Abstract
We have shown previously that stress in the pregnant rat leads to a heightened cardiovascular response to restraint in adult offspring. The present study was undertaken to explore further the persistent cardiovascular effects of prenatal stress, with a focus on peripheral vascular function. Sprague–Dawley female rats were exposed to restraint/bright light three times daily in the last week of pregnancy. Litters from stressed and control females were cross-fostered to control dams to eliminate possible effects of maternal stress on nursing behaviour. At 120 days, offspring cardiovascular variables were measured by radiotelemetry. Reactivity of mesenteric small arteries was assessed by myography, and responses to electrical field stimulation determined. Resting cardiovascular parameters in prenatally stressed (PS) offspring were similar to controls but PS rats showed a greater increase in systolic blood pressure following restraint stress (P < 0.05). Recovery was also prolonged in PS animals compared with controls and was of longer duration in PS females than in PS males (P < 0.05). Adult PS females, but not males, also had elevated basal plasma corticosterone levels in comparison with controls (P < 0.05). Vascular reactivity to neuropeptide Y (P < 0.05) and electrical field stimulation (P < 0.05) in mesenteric arteries was also significantly increased in PS animals. Vascular responses to adrenergic agonists as well as endothelial dilator function did not differ between PS and controls. We conclude that prenatal stress during late gestation has long-lasting effects on cardiovascular responsiveness and vascular reactivity to neuropeptide Y in the offspring.
Numerous studies in man (Barker, 1995; Stein et al. 1996) and experimental animals (Armitage et al. 2004; Seckl & Meaney, 2004; McMillen & Robinson, 2005) provide strong evidence to support the suggestion that the early life environment is a determinant of the adult phenotype. Two distinct branches of research in this field have evolved: one follows the Barker hypothesis and focuses on nutrition and developmental programming of cardiovascular (CV) and metabolic diseases (Barker, 1995), and the other investigates prenatal stress and its effect on offspring stress responses and behavioural development (Weinstock, 2001; Owen et al. 2005; Van den Bergh et al. 2005). Despite apparent divergence in animal models between the types of maternal insult imposed during pregnancy (e.g. stress protocols versus nutritional challenge), it now seems that there may be more commonality in offspring phenotype than originally recognized.
We have previously shown in rodents that prenatal stress has persistent effects on the CV system of the offspring (Igosheva et al. 2004). Exposure of pregnant rats to restraint and bright light during the last week of pregnancy leads to enhanced systolic blood pressure responses (SBP) and blood pressure variability to acute stress, and delayed post-stress recovery. Although the mechanisms of stress-induced hypertension in this model of prenatal stress have not been directly studied, recent reports, using other interventions, have implicated a possible role for altered sympathetic activity in the developmental programming of altered blood pressure responsiveness. The development of the sympathetic nervous system is susceptible to environmental manipulations during early life (Young, 2006) and therefore fetal and neonatal exposures to different stressors may alter sympathetic control of blood pressure later in life. In the rat, prenatal glucocorticoid (GC) exposure (O'Regan et al. 2003) and hypoxia in pregnancy (Peyronnet et al. 2002), leads to exaggerated blood pressure responsiveness to acute stress in the adult offspring, which is associated with increased vascular sensitivity to sympathomimetics (O'Regan et al. 2003) and disturbed metabolism in sympathetic ganglia (Peyronnet et al. 2002). Increased sympathetic activity has also been linked to offspring CV dysfunction associated with maternal malnutrition (Lesage et al. 2002; Fernandez-Twinn et al. 2006) and reduced placental blood flow (Sanders et al. 2004b; Alexander et al. 2005). These observations may have relevance to the model of prenatal stress, since decreased food intake (Matthews, 2002) and reduced uterine blood flow (Morishima et al. 1979) as well as increased plasma concentration of GCs (Seckl & Meaney, 2004) have been reported in stressed dams. The underlying mechanisms in these apparently divergent models of developmental programming could therefore have some common features.
We have previously reported that stress-induced hypertension in the offspring of stressed dams was not associated with permanent alterations in arterial baroreflex function. The central sympathetic outflow to the heart was also unaltered since resting heart rate, as well as the magnitude of heart rate stress responses, were unaffected in PS offspring (Igosheva et al. 2004). Therefore, it is more likely that, if involved, it would be the peripheral rather than central sympathetic pathways that contribute to altered CV function in PS animals.
The present study was undertaken to characterize further the effect of maternal physical and emotional stress on the cardiovascular function of the adult offspring, and to determine whether a permanent modification of vascular adrenergic function may contribute.
Methods
Animals and induction of prenatal stress
All animal care guidelines and animal procedures followed were licensed under the UK Home Office Animal (Scientific Procedures) Act 1986. Virgin female Sprague–Dawley rats 120 days old were purchased from Charles River Laboratories (UK). For breeding, the females were caged with mature males and on the day of conception were randomly allocated to control (n= 10) and stress (n= 10) groups. All dams were maintained under standard conditions with ad libitum access to water and rat chow. Maternal food intake and body weight was monitored daily.
