Abstract
Intracellular recordings were made in vitro from guinea-pig cardiac ganglia to determine whether endogenous neuropeptides such as pituitary adenylate cyclase-activating polypeptide (PACAP) or substance P released during tetanic neural stimulation modulate cardiac neurone excitability and/or contribute to slow excitatory postsynaptic potentials (sEPSPs). When nicotinic and muscarinic receptors were blocked by hexamethonium and atropine, 20 Hz stimulation for 10 s initiated a sEPSP in all innervated neurones. In 40% of the cells, excitability was enhanced after termination of the sEPSP. This suggested that non-cholinergic receptor-mediated mechanisms contributed to the sEPSP and modulated neuronal excitability. Exogenous PACAP and substance P initiated a slow depolarization in the neurones whereas neuronal excitability was only increased by PACAP. When ganglia were treated with the PAC1 antagonist PACAP6-38 (500 nm), the sEPSP evoked by 20 Hz stimulation was reduced by ∼50% and an enhanced excitability occurred in only 10% of the cells. These observations suggested that PACAP released from preganglionic nerve terminals during tetanic stimulation enhanced neuronal excitability and evoked sEPSPs. After addition of 1 nm PACAP to the bath, 7 of 9 neurones exhibited a tonic firing pattern whereas in untreated preparations, the neurons had a phasic firing pattern. PACAP6-38 (500 nm) diminished the increase in excitability caused by 1 nm PACAP so that only 4 of 13 neurones exhibited a tonic firing pattern and the other 9 cells retained a phasic firing pattern. These findings indicate that PACAP can be released by tetanic neural stimulation in vitro and increase the excitability of intrinsic cardiac neurones. We hypothesize that in vivo PACAP released during preganglionic firing may modulate neurotransmission within the intrinsic cardiac ganglia.
Neurones in the guinea pig intracardiac ganglia receive multiple pericellular contacts from extrinsic nerve fibres, many of which can contain a variety of neurotransmitters and/or neuropeptides (Parsons, 2004). For example, substance P-immunoreactive (IR) sensory nerve fibres are present within some, but not all, intrinsic cardiac ganglia (Dalsgaard et al. 1986; Calupca et al. 2000). Also, essentially all of the cholinergic parasympathetic preganglionic fibres terminating on individual cardiac neurones contain pituitary adenylate cyclase-activating polypeptide (PACAP) (Braas et al. 1998; Calupca et al. 2000). Application of substance P and PACAP can depolarize individual guinea pig cardiac neurones; an action mediated primarily through activation of NK3 and PAC1 receptors, respectively (Hardwick et al. 1995, 1997; Braas et al. 1998; Tompkins et al. 2006). Together these data suggest a potential role for these endogenous neuropeptides in modulation of neurotransmission within intrinsic cardiac ganglia.
Repetitive stimulation of interganglionic nerve bundles innervating cardiac ganglia can initiate a slow excitatory postsynaptic potential (sEPSP) (Konishi et al. 1985; Hardwick et al. 1995). A slowly developing depolarization can also be elicited by local application of capsaicin onto individual ganglia (Hardwick et al. 1995). Since capsaicin evokes release of substance P from sensory nerve fibres, previous investigators have suggested that the sEPSP might be generated by substance P released from perineuronal afferent nerve fibres (Konishi et al. 1985; Hardwick et al. 1995).
In addition to eliciting depolarization, neuropeptides, particularly PACAP, are known to affect the excitability of cardiac neurones (Braas et al. 1998; DeHaven & Cuevas, 2004; Tompkins et al. 2006). Even though it is established that a brief period of tetanic neural stimulation can elicit the sEPSP in innervated cells, it is not known whether a change in excitability also occurs following tetanic neural stimulation. Consequently, the present study investigated if high frequency stimulation (HFS) of nerve inputs increases cardiac neuron excitability and if so, whether release of either PACAP or substance P contributed to this effect.
