Abstract
The regulation of ion channels involves more than just modulation of their synthesis and kinetics, as controls on their trafficking and localization are also important. Although the body of knowledge is fairly large, the entire trafficking pathway is not known for any one channel. This review summarizes current knowledge on the trafficking of potassium channels that are expressed in the heart. Our knowledge of channel assembly, trafficking through the Golgi apparatus and on to the surface is covered, as are controls on channel surface retention and endocytosis.
The intracellular trafficking of membrane proteins such as ion channels is complex. These molecules must be synthesized in the endoplasmic reticulum, assembled and processed appropriately, then trafficked and targeted to the membrane or membrane subdomains where they will function. Involved are ER resident proteins like chaperones and glycosylases, microtubules and their associated motors, transport vesicle and Golgi apparatus components, the actin cytoskeleton, myosins, anchoring proteins and more. The specific roles of these various components in the trafficking of ion channels are only beginning to be elucidated, and the entire trafficking pathway is not known for any single channel. This review will examine our knowledge of the trafficking of potassium channels that are expressed in the heart in the context of our knowledge of trafficking mechanisms in general. The generalized path of a channel travelling through a cardiac myocyte will be described from channel synthesis to recycling. Specific knowledge will be highlighted as will many of the gaps in that knowledge. While some results have been confirmed in cardiomyocytes, most work has been conducted using heterologous cells such as HEK293 cells and Xenopus oocytes.
Early events in the endoplasmic reticulum
A channel's life begins in the endoplasmic reticulum. As demonstrated for Kv1.3 – and probably true for most channels – assembly begins concurrently with synthesis on the rough ER (Kosolapov & Deutsch, 2003; Kosolapov et al. 2004; Robinson & Deutsch, 2005; Lu & Deutsch, 2005). Of course, not every nascent channel will assemble properly and quality control checks exist to ensure that only properly folded and assembled channels are exported from the ER. These checks as well as forward trafficking and/or retention signals are incorporated within the channel primary sequences and have profound influences on the fates of the newly synthesized channels. The simplest control on channel fate may relate to the inappropriate exposure of hydrophobic residues to solvent upon misfolding. Such a channel may be retained in the ER simply because it aggregates there with other misfolded proteins. Similarly, hydrophobic exposure to solvent probably promotes degradation of misfolded channels by the proteosome (Asher et al. 2006). This mechanism has been well explored in human ether-à-go-go-related protein (hERG) mutants that are defective in trafficking (Furutani et al. 1999; Gong et al. 2005; Gong et al. 2006; Anderson et al. 2006), some of which, interestingly, can be rescued by hERG-binding drugs (Zhou et al. 1999; Ficker et al. 2002; Paulussen et al. 2002; Gong et al. 2005; Rossenbacker et al. 2005) and/or chemical chaperones (Zhou et al. 1999; Anderson et al. 2006).
Quality control
To deal with problems beyond this probably non-specific aggregation, potassium channels incorporate very specific quality control systems. One such mechanism involves RXR motifs first identified in the KATP channel (Zerangue et al. 1999) and later shown to be functional also in Kir2.1, the major cardiomyocyte inward rectifier (Ma et al. 2001), as well as in hERG (Kupershmidt et al. 2002) and other channels (Chang et al. 1999; Margeta-Mitrovic et al. 2000; Standley et al. 2000). These motifs are hidden in properly folded and assembled channels but promote retention in the ER when they are exposed on the channel surface. RXR motifs are also present in many voltage-gated (Kv) channels, although, with the exception of hERG (Kupershmidt et al. 2002), their functions there have yet to be established. Other motifs, such as dilysine repeats (Harter & Wieland, 1998; Zerangue et al. 2001) also serve to inhibit trafficking out of the ER. The mechanisms by which these motifs prevent export from the endoplasmic reticulum have yet to be established, but some intriguing clues exist.
RXR motifs bind 14-3-3 proteins (Yuan et al. 2003), a family of molecules with diverse functions, thought to promote surface expression of membrane proteins (Shikano et al. 2006). While at first glance this seems inconsistent with a retention role for the RXR motifs, Yuan et al. (2003) have shown that the affinity of the 14-3-3 proteins for the RXR motifs is dramatically higher for tetrameric rather than monomeric constructs. Thus, one possibility is that 14-3-3 proteins promote the export of properly assembled channels. Interestingly, COPI, a component involved in recycling from the Golgi to the ER (Aoe et al. 1998; Nufer & Hauri, 2003), competed with 14-3-3 for RXR perhaps indicating that misassembled channels, with lower 14-3-3 affinity, are returned to the ER by COPI (Yuan et al. 2003). C-terminal dilysine repeats, which also function as ER retention signals (Cosson & Letourneur, 1994), have been shown to bind COPI (Mellman & Warren, 2000; Shikano & Li, 2003) and binding of COPI to a dibasic motif in the twin-pore KCNK3 potassium channel is inhibited by 14-3-3β (O'Kelly et al. 2002). In this case, the competition is indirect; 14-3-3 binding to an adjacent ‘release site’ drives dissociation of the COP protein from the channel (O'Kelly et al. 2002). Another ER retention signal, KDEL (Zerangue et al. 1999), binds to the KDEL receptor in the transport vesicles, and this also targets proteins for Golgi-to-ER recycling (Zhou et al. 2002; Cabrera et al. 2003).
Forward trafficking signals – On to the Golgi
In addition to ER retention/recycling signals, potassium channels harbour forward trafficking signals that promote export from the ER. This is again via ER-to-Golgi transport, a complex and GTP-dependent process, involving COPI and COPII, additional Sec proteins, as well as a pair of Rab proteins and SarI (Lee et al. 2004; Murshid & Presley, 2004). COPII concentrates cargo in the transitional ER and COPI is recruited to the newly formed transport vesicles from where it retrieves recycling, escaped ER- and misfolded proteins back to the ER. Transport is conducted along microtubules and is dependent on the dynein motor (Presley et al. 1997).
Forward trafficking signals in potassium channels are quite diverse. FYCENE serves such a function in Kir2.1 (Ma et al. 2001; Stockklausner et al. 2001), Kv1.4 harbours a VXXSL signal, and Kv1.5 harbours a similar but less effective VXXSN (Zhu et al. 2003; Li et al. 2000). The cyclic nucleotide-binding domains of hERG, ERG3 and HCN2 may also act as forward-trafficking signals (Akhavan et al. 2005) and the Kv1.4 pore appears to harbour a pore-based forward trafficking determinant (Watanabe et al. 2004). The evidence for the latter, though, is consistent also with the absence of a retention motif in Kv1.4 that is present in Kv1.1. It has been suggested that these forward trafficking motifs may interact directly or indirectly with COPII (Ma & January, 2002). Certainly dileucine motifs, which are ubiquitously present in potassium channels and which function as forward trafficking motifs in other membrane proteins, bind to COPII (Nufer et al. 2002). Of course, the diversity in export signals in the various channels implies that a complex scenario is probably operating, leading to differential regulation of channel trafficking. Whether all forward trafficking signals function by promoting ER-to-Golgi transport remains to be established. Also, of course, forward trafficking signals are not the sole promoters of exit from the ER.
