Abstract
The modulation of synaptic transmission by presynaptic ionotropic and metabotropic receptors is an important means to control and dynamically adjust synaptic strength. Even though synaptic transmission and plasticity at the hippocampal mossy fibre synapse are tightly controlled by presynaptic receptors, little is known about the downstream signalling mechanisms and targets of the different receptor systems. In the present study, we identified the cellular signalling cascade by which adenosine modulates mossy fibre synaptic transmission. By means of electrophysiological and optical recording techniques, we found that adenosine activates presynaptic A1 receptors and reduces Ca2+ influx into mossy fibre terminals. Ca2+ currents are directly modulated via a membrane-delimited pathway and the reduction of presynaptic Ca2+ influx can explain the inhibition of synaptic transmission. Specifically, we found that adenosine modulates both P/Q- and N-type presynaptic voltage-dependent Ca2+ channels and thereby controls transmitter release at the mossy fibre synapse.
Synaptic communication between neurons is under precise modulatory control. While only a subset of synapses expresses presynaptic ionotropic receptors, virtually all synaptic terminals in the central nervous system are modulated by metabotropic receptors (Miller, 1998; MacDermott et al. 1999). It is well known that metabotropic receptors are linked to various signal transduction mechanisms via G-proteins. Activation of any of the following four pathways can, in principle, lead to an inhibition of synaptic transmission; however, the specific mechanism differs among synapses (Thompson et al. 1993; Wu & Saggau, 1997).
(1) The cyclic AMP/protein kinase A (cAMP–PKA) cascade has been shown to be involved in inhibition of glutamatergic as well as GABAergic synaptic transmission by adenosine and metabotropic glutamate receptor (mGluR) activation, respectively (Kamiya & Yamamoto, 1997; Bagley et al. 1999). (2) A large number of G-protein-coupled receptors can also activate inwardly rectifying K+ channels (GIRKs) and thereby postsynaptically hyperpolarize neurons. A similar mechanism had also been proposed for presynaptic inhibition via G-protein-coupled receptors, which however, seems unlikely because transgenic animals lacking the GIRK2 gene exhibit normal presynaptic inhibition (Luscher et al. 1997). (3) An alteration of presynaptic Ca2+ influx by direct modulation of voltage-dependent Ca2+ channels (VDCCs) can also influence synaptic transmission. The detailed mechanism of how Ca2+ channels are influenced by neuromodulators has been mainly tested in expression systems and somatic recordings (Bean, 1989; Herlitze et al. 1996). For GABAB receptors, a membrane-delimited pathway has been demonstrated in the giant presynaptic terminal of the calyx of Held (Takahashi et al. 1998; Kajikawa et al. 2001). Whether the same mechanism holds true for adenosine has not been tested so far in central presynaptic nerve terminals. (4) A direct influence of neuromodulators on the release machinery downstream of Ca2+ influx has also been inferred. For example, inhibition of quantal transmitter release by adenosine and GABAB receptors in the absence of Ca2+ influx has been reported in cultured hippocampal pyramidal cells (Scanziani et al. 1992; Scholz & Miller, 1992). Recent reports have also demonstrated that the inhibitory effect of serotonin on transmitter release at the reticulospinal–motoneuron synapse of lampreys is entirely mediated downstream of Ca2+ entry (Blackmer et al. 2001; Gerachshenko et al. 2005).
At the hippocampal mossy fibre synapse, transmitter release, and in some cases also plasticity, is governed by various ionotropic and metabotropic presynaptic receptor systems, including kainate, opioid, GABAA and mGluRs (Scanziani et al. 1992; Kamiya & Ozawa, 1998, 1999; Ruiz et al. 2003; Nicoll & Schmitz, 2005). The neuromodulator adenosine has been shown to effectively depress mossy fibre synaptic transmission (Moore et al. 2003; Fedele et al. 2005; Kukley et al. 2005). However, the downstream transduction pathway for adenosine-mediated inhibition remains unclear.
In the present study, we used electrophysiological and optical recording techniques to identify the mechanism of transmitter release modulation by adenosine at the hippocampal mossy fibre synapse. We have found that adenosine suppresses synaptic transmission by reducing presynaptic Ca2+ influx through activation of presynaptic A1 receptors. In direct bouton recordings we have demonstrated that adenosine most probably acts via a membrane-delimited pathway. Both N- and P/Q-type Ca2+ channels, which contribute to Ca2+ influx for transmitter release at this synapse, are modulated by adenosine. By determining the relationship between presynaptic Ca2+ influx and postsynaptic response amplitudes we have found that the mechanism of action of adenosine on synaptic transmission at the mossy fibre synapse can be explained by modulation of N- and P/Q-type voltage-dependent Ca2+ channels.
Methods
Preparation
For field potential, somatic whole-cell and Ca2+-imaging experiments, hippocampal slices were prepared from C57/Bl6 mice (postnatal days 16–42) as previously described (Schmitz et al. 2003). All experiments were performed according to the animal welfare guidelines of the Charité, Universitätsmedizin Berlin. In brief, the animals were anaesthetized with diethylether, then decapitated and the brains removed. Tissue blocks containing the subicular area and hippocampus were mounted on a Vibratome (Campden Instruments MA752 or DSK DTK-1000) in a chamber filled with ice-cold artificial cerebrospinal fluid (ACSF), containing (mm): NaCl 50 (87 for whole-cell recordings), sucrose 150 (75 for whole-cell recordings), NaHCO3 25, KCl 2.5, NaH2PO4 1, CaCl2 0.5, MgSO4 7 and glucose 10; pH 7.4. Slices were cut at a thickness of 300–400 μm and heated to 35°C for 30 min. The slices were then cooled to room temperature (∼22°C) and transferred to ACSF containing (mm): NaCl 124, NaHCO3 26, glucose 10, KCl 3, CaCl2 2.5, MgSO4 1.3 and NaH2PO4 1.25. All ACSFs were equilibrated with 95% O2–5% CO2. The slices were stored in a submerged chamber for 1–7 h before being transferred to the recording chamber, where they were perfused with ACSF at a rate of 3–4 ml min−1.
For direct recordings from mossy fibre boutons, hippocampal slices were prepared as previously described (Bischofberger et al. 2006). In brief, transverse 300 μm thick slices were cut from the hippocampus of 21- to 24-day-old Wistar rats with a custom-built vibratome (Geiger et al. 2002). For the dissection and storage of slices, a saline solution was used containing (mm); NaCl 64, NaHCO3 25, glucose 10, sucrose 120, KCl 2.5, NaH2PO4 1.25, CaCl2 0.5 and MgCl2 7.
Field potential and postsynaptic whole-cell recordings
Whole-cell voltage-clamp and field excitatory postsynaptic potential (fEPSP) recordings were performed with an Axopatch 700A (Axon Instruments, Union City, CA, USA). Data were digitized (National Instruments BNC-2090) at 5–10 kHz and recorded and analysed with custom-made software in IGOR Pro (WaveMetrics Inc., OR, USA). Patch electrodes (with electrode resistances ranging from 3 to 6 MΩ) were filled with (mm): caesium gluconate 117.5 and 5-QX-314, CsCl 2.5, TEA 10, NaCl 8, Hepes 10, EGTA 0.2, MgATP 4, Na3GTP 0.3 and QX-3145; pH adjusted to 7.3 with CsOH. Series resistance was 14 ± 4 MΩ, and was continuously monitored during the recordings and not allowed to vary by more than 25% during the course of the experiment. No series resistance compensation was used. Field potential recordings were performed with low-resistance patch pipettes filled with external solution placed in stratum lucidum. All recordings were performed at room temperature.
