Abstract
Visual information modulates the balance response evoked by a pure vestibular perturbation (galvanic vestibular stimulation, GVS). Here we investigate two competing hypotheses underlying this visual–vestibular interaction. One hypothesis assumes vision acts in a feedforward manner by altering the weight of the vestibular channel of balance control. The other assumes vision acts in a feedback manner through shifts in the retinal image produced by the primary response. In the first experiment we demonstrate a phenomenon that is predicted by both hypotheses: the GVS-evoked balance response becomes progressively smaller as the amount of visual self-motion information is increased. In the second experiment we independently vary the pre-stimulus and post-stimulus visual environments. The rationale is that feedback effects would depend only upon the post-stimulus visual environment. Although the post-stimulus visual environment did affect later parts of the response (after ∼400 ms), the pre-stimulus visual environment had a strong influence on the size of the early part of the response. We conclude that both feedforward and feedback mechanisms act in concert to modulate the GVS-evoked response. We suggest this dual interaction that we observe between visual and vestibular channels is likely to apply to all sensory channels that contribute to balance control.
The balance system relies upon sensory information to report how the body is moving and therefore its current state of instability. Each sensory system conveys its own particular view of body motion: Head-in-space acceleration (vestibular); relative eye-environment motion (visual); joint rotation (proprioceptive); contact pressure changes (cutaneous). This raises a fundamental question: How is this diverse information combined to stabilize the body? On the one hand, there is a level of independence between sensory channels since any unsteadiness signalled by one sensory channel on its own is sufficient for the balance system to respond. This is known from experiments in which single sensory channels have been perturbed in isolation to evoke balance responses. On the other hand, some of these experiments have also suggested a degree of interdependence between sensory channels. A good example comes from vestibular perturbation studies in which galvanic vestibular stimuli (GVS) are used to evoke balance responses (Fitzpatrick & Day, 2004). The magnitude of the balance response to this stimulus is not fixed but depends upon information signalled by non-vestibular sensory inputs. For instance, GVS produces a larger balance response when visual information is removed (Njiokiktjien & Folkerts, 1971; Smetanin et al. 1990; Britton et al. 1993; Fitzpatrick et al. 1994; Welgampola & Colebatch, 2001; Guerraz & Day, 2005). Similarly, subjects with pathologically reduced somatosensory input show enhanced GVS-evoked responses (Horak & Hlavacka, 2001). The effect is even more dramatic in a deafferented subject with total loss of all large-diameter afferent fibres below the neck (Day & Cole, 2002). This subject's response to GVS was observed to be an order of magnitude larger than in healthy subjects and was approximately a factor of three larger with eyes closed than with them open. In general, the response to a single-channel perturbation is increased when the availability of other sensory information is reduced.
One interpretation of these observations is that the balance system dynamically adjusts the weights of the sensory channels. This is a concept that has been put forward previously as an important principle of motor control in general (Prochazka, 1989), and in particular with respect to multisensory integration for human balance (Nashner & Berthoz, 1978; Horak & Macpherson, 1996; Day & Cole, 2002; Oie et al. 2002; Cenciarini & Peterka, 2006). Here we employ this concept and investigate the following hypothesis: the weights given by the balance system to inputs from each sensory channel vary as a function of the channel's relative acuity for self-motion detection. In this model, all channels have the potential to evoke a balance response but more weight is given to the channel that carries greater or more reliable self-motion information. The proportional representation voting hypothesis proposed by Day & Cole (2002) is one such model. Thus, a loss of a sensory channel will increase the weights of the remaining channels because they carry relatively more self-motion information than with all channels intact. A loss of multiple channels will lead to an even greater weighting of the remaining channels, as in the case of the deafferented, vision-deprived subject described above.
In the first experiment, we tested a prediction of this feedforward hypothesis in the context of vestibular–visual interaction. The hypothesis predicts that the size of response to a fixed vestibular test stimulus should be an inverse function of the amount of self-motion information provided by the visual channel. To investigate this, we measured the whole-body balance response to GVS in subjects standing with their eyes open in four visual environments (Fig. 1): (i) complete blackout, (ii) a single point source of light, (iii) a two-dimensional grid of lights, and (iv) a three-dimensional structure of lights. The environments have been designed to give variable amounts of visual self-motion information. The blackout environment gives no visual information regarding self-motion, whereas a single point source gives minimal information. The 2-D grid gives more information than the point source because it stimulates more of the retina and has a radial structure that can signal body rotation as well as translation. The 3-D structure, although designed to stimulate the same parts of the retina as the 2-D grid, carries greater self-motion information by virtue of parallax, as near and far images move differentially on the retina (Guerraz et al. 2000, 2001). Therefore, the hypothesis predicts that the GVS-evoked response should get progressively smaller as we present richer visual environments. Part of these data has been published in brief form (Day et al. 2002).