Chaperones like Hsp70/Hsc70, Hsp90 and calnexin have been shown to facilitate ER exit of hERG (Ficker et al. 2003; Gong et al. 2006) and Kv1.2 (Manganas & Trimmer, 2004). Very probably, these do so not via an active forward trafficking role but rather by promoting proper folding/assembly of the channels, although, in the case of Hsp70/Hsc70, there may be a role in facilitating vesicular trafficking and membrane fusion as well (Zinsmaier & Bronk, 2001; Clay & Kuzirian, 2002). β-subunits, KChIPs, KChAP and other accessory proteins also bind to their target channels in this locale, promoting forward trafficking via chaperone-like activities (Shi et al. 1996; Wible et al. 1998; Pongs et al. 1999; Kuryshev et al. 2000; Bahring et al. 2001). This is in addition to their roles, in the cases of KChIPs and the β-subunits, as modifiers of channel kinetics (Wible et al. 1998; An et al. 2000; Hanlon & Wallace, 2002; Nerbonne & Guo, 2002; Aimond et al. 2005).
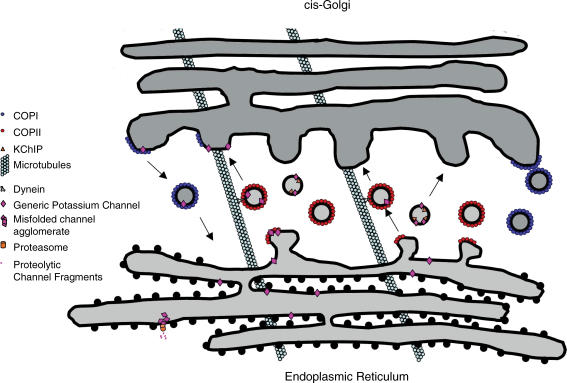
Interestingly, KChIP was recently reported to traffic from the ER to Golgi in vesicles lacking COPII (Hasdemir et al. 2005). Sar1 activity, essential for most ER-to-Golgi traffic (Yoshihisa et al. 1993; Kuge et al. 1994; Gurkan et al. 2006) was not required. That promotion of Kv4.2 expression by the neuron-specific KChIP3 is modulated by GRKs and calcineurin (Ruiz-Gomez et al. 2007) strongly suggests that KChIPs also function downstream of ER-to-Golgi transport and as more than mere chaperones. It will be very interesting to learn what pathway(s) are employed for this trafficking, how it is regulated and whether other channels are transported similarly. Figure 1 summarizes some of the major features of ER-to-Golgi trafficking.
Figure 1. ER to Golgi trafficking of cardiac potassium channels.
Following synthesis on the rough ER, channels which make their way to the transitional ER are recruited into COPII-coated vesicles. They then traffic along microtubules to the cis-Golgi in a dynein-dependent manner. At the cis-Golgi, properly assembled channels are sent on through the organelle. KChIP, accompanied by Kv4.2, is shown trafficking to the Golgi in vesicles lacking COPII. Misassembled channels or those with ‘ER-retention motifs’ are illustrated returning from Golgi apparatus in COPI-coated vesicles. Also illustrated are agglomerated misfolded channels being degraded by a proteasome after translocation out of the ER.
Through the Golgi and on to the sarcolemma
The sorting and targeting of cardiac potassium channels has barely been explored. Nevertheless, it is highly likely that this sorting, like that of other newly synthesized secretory and plasma membrane proteins, begins in the Golgi apparatus (Gu et al. 2001). Glycosylation is completed in the Golgi apparatus, a step important for the surface expression of channels such as EagI (Napp et al. 2005), KATP (Conti et al. 2002), Kv1.4 (Watanabe et al. 2004) and other Kv1-type channels (Khanna et al. 2001; Folco et al. 2004), although the degree to which cardiac potassium channels are sensitive to interference with glycosylation is variable. While the stability of glycosylation-defective hERG channels at the membrane is reduced, glycosylation is not required for hERG expression in heterologous cells (Gong et al. 2002).
Basic sorting to the sarcolemma or to intracellular organelles certainly occurs in the Golgi (specifically, the trans-Golgi network) (Gu et al. 2001), but it is likely that targeting to specific sarcolemma subdomains is effected mainly downstream of this organelle (Cereijido et al. 2003; Mogelsvang & Howell, 2006). We know that Kir2.1 and other inward rectifiers require an intact N-terminal signal for exit from the Golgi when expressed in heterologous cells (Stockklausner & Klocker, 2003), but whether this is a bona fide sorting event is unclear.
In neurons, the targeting of potassium channels is highly specific. Kv4.2, for example, is generally targeted to the distal regions of dendrites, whereas in myelinated neurons, Kv1 channels localize to juxtaparanodal regions (reviewed in Trimmer & Rhodes (2004)). Known to involve various motor proteins, the actin and microtubule cytoskeletons, scaffolding proteins and accessory subunits, just how these various components work together to achieve directed targeting is very poorly understood (reviewed in Lai & January (2006)). Similarly, while specific targeting clearly occurs in cardiac myocytes (see below), we have little insight into the mechanism(s) by which this is effected. Nevertheless, some progress has been made in identifying proteins that enhance forward trafficking and targeting of cardiac potassium channels.
Involvement of MAGUK proteins
The membrane-associated guanylate kinase (MAGUK) protein CASK has been implicated in the targeting of Kir2 channels (Leonoudakis et al. 2004) in heart and brain, where it forms complexes with other PDZ proteins, i.e. SAP97, Veli-1, Veli-3 and Mint1. Expression of a dominant negative CASK mutant disrupts basolateral targeting of these channels in polarized epithelial cells (Leonoudakis et al. 2004); Kir2.2 localizes non-specifically to both the basolateral and the apical membrane, instead. While CASK may indeed be intimately involved in directing potassium channels to their ultimate destinations in the cell, MAGUKs are more generally thought to serve as scaffolding proteins that anchor proteins at their targeted locations (Carnegie & Scott, 2003) rather than targeting the channels, per se. Given that MAGUK complexes associate with motor proteins (Hanada et al. 2000; Naisbitt et al. 2000; Wu et al. 2002), though, the importance of proteins like CASK and SAP97 in intracellular trafficking may be greater than thought.
SAP97 has been implicated in the trafficking of other potassium channels, as well. In heterologous expression systems, Kv1.5 has been reported to interact with SAP97 (Murata et al. 2001; Godreau et al. 2002; Godreau et al. 2003) and to localize to lipid rafts (Martens et al. 2000; Martens et al. 2001) where it forms a tripartite complex with caveolin-3 and SAP97 (Folco et al. 2004). However, in rat and canine cardiac myocytes no evidence of lipid raft localization or of Kv1.5 binding to either SAP97 (Eldstrom et al. 2003) or caveolin-3 could be found (Eldstrom et al. 2006). The interaction of Kv1.5 in heterologous cells may be an artifact of transient overexpression (Mathur et al. 2006), although, if so, the artifact is an interesting one, occurring only in transiently transfected cells and not in stable lines. Nevertheless, SAP97 overexpression increases the levels of Kv1.5 at the cell surface. The mechanism by which this occurs has yet to be elucidated.