Mossy fibres were extracellularly stimulated with patch pipettes filled with external solution placed in the granule cell layer or in the hilus region (see Supplemental material Fig. 1A). The mossy fibre origin of recorded signals was routinely verified by the following procedures, and experiments were discarded if they did not match the criteria. (1) Both in field potential and whole-cell recordings, synaptic response amplitudes were largely increased when the ‘frequency facilitation’ protocol was applied (i.e. a switch in stimulation frequency from 0.05 (0.1 in whole-cell recordings) to 1 Hz (see Supplemental material Fig. 1B and D)). fEPSPs were only accepted if responses showed more than 400% synaptic facilitation. (2) In addition, application of the group II mGluR agonist (2S,2′R)-2-(2′,3′-Dicarboxycyclopropyl)glycine (DCGIV) (0.5–1 μm) at the end of the experiment had to block synaptic transmission. In field potential recordings, synaptic responses were generally completely blocked (see Supplemental material Fig. 1C, n= 15). In a few cases, a repetitive fibre volley was detectable, which was still present with subsequent application of 2,3-Dioxo-6-nitro-1,2,3,4 tetrahydrobenzo[F] quinoxaline-7-sulfonamide disodium salt (NBQX) and could then be subtracted from every trace. In whole-cell recordings, an increase of synaptic failures from 11 ± 3% under control conditions to 86 ± 7% after application of DCGIV was obtained, which was also reflected by an inhibition of synaptic transmission by 94 ± 3% (see Supplemental material Fig. 1E, n= 6).
Presynaptic patch-clamp recordings
Mossy fibre boutons in stratum lucidum of the hippocampal CA3 region were visually identified by their size and location (3–4 μm apparent diameter) using infrared differential interference contrast videomicroscopy. Furthermore, identification was confirmed by the low capacitance (∼1 pF) and the high input resistance (> 1 GΩ; Bischofberger et al. 2006). Patch pipettes were pulled from borosilicate glass tubing (outer diameter, 2.0 mm; wall thickness, 0.7 mm; Hilgenberg, Malsfeld, Germany). Whole-cell voltage-clamp recordings were performed with an Axopatch 200A amplifier. Current signals were filtered at 5 kHz, with a four-pole low-pass Bessel filter and sampled at a frequency of 20 kHz using a CED 1401plus interface (Cambridge Electronic Design, Cambridge, UK) connected to a personal computer. Pulses were generated using custom-made software running under IGOR Pro, which allowed us to apply a previously recorded action potential (AP) as voltage-clamp command. The presynaptic AP waveform was taken from Fig. 5A by Bischofberger et al. (2002). For whole-cell voltage-clamp recordings, we used 5–8 MΩ pipettes filled with a solution containing (mm): CsCl 135, EGTA 10, MgCl2 4, Na2ATP 4, NaGTP 0.3, Disodium phosphocreatine 10 and Hepes 10; pH was adjusted to 7.3 with CsOH. The bath solution contained (mm): NaCl 105, NaHCO3 25, glucose 25, KCl 2.5, NaH2PO4 1.25, CaCl2 2, MgCl2 1 and 2 μm tetrodotoxin (TTX), and 20 mm tetraethylammonium chloride and 5 mm 4-aminopyridine to block voltage-gated Na+ and K+ channels, respectively. Leak and capacitive currents were subtracted using two P/-4 sequences within each protocol. Current traces shown in the figures represent the averages of 5–10 sweeps. The series resistance (typically between 20 and 50 MΩ) was compensated by 80–90% with a lag of 20–30 μs. In some recordings, unclamped tail currents with very slow time course were apparent (these currents may have been generated in neighbouring boutons). These recordings were discarded. All Ca2+ current recordings were done at room temperature.
Figure 5. Adenosine inhibits calcium influx into presynaptic mossy fibre boutons.
A, whole-cell recordings of mossy fibre boutons were performed under visual guidance. Arrows point to mossy fibre boutons, pyr indicates pyramidal neuron. B, application of a 30 ms depolarizing voltage step elicits stable calcium currents in direct voltage-clamp recordings of mossy fibre boutons. Perfusion with 100 μm adenosine leads to a pronounced reduction in amplitude of this calcium current. Traces show averages of five to 10 sweeps each. C, time course of experiment in A. D, adenosine causes a slowing of the activation time constant of mossy fibre calcium currents. Traces depict averages of seven sweeps each (black) and weighted double-exponential fits of the data (grey) for control and in the presence of adenosine. E, summary of reduction of calcium current amplitude and modulation of activation time constant for n= 5 boutons.
Fluorescence measurements
Mossy fibres were locally labelled with a pressure stream of the low-affinity calcium indicator Magnesium Green AM (100 μm, Kd 6 μm; Molecular Probes, Eugene, OR, USA) for photodiode measurements (Regehr & Tank, 1991; Breustedt et al. 2003) or with 200 μm of the calcium indicator Oregon Green BAPTA 1 AM (Kd, 170 nm; Molecular Probes) dissolved in 20% Pluronic/DMSO for confocal imaging experiments. The final DMSO concentration in the injection solution was 5%. Recordings were started 2–3 h after labelling. Epifluorescence was measured with a single photodiode from a spot more than 200 μm away from the loading site with an Olympus LumPlan FI 60 × 0.9 NA water-immersion objective. The signals from the photodiode were low-pass filtered with 1 kHz, digitized at 5 kHz and captured with IGOR Pro software. The change in fluorescence intensity (ΔF) relative to the baseline intensity of fluorescence (F) was calculated as ΔF/F.
For time-lapse confocal recordings, a Yokogawa CSU-22 spinning disc confocal system (BFI Optilas, Puchheim, Germany) was deployed with an Olympus BX-51WI upright microscope and a RedShirt NeuroCCD-SMQ camera (Life Imaging Services, Reinach, Switzerland). Excitation was provided at 488 nm by a Coherent Sapphire 488–50 Laser (Coherent, Utrecht, Netherlands). With the Olympus LumPlan FI 60 × 0.9 NA water immersion objective, the lateral pixel size was 0.4 μm. With the same objective, the pinhole size of the Yokogawa CSU-22 spinning disc confocal corresponded to 1.18 Airy units at 520 nm emission wavelength. Full frames were recorded at 125 Hz. Only boutons in the periphery of the stained structures were chosen to circumvent any crosstalk between adjacent pinholes in thick fluorescent specimen (Egner & Hell, 2005). Boutons were discarded if there was an increase in the exponential decay time constant of the Ca2+ transient > 20% over the time of the recording. For morphological representation of the stained mossy fibre boutons, we switched to a Hamamatsu ORCA ER CCD camera. The lateral pixel size was 0.32 and 0.11 μm at 20 × and 60 × respectively. A frame size of 1344 pixels × 1024 pixels was collected at 1 Hz. Images of single boutons were digitally deconvoluted using the Huygens essential software (SVI imaging, Hilversum, Netherlands).
Analysis and statistics
In presynaptic bouton recordings, Ca2+ currents were evoked by 30 ms voltage steps to 0 mV from a holding potential of −80 mV and current amplitude was measured as the mean current value during the last 20 ms of the voltage pulse. The activation time course of the Ca2+ current I(t) was fitted with either a mono-exponential function:
| (1) |
or with the sum of two exponentials:
| (2) |
with delayed onset accounting for the delay (δ) in Ca2+ channel activation (Bischofberger et al. 2002). According to previous results, δ was constrained ∼200–300 μs. To account for the additional small delay introduced by the lag of series resistance compensation (20–30 μs) and by electronic filtering (5 kHz), the current trace was shifted in time by 100 μs. With this shift, the end of the rectangular current pulse corresponded to the time when the tail current rose to ∼50% of its peak amplitude. The fitted peak current amplitudes (I0, I1 and I2) were either given as absolute values or as relative amplitude contributions A1=I1/(I1+ I2) and A2=I2/(I1+ I2). Accordingly, the amplitude-weighted sum of the activation time constants τ was calculated as τw=A1×τ1+A2×τ2. Time-course fitting was performed using IGOR Pro and coefficient values were adjusted by non-linear least-square fitting based on a built-in Levenberg–Marquardt algorithm.
