Abstract
To re-examine how the basal extracellular concentration of adenosine is regulated in acutely isolated cerebellar slices we have combined electrophysiological and microelectrode biosensor measurements. In almost all cases, synaptic transmission was tonically inhibited by adenosine acting via A1 receptors. By contrast, in most slices, the biosensors did not measure an adenosine tone but did record a spatially non-uniform extracellular tone of the downstream metabolites (inosine and hypoxanthine). Most of the extracellular hypoxanthine arose from the metabolism of inosine by ecto-purine nucleoside phosphorylase (PNP). Adenosine kinase was the major determinant of adenosine levels, as its inhibition increased both adenosine concentration and A1 receptor-mediated synaptic inhibition. Breakdown of adenosine by adenosine deaminase was the major source of the inosine/hypoxanthine tone. However adenosine deaminase played a minor role in determining the level of adenosine at synapses, suggesting a distal location. Blockade of adenosine transport (by NBTI/dipyridamole) had inconsistent effects on basal levels of adenosine and synaptic transmission. Unexpectedly, application of NBTI/dipyridamole prevented the efflux of adenosine resulting from block of adenosine kinase at only a subset of synapses. We conclude that there is spatial variation in the functional expression of NBTI/dipyridamole-sensitive transporters. The increased spatial and temporal resolution of the purine biosensor measurements has revealed the complexity of the control of adenosine and purine tone in the cerebellum.
The purine adenosine is an important neuromodulator, with both excitatory and inhibitory actions within the CNS. This purine molecule is involved in diverse processes including locomotion, sleep and respiration, and provides neuroprotection during hypoxia/ischaemia. Although the basal extracellular levels of adenosine in the brain are low (Newman & McIlwain, 1977; Dunwiddie & Diao, 1994), there is still sufficient to tonically activate high-affinity A1 receptors and produce synaptic inhibition (Dunwiddie & Diao, 1994; Takahashi et al. 1995; Dittman & Regehr, 1996). Regulation of the extracellular level of adenosine in the brain is crucial, since small changes in adenosine levels will affect the degree of synaptic inhibition, and thus modulate neural processing.
The extracellular concentration of adenosine will be determined by the balance of production and elimination. In many cases, the source and mechanism of adenosine release are unclear but could occur via ATP metabolism (released by exocytosis, Edwards et al. 1992; Jo & Schlichter, 1999, or released through gap junction hemi-channels, Arcuino et al. 2002; Stout et al. 2002; Pearson et al. 2005), transport from the cell cytoplasm (Craig & White, 1993; Gu et al. 1995; Sweeney, 1996) or possibly by the exocytosis of adenosine itself (Wall & Dale, 2007). The actions of adenosine are terminated by a combination of metabolism to inosine (by the enzyme adenosine deaminase) and by translocation into neurons or glia by either equilibrative transporters such as ENT1 and ENT2 or concentrative transporters (for review see Noji et al. 2004; Baldwin et al. 2004).
Once internalised, adenosine can be phosphorylated (by adenosine kinase) to form AMP, thus maintaining low levels of intracellular adenosine (for review see Boison, 2006). Because the levels of adenosine are determined by a complex interplay of enzymes and transporters, several studies have investigated the components that contribute to determining extracellular adenosine concentration. In many studies blocking adenosine kinase has a major effect on adenosine concentration, whereas blockade of adenosine deaminase has a much smaller effect (Zhang et al. 1993; Pak et al. 1994; Lloyd & Fredholm, 1995).
The aim of this study is two-fold: firstly to investigate whether recently developed biosensors can be used to determine what controls the basal levels of adenosine in brain slices. Biosensors have inherently better spatial and temporal resolution than other methods such as HPLC or microdialysis, for measuring purine levels. Biosensors have been successfully used to investigate increases in adenosine during episodes of ischaemia, hypoxia and hypercapnia (Dale et al. 2000; Frenguelli et al. 2003, 2007; Dulla et al. 2005) and have been used to measure adenosine release following electrical stimulation (Wall & Dale, 2007). However, measurement of the basal tone of adenosine is more challenging as the concentration of adenosine will be low and may be more difficult to detect against a non-uniform background of downstream adenosine metabolites.
The second aim is to investigate adenosine levels in the cerebellum, since relatively little is known about how the extracellular concentration of adenosine is controlled in this part of the brain. There is growing evidence that adenosine plays an important role in the processing of information by cerebellar circuits. Adenosine is concentrated in the soma and dendrites of cerebellar Purkinje cells (Braas et al. 1986), and adenosine A1 receptors are expressed within the cerebellum (Rivkees et al. 1995). The tonic activation of A1 receptors, producing synaptic inhibition, provides indirect evidence that an extracellular tone of adenosine is present in cerebellar slices (Takahashi et al. 1995; Dittman & Regehr, 1996). The source of this extracellular adenosine is unclear, but in recent studies, Wall & Dale (2007) have directly measured adenosine release in the cerebellum and Beierlein & Regehr (2006) have reported the release of ATP from parallel fibres. The enzymes required for purine metabolism are present in the cerebellum: ecto-ATPase (CD39, Wang & Guidotti, 1998), 5′nucleotidase (Schoen et al. 1987) and adenosine deaminase (Geiger & Nagy, 1986). Nucleoside transporters are also expressed in the cerebellum (Anderson et al. 1999a,b). However, the relative importance of adenosine transport, phosphorylation (by adenosine kinase) and metabolism (by adenosine deaminase) in the regulation of the adenosine tone remains uncertain. Studies of synaptic membranes, cultured glia and homogenised brain tissue suggest that adenosine deaminase activity in the cerebellum is comparable to or higher than in other brain regions (Yamada et al. 1998; Wink et al. 2003; Kukulski et al. 2004). However the physiological relevance of data from such studies is unclear.
Methods
Preparation of cerebellar slices
Parasagittal or transverse slices of cerebellar vermis (400 μm) were prepared from male Wistar rats, at postnatal days 21–28 (P21–28). As described previously (Wall & Usowicz, 1997) and in accordance with the UK Animals (Scientific Procedures) Act 1986, male rats were killed by cervical dislocation and decapitated. The cerebellum was rapidly removed and slices were cut on a Microm HM 650V microslicer in cold (2–4°C) high Mg2+, low Ca2+ aCSF, composed of (mm): 127 NaCl, 1.9 KCl, 7 MgCl2, 0.5 CaCl2, 1.2 KH2PO4, 26 NaHCO3, 10 d-glucose (pH 7.4 when bubbled with 95% O2 and 5% CO2, 300 mosmol l−1). Slices were stored in normal aCSF (1.3 mm MgCl2, 2.4 mm CaCl2) at room temperature for 1–6 h before recording.
Extracellular recording
An individual slice was transferred to a recording chamber, submerged in aCSF and perfused at 6 ml min−1 (30–35°C). Peristaltic pumps were used to pump aCSF in and out of the recording chamber, thus ensuring a constant flow rate. The slice was placed upon a suspended grid to allow perfusion of the slice from above and below and thus reduce the likelihood of hypoxia. Furthermore, all solutions were vigorously bubbled (95% O2/5% CO2) and all tubing had low gas permeability (Tygon). For the stimulation of parallel fibres, square voltage pulses (2–5 V, 200 μs duration) were delivered by an isolated pulse stimulator (model 2100 AM systems Everett WA, USA) via a concentric bipolar metal stimulating electrode (FHC) placed on the surface of the molecular layer in a transverse slice. The recording electrode (an aCSF-filled microelectrode) was placed on the same track along which the parallel fibres travel (‘on-beam’Yuan & Atchison, 1999). A typical extracellular field potential consisted of an initial component which persisted in either 10 μm CNQX or 5 mm kynurenate but was blocked by 1 μm TTX (parallel fibre volley) followed by a component which could be blocked by 1 μm TTX and greatly reduced by either 10 μm CNQX or 5 mm kynurenate. This component is probably produced by parallel fibre-mediated glutamatergic excitatory synaptic currents and subsequent action potentials in Purkinje cells and interneurons (Clark & Barbour, 1997). Parallel fibre (PF) EPSP amplitude was estimated from the CNQX-/kynurenate-sensitive potential, which was measured by subtracting what remained in CNQX/kynurenate from control potentials. Confirmation of PF EPSP identity was achieved by evoking pairs of EPSPs (interval 50 ms) and observing facilitation (Atluri & Regehr, 1996) and by examining the pharmacological profile (inhibition by A1, GABAB and mGlu4R receptor agonists). Pairs of PF EPSPs were evoked at 0.1 Hz, and the amplitude of the first EPSP was used as a measure of adenosine A1 receptor activation. The paired pulse ratio (EPSP2/EPSP1) was used to test for a presynaptic action on release probability. Extracellular recordings were made using an ISO-DAM extracellular amplifier (WPI), filtered at 1 kHz and digitised online (10 kHz) with a Digidata 1322A (Axon Instruments) controlled by 9.2 (Axon pCLAMP).
