Abstract
The positive force–frequency relation, one of the key factors modulating performance of healthy myocardium, has been attributed to an increased Ca2+ influx per unit of time. In failing hearts, a blunted, flat or negative force–frequency relation has been found. In healthy and failing hearts frequency-dependent alterations in Ca2+ sensitivity of the myofilaments, related to different phosphorylation levels of contractile proteins, could contribute to this process. Therefore, the frequency dependency of force, intracellular free Ca2+ ([Ca2+]i), Ca2+ sensitivity and contractile protein phosphorylation were determined in control and monocrotaline-treated, failing rat hearts. An increase in frequency from 0.5 to 6 Hz resulted in an increase in force in control (14.3 ± 3.0 mN mm−2) and a decrease in force in failing trabeculae (9.4 ± 3.2 mN mm−2), whereas in both groups the amplitude of [Ca2+]i transient increased. In permeabilized cardiomyocytes, isolated from control hearts paced at 0 and 9 Hz, Ca2+ sensitivity remained constant with frequency (pCa50: 5.55 ± 0.02 and 5.58 ± 0.01, respectively, P > 0.05), whereas in cardiomyocytes from failing hearts Ca2+ sensitivity decreased with frequency (pCa50: 5.62 ± 0.01 and 5.57 ± 0.01, respectively, P < 0.05). After incubation of the cardiomyocytes with protein kinase A (PKA) this frequency dependency of Ca2+ sensitivity was abolished. Troponin I (TnI) and myosin light chain 2 (MLC2) phosphorylation remained constant in control hearts but both increased with frequency in failing hearts. In conclusion, in heart failure frequency-dependent myofilament Ca2+ desensitization, through increased TnI phosphorylation, contributes to the negative force–frequency relation and is counteracted by a frequency-dependent MLC2 phosphorylation. We propose a novel role for PKC-mediated TnI phosphorylation in modulating the force–frequency relation.
Heart rate is a key factor modulating cardiac performance. Under physiological conditions an increase in stimulation frequency results in enhanced systolic function (Lewartowski & Pytkowski, 1987; Gao et al. 1998; Layland & Kentish, 1999; Stuyvers et al. 2002). This positive force–frequency relation has been attributed to an increased Ca2+ influx in the cardiomyocytes per unit of time via the L-type Ca2+ channels, which increases SR Ca2+ content and promotes Ca2+-induced Ca2+ release (Gao et al. 1998; Layland & Kentish, 1999; Pieske et al. 1999; Stuyvers et al. 2002). However, because of the highly non-linear force–Ca2+ relation, a possible change in myofilament Ca2+ sensitivity, through phosphorylation of contractile proteins, may be present as a contributing factor. For instance, frequency-dependent alterations in intracellular [Ca2+]i could change the Ca2+ sensitivity of the myofilaments by activation of Ca2+-dependent kinases or phosphatases: Ca2+–calmodulin kinase II (CaMK-II) (DeSantiago et al. 2002), the classical Ca2+-dependent protein kinases C (PKCs) (Bowling et al. 1999; Braz et al. 2004), myosin light chain kinase (MLCK) (Tong et al. 2004) and calcineurin (PP2B) (Lim & Molkentin, 1999). Detailed phase-plane analysis of myocardial force and [Ca2+]i in trabeculae of rat (Janssen et al. 2002), rabbit (Varian & Janssen, 2007) and mouse (Gao et al. 1998; Tong et al. 2004) and a study in mouse cardiomyocytes (Antoons et al. 2002) indeed suggested the presence of a frequency-dependent sensitization of the myofilaments.
In failing hearts a blunted, flat or negative force–frequency relation has been observed (Gwathmey et al. 1990; Mulieri et al. 1992; Schwinger et al. 1993; Eising et al. 1994; Pieske et al. 1995; Pieske et al. 1999; Janssen et al. 2000; Brixius et al. 2002; Kogler et al. 2003) and little is known about the impact of frequency-dependent phosphorylation on Ca2+ sensitivity. In several animal models and in human, heart failure is associated with a decrease in the amplitude of the Ca2+ transient (Gwathmey et al. 1990; Pieske et al. 1995) but an increase in Ca2+ sensitivity of the myofilaments (Wolff et al. 1996; Morano et al. 1997; van der Velden et al. 1999, 2001; Kogler et al. 2003). These changes in Ca2+ sensitivity could arise from altered isoform expression but could also be related to different phosphorylation levels of the thin and thick myofilament proteins such as troponin I (TnI) (Bodor et al. 1997; van der Velden et al. 2003b; Tong et al. 2004), troponin T (TnT) (Kameyama et al. 1998), myosin binding protein C (MyBP-C) (Tong et al. 2004) and MLC2 (van der Velden et al. 1999, 2001, 2003a,b). These alterations may alter the force–frequency relation.
We hypothesized that in control and failing hearts the force–frequency relation is determined not only by changes in the intracellular Ca2+ transient but also by frequency-dependent changes in Ca2+ sensitivity of the myofilaments through different phosphorylation levels of contractile proteins. To discriminate between the contribution of Ca2+ handling and myofilament Ca2+ sensitivity, the frequency dependency of force, pressure, intracellular [Ca2+]i, Ca2+ sensitivity and phosphorylation levels of contractile proteins were determined in isolated cardiomyocytes, in trabeculae and in hearts from control and monocrotaline (MCT)-treated failing rats. A single injection of MCT, which induces pulmonary hypertension, causes right ventricular (RV) heart failure (Leineweber et al. 2000; Seyfarth et al. 2000; Korstjens et al. 2002; Kogler et al. 2003; Buermans et al. 2005). This model was chosen because it allows measurements in cardiac trabeculae with dimensions suitable for accurate force and intracellular Ca2+ measurements (Janssen et al. 2002). Our results indicated that not only changes in Ca2+ handling but also changes in Ca2+ sensitivity of the myofilaments, through altered phosphorylation of contractile proteins, contribute to the negative force–frequency relation in failing hearts.
Methods
Design of the study
Male Wistar rats (n= 64) were randomly assigned to two experimental groups. At a body weight of 175 g, animals received a single subcutaneous injection of saline (control) or 80 mg kg−1 monocrotaline (failing). During its first passage through the pulmonary circulation MCT damages the pulmonary endothelium, thereby inducing pulmonary hypertension, causing right ventricular (RV) hypertrophy resulting eventually in RV heart failure (Leineweber et al. 2000; Seyfarth et al. 2000; Korstjens et al. 2002; Kogler et al. 2003; Buermans et al. 2005). The experiments on isolated cardiac trabeculae, isolated cardiomyocytes or isolated hearts were all performed 4 weeks after injection. All protocols were in accordance with the guidelines of the Animal Experimental Welfare Committee of the VU University Medical Center (VUMC).
Force and intracellular Ca2+ measurements
The effects of changes in stimulation frequency on force development and intracellular [Ca2+]i transients were determined in isolated cardiac trabeculae by means of fura 2-AM, as previously described (Lamberts et al. 2002).
Under intraperitonial pentobarbital anaesthesia, the hearts of 22 male Wistar rats (control n= 11, failing n= 11) were quickly removed and perfused via the aorta with a modified Krebs–Henseleit solution (see below) to which 20 mmol l−1 2,3-butanedione monoxime was added. A suitable trabecula from the right ventricle was dissected, transferred to the experimental bath and attached between a force transducer and a micromanipulator. The muscles were constantly superfused with a modified Krebs–Henseleit solution, kept at 27°C and continuously stimulated at 0.5 Hz. The modified Krebs–Henseleit solution consisted of (mmol l−1): 118 NaCl, 4.5 KCl, 1 CaCl2, 0.33 NaH2PO4, 1 MgCl2, 25 NaHCO3 and 10 glucose. The solution was gassed with 95%O2–5%CO2 (pH 7.45). After mounting, the preparations were stimulated for 60 min to allow equilibration. To impose similar stretch levels the muscles in both groups were stretched to the length (Lmax) at which isometric developed force was maximal. The muscles were loaded for 30 min with the cell-permeant acetoxymethyl ester form of the fluorescent intracellular Ca2+ indicator fura 2-AM (Molecular Probes, Eugene, OR, USA; F1221, final concentration 10 μmol l−1) to measure [Ca2+]i transients. During fura 2-AM loading electrical stimulation was turned off and temperature was increased to 37°C. After 30 min of dye washout, during which electrical stimulation was resumed and temperature returned to 27°C, the experimental protocol was started.