In field potential and optical recordings, dose–response curves were fitted with a logistic equation of the form:
| (3) |
with Af being the maximum effect of the drug in percentage and y being the normalized fEPSP or ΔF/F (Origin, OriginLab Corp., Northampton, MA, USA). Throughout the text, average values are expressed as mean ±s.e.m. Statistical significance was tested with a Student's paired t test (an unpaired t-test was used for the data from figure 8), except for the experiments with forskolin, and the PKA agonist and antagonist (Fig. 2), where an ANOVA test was used to compare the three different test groups against the control group with CPA alone, followed by Dunnett's multiple comparison test (Prism software package, Graph Pad; El Camino Real, CA, USA).
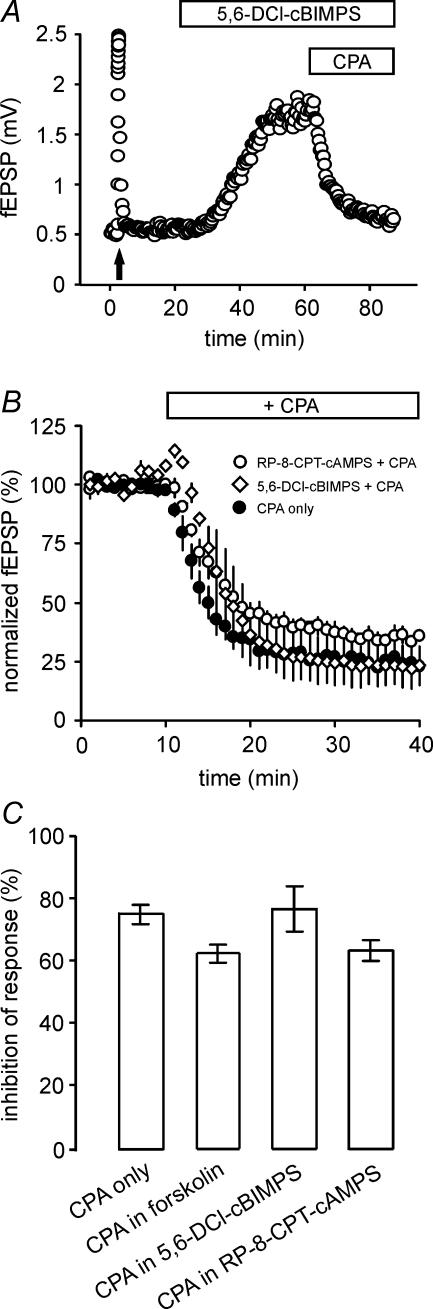
Figure 2. Inhibition of mossy fibre synaptic transmission by adenosine is not mediated via modulation of the cAMP–PKA pathway.
A, pre-application of the membrane-permeable cAMP analogue 5,6-DCl-cBIMPS (100 μm) does not occlude CPA-mediated inhibition of fEPSP amplitudes. Arrow points to frequency facilitation. B, several experiments as in A demonstrate comparable effectivity of A1 receptor agonism by CPA following pre-application of either 5,6-DCl-cBIMPS (n= 4) or the PKA inhibitor RP-8-CPT-cAMPS (100 μm; n= 5). C, summary of results from B and similar experiments with pre-application of forskolin (25 μm; n= 8) to activate adenylyl cyclase. No significant difference in inhibition was found between control values with CPA application alone and the three experimental groups exposed to the different pretreatments (one-way ANOVA with Dunnett's multiple comparison post hoc test, P > 0.05 for all groups).
Drugs
Adenosine, 4-[2-[[6-amino-9-(N-ethyl-β-d-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepro-panoicacid-hydrochloride (CGS), N6-cyclopentyladenosine (CPA), d(−)-2-amino-5-phosphonopentanoic acid (d-AP5), DCGIV, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), forskolin, 1-[2-chloro-6-[[(idophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamid (IB-MECA) and NBQX were obtained from Tocris International (via Biotrend, Cologne, Germany). Cadmium and nickel were from Sigma-Aldrich (Munich, Germany), the toxins ω-agatoxin IVA and ω-conotoxin GVIA were from Peptides International (Louisville KY, USA). Cytochrome c (0.1 mg ml−1) and bovine serum albumine (0.1%) were added to solutions containing toxins. 5,6-Dichloro-1-β-d-ribofuranosylbenzimidazole - 3′, 5′ - cyclic monophosphorothioate (Sp-5,6-DCl-cBIMPS) and 8-(4-chlorophenylthio) adenosine- 3′, 5′- cyclic monophosphorothioate, Rp-isomer (RP-8-CPT-cAMPS) were purchased from BIOLOG Life Science Institute (Bremen, Germany). After each experiment involving the use of DPCPX, the complete perfusion system was exchanged, in order to prevent accumulation of the drug in the tubing, and the recording chamber was cleaned with ethanol.
Results
Adenosine decreases mossy fibre synaptic transmission via A1 receptors
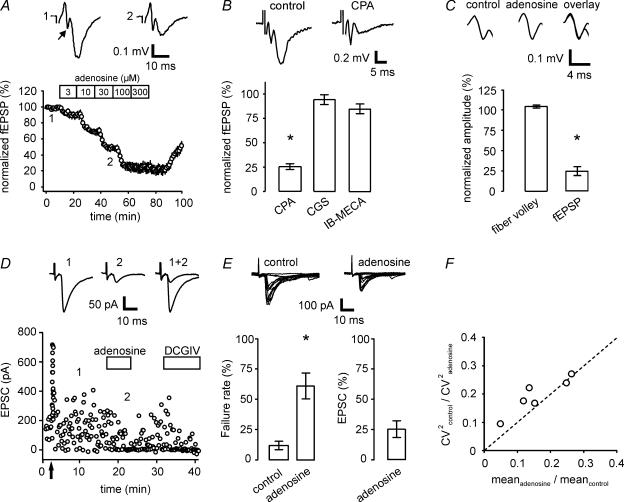
To determine the impact of adenosine on postsynaptic responses, we selectively stimulated mossy fibres with an extracellular electrode and recorded fEPSPs in stratum lucidum of area CA3 (see Supplemental material Fig. 1). Adenosine decreased the fEPSP amplitude in a dose-dependent and reversible manner (Fig. 1A). Maximum suppression was obtained with a concentration of 100 to 300 μm adenosine, reducing the fEPSP to 25.0 ± 5.5% and 22.8 ± 5.9% of control values, respectively. Fitting the dose–response curve with a logistic equation revealed an apparent EC50 of 18 μm for adenosine.
Figure 1. Adenosine inhibits mossy fibre synaptic transmission via presynaptic A1 receptors.
A, application of adenosine decreases fEPSP amplitudes in a concentration-dependent manner. Mean responses are shown with sequential administration of increasing concentrations of adenosine (n= 4 experiments). Averages of 10 sweeps each are depicted in the top row under control conditions and in the presence of 30 μm adenosine for a single example experiment. Arrow points to fibre volley. B, the A1 receptor-specific agonist CPA (300 nm) decreases mossy fibre fEPSP amplitudes to 25% of control values (n= 6). Upper traces depict averages of 10 sweeps each of a representative experiment. In contrast to CPA, application of the specific A2A receptor-specific agonist CGS (200 nm, n= 5) or the A3 receptor-specific agonist IB-MECA (200 nm, n= 4) does not lead to a significant reduction in mossy fibre synaptic transmission. C, adenosine (100 μm) leaves the presynaptic fibre volley unchanged while reducing postsynaptic field potential amplitudes to 25% (n= 5). Representative example of fibre volley in upper traces (average of 10 sweeps each). D, in an example of whole-cell recording of a CA3 pyramidal cell, addition of adenosine (100 μm) leads to reversible reduction of mossy fibre-evoked EPSC amplitude and increased occurrence of failures of transmission. Upper traces are averages of 10 sweeps each. Mossy fibre origin of signal was verified by induction of frequency facilitation (change from 0.1 to 1 Hz stimulation frequency, arrow) and application of DCGIV (1 μm) at the end of the experiment. E, summary of six experiments as in D. Application of adenosine increases failure rate from 11.7% to 60.8%, while decreasing mean EPSC amplitude to 25.4% of control values (including failures; 64.5% excluding failures). Overlays of 20 individual sweeps each are shown for control and in the presence of adenosine in top row. F, the change in the squared coefficient of variation in control versus in the presence of adenosine shows a linear dependence on the change in the mean response amplitude.
