Abstract
Fetal growth is dependent on both the quantity and relative composition of amino acids delivered to the fetal circulation, and impaired placental amino acid supply is associated with restricted fetal growth. Amino acid exchangers can alter the composition, but not the quantity, of amino acids in the intra- and extracellular amino acid pools. In the placenta, exchangers may be important determinants of the amino acid composition in the fetal circulation. This study investigates the substrate specificity of exchange between the placenta and the feto-placental circulation. Maternal–fetal transfer of radiolabelled amino acids and creatinine were measured in the isolated perfused human placental cotyledon. Transfer of l-[14C]serine or l-[14C]leucine, and [3H]glycine, were measured in the absence of amino acids in the fetal circulation (transfer by non-exchange mechanisms) and following 10–20 μmol boluses of unlabelled amino acids into the fetal circulation to provide substrates for exchange (transfer by exchange and non-exchange mechanisms). The ability of fetal arterial boluses of l-alanine and l-leucine to stimulate release of amino acids from the placenta was also determined using HPLC in order to demonstrate the overall pattern of amino acid release. Experiments with radiolabelled amino acids demonstrated increased maternal–fetal transfer of l-serine and l-leucine, but not glycine, following boluses of specific amino acids into the fetal circulation. l-[14C]Leucine, but not l-[14C]serine or [3H]glycine, was transferred from the maternal to the fetal circulation by non-exchange mechanisms also (P < 0.01). HPLC analysis demonstrated that fetal amino acid boluses stimulated increased transport of a range of different amino acids by 4–7 μmol l−1 (P < 0.05). Amino acid exchange provides a mechanism to supply the fetus with amino acids that it requires for fetal growth. This study demonstrates that these transporters have the capacity to exchange micromolar amounts of specific amino acids, and suggests that they play an important role in regulating fetal plasma amino acid composition.
Fetal growth depends on nutrient supply from the mother via the placenta, and inadequate or inappropriate placental nutrient transport impairs fetal development (Constancia et al. 2002; Jansson et al. 2006). Altered fetal amino acid concentrations (Cetin et al. 1988) and impaired placental amino acid transporter activity (Glazier et al. 1997; Jansson et al. 1998) have been associated with reduced fetal growth. Both the quantity and the relative composition of amino acids transported will be important to the fetus, which has specific metabolic requirements.
Amino acid transfer to the fetus is an active process, with fetal plasma amino acids being higher than maternal plasma levels (Philipps et al. 1978). To cross the placenta, amino acids must be transported across both the maternal facing microvillous membrane (MVM) and fetal facing basal membrane (BM) of the placental syncytiotrophoblast. While net amino acid transfer must occur to support fetal growth, aspects of amino acid transport remain unclear, in particular transport across the BM. A working model for the uptake of amino acids by the MVM is that amino acids are actively transported by Na+-dependent amino acid transporters against a concentration gradient into the syncytiotrophoblast (Jansson, 2001). It is less clear how amino acids are transported across the BM into the fetus. Both Na+-dependent transporters and amino acid exchangers are known to be present on the BM. Apart from system N, which is not expressed in placenta (Nakanishi et al. 2001; Broer, 2002), Na+-dependent transporters are thought to mediate amino acid uptake into cells (in the direction of the Na+ gradient maintained by the Na+–K+-ATPase) rather than efflux. Amino acid exchangers exchange an intracellular amino acid for an extracellular amino acid. This may alter the intra- and extracellular composition of amino acids, but cannot explain the increase in size of the fetal amino acid pool. This problem suggests the existence of a BM transporter which mediates efflux from the syncytiotrophoblast (Wagner et al. 2001; Verrey, 2003). This could be a transporter such as LAT3, LAT4 or TAT1 which mediates facilitated diffusion (Bodoy et al. 2005; Ramadan et al. 2006). Alternatively, a leaky BM may allow amino acids to move across it down the placental–fetal concentration gradient (Jansson, 2001).
Amino acid exchangers are hypothesized to mediate directional transepithelial amino acid transfer of specific amino acids (Verrey, 2003). Multiple amino acid exchange transport systems have been identified in the human placenta at the protein or RNA level, some of which have been localized to the BM and MVM of the syncytiotrophoblast using immunohistochemical and functional membrane vesicle preparations (Table 1). Exchangers may therefore mediate the process by which amino acids are taken up into the placental syncytiotrophoblast across the MVM, and transported out across the BM to the fetal compartment. At term, few cytotrophoblasts remain in the human placenta, and the syncytiotrophoblast is the major trophoblast compartment (Ali, 1997). Inside the syncytiotrophoblast, metabolism of amino acids might occur as it does in the placenta of other species (Chung et al. 1998), therefore the amino acids perfused in on the maternal side of the placenta are not necessarily the amino acids that emerge on the fetal side of the placenta. No previous study has demonstrated activity of these exchange transporters in intact tissue or provided any estimate of the capacity of these transporters to exchange amino acids. If exchangers make physiologically relevant changes, i.e. changes that might be expected to alter fetal growth or metabolism, to the fetal amino acid pool, then they may be important determinants of fetal growth.
Table 1.
Amino acid exchangers in the placenta
| Membrane localization | Substrates | ||
|---|---|---|---|
| System | (Cariappa et al. 2003) | Isoform | (Broer, 2002) |
| asc | Unknown (mRNA for both isoforms is expressed) | asc1 | GLY, ALA, SER, CYS, THR |
| asc2 | GLY, ALA, SER, THR | ||
| ASC | BM | ASCT1 | ALA, SER, CYS |
| ASC2T2 | ALA, SER, CYS, THR, GLN | ||
| b0+ | BM | b0+AT | LYS, ARG, ALA, SER, CYS, THR, |
| ASN, GLN, HIS, MET, ISO, LEU, | |||
| VAL, PHE, TYR, TRP, C- | |||
| L | MVM, BM | LAT1 | GLN, HIS, MET, LEU, ISO, |
| VAL, PHE, TYR, TRP | |||
| LAT2 | ALA, SER, CYS, THR, ASN, GLN, HIS | ||
| MET, LEU, ISO, VAL, PHE, TYR, TRP | |||
| y+L | MVM, BM | y+LAT1 | LYS, ARG, GLN, HIS, MET, LEUA, |
| y+LAT2 | LYS, ARG, GLN, HIS, MET, | ||
| LEUA, ALA, CYS | |||
ALA, l-alanine; ARG, l-arginine; ASN, l-asparagine; CYS, l-cysteine; C-, l-cystine; GLU, l-glutamate; GLN, l-glutamine; GLY, glycine; HIS, l-histidine; ISO, l-isoleucine; LEU, l-leucine; LYS, l-lysine; MET, l-methionine; PHE, l-phenylalanine; SER, l-serine; THR, l-threonine; TRP, l-tryptophan; TYR, l-tyrosine; VAL, l-valine; A, y+L influx but not efflux of l-leucine (Chillaron et al. 1996).