Methods
All experiments were performed in vitro on atrial whole mount preparations containing the intrinsic cardiac ganglia from Hartley guinea pigs (mixed sex; 250–700 g). Guinea pigs were killed by halothane overdose followed by exsanguination using animal protocols approved by the University of Vermont Institutional Animal Care and Use Committee and the East Tennessee State University Institutional Animal Care and Use Committee and methods described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering. The heart was quickly removed and placed in cold standard Krebs solution (mm: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, 8 glucose). The pH was maintained at 7.4 by aeration with 95% O2–5% CO2.
Intracellular recording procedures
Atrial whole mount preparations were pinned in a 2.5 ml Sylgard-lined Petri dish and superfused continuously (2–3 ml min−1) with Krebs solution also containing 10 mm Hepes buffer (32 ± 1°C) (Hardwick et al. 1995, 1997; Braas et al. 1998; Tompkins et al. 2006). Intracardiac ganglia were visualized with an inverted microscope equipped with Hoffman optics and individual cardiac neurons were impaled using high impedance borosilicate microelectrodes (2 m KCl-filled; 60–100 MΩ). Active and passive membrane properties were recorded from the impaled neurons using an Axoclamp-2A amplifier coupled with a Digidata 1322A data acquisition system and pCLAMP 8 (Axon Instruments, Union City, CA, USA). Hyperpolarizing current was injected through the recording electrode as needed to maintain a resting membrane potential between −50 and −60 mV. Visually identified nerve bundles innervating a ganglion were stimulated with a bipolar electrode using 1 ms pulses with stimulus intensity ranging between 5 and 150 μA.
For brief local application, PACAP27 (at a concentration of 50 μm) or substance P (at a concentration of 100 μm) was applied by pressure application (Picospritzer, General Valve, Fairfield, NJ, USA) through ∼5 μm-diameter ‘puffer’ pipettes positioned 50–100 μm from the neurone. PACAP27 (1 nm) was also added directly to the bath solution during some experiments. To test for peptide-induced changes in neuronal excitability, 1 s depolarizing current steps (0.1–0.4 nA) were given before and 2–3 min after either exogenous PACAP27 or substance P application or tetanic neural stimulation. Excitability curves were constructed by plotting the number of action potentials generated by increasing stimulus intensities.
Drugs
PACAP27 (referred to simply as PACAP throughout the text) and PACAP6-38 were obtained from American Peptide Co. (Sunnyvale, CA, USA). Substance P was purchased from Sigma (St Louis, MO, USA).
Data analysis
Results are expressed as mean ±s.e.m. In some experiments, control and test results were obtained in the same cell with the results averaged and analysed with Student's paired t test. When results were compared between cells, statistical significance was determined with Student's non-paired t test. Differences with P < 0.05 were considered statistically significant. A χ2 test was used to compare categorical changes in excitability between two groups.
Results
HFS of intracardiac nerve bundles can evoke a sEPSP and can increase cardiac neurone excitability
In initial experiments with untreated whole mount cardiac ganglia preparations, we tested whether a 10 s period of HFS could initiate a sEPSP and/or increase excitability of the innervated intrinsic cardiac neurones. Intracellular recordings were made from individual cardiac neurones prior to, during and following a 20 Hz stimulation of an adjacent nerve bundle. To assess cardiac neurone excitability, a series of 1 s intracellular depolarizing current steps were applied before and after the tetanic stimulation of the nerve bundle. Action potentials were produced in the cardiac neurone following each stimulus applied to the nerve bundle, and a slow depolarization developed after termination of HFS in several cells (Fig. 1A). A sEPSP was observed in all cells in which activation followed HFS of a preganglionic nerve; some cells were antidromically activated which did not produce a sEPSP or a change in excitability (data not shown). In several cells, once the sEPSP ended, the number of action potentials produced by the same constant current depolarizing step applied prior to HFS was markedly greater, indicating that cardiac neurone excitability was also enhanced following tetanic stimulation of the nerve bundle (Fig. 1B). The increase in excitability could last for several minutes.