The adenosine receptor subtypes A1, A2A and A3 have been shown to be expressed in the hippocampus (Fredholm et al. 2001). Here, the A1 receptor-specific agonist CPA (300 nm) reduced fEPSP amplitudes to 24.5 ± 2.9% of control, whereas application of the A2A agonist CGS (200 nm) or the A3 selective agonist IB-MECA (200 nm) did not result in a significant suppression of the fEPSP (CGS, 94.6 ± 5.6%; IB-MECA, 84.6% ± 4.9; Fig. 1B). As reported before (Moore et al. 2003; Fedele et al. 2005; but see Kukley et al. 2005), application of the specific A1 receptor antagonist DPCPX (3, 30 and 200 nm, n= 5, 4 and 5) led to large increases in synaptic transmission (232 ± 26%, 298 ± 39% and 403.3 ± 54.7%, respectively). In the presence of DPCPX, reduction of the fEPSP amplitude by adenosine was fully blocked. Thus, inhibition of synaptic transmission by adenosine can be entirely explained by activation of the A1 receptor subtype, as the application of CPA mimicked and treatment with DPCPX blocked the effect of adenosine.
Adenosine decreases mossy fibre transmission through a presynaptic mechanism
The observed reduction of fEPSP amplitude by adenosine could be caused by (1) a presynaptic reduction of transmitter release, (2) a postsynaptic effect (e.g. through hyperpolarization of the postsynaptic membrane), or (3) both (Proctor & Dunwiddie, 1987). To discriminate between these scenarios we performed the following sets of experiments.
A presynaptic decrease in transmitter release probability will result in a reduced postsynaptic response but is also expected to increase short-term facilitation (Zucker & Regehr, 2002). Here, application of adenosine led to a significant increase in the fifth to first response ratio from 4.4 ± 0.5 to 6.2 ± 1.1 of mossy fibre fEPSP responses to short stimulus trains of five pulses each with interstimulus intervals of 50 ms (see also Toth & McBain, 2000), indicating a presynaptic locus of adenosine action.
Mechanistically, a change in the presynaptic AP waveform could also exert a direct influence on transmitter release (Sabatini & Regehr, 1997; Qian & Saggau, 1999) by changing Ca2+ influx into the presynaptic terminal. In our experiments, however, application of adenosine did not lead to any changes in the extracellularly measured fibre volley, which is a sensitive measure of the presynaptic AP waveform. Fibre volley amplitude and kinetics were unchanged (Fig. 1C), arguing against an adenosine-mediated change of the AP waveform.
A reduction in presynaptic transmitter release is expected to result in a higher incidence of synaptic failures (i.e. presynaptic stimulation without successful synaptic transmission). Therefore, we obtained whole-cell recordings from CA3 pyramidal neurons while applying low-intensity extracellular stimulation of mossy fibres (Fig. 1D). Application of adenosine led to a clear and reversible reduction in evoked EPSC amplitude to 25.4 ± 6.9% of control values. In agreement with this, the rate of failures was significantly increased from 11.7 ± 2.9% under control conditions to 60.8 ± 10.8% in the presence of adenosine (Fig. 1E, P < 0.005, paired t test). Furthermore, the ratio of the squared coefficient of variation (CV2control/CV2adenosine) scaled linearly with the change of the mean response upon application of adenosine (meanadenosine/meancontrol), again arguing for a presynaptic locus of action (Fig. 1F) (Faber & Korn, 1991).
All whole-cell recordings were performed using caesium- and QX314-based intracellular solution to block ion flow through postsynaptic GIRKs, which could have contributed to the reduction in synaptic response amplitudes (Trussell & Jackson, 1985; Proctor & Dunwiddie, 1987). As we did not find any significant change in the holding current upon application of adenosine (−6.8 ± 3.1 pA) and the reduction of response amplitudes is comparable to data from the fEPSP measurements, we can rule out a significant contribution of postsynaptic GIRKs in the action of adenosine.
From the cumulative evidence we conclude that adenosine decreases mossy fibre synaptic transmission entirely through presynaptically localized receptors.
The cAMP–PKA cascade is not required for mediation of the adenosine effects on transmission
Adenosine receptors are coupled to different signal transduction cascades and effector mechanisms (Dunwiddie & Masino, 2001). Specifically the cAMP–PKA signalling pathway has been implicated in mediating some of the neuromodulatory effects of adenosine at the mossy fibre–CA3 pyramidal cell synapse (Kamiya & Yamamoto, 1997; Maccaferri et al. 1998). In our experiments, application of the cAMP analogue Sp-5,6-DCl-cBIMPS (100 μm) led to a strong enhancement of mossy fibre synaptic transmission (Fig. 2A). However, subsequent addition of the A1 receptor agonist CPA (300 nm) with the cAMP analogue still present, reduced the fEPSP to on average 23.4 ± 7.3% (Fig. 2B and C, n= 4). The same holds true for initial application of the adenylyl cyclase activator forskolin (25 μm), where response amplitudes were afterwards reduced to 37.9 ± 3% by CPA (Fig. 2C; n= 8). The latter values are normalized with respect to the fEPSP amplitude in the presence of the cAMP analogue or forskolin alone. Similarly, when we first incubated the slices with the PKA antagonist RP-8-CPT-cAMPS (100 μm, 3–7 h) and subsequently added CPA, the fEPSP was again suppressed to 36.8 ± 3.3% (Fig. 2B and C, n= 5). The effectiveness of incubation with RP-8-CPT-cAMPS could be verified by a significantly reduced impact of forskolin in preincubated slices compared to a control group (increase of response amplitude: control, 386 ± 73%; preincubated slices, 201 ± 11%; n= 4). There was no statistically significant difference between the reduction of fEPSPs with CPA alone or in the presence of the cAMP modulators (Fig. 2C; ANOVA, with Dunnett's multiple comparison test). As modulation of the cAMP–PKA pathway did not interfere with the ability of CPA to depress mossy fibre synaptic transmission, we conclude that this signalling cascade is not required for the effect of adenosine on basal transmitter release.
Adenosine reduces presynaptic Ca2+ influx
Next, we tested whether a reduction of Ca2+ influx into the presynaptic terminal is involved in adenosine-mediated modulation of transmission at the mossy fibre synapse. To probe this question we made use of an optical recording method (Regehr & Tank, 1991; Breustedt et al. 2003). Mossy fibres were selectively labelled with a fluorescent calcium indicator dye and Ca2+ influx was elicited by a single extracellular stimulus in the hilus of the dentate gyrus (Fig. 3A). Application of adenosine (100 μm) or CPA (300 nm) reduced Ca2+ influx to 74 ± 5% (n= 14) and 76 ± 3% (n= 6) of control, respectively (Fig. 3A and B). In a further step, we obtained a dose–response curve for the reduction of presynaptic Ca2+ influx by applying increasing concentrations of adenosine (3, 10, 30 and 100 μm), leading to a reduction in Ca2+ influx to 99 ± 1%, 91 ± 4%, 78 ± 4% and 74 ± 5%, respectively (Fig. 3C). Conversely, application of the A1 receptor antagonist DPCPX (3, 30 and 200 nm) dose-dependently increased the Ca2+ transients (to 137 ± 10%, 150 ± 7% and 177 ± 27%, respectively; n= 5 each).
Figure 3. Adenosine reduces calcium transients in mossy fibre terminals.