Many tissues or metabolic processes require specific amino acids and if transport or synthesis of these amino acids is insufficient fetal growth will be impaired. For this reason the composition of amino acids in the fetal circulation is metabolically important. Although amino acid exchangers cannot increase the size of the fetal amino acid pool they may alter the composition of amino acids within the feto-placental circulation. Exchangers may allow excess fetal amino acids to be transported back to the placenta via exchangers in return for amino acids that are scarce in the fetus. Amino acid exchangers do play a crucial role by accumulating scarce essential amino acids within cells, in exchange for plentiful non-essential amino acids (Broer, 2002). Placental amino acid exchangers could thus play a vital role in maintaining the appropriate amino acid composition to match fetal metabolic demand.
Considerable work has gone into describing which amino acid transport systems are present in the placenta and thus to infer which amino acids are transported. This study takes a different approach by using the dually perfused intact human placental cotyledon to investigate which amino acids are exchanged, rather than which exchangers are present.
Methods
Placentas
Placentas were collected from normal term pregnancies immediately after delivery, following written informed consent and with the approval of the South and West Hampshire Local Research Ethics Committee.
Experimental rationale
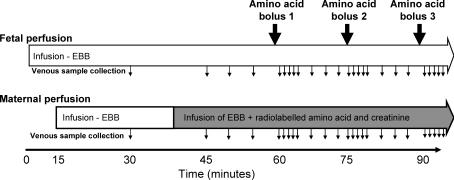
Amino acid transfer into the fetal circulation by exchange was investigated in the isolated perfused placental cotyledon. Following delivery, an intact human placental cotyledon was isolated, and the fetal and maternal circulations were re-established using a physiological buffer solution as described below. Amino acids and creatinine were perfused into the maternal circulation and transfer was indicated by their appearance in the fetal circulation. Amino acid transfer occurs by transporter-mediated mechanisms and passive diffusion, while transfer of creatinine occurs only by passive diffusion. The rate of creatinine transfer (passive diffusion) was used to distinguish between passive and transporter-mediated amino acid transfer. Uni-directional transporters transport an amino acid across a membrane, whereas exchangers exchange one amino acid from one side of the membrane for another on the other side of the membrane. Amino acid exchangers work only if there is amino acid outside the cell for exchange. In our system, amino acids were not perfused into the fetal circulation, so no substrates were available for exchange. Without amino acids in the fetal circulation any transporter-mediated exchange must be mediated by non-exchange mechanisms. To observe exchanger activity, amino acids were added to the fetal circulation. Boluses of specific amino acids were injected into the fetal circulation and an increase in transfer of the amino acids (above that seen before and after the bolus had passed through) indicated exchanger activity. To investigate amino acid specificity of exchange, multiple boluses of different amino acids were administered at 15–20 min intervals (See Fig. 1).
Figure 1. Experimental outline for the perfusion experiments.
The fetal circulation was perfused from time 0 with Earl's bicarbonate buffer (EBB) and, if venous recovery was >95%, the maternal circulation from 15 min. At 40 min, the maternal buffer was changed to a buffer containing radiolabelled amino acids and creatinine, as indicated by the shaded area. Boluses of unlabelled amino acids were injected into the fetal circulation, upstream of the pump, as indicated by the large filled arrows. Although only three boluses are shown in this diagram, up to nine boluses of different amino acids were given in any one experiment. Samples were taken from the maternal and fetal venous circulation as indicated by the small arrows.
Perfusion
Placentas were perfused using perfusion methodology (Schneider et al. 1972), as adapted in our laboratory (Edwards et al. 1993; Brownbill et al. 2000, 2003). Catheters (Portex, UK), 15 cm in length, were inserted in the fetoplacental artery (polythene tubing: i.d. 1.0 mm, o.d. 1.6 mm) and feto-placental vein (PVC tubing: i.d. 2 mm, o.d. 3 mm) of an intact cotyledon, and sutured in place. On the maternal side, five 10 cm lengths of polythene tubing (Portex; i.d. 0.58 mm, o.d. 0.96 mm) were inserted through the decidua and into the intervillous space. The fetal circulation and intervillous space were perfused with a modified Earle's bicarbonate buffer (EBB) (mm): 1.8 CaCl2, 0.4 MgSO4, 116.4 NaCl, 5.4 KCl, 26.2 NaHCO3, 0.9 NaH2PO4, 5.5 glucose, containing 35 g l−1 dextran (MW 64 000–74 000; Sigma Chemical Co., Poole, UK), 0.1% bovine serum albumin, and 5000 IU l−1 heparin, equilibrated with 95% O2–5% CO2 using roller pumps (Watson Marlow, Falmouth, UK) at 6 and 14 ml min−1, respectively. Perfusion of the fetal circulation was established first, and, if fetal venous outflow was >95% of fetal arterial inflow, the maternal-side arterial perfusion with EBB was established 15 min later. Approximately 1 ml samples of fetal and maternal venous outflow were collected.
Creatinine
Creatinine has been shown to have free permeability through the human feto-placental endothelium pores and was used as a marker of paracellular diffusion in the tracer experiments (Illsley et al. 1985). Creatinine was measured using an Infinity Creatinine assay kit (Thermo Electron, Australia) in a microplate read at 490 nm. The intra- and interassay coefficients of variation were 1.9 and 3.8%, respectively.
