our previous study (Krause et al. 2006) showed no effect of EB treatment on Fos-IR in neurosecretory neurons in the hypothalamus. Admittedly, this measure is not equivalent to measuring plasma levels of vasopressin, but we are aware of no studies that have assessed the effect of hypotension on circulating vasopressin in EB-treated rats. Second, vasopressin augmentation of baroreflex responses is eliminated by lesions of the AP (Hasser et al. 1997). If the blunted activation in AP after Isop indicated by attenuated Fos-IR signifies decreased responsiveness of AP neurons to vasopressin, augmented baroreflex HR responses would not be expected. However, it should be noted that responses of AP neurons to vasopressin include both excitation and inhibition, as do subsequent responses in NTS barosensitive neurons (Cai et al. 1994). In this regard, vasopression has been reported to attenuate baroreflex-mediated excitatory HR responses in male rabbits (Luk et al. 1993); thus, additional studies may be necessary to further examine this issue.
For purposes of this analysis, we have concentrated primarily on neural activation in the hindbrain baroreflex pathway and interpreted our results assuming essentially linear, unidirectional information flow. In point of fact, the baroreflex circuit is considerably more complex: reciprocal connections among many of these areas allow feed-forward and feed-back modulation within the hindbrain; in addition, activity at several levels within the hindbrain circuit is modulated by descending input from forebrain areas which, in turn, receive ascending input from these same hindbrain areas (Spyer, 1994). In our previous study (Krause et al. 2006), EB treatment did not affect Isop-induced Fos-IR in the hypothalamic supraoptic (SON) or paraventricular nuclei (PVN) which, on the surface, might imply that differences in neural activation related to baroreflex responses are specific to the hindbrain baroreflex pathway. Interestingly, however, EB treatment also attenuated Fos-IR in the pontine lPBN, one of the major relays for cardiovascular information (Spyer, 1994). It is possible that this effect is attributable to indirect projections from AP to the forebrain via the lPBN (Leslie & Gwyn, 1984). In other words, the attenuated activity in AP after Isop may underlie the attenuation in the lPBN with as yet undetermined consequences for activity in forebrain areas other than the SON and PVN. Alternatively, the attenuated Fos-IR in the lPBN may have implications for activation in the hindbrain baroreflex circuit via descending projections from the lPBN to the NTS (Spyer, 1994). Stimulation of the lPBN decreases baroreflex HR responses (Len & Chan, 2001) and inhibits responses of barosensitive NTS neurons (Hamilton et al. 1981; Felder & Mifflin, 1988; Len & Chan, 1999) and, interestingly, Len & Chan (2001) recently reported that stimulation of lPBN elevates extracellular GABA in the commissural NTS. Thus, similar to the AP, EB attenuation of activity in the lPBN may disinhibit NTS neurons by reducing GABAergic input, thus contributing to normal baroreceptor activation of NTS after Isop despite the reduced depressor response in EB-treated rats. Work by Saleh & Connell (2000, 2003) also has pointed to oestrogen inhibition of neural activity in the PBN as an important factor in oestrogen effects on autonomic functions and, in conjunction with the localization of oestrogen receptors to the lPBN (McEwen, 2002), our findings are consistent with this idea. At present, however, the mechanism(s) underlying attenuated activity in the lPBN remain to be determined, as do the complex interactions among central areas that mediate baroreflex responses to hypotension.
Finally, we chose to examine the effect of EB on activity within the central baroreflex pathway using Fos immunocytochemistry for several important reasons. First, Fos immunolabelling has been extensively employed in studies of cardiovascular function (though primarily in male rats, e.g. Chan & Sawchenko, 1994; Dun et al. 1995; Graham et al. 1995; Potts et al. 1999); as a consequence, much is known about neural activation and patterns of Fos-IR in response to cardiovascular challenges. Second, this technique allows examination of multiple central areas in the same animal, a distinct advantage in analysing activity in a circuit in which a change in one area may, in turn, alter ‘down-stream’ parts of the pathway. Nonetheless, there are limitations to this technique. For example, Fos is not expressed during neuronal inhibition, which may be important in central baroreflex pathways. In fact, not all neurons express Fos; thus, the absence of Fos-IR must be interpreted cautiously. The advantages and limitations of Fos immunolabelling for understanding central pathways in cardiovascular function have been thoughtfully and thoroughly reviewed (e.g. Dampney & Horiuchi, 2003; Dragunow & Faull, 1989) and will not be exhaustively discussed here. However, a more general consideration merits further mention. Specifically, the classic steroidal effects of oestrogen suggest the possibility that the observed differences in central activation might be attributable to oestrogen-mediated changes in gene transcription. We saw no differences in baseline Fos-IR, which would argue against the effects being specific to oestrogen and independent of the hypotension; however, the more telling observation is that EB did not globally alter Fos-IR after Isop. In fact, the EB effect on Fos-IR was specific to two areas within the baroreflex pathway – the AP and the lPBN. Whether these selective differences are attributable to the genomic effects of oestrogen on gene transcription or to non-genomic effects on neuronal excitability remain to be determined.