At 27°C, a force–frequency relation (0.5, 1, 2 and 3 Hz) at 1 mmol l−1 external Ca2+, a force–Ca2+ dose–response curve (0.25, 0.5, 1, 3 mmol l−1 Ca2+) at 0.5 Hz and maximal force (Fmax, at 1 mmol l−1 external Ca2+) attained during post-extrasystolic potentiation (ter Keurs et al. 1987) were determined. Hereafter, the temperature was increased to 37°C and a force–frequency relation (0.5, 1, 2, 3, 4 and 6 Hz) at 1 mmol l−1 Ca2+ and Fmax were determined. During all interventions, force and [Ca2+]i transients were recorded. Force was normalized to cross sectional area calculated from the diameter measured in two perpendicular directions in the middle part of the preparation at Lmax, assuming an ellipsoidal cross-section. The time from stimulus to half-relaxation (tHR) was used as relaxation parameter.
The signal of fura 2 fluorescence at 520 nm following excitation at wavelengths 340 nm and 380 nm was collected with a photomultiplier. In all experiments, the fura 2 signal was at least 5 times above background level (autofluorescence), which was subtracted. In some preparations after switching from 27°C to 37°C, fura 2 leakage precluded accurate measurements of the [Ca2+]i transients at 37°C, so these data were discarded.
Right ventricular pressure measurements
In isolated hearts the RV pressure–frequency relation was determined, as previously described (Lamberts et al. 2007). The hearts of 38 animals (control n= 19, failing n= 19) were rapidly dissected, placed in ice-cold modified Krebs–Henseleit solution and the aorta was cannulated for retrograde Langendorff perfusion at constant coronary perfusion pressure of 100 mmHg at 37°C. The modified Krebs–Henseleit solution had the following composition (mmol l−1): 118.5 NaCl, 4.7 KCl, 1.4 CaCl2, 25 NaHCO3, 1.2 MgCl2, 1.2 KH2PO4 and 11 glucose. The solution was continuously gassed with 95% O2–5% CO2 (pH 7.4).
A drainage cannula was inserted into the apex. The hearts were paced at 5 Hz using two electrodes, one attached to the drain and the other to the right atrium. A custom-made plastic balloon, filled with degassed distilled water, was inserted in the RV. RV pressure was measured using a catheter tip manometer. A pressure–volume relation was recorded to determine the volume (Vmax) at which maximal isovolumic pressure developed. The RV balloon volume was then adjusted to 80% of Vmax.
After mounting, the hearts were left to stabilize for 20 min and a pressure–frequency relation (3, 6, 9 Hz) was determined. During all interventions diastolic and systolic pressures and the maximal rate of force development (+dP/dt) were recorded. The time from stimulus to half-relaxation (tHR) and the ratio between the minimal rate of pressure development (−dP/dt) and developed pressure (Pdev) were taken as relaxation parameters.
Thereafter, control and failing hearts were divided in four subgroups. Three groups (all n= 5) were paced for 60 min at 3, 6 or 9 Hz. In addition in another subgroup (n= 4) hearts were kept quiescent for 60 min by adding 0.37 mmol l−1 lidocaine hydrochloride to the perfusate. After this 60 min period, the hearts of all four groups were immediately placed in ice-cold modified Krebs–Henseleit solution. The free wall of the RV was rapidly dissected, frozen in liquid nitrogen, freeze-dried and stored at −70°C.
In a few experiments, the β-adrenergic receptor blocker atenolol (0.02 mmol l−1) was added to rule out a possible sympathetic effect of electrical stimulation of residual nerve endings in isolated hearts on the force–frequency response, an effect that is distinct from the well-known long-term effect of β-blockers on adrenergic receptor density and sensitivity. We found no effect of atenolol on the force–frequency relation (data not shown).
Ca2+ sensitivity measurements
Frequency-dependent alterations in Ca2+ sensitivity of the contractile apparatus were studied in isolated skinned cardiomyocytes obtained from RV tissue of the above-mentioned isolated control and failing hearts frozen at a pacing frequency of 0 and 9 Hz.
Cardiomyocytes from control and failing hearts were mechanically isolated as previously described (van der Velden et al. 1998). In short, tissue was homogenized and cells were permeabilized with 0.5% Triton and washed. Single cardiomyocytes (n= 10 in each group, from n= 4 hearts in 0 Hz group and n= 5 hearts in 9 Hz group) with uniform striation pattern were selected and glued to thin stainless steel needles with silicon adhesive. Sarcomere length measured in relaxing solution was adjusted to 2.2 μm. Measurements were performed at 15°C. The Ca2+ sensitivity of force was determined by measuring isometric force at maximal [Ca2+] (pCa4.5; pCa =−log[Ca2+]) and submaximal [Ca2+] before and after treatment with the catalytic subunit of protein kinase A (PKA) (100 U ml−1 in relaxing solution with 6 mmol l−1 dithiothreitol (DTT) for 40 min at 20°C). Mean ΔpCa50 values were calculated from the shift in pCa50 upon PKA treatment from individual cardiomyocytes in each group.
Protein analysis
Freeze-dried RV tissue obtained from the control (n= 19) and failing (n= 19) hearts frozen at different pacing frequencies (0, 3, 6 and 9 Hz) was used to determine the frequency-dependent phosphorylation of several contractile proteins.
The phosphorylation levels of myosin light chain 1 (MLC-1), myosin light chain 2 (MLC-2) and troponin T (TnT) were assessed by two-dimensional polyacrylamide gel electrophoresis (2-D-PAGE) (van der Velden et al. 2003b). Samples were treated with trichloroacetic acid to preserve the phosphorylation status of the proteins (Morano et al. 1988) and loaded (200 μg dry weight) on immobiline strips with a pH gradient of 4.0 to 7.0 (Amersham Pharmacia Biotech). In the second dimension, proteins were separated by SDS-PAGE. Gels were stained with Coomassie blue, scanned and analysed using AIDA (Raytest, Germany). The phosphorylation levels were expressed as a percentage of the total protein content.
Phosphorylation of troponin I (TnI) was determined by non-equilibrium isoelectric focusing gel electrophoresis (NEIEF) as described earlier (O'Farrell et al. 1977; Madden, 1995; Kobayashi et al. 2005). The NEIEF gels contained 8 m urea, 5% acrylamide (acrylamide/bis-acrylamide 29: 1; optional 2% Triton X-100), 0.8% ampholyte (3.5–10.0) and 1.2% ampholyte (7.0–9.0) (Amersham, NJ). Sample loading buffer contained 8 mol l−1 urea, 2.5 mol l−1 thiourea, 2 mmol l−1 EDTA, 0.5%, ampholytes (3–10), 4% 3-[(3-cholamidopropyl)dimethylammonio]-propanesulfonate (CHAPS), 10 mmol l−1 DTT, 2 mmol l−1 TBP and a protease inhibitor cocktail. Unlike typical IEF, the lower and upper reservoirs were filled with 20 mol l−1 NaOH and 10 mmol l−1 H3PO4, respectively, and the positive and negative ports were reversed. Electrophoresis was carried out at 100 V for 20 min, 200 V for 50 min followed by 500 V for 10 min without pre-run. For the Western blot, after separation, proteins were transferred to nitrocellulose membrane overnight at 30 V at 4°C. Membranes were blocked for 1 h in 5% non-fat milk in Tris-Buffered Saline Tween-20 (TBST), washed and incubated with monoclonal anti-TnI (1: 5000 in TBST; clone C5 from Research Diagnostics Inc.) for 3 h at room temperature. After washing, they were further incubated with anti-mouse antibody conjugated to horseradish peroxidase Horseradish Peroxidase, correct (HRP). Proteins were visualized with Electrochemiluminescence (ECL) (Amersham) and quantified by densitometric analysis using NIH Image.