Other Kv channels have also been shown to interact with SAP97 (Tiffany et al. 2000) and to do so directly with the closely related PSD95 (Kim et al. 1995; Imamura et al. 2002), although the latter is not expressed in heart (Seeber et al. 2000). Unlike its effect on Kv1.5 though, SAP97 co-expression down-regulates these other Kv channels (Tiffany et al. 2000). While conceivably also an artifact of overexpression, it is certainly possible that SAP97 plays a role in regulating the forward trafficking of these channels as well.
Cytoskeletal players
SAP97 has been shown to bind myosin VI (Wu et al. 2002), a molecular motor implicated in secretion, endoyctosis and submembrane vesicular trafficking along the actin cytoskeleton (Lister et al. 2004). Myosins, tracking along the actin cytoskeleton, are involved mainly in trafficking near the cell surface and it has been long known that disruption of the actin cytoskeleton can have profound effects on potassium channel functional expression (Calaghan et al. 2004). Such disruption dramatically increases the expression of Kv1.5 (Maruoka et al. 2000; Cukovic et al. 2001; Mason et al. 2002) and Kv4.2 (Wang et al. 2004) in both heterologous cells and cardiomyocytes. Similarly, disruption of the microtubule cytoskeleton increases Kv1.5 surface expression (Choi et al. 2005), although microtubule disruption did not affect Kv2.1 expression in heterologous cells (Martens et al. 2000). Long-range vesicular transport generally involves the microtubule cytoskeleton and the kinesin and dynein motors (Karcher et al. 2002).
Kinesins, which track along the microtubule cytoskeleton, have recently been directly implicated in the trafficking of Kv4.2. The neuron-specific kinesin isoform Kif17 was shown to interact with Kv4.2 in brain lysates and dissociated cortical neurons (Chu et al. 2006); expression of a dominant negative Kif17 construct in the neurons blocked surface expression of the channel. Deletion of a previously identified dileucine targeting domain from the channel, though, did not prevent Kv4.2 trafficking but, rather than being restricted to the dendritic tree, these channels were mistargeted and appeared widely throughout the neurons. While Kif17 is not expressed in heart (Setou et al. 2000), it is reasonable to expect that another kinesin isoform is involved in Kv4.2 transport in cardiomyocytes.
Plasma membrane insertion
Whatever the route by which a channel makes its way to the cell surface, it must insert into the sarcolemma once there. Membrane insertion appears to be a conserved process and while the specifics for most channels are unknown, the process is essentially certain to involve SNARE-mediated fusion of exocytotic vesicles with the sarcolemma (Hong, 2005; Jahn & Scheller, 2006). SNAREs are thought to deform membranes, disturbing the hydrophobic–hydrophilic boundary and directly causing fusion (Jahn & Scheller, 2006). Indeed, the exocytotic fusion SNARE proteins SNAP25 and Syntaxin 1A have been implicated in Kv1.1 and Kv2.1 plasma membrane integration (Fili et al. 2001; Ji et al. 2002; MacDonald et al. 2002; Michaelevski et al. 2002; Leung et al. 2003).
Localization, surface retention, recycling and degradation
Potassium channels don't merely traffic non-specifically to the sarcolemma. Instead, individual channel types localize to specific cell surface domains. ERG1 localizes to the transverse tubular network in rat atrial and ventricular myocytes whereas KCNQ1 (KvLQT1) is found in the peripheral sarcolemma and in T-tubules (Rasmussen et al. 2004). Kv4.2, Kir2.1 and TASK-1 are also localized at least in part to T-tubules (Takeuchi et al. 2000; Clark et al. 2001; Jones et al. 2002) and Kv1.5 is highly enriched at the intercalated disk of rat and canine atrial and ventricular myocytes (Mays et al. 1995; Eldstrom et al. 2006), as, in part, is Kv4.2 (Barry et al. 1995) and Nav1.5 (Maier et al. 2002; Kucera et al. 2002). In ventricular myocytes, Kv1.5 is found also in proximity to the Z-lines (Eldstrom et al. 2006). Even in heterologous systems, potassium channels sometimes segregate into distinct cell surface microdomains (O'Connell & Tamkun, 2005). These distinct localizations may result from specific trafficking (see above) or from specialized anchoring in the cell membrane.
A number of candidates exist for mediators of specific targeting/retention of cardiac potassium channel isoforms. In addition to SAP97, caveolin and syntaxin 1A (see above), actin-binding proteins like α-actinin-2 and filamin have been at least circumstantially implicated in channel targeting and anchoring. α-Actinin-2, a molecule that links to the actin cytoskeleton, has been shown to directly bind Kv1.5 (Maruoka et al. 2000; Cukovic et al. 2001). Given that α-actinin-2 antisense RNA increases Kv1.5 surface expression (Maruoka et al. 2000) and the involvement of the actin cytoskeleton in early endosomal trafficking (Jeng & Welch, 2001), it is quite possible that actinin is involved in Kv1.5 endocytosis and/or in maintaining pools of Kv1.5 in vesicles just below the cell surface. Filamin, another molecule that binds the actin cytoskeleton, has been shown to interact with Kv4.2 (Petrecca et al. 2000). Kv4.2 expression is increased by filamin overexpression, suggesting that filamin's role may well be to anchor the channel at the membrane. Yet another actin-binding protein, cortactin, interacts with Kv1.2 (Hattan et al. 2002). Kv1.2 channels defective for cortactin binding express much more poorly in HEK293 cells than do their wild-type equivalents, suggesting a role in channel stabilization at the surface for this protein, as well.
Interestingly, the interaction of Kv1.2 with cortactin can be modulated by tyrosine phosphorylation; activation of the M1 muscarinic acetylcholine receptor dramatically attenuates the interaction of cortactin with the channel (Hattan et al. 2002). Implicating this attenuation with Kv1.2 endocytosis, Nesti et al. (2004) have demonstrated that phosphorylation of a specific Kv1.2 N-terminal tyrosine residue results in rapid internalization of that channel. Incubation of the cells with a dynamin-inhibitory peptide blocked this internalization, confirming the role of endocytosis in this down-regulation of Kv1.2 functional expression.
Dynamin has been recently implicated also in the regulation of Kv1.5 expression (Choi et al. 2005). Dynamin catalyses the scission of endocytic vesicles from the plasma membrane (McClure & Robinson, 1996). It is important for clathrin-dependent and most clathrin-independent endocytosis (Takai et al. 2005) and, perhaps, in modulating actin dynamics at the cell surface (reviewed in Schafer, 2004). Suggesting that ongoing endocytosis is important for the maintenance of normal Kv1.5 expression, Kv1.5 currents are increased in heterologous cells treated with dynamin inhibitory peptide. Immunocytochemistry/confocal microscopy showed that Kv1.5 localized to early endosomes as well as to the cell surface and that dynamin inhibition dramatically reduced the number of these Kv1.5-positive endosomes. A proline-rich SH3 binding domain was found to be essential for internalization of this channel, perhaps implicating tyrosine phosphorylation in Kv1.5 endocytosis, as well.