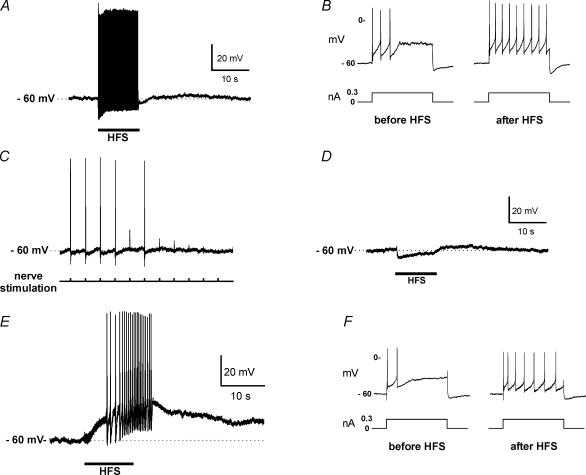
Figure 1. Repetitive neural stimulation can initiate a sEPSP and an increase in cardiac neurone excitability.
A, HFS (20 Hz for 10 s) applied to a nerve fibre connective can elicit action potentials followed by a sEPSP in an innervated cardiac neurone. B, in the same cell as in A, neuronal excitability is enhanced following the sEPSP as evidenced from the increase in number of action potentials elicited by an identical 1 s suprathreshold depolarizing current pulse. C, during exposure to 1 mm hexamethonium, action potential generation elicited by 0.1 Hz stimulation of the nerve bundle is lost as the fast EPSP becomes progressively subthreshold. Hexamethonium was added to the bath just before the beginning of the record. D, when 1 mm hexamethonium was present in the bath solution, HFS elicited a hyperpolarization during the period of stimulation to the nerve connective, followed by a depolarization that persisted after the stimulation had ended. E, when both 1 mm hexamethonium and 1 μm atropine were present in the bath solution, only a sEPSP was elicited by HFS. The sEPSP in some cells was sufficiently large to elicit a burst of action potentials. F, in the same cell as in E, neuronal excitability was enhanced following the sEPSP as evidenced from the increase in number of action potentials elicited by an identical 1 s suprathreshold depolarizing current pulse. In A, C, D and E, the dashed line indicates the resting membrane potential.
Guinea pig cardiac neurones express both nicotinic and muscarinic receptors (Allen & Burnstock, 1990). Activation of muscarinic receptors by neurally released acetylcholine (ACh) could potentially initiate both the sEPSP and increase in excitability. Therefore, experiments were done to test whether the generation of the sEPSP and increase in cardiac neurone excitability following HFS required cholinergic receptor activation. After obtaining a stable intracellular recording, a nearby nerve bundle was stimulated at low frequency (0.1 Hz) to establish that the cell received synaptic input. Then, the neuronal nicotinic receptor antagonist hexamethonium (1 mm) was applied to block the fast excitatory postsynaptic potential (fEPSP) (Fig. 1C). After the fEPSP had been eliminated, HFS of the same nerve bundle produced membrane hyperpolarization during the stimulation period and a slowly developing depolarization after stimulation (Fig. 1D). When atropine (1 μm) was added to the circulating bath solution, the HFS-induced hyperpolarization was eliminated, but the sEPSP remained (data not shown). These observations suggested that for guinea pig cardiac neurones, the HFS-induced sEPSP did not require activation of nicotinic or muscarinic receptors.
In subsequent experiments, preparations were continually bathed in solution containing atropine. Following verification that a cell received synaptic input from an adjacent nerve connective, hexamethonium was added to the bathing solution. With nicotinic and muscarinic receptors blocked, HFS consistently caused a sEPSP, which commenced during stimulation and ranged in amplitude between 2 and 13 mV (mean sEPSP amplitude = 7 ± 1 mV, 11 cells). The duration of the sEPSP ranged from 38 to 118 s (mean sEPSP duration = 61 ± 8 s). With the largest sEPSPs multiple action potentials were elicited (Fig. 1E). In the presence of atropine and hexamethonium, HFS also caused an increase in excitability in 5 of 11 cells, tested after the sEPSP had ended (Fig. 1F).