A, time course of an example experiment showing that adenosine (100 μm) reversibly reduces presynaptic calcium influx. Subsequent application of the specific A1 receptor agonist CPA demonstrates that both agonists affect calcium influx to the same extent. Traces correspond to the numbers in the graph below. B, summary plot for n= 7 experiments illustrating the effect of adenosine on calcium transients. C, the graph depicts the dose–response relationship of the reduction in calcium influx for different adenosine concentrations (3, 10, 30 and 100 μm, n= 4–14 experiments, •). To facilitate comparison, the data for the postsynaptic fEPSP reduction by adenosine from Fig. 1A are partially replotted (○).
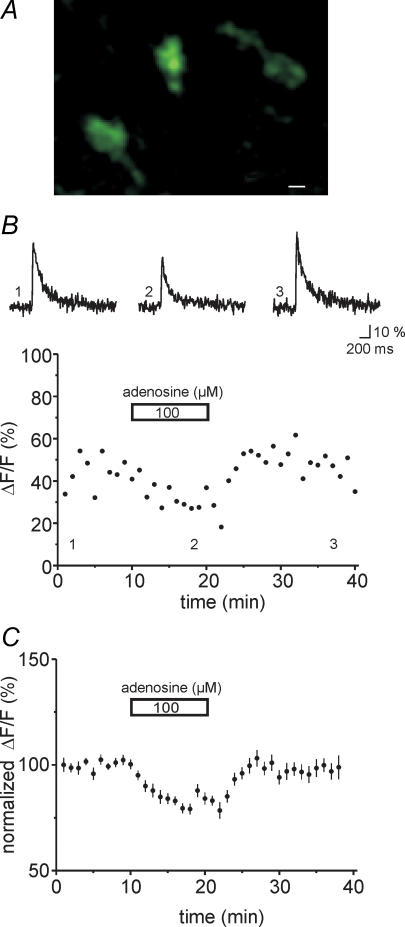
Because those experiments were performed with a single photodiode which lacks spatial resolution, we went on to record Ca2+ transients with a Nipkow spinning disc confocal system in identified single mossy fibre boutons (see Methods). The parallel scanning pattern permits a higher full-frame acquisition speed, so a sensitive CCD camera can be used as a sensor. Parallel scanning also results in longer pixel dwell times, distributing the integrated illumination differently over time and space as compared to single-point scanning. These two factors result in a lower phototoxicity at a given signal-to-noise rate when compared to conventional single-point scanning confocal systems (Wang et al. 2005), making this system especially suited for long-term recordings. Boutons could be readily resolved with this approach (Fig. 4A). Again, application of adenosine (100 μm) reduced Ca2+ transients in the mossy fibre boutons to 82 ± 1% of control (Fig. 4B and C, n= 22 boutons, six slices) in good agreement with the photodiode measurements. Ca2+ transients completely recovered upon washout of adenosine.
Figure 4. Adenosine decreases calcium transients in calcium imaging for single boutons.
A, the photograph shows three individual mossy fibre boutons in stratum lucidum. Fibres were labelled with the calcium indicator dye Oregon Green BAPTA-1-AM and the picture was taken with a Hamamastsu ORCA ER camera. Scale bar corresponds to 1 μm. B, illustration of the effect of adenosine in an example fluorometric recording from a single mossy fibre bouton. Traces were taken at time points indicated by the numbers in the graph; averages of five sweeps each. C, the graph displays a summary of the reversible effect of adenosine (100 μm) for n= 19–22 boutons.
Adenosine modulates presynaptic Ca2+ channel gating
Ca2+ influx reduction can be achieved by modulation of presynaptic Ca2+ channels through direct binding of G-protein βγ subunits (G-βγ) (Herlitze et al. 1996; Catterall, 2000; Kajikawa et al. 2001). To test this possibility, we made direct patch-clamp recordings of Ca2+ currents from mossy fibre boutons (Geiger & Jonas, 2000; Bischofberger et al. 2002). Rectangular voltage pulses to 0 mV from a holding potential of −80 mV revealed large Ca2+ currents with an amplitude of 114.5 ± 17.9 pA (Fig. 5B, n= 5), similar to previously published values (Bischofberger et al. 2002). Application of 100 μm adenosine significantly reduced the current amplitude to 91.5 ± 1.6% of the control value (Fig. 5C and E, P < 0.01, n= 5).
Slowing of the activation rate is one of the hallmarks of G-protein-mediated inhibition of Ca2+ channels (Catterall, 2000). Therefore, we analysed the activation kinetics of the Ca2+ channels in the presence of adenosine which indeed introduced a prominent slow component in the activation time course (Fig. 5D and E). Fitting the Ca2+ channel activation with the sum of two exponentials revealed a fast and slow time constant of 0.85 ± 0.25 ms (75.0%) and 9.75 ± 3.37 ms (25.0%), leading to a weighted time constant (τw) of 2.55 ± 0.35 ms (Fig. 5E, n= 5). This was about two times slower than the activation in control solution, with time constants of τ= 1.17 ± 0.15 ms and τw= 1.39 ± 0.19 ms (n= 5) when fitted with a mono-exponential or bi-exponential function, respectively.
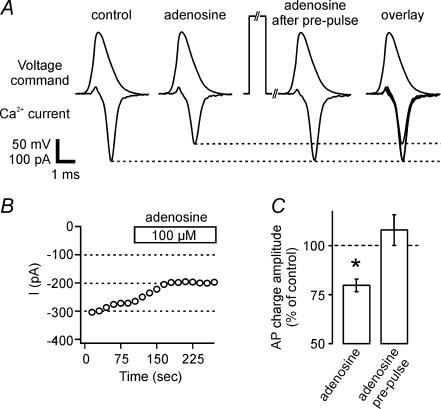
Ca2+ channel kinetics appear to be optimized to produce a transient Ca2+ influx with large amplitude and brief time course in response to action potentials invading the mossy fibre terminals (Bischofberger et al. 2002). The large Ca2+ influxes are critically important in eliciting transmitter release and the extent of Ca2+ current inhibition by adenosine might be more pronounced during short AP-like waveforms than during rectangular activation pulses. We used a previously recorded AP waveform as a voltage-clamp command to elicit more physiological Ca2+ currents (see Methods and Bischofberger et al. 2002). This waveform evoked a large and brief Ca2+ influx with a peak amplitude of 175.7 ± 37.5 pA and a half-duration of 637 ± 116 μs (Fig. 6A, n= 4), as previously described. Application of 100 μm adenosine substantially reduced the Ca2+ influx to 79.9 ± 3% of control (Fig. 6A–C, n= 4), as estimated by the current integral of 98 ± 9 fC in the presence of adenosine versus 123 ± 12 fC in control solution.
Figure 6. Action potential-evoked calcium currents are decreased by adenosine in a membrane-delimited manner.
A, application of a previously recorded action potential (AP) waveform as voltage command elicits fast and robust inward calcium currents at direct recordings at mossy fibre boutons, which are significantly inhibited by application of 100 μm adenosine. This decrease in current amplitude completely recovered with the application of a 50 ms prepulse depolarization to +80 mV shortly (10 ms) before the AP. Traces depict averages of five to 10 sweeps each. B, time course of experiment in A without prepulses. C, summary of reduction in AP-evoked charge and recovery by prepulse in n= 4 boutons.
As Ca2+ channel modulation by G-βγ is voltage-dependent, positive voltage pulses transiently relieve the G-protein-mediated inhibition (Herlitze et al. 1996). Therefore, we depolarized the boutons for 50 ms to +80 mV shortly before (10 ms) the test pulse which caused an enhancement of Ca2+ influx to 118 ± 3% in control conditions. During inhibition of the Ca2+ currents by adenosine, Ca2+ influx markedly increased after the prepulse, leading to a recovery of the current influx (Fig. 6A). Consistent with these findings, Ca2+ current amplitude during rectangular voltage steps to 0 mV also recovered back to control values after a prepulse to +80 mV. Moreover, the activation time constant τ under these conditions was accelerated to 0.98 ± 0.17 ms (83.5 ± 7.8% of control). Taken together, these data indicate that adenosine directly modulates presynaptic Ca2+ channel gating in mossy fibre boutons.