Purine biosensors
Sensors were obtained from Sarissa Biomedical Ltd (Coventry UK). In brief, the adenosine sensor consisted of three entrapped enzymes (adenosine deaminase, AD, purine nucleoside phosphorylase, PNP and xanthine oxidase, XO), within a matrix that was deposited around a 25–50 μm platinum or platinum/iridium (90/10) wire (Llaudet et al. 2003). The biosensor had an exposed length of ∼500 μm that was screened with an inner permselectivity layer to greatly reduce responses to electro-active interferents (such as 5-HT, noradrenaline, dopamine and ascorbate). It was then coated with enzymes to make it capable of detecting purines. Five types of purine biosensor were used in this study to identify released substances. Firstly, a screened null sensor, possessing the deposition matrix but no enzymes, was used to control for the release of any non-specific electro-active interferents. Secondly, screened biosensors containing just XO (only responsive to hypoxanthine, HYPO), PNP and XO (responsive to inosine and hypoxanthine, INO) and PNP, XO and AD (responsive to inosine, hypoxanthine and adenosine, ADO) were used. The difference signal between these three types of biosensors gave the specific hypoxanthine, inosine and adenosine signals. Matching the sizes and sensitivities of the biosensor types as well as careful positioning into or above the slice was vital to optimise the differential recordings. The screened ATP biosensor (Llaudet et al. 2005) consisted of the entrapped enzymes glycerol kinase (GK) and glycerol-3-phosphate oxidase (G3POx). Glycerol 2 mm was included in solutions, as glycerol is a co-substrate required for ATP detection. A full description of the properties of the biosensors has been published. They show a linear response to increasing concentration of analyte, are fast to respond and have a 10–90% rise time of less than 10 s (Llaudet et al. 2003, 2005).
The biosensors were either carefully inserted (at an angle of ∼70 deg) either into the molecular layer or positioned just above the surface of the slice (either at an angle of ∼70 deg or bent so their longitudinal surface was parallel to the slice surface). Biosensors were calibrated with known concentrations (10 μm) of adenosine, inosine, hypoxanthine and ATP. Calibration was performed before the slice was present in the perfusion chamber and after the slice had been removed; this allowed measurement of any reduction in sensitivity during the experiment.
To quantify the concentrations of adenosine, inosine and hypoxanthine, it was assumed that the concentrations of these purines were homogenous in the slice and thus the calibrated signals can be subtracted from one another. The HYPO sensor will only detect hypoxanthine and thus this signal can be subtracted from the signal on the INO sensor to give the inosine signal. The signal from the INO sensor (comprising inosine and hypoxanthine) can be subtracted from the signal on the ADO sensor to give the specific adenosine signal. For example, assuming identical sensitivity for the ADO, INO and HYPO sensors and that 10 μm of each analyte (adenosine, inosine or hypoxanthine) produces 3000 pA. Clearly the ADO sensor will produce a current of 3000 pA in response to 10 μm adenosine, 10 μM inosine or 10 μm hypoxanthine, whereas the HYPO sensor will only respond to hypoxanthine. If upon exposure to tissue, the HYPO sensor produces a current of 300 pA, then the hypoxanthine concentration in the tissue is (300 pA/3000 pA × 10 μm) = 1 μm. If The INO sensor produces a current of 700 pA, then subtracting the current due to hypoxanthine (300 pA) leaves 400 pA which is the current due to inosine detection. Thus the inosine concentration in the tissue is (400 pA/3000 pA × 10 μm) = 1.3 μm. The signal from the INO sensor can then be subtracted (in a similar way) from the ADO sensor to give the specific adenosine signal. In reality, because the sensitivity of sensors to inosine and hypoxanthine will differ slightly, this has to be taken into account during subtraction by appropriate weighting. Note that this differential subtraction procedure only gives valid results if the metabolites are distributed in a uniform identical manner. If this is not the case, for example there are concentration ‘hotspots’, it will be virtually impossible to place the different sensors at identical relative locations with respect to the hotspots. Hence the signals will not be equivalent from the different sensors. Thus the detection of a purine tone will be accurate but its subdivision into concentrations of adenosine, inosine and hypoxanthine may be less so. Sensor signals were acquired at 1 kHz with a Digidata 1322A or a MiniDigi (Axon) using Pclamp 9.2 or Axoscope 9.2 (Axon). All values are mean ± SEM.
Drugs
All drugs were made up as 10–100 mm stock solutions, stored frozen and then thawed and diluted with aCSF on the day of use. Iodotubercidin, pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt (PPADs), suramin, Evans Blue, α,β-methylene-ADP, adenosine, inosine, hypoxanthine, 6-[(4-nitrobenzyl)thiol]-9-β-d-ribofuranosylpurine (NBTI) 8-cyclopentyl-theophylline (8CPT) and dipyridamole were purchased from Sigma. Erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), ARL67156 and d(−)-2-amino-5-phosphonopentanoic acid (AP5) were purchased from Tocris-Cookson. ATP was purchased from Roche.
Results
Parallel fibre EPSPs are tonically inhibited by adenosine in the majority of slices
Previous studies have shown that A1 adenosine receptors on parallel fibre–Purkinje cell synapses tonically inhibit synaptic transmission, suggesting the presence of an extracellular adenosine tone (Kocsis et al. 1984; Takahashi et al. 1995; Dittman & Regehr, 1996). To confirm the presence of extracellular adenosine in our cerebellar slices, we have studied parallel fibre–Purkinje cell excitatory postsynaptic potentials (PF EPSPs). The identity of PF EPSPs was confirmed by the presence of paired pulse facilitation (PPF, with a 50 ms interval the paired pulse ratio was 1.3 ± 0.01, n= 20, Fig. 1A) and the sensitivity of PF EPSPs to the glutamate receptor antagonists CNQX (10 μM) or kynurenic acid (5 mm, Fig. 1B).
Figure 1. Endogenous adenosine inhibits parallel fibre to Purkinje cell synaptic transmission.
A, average of 50 pairs (interval 50 ms) of parallel fibre–Purkinje cell (PF) EPSPs illustrating paired pulse facilitation (greater amplitude of second EPSP compared to first). PF EPSPs were averaged by aligning on the stimulus artefact. B, the A1 receptor antagonist 8CPT (1 μm) increased PF EPSP amplitude (31%) demonstrating tonic activation of A1 receptors. PF EPSPs were blocked by CNQX (10 μm), confirming their production through glutamate receptor activation. Graph plots the amplitude of individual PF EPSPs against time. C, The antagonist 8CPT (1 μm) decreased the paired pulse ratio, confirming a presynaptic site of action. Graph plots the paired pulse ratio against time for the PF EPSPs in B. The line is the mean paired pulse ratio for five EPSPs. D, adenosine (100 μM) reversibly decreased PF EPSP amplitude (48%). Graph plots the amplitude of individual PF EPSPs against time. E, adenosine produced a marked increase in the paired pulse ratio, indicating a presynaptic site of action. Graph plots the paired pulse ratio against time for the PF EPSPs in D. The line is the mean paired pulse ratio for three EPSPs. F, graph summarising the actions of purines on PF EPSP amplitude (n= 5–12).