Prenatal stress protocol
Restraint stress was performed from embryonic day 15 until day 21. The stress protocol involved placing the pregnant female in a plexiglas restraint tube (19 cm × 6 cm × 9 cm) over which was poised two 100 W flood lights. Three 30 min stress interventions were conducted on each day at 9.00, 13.00 and 16.00 h. Control dams were left undisturbed throughout gestation.
This protocol has been employed in several previous studies including our own and shown to significantly affect cardiovascular (Igosheva et al. 2004) and neuroendocrine stress reactivity in adult offspring (Szuran et al. 2000; Sternberg & Ridgway, 2003).
Two days post-partum, all offspring were cross-fostered to recently parturient control dams and litters were adjusted to eight pups with equal numbers of males and females. Groups studied were: (1) offspring born to stressed dams and suckled by non-stressed mothers (PS, n= 20, 10 offspring of each sex); (2) offspring born to non-stressed dams and suckled by foster non-stressed dams (C, n= 20, 10 offspring of each sex). All offspring were weaned at 21 days of age. Food intake and body weight were monitored weekly. Two animals of each sex from any one litter were studied at a given age, and these were randomly chosen to remove any litter effects.
Determination of cardiovascular function by radiotelemetry
At 120 days of age, animals were instrumented with a biocompatible radio-telemetry probe (Data Science International, Arden Hills, MN, USA) and blood pressure (systolic, diastolic and mean pressure), heart rate and activity were measured using the Dataquest IV system (Data Sciences International, Arden Hills, USA) as previously described (Khan et al. 2003). The rats were anaesthetized by isofluorane inhalation (4%) and administered preoperative buprenorphine (0.1 mg kg−1 subcutaneous, Alstoe Animal Health, York, UK). Following a routine laparotomy, the flexible catheter of the radiotelemetry probe was surgically implanted into the descending abdominal aorta. The depth of anaesthesia was monitored by testing for the pedal pinch reflex. Post-operatively, the animal was placed in a recovery chamber pre-heated to 30°C for 1 h. On recovery, rats were housed in individual cages and collection of data for analysis commenced 1 week after surgery. Variables were recorded over 10 s intervals every 5 min for 1 week. To assess acute responses to restraint stress, CV parameters were also recorded continuously for 30 min after placing the animal in the restraint cylinder and 120 min after returning the animal to its home cage.
Determination of isolated artery function
Small artery function was assessed in the same animals used for telemetric recording of cardiovascular function. Animals were fasted overnight (12 h). On the following morning, animals were killed by a rising concentration of CO2 between 08.00 and 09.00 h. Organs were quickly removed from all animals, weighed and snap frozen in liquid nitrogen and stored at −80°C. Blood samples for measurement of corticosterone were obtained by cardiac puncture, and plasma was stored at −70°C before analysis. Third order mesentery arteries were dissected, mounted in physiological salt solution (PSS (mm); NaCl 119, CaCl2 2.5, KCl 4.7, MgSO4 7 H2O 1.17, NaHCO3 25, KH2PO4 1.18, EDTA 0.025, d-(+)-glucose 6) on a small vessel myograph (Model 610M, Danish Myotechnology, Denmark; Mulvany & Halpern, 1977). Arteries were maintained at 37°C, in PSS gassed with 95% oxygen–5% CO2 mixture to give a pH of 7.4 and subjected to a standard normalization procedure and run-up protocol as previously described (Mulvany & Halpern, 1977). Vascular function was assessed by cumulative concentration–response curves to noradrenaline (NA, 10−8–10−6m), phenylephrine (PE, 10−9–10−5m), the β1-adrenoreceptor agonist, dobutamine (10−9–10−5m), acetylcholine (10−9–10−5m) and aqueous nitric oxide (10−7–10−4m). Responses to neuropeptide Y (NPY, 10−9–10−6m) and vasodilators were determined in arteries pre-activated with NA (80% of maximal constriction). Electrical field stimulation (EFS) was performed by passing a stimulating current across specially adapted myograph jaws fitted with platinum electrodes (Danish Myotechnology, Denmark). Arteries were subjected to 0.3 ms pulse, 20 s trains of 40 mA current stimulation using a bipolar current with 3 min between each train (electrical stimulator CS200, Danish Myotechnology, Denmark). The arteries were stimulated at frequencies of 4, 8, 16 and 32 Hz and a frequency–tension response relationship was recorded. In all experiments two arteries from each rat were investigated simultaneously and the results expressed as the average response.
Corticosterone assay
Plasma corticosterone (CS) was evaluated using a commercial double antibody radioimmunoassay kit (Immunodiagnostic Systems, Boldon, UK).
Statistical analysis
Results are expressed as the mean ±s.e.m. with P < 0.05 considered significant. Gestational length, litter size, birth weight, organ weight, body weight and the basal plasma corticosterone concentration were compared between groups by unpaired Student's t test. Radio-telemetric data were expressed as the mean value over 12 h night (active) and day (inactive) periods and analysed by repeated measures analysis of variance (ANOVA). Cardiovascular stress responses were expressed as a percentage change from baseline for both stress and recovery periods and evaluated using mixed design repeated measures ANOVA. Responses to constrictor agonists were expressed as active wall tension (mN mm−1). NPY and dilator responses were expressed as percentage of NE-induced constrictor tone. Concentration–response curves were analysed by fitting individual concentration–response data to a sigmoidal logistic curve using Graphpad Prism version 2.01 (Graphpad Software SanDiego, CA, USA). All statistical analyses were performed using Statistica version 5.0 for Windows (Statsoft Inc., Tulsa, OK, USA).