In addition, the phosphorylation levels of myosin binding protein C (MyBP-C) MLC1, MLC2, TnT and TnI were assessed by phosphospecific staining. Proteins were separated on a one-dimensional 12% SDS-PAGE and the gels were stained with Pro-Q Diamond phosphoprotein stain (Molecular Probes) (Jweied et al. 2005). After visualization of the phosphoproteins, the same gel was stained with colloidal Coomassie blue to verify equal protein loading in each lane. Images were quantified by using ImageQuant analysis software (Bio-Rad). Pro-Q Diamond signals were normalized to total actin protein content.
The amount of PKCα protein and phosphorylation levels were assessed by gel electrophoresis and stained with antibodies for PKCα (dilution 1: 500, Research & Diagnostics Antibodies (R & D) Systems) and phosphorylated PKCα at Ser657 (dilution 1: 200, Santa Cruz Biotechnology). The immunoreactive bands were visualized by chemiluminescence and quantified with AIDA software. Protein content was determined by Ponceau staining. The amount of phosphorylation of the blots was normalized to the actin levels on the Ponceau-stained blot. To circumvent difficulties in the analysis of experimental groups between different gels we applied on a gel with 12 lanes/slots 2 times five samples (n= 10) of failing hearts at 0 Hz or 9 Hz stimulation frequencies, respectively. On the same gel two additional samples were applied, which each consisting of a pool of five control hearts samples paced at 0 Hz or 9 Hz, respectively. This procedure was repeated on a second gel for control and failing hearts, but on this second gel the individual control heart samples were applied and the failing heart samples were pooled.
Analysis and statistics
Statistical differences within groups were tested with one- or two-way ANOVA followed by a Bonferroni post hoc test; P < 0.05 was considered significant. All data are expressed as mean ±s.e.m., with n indicating the number of animals per group, unless indicated otherwise. Fura 2 signals of five consecutive contractions were averaged to obtain accurate [Ca2+]i transients. The force–pCa relation was fitted to the following Hill equation:
where F is steady state force, Fo denotes the steady state force at saturating Ca2+ concentration determined at pCa = 4.5, nH represents the steepness of the relationship, and Ca50 or pCa50 represent the Ca2+ concentration at which force is half of Fo.
Results
MCT-induced right ventricular heart failure
On the day of the experiment, MCT-treated animals had an almost 2-fold increased RV weight, an almost 3-fold increased RV weight/body weight ratio and a 2-fold increased RV/LV weight ratio in comparison to the controls, indicating marked RV hypertrophy (Table 1). Furthermore, MCT-treated animals displayed a progressive loss of body weight during several days before killing and showed an increased lung weight (Table 1) with pleural effusion (fluid in the lungs), indicative of congestive heart failure (Leineweber et al. 2000; Seyfarth et al. 2000; Buermans et al. 2005). RV hypertrophy was also reflected in the cross-sectional area of the trabeculae studied: 0.031 ± 0.004 mm2 in the control and 0.046 ± 0.009 mm2 in the failing group. Although, in the present study, an increase in LV/body weight ratio was observed (Table 1), this increase does not reflect LV hypertrophy, but is mainly due to the fact of increased body weight of the MCT-treated rats with unaltered LV weight (Leineweber et al. 2000; Seyfarth et al. 2000).
Table 1.
Body weight and wet lung and ventricle weights on the day of the experiment
| Control (n= 11) | Failing (n= 10) | |
|---|---|---|
| BW (g) | 333 ± 10 | 231 ± 8* |
| LW (g) | 1.63 ± 0.05 | 2.35 ± 0.16* |
| RV (mg) | 220 ± 15 | 412 ± 20* |
| RV/BW (mg g−1) | 0.66 ± 0.04 | 1.81 ± 0.11* |
| LV (mg) | 968 ± 69 | 839 ± 55 |
| LV/BW (mg g−1) | 2.90 ± 0.18 | 3.65 ± 0.20 |
| RV/LV | 0.23 ± 0.01 | 0.50 ± 0.03* |
BW, body weight; LW, lung weight; RV, right ventricle weight; LV, weight of left ventricle + septum. Values are expressed as means ±s.e.m.
P < 0.05 versus control.
Frequency dependency of force and intracellular Ca2+
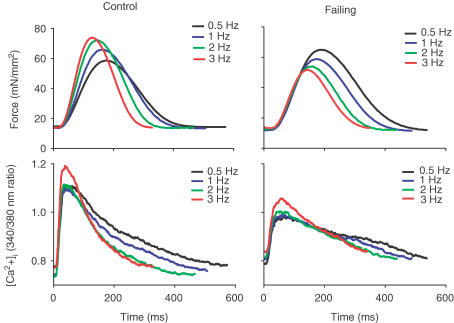
Force and [Ca2+]i transients obtained at different stimulation frequencies for control and failing trabeculae are shown in Fig. 1. In Fig. 2 the averaged diastolic and systolic force and [Ca2+]i levels are shown.
Figure 1. Averaged force twitches (upper panels, n= 10) and [Ca2+]i transients (lower panels) at different stimulation frequencies from control (left panels) and failing (right panels) right ventricular trabeculae.
In the control group a positive force–frequency relation with increased frequency-dependent acceleration of relaxation (of force and Ca2+ transient) exists, while in the failing group a negative force–frequency relation with a preserved frequency-dependent acceleration of relaxation was observed.
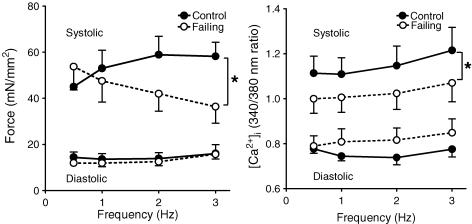
Figure 2. Averaged diastolic (lower values) and peak systolic (upper values) force (left panel) and [Ca2+]i (right panel) at different stimulation frequencies at 1 mmol l−1 Ca2+ from control (n= 10) and failing (n= 10) right ventricular trabeculae.
In controls, systolic force and [Ca2+]i increased with frequency, whereas in failing muscles, systolic force decreased and [Ca2+]i increased with frequency. In both groups, diastolic force and diastolic [Ca2+]i remained constant. Values are expressed as means ±s.e.m.*P < 0.05 versus control group.
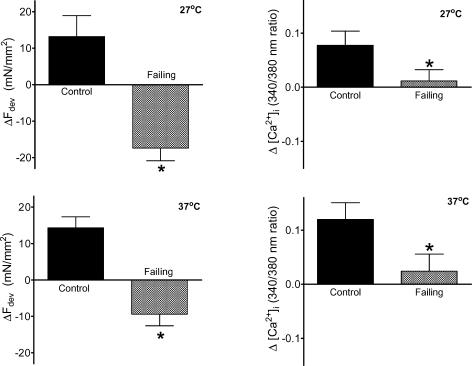
A positive force–frequency relation was observed in control trabeculae, which was accompanied by a frequency-dependent increase in systolic [Ca2+]i, while diastolic force and diastolic [Ca2+]i remained constant. Failing trabeculae clearly displayed a negative systolic force–frequency relation, while diastolic force remained constant. In the failing group systolic [Ca2+]i was depressed compared with control, but peak systolic [Ca2+]i increased with frequency. Diastolic [Ca2+]i tended to be elevated (P= 0.07) in the failing trabeculae compared with controls, but was not affected by frequency. The disparity of the force–frequency and [Ca2+]i–frequency relations in the failing group suggests the presence of frequency-dependent changes in myofilament Ca2+ sensitivity. This is further illustrated in Fig. 3 (upper panel) where the impact of an increase in frequency on developed force and the amplitude of the [Ca2+]i transient is shown: the decrease in developed force with increasing pacing frequency in the failing muscle is accompanied by a small increase in the amplitude of [Ca2+]i rather than a decrease. Figure 3 shows that the magnitudes of the frequency-dependent effects in Ca2+ sensitivity at 27°C (upper panel) and 37°C (lower panel) were very similar.
Figure 3. Changes in developed force (left panels) and changes in the amplitude of the [Ca2+]i transient (right panels) with an increase in stimulation frequency from 0.5 to 3 Hz at 27°C (upper panels) and from 0.5 to 6 Hz at 37°C (lower panels) from control (n= 10) and failing (n= 10) right ventricular trabeculae.