Both PACAP and substance P depolarize the cardiac neurons, but only PACAP causes an increase in excitability
Earlier studies had shown that PACAP and substance P work through distinct receptors to depolarize guinea pig cardiac neurones (Hardwick et al. 1997; Braas et al. 1998). We have confirmed these earlier results. Brief puffer application (500 ms) of either PACAP or substance P elicited a slow depolarization (Fig. 2A and C). The mean amplitude of the depolarization was comparable for both peptides although substance P more consistently initiated depolarization (Fig. 2E). Neuronal excitability was assessed in the same cells before exposure to peptide and again following termination of the agonist-induced depolarization. As illustrated in Fig. 2B and D, excitability was enhanced following application of PACAP, but not after substance P. Excitability curves were determined for multiple cells by intracellular stimulation at several current intensities before and after application of either PACAP or substance P (Fig. 2F). The number of action potentials generated prior to peptide application ranged between one and two regardless of current amplitude (from 0.1 to 0.4 nA). No additional action potentials occurred after application of substance P, whereas following PACAP application, the number of action potentials increased progressively with increased stimulus intensity.
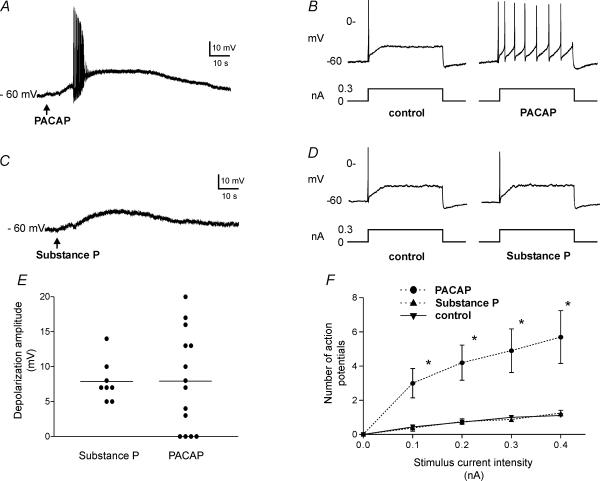
Figure 2. Application of PACAP or substance P can initiate depolarization, but only PACAP increases cardiac neurone excitability.
A, local puffer application of PACAP, indicated by arrow, could initiate a slow depolarization in the cardiac neurones. In many cells, a burst of action potentials was generated during the rising phase of the depolarization. B, in the same cell as in A, membrane excitability was enhanced following PACAP application as evidenced by the increased number of action potentials elicited by a similar 1 s suprathreshold depolarizing current pulse as given prior to peptide application. C, local puffer application of substance P, indicated by arrow consistently initiated a slow depolarization. D, in the same cell as C, there was no change in excitability (determined using a similar 1 s suprathreshold depolarizing current pulse) evident following substance P application. E, the mean amplitude of the depolarization produced in different cells by PACAP or substance P was similar. However, the range of depolarization was noticeably greater for PACAP than for substance P. F, a graph demonstrating that after PACAP application, the number of action potentials elicited by 1 s depolarizing current pulses increased with increasing stimulus strength. Data points represent mean ±s.e.m. from 10 cells following PACAP application (•). In contrast, prior to peptide application or following substance P application (n= 9 cells, ▴), the number of action potentials produced did not change markedly with increasing stimulus strength. The control curve is the average response obtained from 18 cells prior to peptide application (▾). The asterisks indicate that the number of action potentials produced was significantly greater following PACAP application than prior to peptide application.