Once internalized, a channel must eventually be either recycled to the membrane or degraded. In the same study that implicated the SH3 binding domain in Kv1.5 endocytosis, the dynein motor was shown also to profoundly affect Kv1.5 surface expression. Similar to its effects on the ClC-2 chloride channel (Dhani et al. 2003), dynein inhibition increased Kv1.5 surface expression as assayed both physically and electrophysiologically (Choi et al. 2005). These increases in Kv1.5 functional expression matched those obtained with the dynamin-inhibitory peptide. Dynein is a molecular motor required for retrograde trafficking of cargo along the microtubule cytoskeleton. Very probably, interference with this retrograde trafficking prevented the trafficking of newly formed endosomes and these endosomes, unable to internalize further, either reintegrated into the sarcolemma or interfered with the further endocytosis of the channel, thus increasing Kv1.5 net surface expression.
Fates unknown
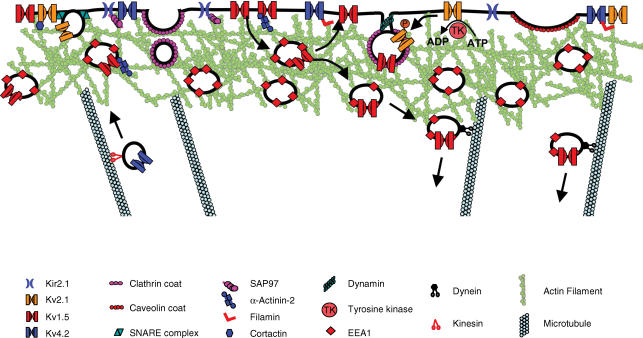
Beyond the apparent role of dynein in modulation of Kv1.5 surface expression, little is known about the fate of cardiac potassium channels following internalization. Probably many recycle to the sarcolemma and others are targeted for degradation, perhaps with ubiquitination playing an important role in the determination of a channel's fate (Lin et al. 2005; Chapman et al. 2005; Kato et al. 2005). Future work with Rab proteins, etc., will be necessary to identify the compartments to which various potassium channels segregate, not only after endocytosis, but throughout the trafficking process. A summary of our present knowledge concerning near-cell surface trafficking of cardiac potassium channels is presented in Fig. 2.
Figure 2. Potassium channel dynamics near the cell surface.
Interactions of several cardiac potassium channels with components of the trafficking machinery near the sarcolemma are illustrated. Early endosomes travel through the cortical actin cytoskeleton and are pictured as either recycled to the sarcolemma or transferred to the dynein motor for further internalization. Anterograde trafficking of Kv4.2 is illustrated as involving kinesin based on the known interaction of Kv4.2 with Kif17 in neurons. The involvement of clathrin-coated pits in potassium channel endocytosis is hypothesized on the basis of the ubiquitous presence of dileucine motifs in the channels and the known interaction of Kir2.1 with clathrin. Kv1.5 and Kv2.1 are illustrated in the process of internalization: Kv1.2 in response to tyrosine phosphorylation and Kv1.5 on the basis of the known role for dynamin in its internalization. Other interactions are as described in the text of this review.
Still more questions
Many questions remain about potassium channel trafficking in the heart. Are pathways shared by most potassium channels or are different pathways utilized for each? Is Hsc70 involved in channel endocytosis as well as forward trafficking? What other molecules are involved in trafficking and targeting? How is trafficking regulated? By what mechanisms do drugs that promote or inhibit potassium channel trafficking (Cordes et al. 2005; Kuryshev et al. 2005; Anderson et al. 2006; Gong et al. 2006; Rajamani et al. 2006; Sun et al. 2006) operate? The study of cardiac ion channel trafficking is a young and growing field. Undoubtedly the processes are complex and intertwined. Much is to be gained both intellectually and clinically in deciphering the trafficking of these channels.
References
- Aimond F, Kwak SP, Rhodes KJ, Nerbonne JM. Accessory Kv β1 subunits differentially modulate the functional expression of voltage-gated K+ channels in mouse ventricular myocytes. Circ Res. 2005;96:451–458. doi: 10.1161/01.RES.0000156890.25876.63. [DOI] [PubMed] [Google Scholar]
- Akhavan A, Atanasiu R, Noguchi T, Han W, Holder N, Shrier A. Identification of the cyclic-nucleotide-binding domain as a conserved determinant of ion-channel cell-surface localization. J Cell Sci. 2005;118:2803–2812. doi: 10.1242/jcs.02423. [DOI] [PubMed] [Google Scholar]
- An WF, Bowlby MR, Bett M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong QM, Zhou ZF, Ackerman MJ, January CT. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- Aoe T, Lee AJ, van Donselaar E, Peters PJ, Hsu VW. Modulation of intracellular transport by transported proteins: Insight from regulation of COPI-mediated transport. Proc Natl Acad Sci U S A. 1998;95:1624–1629. doi: 10.1073/pnas.95.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G, Reuven N, Shaul Y. 20S proteasomes and protein degradation ‘by default’. Bioessays. 2006;28:844–849. doi: 10.1002/bies.20447. [DOI] [PubMed] [Google Scholar]
- Bahring R, Dannenberg J, Peters HC, Leicher T, Pongs O, Isbrandt D. Conserved Kv4 N-terminal domain critical for effects of Kv channel-interacting protein 2.2 on channel expression and gating. J Biol Chem. 2001;276:23888–23894. doi: 10.1074/jbc.M101320200. [DOI] [PubMed] [Google Scholar]
- Barry DM, Trimmer JS, Merlie JP, Nerbonne JM. Differential expression of voltage-gated K+ channel subunits in adult rat heart: Relation to functional K+ channels. Circ Res. 1995;77:361–369. doi: 10.1161/01.res.77.2.361. [DOI] [PubMed] [Google Scholar]
- Cabrera M, Muniz M, Hidalgo J, Vega L, Martin ME, Velasco A. The retrieval function of the KDEL receptor requires PKA phosphorylation of its C-terminus. Mol Biol Cell. 2003;14:4114–4125. doi: 10.1091/mbc.E03-04-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaghan SC, Le Guennec JY, White E. Cytoskeletal modulation of electrical and mechanical activity in cardiac myocytes. Prog Biophys Mol Biol. 2004;84:29–59. doi: 10.1016/s0079-6107(03)00057-9. [DOI] [PubMed] [Google Scholar]
- Carnegie GK, Scott JD. A-kinase anchoring proteins and neuronal signaling mechanisms. Genes Dev. 2003;17:1557–1568. doi: 10.1101/gad.1095803. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Shoshani L, Garcia-Villegas MR. Membrane targeting. Prog Biophys Mol Biol. 2003;81:81–115. doi: 10.1016/s0079-6107(02)00047-0. [DOI] [PubMed] [Google Scholar]
- Chang XB, Cui LY, Hou YX, Jensen TJ, Aleksandrov AA, Mengos A, Riordan JR. Removal of multiple arginine-framed trafficking signals overcomes misprocessing of Delta F508 CFTR present in most patients with cystic fibrosis. Mol Cell. 1999;4:137–142. doi: 10.1016/s1097-2765(00)80196-3. [DOI] [PubMed] [Google Scholar]