We confirmed previous reports (Takahashi et al. 1995; Dittman & Regehr, 1996) that inhibitory A1 receptors on parallel fibre–Purkinje cell synapses are tonically activated in slices, as application of A1 receptor antagonists enhanced PF EPSP amplitude (1 μm 8CPT or 200 μM theophylline 46.1 ± 8.6% increase) and significantly reduced the paired pulse ratio (from 1.3 ± 0.04 to 1.2 ± 0.03, Fig. 1B and C) in 17 out of 19 slices. In two slices, the antagonists had no effect on PF EPSP amplitude. The continual activation of A1 receptors is probably a consequence of the presence of endogenous adenosine, since application of adenosine (100 μm) reduced PF EPSP amplitude by 60.5 ± 4.8% and increased the paired pulse ratio from 1.3 ± 0.06 to 1.6 ± 0.08, (n= 12, Fig. 1D and E). As the applied adenosine could be metabolised to inosine and hypoxanthine, the activity of these metabolites on synaptic transmission was assessed. As expected, neither inosine nor hypoxanthine (100 μm) had any significant effect on either the PF EPSP amplitude or the paired pulse ratio (n= 5, Fig. 1F). ATP (100 μm) also reduced EPSP amplitude (50 ± 6.5%). Since this inhibition was blocked by A1 receptor antagonists (n= 6), ATP must be metabolised to adenosine. Our results confirm previous reports that there is a tone of extracellular adenosine in the cerebellum which inhibits synaptic transmission and is present in the majority (∼90%) of slices.
Biosensors detect adenosine metabolites but not adenosine in most cerebellar slices
We have used microelectrode biosensors to directly measure the concentration of extracellular adenosine present in cerebellar slices. The use of different sensors allows the detection of ATP (a possible source of adenosine), adenosine and the adenosine metabolites (inosine and hypoxanthine). A null sensor was also present to control for any non-specific electro-active interferents. To verify our findings we used two different methods. Firstly, we measured the current produced when purine biosensors were moved close to the slice surface (Fig. 2A). This method has the advantage that it is non-invasive and thus any purines detected do not result from biosensor-mediated tissue damage. Movement of null sensors (with no enzyme cascade, n= 10) close to the slice surface produced either no current or a small negative current; thus any electro-interferents released from the slice are not detected by the biosensors. The signal on ATP biosensors (n= 10) was no different from that on null sensors (Fig. 2A), and thus the level of ATP at the slice surface is below the limits of detection by the biosensors (∼60 nm). In contrast, large currents were measured when ADO, INO and HYPO biosensors were moved close to the slice surface (Fig. 2A). After calibration and subtraction (Fig. 2B), to determine the specific analyte concentration (see Methods), adenosine was only detected in a single slice (1 out of 12 slices at 1.5 μm), whereas hypoxanthine was detected in all slices (0.8 ± 0.2 μm, 12 out of 12 slices) and inosine was detected in 50% of slices (0.5 ± 0.1 μm, in 6 slices, Fig. 2B).
Figure 2. Adenosine metabolites, but not adenosine, can be measured in the extracellular space of cerebellar slices.
A, example of biosensor traces from an experiment to measure the concentration of purines at the slice surface. Superimposed traces from ADO, INO, HYPO and ATP sensors. After the biosensors were moved close to the slice surface there was a rapid increase in the baseline current of ∼300–500 pA on the ADO, INO and HYPO sensors. Moving the ATP biosensor close to the slice produced a small drop in the baseline current. B, the traces from A were normalised by calibration and subtracted to give the amounts of adenosine, inosine and hypoxanthine detected at the slice surface (see Methods). C, graph plots the mean concentration of purines measured at the surface of 12 cerebellar slices. Currents were scaled by sensor calibration and then subtracted (as in B). D, examples of experiments where biosensors were used to measure the purine concentration within slices. Biosensors were carefully pushed into slices, left in position for ∼30 min and then withdrawn. The top panel shows the response following removal of an ATP sensor from a slice. There is a small sustained increase in current (similar to that observed on null sensors) and thus no ATP was detected. In contrast when an inosine (INO) sensor was removed there was a sustained fall in current as a result of terminating purine detection (bottom panel). The transient upward deflections (arrows) are presumably due to cell damage and the release and resultant metabolism of ATP. E, graph plotting the mean concentration of purines measured in the extracellular space of 16 cerebellar slices. Currents were scaled by sensor calibration and then subtracted (see Methods).
The second method measured the extracellular concentration of purines within the cerebellum, by carefully inserting purine biosensors into the slice. After 30 min biosensors were removed from the tissue and the resultant deflection in baseline current was used as a measure of purine concentration within the slice. If extracellular purines are present, removal of the biosensor from the slice should result in a rapid fall in baseline, as the purines will no longer be measured. This method avoids the detection of purine release due to tissue damage during sensor insertion. Removal of null sensors produced either no current deflection or a small positive current deflection. The response of ATP biosensors was no different from null sensors (32–34°C n= 20 slices, room temperature n= 10 slices, Fig. 2D), and thus no ATP tone could be detected within the tissue. However clear falls in baseline current were observed following the removal of ADO, INO and HYPO biosensors from slices (n= 6 slices, Fig. 2D). After calibration and subtraction, hypoxanthine was detected in 81% of the slices (1.8 ± 0.3 μm, n= 13 slices), inosine was detected in 50% of the slices (2.5 ± 1.1 μm, n= 8 slices) and adenosine was detected in only 12.5% of the slices (1.2 ± 0.5 μm, n= 2 slices Fig. 2E). Thus both methods of purine measurement gave similar results: no ATP was detected, an adenosine tone was occasionally present and most slices exhibited a tone for inosine and or hypoxanthine.
The inability to reliably detect adenosine with the biosensors contrasts with electrophysiological studies on synaptic transmission that demonstrate an extracellular adenosine tone in 90% of cases. Adenosine could be metabolised to inosine and hypoxanthine before it reaches the sensors, and may thus be present only in the restricted space around parallel fibre synapses. However it is very unlikely that the sensors will not sample from this compartment, as the synapses are present at a high density in the molecular layer.
A more likely explanation for this discrepancy is that the subtraction procedure used to measure adenosine is inaccurate. This could arise from a non-uniform distribution of the analytes adenosine, inosine and hypoxanthine, which would reduce the accuracy of the subtraction procedure (see Methods) In many cases, subtraction of the calibrated INO sensor signals implied a negative concentration of adenosine (clearly impossible), strongly indicating non-uniform concentrations of inosine and hypoxanthine. Our data suggest considerable variation in the levels of inosine (0–0.6 μm) and hypoxanthine (range 0.1–3 μm) between slices. If this reflects hotspots of release/production, depending on enzyme location relative to sensor position, then the differential measurement procedure will not give accurate concentrations of adenosine and inosine.
Exogenous ATP and adenosine are metabolised through to hypoxanthine
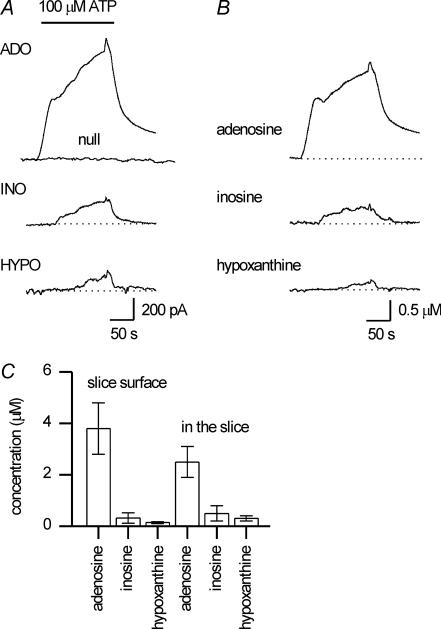
To test whether: (1) adenosine can be metabolised to inosine and hypoxanthine (to give the observed purine tone), (2) adenosine can be detected in cerebellar slices, and (3) there are hotspots of extracellular purine metabolism, exogenous ATP was applied to slices and the real-time production of metabolites was measured using ADO, INO and HYPO biosensors. As before, this experiment was carried out in two ways: firstly non-invasively with sensors placed close to the slice surface, and secondly with the biosensors placed within the slice. The inflow was changed from control saline to 100 μm ATP for 5 min. The exact duration of the ATP application is not known (as the 5 min includes the time taken for the ATP to reach the bath and exchange with the bath solution). However, because the flow rate is constant, the applications will be reproducible. Application of ATP rapidly resulted in the detection of 3.8 ± 1 μm adenosine, 0.3 ± 0.2 μm inosine and 0.14 ± 0.03 μm hypoxanthine at the slice surface (n= 6, Fig. 3). Similar results were found when the biosensors were placed within the slice (2.5 ± 0.6 μm adenosine, 0.5 ± 0.3 μm inosine and 0.3 ± 0.1 μm hypoxanthine, n= 7, Fig. 3C). In all slices, after subtraction, there was a positive amount of adenosine demonstrating that the subtraction procedure was successful. This confirms that biosensors can be used to measure adenosine in cerebellar slices.