When the PAC1 receptor antagonist PACAP6-38 was present, both the HFS-induced sEPSP and increased excitability were reduced
Based on these results, we postulated that the increase in excitability following HFS could be caused by the release of PACAP from preganglionic nerve terminals, but most likely not by substance P released from afferent fibres. To evaluate this possibility, we tested whether HFS elicited a sEPSP and an increase in excitability when the PAC1 receptor antagonist PACAP6-38 (500 nm) was present in the bath solution along with hexamethonium and atropine. In the presence of PACAP6-38, the mean amplitude of the sEPSP was significantly smaller (3 ± 1 mV, 10 cells) than in the absence of PACAP6-38 (7 ± 1 mV, 11 cells), and no depolarization was evident after HFS in 2 of the 10 cells. Furthermore, excitability was unchanged in 9 out of 10 neurons after HFS in the presence of PACAP6-38 and the remaining cell showed only a small increase. These results provided further evidence that PACAP released from preganglionic nerve terminals by HFS can modulate cardiac neurone excitability and contribute to the generation of the sEPSP.
A low concentration of PACAP in the bathing solution causes a sustained increase in the excitability of cardiac neurons
Our results indicate that PACAP can be released from preganglionic terminals during HFS. We hypothesized that low levels of PACAP might be released in vivo during ongoing vagal activity and have a role in regulating cardiac neurone excitability. When the whole mount preparation is prepared for in vitro experiments, the vagal input is severed and the ongoing vagal tone is lost. In these preparations, ∼90% of the cardiac neurones exhibit a phasic-like pattern of action potential firing when tested with depolarizing current steps (Edwards et al. 1995; Hardwick et al. 1997; Braas et al. 1998; Tompkins et al. 2006). Thus, we hypothesize that in vivo there may be a low level of PACAP present, which could enhance cell excitability. Consequently, we tested in four whole mount preparations whether 1 nm PACAP added to the bathing solution affected the firing pattern of the intrinsic cardiac neurones. In three cells, we tested excitability prior to and following PACAP application. All three neurones were phasic prior to PACAP and two of these three neurons exhibited a tonic-like firing pattern after a few minutes exposure to PACAP. Results from one of these neurones are shown in Fig. 3A. Five of six other neurones tested during a 1–3 h sustained exposure to PACAP also exhibited a tonic-like firing pattern. So in total, 7 of 9 cardiac neurones exhibited a tonic-like firing pattern during sustained exposure to 1 nm PACAP (Fig. 3B).
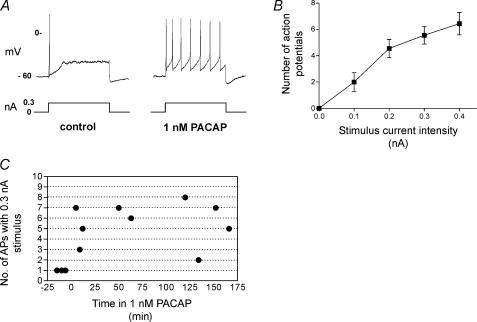
Figure 3. An increased excitability was sustained during bath application of 1 nm PACAP.
A, a recording from the same cell obtained prior to and 5 min after bath application of 1 nm PACAP. Note that the firing pattern elicited with a 1 s depolarizing pulse shifted from phasic to tonic-like during exposure to 1 nm PACAP. B, an excitability curve demonstrating that during continued exposure to 1 nm PACAP, the number of action potentials elicited by 1 s depolarizing current pulses increased with increased stimulus strength (averaged data from 9 cells that were evaluated at different times over a 5–166 min exposure to PACAP). C, the number of action potentials produced by a 0.3 nA stimulus in different cells are plotted as a function of time in 1 nm PACAP. The results show that neuronal excitability remained elevated during the sustained exposure to PACAP.
We also tested in four other whole mount preparations whether the presence of 500 nm PACAP6-38 could suppress the PACAP-induced shift in firing pattern. Four cells were tested in these preparations prior to PACAP application. All four exhibited a phasic firing pattern. Out of 13 cells exposed to 500 nm PACAP6-38 along with 1 nm PACAP, only 4 exhibited a tonic-like firing pattern. The remaining 9 cells retained a phasic-like firing pattern comparable to untreated cells. Thus, the change in excitability produced by PACAP, when PACAP6-38 was present, was significantly less than when PACAP was applied alone (P= 0.03, χ2 test).