Figure 1. Geometrical configuration of coloured LEDs used to generate the four visual environments.
Subjects stood in a blacked-out room whilst viewing no LEDs (vis0), a single blue LED (vis1), a two-dimensional grid of alternating red and green LEDS (vis2), or a three-dimensional structure of red and green LEDs (vis3).
In the second experiment we tested an alternative hypothesis involving a visual feedback mechanism that predicts similar effects of visual self-motion information. Visual feedback mechanisms come into play as the GVS-evoked body movement response causes a shift in the retinal image. The visual flow resulting from the moving retinal image has the potential to produce a visually evoked whole-body response similar to that produced by a moving visual environment (Lestienne et al. 1977; Bronstein & Buckwell, 1997). The direction of such a secondary visually evoked response would act effectively to attenuate the primary vestibularly evoked response. Furthermore, it is likely that the size of the visually evoked response would co-vary with the self-motion acuity provided by the visual environment. At first glance it might seem possible to distinguish feedback from re-weighting mechanisms by the latency of the effect. Feedback requires a finite movement duration and neural transport delays before the process can start, whereas re-weighting processes would have no such lag. However, this reasoning is complicated by the fact that GVS produces eye movements as well as body movements (Pfaltz, 1970; Quarck et al. 1998; Zink et al. 1998; Schneider et al. 2002; Séverac Cauquil et al. 2003). The visual flow from an involuntary eye movement may also produce a compensatory whole-body response, but one that overlaps with the earliest part of the vestibular response. This is because the latency of eye movement (< 50 ms; Séverac Cauquil et al. 2003) is much shorter than the latency of body movement (∼200 ms). Therefore, in the second experiment we attempted to distinguish a visual feedback mechanism from a re-weighting process in the following way. Subjects stood in one of two visual environments (blackout or 3-D structure) that could sometimes switch around the time of GVS onset. This enabled us to compare GVS-evoked responses that start from the same visual baseline but with radically different amounts of visual feedback during stimulation, and vice versa. We argue that re-weighting effects would be sensitive to pre-stimulus baseline visual information, whereas feedback effects would depend only upon the visual information available during stimulation. The results suggest that visual re-weighting and feedback mechanisms both play a role in shaping the balance response to a pure vestibular perturbation.
Methods
Subjects
Twenty-one healthy subjects consented to participate according to guidelines of the local ethics committee and to the Declaration of Helsinki. All subjects had no history of vestibular, orthopaedic or neuromuscular disease. Ten subjects (7 males, 3 females), ranging in age from 24 to 49 years (mean (s.d.): 30.8 years (7.7 years)), participated in experiment 2a. Eleven different subjects (6 male, 5 female), ranging in age from 24 to 51 years: 29.8 years (8.0 years)), participated in experiment 2b. Ten of these 21 subjects participated in experiment 1.
Galvanic stimulation
A custom-built constant-current stimulator was used for galvanic vestibular stimulation (GVS). Bipolar, binaural stimuli were applied via 3 cm diameter electrodes adhered to the mastoid processes. GVS consisted of a rectangular current profile of 0.5 mA lasting for 4 s.
Visual display
The visual display system consisted of small (2 mm diameter) coloured light-emitting diodes (LEDs) suspended on fine wires on two vertical planes separated by 1 m (Fig. 1). The back plane could be seen through the front plane (which was mainly empty space). The front plane contained a square grid of 121 LEDs (11 × 11) spaced 25 mm apart. They were alternately coloured red and green except for a blue central LED. The back plane contained a grid of 60 green LEDs spaced 150 mm apart. The geometry was such that for a subject standing 50 cm in front of the display, the green LEDs of the back plane appeared approximately at the same retinal locations as the green LEDs of the front plane. The green LEDs in the back plane were brighter than those in the front plane to account for their greater distance from the subject's eyes.
The LEDs were illuminated in different combinations to provide four different visual environments. A 3-D visual environment (vis3) was produced by illuminating the green rear LEDs, the red front LEDs and the blue central fixation LED. The green rear LEDs appeared in the gaps between the red front LEDs. This was reduced to a 2-D visual environment (vis2), without substantially altering the retinal image, by turning off the rear green LEDs and turning on the front green LEDs together with the front red LEDs and blue central LED. The visual information was reduced further by turning off all LEDs apart from the blue central fixation LED (vis1). Finally, all LEDs were turned off to give the fourth condition of no visual information (vis0).