In summary, the present study shows that EB reduces the depressor response to Isop in OVX rats, and that the reduction is accompanied by a compensatory increase in HR similar to that of rats in which MAP decreased nearly two-fold more. Although peripheral effects of EB also could contribute to this effect, the actions of EB to modify activation in the central baroreflex pathway may be important in the observed EB enhancement of baroreflex responses. We show for the first time that EB treatment selectively attenuates Fos-IR, an indication of neuronal activation, in the AP and lPBN after Isop. In contrast, Isop increased Fos-IR in the NTS and RVLM (see also Chan & Sawchenko, 1994; Dun et al. 1995; Graham et al. 1995; Curtis et al. 1999), areas associated with baroreceptor afferent input and sympathoexcitation, respectively, and the increase was not altered by EB. We also show for the first time that the body weight loss associated with EB treatment does not underlie these effects, as OVX rats that were food restricted to produce weight loss similar to that which occurs with EB treatment, did not differ from OVX rats that were not given EB.
Within the central nervous system, oestrogen acts at specific membrane-bound receptors to produce rapidly occurring nongenomic effects, in addition to the classic steroid effects to alter gene transcription via actions at intranuclear receptors. We cannot distinguish between these mechanisms based on the present observations; however, it seems clear that oestrogen effects on compensatory responses to cardiovascular challenges involve differences in neuronal activation in specific areas within central baroreflex pathways. We therefore propose that EB-mediated decrease in neural activation in AP and/or lPBN after Isop alters activation in the baroreflex circuit which ultimately results in enhanced compensatory tachycardia, notwithstanding reduced depressor responses. More specifically, we propose that EB attenuation of neural responses in the AP which, based on our previous studies showing that EB reduces AT1 receptor mRNA in a forebrain circumventricular organ, may be attributable to decreased responsiveness to circulating AngII, or in the lPBN which may be attributable to alterations in neural signals relayed to the lPBN, reduces GABAergic inhibition of the NTS. This disinhibition allows activation in the NTS despite reduced baroreceptor input and, in turn, leads to activation of the RVLM and thus to increased HR.
Acknowledgments
This work was supported by NIH DK063754 (EGK), DC006360 (KSC), and DC04785 (RJC).
References
- Babic T, Ciriello J. Medullary and spinal cord projections from cardiovascular responsive sites in the rostral ventromedial medulla. J Comp Neurol. 2004;469:391–412. doi: 10.1002/cne.11024. [DOI] [PubMed] [Google Scholar]
- Badoer E, McKinley MJ, Oldfield BJ, McAllen RM. A comparison of hypotensive and non-hypotensive hemorrhage on Fos expression in spinally projecting neurons of the paraventricular nucleus and rostral ventrolateral medulla. Brain Res. 1993;610:216–223. doi: 10.1016/0006-8993(93)91403-f. [DOI] [PubMed] [Google Scholar]
- Brown L, Hoong I, Doggrell SA. The heart as a target for oestrogens. Heart Lung Circ. 2000;9:113–125. doi: 10.1046/j.1444-2892.2000.009003113.x. [DOI] [PubMed] [Google Scholar]
- Buller KM, Smith DW, Day TA. Differential recruitment of hypothalamic neuroendocrine and ventrolateral medulla catecholamine cells by non-hypotensive and hypotensive hemorrhages. Brain Res. 1999;834:42–54. doi: 10.1016/s0006-8993(99)01539-5. [DOI] [PubMed] [Google Scholar]
- Cai Y, Hay M, Bishop VS. Stimulation of area postrema by vasopressin and angiotensin II modulates neuronal activity in the nucleus tractus solitarius. Brain Res. 1994;647:242–248. doi: 10.1016/0006-8993(94)91323-4. [DOI] [PubMed] [Google Scholar]
- Chan RK, Sawchenko PE. Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J Comp Neurol. 1994;348:433–460. doi: 10.1002/cne.903480309. [DOI] [PubMed] [Google Scholar]
- Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB. Oestrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen2.Lewis rat. Hypertension. 2003;42:781–786. doi: 10.1161/01.HYP.0000085210.66399.A3. [DOI] [PubMed] [Google Scholar]
- Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Oestrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by oestrogen. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. Erratum in J Clin Invest103, 1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric O, Susic D. Effect of isoproterenol on blood pressure and heart rate in different phases of the oestrous cycle. Endokrinologie. 1980;76:274–278. [PubMed] [Google Scholar]
- Contreras RJ, Wong DL, Henderson R, Curtis KS, Smith JC. High dietary NaCl early in development enhances mean arterial pressure of adult rats. Physiol Behav. 2000;71:173–181. doi: 10.1016/s0031-9384(00)00331-0. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Cunningham JT, Heesch CM. Fos expression in brain stem nuclei of pregnant rats after hydralazine-induced hypotension. Am J Physiol Regul Integr Comp Physiol. 1999;277:R532–R540. doi: 10.1152/ajpregu.1999.277.2.R532. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Krause EG, Contreras RJ. Fos expression in non-catecholaminergic neurons in medullary and pontine nuclei after volume depletion induced by polyethylene glycol. Brain Res. 2002;948:149–154. doi: 10.1016/s0006-8993(02)03051-2. [DOI] [PubMed] [Google Scholar]
- Da Costa LS, de Oliveira MA, Rubim VS, Wajngarten M, Aldrighi JM, Rosano GM, Neto CD, Gebara OC. Effects of hormone replacement therapy or raloxifene on ambulatory blood pressure and arterial stiffness in treated hypertensive postmenopausal women. Am J Cardiol. 2004;94:1453–1456. doi: 10.1016/j.amjcard.2004.07.153. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol. 2003;71:359–384. doi: 10.1016/j.pneurobio.2003.11.001. Review. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. Review. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Shen E, Tang H, Huang R, Chiu TH. c-fos expression as a marker of central cardiovascular neurons. Biol Signals. 1995;4:117–123. doi: 10.1159/000109431. Review. [DOI] [PubMed] [Google Scholar]
- el-Mas MM, Abdel-Rahman AA. Oestrogen enhances baroreflex control of heart rate in conscious ovariectomized rats. Can J Physiol Pharmacol. 1998;76:381–386. [PubMed] [Google Scholar]
- Emdin M, Gastaldelli A, Muscelli E, Macerata A, Natali A, Camastra S, Ferrannini E. Hyperinsulinemia and autonomic nervous system dysfunction in obesity: effects of weight loss. Circulation. 2001;103:513–519. doi: 10.1161/01.cir.103.4.513. [DOI] [PubMed] [Google Scholar]
- Felder RB, Mifflin SW. Modulation of carotid sinus afferent input to nucleus tractus solitarius by parabrachial nucleus stimulation. Circ Res. 1988;63:35–49. doi: 10.1161/01.res.63.1.35. [DOI] [PubMed] [Google Scholar]
- Flanagan-Cato LM, King JF, Blechman JG, O'Brien MP. Oestrogen reduces cholecystokinin-induced c-Fos expression in the rat brain. Neuroendocrinology. 1998;67:384–391. doi: 10.1159/000054337. [DOI] [PubMed] [Google Scholar]
- Graham JC, Hoffman GE, Sved AF. c-Fos expression in brain in response to hypotension and hypertension in conscious rats. J Auton Nerv Syst. 1995;55:92–104. doi: 10.1016/0165-1838(95)00032-s. [DOI] [PubMed] [Google Scholar]
- Hamilton RB, Ellenberger H, Liskowsky D, Schneiderman N. Parabrachial area as mediator of bradycardia in rabbits. J Auton Nerv Syst. 1981;4:261–281. doi: 10.1016/0165-1838(81)90049-7. [DOI] [PubMed] [Google Scholar]
- Harrison-Bernard LM, Raij L. Postmenopausal hypertension. Curr Hypertens Rep. 2000;2:202–207. doi: 10.1007/s11906-000-0083-2. Review. [DOI] [PubMed] [Google Scholar]
- Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity. Hypertension. 2003;2:1157–1163. doi: 10.1161/01.HYP.0000102180.13341.50. [DOI] [PubMed] [Google Scholar]
- Hartley DE, Dickson SL, Forsling ML. Plasma vasopressin concentrations and Fos protein expression in the supraoptic nucleus following osmotic stimulation or hypovolaemia in the ovariectomized rat: effect of oestradiol replacement. J Neuroendocrinol. 2004;16:191–197. doi: 10.1111/j.0953-8194.2004.01150.x. [DOI] [PubMed] [Google Scholar]