Maternal tracer experiments – dose-dependent amino acid exchange
Following 40 min of initial perfusion, the maternal arterial perfusion was switched to EBB containing 100 μm each of l-serine and glycine, 0.6 μm l-[14C]serine, 20 μm[3H]glycine and 1.8 mm creatinine (n= 5). Samples were collected every 5 min for 60 min. From 60 min, 10 μmol l-alanine, 20 μmol l-glutamate and 20 μmol l-alanine, prepared in 3 ml of EBB, were each administered as a separate bolus to the fetal inflow line via an injection point prior to the pump (to avoid pressure changes) over a 30 s period at 20 min intervals. The arterial boluses were followed by fetal and maternal venous sampling at +1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 13 and 17 min. Given in this way, the bolus was diluted 2.8-fold as it went through the pump and the tubing prior to entering the placental circulation. So a 20 μmol bolus gave a peak concentration 2.4 mmol l−1, and for the 10 μmol bolus the peak concentration was 1.2 mmol l−1. Appearance of l-[14C]serine and [3H]glycine were measured as counts per minute (c.p.m.) in maternal and fetal venous samples (0.5 ml sample in 8 ml; Optiphase HiSafe 2; Perkin Elmer, The Netherlands) by liquid scintillation counting (Tricarb 2100TR; Perkin Elmer Life Sciences, UK).
Maternal tracer experiments – substrate-specific amino acid exchange
Following 40 min of initial perfusion, the maternal arterial perfusion was switched to EBB containing either (a) 50 μm each of l-serine and glycine, 0.6 μm l-[14C]serine, 20 μm[3H]glycine and 1.8 mm creatinine (n= 5), or (b) 50 μm each of l-leucine and glycine, 0.6 μm l-[14C]leucine and 1.8 mm creatinine (n= 5). Sampling was continued every 5 min until 60 min.
From 60 min, each of the following amino acids or system specific amino acid analogues: l-serine, l-lysine, l-leucine, glycine, l-threonine, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH; a system L substrate), l-glutamine, l-tryptophan and l-glutamate, were administered as a separate bolus (12.5 μmol in 1.5 ml EBB) to the fetal inflow line via an injection point prior to the pump over a 15 s period. Given in this way, the bolus was diluted 7.7-fold as it went through the pump and the tubing prior to entering placental circulation. So a 12.5 μmol bolus in 1.5 ml gave a peak concentration of 1.0 mmol l−1. The boluses were given in random order at 15 min intervals followed by fetal and maternal venous sampling at +1, 2, 3, 4, 5, 8, 11 and 14 min. Amino acid boluses were chosen to cover the range of amino acid exchangers in the placenta, and l-glutamate was included as a negative control as it is not exchanged by any exchanger expressed in the placenta.
Appearance of l-[14C]serine or l-[14C]leucine, and [3H]glycine, were measured in maternal and fetal venous samples (0.5 ml in 8 ml scintillation fluid) by liquid scintillation counting.
Accumulation of radiolabel within the cotyledon
Cotyledons from the specificity of exchange experiments were homogenized in a blender with water (1:2, tissue:water). The debris was spun down, and radiolabel in the supernatant was determined by liquid scintillation counting (0.5 ml in 8 ml scintillation fluid). This was used to determine the accumulation of the maternal amino acid (nmol g−1) within the cotyledon.
Column extraction
To establish whether the radiolabel was still attached to the amino acid, and that the amino acid had not been metabolized, the amino acids were separated from the non-amino acid component of the sample. Samples were acidified with an equal volume of 1 m acetic acid, and 1 ml was applied to the column containing 1 ml Dowex 50W×8 cation exchanger. The sample was washed through with 4 ml of H2O and collected to determine unbound counts. Bound amino acids were eluted from the column with 4 ml 3 m ammonia. Appearance of 14C and 3H were measured in the eluted amino acid and non-amino acid samples (0.5 ml in 8 ml scintillation fluid) by liquid scintillation counting.
Determining range and concentration of exchanged amino acid by HPLC
The tracer studies allowed us to determine the effect of a fetal amino acid bolus on one or two maternal amino acids per experiment, but did not allow us to observe the overall pattern of amino acid release. For this reason HPLC was used to measure the release of amino acids into the fetal circulation following l-alanine and l-leucine boluses. HPLC also provided a direct quantitative measure of the concentrations of amino acids released.
Placental cotyledons (n= 5) had the fetal and maternal circulation perfused with EBB. Following 160 min of initial perfusion, fetal arterial boluses of 12.5 μmol l-alanine and 12.5 μmol l-leucine prepared in 3 ml of EBB were administered to the fetal inflow line over a 30 s period. The boluses were given at 20 min intervals followed by fetal and maternal venous sampling at +1, 2, 3, 4, 5, 10, 15 and 19 min. Samples were stored at −80°C before HPLC analysis.
HPLC
Amino acid concentrations were measured by HPLC (Godel et al. 1991) with fluorescence detection using nor-valine as an internal standard. Perfusate plus an equal volume of 6% sulphosalicylic acid containing 400 μmol l−1 nor-valine (Sigma, UK) was centrifuged to remove protein, then the supernatant underwent precolumn derivatization with o-phthaldialdehyde/3-mercaptopropionic acid at pH 9.2 (Gilson 231 programmable autosampler; Anachem, UK). After reaction for 100 s at room temperature, a 20 μl sample was injected into the HPLC system. A binary solvent system was used: solvent A, 0.1 m di-sodium hydrogen phosphate adjusted to pH 6.2 with propionic acid, methanol and tetrahydrofuran in ratio 460:40:5; solvent B, water, methanol, acetonitrile (Fisher, UK) in ratio 4:3:3. The amino acid internal standard (nor-valine) peak area ratio was calculated, and samples were quantified by comparison to the area ratios of known amino standards. The coefficient of variation of the amino acid analysis was 2–5%.
Data analysis
Data are presented as means ±s.e.m. In the HPLC experiments, the amino acid response (μmol l−1) to an amino acid bolus was expressed as change from baseline. In the maternal tracer experiments: loss of amino acids from the maternal circulation = 100 − (maternal venous sample in c.p.m. as a percentage of maternal arterial sample in c.p.m.); gain of amino acids into the fetal circulation = fetal venous sample in c.p.m. as a percentage of maternal arterial sample in c.p.m. Release of labelled amino acid into the fetal circulation (c.p.m.) following a fetal amino acid bolus was expressed as area under the response curve (AUC) calculated using the trapezium rule:
where there are n+ 1 measurements yi (c.p.m.) at times ti (min). AUC was determined over the 10 min period following the injection of the bolus with two measurements prior to this (e.g. −4 and −1 min) and following this (e.g. +11 and +14 min) used to calculate the baseline. Measurements of c.p.m. in the maternal perfusate allowed this value to be converted to nanomoles.