The visual display was mounted in a completely blacked-out room. Its frame was painted black and the walls of the room were draped with black material. The intensity of the LEDs was reduced to a level such that when they were illuminated they were the only objects visible to the subject.
Whole-body recordings
Subjects stood on a force plate (type 9281B, Kistler Instrumente AG, CH-8408 Winterthur, Switzerland), which registered ground reaction force in three dimensions, facing the visual display system. The position of the display was adjusted such that its centre was at the level of the eyes and its front plane 50 cm from the eyes. Motion of the body was measured in three dimensions using an opto-electronic motion analysis system (Selspot II), which tracked the movement of a number of infra-red-emitting diodes fixed to various sites on the body. We present data only from the marker fixed to the skin overlying the C7 spinous process. The position and force data were collected with a sampling frequency of 100 Hz.
Protocol
Experiment 1: Influence of content of visual environment
At the start of a trial, one of the four visual environments was selected pseudo-randomly and the corresponding LEDs were illuminated. After 3 s an auditory cue (1 kHz, 100 ms) was sounded which acted as a signal for the subject to stand still. This was followed by a random delay of 1–3.5 s after which data collection began and lasted for a period of 12 s. In two-thirds of trials, GVS was applied for 4 s starting 4 s after the beginning of data collection. At the end of a trial the visual environment was switched to the default single LED (vis1), which cued the subjects to relax. A new trial began after an interval of approximately 10 s.
The four visual environments (vis0, vis1, vis2 and vis3) were paired with each of the three GVS conditions (no stimulus, anode right, anode left) giving 12 conditions in total. Each condition was repeated 6 times in pseudo-random order giving a total of 72 trials per subject.
Experiment 2: Influence of visual feedback
The 2-D grid (vis2) was illuminated continuously between trials. At 1–3.5 s prior to the start of a trial the visual scene was changed to either blackout (vis0) or 3-D (vis3) conditions. This acted as a cue to subjects that a trial had started and that they were to remain still. There were four visual conditions. In half the trials the visual scene remained constant throughout (vis0 or vis3). In the remaining half of trials the visual scene was switched partway through the trial. Either the 3-D scene was switched to blackout (vis3to0) or vice versa (vis0to3). In two thirds of trials, 0.5 mA GVS was applied for 4 s starting 4 s after the beginning of the trial. Each of the three GVS conditions (no stimulus, anode right, anode left) was paired with each of the four visual conditions to give a total of 12 experimental conditions. The 12 conditions were presented in pseudo-random order. Trials were recorded in three blocks of 24 trials (72 trials total; 6 per condition) with 5 min rests between each block.
Two timings of visual scene change relative to GVS onset were investigated in two separate experiments on different subjects. In the first (experiment 2a), the visual scene was switched 150 ms after the onset of GVS. This timing was chosen on the basis that the scene change would occur before the onset of GVS-evoked head or body movement. In the second series of experiments (experiment 2b) the visual scene was switched at the same time as GVS onset. This ensured that the scene change occurred before any GVS-evoked movements of the eye.
Data analysis
For each subject, the marker positions and ground reaction forces were averaged across trials for each condition. Responses to the two polarities of stimulation were oppositely directed but otherwise symmetrical. They were therefore averaged together after inversion of the responses obtained with the anode left. Measurements were made on these averaged responses.
An early manifestation of the response to GVS is the pulse of force produced between the feet and the ground. This is created by the stimulus-evoked change in motor drive to the various leg muscles (Day et al. 1997), and acts to accelerate the body in a specific direction. With the head facing forward, this change in ground reaction force produces a lateral motion of the body in a direction towards the anodal ear, as shown in many previous studies (reviewed in Fitzpatrick & Day, 2004). Due to the large inertia of the human body, much of the force response occurs before there is appreciable body displacement (see Fig. 2B). Therefore, our analyses focus mainly on the lateral component of the horizontal force pulse response. Measurements were made on the averaged force and displacement records of each subject at specific times after stimulus onset after first subtracting the mean pre-stimulus signal amplitude obtained from the initial 4 s of each average. We measured the force response at 400 ms, which we considered to be a time that would provide an early yet robust measure of the response. The displacement was measured at a later time of 2 s when the sway was well developed but before any compensatory reversals of direction had occurred.
Figure 2. Effect of visual environment on group mean lateral responses.