Figure 3. Exogenous ATP is metabolised by cerebellar slices.
A, example traces from adenosine (ADO), null, inosine (INO) and hypoxanthine (HYPO) biosensors placed on the surface of a cerebellar slice. Following application of 100 μm ATP, currents were produced on all the sensors except the null. B, the traces from A, calibrated and subtracted illustrating the metabolism of ATP to adenosine, inosine, and hypoxanthine. C, graph plots the mean concentration of ATP metabolites measured at the surface of slices (n= 6) and within slices (n= 7) following application of 100 μm ATP.
To test that the inosine arose from the metabolism of adenosine and that hypoxanthine arose from inosine breakdown, adenosine and inosine were applied to slices. Following a 1 min application of 100 μm adenosine, 3.2 ± 0.7 μm inosine and 0.3 ± 0.06 μm hypoxanthine were detected (n= 5) and 100 μm inosine produced 0.7 ± 0.1 μm hypoxanthine (n= 5). These results demonstrate that cerebellar slices contain the enzymes that rapidly break down adenosine to hypoxanthine (adenosine deaminase and purine nucleoside phosphorylase). Thus it is feasible for the inosine/hypoxanthine tone to arise from the breakdown of endogenous adenosine. The breakdown of ATP to adenosine suggests that the extracellular metabolism of ATP could be a potential source of adenosine.
There was considerable variation in the relative quantities of the metabolites detected following ATP application. At the surface of the slice, the proportion of ATP metabolites (relative proportions of adenosine, inosine and hypoxanthine) ranged from 25% to 91% for adenosine, (mean 84 ± 7%), inosine accounted for 0–60% (mean 11 ± 6%) and hypoxanthine accounted for 0–14% (mean 5 ± 1%). Similar results were observed with biosensors placed within slices. This suggests that the distribution of metabolising enzymes within slices is not uniform and that the positioning of the biosensors relative to the enzymes may dictate the proportions of metabolites detected. This strengthens the argument that the inability to detect basal levels of adenosine using biosensors results from a non-homogenous distribution of inosine/hypoxanthine in the cerebellum.
Extracellular purine nucleoside phosphorylase (PNP) is present in the cerebellum
The conversion of inosine to hypoxanthine is catalysed by purine nucleoside phosphorylase (PNP) which is an important enzyme in the salvage of nucleotide bases (Bzowska et al. 2000). Although PNP is considered an intracellular enzyme it has been described in rat CSF (Silva et al. 2004). The production of hypoxanthine following ATP, adenosine or inosine application suggests that an extracellular form of PNP is expressed in the cerebellum. To confirm the extracellular expression of PNP, we used a specific inhibitor of PNP, immucullin H (Horenstein & Schramm, 1993). Immucullin H (200 nm) caused a 66 ± 3% reduction in the production of hypoxanthine (following changing the inflow from control saline to 100 μm inosine for 2.5 min) as measured by a HYPO biosensor laid on the surface of the slice (n= 3, Fig. 4A). Immucullin H had no effect on the responsiveness of the HYPO sensor to hypoxanthine, and HYPO sensors could not detect inosine (Fig. 4B)
Figure 4. Extracellular purine nucleoside phosphorylase (PNP) metabolises inosine to hypoxanthine in cerebellar slices.
A, record from a HYPO biosensor placed on the surface of a cerebellar slice (the sensor was bent so that it was laid parallel to the slice surface, increasing the sensitivity of purine detection). Application of 100 μm inosine (bar) produced a large current on the sensor due to rapid conversion of inosine to hypoxanthine. Application of the PNP inhibitor, immucillin H (200 nm), markedly reduced the conversion of inosine to hypoxanthine. Following wash there was recovery in the conversion of inosine to hypoxanthine. B, the same sensor as A, with no slice present. The sensor responds to hypoxanthine but does not respond to inosine.
What determines the extracellular concentration of adenosine in the cerebellum?
Adenosine kinase
Adenosine kinase is a key intracellular enzyme that phosphorylates adenosine to form AMP and thus maintains low levels of intracellular adenosine helping to prevent efflux via equilibrative transporters (for review see Boison, 2006). To assess whether adenosine kinase is important in the cerebellum, we blocked the enzyme adenosine kinase with iodotubercidin and measured the effects on synaptic transmission and the extracellular concentration of adenosine. Application of iodotubercidin (1–2 μm) reliably inhibited PF EPSPs (9 out of 10 slices, 51 ± 2%, Fig. 5A) and increased the paired pulse ratio (from 1.58 ± 0.06 to 1.86 ± 0.02) indicating a presynaptic action. The effects of iodotubercidin were reversed by 8CPT (1 μm) and the mean increase in PF EPSP amplitude was much larger (168 ± 21%) than in control (46.1%) indicating increased A1 receptor activation. Iodotubercidin was without effect in only one slice (although EPSP amplitude was increased by 8CPT, suggesting the presence of an adenosine tone). Simultaneous biosensor measurements reliably detected an increased level of purines following iodotubercidin application (8 out of 10 slices, Fig. 5B). After subtraction, the concentration of adenosine was increased by 0.68 ± 0.1 μm and inosine was increased by 0.37 ± 0.2 μm. The consistent increase in adenosine suggests that adenosine kinase is an important and global mechanism for determining the extracellular concentration of purines in cerebellar slices. This also demonstrates that our method of measuring adenosine is reliable and that the variable results obtained with other manipulations of adenosine concentration must be due to biological not technical mechanisms.
Figure 5. Inhibition of adenosine kinase increases the extracellular concentration of adenosine.
A, the adenosine kinase inhibitor iodotubercidin (1 μm) caused a reduction in PF EPSP amplitude (∼50%) which was reversed by the A1 receptor antagonist 8CPT (1 μm). At the end of the experiment, PF EPSPs were blocked by kynurenate (5 mm). Graph plots the amplitude of individual PF EPSPs against time. Inset, iodotubercidin (1 μm) caused an increase in the paired pulse ratio, confirming presynaptic inhibition due to adenosine efflux. Graph plots the paired pulse ratio against time for the PF EPSPs in A. The average paired pulse ratio for five EPSPs is plotted. B, trace from an ADO and INO sensor placed within the same slice as A. Application of iodotubercidin (1 μm) produced a current on the ADO sensor with little effect on the INO sensor. After calibration there was a net increase in the extracellular adenosine concentration of ∼0.5 μm. C, the equilibrative transport inhibitors NBTI (5 μm) and dipyridamole (10 μm) inhibited PF EPSP amplitude and occluded the effects of iodotubercidin. Although iodotubericidin (2 μm) had no effect on PF EPSP amplitude (in the presence of NBTI/dipyridamole), the adenosine receptors were not saturated as application of adenosine (100 μm) produced increased inhibition. The modulation of PF EPSPs was reversed by block of A1 receptors (1 μm 8CPT), and EPSPs were blocked by kynurenate (5 mm) at the end of the experiment. D, NBTI (5 μm) and dipyridamole (10 μm) caused a small inhibition of PF EPSP amplitude but did not occlude the effects of iodotubercidin. Application of 2 μm iodotubericidin (in the presence of NBTI/dipyridamole) markedly inhibited EPSP amplitude. The modulation of PF EPSPs was reversed by block of A1 receptors (1 μm 8CPT), and EPSPs were blocked by 5 mm kynurenate at the end of the experiment.
