Abstract
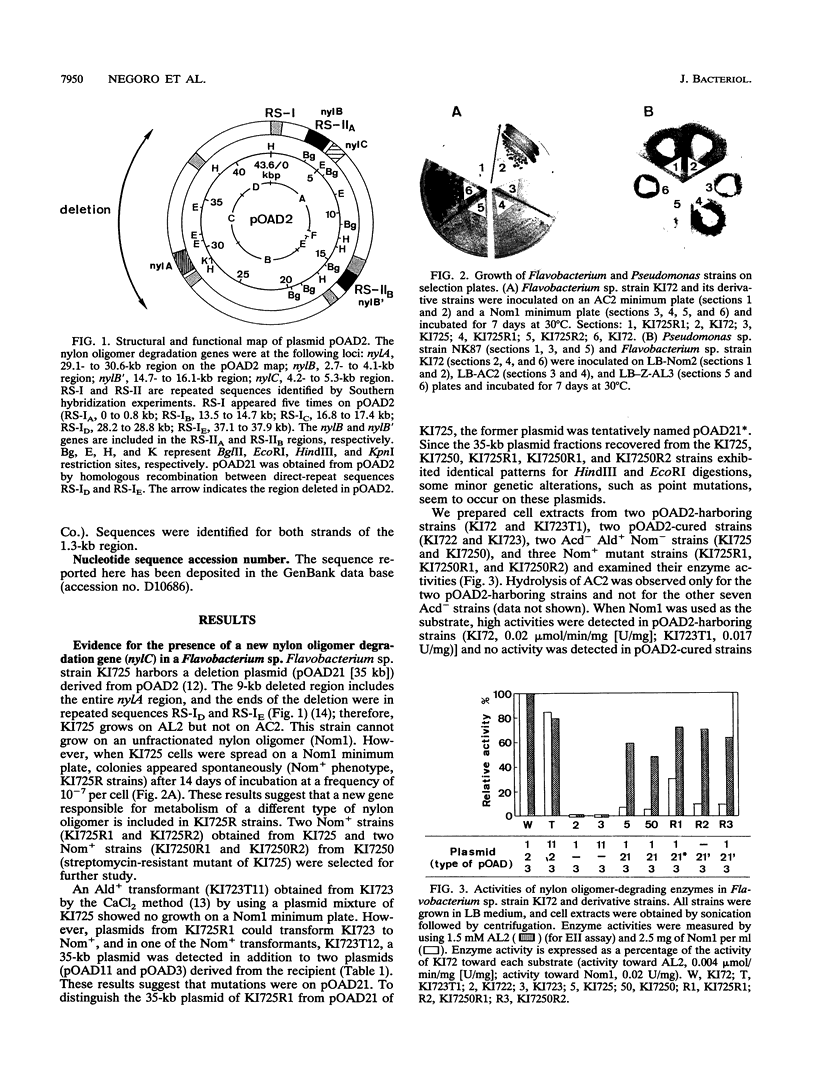
Flavobacterium sp. strain KI725 harbors plasmid pOAD21, a derivative of nylon oligomer-degradative plasmid pOAD2, in which all of nylA (the gene for 6-aminohexanoate cyclic dimer hydrolase [EI]) was deleted but nylB (the gene for 6-aminohexanoate dimer hydrolase [EII]) was retained. KI725 showed no growth on unfractionated nylon oligomers (Nom1) obtained from a nylon factory as a sole carbon and nitrogen source (Nom1 minimum plate). Extracts of KI725 cells possessed hydrolytic activity for Nom1 (approximately 5% of the activity of KI72), but pOAD2-cured strains (KI722 and KI723) showed no activity. KI725R strains which grew on the Nom1 minimum plate were spontaneously isolated from KI725 at a frequency of 10(-7) per cell. Activity toward Nom1 was enhanced in KI725R strains (10 to 30% of the activity of KI72). This new Nom1 degrading enzyme (EIII, the nylC gene product) hydrolyzed not only Nom1 but also the N-carbobenzoxy-6-aminohexanoate trimer, a substrate which was not hydrolyzed by either EI or EII. Cloning and sequence analysis showed that the nylC gene is located close to nylB on pOAD21 and is a 1,065-bp open reading frame corresponding to 355 amino acid residues. The nucleotide sequence of the nylC gene and the deduced amino acid sequence of EIII had no detectable homology with the sequences of nylA (EI) and nylB (EII).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of Lambda Lysogenicity during Bacterial Recombination in Escherichia Coli K12. Genetics. 1954 Jul;39(4):429–439. doi: 10.1093/genetics/39.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Kanagawa K., Negoro S., Takada N., Okada H. Plasmid dependence of Pseudomonas sp. strain NK87 enzymes that degrade 6-aminohexanoate-cyclic dimer. J Bacteriol. 1989 Jun;171(6):3181–3186. doi: 10.1128/jb.171.6.3181-3186.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S., Negoro S., Muramatsu M., Bisaria V. S., Sawada S., Okada H. 6-Aminohexanoic acid cyclic dimer hydrolase. A new cyclic amide hydrolase produced by Achromobacter guttatus KI74. Eur J Biochem. 1977 Nov 1;80(2):489–495. doi: 10.1111/j.1432-1033.1977.tb11904.