Reduction of Ca2+ influx can explain adenosine effects
Does the reduction of Ca2+ influx into presynaptic terminals explain the depression of synaptic response amplitudes by adenosine, or do other mechanisms downstream of Ca2+ influx have to be considered? A highly non-linear dependence of transmitter release from Ca2+ influx has been demonstrated in various neural preparations (Dodge & Rahamimoff, 1967; Mintz et al. 1995). We determined the power function of transmitter release for the mossy fibre synapse by selectively manipulating Ca2+ influx into the presynaptic mossy fibre terminals. Both mossy fibre evoked fEPSPs and presynaptic Ca2+ influx were measured under these conditions (Fig. 7A). The non-linear relationship between fEPSP and Ca2+ influx could be readily fitted with an equation of the form:
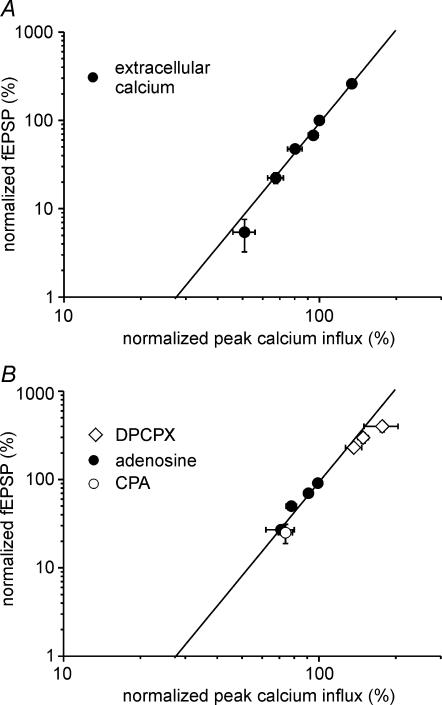
We obtained the best fit with n= 3.5. Next we compared the data obtained from treatments with adenosine, the A1 receptor agonist CPA and the antagonist DPCPX with the power function for transmitter release (Fig. 7B). All the data points corresponded well with the power function fit. Therefore, the influence of adenosine on transmission at the mossy fibre synapse can be explained by a modulation of presynaptic Ca2+ channels.
Figure 7. Modulation of presynaptic calcium channels can explain adenosine-mediated modulation of transmission.
A, the relationship between calcium influx and fEPSP is shown for a manipulation exclusively affecting presynaptic calcium influx. Changing the extracellular calcium concentration from 2.5 to 2, 1.5, 1, 0.5 and 6 mm leads to differential alterations in fEPSP and calcium transient amplitudes (n= 5–6 experiments each). All values are normalized to the standard extracellular recording solution containing 2.5 mm calcium and 1.3 mm magnesium. The continuous line represents the fit of the data by a power function with an exponent of 3.5. B, data of the effects of adenosine (3, 10, 30 and 100 μm), CPA (100 nm) and DPCPX (3, 30 and 200 nm) on calcium influx and fEPSP are compared to the power function fit from the graph in A.
Role of different VDCC subtypes in adenosine-mediated inhibition of transmission
At the hippocampal mossy fibre synapse, transmission is mediated by P/Q- and N-type Ca2+ channels (Castillo et al. 1994; Kamiya & Ozawa, 1998; Dietrich et al. 2003; Breustedt et al. 2003). To test whether adenosine selectively modulates a single VDCC subtype (Yawo & Chuhma, 1993; Umemiya & Berger, 1994) or affects multiple VDCCs (Dittman & Regehr, 1996), we performed the following set of experiments. Application of the specific P/Q-type channel blocker ω-agatoxin-IVA (1 μm) reduced presynaptic Ca2+ influx by 47 ± 2% (Fig. 8A, n= 5). In the presence of adenosine, addition of ω-agatoxin-IVA further reduced Ca2+ influx by only 25 ± 3% (Fig. 8B, n= 7, P < 0.05 for control condition versus in the presence of adenosine). Ca2+ influx through N-type VDCCs was reduced by ω-conotoxin-GVIA (1 μm) by 20 ± 4%. Pre-application of adenosine decreased the reduction by ω-agatoxin-IVA of Ca2+ influx to 7 ± 3% (Fig. 8C–E, n= 7 each, P < 0.05, for control condition versus in the presence of adenosine). From these results we calculated the fraction of each Ca2+ channel subtype blocked by adenosine. Adenosine significantly reduced Ca2+ influx through P/Q-type Ca2+ channels by 46% and N-type channels by 57% (Fig. 8F, P < 0.05). Thus, adenosine modulates both P/Q- and N-type VDCCs to reduce Ca2+ influx at hippocampal mossy fibre boutons.
Figure 8. Adenosine inhibits N-type and P/Q-type voltage-dependent calcium channels.
A, example experiment showing the reduction of calcium influx by the P/Q-type calcium channel antagonist ω-agatoxin-IVA (1 μm) by 42%. B, shows a representative experiment where in the presence of adenosine (100 μm) addition of ω-agatoxin-IVA leads to a further reduction of the calcium transient by 20%. C, single experiment demonstrating the reduction of calcium influx by 15% with the N-type calcium channel blocker ω-conotoxin-GVIA (1 μm). D, in the continuous presence of adenosine (100 μm) ω-conotoxin-GVIA reduces calcium transients by an additional 9%. In A–D the traces above the graphs are averages of five consecutive responses taken at the time points indicated in the time plots below. E, the bar diagram depicts the reduction of calcium influx into the mossy fibre terminals by the two antagonists ( ) and the reduced inhibition of calcium influx by the antagonists in the presence of adenosine (
) and the reduced inhibition of calcium influx by the antagonists in the presence of adenosine ( ). F, shows the inhibition of calcium influx through N- and P/Q-type calcium channels by adenosine. *Significant difference, P < 0.05.
). F, shows the inhibition of calcium influx through N- and P/Q-type calcium channels by adenosine. *Significant difference, P < 0.05.
Discussion
In this study, we have elucidated the transduction pathway by which adenosine reduces synaptic transmission at hippocampal mossy fibre synapses. We provide evidence that adenosine decreases synaptic responses via presynaptic A1 receptors involving a direct inhibition of two types of voltage-dependent Ca2+ channels.
The A1 receptor subtype couples to both Gi- and Go-proteins in different systems, enabling a number of transduction mechanisms for imposing inhibition of synaptic transmission. These include inhibition of adenylyl cyclase, activation of GIRKs, inhibition of Ca2+ channels and activation of phospholipase C (for review see Dunwiddie & Masino, 2001). In principle, adenosine could therefore act at a presynaptic or a postsynaptic locus of the mossy fibre synapse, or even – by combination of several mechanisms – at both sites.
Our experiments present several lines of positive evidence for a presynaptic locus of action. (1) Short-term plasticity of the synaptic responses was changed after the application of adenosine, in that the fEPSP amplitude ratio of the fifth to the first response was significantly increased (Zucker & Regehr, 2002). (2) In whole-cell voltage-clamp measurements, application of adenosine significantly increased the rate of transmission failures upon synaptic stimulation, indicating lowered presynaptic release. (3) We found a linear dependence of changes in the squared coefficient of variation and mean response amplitude of synaptic events (Faber & Korn, 1991). (4) Direct patch-clamp recordings from mossy fibre boutons as well as optical measurements showed a reduction of presynaptic Ca2+ currents and Ca2+ influx upon adenosine application. We therefore infer that adenosine mediates inhibition of transmission at the mossy fibre synapse by acting on presynaptic adenosine receptors.