- Hasser EM, Bishop VS, Hay M. Interactions between vasopressin and baroreflex control of the sympathetic nervous system. Clin Exp Pharmacol Physiol. 1997;24:102–108. doi: 10.1111/j.1440-1681.1997.tb01791.x. Review. [DOI] [PubMed] [Google Scholar]
- Hasser EM, Cunningham JT, Sullivan MJ, Curtis KS, Blaine EH, Hay M. Area postrema and sympathetic nervous system effects of vasopressin and angiotensin II. Clin Exp Pharmacol Physiol. 2000;27:432–436. doi: 10.1046/j.1440-1681.2000.03261.x. Review. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Wilson PW. Risk factors that attenuate the female coronary disease advantage. Arch Intern Med. 1995;155:57–61. [PubMed] [Google Scholar]
- Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ. Oestrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav. 2003;79:267–274. doi: 10.1016/s0031-9384(03)00095-7. [DOI] [PubMed] [Google Scholar]
- Krause EG, Curtis KS, Stincic TL, Markle JP, Contreras RJ. Ooestrogen and weight loss decrease isoproterenol-induced Fos immunoreactivity and angiotensin type 1 mRNA in the subfornical organ of female rats. J Physiol. 2006;573:251–262. doi: 10.1113/jphysiol.2006.106740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Len WB, Chan JY. Glutamatergic projection to RVLM mediates suppression of reflex bradycardia by parabrachial nucleus. Am J Physiol Heart Circ Physiol. 1999;275:H1482–H1492. doi: 10.1152/ajpheart.1999.276.5.H1482. [DOI] [PubMed] [Google Scholar]
- Len WB, Chan JY. GABAergic neurotransmission at the nucleus tractus solitarii in the suppression of reflex bradycardia by parabrachial nucleus. Synapse. 2001;42:27–39. doi: 10.1002/syn.1096. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Neuroscience. 1998;82:827–841. doi: 10.1016/s0306-4522(97)00328-x. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Gwyn DG. Neuronal connections of the area postrema. Fed Proc. 1984;43:2941–2943. Review. [PubMed] [Google Scholar]
- Li HY, Bian JS, Kwan YW, Wong TM. Enhanced responses to 17β-estradiol in rat hearts treated with isoproterenol: involvement of a cyclic AMP-dependent pathway. J Pharmacol Exp Ther. 2000;293:592–598. [PubMed] [Google Scholar]
- Luk J, Ajaelo I, Wong V, Wong J, Chang D, Chou L, Reid IA. Role of V1 receptors on the baroreflex control of heart rate. Am J Physiol Regul Integr Comp Physiol. 1993;265:R524–R529. doi: 10.1152/ajpregu.1993.265.3.R524. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Coirini H, Schumaker M, Pfaff DW, McEwen BS, Schwartz-Giblin S. Ovarian steroid modulation of [3H]muscimol binding in the spinal cord of the rat. Brain Res. 1991;556:321–323. doi: 10.1016/0006-8993(91)90323-n. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Oestrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. Review. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. Review. [DOI] [PubMed] [Google Scholar]
- Mohamed MK, El-Mas MM, Abdel-Rahman AA. Oestrogen enhancement of baroreflex sensitivity is centrally mediated. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1030–R1037. doi: 10.1152/ajpregu.1999.276.4.R1030. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Takano Y, Eguchi K, Migita K, Saito R, Tsujimoto G, Kamiya H. Modulation of the arterial baroreceptor reflex by the vasopressin receptor in the area postrema of the hypertensive rats. Neurosci Lett. 1997;226:179–182. doi: 10.1016/s0304-3940(97)00274-7. [DOI] [PubMed] [Google Scholar]
- Pamidimukkala J, Hay M. 17β-Estradiol inhibits angiotensin II activation of area postrema neurons. Am J Physiol Heart Circ Physiol. 2003a;285:H1515–H1520. doi: 10.1152/ajpheart.00174.2003. [DOI] [PubMed] [Google Scholar]
- Pamidimukkala J, Hay M. Oestrogen modulation of baroreflex function in conscious mice. Am J Physiol Regul Integr Comp Physiol. 2003b;284:R983–R989. doi: 10.1152/ajpregu.00761.2001. [DOI] [PubMed] [Google Scholar]
- Pamidimukkala J, Xue B, Newton LG, Lubahn DB, Hay M. Oestrogen receptor-α mediates oestrogen facilitation of baroreflex heart rate responses in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H1063–H1070. doi: 10.1152/ajpheart.01163.2003. [DOI] [PubMed] [Google Scholar]