Discussion
The present study demonstrates for the first time that a 10 s period of tetanic neural stimulation of associated input projections can elicit both a sEPSP and an increase in excitability in guinea pig cardiac neurones. We also show that these two effects of HFS do not require cholinergic receptor activation. Instead, under the conditions of the present experiments with both nicotinic and muscarinic receptors blocked, the depolarization and increased excitability must be elicited by another neurotransmitter(s) released by HFS. Thus, the sEPSP and the increase in excitability observed in our experiments cannot be attributed to a muscarinic-induced inhibition of the M current (Brown, 1988).
Following nicotinic receptor blockade, HFS generally evoked a hyperpolarization, which was mediated by activation of muscarinic receptors. A neurally evoked IPSP was originally identified in mudpuppy intracardiac neurones (Hartzell et al. 1977) and later also observed in mammalian cardiac neurones (Xi-Moy et al. 1993). For instance, a muscarinic receptor-mediated hyperpolarization or outward current has also been recorded in neonatal rat and guinea pig cultured cardiac neurones (Allen & Burnstock, 1990; Beker et al. 2003).
Exogenous application of PACAP and substance P elicited depolarization, but only PACAP increased cardiac neurone excitability. This observation and the fact that the PAC1 antagonist effectively inhibited the increase in HFS-induced excitability suggested that PACAP released from preganglionic nerve terminals mediated this effect.
Both substance P-IR fibres and PACAP-IR fibres can innervate guinea pig cardiac ganglia (Dalsgaard et al. 1986; Braas et al. 1998). PACAP, which is contained in the cholinergic preganglionic terminals, is present in fibres innervating virtually all guinea pig cardiac neurons (Calupca et al. 2000). Conversely, substance P-IR fibres commonly innervate only a subpopulation of ganglia (Calupca et al. 2000). Thus, it is quite likely that PACAP-IR fibres are present in more nerve connectives and at a greater abundance than substance P-IR fibres. Therefore, we reasoned that PACAP released from preganglionic nerve terminals during HFS was more likely to be responsible for initiation of the sEPSP in most intrinsic cardiac neurones. However, we cannot exclude the possibility that SP released from afferent fibres could contribute to the generation of the sEPSP in some cells under the conditions of these experiments.
PACAP6-38 at a concentration of 500 nm did not always eliminate the sEPSP. We propose three interpretations of this observation. First, the release of substance P from afferent fibres might have contributed to HFS-induced generation of the sEPSP in some cells. Second, given that there is heterogeneity within the population of intrinsic cardiac neurones, not all cells respond similarly to PACAP. Third, at 500 nm, PACAP6-38 might not completely block all PAC1 receptors. The latter observation is consistent with the observation that PACAP6-38 suppressed, but did not eliminate, the PACAP-induced increase in excitability when the peptide was added to the bathing solution.
Application of PACAP can depolarize and increase excitability of guinea pig myenteric neurones (Ermilov et al. 2004). In addition, PACAP released during nerve stimulation or reflex activation of the colon can depolarize these neurones, an effect blunted by PAC1 receptor antagonists. Thus, in the guinea pig, release of endogenous PACAP at different autonomic synapses apparently can alter the properties of the postsynaptic cells.
In conclusion, the present results demonstrate that endogenous PACAP, which is released from preganglionic parasympathetic nerve terminals during repetitive nerve stimulation, contributes to the generation of the sEPSP and can modulate excitability of guinea pig intrinsic cardiac neurones. Furthermore, in the present study, when 1 nm PACAP was included in the bath solution, cardiac neurone excitability was consistently enhanced and this effect was maintained for the duration (1–3 h) of exposure. Thus, we hypothesize that in vivo during ongoing vagal preganglionic fibre firing, low levels of PACAP may be released and could regulate the excitability state of cardiac neurones.
Acknowledgments
This work was supported by National Institutes of Health Grants, HL-54633 to D.B.H., HL-71830 to J.L.A., and HL-65481 and P20 RR-16435 to R.L.P.