- Chapman H, Ramstrom C, Korhonen L, Laine M, Wann KT, Lindholm D, Pasternack M, Tornquist K. Downregulation of the HERG (KCNH2) K+ channel by ceramide: evidence for ubiquitin-mediated lysosomal degradation. J Cell Sci. 2005;118:5325–5334. doi: 10.1242/jcs.02635. [DOI] [PubMed] [Google Scholar]
- Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res. 2005;97:363–371. doi: 10.1161/01.RES.0000179535.06458.f8. [DOI] [PubMed] [Google Scholar]
- Chu PJ, Rivera JF, Arnold DB. A role for Kif17 in transport of Kv4.2. J Biol Chem. 2006;281:365–373. doi: 10.1074/jbc.M508897200. [DOI] [PubMed] [Google Scholar]
- Clark RB, Tremblay A, Melnyk P, Allen BG, Giles WR, Fiset C. T-tubule localization of the inward-rectifier K+ channel in mouse ventricular myocytes: a role in K+ accumulation. J Physiol. 2001;537:979–992. doi: 10.1111/j.1469-7793.2001.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay JR, Kuzirian A. Trafficking of axonal K+ channels: Potential role of Hsc70. J Neurosci Res. 2002;67:745–752. doi: 10.1002/jnr.10182. [DOI] [PubMed] [Google Scholar]
- Conti LR, Radeke CM, Vandenberg CA. Membrane targeting of ATP-sensitive potassium channel – Effects of glycosylation on surface expression. J Biol Chem. 2002;277:25416–25422. doi: 10.1074/jbc.M203109200. [DOI] [PubMed] [Google Scholar]
- Cordes JS, Sun ZQ, Lloyd DB, Bradley JA, Opsahl AC, Tengowski MW, Chen X, Zhou J. Pentamidine reduces hERG expression to prolong the QT interval. Br J Pharmacol. 2005;145:15–23. doi: 10.1038/sj.bjp.0706140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic-reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Cukovic D, Lu GWK, Wible B, Steele DF, Fedida D. A discrete amino terminal domain of Kv1.5 and Kv1.4 potassium channels interacts with the spectrin repeats of α-actinin-2. FEBS Letts. 2001;498:87–92. doi: 10.1016/s0014-5793(01)02505-4. [DOI] [PubMed] [Google Scholar]
- Dhani SU, Mohammad-Panah R, Ahmed N, Ackerley C, Ramjeesingh M, Bear CE. Evidence for a functional interaction between the ClC-2 chloride channel and the retrograde motor dynein complex. J Biol Chem. 2003;278:16262–16270. doi: 10.1074/jbc.M209828200. [DOI] [PubMed] [Google Scholar]
- Eldstrom J, Choi WS, Steele DF, Fedida D. SAP97 increases Kv1.5 currents through an indirect N-terminal mechanism. FEBS Letts. 2003;547:205–211. doi: 10.1016/s0014-5793(03)00668-9. [DOI] [PubMed] [Google Scholar]
- Eldstrom J, Van Wagoner DR, Moore ED, Fedida D. Localization of Kv1.5 channels in rat and canine myocyte sarcolemma. FEBS Letts. 2006;580:6039–6046. doi: 10.1016/j.febslet.2006.09.069. [DOI] [PubMed] [Google Scholar]
- Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ Res. 2003;92:e87–100. doi: 10.1161/01.RES.0000079028.31393.15. [DOI] [PubMed] [Google Scholar]
- Ficker E, Obejero-Paz CA, Zhao S, Brown AM. The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (HERG) mutations. J Biol Chem. 2002;277:4989–4998. doi: 10.1074/jbc.M107345200. [DOI] [PubMed] [Google Scholar]
- Fili O, Michaelevski I, Bledi Y, Chikvashvili D, Singer-Lahat D, Boshwitz H, Linial M, Lotan I. Direct interaction of a brain voltage-gated K+ channel with syntaxin 1A: Functional impact on channel gating. J Neurosci. 2001;21:1964–1974. doi: 10.1523/JNEUROSCI.21-06-01964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco EJ, Liu GX, Koren G. Caveolin-3 and SAP97 form a scaffolding protein complex that regulates the voltage-gated potassium channel Kv1.5. Am J Physiol Heart Circ Physiol. 2004;287:H681–H690. doi: 10.1152/ajpheart.00152.2004. [DOI] [PubMed] [Google Scholar]
- Furutani M, Trudeau MC, Hagiwara N, Seki A, Gong Q, Zhou Z, Imamura S-I, Nagashima H, Kasanuki H, Takao A, Momma K, January CT, Robertson GA, Matsuoka R. Novel mechanism associated with an inherited cardiac arrhythmia. Defective protein trafficking by the mutant HERG (G601S) potassium channel. Circulation. 1999;99:2290–2294. doi: 10.1161/01.cir.99.17.2290. [DOI] [PubMed] [Google Scholar]
- Godreau D, Vranckx R, Maguy A, Goyenvalle C, Hatem SN. Different isoforms of synapse-associated protein, SAP97, are expressed in the heart and have distinct effects on the voltage-gated K+ channel Kv1.5. J Biol Chem. 2003;278:47046–47052. doi: 10.1074/jbc.M308463200. [DOI] [PubMed] [Google Scholar]
- Godreau D, Vranckx R, Maguy A, Rucker-Martin C, Goyenvalle C, Abdelshafy S, Tessier S, Couétil J-P, Hatem SN. Expression, regulation and role of the MAGUK protein SAP-97 in human atrial myocardium. Cardiovasc Res. 2002;56:433–442. doi: 10.1016/s0008-6363(02)00602-8. [DOI] [PubMed] [Google Scholar]
- Gong QM, Anderson CL, January CT, Zhou ZF. Role of glycosylation in cell surface expression and stability of HERG potassium channels. Am J Physiol Heart Circ Physiol. 2002;283:H77–H84. doi: 10.1152/ajpheart.00008.2002. [DOI] [PubMed] [Google Scholar]
- Gong QM, Jones MA, Zhou ZF. Mechanisms of pharmacological rescue of trafficking-defective hERG mutant channels in human long QT syndrome. J Biol Chem. 2006;281:4069–4074. doi: 10.1074/jbc.M511765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong QM, Keeney DR, Molinari M, Zhou ZF. Degradation of trafficking-defective long QT syndrome type II mutant channels by the ubiquitin-proteasome pathway. J Biol Chem. 2005;280:19419–19425. doi: 10.1074/jbc.M502327200. [DOI] [PubMed] [Google Scholar]
- Gu F, Crump CM, Thomas G. Trans-Golgi network sorting. Cell Mol Life Sci. 2001;58:1067–1084. doi: 10.1007/PL00000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkan C, Stagg SM, LaPointe P, Balch WE. The COPII cage: unifying principles of vesicle coat assembly. Nat Rev Mol Cell Biol. 2006;7:727–738. doi: 10.1038/nrm2025. [DOI] [PubMed] [Google Scholar]
- Hanada T, Lin LH, Tibaldi EV, Reinherz EL, Chishti AH. GAKIN, a novel kinesin-like protein associates with the human homologue of the Drosophila discs large tumor suppressor in T lymphocytes. J Biol Chem. 2000;275:28774–28784. doi: 10.1074/jbc.M000715200. [DOI] [PubMed] [Google Scholar]
- Hanlon MR, Wallace BA. Structure and function of voltage-dependent ion channel regulatory β subunits. Biochemistry. 2002;41:2886–2894. doi: 10.1021/bi0119565. [DOI] [PubMed] [Google Scholar]