In the control group as well as in the failing muscles, the frequency-dependent increase in developed force was accompanied by an increase in amplitude of [Ca2+]i, although the amplitude of [Ca2+]i was decreased in the failing muscles compared with controls. Values are expressed as means ±s.e.m.*P < 0.05 versus control group.
The relaxation of force was slowed in failing muscle (time to half-relaxation (tHR) at 0.5 Hz at 27°C: control 282 ± 9 ms; failing 314 ± 10 ms, P < 0.05; and at 37°C: control 130 ± 4 ms; failing 152 ± 5 ms, P < 0.05, all groups n= 10) and was accompanied in the failing muscles with prolonged relaxation of the [Ca2+]i transient (Fig. 1). Although relaxation was slower, the frequency-dependent acceleration of relaxation remained and was even more pronounced in the failing trabeculae than in control muscles (change in time to half-relaxation from 0.5 to 3 Hz at 27°C: control 78 ± 5 ms; failing 92 ± 6 ms, P < 0.05; and from 0.5 to 6 Hz at 37°C: control 21 ± 3 ms; failing 39 ± 11 ms, P= 0.07).
Maximal force (Fmax) attained during post-extrasystolic potentiation was not affected in the failing muscles (control 75.2 ± 7.4 mN mm−2, failing 62.7 ± 9.6 mN mm−2, P > 0.05, n= 10).
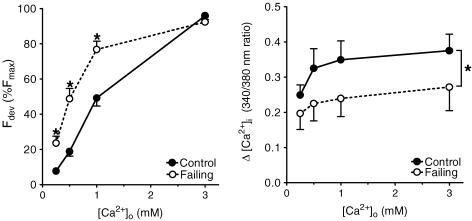
Figure 4 shows the normalized force–external [Ca2+]o relation (left panel) and the amplitude of intracellular [Ca2+]i (right panel) for both groups. Developed force as well as the amplitude of [Ca2+]i increased with an increase in external [Ca2+]o in both groups. The leftward shift in the force–[Ca2+]o relation in failing compared with control muscles, indicating increased sensitivity of the failing cardiomyocytes to external calcium, was accompanied by a decreased amplitude of the [Ca2+]i transient.
Figure 4. Effect of external [Ca2+]o on developed force (left panel, normalized to Fmax) and the amplitude of intracellular [Ca2+]i (right panel) at 0.5 Hz and 27°C from control (n= 10) and failing (n= 10) right ventricular trabeculae.
A leftward shift in the force–[Ca2+]o relation in the failing group indicates an increased sensitivity of the cardiomyocytes to external calcium, which is accompanied by a decreased amplitude of [Ca2+]i as compared with control preparations. Force and amplitude of [Ca2+]i increased with [Ca2+]o in both groups. Values are expressed as means ±s.e.m.*P < 0.05 versus control group.
Frequency dependency of RV pressure
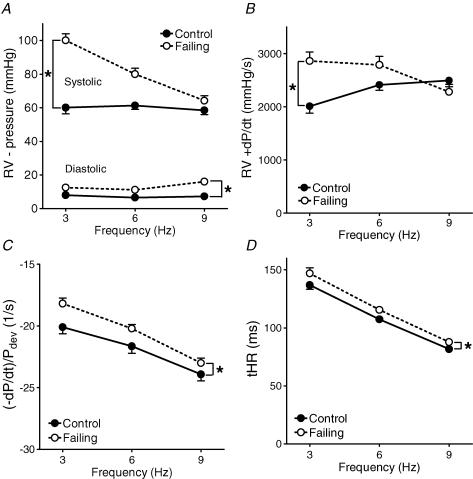
The upper panels of Fig. 5 show that in the control hearts a flat RV pressure–frequency relation existed and that +dP/dtmax, an often-used index of contractility in isolated hearts, increased with stimulation frequency. In failing hearts the pressure–frequency and +dP/dtmax–frequency relation were both negative, similar to the force–frequency relations in the isolated trabeculae (Figs 1 and 2). The lower panels of Fig. 5 show that in the failing hearts relaxation (parameters (−dP/dt)/Pdev and tHR) was slower compared with the control hearts, while the frequency-dependent acceleration of relaxation remained.
Figure 5. Averaged diastolic and systolic RV pressures (A), +dP/dt (B), (−dP/dt)/Pdev (C) and tHR (D) at different stimulation frequencies in control and failing Langendorff perfused hearts.
In the control group a flat pressure–frequency relation and positive +dP/dtmax–frequency relation was found, whereas in the failing group a negative pressure–frequency and +dP/dtmax–frequency relation was found. In the failing group, relaxation (C and D) was slower, but the frequency-dependent acceleration of relaxation remained. Values are expressed as means ±s.e.m.*P < 0.05 versus control.
Frequency dependency of Ca2+ sensitivity
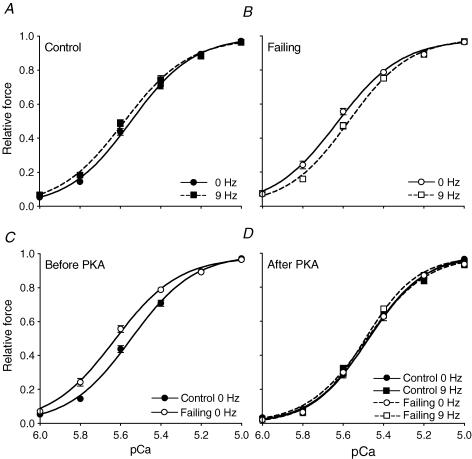
To directly test whether the Ca2+ sensitivity of the myofilaments is frequency dependent, isometric force–pCa relations were determined in isolated skinned cardiomyocytes from quiescent control and failing hearts and after pacing at 9 Hz.
Figure 6A shows that in control cardiomyocytes the force–pCa curve was not affected by an increase in frequency from 0 Hz to 9 Hz. The pCa50 values (Table 2) were not significantly different, indicating that Ca2+ sensitivity was not altered. In contrast, in failing cardiomyocytes an increase in frequency from 0 Hz to 9 Hz reduced Ca2+ sensitivity as reflected in the rightward shift of the force–pCa relation and the decreased pCa50 values (Fig. 6B, Table 2).
Figure 6. Isometric force–pCa relations in isolated skinned cardiomyocytes from control (A) and failing (B) quiescent (0 Hz) or stimulated (9 Hz) right ventricles before (C) and after (D) PKA treatment.
In the failing cardiomyocytes the force–pCa curve shifted to the right with an increase in frequency. At 0 Hz, the force–pCa relation was shifted leftward in failing compared with control cardiomyocytes. After PKA treatment the force–pCa curves were similar for all groups. Values are expressed as means ±s.e.m. from n= 10 cardiomyocytes in each group.
Table 2.
pCa50, ΔpCa50 and nH values before and after PKA treatment
| Control | Failing | |||
|---|---|---|---|---|
| Before PKA | pCa50 | 0 Hz | 5.55 ± 0.02 | 5.62 ± 0.01* |
| 9 Hz | 5.58 ± 0.01 | 5.57 ± 0.01† | ||
| nH | 0 Hz | 2.87 ± 0.10 | 2.70 ± 0.13 | |
| 9 Hz | 2.68 ± 0.12 | 2.79 ± 0.07 | ||
| After PKA | pCa50 | 0 Hz | 5.48 ± 0.01 | 5.47 ± 0.01 |
| 9 Hz | 5.48 ± 0.02 | 5.49 ± 0.01 | ||
| ΔpCa50 | 0 Hz | 0.08 ± 0.01 | 0.15 ± 0.01* | |
| 9 Hz | 0.10 ± 0.01 | 0.08 ± 0.01† | ||
| nH | 0 Hz | 3.12 ± 0.12 | 3.19 ± 0.14 | |
| 9 Hz | 2.90 ± 0.12 | 3.24 ± 0.15 |
Values are expressed as means ±s.e.m. from n= 10 cardiomyocytes in each group.
P < 0.05 versus control
P < 0.05 versus 0 Hz. Mean ΔpCa50 values were calculated from the shift in pCa50 upon PKA treatment from individual cardiomyocytes in each group.