From top: lateral displacement of the neck marker at C7, lateral ground reaction force and GVS timing. Positive deflections are in the direction of the anodal ear. The visual environments were vis0 (black), vis1 (blue), vis2 (green) or vis3 (red). The same data are shown in A and B on different time scales.
In experiment 1, spontaneous body sway was measured from control trials in which GVS was not applied. First, the 3-D path of the marker at the level of C7 was computed after averaging every 10 data points to reduce both the noise and the effective sampling frequency to 10 Hz. The total length of this path during the trial was measured and divided by the trial duration of 12 s to give a mean sway speed. For each subject, the resulting sway speeds were averaged across all control trials of each visual condition.
The data were analysed statistically using a repeated-measures ANOVA (general linear model, SPSS) involving one factor (vision) with four levels (experiment 1: vis0, vis1, vis2, vis3; experiment 2: vis0, vis3, vis0to3, vis3to0). Planned analysis of contrasts were performed between three pre-selected pairs of the four levels. For experiment 1 the contrasts were planned to test whether successive levels of visual content have progressive effects. Thus, vis0 was contrasted with vis1, vis1 with vis2, and vis2 with vis3. For experiment 2 the contrasts were planned to test (a) whether the response is governed by the unswitched visual environments (vis0 versus vis3) as in experiment 1, and (b) whether there is an effect of vision over and above visual feedback. For this we contrasted switched and unswitched environments in which the feedback environment was the same but the initial environment was different (vis0 versus vis3to0; vis3 versus vis0to3). The rationale is that if differences between responses are due solely to visual feedback, there should be no difference between conditions in which the feedback environment is identical.
Results
Galvanic vestibular stimulation (GVS) caused subjects to sway laterally in a direction towards the anodal ear, the body coming to rest at a position that was tilted away from its normal upright posture (Fig. 2A). When the stimulating current was turned off, subjects returned to an upright position. These movements were initiated by the exertion of lateral forces on the ground that acted to accelerate the body sideways. The earliest of these force changes are shown in more detail in Fig. 2B. There was a small initial force deflection followed by a larger deflection in the opposite direction, with the latter producing the observed body sway. These two force changes correspond to the early- and medium-latency electromyographic responses that have been described (Britton et al. 1993; Fitzpatrick et al. 1994).
Influence of visual content
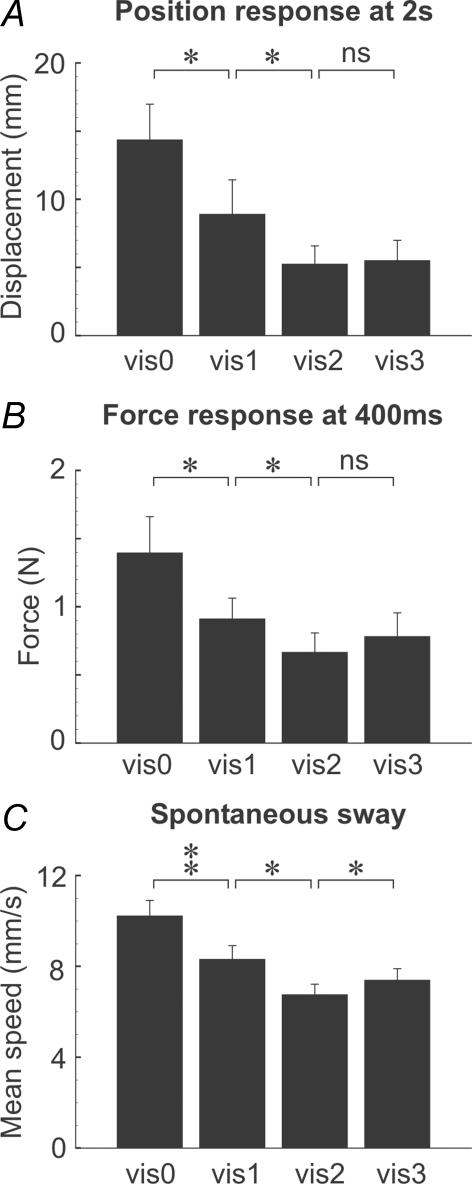
The degree of visual self-motion information had a strong effect on the mean speed of spontaneous body sway in the trials in which no stimulus was given (F3,27= 20.33, P < 0.001). As shown in Fig. 3C, analysis of contrasts revealed that spontaneous sway speed was significantly less in the vis1 than the vis0 environment (F1,9= 26.41, P= 0.001), and less in the vis2 than the vis1 environment (F1,9= 8.34, P= 0.018). However, there was a marginally significant increase in sway speed for the vis3 compared with the vis2 environment (F1,9= 5.25, P= 0.048).