To investigate whether the equilibrative transporters mediate the increase in adenosine tone following block of adenosine kinase, we applied NBTI/dipyridamole (to block ENT1/ENT2) prior to application of iodotubercidin (to inhibit adenosine kinase). NBTI and dipyridamole have been used to block ENT1 and ENT2 in a number of studies (Dunwiddie & Diao, 1994; Frenguelli et al. 2007). In four out of eight slices, the inhibitory actions of iodotubercidin were almost completely occluded by the prior application of NBTI/dipyridamole (Fig. 5C). At these synapses the equilibrative transport of adenosine was abolished and thus the increase in intracellular adenosine concentration (as a result of adenosine kinase inhibition) did not result in adenosine efflux. In the remaining four slices, NBTI/dipyridamole did not occlude the actions of iodotubercidin (Fig. 5D). Presumably in these slices, the equilibrative transport of adenosine was not effectively blocked and thus the increase in intracellular adenosine concentration (as a result of adenosine kinase inhibition) still resulted in an efflux of adenosine. These results suggest heterogeneous expression of synaptic adenosine transporters with different proportions of NBTI-/dipyridamole-sensitive transporters expressed at different synapses.
Equilibrative transporters
We further tested the role of equilibrative transporters (ENT1 and ENT2) in the control of the adenosine tone. Adenosine can be removed from the extracellular space by transport into neurons and glia. Long applications (30 min) of NBTI (5 μm) and dipyridamole (10 μm) had variable effects on PF EPSP amplitude and biosensor measurements (n= 18 slices). Results could be divided into three categories: in nine slices, PF EPSP amplitude was decreased (47 ± 8%) with an increase in PPF (from 1.6 ± 0.1 to 1.9 ± 0.1) and a large increase in EPSP amplitude following 8CPT (1 μm) application (180 ± 20%vs. 46.1 ± 8.6%, Fig. 6A), indicating increased A1 receptor activation. However there was no shift in the baseline current measured by purine biosensors, suggesting no change in the bulk concentration of purines within the slice (Fig. 6B). In one additional slice, as well as reducing PF EPSP amplitude, the block of adenosine uptake also increased the purine (inosine/hypoxanthine) concentration measured by the biosensor (Fig. 6C). In the final eight slices, blocking adenosine transport had little or no effect on either PF EPSP amplitude (decrease 3 ± 6%, PPF 1.56 ± 0.1 vs. 1.5 ± 0.1) or biosensor baseline current. However application of 8CPT (1 μm) still increased EPSP amplitude by 55 ± 17% (vs. 46.1 ± 8.6% in control) suggesting that a baseline level of adenosine was present in these synapses (Fig. 6D). These highly variable data do not reflect technical deficiencies of measurement, as iodotubercidin reliably evokes changes in adenosine tone. Rather they probably reflect that NBTI-/dipyridamole-sensitive transporters determine the concentration of purines at only a subset of parallel fibre to Purkinje cell synapses.
Figure 6. Blocking equilibrative transport has heterogeneous effects.
A, application of ATP (100 μm) caused a reversible inhibition of PF EPSP amplitude. Block of equilibrative transport with 5 μm NBTI and 10 μm dipyridamole caused a reduction in PF EPSP amplitude, which was reversed by block of A1 receptors with 8CPT (1 μm). PF EPSPs were blocked by CNQX at the end of the experiment. Graph plots the amplitude of individual PF EPSPs against time. Inset, application of ATP (*) and NBTI/dipyridamole caused an increase in the paired pulse ratio, confirming a presynaptic site of action. Graph plots the paired pulse ratio against time for the PF EPSPs in A. The average paired pulse ratio for five EPSPs is plotted. B, trace from an ADO biosensor present in the same slice as A. Application of 100 μm ATP caused an increase in adenosine concentration (as a result of metabolism), but 5 μM NBTI/10 μm dipyridamole had no effect. C, trace from an ADO biosensor in a different slice. Application of 5 μm NBTI/10 μm dipyridamole produced a current on the sensor, suggesting an increase in the concentration of purines in the bulk of the slice. D, application of ATP (100 μm) caused a reduction in PF EPSP amplitude, but 5 μm NBTI/10 μm dipyridamole had no effect. There was also no effect on the paired pulse ratio (not illustrated). There is an adenosine tone, as block of A1 receptors with 8CPT (1 μm) increased PF EPSP amplitude. PF EPSPs were blocked by CNQX (10 μm) at the end of the experiment.
Adenosine deaminase
As well as being an intracellular enzyme, adenosine deaminase can convert extracellular adenosine to inosine. As our biosensor measurements routinely detect an extracellular inosine and hypoxanthine tone, we have investigated the contribution of adenosine deaminase to this tone by means of simultaneous biosensor and PF EPSP recordings in the presence of EHNA, a selective blocker of adenosine deaminase (Agarwal, 1982).
Although the increase in adenosine concentration cannot be measured directly (biosensor detection of adenosine uses adenosine deaminase), the effects of EHNA can be monitored via changes in PF EPSP amplitude and by changes in inosine/hypoxanthine concentration. EHNA (20 μm) is effective at inhibiting adenosine deaminase as it blocks the adenosine deaminase present on the adenosine biosensor, with no effect on the detection of inosine and hypoxanthine (Wall & Dale, 2007). Application of EHNA had only minor effects on PF EPSPs: the mean PF EPSP amplitude was slightly reduced (11 ± 5%) and the paired pulse ratio was increased (from 1.4 ± 0.07 to 1.6 ± 0.07, n= 7, Fig. 7A and B). Furthermore, application of 1 μm 8CPT (in the presence of EHNA) produced a significantly larger increase in PF EPSP amplitude than in control (84 ± 19%vs. 46.1 ± 8.6%), demonstrating greater A1 receptor activation. Simultaneous biosensor recordings revealed a fall in the extracellular levels of inosine and hypoxanthine, which was coincident with the effect on PF EPSP amplitude (Fig. 7C) There was a fall in hypoxanthine concentration of 1.2 ± 0.7 μm (7 out of 7 slices) and a fall in inosine concentration of 0.3 ± 0.2 μm (4 out of 7 slices). As the basal concentration of hypoxanthine in the slices is 1.8 μm, then at least 66% of the hypoxanthine arises from the metabolism of adenosine. The remaining hypoxanthine could arise from other sources such as inosine monophosphate. Although ecto-adenosine deaminase does contribute to the inosine/hypoxanthine tone, it does not appear to be the major regulator of the adenosine tone.
Figure 7. The metabolism of adenosine contributes to the extracellular inosine/hypoxanthine tone but has little effect on synaptic transmission.
A, the adenosine deaminase inhibitor EHNA (20 μm) caused a reduction in EPSP amplitude (∼17%), which was reversed by block of A1 receptors with 8CPT (1 μm). EPSPs were blocked at the end of the experiment with 5 mm kynurenate. Graph plots the amplitude of individual PF EPSPs against time. B, EHNA caused an increase in the paired pulse ratio whereas 8CPT reduced the ratio, confirming a presynaptic site of action. Graph plots the paired pulse ratio against time for the PF EPSPs in A. The average paired pulse ratio for five EPSPs is plotted. C, trace from an INO sensor placed within the same slice as A. Application of EHNA caused a fall in the concentration of the adenosine metabolites inosine/hypoxanthine.
Endogenous sources of extracellular adenosine
We have recently demonstrated that action potential-dependent adenosine release occurs in response to short trains of electrical stimuli in the molecular layer (Wall & Dale, 2007). If this adenosine were to be metabolised to inosine/hypoxanthine then it could contribute to the extracellular purine tone. We therefore stimulated in the molecular layer and recorded the release of adenosine (with an ADO sensor) and measured any breakdown of the released adenosine to hypoxanthine (with a HYPO sensor). Following stimulation there was a rapid release of adenosine (as previously described, Wall & Dale, 2007) measured on the ADO sensor, followed by a much slower build-up of hypoxanthine detected by the HYPO sensor (n= 6, Fig. 8A). If the entire ADO sensor signal was due to adenosine, then 360 ± 100 nm adenosine was released, which was metabolised to 45 ± 58 nm hypoxanthine. Both the adenosine and hypoxanthine signals were blocked following addition of TTX to block action potentials (1 μm, n= 3). If the action potential-dependent release of adenosine occurs spontaneously, then it could contribute to the inosine/hypoxanthine tone measured in slices. Blocking action potential adenosine release with TTX (1 μm) produced a small fall (∼15 pA) in the baseline current of the ADO biosensor in three out of ten slices, suggesting that activity-dependent adenosine is only a minor contributor to the purine tone in the slice. However in vivo this source of adenosine may be more important, as there is much greater parallel fibre activity.