A1 receptors were shown to be negatively coupled to the cAMP–PKA signalling cascade in several systems (van Calker et al. 1978; Ebersolt et al. 1983). In immature hippocampal CA1 neurons, adenosine modulates transmitter release via the PKA pathway, as PKA antagonists block adenosine-mediated reduction of miniature IPSCs (Jeong et al. 2003). At the hippocampal mossy fibre synapse, basal transmitter release and synaptic plasticity crucially depend on activity of the cAMP–PKA cascade (Weisskopf et al. 1994; Huang et al. 1994; Tzounopoulos et al. 1998). Moreover, the adenylyl cyclase activator forskolin reduces the suppression of mossy fibre synaptic transmission by the group II mGluR agonist DCGIV (Kamiya & Yamamoto, 1997). It is interesting that this effect is target specific, as mGluR depression of transmission of mossy fibre synapses onto CA3 interneurons does not involve cAMP-dependent pathways (Maccaferri et al. 1998). In this study, however, we could not detect a direct link between adenosine action and the presynaptic cAMP–PKA transduction pathway in modulating single action potential-evoked transmitter release. Interference with this signalling pathway by pre-application of forskolin and the PKA agonist SP-5,6-cBIMPS did not significantly occlude and the antagonist RP-8-CPT-cAMPS did not inhibit the efficacy of the A1 receptor agonist CPA. Thus, adenosine-mediated inhibition of basal synaptic transmission must be coupled to another transduction mechanism at the mossy fibre synapse.
An efficient way for neuromodulators to influence synaptic transmission is the reduction of presynaptic Ca2+ influx, which has been demonstrated for different modulators in various preparations (Wu & Saggau, 1997). Indeed, our electrophysiological and optical recording experiments provide evidence that adenosine reduces Ca2+ influx into mossy fibre boutons. Both single photodiode measurements and recordings from identified boutons with a Nipkow spinning disc confocal system show a roughly 20% reduction of Ca2+ influx by saturating concentrations of adenosine. In addition, Ca2+ currents induced by action potential-like waveforms into mossy fibre terminals were also attenuated by 20%, confirming the consistance of fluorometric and electrophysiological recordings.
The release sites at the mossy fibre synapse are driven by overlapping domains of multiple Ca2+ channels, as the effects of P/Q- and N-type Ca2+ channel blockers add linearly with respect to presynaptic Ca2+ influx but add non-linearly with respect to the effect on synaptic transmission (Castillo et al. 1994; Dietrich et al. 2003; Breustedt et al. 2003). Furthermore, the average number of Ca2+ channels that open during action potentials was estimated to be ∼20 per release site (Bischofberger et al. 2002). Finally, synaptic transmission at the mossy fibre–CA3 synapse is sensitive to the slow calcium buffer EGTA (Salin et al. 1996; Blatow et al. 2003), indicating a relatively large distance between Ca2+ channels and calcium sensor in the range of several hundred nanometers, consistent with overlapping calcium microdomains (Meinrenken et al. 2002). Given these conditions at the mossy fibre synapse, both a reduction in calcium flux per channel and a reduction in the number of open Ca2+ channels is expected to result in a non-linear relationship between Ca2+ influx and EPSP (Mintz et al. 1995). In our experiments, the effects of CPA and different concentrations of adenosine and DPCPX on fEPSP and presynaptic Ca2+ influx amplitudes could be readily approximated by a power function and fitted well with our measured non-linear calcium dependence of transmitter release for the mossy fibre synapse (with n= 3.5). Taken together, we conclude that inhibition of presynaptic voltage-dependent Ca2+ channels can explain the reduction of synaptic transmission by adenosine. A reduction of Ca2+ currents by adenosine, which could fully explain the reduction of EPSC amplitudes, was also described in direct presynaptic recordings at the calyx of Held in immature rats (Kimura et al. 2003).
Both N- and P/Q-type voltage-dependent Ca2+ channels contribute to presynaptic Ca2+ influx and subsequent transmitter release at the mossy fibre synapse (Castillo et al. 1994; Kamiya & Ozawa, 1999). By contrast, Ca2+ influx through L- or R-type Ca2+ channels is not involved in transmitter release (Castillo et al. 1994; Kamiya & Ozawa, 1998; Dietrich et al. 2003; Breustedt et al. 2003; but see Gasparini et al. 2001). In good agreement with previous studies, we found that N- and P/Q-type Ca2+ channels contribute to approximately 20% and 47% of Ca2+ influx, respectively (Miyazaki et al. 2005). A selective preference for inhibition of N-type VDCCs by adenosine has been reported at hippocampal Schaffer collateral synapses (Wu & Saggau, 1994), rat brainstem interneurons (Umemiya & Berger, 1994) and chick ciliary ganglion neurons (Yawo & Chuhma, 1993). On the other hand, an inhibition of multiple VDCC subtypes by neuromodulators has been described at the cerebellar parallel fibre synapse for endocannabinoids, GABA and adenosine (Dittman & Regehr, 1996; Brown et al. 2004). Using specific toxins for the different channel subtypes we found that adenosine inhibits presynaptic Ca2+ influx through N- and P/Q-type Ca2+ channels to a similar extent by 57% and 46%, respectively, arguing for the use of multiple Ca2+ channel subtypes to influence transmitter release.
Direct G-protein βγ subunit-mediated modulation of Ca2+ channels has been almost exclusively described in expression systems and neuronal somata (Bean, 1989; Herlitze et al. 1996). This is because presynaptic terminals are largely inaccessible to direct electrophysiological recordings. A major advancement was made with recordings from the giant presynaptic terminal of the calyx of Held (Forsythe, 1994), where a role for βγ subunits in GABAB receptor-mediated modulation could be established (Takahashi et al. 1998; Kajikawa et al. 2001). A technique has been developed to obtain patch-clamp recordings from identified hippocampal mossy fibre boutons in slice preparations. With this method, functions of intrinsic conductances in transmission and plasticity at this central synapse could be described (Geiger & Jonas, 2000; Bischofberger et al. 2002). Using direct bouton recordings, we observed two key features of G-protein-mediated inhibition of Ca2+ currents by adenosine: (1) the slowing of the Ca2+ channel activation rate; and (2) the relief from block by strong depolarisation (Bean, 1989; Herlitze et al. 1996; Patil et al. 1996). These results strongly indicate that the activation of adenosine receptors leads to inhibition of Ca2+ channels via a membrane-delimited pathway.
In summary, our results provide evidence that adenosine reduces synaptic transmission at the hippocampal mossy fibre synapse through a presynaptic mechanism involving inhibition of voltage-dependent Ca2+ channels by a membrane-delimited pathway. On first sight, this conclusion is in apparent opposition to two earlier reports, which concluded that adenosine acts downstream of Ca2+ influx by monitoring spontaneous synaptic activity (Scanziani et al. 1992; Scholz & Miller, 1992). Interestingly, a recent study reports that stimulus-evoked and spontaneous transmitter release recruit synaptic vesicles from different pools (Sara et al. 2005). Whether this selective recruitment from distinct vesicle pools for spontaneous and evoked release also holds true for the mossy fibre synapse and whether adenosine differentially affects these vesicle pools remains to be determined in future studies.
Acknowledgments
This work was supported by the Emmy-Noether Program (SCHM 1383/4–1), deutsche Forschungsgemeinschaft (DFG) grants SFB 618 and SFB 665, and the Bernstein Center for Computational Neuroscience Berlin (BMBF). We thank Peter Jonas for critically reading the manuscript and Susanne Walden and Anke Schönherr for excellent technical assistance.
Supplemental material
The supplementary figure contains additional data regarding the pharmacological and short-termplasticity criteria used to select for mossy fibre inputs.