Figure 3. Effect of visual environment on group mean summary responses.
Histograms show mean (+s.e.m.) GVS-evoked response in the direction of the anodal ear as measured from: A, neck (C7) position at 2 s latency, and B, ground reaction force at 400 ms latency. C shows mean speed of spontaneous sway without GVS. Statistical contrasts shown by brackets (*P < 0.05; **P < 0.01).
The degree of visual self-motion information also affected the response to GVS. Lateral displacement of the body at the level of the neck (C7) was measured at 2 s post-stimulus onset (Fig. 2A). At this time, displacement was strongly affected by the visual environment (F3,27= 9.47, P < 0.001). Contrast analysis (Fig. 3A) showed that displacement was significantly less for vis1 than for vis0 (F1,9= 8.16, P= 0.019), and less for vis2 than vis1 (F1,9= 5.94, P= 0.038). Differences between vis3 and vis2 were not significant (F1,9= 0.15, P > 0.05).
Force response measurements at an earlier post-stimulus time of 400 ms showed a similar pattern to that of later body displacement (Figs 2B and 3B). Force was strongly affected by visual environment (F3,27= 7.53, P= 0.001). Analysis of contrasts showed that the force response was less for vis1 than vis0 (F1,9= 6.59, P= 0.030), and less for vis2 than vis1 (F1,9= 6.80, P= 0.028). There was no significant difference between vis3 and vis2 (F1,9= 1.11, P > 0.05).
Visual feedback versus feedforward mechanisms
To differentiate between visual feedback and feedforward influences on the effects described above, we switched the visual environment around the time of GVS onset in two separate experiments. The visual environment was switched either before body movement (experiment 2a) or before eye movement (experiment 2b). The mean responses obtained under the various visual conditions are shown in Fig. 4A–D. These responses suggest a complex interaction with the visual environment.
Figure 4. Effect of switching the visual environment on group mean responses to GVS.
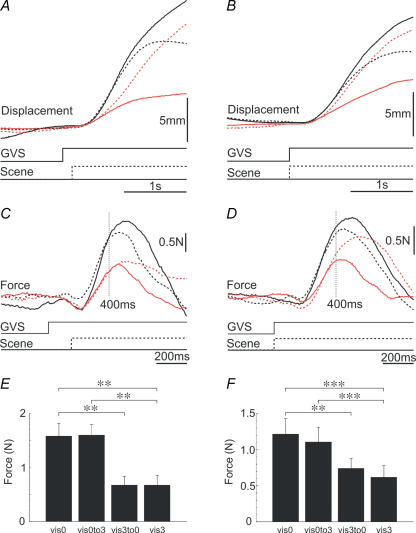
Traces show mean C7 lateral displacement (top panels) and mean lateral ground reaction force (middle panels; note different time scale) to unswitched (vis0, continuous black lines; vis3, continuous red lines) and switched (vis0to3, dashed black lines; vis3to0, dashed red lines) environments. Positive deflections are in the direction of the anodal ear. GVS indicates time of stimulation. Scene indicates time of visual switch. Lower histograms show mean (+s.e.m.) force response magnitude at 400 ms latency for the four visual conditions. Statistical contrasts shown by brackets (**P < 0.01; ***P < 0.001). The scene switch occurred either 150 ms (A, C and E) or 0 ms (B, D and F) after GVS onset.
Lateral motion of the body (Fig. 4A and B) appeared to be governed partially by the pre-stimulus visual environment, initially moving faster when it had been dark (black traces) than light (red traces). The subsequent effect of visual feedback can clearly be seen in these body displacement traces. When the 3-D scene was present during stimulation the body became stabilized by 2 s, whereas in the blackout environment it was still moving laterally. This caused the traces to cross over for the two switched conditions (red and black dashed traces).
A visual feedforward effect is suggested by the force records (Fig. 4C and D). As in experiment 1, when the visual condition was not switched the response was larger in the dark (vis0, continuous black trace) than in the light (vis3, continuous red trace). Switching the visual environment partway through the trial had a further effect. Switching from the dark to the light (vis0to3, dashed black trace) attenuated the response, whereas switching from the light to the dark (vis3to0, dashed red trace) augmented the response. However, these switching effects occurred relatively late in the traces. In contrast, the early part of the force response (until approximately 400 ms after GVS onset) appeared to be determined almost exclusively by the pre-stimulus visual environment.