Results
Effect of prenatal stress on maternal and offspring parameters
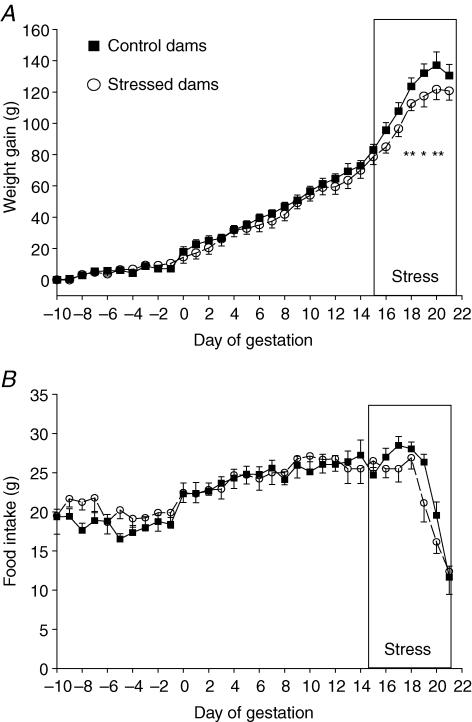
Body weight increased during pregnancy in both control and stressed dams (F= 181, P < 0.001, ANOVA). Restraint stress during the last week of pregnancy had a significant effect on the weight gain of dams (F= 5.1; P < 0.05, ANOVA) with stressed dams exhibiting a lower increase in weight from gestation day 16 (GD 16) to GD 21 (Fig. 1A). There was a trend for the stressed dams to have a lower food intake than controls but this did not reach statistical significance (F= 3.8, P= 0.07, by repeated measures ANOVA, Fig. 1B).
Figure 1. Body weight change and food intake.
Body weight change (A) and food intake (B) in control (▪, n= 10) and stressed mothers (^, n= 10) 10 days before and during pregnancy. *P < 0.05 control versus stressed dams.
Prenatal stress had no effect on gestational length (control 22 ± 0.3 days, stressed 21.6 ± 0.5 days), litter size (control 12.5 ± 0.9 pups, stressed 12.6 ± 0.5 pups) and the sex ratios of the litters. At birth the body weights of the offspring born to stressed mothers were not different from those of control mothers (control 7.4 ± 0.6 g versus stressed 6.7 ± 0.4 g, P= 0.34). Prenatal stress did not affect weight gain in the offspring during both pre- and post-weaning periods.
No significant effect of PS was observed on body and organ weights of the adult offspring at 120 days of age (data not shown).
The basal plasma corticosterone concentration did not differ between control females and control males. ANOVA analysis of the basal corticosterone concentrations revealed a significant prenatal stress–gender interaction (F= 6.5, P < 0.05) attributable to a higher concentration in the adult PS female offspring compared with control females (PS, 161 ± 21 ng ml−1, n= 10 versus C, 107 ± 14 ng ml−1, n= 10, P < 0.05). The basal plasma CS concentration did not differ between PS and control males (PS, 70 ± 5 ng ml−1, n= 10 versus C, 82 ± 11 ng ml−1, n= 10). Thus, the effect of prenatal stress was gender-specific (F= 19.3, P < 0.001) with PS females having a significantly higher CS plasma concentration than PS males (PS, females 161 ± 21 ng ml−1, n= 10 versus PS, males 70 ± 5 ng ml−1, n= 10).
Effects of prenatal stress on cardiovascular function
Twenty four hour radiotelemetry monitoring of offspring blood pressure, heart rate and activity
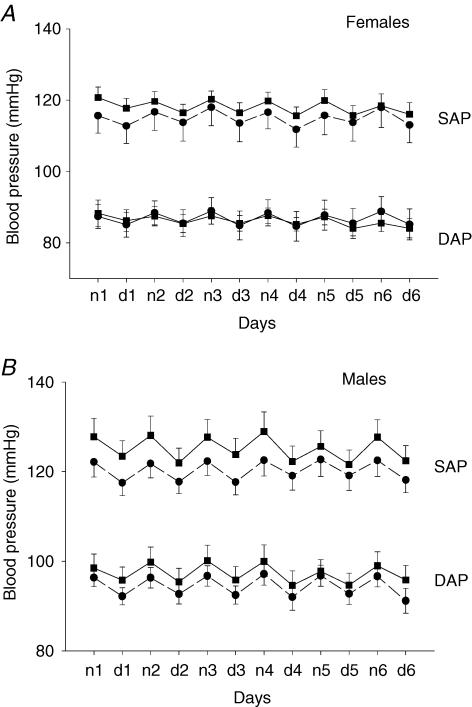
At 120 days of age, basal systolic, diastolic arterial pressure (Fig. 2A and B) and heart rate did not differ between the groups. The diurnal variation of cardiovascular parameters and motor activity were also found to be similar in PS offspring and controls.
Figure 2. Systolic and diastolic blood pressures.