Data were compared to glutamate using ANOVA and a Dunnetts post hoc test (SPSS, Chicago, Illinois, USA). Significance was accepted when P < 0.05.
Results
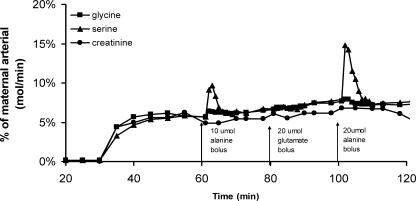
Mean perfused cotyledon size was 26.7 ± 2.3 g, mean fetal venous outflow was 98.9 ± 0.5% of fetal arterial inflow, and unidirectional materno-fetal clearance of creatinine normalized to wet cotyledon weight was 30.9 ± 4.0 μl min−1 g−1. These values represent all placental cotyledons, as no differences occurred between experimental sets. An example experiment showing the appearance of substrates in the fetal venous circulation following perfusion into the maternal artery is shown in Fig. 2.
Figure 2. Representative perfusion experiment showing l-[14C]serine, [3H]glycine and creatinine in the fetoplacental venous perfusate as a percentage of substrate perfused into the maternal circulation.
Perfusion of l-serine glycine and creatinine into the maternal arterial circulation was started at 40 min from the start of fetal perfusion. At 60, 80 and 100 min, 3 ml boluses containing 10 μmol l-alanine, 20 μmol l-glutamate, and 20 μmol l-alanine, respectively, were injected into the fetal artery over 30 s.
Maternal tracer experiments – dose-dependent amino acid exchange
Placental uptake of l-serine and glycine from the maternal circulation in the dose-dependency experiments
At baseline (10 min period before each amino acid bolus) the proportion of creatinine taken up by the placenta from the maternal circulation was 11.2 ± 2.9%; as compared with this, a greater proportion of maternal l-serine (22.2 ± 1.5% of maternal perfusate (in nmol min−1), P < 0.01) was taken up by the placenta from the maternal circulation but there was no significant difference in glycine uptake (14.3 ± 0.9%).
Release of l-serine and glycine into the feto-placental circulation in the dose-dependency experiments
At baseline there were no significant differences in the proportions of maternal l-serine (8.6 ± 0.5% of maternal perfusate (in nmol min−1)) or glycine (8.3 ± 1.0%) transferred from the maternal to the fetal circulation as compared with creatinine transfer (8.2 ± 1.1%).
Dose-dependent amino acid exchange
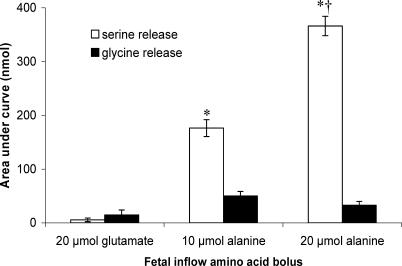
Following a fetal bolus of 20 μmol l-glutamate there was no increase in either l-serine or glycine transfer compared with baseline (n= 5; Fig. 3). Following fetal boluses of 10 or 20 μmol l-alanine, there were significant increases in the amount of l-serine appearing in fetal venous outflow (P < 0.01 compared with 20 μmol l-glutamate; Fig. 4). The amount of l-serine appearing in the fetal circulation following the 20 μmol bolus was significantly greater than that following the 10 μmol bolus (P < 0.01; Fig. 3).
Figure 3. l-Serine and glycine released into the fetal circulation following fetal arterial bolus of l-glutamate or l-alanine.
Data (mean ±s.e.m., n= 5) are presented as area under the curve (AUC). l-Glutamate is not a substrate for the amino acid exchangers that transport l-serine and glycine. *P < 0.05, AUC is significantly different from l-glutamate. †P < 0.05, l-serine release is significantly different from that seen after a 10 μmol l-alanine bolus.
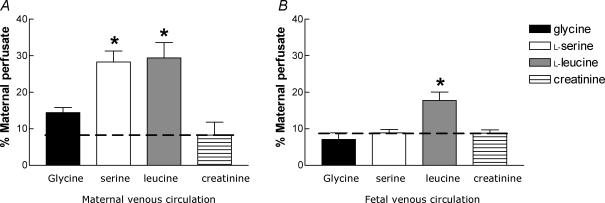
Figure 4. Maternal uptake and placental transfer of amino acids and creatinine.
A, loss from the maternal circulation; B, release into the fetal venous circulation of l-serine, l-leucine, glycine and creatinine (mean ±s.e.m., n= 5) as a percentage of substrate perfused into the maternal circulation. As creatinine is not taken up by cells, its transfer represents the rate of paracellular diffusion. Uptake or release above that of creatinine (indicated by the dashed line) indicates active transport. *P < 0.01, significantly different to their respective creatinine measurements (l-serine and glycine were in separate experiments to l-leucine).
Following fetal amino acid boluses, maternal venous l-serine and glycine levels were not significantly different from baseline. The AUCs for maternal venous l-serine following 20 μmol l-glutamate, 10 μmol l-alanine and 20 μmol l-alanine were 9.9 ± 12.0, 3.4 ± 14.9 and 0.8 ± 9.7 nmol, respectively (n= 5). The AUCs for maternal venous glycine following 20 μmol l-glutamate, 10 μmol l-alanine and 20 μmol l-alanine were 3.2 ± 3.7, 0.7 ± 11.7 and −0.4 ± 8.1 nmol, respectively (n= 5).
Maternal tracer experiments – substrate-specific amino acid exchange
Placental uptake of l-leucine, l-serine and glycine from the maternal circulation in the substrate specificity experiments
At baseline (10 min before the first amino acid bolus) a greater proportion of maternal l-leucine and l-serine was taken up by the placenta from the maternal circulation as compared with creatinine uptake (P < 0.01, Fig. 4A), but there was no significant difference in glycine uptake.
Release of l-leucine, l-serine and glycine into the feto-placental circulation in the substrate-specificity experiments
At baseline, a significantly greater proportion of maternal l-leucine, but not l-serine or glycine, was transferred from the maternal to the fetal circulation as compared with creatinine transfer (P < 0.01, Fig. 4B).