The statistical analysis of the force response at 400 ms post-stimulus confirmed these impressions (Fig. 4E and F). For both experiments, the visual environment had strong effects on this early measure of response (experiment 1a, F3,27= 16.63, P < 0.001; experiment 1b, F3,30= 16.63, P < 0.001). First, we contrasted the two unswitched environments, which showed that the response was larger in the dark than in the light (vis0 versus vis3: experiment 2a, F1,9= 13.76, P= 0.005; experiment 2b, F1,10= 38.83, P < 0.001). To investigate whether this effect can be explained solely on the basis of a visual feedback mechanism, we contrasted switched and unswitched environments in which the feedback environment was identical but the initial environment was different. These contrasts showed highly significant differences (vis0to3 versus vis3: experiment 2a, F1,9= 23.76, P= 0.001; experiment 2b, F1,10= 26.73, P < 0.001; vis3to0 versus vis0: experiment 2a, F1,9= 23.07, P= 0.001; experiment 2b, F1,10= 15.23, P= 0.003). Thus, the attenuating effect of visual information on the GVS response cannot be due solely to a visual feedback mechanism.
Discussion
The sensory-weighting hypothesis outlined in the introduction predicts an inverse relationship between the size of a vestibularly evoked balance response and the acuity of visual self-motion information. Visual environments with greater self-motion acuity increase body stabilization and reduce spontaneous body sway (Paulus et al. 1984). Therefore, the graded effect that our visual environments had on spontaneous sway indicate that we succeeded in varying visual self-motion acuity. A single point source was more effective than no vision at all, and a 2-D grid of point sources was more effective than a single source. However, the 3-D structure, contrary to expectation, was slightly less effective than the 2-D grid. One possible explanation for this is that the 3-D structure produced a slight visual disturbance in some subjects; after prolonged viewing, an illusory reversal of the 3-D display was sometimes perceived in which the back-plane of lights appeared in front of the front-plane. There may also have been a ceiling effect with maximum stabilization occurring with a 2-D environment. Nevertheless, our environments were sufficiently graded to produce a usable range of self-motion information. As predicted by the sensory-weighting hypothesis, the size of response to GVS was similarly graded according to this effectiveness in signalling self-motion.
The alternative hypothesis invokes visual feedback mechanisms. The visual signals supplying such a process could arise either from vestibularly evoked head-in-space movement or from vestibularly evoked eye-in-head movement. In standing subjects, the head-in-space response is a roll tilt plus lateral linear translation, both occurring in the direction of the anodal ear (Day et al. 1997). The ocular response is dominated by torsion in which the top of the eye rotates towards the anodal ear (Zink et al. 1997, 1998; Watson et al. 1998; Kleine et al. 1999; Schneider et al. 2000, 2002; Séverac Cauquil et al. 2003), plus a smaller horizontal component in which the eyes deviate horizontally towards the anodal ear (Pfaltz, 1970; Breson et al. 1971; Zink et al. 1997, 1998; Quarck et al. 1998; Karlberg et al. 2000; Séverac Cauquil et al. 2003). Therefore, for both head-in-space and eye-in-head movements, the direction of compensatory response to the visual disturbance would be a counter-rotation and translation away from the anodal ear. Such movements would act to oppose the primary GVS whole-body response.
A visual perturbation arising from head-in-space motion can alter only the later parts of the GVS response because of the long latency of body displacement (∼200 ms) and the further delays due to nerve conduction and central processing of the visual input. However, visual inputs arising from eye movements, which are known to have a much shorter latency of around 50 ms (Séverac Cauquil et al. 2003), could affect even an early part of the sway response that is produced by leg muscle EMG change at ∼120 ms (medium-latency response; Britton et al. 1993). The result of switching the visual environment suggests that feedback effects do indeed contribute to the overall behaviour but that they have little effect before approximately 400 ms, as judged from the separation of traces in Fig. 4C and D. Interestingly the traces seem to separate slightly earlier when the visual scene was switched earlier. This may reflect a feedback effect coming from eyes-in-head movement, but it need not. For example, a feedforward gain control mechanism, which is linked to the current visual environment, would undergo a dynamic adjustment when the visual environment suddenly changes. Although we do not know the dynamics of such a gain change, it would be reasonable to assume that the further in time one goes from the switching event the more the gain will reflect the new switched visual environment. The earlier divergence of traces with an earlier switch may simply reflect the dynamics of such a gain change rather than an eyes-in-head visual feedback effect.