Systolic and diastolic blood pressures in female (A) and male (B) offspring of control (▪, n= 10) and stressed dams (•, n= 10) at 120 days of age. Values are expressed as the mean ±s.e.m. of 12 h night (n) and day (d) 12 h averages over 6 days (1–6).
Cardiovascular responses to restraint stress and recovery in control and PS rats
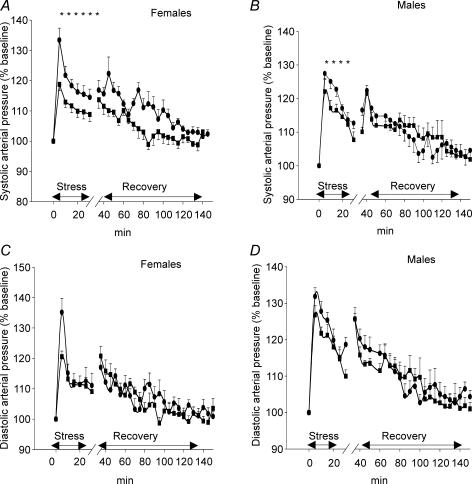
Figure 3A and B shows changes from baseline in systolic blood pressure (SBP) in PS and control rats during restraint stress and the recovery period. ANOVA analysis revealed a marked difference in SBP stress responses between the groups (prenatal treatment F= 5, P < 0.05; prenatal treatment–time interaction F= 3.5, P < 0.05). The magnitude of SBP stress-induced responses was significantly higher in PS rats than in controls. There was no significant effect of gender on SBP stress responsiveness. However, PS females showed a protracted duration of the SBP stress response compared with PS males (30 min versus 20 min, respectively). SBP responses during the post-stress period were also modified by prenatal stress (F= 4.4, P < 0.05, ANOVA). Recovery to baseline SBP after return to the cage was delayed in PS rats in comparison with controls: increased SBP values were sustained for 95 min in PS rats compared with only 45 min in controls. There was a significant interaction of sex and time (F= 3.3, P < 0.05, ANOVA) in PS group indicating that PS females had higher SBP responses than PS males during recovery (P < 0.01, Duncan's post hoc test).
Figure 3. Changes in systolic and diastolic blood pressure.
Changes in systolic (A and B) and diastolic blood (C and D) pressure during 30 min of restraint and for 120 min following return to the home cage in the adult offspring of control (▪, n= 10) and stressed dams (•, n= 10). Data are expressed as the mean ±s.e.m. PS rats showed a greater increase in systolic arterial pressure (SAP) following restraint stress and extended SAP responses during recovery compared with controls (95 min versus 45 min). Diastolic arterial pressure (DAP) responses during stress and recovery did not differ between the groups. *P < 0.05 PS versus controls (repeated measures ANOVA).
As shown in Fig. 3C and D restraint stress induced a significant but transient increase in diastolic blood pressure (DBP) in all animals (F= 27, P < 0.001, ANOVA). ANOVA revealed a significant effect of gender (F= 5, P < 0.05) with male rats having higher DBP stress responses than females regardless of prenatal conditions. There was no effect of prenatal stress on DBP responses during either the restraint stress or recovery period.
No effect of prenatal treatment or gender was found for HR responses during either the stress or the post-stress periods.
Effect of prenatal stress on peripheral vascular function
Constrictor responses of mesenteric arteries to adrenergic agonists and neuropeptide Y (NPY)
Baseline diameters (μm) of the arteries were as follows: control females 203 ± 10, PS females 206 ± 7, control males 215 ± 6 and PS males 218 ± 7.
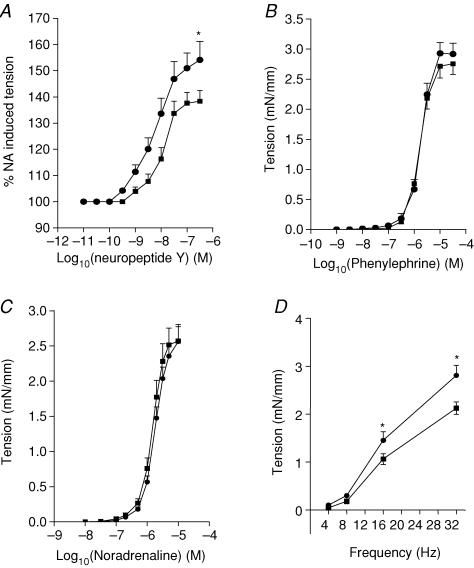
There were no gender differences in NPY-induced vasoconstriction and so data from females and males were pooled for further analysis. Whilst the sensitivity to NPY was not significantly different among the groups (EC50: C: −7.7 ± 0.2, PS −7.9 ± 0.2), PS offspring showed enhanced reactivity to NPY across the entire concentration range compared with controls (F= 4.5, P < 0.05, ANOVA, Fig. 4A)
Figure 4. Contractile responses.