Substrate-specific amino acid exchange
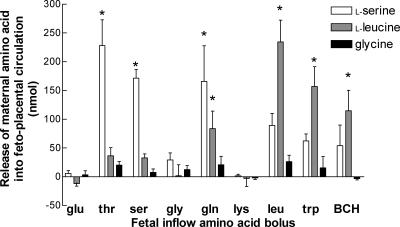
In response to fetal boluses of specific amino acids, maternal l-serine and l-leucine, but not glycine, were released into the feto-placental circulation (Fig. 5). Following fetal boluses of 12.5 μmol l-threonine, l-serine or l-glutamine, there were significant increases in the amount of l-serine appearing in fetal venous outflow (P < 0.01 compared with 12.5 μmol l-glutamate, Fig. 5). Following fetal boluses of 12.5 μmol l-leucine, l-tryptophan or BCH, there were significant increases in the amount of l-leucine appearing in fetal venous outflow (P < 0.01 compared with 12.5 μmol l-glutamate, Fig. 5).
Figure 5. l-Serine, l-leucine and glycine release into the fetal circulation following a fetal amino acid bolus.
Data (mean ±s.e.m., n= 5) are presented as AUC. *P < 0.05, the AUC is different from that of l-glutamate, which is not a substrate for amino acid exchangers.
Maternal venous l-serine, l-leucine and glycine levels following fetal amino acid boluses were not significantly different from those following a l-glutamate bolus (data not shown).
Determining range and concentration of exchanged amino acid by HPLC
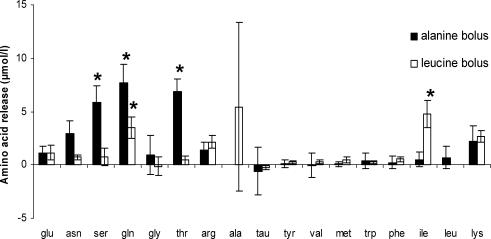
Following a fetal bolus of 12.5 μmol l-alanine, there were significant increases in the amounts of l-serine, l-glutamine and l-threonine appearing in fetal venous outflow (P < 0.05 compared with the change in the appearance of l-glutamate, Fig. 6). Following a fetal bolus of 12.5 μmol l-leucine, there were significant increases in the amounts of l-glutamine and l-isoleucine appearing in fetal venous outflow (P < 0.05 compared with appearance of l-glutamate, Fig. 6).
Figure 6. Amino acids released into the fetal circulation following a fetal amino acid bolus of l-alanine and l-leucine.
HPLC data (mean ±s.e.m., n= 5) presented as change from baseline. *P < 0.05, the amino acid release is different from that of l-glutamate, which is not a substrate for amino acid exchangers. l-Alanine and l-leucine bars have been removed following their respective boluses.
Accumulation of radiolabel within the cotyledon
Accumulation of label within the cotyledon was determined for the substrate specificity experiments (n= 5). There was accumulation of 126.7 ± 25.2 nmol g−1l-serine, 44.9 ± 16.2 nmol g−1 glycine and 55.2 ± 14.1 nmol g−1l-leucine. Accumulation of l-serine was significantly greater than for glycine or l-leucine (P < 0.001).
Amino acid metabolism by the placenta
Amino acids in the infused buffer and fetal venous exudate were bound to a column to determine how much of the label was incorporated within amino acids. For l-serine and l-leucine, the proportion of radiolabel binding to the column (i.e. incorporated within amino acids) going into the placenta was not significantly different to that coming out the fetal vein, indicating that they were not being metabolized in the placenta (one-way ANOVA; Table 2). For glycine, there was a significant fall in the proportion of radiolabel binding the column in the fetal perfusate coming out of the placenta compared with that going in, suggesting that it may be being metabolized within the placenta (P < 0.001, Mann–Whitney U test; Table 2).
Table 2.
Proportion of radiolabel incorporated in amino acids within the maternal perfusate and fetal venous outflow
| Amino acid | Maternal perfusate (n= 4) | Baseline perfusate (n= 4) | Following boli (n= 4) |
|---|---|---|---|
| l-[14C]Serine | 98.3 ± 0.5% | 97.3 ± 0.9% | 97.6 ± 0.7% |
| l-[14C]Leucine | 97.1 ± 1.8% | 92.5 ± 2.6% | 96.6 ± 1.6% |
| [3H]Glycine | 87.0 ± 0.4% | 77.9 ± 3.2%* | NA |
Data are means ±s.e.m. NA, not applicable. There was no significant difference between maternal perfusate, baseline or samples taken following a bolus of l-serine or l-leucine, one-way ANOVA.
P < 0.001, baseline significantly different to maternal perfusate for glycine, Mann–Whitney U test.
Discussion
This study demonstrates substrate-specific amino acid exchange between the placenta and the feto-placental circulation. Experiments with radiolabelled amino acids demonstrated significant increases in maternal–fetal transfer of l-serine and l-leucine, but not glycine, following boluses of specific amino acids into the fetal circulation. The HPLC studies demonstrated that following a fetal amino acid bolus there was exchange of a select range of amino acids, they also provide a direct measure of the change in amino acid concentrations due to exchange.
Amino acid release from the syncytiotrophoblast into the feto-placental circulation following a fetal arterial amino acid bolus strongly indicates exchange. This is supported by the observations that the exchange was substrate specific, dose dependent and not associated with a corresponding loss of substrate from the maternal circulation. The radiolabel studies showed increases in the amount of maternal l-serine appearing in fetal venous outflow in response to fetal boluses of l-threonine, l-serine or l-glutamine, and increases in the amount of maternal l-leucine appearing in fetal venous outflow following fetal boluses of l-leucine, l-tryptophan or BCH. This provides further insight into which amino acid transporters are functioning in the BM of the intact human placenta. The radiolabel in the fetal circulation was shown by column chromatography to be incorporated in amino acids, and not a breakdown product. Therefore, although the net amount of a particular amino acid delivered to the fetal circulation is determined by transplacental flux from the maternal to fetal circulation, backflux from the fetal circulation to the placenta and the flux of amino acid derived from placental protein breakdown and placental amino acid synthesis, these maternal tracer experiments specifically measure the transport of the maternal (labelled) amino acid.