The response varied with pre-stimulus visual environment even when the post-stimulus feedback environments were the same. Thus, the results point to an additional feedforward modulation of vestibular-evoked balance responses. The sensory-weighting hypothesis provides a rationale for such feedforward effects, and is attractive for many reasons: (1) It explains the large changes in GVS response observed in the chronically deafferented subject IW (Day & Cole, 2002); (2) it is consistent with results from other experiments that have suggested strongly that dynamic re-weighting occurs between visual and tactile sensory channels (Oie et al. 2002), and between vestibular and somatosensory channels (Cenciarini & Peterka, 2006) for balance control; (3) it is consistent with the reciprocal visual–vestibular weighting postulated for self-motion perceptual processes (Brandt et al. 1998). However, other possible explanations are not ruled out by the present experiments. For example, the response modulation could have occurred as an indirect effect through a change in some other body state caused by the visual environment. One candidate would be an indirect effect through changes in spontaneous sway, which necessarily accompanied the changes in visual environment. Such an effect is unlikely to be through simple summation of a constant response superimposed upon a greater or lesser baseline. The spontaneous sway would have been directionally uncorrelated with the evoked response and so would have been just as likely to subtract from the response as to augment it. Equally, the greater fluctuations in muscle excitability associated with increased sway would be uncorrelated with the stimulus. It is feasible though that the increase in head motion with greater body sway could act indirectly to up-regulate the vestibular contribution to balance control. Indirect mechanisms of this sort need to be tested empirically against the direct sensory-weighting hypothesis in future experiments.
In conclusion, the results show that visual information affects the processing of vestibular information for the control of human balance. This vestibular–visual interaction engages at least two independent mechanisms: one in which current visual information either directly or indirectly alters the gain of the vestibulo-motor pathway, and one in which activity in that pathway is altered on the basis of changing visual feedback information. We suggest this dual interaction that we observe between visual and vestibular channels is likely to apply to all sensory channels that contribute to balance control.
Acknowledgments
This work was funded by The Medical Research Council. Mr Richard Bedlington (deceased) provided expert technical assistance and constructed the visual display and vestibular stimulation apparatus.
References
- Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual–vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain. 1998;121:1749–1758. doi: 10.1093/brain/121.9.1749. [DOI] [PubMed] [Google Scholar]
- Breson K, Elberling C, Fangel J. Galvanic nystagmography. Acta Otolaryngol. 1971;71:449–455. doi: 10.3109/00016487109125388. [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Buckwell D. Automatic control of postural sway by visual motion parallax. Exp Brain Res. 1997;113:243–248. doi: 10.1007/BF02450322. [DOI] [PubMed] [Google Scholar]
- Cenciarini M, Peterka RJ. Stimulus-dependent changes in the vestibular contribution to human postural control. J Neurophysiol. 2006;95:2733–2750. doi: 10.1152/jn.00856.2004. [DOI] [PubMed] [Google Scholar]
- Day BL, Cole J. Vestibular-evoked postural responses in the absence of somatosensory information. Brain. 2002;125:2081–2088. doi: 10.1093/brain/awf212. [DOI] [PubMed] [Google Scholar]
- Day BL, Guerraz M, Cole J. Sensory interactions for human balance control revealed by galvanic vestibular stimulation. Adv Exp Med Biol. 2002;508:129–137. doi: 10.1007/978-1-4615-0713-0_16. [DOI] [PubMed] [Google Scholar]
- Day BL, Severac Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol. 1997;500:661–672. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Guerraz M, Day BL. Expectation and the vestibular control of balance. J Cog Neurosci. 2005;17:463–469. doi: 10.1162/0898929053279540. [DOI] [PubMed] [Google Scholar]
- Guerraz M, Gianna C, Burchill P, Gresty MA, Bronstein AM. Effect of visual surrounding motion on body sway in a 3 D environment. Percept Psychophys. 2001;63:47–58. doi: 10.3758/bf03200502. [DOI] [PubMed] [Google Scholar]