Figure 8. What is the source of endogenous adenosine?
A, superimposed traces from ADO and HYPO biosensors placed parallel to the molecular layer surface. Following stimulation (5 V, 20 Hz, 10 s) in the molecular layer, adenosine is released and a proportion is metabolised to hypoxanthine. The traces are normalised by the biosensor calibration, assuming that all ADO biosensor signal results from adenosine detection. B, application of α,β-methylene-ADP (100 μm) and ARL67156 (100 μm), to reduce conversion of ATP to adenosine, had no effect on PF EPSP amplitude. However, application of 8CPT (1 μm) increased PF EPSP amplitude, demonstrating that synaptic A1 receptors were activated by endogenous adenosine. Graph plots the amplitude of individual PF EPSPs against time. C, trace from ADO biosensor positioned in the same slice as A. Application of 100 μmα,β-methylene-ADP and ARL67156 had no effect on the baseline current. D, application of α,β-methylene-ADP (100 μm) and ARL67156 (100 μm) increased PF EPSP amplitude. Upon wash there was a transient reduction in PF EPSP amplitude below control levels. E, trace from ADO biosensor positioned in the same slice as D. Addition of α,β-methylene-ADP and ARL67156 caused a reduction in the baseline current (reduction in the conversion of ATP to adenosine, coincident with increase in PF EPSP amplitude). Upon wash there was a transient increase in the level of adenosine, which coincided with the inhibition of PF EPSP amplitude.
We next tested whether release of ATP is the source of endogenous adenosine (and the inosine/hypoxanthine tone). Under control conditions we could not measure any extracellular ATP (in or above the slice), suggesting either that little ATP is released or that it is rapidly metabolised. We thus inhibited the metabolism of ATP with ARL67156 (100 μm), an inhibitor of ecto-ATPase (Crack et al. 1995). Following ARL67156 application, there was still no difference between ATP and null sensors at either room temperature (n= 15) or 33–35°C (n= 20). Similar results were found with other ecto-ATPase inhibitors: PPADS (10–100 μm), suramin (100 μm), dipyridamole (10 μm, Connolly & Duley, 2000) and Evans Blue (100 μm). We have previously shown that the agents ARL67156 and Evans Blue are effective inhibitors of ATP breakdown by cerebellar slices (Wall & Dale, 2007).
An alternative approach to directly observing the release of ATP is to inhibit the conversion of ATP to adenosine and thus measure a fall in adenosine concentration (measured as an increase in PF EPSP amplitude and by a fall in inosine/hypoxanthine levels). The ecto-5′-nucelotidase inhibitor α,β-methylene-ADP was used to reduce the conversion of AMP to adenosine (effective in the cerebellum, Wall & Dale, 2007). To increase the likelihood of reducing the conversion of ATP to adenosine, α,β-methylene-ADP (100 μm) was applied in combination with ARL67156 (100 μm). In the majority of slices (n= 8) there was no effect on either PF EPSP amplitude or biosensor current (Fig. 8B and C). In four slices there was a small reversible decrease in inosine/hypoxanthine levels, as detected by a fall in the baseline current measured by biosensors (mean fall in current 49 ± 35 pA, Fig. 8E). In three out of the four slices this was accompanied by a small increase in PF EPSP amplitude (mean increase 8.6 ± 5.8%, Fig. 8D). Upon wash there was a transient increase in adenosine concentration, presumably as accumulated ADP and AMP are rapidly broken down to adenosine by the now uninhibited enzymes. This was measured by biosensors as a current increase of 56 ± 23 pA and by a transient reduction in PF EPSP amplitude of 36.6 ± 11% (Fig. 8D and E). We therefore find that metabolism of ATP may contribute to the production of adenosine in some slices, but that in many slices this does not appear to be the source of the purine tone.
Discussion
Many studies have demonstrated the presence of an adenosine tone in the CNS (including the cerebellum, Takahashi et al. 1995; Dittman & Regehr, 1996). We have confirmed this by examining synaptic transmission. Nevertheless, direct measurements with biosensors failed to reliably detect an extracellular adenosine tone in the cerebellum. Instead we demonstrated the presence of an extracellular inosine and hypoxanthine tone that arises (at least in part) from the breakdown of adenosine. The conversion of adenosine to hypoxanthine requires the extracellular expression of adenosine deaminase and purine nucleoside phosphorylase (PNP). The inhibition of the conversion of inosine to hypoxanthine by the specific PNP inhibitior immucullin-H confirms the extracellular expression of PNP. This is unexpected since PNP is usually considered an intracellular enzyme (Bzowska et al. 2000). Interestingly, astrocytes cultured from neonatal cerebellum also metabolise exogenous ATP to hypoxanthine (Wink et al. 2003), suggesting that the adenosine metabolising enzymes could be expressed by glia. The rationale for the conversion of adenosine to inosine seems obvious: the conversion of active (adenosine) to inactive (inosine) molecules. Although inosine is often considered inert, its metabolism to hypoxanthine suggests that it may be functionally active and plays some role in the cerebellum. There is growing support for inosine being active as it appears to play a role in the immune system and may be neuroprotective (Hasko et al. 2004).
Inability to directly detect endogenous adenosine with biosensors
In common with findings in other studies (Takahashi et al. 1995; Dittman & Regehr, 1996), parallel fibre–Purkinje cell synaptic transmission is tonically inhibited by endogenous adenosine in cerebellar slices. However we have been unable to directly measure this extracellular adenosine using biosensors. A similar finding has been reported in the hippocampus where an adenosine tone was not detectable in three out of four slices, although tonic adenosine-mediated synaptic inhibition was present (Frenguelli et al. 2007). It is unlikely that the levels of adenosine are below the levels of detection, as pharmacological experiments have estimated that 200–400 nm adenosine is present in the extracellular space of the brain (Dunwiddie & Diao, 1994). This concentration of adenosine would give a current on an ADO biosensor of 60–120 pA, which in normal conditions would easily be detected. There are two possibilities which would explain this inability to detect adenosine: firstly, adenosine is metabolised to inosine/hypoxanthine before it reaches the sensor. It could be argued that the sensor is further from the adenosine release sites than the A1 receptors and thus breakdown in or around the synapses occurs before any purines reach the biosensor. However the large density of parallel fibre synapses in the molecular layer makes it unlikely that the sensor does not sample from the synaptic compartment. Furthermore, blockade of extracellular breakdown (with EHNA) had little effect on synaptic inhibition, suggesting that it is not the major mechanism of removing adenosine. Dilution of adenosine before detection was also unlikely, as biosensors were inserted through the slice and the sensing area of the biosensors was in intimate contact with the tissue. Secondly, it is more likely that a non-homogenous distribution of inosine and hypoxanthine in the extracellular space prevented reliable measurement of adenosine for technical reasons. If the levels of inosine/hypoxanthine are not equivalent for each sensor (ADO, INO and HYPO), then upon subtraction an inaccurate adenosine concentration will be measured. A failure of subtraction will result in either an overestimate or underestimate of the adenosine concentration. Subtraction of the calibrated inosine and hypoxanthine signals gave either a negative adenosine signal or a large adenosine signal (1–2 μm). The presence of a non-uniform distribution of inosine and hypoxanthine is supported by the observations that (1) there is a large variation in the amount of inosine and hypoxanthine measured in different slices, and (2) movement of sensors to different parts of the slice detected differing amounts of inosine and hypoxanthine following ATP application (M.J. Wall and N. Dale, unpublished observations). A heterogeneous distribution of inosine/hypoxanthine could stem from the distribution of adenosine-metabolising enzymes. If the enzymes are clustered (rather than spread uniformly), then the positioning of the sensors relative to the enzymes will determine the amounts of inosine and hypoxanthine detected. Future work is required to test directly for the clustering of enzymes. Despite these limitations we reliably observed a purine tone. Furthermore, when we used iodotubercidin (to block adenosine kinase) there was a global increase in the adenosine tone, which was reliably detected by the biosensors.