The radiolabel experiments were supported by the HPLC experiments, which showed exchange of a range of different amino acids from within the placental syncytiotrophoblast to the fetal circulation following fetal amino acid boluses. The HPLC experiments demonstrated specific and distinct profiles: l-alanine exchanged for l-glutamine, l-threonine and l-serine, whereas l-leucine exchanged for l-glutamine and l-isoleucine. The HPLC data indicate that amino acid exchangers have the capacity to alter the amino acid composition of certain amino acids within the feto-placental circulation by 5–7 μmol l−1. This could be an important determinant of fetal plasma amino acid composition as the average umbilical arterial–venous (AV) difference for amino acids in humans is around 9 μmol l−1 with a range of −8.35 to 32.29 μmol l−1 (Cetin et al. 1988) and amino acid accretion rates in the term fetus are between 0.5 and 6.5 mmol day−1 (average 2 mmol day−1) which equates to a required umbilical AV difference of 1–13 μmol l−1 (Widdowson et al. 1979). However, it is unclear whether the concentrations of specific amino acids would be altered to the same degree in the presence of physiological concentrations of amino acids.
Exchangers may allow amino acids with a greater abundance in the fetal circulation to be transported back to the placenta in return for amino acids that are less abundant in the fetal circulation, particularly essential amino acids that cannot be synthesized by the fetus. This may be a mechanism to maintain the appropriate levels of specific amino acids vital for fetal protein accretion, as metabolic precursors and for energy metabolism. Certain tissues preferentially metabolize specific amino acids, for instance l-glutamine (Newsholme et al. 1985; Jungas et al. 1992), and as amino acids are not necessarily supplied in the quantities that they are required the feto-placental unit must have mechanisms to alter the composition of the amino acid pool.
Not all the potential amino acid exchanges that might have been expected to occur were observed. Most notably, no glycine exchange was observed despite its metabolic importance to the fetus (Jackson, 1991) and its high levels in fetal plasma. Glycine could potentially be transported to the fetus by the exchanger LAT2, which mediates glycine efflux and is thought to be localized on the BM (Kudo & Boyd, 2001) or by system asc, although the latter has not been localized to the BM (Fukasawa et al. 2000; Chairoungdua et al. 2001). A combination of lower uptake of maternal glycine, greater effective intracellular tracer dilution (due to lower glycine uptake and an intracellular glycine concentration twice that of l-serine; Philipps et al. 1978), metabolism of glycine by the placenta and a more restricted range of lower-affinity BM exchangers could all contribute to the lack of glycine transport by exchange. Consistent with the findings of this study, uptake of glycine into MVM vesicles is lower than for l-serine (Lewis et al. 2006).
Several combinations of amino acids, other than those involving glycine, were also predicted to exchange based on our understanding of the affinities of amino acid transport systems. Fetal l-threonine would be expected to exchange for placental l-leucine via system L (LAT2) and system b0+ as they are both substrates for these exchangers, and both system L and b0+ activities have previously been reported in BM vesicle preparations (Furesz & Smith, 1997; Kudo & Boyd, 2001). Fetal l-lysine would be expected to exchange for placental l-serine via system b0+, and for placental l-leucine via system b0+ and possibly system y+L, as system y+L activity has also been shown in BM vesicle preparations (Ayuk et al. 2000). System y+L isoforms y+LAT1 (Chillaron et al. 1996) and y+LAT2 (Broer et al. 2000) have a low ability to mediate l-leucine efflux, which may explain our observations; however, it is unclear why we did not see exchange indicative of system b0+. Fetal l-leucine, l-tryptophan and BCH would be expected to exchange for placental l-serine via LAT2 (there was some release apparent here but it was not statistically significant). Further anomalies were observed with our HPLC data, where l-leucine stimulated exchange of l-isoleucine but not l-valine, two amino acids transported by LAT2. Therefore, in our system, there were discrepancies between what was observed and what might have been expected for systems L (LAT2), b0+ and possibly y+L. As we have shown that the system-L-specific substrate BCH stimulates exchange, we know that at least one system L isoform is active in our preparation. The pattern of exchange we observed would be more consistent for LAT1 than for LAT2, but it is LAT2 that is reported to be localized to the BM (Kudo & Boyd, 2001).
While this experiment does not directly demonstrate which placental cell type mediates amino acid exchange, we believe it is primarily the syncytiotrophoblast. This has a large surface area directly exposed to substrate in the maternal circulation, making it the most likely site for uptake. Given that the peak release following an amino acid bolus was 15–20% of the maternal concentration it is unlikely that endothelial cells could take up enough maternal substrate to replenish it in the experiments with multiple consecutive fetal boluses. The rapid appearance of l-serine and l-leucine in the fetal circulation following the fetal boluses suggests that the fetal endothelium does not provide a significant barrier to the transport of amino acids. Peak bolus delivery was at 2.5 min with 30 s sampling, and peak l-serine and l-leucine release following a bolus was at 3 min with 1 min sampling. Transit through the endothelial layer, exchange with the BM, transit back to the capillary and transit through the cotyledon must all occur in under 25 s, and potentially much less. To investigate this further, future studies require an increased sampling frequency and a marker to determine transit time through the cotyledon.
l-Serine and glycine were not actively transported from the placenta to the feto-placental circulation. This also suggests that there is no non-specific amino acid diffusion from the placenta into the feto-placental circulation. In sheep maternal l-serine is converted to glycine within the placenta by the enzyme serine hydroxymethyltransferase (SHMT) and this glycine, not maternal glycine, is transported to the fetus (Chung et al. 1998). If this were the case in humans we would not necessarily expect to see transport of glycine in this model and what we thought was glycine may actually be l-serine. However, we have previously demonstrated that in the human placenta SHMT activity is 24 times lower than in the sheep, and we do not think that this is a major metabolic pathway in human placenta (Lewis et al. 2005).