- Guerraz M, Sakellari V, Burchill P, Bronstein AM. Influence of motion parallax in the control of spontaneous body sway. Exp Brain Res. 2000;131:244–252. doi: 10.1007/s002219900307. [DOI] [PubMed] [Google Scholar]
- Horak FB, Hlavacka F. Somatosensory loss increases vestibulospinal sensitivity. J Neurophysiol. 2001;86:575–585. doi: 10.1152/jn.2001.86.2.575. [DOI] [PubMed] [Google Scholar]
- Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 255–292. section 12. [Google Scholar]
- Karlberg M, McGarvie L, Magnusson M, Aw ST, Halmagyi GM. The effects of galvanic stimulation on the human vestibulo-ocular reflex. Neuroreport. 2000;11:3897–3901. doi: 10.1097/00001756-200011270-00058. [DOI] [PubMed] [Google Scholar]
- Kleine JF, Guldin WO, Clarke AH. Variable otolith contribution to the galvanically induced vestibulo-ocular reflex. Neuroreport. 1999;10:1143–1148. doi: 10.1097/00001756-199904060-00044. [DOI] [PubMed] [Google Scholar]
- Lestienne F, Soechting JF, Berthoz A. Postural readjustments induced by linear motion of visual scenes. Exp Brain Res. 1977;28:363–384. doi: 10.1007/BF00235717. [DOI] [PubMed] [Google Scholar]
- Nashner L, Berthoz A. Visual contribution to rapid motor responses during postural control. Brain Res. 1978;150:403–407. doi: 10.1016/0006-8993(78)90291-3. [DOI] [PubMed] [Google Scholar]
- Njiokiktjien C, Folkerts JF. Displacement of the body's centre of gravity at galvanic stimulation of the labyrinth. Conf Neurol. 1971;33:46–54. doi: 10.1159/000103102. [DOI] [PubMed] [Google Scholar]
- Oie KS, Kiemel T, Jeka JJ. Multisensory fusion: simultaneous re-weighting of vision and touch for the control of human posture. Cog Brain Res. 2002;14:164–176. doi: 10.1016/s0926-6410(02)00071-x. [DOI] [PubMed] [Google Scholar]
- Paulus WM, Straube A, Brandt Th. Visual stabilization of posture. Physiological stimulus characteristics and clinical aspects. Brain. 1984;107:1143–1163. doi: 10.1093/brain/107.4.1143. [DOI] [PubMed] [Google Scholar]
- Pfaltz CR. The diagnostic importance of nystagmography in the galvanic test. In: Stahle J, editor. Vestibular Function on Earth and in Space. Oxford New York: Pergamon; 1970. pp. 187–199. [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol. 1989;33:281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Quarck G, Etard O, Normand H, Pottier M, Denise P. Low intensity galvanic vestibulo-ocular reflex in normal subjects. Neurophysiol Clin. 1998;28:413–422. doi: 10.1016/S0987-7053(99)80025-2. [DOI] [PubMed] [Google Scholar]
- Schneider E, Glasauer S, Dieterich M. Central processing of human ocular torsion analyzed by galvanic vestibular stimulation. Neuroreport. 2000;11:1559–1563. [PubMed] [Google Scholar]
- Schneider E, Glasauer S, Dieterich M. Comparison of human ocular torsion patterns during natural and galvanic vestibular stimulation. J Neurophysiol. 2002;87:2064–2073. doi: 10.1152/jn.00558.2001. [DOI] [PubMed] [Google Scholar]
- Séverac Cauquil A, Faldon M, Popov K, Day BL, Bronstein AM. Short-latency eye movements evoked by near-threshold galvanic vestibular stimulation. Exp Brain Res. 2003;148:414–418. doi: 10.1007/s00221-002-1326-z. [DOI] [PubMed] [Google Scholar]
- Smetanin AB, Popov KE, Shlykov VYu. Changes in vestibular postural response determined by information content of visual feedback. Neirofiziologiya. 1990;22:80–87. [PubMed] [Google Scholar]
- Watson SRD, Brizuela AE, Curthoys IS, Curthoys IS, Colebatch JG, Macdougall HG, Halmagyi GM. Maintained ocular torsion produced by bilateral and unilateral galvanic (DC) vestibular stimulation in humans. Exp Brain Res. 1998;122:453–458. doi: 10.1007/s002210050533. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Vestibulospinal reflexes: quantitative effects of sensory feedback and postural task. Exp Brain Res. 2001;139:345–353. doi: 10.1007/s002210100754. [DOI] [PubMed] [Google Scholar]
- Zink R, Bucher SF, Weiss A, Brandt Th, Dieterich M. Effects of galvanic vestibular stimulation on otolithic and semicircular canal eye movements and perceived vertical. Electroencephalogr clin. Neurophysiol. 1998;107:200–205. doi: 10.1016/s0013-4694(98)00056-x. [DOI] [PubMed] [Google Scholar]
- Zink R, Steddin S, Weiss A, Brandt T, Dieterich M. Galvanic vestibular stimulation in humans: effects on otolith function in roll. Neurosci Lett. 1997;232:171–174. doi: 10.1016/s0304-3940(97)00610-1. [DOI] [PubMed] [Google Scholar]