The force–pCa curve from quiescent failing cardiomyocytes was significantly shifted to the left compared with the curve from quiescent control cardiomyocytes (Fig. 6C, Table 2), indicating an increase in basal Ca2+ sensitivity in failing myocardium. In all groups the force–pCa curve was shifted rightwards to a similar end-value after PKA treatment (Fig. 6D, Table 2), suggesting that the frequency-dependent changes in Ca2+ sensitivity observed predominantly result from differences in phosphorylation levels of TnI and/or MyBP-C. The steepness of the curves, as indicated by the Hill coefficients, did not differ between groups (Table 2). The maximum force generated by the control and failing cardiomyocytes was very similar (Control: 22.0 ± 1.5 kN mm−2versus Failing: 25.4 ± 1.7 kN mm−2, P= 0.15).
Frequency dependency of protein phosphorylation
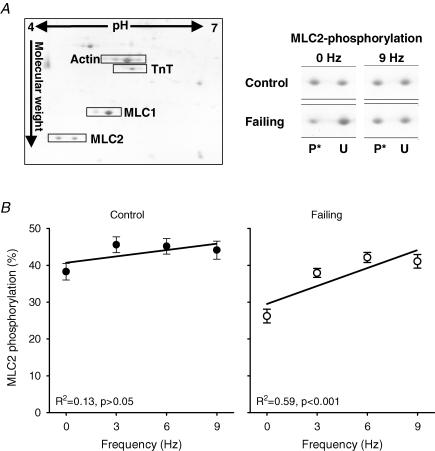
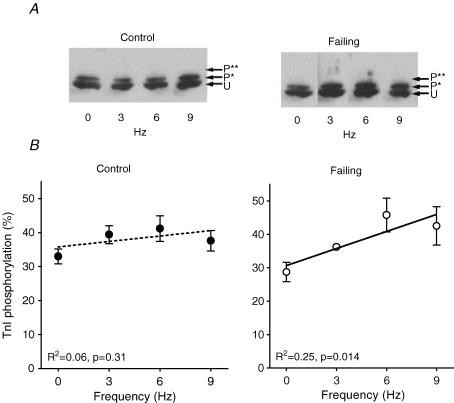
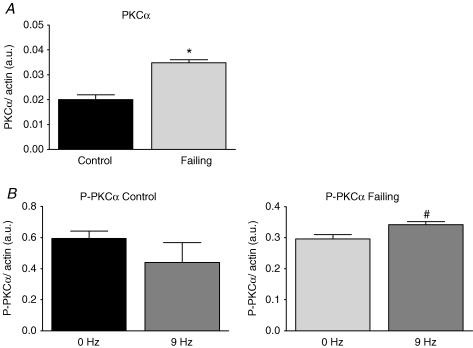
In Fig. 7A an example of a Coomassie stained 2-D SDS-PAGE gel (left panel) is shown of a control heart paced at 3 Hz. The specific MLC2 sections of control and failing hearts at 0 and 9 Hz are shown in the right panel of Fig. 7A. Figure 7B and the included linear regression lines show that MLC2 phosphorylation increases with frequency in failing hearts, but not in control hearts (n= 5 samples per frequency in control and failing groups, with 2 runs per sample, which yielded an overall reproducibility of 4.5 ± 0.4%). The mean phosphorylation levels of MLC2 in the range of frequencies studied were less in failing hearts (37.4 ± 1.6%, P < 0.05) than in control hearts (43.6 ± 1.3%). Figure 8A shows an example of a NEIEF gel from samples of the control (left panel) and failing group (right panel). Figure 8B and the included linear regression lines show that TnI phosphorylation increases with frequency in failing hearts, but not in control hearts (Control: 0 Hz (n= 4); 3, 6 and 9 Hz (n= 5); Failing: 3 Hz (n= 5); 0, 6 and 9 Hz (n= 6), with a total average of 3.6 ± 0.2 repeated measurements per sample, which yielded an overall reproducibility of 6.5 ± 0.7%). The mean levels of TnI phosphorylation in the range of frequencies studied were not different between both groups (36.7 ± 2.0%versus 37.5 ± 1.4% phosphorylation, control and failing, respectively). The differences in phosphorylation levels of MLC2 and TnI found using the Pro-Q-Diamond stain were consistent with the differences found on the 2-D and NEIEF gel, respectively. Analysis of the MLC1 and TnT spots on the 2-D gels and of MLC1, TnT and MyBP-C on the Pro-Q Diamond gels revealed no frequency dependency of their phosphorylation levels and also no differences between control and failing hearts (data not shown). In Fig. 9A is shown the total amount of protein expression of PKCα of control (pooled, n= 10) and failing hearts (individual, n= 10). In the failing hearts, the amount of PKCα protein expression is significantly increased compared with controls. On a second gel, with control (individual, n= 10) and failing hearts (pooled, n= 10), a similar increase in the amount of PKCα protein expression was found (data not shown). In Fig. 9B is shown the phosphorylation levels of PKCα, normalized to the amount of PKCα protein, of control and failing hearts at 0 or 9 Hz stimulation frequencies. The phosphorylation level of PKCα was increased by frequency in the failing hearts (P < 0.05), but not in controls.
Figure 7. MLC2 phosphorylation at different stimulation frequencies in control and failing hearts.
A, total coomassie stained 2-D gel of RV tissue of a control heart at 3 Hz (left panel) and specific MLC2 sections (right panel) of control and failing hearts at 0 and 9 Hz. B, frequency dependency of MLC2 in failing hearts but not in control. Abbreviations used: MLC2, myosin light chain 2; U, unphosphorylated; and P*, phosphorylated. Values are expressed as means ±s.e.m., n= 5 per frequency.
Figure 8. Tnl phosphorylation at different stimulation frequencies in control and failing hearts.
A, Western immunoblot of a NEIEF gel, stained with TnI antibody of failing hearts. B, frequency dependency of TnI in failing hearts but not in control. Abbreviations used: TnI, troponin I; U, unphosphorylated; P*, monophosphorylated; and P**, biphosphorylated. Values are expressed as means ±s.e.m. (Control: 0 Hz (n= 4); 3, 6 and 9 Hz (n= 5); Failing: 3 Hz (n= 5), 0, 6 and 9 Hz (n= 6).
Figure 9. Expression and phosphorylation levels of PKCα in quiescent (0 Hz) and stimulated (9 Hz) control and failing hearts.
A, increased expression of PKCα in failing hearts compared with control. B, frequency dependency of PKC phosphorylation in failing hearts but not in control. Abbreviations used: PKCα, protein kinase C α; P-PKCα, phosphorylated PKCα. Values are expressed as means ±s.e.m.*P < 0.05 versus control, #P < 0.05 versus 0 Hz.
Discussion
In the control group a positive force–frequency relation was found with a considerable increase in amplitude of the [Ca2+]i transient with frequency, while Ca2+ sensitivity of force, measured in permeabilized cardiomyocytes, remained constant. In the failing group, the negative force–frequency relation was accompanied by a small increase in amplitude of the [Ca2+]i transients with frequency and a frequency-dependent decrease in Ca2+ sensitivity. Thus, frequency-dependent Ca2+ desensitization of the myofilaments contributes to the negative force–frequency relation in failing rat myocardium This frequency-dependent Ca2+ desensitization was associated with a frequency-dependent increase in phosphorylation of TnI, MLC2 and PKCα.
Frequency dependency of force and phosphorylation in the control group
Under physiological conditions, a positive force–frequency relation is found in a number of mammalian species including man. The increase in sarcolemmal Ca2+ influx per unit of time, resulting in increased SR Ca2+ loading, is well established as an underlying mechanism for the increase in myocardial force (Gao et al. 1998; Layland & Kentish, 1999; Pieske et al. 1999; Stuyvers et al. 2002). However, because of the highly non-linear force–Ca2+ relation, a possible change in myofilament Ca2+ sensitivity, through phosphorylation of contractile proteins, cannot be ruled out as a contributing factor. For instance, experiments on rabbit septal preparations revealed a significant frequency-dependent increase in MLC2 phosphorylation (Silver et al. 1986; Sweeney et al. 1993). Also, a positive correlation was found between heart rate and MLC2 phosphorylation in rats studied at different levels of treadmill exercise or β-adrenergic stimulation and inhibition (Fitzsimons et al. 1989). More recently, a decreased Ca2+ sensitivity was observed in rabbit trabeculae at higher frequencies, which was accompanied by changes in MLC2 and TnI phosphorylation (Varian & Janssen, 2007).