References
- Allen TGJ, Burnstock G. M1 and M2 muscarinic receptors mediate excitation and inhibition of guinea-pig intracardiac neurons in culture. J Physiol. 1990;422:463–480. doi: 10.1113/jphysiol.1990.sp017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beker F, Weber M, Fink RHA, Adams DJ. Muscarinic and nicotinic ACh receptor activation differentially mobilize Ca2+ in rat intracardiac ganglion neurons. J Neurophysiol. 2003;90:1956–1964. doi: 10.1152/jn.01079.2002. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci. 1998;18:9766–9779. doi: 10.1523/JNEUROSCI.18-23-09766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. M-currents: an update. Trends Neurosci. 1988;11:294–299. doi: 10.1016/0166-2236(88)90089-6. [DOI] [PubMed] [Google Scholar]
- Calupca MA, Vizzard MA, Parsons RL. Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol. 2000;423:26–39. [PubMed] [Google Scholar]
- Dalsgaard CJ, Franco-Cereceda A, Saria A, Lundberg JM, Theodossson-Norheim E, Hokfelt T. Distribution and origin of substance P- and neuropeptide Y-immunoreactive nerves in the guinea pig heart. Cell Tissue Res. 1986;243:477–485. doi: 10.1007/BF00218054. [DOI] [PubMed] [Google Scholar]
- DeHaven WI, Cuevas J. VPAC receptor modulation of neuroexcitability in intracardiac neurons: dependence on intracellular calcium mobilization and synergistic enhancement by PAC1 receptor activation. J Biol Chem. 2004;279:40609–40621. doi: 10.1074/jbc.M404743200. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GDS, Klemm MF, Steele P. Different types of ganglion cells in the cardiac plexus of guinea-pigs. J Physiol. 1995;486:453–471. doi: 10.1113/jphysiol.1995.sp020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermilov LG, Schmalz PF, Miller SM, Szurszewski JH. PACAP modulation of the colon-inferior mesenteric ganglion reflex in the guinea pig. J Physiol. 2004;560:231–247. doi: 10.1113/jphysiol.2004.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JC, Mawe GM, Parsons RL. Evidence for afferent fiber innervation of parasympathetic neurons of the guinea pig cardiac ganglion. J Auton Nerv Syst. 1995;53:166–174. doi: 10.1016/0165-1838(94)00182-j. [DOI] [PubMed] [Google Scholar]
- Hardwick JC, Mawe GM, Parsons RL. Tachykinin-induced activation of non-specific cation conductance via NK3 neurokinin receptors in guinea-pig intracardiac neurons. J Physiol. 1997;504:65–74. doi: 10.1111/j.1469-7793.1997.065bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell HC, Kuffler SW, Stickgold R, Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977;271:817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Okamoto T, Otsuka M. Substance P as a neurotransmitter released from peripheral branches of primary afferent neurons producing slow synaptic excitation in autonomic ganglion cells. In: Jordan CC, Oehme P, editors. Substance P. Metabolism and Biological Action. Philadelphia, PA, USA: Taylor & Francis; 1985. pp. 121–136. [Google Scholar]
- Parsons RL. Mammalian cardiac ganglia as local integration centers: histochemical and electrophysiological evidence. In: Dun N, Machado B, Pilowsky P, editors. Mechanisms of Cardiovascular Regulation. Norwell, MA, USA: Kluwer Academic Publishers; 2004. pp. 335–356. chap. 15. [Google Scholar]
- Tompkins JD, Hardwick JC, Locknar SA, Merriam LA, Parsons RL. Ca2+ influx, but not Ca2+ release from internal stores, is required for the PACAP-induced increase in excitability in guinea pig intracardiac neurons. J Neurophysiol. 2006;95:2134–2142. doi: 10.1152/jn.01077.2005. [DOI] [PubMed] [Google Scholar]
- Xi-Moy SX, Randall WC, Wurster RD. Nicotinic and muscarinic synaptic transmission in canine intracardiac ganglion cells innervating the sinoatrial node. J Auton Nerv Syst. 1993;42:201–214. doi: 10.1016/0165-1838(93)90365-2. [DOI] [PubMed] [Google Scholar]