Control of purine levels in cerebellum compared to other brain regions
The levels of adenosine in the cerebellum are controlled by the balance between the rates of production and elimination. The tone of inosine and hypoxanthine arises (at least in part) from adenosine metabolism. What is the source of this adenosine? The two major possibilities are production via extracellular ATP breakdown (for review see Zimmermann, 2000) or direct release (either by transport or by exocytosis). Although we have used several different methods to distinguish between these possibilities, the major source of adenosine remains uncertain. It has not been possible to directly measure ATP in cerebellar slices with biosensors (limit of detection ∼60 nm), and reducing ATP breakdown (with ecto-ATPase inhibitors) did not reveal an ATP signal. Thus either the ATP tone described by Brockhaus et al. (2004) (detected indirectly by its effect on the spontaneous activity of inhibitory interneurons) must be too small to detect by our methods, or the breakdown of ATP is very rapid and not prevented by the inhibitors. However, slowing the conversion of ATP to adenosine did reduce the concentration of adenosine metabolites in some slices. However, the reduction was small (15%) and the effect on EPSP amplitude was minor (10% increase vs. 46% inhibition). Thus the contribution of ATP metabolism to the purine tone appears minor. This contrasts with the hippocampus, where metabolism of ATP is a major contributor to the purine tone (Frenguelli et al. 2007; Pascual et al. 2005).
We have recently described the release of adenosine in response to focal electrical stimulation of the molecular layer, a process which is both Ca2+ and TTX sensitive (Wall & Dale, 2007). Blocking any spontaneous release of action potential-dependent adenosine release with TTX had only a small effect on the inosine/hypoxanthine tone. However, in vivo, the activity of parallel fibres is much greater and thus this source of adenosine may be much more important.
Elimination of adenosine occurs by a combination of two processes: metabolism (by adenosine deaminase) to inactive metabolites and transport into neurons and glia followed by phosphorylation to AMP (by adenosine kinase). Using pharmacological agents, we have investigated which of these components is more important in determining extracellular adenosine levels in the cerebellum. Changes in adenosine levels were assessed by comparing synaptic inhibition with direct measurement of purines via biosensors. Blocking adenosine deaminase had minor effects on parallel fibre–Purkinje cell synaptic transmission (∼10% reduction), but did reduce the levels of extracellular inosine and hypoxanthine (∼50%) measured by biosensors. The small effect of EHNA on synaptic transmission suggests that extracellular metabolism is not the major mechanism controlling synaptic adenosine concentration, and adenosine deaminase maybe located outside the synapses. Similar small effects of EHNA on synaptic transmission in the hippocampus have also been observed (Dunwiddie & Diao, 1994; Pak et al. 1994). The effect of EHNA on the inosine/hypoxanthine tone demonstrates that around half of the tone arises from adenosine metabolism (the rest may arise from other sources such as inosine monophosphate). As the tone of inosine/hypoxanthine is ∼1–2 μm, the adenosine concentration would be expected to increase by 0.5–1 μm, which should markedly increase synaptic inhibition. The small effect of EHNA on synaptic transmission suggests that any increase in adenosine is presumably diminished by greater equilibrative transport into neurons and glia around synapses.
In contrast to the minor effects of blocking metabolism, inhibition of adenosine kinase produced a marked decrease (∼50%) in PF EPSP amplitude in the majority of slices, accompanied by a simultaneous increase in adenosine concentration detected by biosensors. The agreement of biosensor measurements with the increase in synaptic inhibition suggests that blocking adenosine kinase produces a global increase in adenosine concentration. Presumably blockade of adenosine kinase causes an increase in the intracellular concentration of adenosine and a resultant efflux of adenosine via equilibrative transporters (see Boison, 2006). The greater importance of adenosine kinase activity compared to extracellular adenosine metabolism has been observed in other brain regions (Zhang et al. 1993; Pak et al. 1994; Lloyd & Fredholm, 1995).
The role of equilibrative transport in determining adenosine concentration was assessed by blocking ENT1 and ENT2. In ∼50% of parallel fibre–Purkinje cell synapses, synaptic transmission was markedly inhibited (∼45%) as a result of the build-up of adenosine following the inhibition of adenosine transport. However (apart from one slice), no increase in adenosine concentration could be measured by biosensors. The effects of blocking equilibrative transport were very inconsistent (compared to blocking adenosine kinase). We can exclude any technical difficulties in measuring an adenosine increase, as adenosine was reliably detected following adenosine kinase inhibition. Also, after removing the biosensors from the tissue there was very little reduction in sensitivity. Thus the biosensors retain their ability to detect adenosine throughout the experiment. Furthermore, similar biosensors could reliably detect an increase in adenosine in hippocampal slices following application of NBTI/dipyridamole (Frenguelli et al. 2007). The most likely explanation is that equilibrative transport in the cerebellum is not always effectively blocked by application of NBTI/dipyridamole. This is supported by the observation that we cannot always occlude the effects of iodotubercidin by prior application of NBTI/dipyridamole. Heterogeneous expression of ENT1 and ENT2 could lead to variation in the actions of NBTI/dipyridamole. Although rat ENT1 is effectively blocked by NBTI, rat ENT2 is only weakly blocked by NBTI and dipyridamole (Baldwin et al. 2004).Thus variable expression of ENT2 at parallel fibre synapses, could plausibly account for the observed effects of NBTI/dipyridamole. Alternatively, differential phosphorylation of ENT1 and or ENT2 at different synapses could change the proportion of transport via ENT1 or ENT2 (Stolk et al. 2005). It is also possible that there is heterogeneous expression of another transporter (other than ENT1 and ENT2) that is insensitive to NBTI/dipyridamole. The variation in effect of NBTI/dipyridamole between different slices suggests that any rise in adenosine concentration is localised and does not spread between all synapses. This is supported by the observation that an increase in purine concentration (following block of equilibrative transport) could only be measured by biosensors in one slice. Although antibody staining has revealed diffuse expression of the transporters in the molecular layer, granule cell layer and Purkinje cell bodies (Anderson et al. 1999a,b), the density of ENT1 and ENT2 relative to each other and to individual synapses is currently unclear.
In conclusion, we have demonstrated that a combination of biosensor measurements and electrophysiology can be used to determine how the basal extracellular levels of adenosine are regulated. Using these methods, we have documented an inosine/hypoxanthine tone (undetectable by electrophysiology alone) that has a heterogeneous distribution (which would not be observed with either HPLC or microdialysis). The control of extracellular adenosine concentration in the cerebellum appears comparable to that in other brain regions where the activity of adenosine kinase is the major determining factor. However the variable effects of blocking adenosine transport (on both synaptic inhibition and biosensor measurements) suggest that the transporters display an unexpected non-uniform distribution relative to synapses.
Acknowledgments
We thank Dr Enrique Llaudet and Shakila Bibi of Sarissa Biomedical Ltd for biosensor manufacture. We thank V. Schramm for the generous gift of immucullin H. This work was supported by the Wellcome Trust (M.J.W. and N.D.) and the BBSRC.