Our experiments on permeabilized isolated isometric contracting cardiomyocytes (Fig. 6A, Table 2) revealed that Ca2+ sensitivity was not affected by an increase in frequency in the control group. Moreover, our data revealed that phosphorylation levels of MLC1, MLC2, MyBP-C, TnT and TnI of isovolumic contracting control hearts were not frequency dependent. This is in line with the results of Takimoto et al. (2004) who showed in transgenic mice with aspartic acid substitutions for the serine sites targeted by PKA on TnI, mimicking constitutive phosphorylation at the PKA sites, that the frequency-dependent increase in isometrically contracting trabeculae was not affected. In our study, in isometrically contracting control cardiomyocytes, PKA treatment, which does not affect MLC2 phosphorylation (Strang et al. 1994), induced a shift in Ca2+ sensitivity which was similar in quiescent and in paced (9 Hz) hearts (Fig. 6C and D). This supports the concept that TnI phosphorylation may enhance the frequency-dependent regulation of cardiac contraction in the in vivo working heart, but not in isometric contractions (Layland & Kentish, 2002; Takimoto et al. 2004). Recently, it was also demonstrated in transgenic mice, which expressed a non-phosphorylatable MLC2 protein, that phosphorylation of MLC2 was not an essential determinant of the force–frequency relation (Dias et al. 2006).
Thus, our experiments in control hearts are consistent with the notion that the positive force–frequency relation is mainly due to an increase in sarcolemmal Ca2+ influx per unit of time and that alterations in Ca2+ sensitivity, through phosphorylation of contractile proteins, do not contribute to the positive force–frequency relation in healthy myocardium.
Frequency dependency of force and phosphorylation in the failing group
The negative, blunted or flat force–frequency relation is a hallmark of the failing heart (Gwathmey et al. 1990; Mulieri et al. 1992; Schwinger et al. 1993; Eising et al. 1994; Pieske et al. 1995, 1999; Janssen et al. 2000; Brixius et al. 2002; Kogler et al. 2003), and has mainly been attributed to alterations in Ca2+ handling (Pieske et al. 1999). Altered Ca2+ homeostasis in failing hearts was evident from a reduced amplitude of the [Ca2+]i transient (Fig. 2), an increased sensitivity of developed force to extracellular calcium (Fig. 4) and an elevated systolic function at low pacing frequencies (Figs 4 and 5). The half-times of relaxation of force of the trabeculae and isolated hearts were prolonged, consistent with the deceleration of Ca2+ re-uptake and Ca2+ extrusion (Pieske et al. 1999; Bers et al. 2003), which was supported by the prolonged relaxation of the [Ca2+]i transients in the failing muscles (Fig. 1). The maximal forces (Fmax) attained in trabeculae during post-extrasystolic potentiation and in skinned cardiomyocytes were the same in control and failing hearts. Together with the increased force at low stimulation frequencies in failing trabeculae this indicates that the contractile capacity of failing cardiomyocytes is not impaired but that developed force is closer to saturation, which reduces contractile reserve (Korstjens et al. 2002; Kogler et al. 2003), probably due to enhanced SR load (Pieske et al. 1999; Bers et al. 2003).
In our animals with heart failure, the Ca2+ sensitivity was increased under basal conditions, which is consistent with findings in the MCT model (Kogler et al. 2003) and in human failing myocardium (van der Velden et al. 2003a,b). Interestingly, in failing trabeculae the net influx of Ca2+ per unit of time increased with an increase in frequency (Fig. 3) and the relaxation of the [Ca2+]i transient was prolonged (Fig. 1). Thus, with Ca2+ sensitivity increased, and force not yet saturated (Fig. 4) in failing hearts this would be expected to accentuate the frequency-dependent alterations in Ca2+ handling resulting in increased myocardial force at higher frequencies. Nevertheless the force–frequency relation is negative and this reflects a frequency-dependent desensitization of the myofilaments.
The increased basal Ca2+ sensitivity in failing myocardium cannot be explained by alterations in phosphorylation of MLC2 or TnI. Recently, the importance of site-specific alterations in phosphorylation of TnI on the force–frequency relation was demonstrated in transgenic mice (Bilchick et al. 2007). Hence, the most likely explanation is that the intricate alterations in phosphatases and kinases involved result in a specific pattern of phosphorylation, and that also the location of phosphorylation site(s) may be important to explain the functional effects.
Frequency-induced changes in contraction and Ca2+ handling can be different at temperatures lower than body temperature (Layland & Kentish, 1999; Janssen et al. 2002); however, in our trabeculae at 27 and 37°C we found qualitatively similar results in the frequency-induced changes in force and [Ca2+]i. Thus, the frequency-dependent Ca2+ desensitization of the contractile apparatus also exists in failing hearts at body temperature. This is confirmed by the direct Ca2+ sensitivity measurements in the isolated cardiomyocytes in this study (Fig. 6B, Table 2).
As mentioned above, a frequency-dependent increase in MLC2 phosphorylation has been found in healthy myocardium (Silver et al. 1986; Fitzsimons et al. 1989; Sweeney et al. 1993). From our study it is clear that the frequency-dependent increase in MLC2 phosphorylation is also present in failing rat myocardium (Fig. 7B). This frequency dependence of MLC2 phosphorylation may originate from a CaMK-II-dependent increase in MLCK activity. MLC2 phosphorylation results in a stereospecific increase in the probability of force-generating crossbridge formation (Sweeney et al. 1993; Olsson et al. 2004). However, since MLC2 phosphorylation leads to increased Ca2+ sensitivity (Sweeney et al. 1993; Olsson et al. 2004), it cannot explain the frequency-dependent Ca2+ desensitization observed in failing cardiomyocytes. Thus, the frequency-dependent phosphorylation of MLC2 counteracts a more potent mechanism responsible for the negative force–frequency relation in heart failure.
The frequency dependence of Ca2+ sensitivity of the contractile apparatus in failing cardiomyocytes was abolished by PKA treatment (Fig. 6D). TnI and myosin binding protein C (MyBP-C) represent the main targets for PKA (Strang et al. 1994), and phosphorylation of both proteins is accompanied by a decreased Ca2+ sensitivity (Garvey et al. 1988; Harris et al. 2002). In our failing hearts, MyBP-C phosphorylation remained constant, while TnI phosphorylation increased with frequency (Fig. 8B). This indicates that phosphorylation of TnI and not MyBP-C is responsible for the frequency-dependent Ca2+ desensitization in failing myocardium. Furthermore, in quiescent cardiomyocytes the shift upon PKA treatment was larger in the failing group than in controls. However, at 9 Hz the PKA-induced shift was not different between failing and controls. This is in agreement with the observation that β-adrenergic stimulation blunts the force–frequency relation in failing myocardium (Schwinger et al. 1993; Eising et al. 1994). The absence of a significant shift in Ca2+ sensitivity by PKA in failing hearts is not in conflict with the modest frequency-dependent increase in MLC2 phosphorylation (26.2 ± 1.9% at 0 Hz to 41.1 ± 1.9% at 9 Hz), as was found by others in rat (Olsson et al. 2004) and mice (Stelzer et al. 2006). Together these results indicate that the functional implications of the increase in MLC2 phosphorylation observed is only modest, as can be deduced also from the results observed in transgenic mice with a non-phosphorylatable MLC2 protein (Dias et al. 2006).
What could be the reason for the frequency-dependent increase in TnI phosphorylation and why is it evident only in failing hearts? Since the time-averaged intracellular Ca2+ concentration increases with pacing frequency, the answer to these questions should reside in an altered balance of Ca2+-dependent kinase or phosphatase activity. Hence the main candidates would be the classical Ca2+-dependent isoforms of PKC and the Ca2+-dependent phosphatase calcineurin (PP2B). Both enzymes are up-regulated in heart failure (Bowling et al. 1999; Lim & Molkentin, 1999; Braz et al. 2004) and thus their contribution may be more conspicuous in failing than in control hearts. In agreement with these findings, our study clearly shows an increase in protein expression of the Ca2+-dependent PKCα isoform in failing hearts (Fig. 9A).