Contractile responses to neuropeptide Y (A), phenylephrine (B), noradrenaline (C) and electrical field stimulation (D) in small mesenteric arteries from control (▪, n= 10) and prenatally stressed (•, n= 10) rats. Responses to neuropeptide Y were determined on the arteries pre-activated with NA (80% of maximal constriction) and expressed as percentage of NA-induced tension. Significance was assessed by ANOVA: *P < 0.05 controls versus PS animals. The level of NA-induced pre-constrictor tension (mN mm−1) were not different between experimental groups: control females 2.1 ± 0.2 (s.e.m.), PS females 2.06 ± 0.26, control males 2.14 ± 0.35, PS males 2.18 ± 0.13.
There were no differences in dose–response curves to either noradrenaline or phenylephrine between the groups (Fig. 4B and C).
Electrical field stimulation (EFS) induced a frequency-dependent vasoconstriction in all rats (F= 23, P < 0.001; ANOVA; Fig. 4D). Gender did not have a significant effect on EFS-induced vasoconstriction and so data from females and males were pooled for further analysis. ANOVA revealed a significant effect of prenatal stress (F= 5.7, P < 0.05, ANOVA) and a significant prenatal conditions–frequency interaction (F= 5, P < 0.01, ANOVA). Post hoc comparisons confirmed that contractions induced by 16 Hz and 32 Hz EFS were significantly enhanced in arteries from PS offspring compared with controls.
Endothelium-dependent relaxation
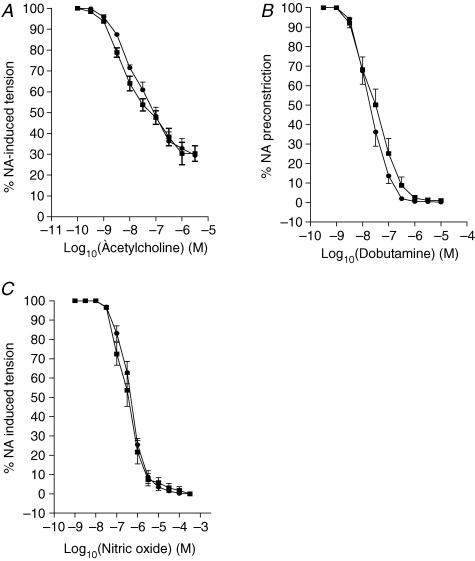
Intrauterine stress did not alter vasodilator responses to either acetylcholine or dobutamine in mesenteric arteries (Fig. 5A and B).
Figure 5. Relaxing responses.
Relaxing responses to acetylcholine (A), dobutamine (B) and nitric oxide (C) in small mesenteric arteries from control (▪, n= 10) and prenatally stressed (•, n= 10) offspring rats.
Endothelium-independent relaxation
Vasodilator responses to nitric oxide were similar in PS and control rats. (Fig. 5C).
Discussion
This study has confirmed our previous findings of exaggerated SBP responses to stress in the offspring of rat dams subjected to stress in pregnancy (Igosheva et al. 2004) and provides new insight into underlying mechanisms. We report an association between prenatal stress and an enhanced constrictor response to NPY and also to perivascular nerve stimulation of mesenteric arteries. PS females demonstrated greater prolongation of SBP both during stress and recovery and higher basal plasma corticosterone concentration than PS males. Since all offspring were fostered to non-stressed dams, these abnormalities could not have been due to altered maternal nursing behaviour (Champagne & Meaney, 2006) but must have arisen as a direct physiological consequence of maternal stress in late pregnancy.
In our previous study (Igosheva et al. 2004) arterial blood pressure in the adult PS rats was measured directly using the indwelling fluid-filled catheter method which allows continuous recording of haemodynamic parameters in the conscious animal. However, this method requires that the rat is semi-restrained and potentially stressed. Moreover, catheter patency limitations prevent long-term measurements (Butz & Davisson, 2001). Radiotelemetry has the benefit of long-term assessment of cardiovascular parameters in unrestrained animals in a stress-free environment, and thus has considerable advantage over methods hitherto used in studies of fetal programming (Bertram & Hanson, 2001). The use of telemetry in the present study enabled us to undertake more accurate and reliable testing of cardiovascular function and confirm our previous findings of altered cardiovascular function in PS animals. As in our previous study, prenatal stress did not affect resting haemodynamic parameters in adulthood. Other recent studies of the developmental programming of CV function have also shown no change in basal blood pressure in the adult offspring of rat dams subjected to undernutrition (Holemans et al. 1999), hypoxia (Peyronnet et al. 2002) or uterine artery ligation (Jansson & Lambert, 1999). However, elevated basal blood pressure has been reported in adult offspring of rats prenatally exposed to high dose of synthetic GC (Levitt et al. 1996) and this may suggest a different mechanistic pathway (O'Regan et al. 2004). In common with the present study, it has been shown in a number of animal models that offspring exposed prenatally to an adverse environment only demonstrate altered CV function when challenged (Li et al. 2003; Molnar et al. 2003; Louey & Thornburg, 2005). The results of this study confirm and extend our previous findings (Igosheva et al. 2004) on this ‘silent programming’ of cardiovascular function in the prenatal stress protocol. Thus, PS animals showed no changes in basal CV parameters compared with controls, but significant elevation in SBP only when challenged by acute restraint stress. This is similar to the enhanced blood pressure responsiveness to stress in the offspring of rat dams subjected to protein undernutrition (Tonkiss et al. 1998) or hypoxia (Peyronnet et al. 2002). Our data also show a sex-specific effect of prenatal stress on blood pressure responsiveness to stress. Consistent with our previous findings, PS females had more prolonged SBP responses to restraint stress than PS males and showed delayed recovery. Greater female susceptibility to programming of CV function has also been observed in offspring of rat dams fed a high fat diet (Khan et al. 2003; Khan, 2004) and those exposed to synthetic GCs prenatally (O'Regan et al. 2004). The present experiments also demonstrate the sex-specific effects of prenatal stress on basal plasma corticosterone concentration. Adult PS females, but not males, have higher basal plasma corticosterone levels in comparison with controls. Elevated basal plasma corticosterone concentration in female rats born to PS dams has been reported in a number of studies (Weinstock et al. 1992; McCormick et al. 1995; Ward et al. 2000) consistant with adrenal hypertrophy (Ward et al. 2000). Others have shown that prenatal stress differentially affects adult hippocampal corticosteroid receptor density, with permanently decreased numbers of hippocampal glucocorticoid receptors and mineralocorticoid receptors in prenatally stressed females (Koehl et al. 1999; Szuran et al. 2000), whereas no differences in hippocampal glucocorticoid binding was apparent in male offspring (Szuran et al. 2000); this provides a potential explanation in the present study. It is also possible that prenatal stress could affect the level of corticostereone binding globulin in females but not in males (McCormick et al. 1995).