References
- Agarwal RP. Inhibitors of adenosine deaminase. Pharmacol Ther. 1982;17:399–429. doi: 10.1016/0163-7258(82)90023-7. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Baldwin SA, Young JD, Cass CE, Parkinson FE. Distribution of mRNA encoding a nitrobenzylthioinosine-insensitive nucleoside transporter (ENT2) in rat brain. Brain Res Mol Brain Res. 1999a;70:293–297. doi: 10.1016/s0169-328x(99)00164-3. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Xiong W, Geiger JD, Young JD, Cass CE, Baldwin SA, Parkinson FE. Distribution of equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporters (ENT1) in brain. J Neurochem. 1999b;73:867–873. doi: 10.1046/j.1471-4159.1999.0730867.x. [DOI] [PubMed] [Google Scholar]
- Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin SA, Beal PR, Yao SYM, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Regehr WG. Brief bursts of parallel fiber activity trigger calcium signals in Bergmann glia. J Neurosci. 2006;26:6958–6967. doi: 10.1523/JNEUROSCI.0613-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Braas KM, Newby AC, Wilson VS, Snyder SH. Adenosine-containing neurons in the brain localised by immunocytochemistry. J Neurosci. 1986;6:1952–1961. doi: 10.1523/JNEUROSCI.06-07-01952.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J, Dressel D, Herold S, Deimer JW. Purinergic modulation of synaptic input to Purkinje neuons in rat cerebellar slices. Eur J Neurosci. 2004;19:2221–2230. doi: 10.1111/j.0953-816X.2004.03325.x. [DOI] [PubMed] [Google Scholar]
- Bzowska A, Kulikowska E, Shugar D. Purine nucleoside phosphorylase: properties, functions and clinical aspects. Pharmacol Ther. 2000;88:349–425. doi: 10.1016/s0163-7258(00)00097-8. [DOI] [PubMed] [Google Scholar]
- Clark BA, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices. J Physiol. 1997;502:335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly GP, Duley JA. Ecto-nucleotidase of cultured rat superior cervical ganglia: dipyridamole is a novel inhibitor. Eur J Pharmacol. 2000;397:271–277. doi: 10.1016/s0014-2999(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Crack BE, Pollard CE, Beukers MW, Roberts SM, Hunt SF, Ingall AH, McKechnie KCW, Ijzerman AP, Leff P. Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br J Pharmacol. 1995;114:475–481. doi: 10.1111/j.1476-5381.1995.tb13251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CG, White TD. N-methyl-D-aspartate and non-N-methyl-d-aspartate-evoked adenosine release from rat cortical slices: distinct purinergic sources and mechanisms of release. J Neurochem. 1993;60:1073–1080. doi: 10.1111/j.1471-4159.1993.tb03256.x. [DOI] [PubMed] [Google Scholar]
- Dale N, Pearson T, Frenguelli BG. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J Physiol. 2000;526:143–155. doi: 10.1111/j.1469-7793.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;48:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L. Extracellular adenosine concentration in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J Pharmacol Exp Ther. 1994;268:537–545. [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Frenguelli BG, Llaudet E, Dale N. High-resolution real-time recording with microelectrode biosensors reveals novel aspects of adenosine release during hypoxia in rat hippocampal slices. J Neurochem. 2003;86:1506–1515. doi: 10.1046/j.1471-4159.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischemia in the mammalian hippocampus. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2006.04425.x. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JD, Nagy JI. Distribution of adenosine deaminase activity in rat brain and spinal cord. J Neurosci. 1986;6:2707–2714. doi: 10.1523/JNEUROSCI.06-09-02707.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JG, Foga IO, Parkinson FE, Geiger JD. Involvement of bidirectional adenosine transporters in the release of l-[3H]adenosine from rat brain synaptosomal preparations. J Neurochem. 1995;64:2105–2110. doi: 10.1046/j.1471-4159.1995.64052105.x. [DOI] [PubMed] [Google Scholar]
- Hasko G, Sitkovsky MV, Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Horenstein BA, Schramm VL. Correlation of the molecular eletrostatic potential surface of an enzymatic transition state with novel transition-state inhibitors. Biochemistry. 1993;32:9917–9925. doi: 10.1021/bi00089a007. [DOI] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Eng DL, Bhisitkul RB. Adenosine selectively blocks parallel-fiber-mediated synaptic potentials in rat cerebellar cortex. Proc Natl Acad Sci U S A. 1984;81:6531–6534. doi: 10.1073/pnas.81.20.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukulski F, Sévigny J, Komoszyński M. Comparative hydrolysis of extracellular adenine nucleotides and adenosine in synaptic membranes from porcine brain cortex, hippocampus, cerebellum and medulla oblongata. Brain Res. 2004;1030:49–56. doi: 10.1016/j.brainres.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Llaudet E, Botting N, Crayston J, Dale N. A three enzyme microelectrode sensor for detecting purine release from central nervous system. Biosens Bioelectron. 2003;18:43–52. doi: 10.1016/s0956-5663(02)00106-9. [DOI] [PubMed] [Google Scholar]
- Llaudet E, Hatz S, Droniou M, Dale N. Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal Chem. 2005;77:3267–3273. doi: 10.1021/ac048106q. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Fredholm BB. Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem Int. 1995;26:387–395. doi: 10.1016/0197-0186(94)00144-j. [DOI] [PubMed] [Google Scholar]
- Newman ME, McIlwain H. Adenosine as a constituent of the brain and of isolated cerebral tissues and its relationship to the generation of cyclic AMP. Biochem J. 1977;164:131–137. doi: 10.1042/bj1640131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji T, Karaswa A, Kusaka H. Adenosine uptake inhibitors. Eur J Pharmacol. 2004;495:1–16. doi: 10.1016/j.ejphar.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Pak MA, Haas HL, Decking UK, Schrader J. Inhibition of adenosine kinase increases endogenous adenosine and depresses neuronal activity in hippocampal slices. Neuropharmacology. 1994;33:1049–1053. doi: 10.1016/0028-3908(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Price SL, Zhou FC. Immunohistochemical detection of A1 adenosine receptors in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum and basal ganglia. Brain Res. 1995;677:193–203. doi: 10.1016/0006-8993(95)00062-u. [DOI] [PubMed] [Google Scholar]
- Schoen SW, Graeber MB, Reddington M, Kreutzberg GW. Light and electron microscopical immunocytochemistry of 5′-nucleotidase in rat cerebellum. Histochemistry. 1987;87:107–113. doi: 10.1007/BF00533394. [DOI] [PubMed] [Google Scholar]
- Silva RG, Santos DS, Basso LA, Oses JP, Wofchuk S, Portela LV, Souza DO. Purine nucleoside phosphorylase activity in rat cerebrospinal fluid. Neurochem Res. 2004;10:1831–1835. doi: 10.1023/b:nere.0000042209.02324.98. [DOI] [PubMed] [Google Scholar]
- Stolk M, Cooper E, Vilk G, Litchfield DW, Hammond JR. Subtype-specific regulation of equilibrative nucleoside transporters by protein kinase CK2. Biochem J. 2005;386:281–289. doi: 10.1042/BJ20041571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Sweeney MI. Adenosine release and uptake in cerebellar granule neurons both occur via an equilibrative nucleoside carrier that is modulated by G proteins. J Neurochem. 1996;67:81–88. doi: 10.1046/j.1471-4159.1996.67010081.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kovalchuk Y, Atwell D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar fiber and parallel fiber to Purkinje cell synapses. J Neurosci. 1995;15:5693–5702. doi: 10.1523/JNEUROSCI.15-08-05693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Dale N. Auto-inhibition of parallel fibre-Purkinje cell synapses by activity dependent adenosine release. J Physiol. 2007 doi: 10.1113/jphysiol.2006.126417. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci. 1997;9:533–548. doi: 10.1111/j.1460-9568.1997.tb01630.x. [DOI] [PubMed] [Google Scholar]
- Wang TF, Guidotti G. Widespread expression of ecto-apyrase (CD39) in the central nervous system. Brain Res. 1998;790:318–321. doi: 10.1016/s0006-8993(97)01562-x. [DOI] [PubMed] [Google Scholar]
- Wink MR, Braganhol E, Tamajusuku ASK, Casali EA, Karl J, Barreto-Chaves ML, Sarkis JJF, Battastini AMO. Extracellular adenine nucleotides metabolism in astrocyte cultures from different brain regions. Neurochem Int. 2003;43:621–628. doi: 10.1016/s0197-0186(03)00094-9. [DOI] [PubMed] [Google Scholar]
- Yamada T, Kobayashi T, Okada Y. The effects of simulated ischemia on the levels of adenosine and its metabolites in slices of cerebellum, superior colliculus and hippocampus in the guinea pig –in vitro study. Brain Res. 1998;787:220–225. doi: 10.1016/s0006-8993(97)01482-0. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Comparative effects of methylmercury on parallel-fiber and climbing fiber responses of rat cerebellar slices. J Pharmacol Exp Ther. 1999;288:1015–1025. [PubMed] [Google Scholar]
- Zhang G, Franklin PH, Murray TF. Manipulation of endogenous adenosine in the rat prepiriform cortex modulates seizure susceptibility. J Pharmacol Exp Ther. 1993;264:1415–1424. [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]