Evidence suggests that PKCs phosphorylate not only the PKC-specific sites on TnI (Ser-42, Ser-44 and Thr-143), but also the PKA sites (Ser-23 and Ser-24) (Swiderek et al. 1990; Noland et al. 1995; Kobayashi et al. 2005). Recently, in transgenic mice with mutated PKA-TnI sites, to mimic dephosphorylation, and PKC sites mutated, to mimic constitutive phosphorylation, a blunted force–frequency response was found (Bilchick et al. 2007). Our study revealed a modest but significant increase in the phosphorylation level of PKCα with frequency in the failing hearts (Fig. 9B). This suggests a novel role for PKC-mediated TnI phosphorylation in modulating the force–frequency relation. Altered expression of PP2B might be involved as well, but only if this phosphatase would relieve the inhibition of a kinase involved in the phosphorylation of TnI, a route which might involve PKCs as well.
Conclusion
In control hearts, the positive force–frequency relation is primarily due to an increase in Ca2+ influx per unit of time. In failing rat hearts, frequency-dependent Ca2+ desensitization of the myofilaments together with alterations in Ca2+ homeostasis contributes to the negative force–frequency relation in failing rat myocardium. This frequency-dependent Ca2+ desensitization was associated with a frequency-dependent increase in phosphorylation of TnI and was counteracted by a frequency-dependent increase in MLC2 phosphorylation. This suggests that the negative force–frequency relation can be opposed by Ca2+ sensitizers and may explain why levosimendan improves the negative force–frequency relation in human failing myocardium (Janssen et al. 2000; Brixius et al. 2002) and acts more pronounced at higher heart rates (Janssen et al. 2000).
Acknowledgments
Supported, in part, by NIH grants HL-62426 (project 4) and HL-75494 (PdT).
References
- Antoons G, Mubagwa K, Nevelsteen I, Sipido KR. Mechanisms underlying the frequency dependence of contraction and [Ca2+]i transients in mouse ventricular myocytes. J Physiol. 2002;543:889–898. doi: 10.1113/jphysiol.2002.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res. 2003;93:487–490. doi: 10.1161/01.RES.0000091871.54907.6B. [DOI] [PubMed] [Google Scholar]
- Bilchick KC, Duncan JG, Ravi R, Takimoto E, Champion HC, Gao WD, Stull LB, Kass DA, Murphy AM. Heart failure-associated alterations in troponin I phosphorylation impair ventricular relaxation-afterload and force-frequency responses and systolic function. Am J Physiol Heart Circ Physiol. 2007;292:H318–H325. doi: 10.1152/ajpheart.00283.2006. [DOI] [PubMed] [Google Scholar]
- Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation. 1997;96:1495–1500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, et al. PKC-α regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- Brixius K, Reicke S, Schwinger RH. Beneficial effects of the Ca2+ sensitizer levosimendan in human myocardium. Am J Physiol Heart Circ Physiol. 2002;282:H131–H137. doi: 10.1152/ajpheart.2002.282.1.H131. [DOI] [PubMed] [Google Scholar]
- Buermans HP, Redout EM, Schiel AE, Musters RJ, Zuidwijk M, Eijk PP, van Hardeveld C, Kasanmoentalib S, Visser FC, Ylstra B, Simonides WS. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics. 2005;21:314–323. doi: 10.1152/physiolgenomics.00185.2004. [DOI] [PubMed] [Google Scholar]
- DeSantiago J, Maier LS, Bers DM. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. J Mol Cell Cardiol. 2002;34:975–984. doi: 10.1006/jmcc.2002.2034. [DOI] [PubMed] [Google Scholar]
- Dias FA, Walker LA, Arteaga GM, Walker JS, Vijayan K, Pena JR, Ke Y, Fogaca RT, Sanbe A, Robbins J, Wolska BM. The effect of myosin regulatory light chain phosphorylation on the frequency-dependent regulation of cardiac function. J Mol Cell Cardiol. 2006;41:330–339. doi: 10.1016/j.yjmcc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Eising GP, Hammond HK, Helmer GA, Gilpin E, Ross J., Jr Force-frequency relations during heart failure in pigs. Am J Physiol Heart Circ Physiol. 1994;267:H2516–H2522. doi: 10.1152/ajpheart.1994.267.6.H2516. [DOI] [PubMed] [Google Scholar]
- Fitzsimons DP, Bodell PW, Baldwin KM. Phosphorylation of rodent cardiac myosin light chain 2: effects of exercise. J Appl Physiol. 1989;67:2447–2453. doi: 10.1152/jappl.1989.67.6.2447. [DOI] [PubMed] [Google Scholar]
- Gao WD, Perez NG, Marban E. Calcium cycling and contractile activation in intact mouse cardiac muscle. J Physiol. 1998;507:175–184. doi: 10.1111/j.1469-7793.1998.175bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey JL, Kranias EG, Solaro RJ. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. Biochem J. 1988;249:709–714. doi: 10.1042/bj2490709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey JK, Slawsky MT, Hajjar RJ, Briggs GM, Morgan JP. Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest. 1990;85:1599–1613. doi: 10.1172/JCI114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, Powers PA, Moss RL. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res. 2002;90:594–601. doi: 10.1161/01.res.0000012222.70819.64. [DOI] [PubMed] [Google Scholar]
- Janssen PM, Datz N, Zeitz O, Hasenfuss G. Levosimendan improves diastolic and systolic function in failing human myocardium. Eur J Pharmacol. 2000;404:191–199. doi: 10.1016/s0014-2999(00)00609-9. [DOI] [PubMed] [Google Scholar]
- Janssen PM, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am J Physiol Heart Circ Physiol. 2002;282:H499–H507. doi: 10.1152/ajpheart.00595.2001. [DOI] [PubMed] [Google Scholar]
- Jweied EE, McKinney RD, Walker LA, Brodsky I, Geha AS, Massad MG, Buttrick PM, de Tombe PP. Depressed cardiac myofilament function in human diabetes mellitus. Am J Physiol Heart Circ Physiol. 2005;289:H2478–H2483. doi: 10.1152/ajpheart.00638.2005. [DOI] [PubMed] [Google Scholar]
- Kameyama T, Chen Z, Bell SP, VanBuren P, Maughan D, LeWinter MM. Mechanoenergetic alterations during the transition from cardiac hypertrophy to failure in Dahl salt-sensitive rats. Circulation. 1998;98:2919–2929. doi: 10.1161/01.cir.98.25.2919. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Yang X, Walker LA, Van Breemen RB, Solaro RJ. A non-equilibrium isoelectric focusing method to determine states of phosphorylation of cardiac troponin I: identification of Ser-23 and Ser-24 as significant sites of phosphorylation by protein kinase C. J Mol Cell Cardiol. 2005;38:213–218. doi: 10.1016/j.yjmcc.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Kogler H, Hartmann O, Leineweber K, Nguyen van P, Schott P, Brodde OE, Hasenfuss G. Mechanical load-dependent regulation of gene expression in monocrotaline induced right ventricular hypertrophy in the rat. Circ Res. 2003;93:230–237. doi: 10.1161/01.RES.0000085042.89656.C7. [DOI] [PubMed] [Google Scholar]
- Korstjens IJ, Rouws CH, van der Laarse WJ, Van der Zee L, Stienen GJM. Myocardial force development and structural changes associated with monocrotaline induced cardiac hypertrophy and heart failure. J Muscle Res Cell Motil. 2002;23:93–102. doi: 10.1023/a:1019988815436. [DOI] [PubMed] [Google Scholar]
- Lamberts RR, Vaessen RJ, Westerhof N, Stienen GJM. Right ventricular hypertrophy causes impairment of left ventricular diastolic function in the rat. Basic Res Cardiol. 2007;102:19–27. doi: 10.1007/s00395-006-0620-5. [DOI] [PubMed] [Google Scholar]
- Lamberts RR, Van Rijen MH, Sipkema P, Fransen P, Sys SU, Westerhof N. Coronary perfusion and muscle lengthening increase cardiac contraction: different stretch triggered mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H1515–H1522. doi: 10.1152/ajpheart.00113.2002. [DOI] [PubMed] [Google Scholar]