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Terada T., Taniguchi T., Takene Y., Masuda S., Matsunaga N., Okada H. Purification and characterization of 6-aminohexanoic-acid-oligomer hydrolase of Flavobacterium sp. Ki72. Eur J Biochem. 1981 Jun 1;116(3):547–551. doi: 10.1111/j.1432-1033.1981.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Negoro S., Mitamura T., Oka K., Kanagawa K., Okada H. Determination of the active-site serine of 6-aminohexanoate-dimer hydrolase. Eur J Biochem. 1989 Nov 20;185(3):521–524. doi: 10.1111/j.1432-1033.1989.tb15144.x. [DOI] [PubMed] [Google Scholar]
- Negoro S., Nakamura S., Kimura H., Fujiyama K., Zhang Y. Z., Kanzaki N., Okada H. Construction of hybrid genes of 6-aminohexanoic acid-oligomer hydrolase and its analogous enzyme. Estimation of the intramolecular regions important for the enzyme evolution. J Biol Chem. 1984 Nov 25;259(22):13648–13651. [PubMed] [Google Scholar]
- Negoro S., Nakamura S., Okada H. DNA-DNA hybridization analysis of nylon oligomer-degradative plasmid pOAD2: identification of the DNA region analogous to the nylon oligomer degradation gene. J Bacteriol. 1984 May;158(2):419–424. doi: 10.1128/jb.158.2.419-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoro S., Shinagawa H., Nakata A., Kinoshita S., Hatozaki T., Okada H. Plasmid control of 6-aminohexanoic acid cyclic dimer degradation enzymes of Flavobacterium sp. KI72. J Bacteriol. 1980 Jul;143(1):238–245. doi: 10.1128/jb.143.1.238-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoro S., Taniguchi T., Kanaoka M., Kimura H., Okada H. Plasmid-determined enzymatic degradation of nylon oligomers. J Bacteriol. 1983 Jul;155(1):22–31. doi: 10.1128/jb.155.1.22-31.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Negoro S., Kimura H., Nakamura S. Evolutionary adaptation of plasmid-encoded enzymes for degrading nylon oligomers. Nature. 1983 Nov 10;306(5939):203–206. doi: 10.1038/306203a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Fukuyama S., Kanzaki N., Kanagawa K., Negoro S., Okada H. High homology between 6-aminohexanoate-cyclic-dimer hydrolases of Flavobacterium and Pseudomonas strains. J Bacteriol. 1989 Jun;171(6):3187–3191. doi: 10.1128/jb.171.6.3187-3191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]