Online supplemental material for this paper can be accessed at:http://jp.physoc.org/cgi/content/full/jphysiol.2007.132613/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.132613
References
- Bagley EE, Vaughan CW, Christie MJ. Inhibition by adenosine receptor agonists of synaptic transmission in rat periaqueductal grey neurons. J Physiol. 1999;516:219–225. doi: 10.1111/j.1469-7793.1999.219aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Li L, Geiger J, Jonas P. Patch-clamp recordings from mossy fiber terminals in hippocampal slices. Nat Protoc. 2006;1:2075–2081. doi: 10.1038/nprot.2006.312. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Geiger JR, Jonas P. Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons. J Neurosci. 2002;22:10593–10602. doi: 10.1523/JNEUROSCI.22-24-10593.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G protein βγ subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–297. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. doi: 10.1016/s0896-6273(03)00196-x. [DOI] [PubMed] [Google Scholar]
- Breustedt J, Vogt KE, Miller RJ, Nicoll RA, Schmitz D. α1E-containing Ca2+ channels are involved in synaptic plasticity. Proc Natl Acad Sci U S A. 2003;100:12450–12455. doi: 10.1073/pnas.2035117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Dietrich D, Kirschstein T, Kukley M, von der Pereverzev ABC, Schneider T, Beck H. Functional specialization of presynaptic Cav2.3 Ca2+ channels. Neuron. 2003;39:483–496. doi: 10.1016/s0896-6273(03)00430-6. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Jr, Rahamimoff R. On the relationship between calcium concentration and the amplitude of the end-plate potential. J Physiol. 1967;189:90P–92P. [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Ebersolt C, Premont J, Prochiantz A, Perez M, Bockaert J. Inhibition of brain adenylate cyclase by A1 adenosine receptors: pharmacological characteristics and locations. Brain Res. 1983;267:123–129. doi: 10.1016/0006-8993(83)91045-4. [DOI] [PubMed] [Google Scholar]
- Egner A, Hell SW. Fluorescence microscopy with super-resolved optical sections. Trends Cell Biol. 2005;15:207–215. doi: 10.1016/j.tcb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991;60:1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Guttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gasparini S, Kasyanov AM, Pietrobon D, Voronin LL, Cherubini E. Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J Neurosci. 2001;21:8715–8721. doi: 10.1523/JNEUROSCI.21-22-08715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Bischofberger J, Vida I, Frobe U, Pfitzinger S, Weber HJ, Haverkampf K, Jonas P. Patch-clamp recording in brain slices with improved slicer technology. Pflugers Arch. 2002;443:491–501. doi: 10.1007/s00424-001-0735-3. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Gerachshenko T, Blackmer T, Yoon EJ, Bartleson C, Hamm HE, Alford S. Gβγ acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci. 2005;8:597–605. doi: 10.1038/nn1439. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Jang IS, Nabekura J, Akaike N. Adenosine A1 receptor-mediated presynaptic inhibition of GABAergic transmission in immature rat hippocampal CA1 neurons. J Neurophysiol. 2003;89:1214–1222. doi: 10.1152/jn.00516.2002. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, Saitoh N, Takahashi T. GTP-binding protein βγ subunits mediate presynaptic calcium current inhibition by GABAB receptor. Proc Natl Acad Sci U S A. 2001;98:8054–8058. doi: 10.1073/pnas.141031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J Physiol. 1998;509:833–845. doi: 10.1111/j.1469-7793.1998.833bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Dual mechanism for presynaptic modulation by axonal metabotropic glutamate receptor at the mouse mossy fibre-CA3 synapse. J Physiol. 1999;518:497–506. doi: 10.1111/j.1469-7793.1999.0497p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Yamamoto C. Phorbol ester and forskolin suppress the presynaptic inhibitory action of group-II metabotropic glutamate receptor at rat hippocampal mossy fibre synapse. Neuroscience. 1997;80:89–94. doi: 10.1016/s0306-4522(97)00098-5. [DOI] [PubMed] [Google Scholar]
- Kimura M, Saitoh N, Takahashi T. Adenosine A1 receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. J Physiol. 2003;553:415–426. doi: 10.1113/jphysiol.2003.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Schwan M, Fredholm BB, Dietrich D. The role of extracellular adenosine in regulating mossy fiber synaptic plasticity. J Neurosci. 2005;25:2832–2837. doi: 10.1523/JNEUROSCI.4260-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Toth K, McBain CJ. Target-specific expression of presynaptic mossy fiber plasticity. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Meinrenken CJ, Borst JG, Sakmann B. Calcium secretion coupling at calyx of held governed by nonuniform channel-vesicle topography. J Neurosci. 2002;22:1648–1667. doi: 10.1523/JNEUROSCI.22-05-01648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Ishizuka T, Yawo H. Synapse-to-synapse variation of calcium channel subtype contributions in large mossy fiber terminals of mouse hippocampus. Neuroscience. 2005;136:1003–1014. doi: 10.1016/j.neuroscience.2005.08.049. [DOI] [PubMed] [Google Scholar]
- Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2003;100:14397–14402. doi: 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Patil PG, de Leon M, Reed RR, Dubel S, Snutch TP, Yue DT. Elementary events underlying voltage-dependent G-protein inhibition of N-type calcium channels. Biophys J. 1996;71:2509–2521. doi: 10.1016/S0006-3495(96)79444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor WR, Dunwiddie TV. Pre- and postsynaptic actions of adenosine in the in vitro rat hippocampus. Brain Res. 1987;426:187–190. doi: 10.1016/0006-8993(87)90441-0. [DOI] [PubMed] [Google Scholar]
- Qian J, Saggau P. Modulation of transmitter release by action potential duration at the hippocampal CA3-CA1 synapse. J Neurophysiol. 1999;81:288–298. doi: 10.1152/jn.1999.81.1.288. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Tank DW. Selective fura-2 loading of presynaptic terminals and nerve cell processes by local perfusion in mammalian brain slice. J Neurosci Methods. 1991;37:111–119. doi: 10.1016/0165-0270(91)90121-f. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampal mossy fibers. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci. 1997;17:3425–3435. doi: 10.1523/JNEUROSCI.17-10-03425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Breustedt J, Nicoll RA. Presynaptic kainate receptors impart an associative property to hippocampal mossy fiber long-term potentiation. Nat Neurosci. 2003;6:1058–1063. doi: 10.1038/nn1116. [DOI] [PubMed] [Google Scholar]
- Scholz KP, Miller RJ. Inhibition of quantal transmitter release in the absence of calcium influx by a G protein-linked adenosine receptor at hippocampal synapses. Neuron. 1992;8:1139–1150. doi: 10.1016/0896-6273(92)90134-y. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kajikawa Y, Tsujimoto T. G-protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor. J Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Capogna M, Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- Toth K, McBain CJ. Target-specific expression of pre- and postsynaptic mechanisms. J Physiol. 2000;525:41–51. doi: 10.1111/j.1469-7793.2000.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO, Jackson MB. Adenosine-activated potassium conductance in cultured striatal neurons. Proc Natl Acad Sci U S A. 1985;82:4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Janz R, Sudhof TC, Nicoll RA, Malenka RC. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron. 1998;21:837–845. doi: 10.1016/s0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Activation of adenosine A1 and A2 receptors differentially modulates calcium channels and glycinergic synaptic transmission in rat brainstem. Neuron. 1994;13:1439–1446. doi: 10.1016/0896-6273(94)90429-4. [DOI] [PubMed] [Google Scholar]
- van Calker D, Muller M, Hamprecht B. Adenosine inhibits the accumulation of cyclic AMP in cultured brain cells. Nature. 1978;276:839–841. doi: 10.1038/276839a0. [DOI] [PubMed] [Google Scholar]
- Wang E, Babbey CM, Dunn KW. Performance comparison between the high-speed Yokogawa spinning disc confocal system and single-point scanning confocal systems. J Microsc. 2005;218:148–159. doi: 10.1111/j.1365-2818.2005.01473.x. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Yawo H, Chuhma N. Preferential inhibition of ω-conotoxin-sensitive presynaptic Ca2+ channels by adenosine autoreceptors. Nature. 1993;365:256–258. doi: 10.1038/365256a0. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary figure contains additional data regarding the pharmacological and short-termplasticity criteria used to select for mossy fibre inputs.
Online supplemental material for this paper can be accessed at:http://jp.physoc.org/cgi/content/full/jphysiol.2007.132613/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.132613