- Layland J, Kentish JC. Positive force- and [Ca2+]i-frequency relationships in rat ventricular trabeculae at physiological frequencies. Am J Physiol Heart Circ Physiol. 1999;276:H9–H18. doi: 10.1152/ajpheart.1999.276.1.H9. [DOI] [PubMed] [Google Scholar]
- Layland J, Kentish JC. Myofilament-based relaxant effect of isoprenaline revealed during work-loop contractions in rat cardiac trabeculae. J Physiol. 2002;544:171–182. doi: 10.1113/jphysiol.2002.022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leineweber K, Seyfarth T, Brodde OE. Chamber-specific alterations of noradrenaline uptake (uptake1) in right ventricles of monocrotaline-treated rats. Br J Pharmacol. 2000;131:1438–1444. doi: 10.1038/sj.bjp.0703698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewartowski B, Pytkowski B. Cellular mechanism of the relationship between myocardial force and frequency of contractions. Prog Biophys Mol Biol. 1987;50:97–120. doi: 10.1016/0079-6107(87)90005-8. [DOI] [PubMed] [Google Scholar]
- Lim HW, Molkentin JD. Calcineurin and human heart failure. Nat Med. 1999;5:246–247. doi: 10.1038/6430. [DOI] [PubMed] [Google Scholar]
- Madden MS. Reverse isoelectric focusing procedure resolves charge variants of basic proteins. Anal Biochem. 1995;229:203–206. doi: 10.1006/abio.1995.1403. [DOI] [PubMed] [Google Scholar]
- Morano I, Arndt H, Gartner C, Ruegg JC. Skinned fibers of human atrium and ventricle: myosin isoenzymes and contractility. Circ Res. 1988;62:632–639. doi: 10.1161/01.res.62.3.632. [DOI] [PubMed] [Google Scholar]
- Morano I, Hadicke K, Haase H, Bohm M, Erdmann E, Schaub MC. Changes in essential myosin light chain isoform expression provide a molecular basis for isometric force regulation in the failing human heart. J Mol Cell Cardiol. 1997;29:1177–1187. doi: 10.1006/jmcc.1996.0353. [DOI] [PubMed] [Google Scholar]
- Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85:1743–1750. doi: 10.1161/01.cir.85.5.1743. [DOI] [PubMed] [Google Scholar]
- Noland TA, Jr, Guo X, Raynor RL, Jideama NM, Averyhart-Fullard V, Solaro RJ, Kuo JF. Cardiac troponin I mutants. Phosphorylation by protein kinases C and A and regulation of Ca2+-stimulated MgATPase of reconstituted actomyosin S-1. J Biol Chem. 1995;270:25445–25454. doi: 10.1074/jbc.270.43.25445. [DOI] [PubMed] [Google Scholar]
- O'Farrell PZ, Goodman HM, O'Farrell PH. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977;12:1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL. Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. Am J Physiol Heart Circ Physiol. 2004;287:H2712–H2718. doi: 10.1152/ajpheart.01067.2003. [DOI] [PubMed] [Google Scholar]
- Pieske B, Kretschmann B, Meyer M, Holubarsch C, Weirich J, Posival H, Minami K, Just H, Hasenfuss G. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92:1169–1178. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- Schwinger RH, Bohm M, Muller-Ehmsen J, Uhlmann R, Schmidt U, Stablein A, Uberfuhr P, Kreuzer E, Reichart B, Eissner HJ. Effect of inotropic stimulation on the negative force-frequency relationship in the failing human heart. Circulation. 1993;88:2267–2276. doi: 10.1161/01.cir.88.5.2267. [DOI] [PubMed] [Google Scholar]
- Seyfarth T, Gerbershagen HP, Giessler C, Leineweber K, Heinroth-Hoffmann I, Ponicke K, Brodde OE. The cardiac β-adrenoceptor-G-protein(s)-adenylyl cyclase system in monocrotaline-treated rats. J Mol Cell Cardiol. 2000;32:2315–2326. doi: 10.1006/jmcc.2000.1262. [DOI] [PubMed] [Google Scholar]
- Silver PJ, Buja LM, Stull JT. Frequency-dependent myosin light chain phosphorylation in isolated myocardium. J Mol Cell Cardiol. 1986;18:31–37. doi: 10.1016/s0022-2828(86)80980-4. [DOI] [PubMed] [Google Scholar]
- Stelzer JE, Patel JR, Moss RL. Acceleration of stretch activation in murine myocardium due to phosphorylation of myosin regulatory light chain. J Gen Physiol. 2006;128:261–272. doi: 10.1085/jgp.200609547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang KT, Sweitzer NK, Greaser ML, Moss RL. β-Adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circ Res. 1994;74:542–549. doi: 10.1161/01.res.74.3.542. [DOI] [PubMed] [Google Scholar]
- Stuyvers BD, McCulloch AD, Guo J, Duff HJ, ter Keurs HE. Effect of stimulation rate, sarcomere length and Ca2+ on force generation by mouse cardiac muscle. J Physiol. 2002;544:817–830. doi: 10.1113/jphysiol.2002.024430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol Cell Physiol. 1993;264:C1085–C1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Swiderek K, Jaquet K, Meyer HE, Schachtele C, Hofmann F, Heilmeyer LM., Jr Sites phosphorylated in bovine cardiac troponin T and I. Characterization by 31P-NMR spectroscopy and phosphorylation by protein kinases. Eur J Biochem. 1990;190:575–582. doi: 10.1111/j.1432-1033.1990.tb15612.x. [DOI] [PubMed] [Google Scholar]
- Takimoto E, Soergel DG, Janssen PM, Stull LB, Kass DA, Murphy AM. Frequency- and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase A phosphorylation sites. Circ Res. 2004;94:496–504. doi: 10.1161/01.RES.0000117307.57798.F5. [DOI] [PubMed] [Google Scholar]
- ter Keurs HE, Schouten VJ, Bucx JJ, Mulder BM, de Tombe PP. Excitation-contraction coupling in myocardium: implications of calcium release and Na+-Ca2+ exchange. Can J Physiol Pharmacol. 1987;65:619–626. doi: 10.1139/y87-104. [DOI] [PubMed] [Google Scholar]
- Tong CW, Gaffin RD, Zawieja DC, Muthuchamy M. Roles of phosphorylation of myosin binding protein-C and troponin I in mouse cardiac muscle twitch dynamics. J Physiol. 2004;558:927–941. doi: 10.1113/jphysiol.2004.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden J, Klein LJ, van der Bijl M, Huybregts MA, Stooker W, Witkop J, Eijsman L, Visser CA, Visser FC, Stienen GJM. Force production in mechanically isolated cardiac myocytes from human ventricular muscle tissue. Cardiovasc Res. 1998;38:414–423. doi: 10.1016/s0008-6363(98)00019-4. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Klein LJ, van der Bijl M, Huybregts MA, Stooker W, Witkop J, Eijsman L, Visser CA, Visser FC, Stienen GJM. Isometric tension development and its calcium sensitivity in skinned myocyte-sized preparations from different regions of the human heart. Cardiovasc Res. 1999;42:706–719. doi: 10.1016/s0008-6363(98)00337-x. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Klein LJ, Zaremba R, Boontje NM, Huybregts MA, Stooker W, Eijsman L, de Jong JW, Visser CA, Visser FC, Stienen GJM. Effects of calcium, inorganic phosphate, and pH on isometric force in single skinned cardiomyocytes from donor and failing human hearts. Circulation. 2001;104:1140–1146. doi: 10.1161/hc3501.095485. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Papp Z, Boontje NM, Zaremba R, de Jong JW, Janssen PM, Hasenfuss G, Stienen GJM. The effect of myosin light chain 2 dephosphorylation on Ca2+ sensitivity of force is enhanced in failing human hearts. Cardiovasc Res. 2003a;57:505–514. doi: 10.1016/s0008-6363(02)00662-4. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K, Stienen GJM. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003b;57:37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Varian KD, Janssen PM. Frequency dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol. 2007;292:H2212–2219.. doi: 10.1152/ajpheart.00778.2006. [DOI] [PubMed] [Google Scholar]
- Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered β-adrenergically mediated protein phosphorylation. J Clin Invest. 1996;98:167–176. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]