This study demonstrated efflux of l-leucine from the placenta into the feto-placental circulation that did not appear to be mediated by exchangers. This is consistent with in vivo studies in human pregnancies which use stable isotope methodology to show that transplacental transfer of l-leucine is rapid, whereas that of glycine is more limited (Cetin et al. 1995). It is not yet clear which amino acid transporter is mediating this non-exchange l-leucine efflux. One strong candidate is LAT4, which is expressed in placenta and is thought to mediate facilitated diffusion of specific amino acids including leucine (Bodoy et al. 2005). Another possibility is transport by TAT1, a bidirectional transporter of aromatic amino acids which is expressed in placenta and has also been shown to have some leucine efflux activity (Ramadan et al. 2006).
However this l-leucine is being transported across the BM, it is resulting in a quantitative increase in the amount of amino acids in the fetal circulation. Amino acid exchangers on the BM of the syncytiotrophoblast will then be able to exchange this l-leucine for other amino acids for which no BM efflux mechanisms exist, resulting in qualitative change in the fetal plasma amino acid pool.
Transport of l-leucine may be important for fetal growth as in vivo human studies show that intrauterine growth restricted (IUGR) human fetuses have impaired l-leucine, but not glycine, transplacental flux (Paolini et al. 2001), and studies using MVM and BM vesicles show poor l-leucine uptake in vesicles from IUGR pregnancies (Jansson et al. 1998). IUGR is commonly associated with a marked decrease in system A activity in the MVM (Mahendran et al. 1993; Glazier et al. 1997; Jansson et al. 2002). System A activity will transport amino acids into the syncytiotrophoblast creating gradients that are used by exchangers to drive uptake of other amino acids including l-leucine (Jansson et al. 1998). If system A is not working efficiently, the gradients of amino acids required for exchange across the MVM, and possibly also efflux across the BM via facilitated diffusion through LAT4 and TAT1, may not be generated. Interestingly, maternal amino acid supplementation in IUGR pregnancies elevated umbilical vein concentrations of l-leucine, l-serine and glycine following amino acid infusion into the maternal circulation (Ronzoni et al. 2002). While the current study demonstrated transporter-mediated routes for l-leucine and l-serine transfer to the fetus, it is unclear how glycine would get across the placenta other than by paracellular routes.
The differences in uptake and release of the three amino acids in the present study correspond with the measurements of label retained within the cotyledon. There was enrichment of l-serine above that of glycine and l-leucine, indicating that although l-serine uptake from the maternal circulation was equivalent to that of l-leucine, the l-leucine was being transferred to the fetal circulation preventing its build up within the placenta. In contrast, glycine enrichment was lower than for l-serine, and this corresponds with the lower placental glycine uptake.
In summary, while amino acid exchangers have been functionally and immunologically localized to the BM, this is the first demonstration that exchangers are functional in the intact placenta, and of the capacity of exchangers to mediate amino acid exchange. Amino acid exchangers in the placenta may function to take up amino acids required in lower quantities by the fetus in exchange for placental amino acids for which the fetus has a high metabolic requirement. l-Leucine transport across the placenta to the fetus by mechanisms other than exchange was also observed, which may provide a driver for net placental amino acid efflux across the BM. Further studies are now needed to determine the specificity of exchange for a wider range of amino acids, and to determine which amino acids other than l-leucine are transported out of the placenta by non-exchange efflux mechanisms. Until we determine which amino acids are transported to the fetus by unidirectional efflux from the placenta, we cannot fully understand the role of amino acid exchange in relation to observed umbilical AV differences of amino acids in the fetal circulation (Hayashi et al. 1978; Cetin et al. 1988).
Acknowledgments
We would like to acknowledge midwives at the Princess Anne Hospital, Southampton, for their help in collecting placentas, and The Henry Smith Charity for funding this work. M.A.H. is supported by the British Heart Foundation.
References
- Ali KZ. Stereological study of the effect of altitude on the trophoblast cell populations of human term placental villi. Placenta. 1997;18:447–450. doi: 10.1016/s0143-4004(97)80046-x. [DOI] [PubMed] [Google Scholar]
- Ayuk PT, Sibley CP, Donnai P, D'Souza S, Glazier JD. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol. 2000;278:C1162–C1171. doi: 10.1152/ajpcell.2000.278.6.C1162. [DOI] [PubMed] [Google Scholar]
- Bodoy S, Martin L, Zorzano A, Palacin M, Estevez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Broer S. Adaptation of plasma membrane amino acid transport mechanisms to physiological demands. Pflugers Arch. 2002;444:457–466. doi: 10.1007/s00424-002-0840-y. [DOI] [PubMed] [Google Scholar]
- Broer A, Wagner CA, Lang F, Broer S. The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem J. 2000;349:787–795. doi: 10.1042/bj3490787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownbill P, Bell NJ, Woods RJ, Lowry PJ, Page NM, Sibley CP. Neurokinin B is a paracrine vasodilator in the human fetal placental circulation. J Clin Endocrinol Metab. 2003;88:2164–2170. doi: 10.1210/jc.2002-021727. [DOI] [PubMed] [Google Scholar]
- Brownbill P, Mahendran D, Owen D, Swanson P, Thornburg KL, Nelson DM, Sibley CP. Denudations as paracellular routes for a-fetoprotein and creatinine across the human syncytiotrophoblast. Am J Physiol Regul Integr Comp Physiol. 2000;278:R677–R683. doi: 10.1152/ajpregu.2000.278.3.R677. [DOI] [PubMed] [Google Scholar]
- Cariappa R, Heath-Monnig E, Smith CH. Isoforms of amino acid transporters in placental syncytiotrophoblast: plasma membrane localization and potential role in maternal/fetal transport. Placenta. 2003;24:713–726. doi: 10.1016/s0143-4004(03)00085-7. [DOI] [PubMed] [Google Scholar]