- Harter C, Wieland FT. A single binding site for dilysine retrieval motifs and p23 within the gamma subunit of coatomer. Proc Natl Acad Sci U S A. 1998;95:11649–11654. doi: 10.1073/pnas.95.20.11649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasdemir B, Fitzgerald DJ, Prior IA, Tepikin AV, Burgoyne RD. Traffic of Kv4 K+ channels mediated by KChlP1 is via a novel post-ER vesicular pathway. J Cell Biol. 2005;171:459–469. doi: 10.1083/jcb.200506005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattan D, Nesti E, Cachero TG, Morielli AD. Tyrosine phosphorylation of Kv1.2 modulates its interaction with the actin-binding protein cortactin. J Biol Chem. 2002;277:38596–38606. doi: 10.1074/jbc.M205005200. [DOI] [PubMed] [Google Scholar]
- Hong WJ. SNARES and traffic. Biochim Biophys Acta Mol Cell Res. 2005;1744:120–144. doi: 10.1016/j.bbamcr.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Imamura F, Maeda S, Doi T, Fujiyoshi Y. Ligand binding of the second PDZ domain regulates clustering of PSD-95 with the Kv1.4 potassium channel. J Biol Chem. 2002;277:3640–3646. doi: 10.1074/jbc.M106940200. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs – engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jeng RL, Welch MD. Cytoskeleton: actin and endocytosis – no longer the weakest link. Curr Biol. 2001;11:R691–R694. doi: 10.1016/s0960-9822(01)00410-9. [DOI] [PubMed] [Google Scholar]
- Ji JZ, Tsuk S, Salapatek AMF, Huang XH, Chikvashvili D, Pasyk EA, Kang YH, Sheu L, Tsushima R, Diamant N, Trimble WS, Lotan I, Gaisano HY. The 25-kDa synaptosome-associated protein (SNAP-25) binds and inhibits delayed rectifier potassium channels in secretory cells. J Biol Chem. 2002;277:20195–20204. doi: 10.1074/jbc.M201034200. [DOI] [PubMed] [Google Scholar]
- Jones SA, Morton MJ, Hunter M, Boyett MR. Expression of TASK-1, a pH-sensitive twin-pore domain K+ channel, in rat myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H181–H185. doi: 10.1152/ajpheart.00963.2001. [DOI] [PubMed] [Google Scholar]
- Karcher RL, Deacon SW, Gelfand VI. Motor–cargo interactions: the key to transport specificity. Trends Cell Biol. 2002;12:21–27. doi: 10.1016/s0962-8924(01)02184-5. [DOI] [PubMed] [Google Scholar]
- Kato M, Ogura K, Miaki J, Sasaki N, Taniguchi S, Igawa O, Yoshida A, Hoshikawa Y, Murata M, Nanba E, Kurata Y, Kawata Y, Ninomiya H, Morisaki T, Kitakaze M, Isatome I. Evidence for proteasomal degradation of Kv1.5 channel protein. Biochem Biophys Res Commun. 2005;337:343–348. doi: 10.1016/j.bbrc.2005.09.053. [DOI] [PubMed] [Google Scholar]
- Khanna R, Myers MP, Lainé M, Papazian DM. Glycosylation increases potassium channel stability and surface expression in mammalian cells. J Biol Chem. 2001;276:34028–34034. doi: 10.1074/jbc.M105248200. [DOI] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kosolapov A, Deutsch C. Folding of the voltage-gated K+ channel T1 recognition domain. J Biol Chem. 2003;278:4305–4313. doi: 10.1074/jbc.M209422200. [DOI] [PubMed] [Google Scholar]
- Kosolapov A, Tu L, Wang J, Deutsch C. Structure acquisition of the T1 domain of Kv1.3 during biogenesis. Neuron. 2004;44:295–307. doi: 10.1016/j.neuron.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Kucera JP, Rohr S, Rudy Y. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ Res. 2002;91:1176–1182. doi: 10.1161/01.res.0000046237.54156.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, Ravazzola M, Tanigawa G, Rothman JE, Balch WE. Sar1 promotes vesicle budding from the endoplasmic-reticulum but not golgi compartments. J Cell Biol. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupershmidt S, Yang T, Chanthaphaychith S, Wang Z, Towbin JA, Roden DM. Defective human ether-a-go-go-related gene trafficking linked to an endoplasmic reticulum retention signal in the C terminus. J Biol Chem. 2002;277:27442–27448. doi: 10.1074/jbc.M112375200. [DOI] [PubMed] [Google Scholar]
- Kuryshev YA, Ficker E, Wang L, Hawryluk P, Dennis AT, Wible BA, Brown AM, Kang JS, Chen XL, Sawamura K, Reynolds W, Rampe D. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther. 2005;312:316–323. doi: 10.1124/jpet.104.073692. [DOI] [PubMed] [Google Scholar]
- Kuryshev YA, Gudz TI, Brown AM, Wible BA. KChAP as a chaperone for specific K+ channels. Am J Physiol Cell Physiol. 2000;278:C931–C941. doi: 10.1152/ajpcell.2000.278.5.C931. [DOI] [PubMed] [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- Lee MCS, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Ann Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Conti LR, Radeke CM, McGuire LMM, Vandenberg CA. A multiprotein trafficking complex composed of SAP97, CASK, Veli, and Mint1 is associated with inward rectifier Kir2 potassium channels. J Biol Chem. 2004;279:19051–19063. doi: 10.1074/jbc.M400284200. [DOI] [PubMed] [Google Scholar]
- Leung YM, Kang YH, Gao XD, Xia FZ, Xie HL, Sheu L, Tsuk S, Lotan I, Tsushima RG, Gaisano HY. Syntaxin 1A binds to the cytoplasmic C terminus of Kv2.1 to regulate channel gating and trafficking. J Biol Chem. 2003;278:17532–17538. doi: 10.1074/jbc.M213088200. [DOI] [PubMed] [Google Scholar]
- Li D, Takimoto K, Levitan ES. Surface expression of Kv1 channels is governed by a C-terminal motif. J Biol Chem. 2000;275:11597–11602. doi: 10.1074/jbc.275.16.11597. [DOI] [PubMed] [Google Scholar]
- Lin DH, Sterling H, Wang ZJ, Babilonia E, Yang BF, Dong K, Hebert SC, Giebisch G, Wang WH. ROMK1 channel activity is regulated by monobuquitination. Proc Natl Acad Sci U S A. 2005;102:4306–4311. doi: 10.1073/pnas.0409767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister I, Roberts R, Schmitz S, Walker M, Trinick J, Veigel C, Buss F, Kendrick-Jones J. Myosin VI: a multifunctional motor. Biochem Soc Trans. 2004;32:685–688. doi: 10.1042/BST0320685. [DOI] [PubMed] [Google Scholar]
- Lu JL, Deutsch C. Secondary structure formation of a transmembrane segment in Kv channels. Biochemistry. 2005;44:8230–8243. doi: 10.1021/bi050372q. [DOI] [PubMed] [Google Scholar]
- Ma D, Jan LY. ER transport signals and trafficking of potassium channels and receptors. Curr Opin Neurobiol. 2002;12:287–292. doi: 10.1016/s0959-4388(02)00319-7. [DOI] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, Jan LY. Role of ER export signals in controlling surface potassium channel numbers. Science. 2001;291:316–319. doi: 10.1126/science.291.5502.316. [DOI] [PubMed] [Google Scholar]
- McClure SJ, Robinson PJ. Dynamin, endocytosis and intracellular signalling. Mol Membr Biol. 1996;13:189–215. doi: 10.3109/09687689609160598. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Wang GT, Tsuk S, Dodo C, Kang YH, Tang L, Wheeler MB, Cattral MS, Lakey JRT, Salapatek AMF, Lotan I, Gaisano HY. Synaptosome-associated protein of 25 kilodaltons modulates Kv2.1 voltage-dependent K+ channels in neuroendocrine islet β-cells through an interaction with the channel N terminus. Mol Endocrinol. 2002;16:2452–2461. doi: 10.1210/me.2002-0058. [DOI] [PubMed] [Google Scholar]
- Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci U S A. 2002;99:4073–4078. doi: 10.1073/pnas.261705699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Trimmer JS. Calnexin regulates mammalian Kv1 channel trafficking. Biochem Biophys Res Commun. 2004;322:577–584. doi: 10.1016/j.bbrc.2004.06.182. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Martens JR, Navarro-Polanco R, Coppock EA, Nishiyama A, Parshley L, Grobaski T, Tamkun MM. Differential targeting of Shaker-like potassium channels to lipid rafts. J Biol Chem. 2000;275:7443–7446. doi: 10.1074/jbc.275.11.7443. [DOI] [PubMed] [Google Scholar]
- Martens JR, Sakamoto N, Sullivan SA, Grobaski TD, Tamkun MM. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv1.5 to caveolae. J Biol Chem. 2001;276:8409–8414. doi: 10.1074/jbc.M009948200. [DOI] [PubMed] [Google Scholar]
- Maruoka ND, Steele DF, Au BPY, Dan P, Zhang X, Moore EDW, Fedida D. α-Actinin-2 couples to cardiac Kv1.5 channels, regulating current density and channel localization in HEK cells. FEBS Letts. 2000;473:188–194. doi: 10.1016/s0014-5793(00)01521-0. [DOI] [PubMed] [Google Scholar]
- Mason HS, Latten MJ, Godoy LD, Horowitz B, Kenyon JL. Modulation of Kv1.5 currents by protein kinase A, tyrosine kinase, and protein tyrosine phosphatase requires an intact cytoskeleton. Mol Pharmacol. 2002;61:285–293. doi: 10.1124/mol.61.2.285. [DOI] [PubMed] [Google Scholar]
- Mathur R, Choi WS, Eldstrom J, Wang Z, Kim J, Steele DF, Fedida D. A specific N-terminal residue in Kv1.5 is required for upregulation of the channel by SAP97. Biochem Biophys Res Commun. 2006;342:1–8. doi: 10.1016/j.bbrc.2006.01.110. [DOI] [PubMed] [Google Scholar]
- Mays DJ, Foose JM, Philipson LH, Tamkun MM. Localization of the Kv1.5 K+ channel protein in explanted cardiac tissue. J Clin Invest. 1995;96:282–292. doi: 10.1172/JCI118032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Warren G. The road taken: Past and future foundations of membrane traffic. Cell. 2000;100:99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- Michaelevski L, Chikvashvili D, Tsuk S, Fili O, Lohse MJ, Singer-Lahat D, Lotan I. Modulation of a brain voltage-gated K+ channel by syntaxin 1A requires the physical interaction of Gβγ with the channel. J Biol Chem. 2002;277:34909–34917. doi: 10.1074/jbc.M203943200. [DOI] [PubMed] [Google Scholar]
- Mogelsvang S, Howell KE. Global approaches to study Golgi function. Curr Opin Cell Biol. 2006;18:438–443. doi: 10.1016/j.ceb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Murata M, Buckett PD, Zhou J, Brunner M, Folco E, Koren G. SAP97 interacts with Kv1.5 in heterologous expression systems. Am J Physiol Heart Circ Physiol. 2001;281:H2575–H2584. doi: 10.1152/ajpheart.2001.281.6.H2575. [DOI] [PubMed] [Google Scholar]
- Murshid A, Presley JF. ER-to-Golgi transport and cytoskeletal interactions in animal cells. Cell Mol Life Sci. 2004;61:133–145. doi: 10.1007/s00018-003-3352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt S, Valtschanoff J, Allison DW, Sala C, Kim E, Craig AM, Weinberg RJ, Sheng M. Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin-V and dynein. J Neurosci. 2000;20:4524–4534. doi: 10.1523/JNEUROSCI.20-12-04524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napp J, Monje F, Stuhmer W, Pardo LA. Glycosylation of Eag1 (Kv10.1) potassium channels – Intracellular trafficking and functional consequences. J Biol Chem. 2005;280:29506–29512. doi: 10.1074/jbc.M504228200. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Guo WN. Heterogeneous expression of voltage-gated potassium channels in the heart: Roles in normal excitation and arrhythmias. J Cardiovasc Electrophysiol. 2002;13:406–409. doi: 10.1046/j.1540-8167.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- Nesti E, Everill B, Morielli AD. Endocytosis as a mechanism for tyrosine kinase dependent suppression of a voltage gated potassium channel. Mol Biol Cell. 2004;15:4073–4088. doi: 10.1091/mbc.E03-11-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nufer O, Guldbrandsen S, Degen M, Kappeler F, Paccaud JP, Tani K, Hauri HP. Role of cytoplasmic C-terminal amino acids of membrane proteins in ER export. J Cell Sci. 2002;115:619–628. doi: 10.1242/jcs.115.3.619. [DOI] [PubMed] [Google Scholar]
- Nufer O, Hauri HP. ER export: Call 14-3-3. Curr Biol. 2003;13:R391–R393. doi: 10.1016/s0960-9822(03)00318-x. [DOI] [PubMed] [Google Scholar]
- O'Connell KMS, Tamkun MM. Targeting of voltage-gated potassium channel isoforms to distinct cell surface microdomains. J Cell Sci. 2005;118:2155–2166. doi: 10.1242/jcs.02348. [DOI] [PubMed] [Google Scholar]
- O'Kelly I, Butler MH, Zilberberg N, Goldstein SAN. Forward transport: 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 2002;111:577–588. doi: 10.1016/s0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- Paulussen A, Raes A, Matthijs G, Snyders DJ, Cohen N, Aerssens J. A novel mutation (T65P) in the PAS domain of the human potassium channel HERG results in the long QT syndrome by trafficking deficiency. J Biol Chem. 2002;277:48610–48616. doi: 10.1074/jbc.M206569200. [DOI] [PubMed] [Google Scholar]
- Petrecca K, Miller DM, Shrier A. Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin. J Neurosci. 2000;20:8736–8744. doi: 10.1523/JNEUROSCI.20-23-08736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O, Leicher T, Berger M, Roeper J, Bahring R, Wray D, Giese KP, Silva AJ, Storm JF. Functional and molecular aspects of voltage-gated K+ channel β subunits. Ann N Y Acad Sci. 1999;868:344–355. doi: 10.1111/j.1749-6632.1999.tb11296.x. [DOI] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Rajamani S, Eckhardt LL, Valdivia CR, Klemens CA, Gillman BM, Anderson CL, Holzem KM, Delisle BP, Anson D, Makielski JC, January CT. Drug–induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. Br J Pharmacol. 2006;149:481–489. doi: 10.1038/sj.bjp.0706892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HB, Moller M, Knaus HG, Jensen BS, Olesen SP, Jorgensen NK. Subcellular localization of the delayed rectifier K+ channels KCNQ1 and ERG1 in the rat heart. Am J Physiol Heart Circ Physiol. 2004;286:H1300–H1309. doi: 10.1152/ajpheart.00344.2003. [DOI] [PubMed] [Google Scholar]