- Papas S, Ferguson AV. Electrophysiological evidence of baroreceptor input to area postrema. Am J Physiol Regul Integr Comp Physiol. 1991;261:R9–R13. doi: 10.1152/ajpregu.1991.261.1.R9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Peysner K, Forsling ML. Effect of ovariectomy and treatment with ovarian steroids on vasopressin release and fluid balance in the rat. J Endocrinol. 1990;124:277–284. doi: 10.1677/joe.0.1240277. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Milner TA. Cellular substrates for interactions between neurons containing phenylethanolamine N-methyltransferase and GABA in the nuclei of the solitary tracts. J Comp Neurol. 1989;286:243–259. doi: 10.1002/cne.902860209. [DOI] [PubMed] [Google Scholar]
- Potts PD, Polson JW, Hirooka Y, Dampney RA. Distribution of neurons projecting to the rostral ventrolateral medullary pressor region that are activated by sustained hypotension. Neuroscience. 1999;89:1319–1329. doi: 10.1016/s0306-4522(98)00399-6. [DOI] [PubMed] [Google Scholar]
- Rissanen P, Franssila-Kallunki A, Rissanen A. Cardiac parasympathetic activity is increased by weight loss in healthy obese women. Obes Res. 2001;9:637–643. doi: 10.1038/oby.2001.84. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ. 17β-Estradiol modulates baroreflex sensitivity and autonomic tone of female rats. J Auton Nerv Syst. 2000;80:148–161. doi: 10.1016/s0165-1838(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ. Oestrogen-induced autonomic effects are mediated by NMDA and GABAA receptors in the parabrachial nucleus. Brain Res. 2003;973:161–170. doi: 10.1016/s0006-8993(03)02432-6. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ, Saleh MC. Acute injection of 17β-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. Auton Neurosci. 2000a;84:78–88. doi: 10.1016/s1566-0702(00)00196-x. [DOI] [PubMed] [Google Scholar]
- Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central oestrogen injection in ovariectomized female rats. Brain Res. 2000b;879:105–114. doi: 10.1016/s0006-8993(00)02757-8. [DOI] [PubMed] [Google Scholar]
- Schlenker EH, Hasen SN. Sex-specific densities of oestrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res. 2006;1123:89–100. doi: 10.1016/j.brainres.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Pfaff DW, McEwen BS. Light-dark differences in behavioral sensitivity to oxytocin. Behav Neurosci. 1991;105:487–492. doi: 10.1037//0735-7044.105.3.487. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of oestrogen receptor-alpha and – beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Spyer KM. Annual review prize lecture. Central nervous mechanisms contributing to cardiovascular control. J Physiol. 1994;474:1–19. doi: 10.1113/jphysiol.1994.sp019997. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Sved AF, Stricker EM. Role of renin-angiotensin system in hypotension-evoked thirst: studies with hydralazine. Am J Physiol Regul Integr Comp Physiol. 2000;279:R576–R585. doi: 10.1152/ajpregu.2000.279.2.R576. [DOI] [PubMed] [Google Scholar]
- Thawornkaiwong A, Preawnim S, Wattanapermpool J. Upregulation of β1-adrenergic receptors in ovariectomized rat hearts. Life Sci. 2003;72:1813–1824. doi: 10.1016/s0024-3205(02)02473-6. [DOI] [PubMed] [Google Scholar]
- Tremollieres FA, Pouilles JM, Ribot CA. A prospective two-year study of progestin given alone in postmenopausal women: effect on lipid and metabolic parameters. Am J Obstet Gynecol. 1995;173:85–89. doi: 10.1016/0002-9378(95)90174-4. [DOI] [PubMed] [Google Scholar]
- Xue B, Gole H, Pamidimukkala J, Hay M. Role of the area postrema in angiotensin II modulation of baroreflex control of heart rate in conscious mice. Am J Physiol Heart Circ Physiol. 2003;284:H1003–H1007. doi: 10.1152/ajpheart.00793.2002. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Saleh MC. Inhibitory effect of 17beta-estradiol in the parabrachial nucleus is mediated by GABA. Brain Res. 2001;911:116–124. doi: 10.1016/s0006-8993(01)02699-3. [DOI] [PubMed] [Google Scholar]