Although vascular function has been investigated in nutritional (Taylor et al. 2004; Khan et al. 2005) and hypoxic (Ruijtenbeek et al. 2002; Sanders et al. 2004a) models of developmental programming of CV function, this is, to our knowledge, the first report of the effects of maternal stress on the peripheral vascular responses of the offspring. Here we show that PS resulted in permanent alterations in agonist-induced reactivity of offspring mesenteric arteries confined to enhanced contractile responses to NPY.
We have also shown that PS increased neurogenic contractions induced by EFS in a frequency-dependent manner. The high-frequency stimulations (16 and 32 Hz), mimicking neurotransmission at high level of sympathetic nerve activity (Han et al. 1998b), evoked higher contractions in arteries from PS offspring compared with controls whereas low-frequency stimulations had similar effects on mesenteric arteries from control and PS animals.
EFS-induced vascular contractions arise from activation of perivascular sympathetic nerves, the major associated neurotransmitters being NA and NPY (Donoso et al. 1997). As vasoconstrictor responses to exogenous NA and phenylephrine did not differ between the groups this suggests that adrenergic sensitivity of mesenteric arteries is unaltered in PS offspring and is unlikely to contribute to the enhanced constrictor response to high-frequency EFS. Furthermore, NPY is known to be preferentially released by high-frequency stimulation and/or during stimulation that leads to a higher degree of sympathetic nerve activity (Chronwall & Zukowska, 2004). Therefore, it is plausible that the enhanced vasoconstriction upon intense EFS in the PS rats is predominantly mediated by activation of NPY neurotransmission. Whilst this is a likely explanation other mechanisms cannot be ruled out including altered neuronal reuptake of NA, or density of perivascular sympathetic nerves or of presynaptic α2-adrenoreceptor. Vascular smooth muscle is also innervated by calcitonin gene-related peptide (CGRP)-containing vasodilator sensory–motor nerve fibres and it is possible that reduced CGRP-induced vasodilatation could play a role.
However, the enhanced sensitivity of mesenteric arteries from PS animals to NPY is supportive of a central role for the NPY constrictor pathway in the ‘programmed’ vascular anomalies observed. Although not determined here, alterations in peripheral NPY Y1 receptor density could contribute as these receptors are implicated in stress-induced mesenteric vasoconstriction in rats as assessed in vivo by Doppler blood flow probes (Zukowska-Grojec et al. 1996), in elevation of the pressor response to acute stress (Han et al. 1998a; Carrasco & Van de Kar, 2003) and in the pathogenesis of hypertension (Westfall, 2006). In studies of developmental programming, alterations in the NPY-dependent pathway have been implicated in sheep models of vascular dysfunction induced by prenatal acute hypoxaemia (Fletcher et al. 2003), maternal undernutrition (Warnes et al. 1998) and synthetic GC exposure (Fletcher et al. 2000). Neuropeptide Y binding to NPY Y1 receptors activates different signalling pathways that regulate constriction of vascular smooth muscle. One of these pathways is linked to inhibition of adenylyl cyclase (Prieto et al. 2000). Since in the present study constrictor responses to NPY were determined on the arteries pre-activated with NA, differences in arterial reactivity to NPY between PS and control animals may probably be ascribed to differences in β-adrenoreceptor-mediated adenylyl cyclase activation. As vascular responses to the β-adrenergic agonist dobutamine did not differ between control and PS animals it is unlikely that increased β-adrenoreceptor-mediated adenylyl cyclase activation could contribute to the enhanced constrictor response to NPY in PS animals. Up-regulation of peripheral NPY Y1 receptors is therefore a plausible explanation for the enhanced peripheral vasoconstrictor responses to exogenous NPY and EFS-induced stimulation in PS animals. Alternatively changes in activity or expression of dipeptidil-peptidase IV could occur, resulting in alteration in NPY breakdown and NPY receptor binding. This requires interrogation in future studies in which determination of receptor density coupled with the use of dipeptidil-peptidase IV and NPY Y1 receptor antagonists would enable the mechanisms to be unravelled.