- Robinson JM, Deutsch C. Coupled tertiary folding and oligomerization of the T1 domain of Kv channels. Neuron. 2005;45:223–232. doi: 10.1016/j.neuron.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Rossenbacker T, Mubagwa K, Jongbloed RJ, Vereecke J, Devriendt K, Gewillig M, Carmeliet E, Collen D, Heidbuchel H, Carmeliet P. Novel mutation in the Per-Arnt-Sim domain of KCNH2 causes a malignant form of long-QT syndrome. Circulation. 2005;111:961–968. doi: 10.1161/01.CIR.0000156327.35255.D8. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gomez A, Mellstrom B, Tornero D, Morato E, Savignac M, Holguin H, Aurrekoetxea K, Gonzalez P, Gonzalez-Garcia C, Cena V, Mayor F, Naranjo JR. G protein-coupled receptor kinase 2-mediated phosphorylation of downstream regulatory element antagonist modulator regulates membrane trafficking of Kv4.2 potassium channel. J Biol Chem. 2007;282:1205–1215. doi: 10.1074/jbc.M607166200. [DOI] [PubMed] [Google Scholar]
- Schafer DA. Regulating actin dynamics at membranes: a focus on dynamin. Traffic. 2004;5:463–469. doi: 10.1111/j.1600-0854.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Seeber S, Becker K, Rau T, Eschenhagen T, Becker CM, Herkert M. Transient expression of NMDA receptor subunit NR2B in the developing rat heart. J Neurochem. 2000;75:2472–2477. doi: 10.1046/j.1471-4159.2000.0752472.x. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Shi GY, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. βSubunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- Shikano S, Coblitz B, Wu M, Li M. 14-3-3 proteins: regulation of endoplasmic reticulum localization and surface expression of membrane proteins. Trends Cell Biol. 2006;16:370–375. doi: 10.1016/j.tcb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Shikano S, Li M. Membrane receptor trafficking: Evidence of proximal and distal zones conferred by two independent endoplasmic reticulum localization signals. Proc Natl Acad Sci U S A. 2003;100:5783–5788. doi: 10.1073/pnas.1031748100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley S, Roche KW, McCallum J, Sans N, Wenthold RJ. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron. 2000;28:887–898. doi: 10.1016/s0896-6273(00)00161-6. [DOI] [PubMed] [Google Scholar]
- Stockklausner C, Klocker N. Surface expression of inward rectifier potassium channels is controlled by selective Golgi export. J Biol Chem. 2003;278:17000–17005. doi: 10.1074/jbc.M212243200. [DOI] [PubMed] [Google Scholar]
- Stockklausner C, Ludwig J, Ruppersberg JP, Klöcker N. A sequence motif responsible for ER export and surface expression of Kir2.0 inward rectifier K+ channels. FEBS Letts. 2001;493:129–133. doi: 10.1016/s0014-5793(01)02286-4. [DOI] [PubMed] [Google Scholar]
- Sun HY, Liu XD, Xiong QJ, Shikano S, Li M. Chronic inhibition of cardiac Kir2.1 and hERG potassium channels by celastrol with dual effects on both ion conductivity and protein trafficking. J Biol Chem. 2006;281:5877–5884. doi: 10.1074/jbc.M600072200. [DOI] [PubMed] [Google Scholar]
- Takai K, Yoshida Y, Yamada H. Regulatory mechanisms of dynamin-dependent endocytosis. J Biochem. 2005;137:243–247. doi: 10.1093/jb/mvi052. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takagishi Y, Yasui K, Murata Y, Toyama J, Kodama I. Voltage-gated K+ channel, Kv4.2, localizes predominantly to the transverse-axial tubular system of the rat myocyte. J Mol Cell Cardiol. 2000;32:1361–1369. doi: 10.1006/jmcc.2000.1172. [DOI] [PubMed] [Google Scholar]
- Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K+ channel surface expression and clustering. J Cell Biol. 2000;148:147–158. doi: 10.1083/jcb.148.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Ann Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Wang Z, Eldstrom JR, Jantzi J, Moore ED, Fedida D. Increased focal Kv4.2 channel expression at the plasma membrane is the result of actin depolymerization. Am J Physiol Heart Circ Physiol. 2004;286:H749–H759. doi: 10.1152/ajpheart.00398.2003. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Zhu J, Recio-Pinto E, Thornhill WB. Glycosylation affects the protein stability and cell surface expression of Kv1.4 but not Kv1.1 potassium channels. A pore region determinant dictates the effect of glycosylation on trafficking. J Biol Chem. 2004;279:8879–8885. doi: 10.1074/jbc.M309802200. [DOI] [PubMed] [Google Scholar]
- Wible BA, Yang Q, Kuryshev YA, Accili EA, Brown AM. Cloning and expression of a novel K+ channel regulatory protein, KChAP. J Biol Chem. 1998;273:11745–11751. doi: 10.1074/jbc.273.19.11745. [DOI] [PubMed] [Google Scholar]
- Wu HJ, Nash JE, Zamorano P, Garner CC. Interaction of SAP97 with minus-end-directed actin motor myosin VI – Implications for AMPA receptor trafficking. J Biol Chem. 2002;277:30928–30934. doi: 10.1074/jbc.M203735200. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic-reticulum. Science. 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]
- Yuan H, Michelsen K, Schwappach B. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Curr Biol. 2003;13:638–646. doi: 10.1016/s0960-9822(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Malan MJ, Fried SR, Dazin PF, Jan YN, Jan LY, Schwappach B. Analysis of endoplasmic reticulum trafficking signals by combinatorial screening in mammalian cells. Proc Natl Acad Sci U S A. 2001;98:2431–2436. doi: 10.1073/pnas.051630198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Zhou ZF, Gong QM, January CT. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome – Pharmacological and temperature effects. J Biol Chem. 1999;274:31123–31126. doi: 10.1074/jbc.274.44.31123. [DOI] [PubMed] [Google Scholar]
- Zhou JS, Shin HG, Yi JX, Shen WZ, Williams CP, Murray KT. Phosphorylation and putative ER retention signals are required for protein kinase A-mediated potentiation of cardiac sodium current. Circ Res. 2002;91:540–546. doi: 10.1161/01.res.0000033598.00903.27. [DOI] [PubMed] [Google Scholar]
- Zhu J, Watanabe I, Gomez B, Thornhill WB. Trafficking of Kv1.4 potassium channels: interdependence of a pore region determinant and a cytoplasmic C-terminal VXXSL determinant in regulating cell-surface trafficking. Biochem J. 2003;375:761–768. doi: 10.1042/BJ20030885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinsmaier KE, Bronk P. Molecular chaperones and the regulation of neurotransmitter exocytosis. Biochem Pharmacol. 2001;62:1–11. doi: 10.1016/s0006-2952(01)00648-7. [DOI] [PubMed] [Google Scholar]