- Cetin I, Marconi AM, Baggiani AM, Buscaglia M, Pardi G, Fennessey PV, Battaglia FC. In vivo placental transport of glycine and leucine in human pregnancies. Pediatr Res. 1995;37:571–575. doi: 10.1203/00006450-199505000-00002. [DOI] [PubMed] [Google Scholar]
- Cetin I, Marconi AM, Bozzetti P, Sereni LP, Corbetta C, Pardi G, Battaglia FC. Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am J Obstet Gynecol. 1988;158:120–126. doi: 10.1016/0002-9378(88)90792-2. [DOI] [PubMed] [Google Scholar]
- Chairoungdua A, Kanai Y, Matsuo H, Inatomi J, Kim DK, Endou H. Identification and characterization of a novel member of the heterodimeric amino acid transporter family presumed to be associated with an unknown heavy chain. J Biol Chem. 2001;276:49390–49399. doi: 10.1074/jbc.M107517200. [DOI] [PubMed] [Google Scholar]
- Chillaron J, Estevez R, Mora C, Wagner CA, Suessbrich H, Lang F, Gelpi JL, Testar X, Busch AE, Zorzano A, Palacin M. Obligatory amino acid exchange via systems bo,+-like and y+L-like. A tertiary active transport mechanism for renal reabsorption of cystine and dibasic amino acids. J Biol Chem. 1996;271:17761–17770. doi: 10.1074/jbc.271.30.17761. [DOI] [PubMed] [Google Scholar]
- Chung M, Teng C, Timmerman M, Meschia G, Battaglia FC. Production and utilization of amino acids by ovine placenta in vivo. Am J Physiol Endocrinol Metab. 1998;274:E13–E22. doi: 10.1152/ajpendo.1998.274.1.E13. [DOI] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- Edwards D, Jones CJ, Sibley CP, Nelson DM. Paracellular permeability pathways in the human placenta: a quantitative and morphological study of maternal–fetal transfer of horseradish peroxidase. Placenta. 1993;14:63–73. doi: 10.1016/s0143-4004(05)80249-8. [DOI] [PubMed] [Google Scholar]
- Fukasawa Y, Segawa H, Kim JY, Chairoungdua A, Kim DK, Matsuo H, Cha SH, Endou H, Kanai Y. Identification and characterization of a Na+-independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral d- and l-amino acids. J Biol Chem. 2000;275:9690–9698. doi: 10.1074/jbc.275.13.9690. [DOI] [PubMed] [Google Scholar]
- Furesz TC, Smith CH. Identification of two leucine-sensitive lysine transport activities in human placental basal membrane. Placenta. 1997;18:649–655. doi: 10.1016/s0143-4004(97)90006-0. [DOI] [PubMed] [Google Scholar]
- Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42:514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- Godel H, Graser T, Foldi P, Pfaender P, Furst P. Measurement of free amino acids in human biological fluids by high-performance liquid chromatography. J Chromatogr. 1991;564:81–91. doi: 10.1016/s0021-9673(01)89028-2. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Sanada K, Sagawa N, Yamada N, Kido K. Umbilical vein–artery differences of plasma amino acids in the last trimester of human pregnancy. Biol Neonate. 1978;34:11–18. doi: 10.1159/000241099. [DOI] [PubMed] [Google Scholar]
- Illsley NP, Hall S, Penfold P, Stacey TE. Diffusional permeability of the human placenta. Contrib Gynecol Obstet. 1985;13:92–97. [PubMed] [Google Scholar]
- Jackson AA. The glycine story. Eur J Clin Nutr. 1991;45:59–65. [PubMed] [Google Scholar]
- Jansson T. Amino acid transporters in the human placenta. Pediatr Res. 2001;49:141–147. doi: 10.1203/00006450-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Downregulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ylven K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- Jungas RL, Halperin ML, Brosnan JT. Quantitative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol Rev. 1992;72:419–448. doi: 10.1152/physrev.1992.72.2.419. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA. Characterisation of l-tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles. J Physiol. 2001;531:405–416. doi: 10.1111/j.1469-7793.2001.0405i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RM, Glazier JD, Greenwood SL, Bennet EJ, Godfrey KM, Jackson AA, Sibley CP, Cameron IT, Hanson MA. l-Serine uptake by human placental microvillous membrane vesicles. Placenta. 2006;28:445–253. doi: 10.1016/j.placenta.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Godfrey KM, Jackson AA, Cameron IT, Hanson MA. Low serine hydroxymethyltransferase activity in the human placenta has important implications for fetal glycine supply. J Clin Endocrinol Metab. 2005;90:1594–1598. doi: 10.1210/jc.2004-0317. [DOI] [PubMed] [Google Scholar]
- Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34:661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Sugawara M, Huang W, Martindale RG, Leibach FH, Ganapathy ME, Prasad PD, Ganapathy V. Structure, function, and tissue expression pattern of human SN2, a subtype of the amino acid transport system N. Biochem Biophys Res Commun. 2001;281:1343–1348. doi: 10.1006/bbrc.2001.4504. [DOI] [PubMed] [Google Scholar]
- Newsholme EA, Crabtree B, Ardawi MS. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J Exp Physiol. 1985;70:473–489. doi: 10.1113/expphysiol.1985.sp002935. [DOI] [PubMed] [Google Scholar]
- Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2001;86:5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- Philipps AF, Holzman IR, Teng C, Battaglia FC. Tissue concentrations of free amino acids in term human placentas. Am J Obstet Gynecol. 1978;131:881–887. doi: 10.1016/s0002-9378(16)33136-2. [DOI] [PubMed] [Google Scholar]
- Ramadan T, Camargo SM, Summa V, Hunziker P, Chesnov S, Pos KM, Verrey F. Basolateral aromatic amino acid transporter TAT1 (Slc16a10) functions as an efflux pathway. J Cell Physiol. 2006;206:771–779. doi: 10.1002/jcp.20531. [DOI] [PubMed] [Google Scholar]
- Ronzoni S, Marconi AM, Paolini CL, Teng C, Pardi G, Battaglia FC. The effect of a maternal infusion of amino acids on umbilical uptake in pregnancies complicated by intrauterine growth restriction. Am J Obstet Gynecol. 2002;187:741–746. doi: 10.1067/mob.2002.124291. [DOI] [PubMed] [Google Scholar]
- Schneider H, Panigel M, Dancis J. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am J Obstet Gynecol. 1972;114:822–828. doi: 10.1016/0002-9378(72)90909-x. [DOI] [PubMed] [Google Scholar]
- Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445:529–533. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- Wagner CA, Lang F, Broer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol. 2001;281:C1077–C1093. doi: 10.1152/ajpcell.2001.281.4.C1077. [DOI] [PubMed] [Google Scholar]
- Widdowson EM, Southgate DAT, Hey NT. Body composition of the fetus and infant. In: Visser HKA, editor. Nutrition and Metabolism of the Fetus. The Hague: Matinus Nigoff; 1979. pp. 169–177. [Google Scholar]