In summary, this study provides new evidence for the hypothesis that maternal stress in pregnancy can program enhanced cardiovascular responsiveness to stress. The increased vascular contractile responses to NPY occurred in conjunction with enhanced blood pressure responsiveness to stress in PS offspring. We propose that maternal stress in pregnancy may affect the NPY pathway and that the persistently elevated vascular sensitivity to NPY may account for the stress-induced systemic hypertension in the rat model of prenatal stress. Future studies will test this relationship in a different cohort of animals. Subsequent investigations should also include detailed evaluation of mechanisms of NPY-induced vasoconstriction using diverse pharmacological tools. Further understanding of how prenatal stress can influence central and peripheral mechanisms implicated in the regulation of cardiovascular responses to stress may provide insight into the risk factors that determine susceptibility to cardiovascular diseases in later life.
Acknowledgments
This work was supported by grants from the Institute of Obstetrics and Gynaecology Trust and from the Civilian Research and Development Foundation and the Ministry of Education of the Russian Federation.
References
- Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45:754–758. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull. 2001;60:103–121. doi: 10.1093/bmb/60.1.103. [DOI] [PubMed] [Google Scholar]
- Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics. 2001;5:89–97. doi: 10.1152/physiolgenomics.2001.5.2.89. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Chronwall BM, Zukowska Z. Neuropeptide Y, ubiquitous and elusive. Peptides. 2004;25:359–363. doi: 10.1016/j.peptides.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Donoso MV, Brown N, Carrasco C, Cortes V, Fournier A, Huidobro-Toro JP. Stimulation of the sympathetic perimesenteric arterial nerves releases neuropeptide Y potentiating the vasomotor activity of noradrenaline: involvement of neuropeptide Y-Y1 receptors. J Neurochem. 1997;69:1048–1059. doi: 10.1046/j.1471-4159.1997.69031048.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ekizoglou S, Wayman A, Petry CJ, Ozanne SE. Maternal low-protein diet programs cardiac β-adrenergic response and signaling in 3-mo-old male offspring. Am J Physiol Regul Integr Comp Physiol. 2006;291:R429–R436. doi: 10.1152/ajpregu.00608.2005. [DOI] [PubMed] [Google Scholar]
- Fletcher AJ, Gardner DS, Edwards CM, Fowden AL, Giussani DA. Cardiovascular and endocrine responses to acute hypoxaemia during and following dexamethasone infusion in the ovine fetus. J Physiol. 2003;549:271–287. doi: 10.1113/jphysiol.2002.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AJ, Goodfellow MR, Forhead AJ, Gardner DS, McGarrigle HH, Fowden AL, Giussani DA. Low doses of dexamethasone suppress pituitary-adrenal function but augment the glycemic response to acute hypoxemia in fetal sheep during late gestation. Pediatr Res. 2000;47:684–691. doi: 10.1203/00006450-200005000-00021. [DOI] [PubMed] [Google Scholar]
- Han S, Chen X, Cox B, Yang CL, Wu YM, Naes L, Westfall T. Role of neuropeptide Y in cold stress-induced hypertension. Peptides. 1998a;19:351–358. doi: 10.1016/s0196-9781(97)00297-0. [DOI] [PubMed] [Google Scholar]
- Han S, Yang CL, Chen X, Naes L, Cox BF, Westfall T. Direct evidence for the role of neuropeptide Y in sympathetic nerve stimulation-induced vasoconstriction. Am J Physiol Heart Circ Physiol. 1998b;274:H290–H294. doi: 10.1152/ajpheart.1998.274.1.H290. [DOI] [PubMed] [Google Scholar]
- Holemans K, Gerber R, Meurrens K, De Clerck F, Poston L, Van Assche FA. Maternal food restriction in the second half of pregnancy affects vascular function but not blood pressure of rat female offspring. Br J Nutr. 1999;81:73–79. [PubMed] [Google Scholar]
- Igosheva N, Klimova O, Anishchenko T, Glover V. Prenatal stress alters cardiovascular responses in adult rats. J Physiol. 2004;557:273–285. doi: 10.1113/jphysiol.2003.056911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T, Lambert GW. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3–4 months of age. J Hypertens. 1999;17:1239–1248. doi: 10.1097/00004872-199917090-00002. [DOI] [PubMed] [Google Scholar]
- Khan IY. Maternal and Fetal Research Unit, Department of Women's Health. London: Guy's, King's and St Thomas' School of Medicine, King's College; 2004. In utero programming of vascular dysfunction and hypertension by raised dietary fat; p. 321. [Google Scholar]
- Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R127–R133. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Lesage J, Dufourny L, Laborie C, Bernet F, Blondeau B, Avril I, Breant B, Dupouy JP. Perinatal malnutrition programs sympathoadrenal and hypothalamic-pituitary-adrenal axis responsiveness to restraint stress in adult male rats. J Neuroendocrinol. 2002;14:135–143. doi: 10.1046/j.0007-1331.2001.00753.x. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Invest. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Louey S, Thornburg KL. The prenatal environment and later cardiovascular disease. Early Hum Dev. 2005;81:745–751. doi: 10.1016/j.earlhumdev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- Molnar J, Howe DC, Nijland MJ, Nathanielsz PW. Prenatal dexamethasone leads to both endothelial dysfunction and vasodilatory compensation in sheep. J Physiol. 2003;547:61–66. doi: 10.1113/jphysiol.2002.032565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima HO, Yeh MN, James LS. Reduced uterine blood flow and fetal hypoxemia with acute maternal stress: experimental observation in the pregnant baboon. Am J Obstet Gynecol. 1979;134:270–275. doi: 10.1016/s0002-9378(16)33032-0. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- O'Regan D, Kenyon CJ, Brooker G, Mullins JJ, Seckl JR, Holmes MC. Sympathetic responsivity; the origin of programmable hypertension. J Human Hypertension. 2003;17:S1. [Google Scholar]
- O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programmes gender specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Peyronnet J, Dalmaz Y, Ehrstrom M, Mamet J, Roux JC, Pequignot JM, Thoren HP, Lagercrantz H. Long-lasting adverse effects of prenatal hypoxia on developing autonomic nervous system and cardiovascular parameters in rats. Pflugers Arch. 2002;443:858–865. doi: 10.1007/s00424-001-0766-9. [DOI] [PubMed] [Google Scholar]
- Prieto D, Buus CL, Mulvany MJ, Nilsson H. Neuropeptide Y regulates intracellular calcium through different signalling pathways linked to a Y1-receptor in rat mesenteric small arteries. Br J Pharmacol. 2000;129:1689–1699. doi: 10.1038/sj.bjp.0703256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijtenbeek K, Kessels CG, Villamor E, Blanco CE, De Mey JG. Direct effects of acute hypoxia on the reactivity of peripheral arteries of the chicken embryo. Am J Physiol Regul Integr Comp Physiol. 2002;283:R331–R338. doi: 10.1152/ajpregu.00675.2001. [DOI] [PubMed] [Google Scholar]
- Sanders M, Fazzi G, Janssen G, Blanco C, De Mey J. Prenatal stress changes rat arterial adrenergic reactivity in a regionally selective manner. Eur J Pharmacol. 2004a;488:147–155. doi: 10.1016/j.ejphar.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Sanders MW, Fazzi GE, Janssen GM, de Leeuw PW, Blanco CE, De Mey JG. Reduced uteroplacental blood flow alters renal arterial reactivity and glomerular properties in the rat offspring. Hypertension. 2004b;43:1283–1289. doi: 10.1161/01.HYP.0000127787.85259.1f. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Stein CE, Fall CH, Kumaran K, Osmond C, Cox V, Barker DJ. Fetal growth and coronary heart disease in south India. Lancet. 1996;348:1269–1273. doi: 10.1016/s0140-6736(96)04547-3. [DOI] [PubMed] [Google Scholar]
- Sternberg WF, Ridgway CG. Effects of gestational stress and neonatal handling on pain, analgesia, and stress behavior of adult mice. Physiol Behav. 2003;78:375–383. doi: 10.1016/s0031-9384(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Taylor PD, Khan IY, Hanson MA, Poston L. Impaired EDHF-mediated vasodilatation in adult offspring of rats exposed to a fat-rich diet in pregnancy. J Physiol. 2004;558:943–951. doi: 10.1113/jphysiol.2002.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkiss J, Trzcinska M, Galler JR, Ruiz-Opazo N, Herrera VL. Prenatal malnutrition-induced changes in blood pressure: dissociation of stress and nonstress responses using radiotelemetry. Hypertension. 1998;32:108–114. doi: 10.1161/01.hyp.32.1.108. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29:237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Ward HE, Johnson EA, Salm AK, Birkle DL. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol Behav. 2000;70:359–366. doi: 10.1016/s0031-9384(00)00270-5. [DOI] [PubMed] [Google Scholar]
- Warnes KE, Morris MJ, Symonds ME, Phillips ID, Clarke IJ, Owens JA, McMillen IC. Effects of increasing gestation, cortisol and maternal undernutrition on hypothalamic neuropeptide Y expression in the sheep fetus. J Neuroendocrinol. 1998;10:51–57. doi: 10.1046/j.1365-2826.1998.00172.x. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Westfall TC. Neuropeptide Y and sympathetic control of vascular tone in hypertension. EXS. 2006;95:89–103. doi: 10.1007/3-7643-7417-9_6. [DOI] [PubMed] [Google Scholar]
- Young JB. Developmental origins of obesity: a sympathoadrenal perspective. Int J Obes (Lond) 2006;30(Suppl. 4):S41–S49. doi: 10.1038/sj.ijo.0803518. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Dayao EK, Karwatowska-Prokopczuk E, Hauser GJ, Doods HN. Stress-induced mesenteric vasoconstriction in rats is mediated by neuropeptide Y Y1 receptors. Am J Physiol Heart Circ Physiol. 1996;270:H796–H800. doi: 10.1152/ajpheart.1996.270.2.H796. [DOI] [PubMed] [Google Scholar]