Abstract
Age-related physiological and morphological changes of muscle spindles were examined in rats (male Fischer 344/DuCrj: young, 4–13 months; middle-aged, 20–22 months; old, 28–31 months). Single afferent discharges of the muscle spindles in gastrocnemius muscles were recorded from a finely split dorsal root during ramp-and-hold (amplitude, 2.0 mm; velocity, 2–20 mm s−1) or sinusoidal stretch (amplitude, 0.05–1.0 mm; frequency, 0.5–2 Hz). Respective conduction velocities (CVs) were then measured. After electrophysiological experimentation, the muscles were dissected. The silver-impregnated muscle spindles were teased and then analysed using a light microscope. The CV and dynamic response to ramp-and-hold stretch of many endings were widely overlapped in old rats because of the decreased CV and dynamic response of primary endings. Many units in old rats showed slowing of discharge during the release phase under ramp-and-hold stretch and continuous discharge under sinusoidal stretch, similarly to secondary endings in young and middle-aged rats. Morphological studies revealed that primary endings of aged rat muscle spindles were less spiral or non-spiral in appearance, but secondary endings appeared unchanged. These results suggest first that primary muscle spindles in old rats are indistinguishable from secondary endings when determined solely by previously used physiological criteria. Secondly, these physiological results reflect drastic age-related morphological changes in spindle primary endings.
Postural sway and gait stability deterioration that occur with ageing have been studied extensively (Overstall et al. 1977; Alexander, 1994; Hausdorff et al. 1997; Hurley et al. 1998). It has been considered that peripheral proprioceptors, particularly muscle spindles, play an important role in the detection of both passive and active movements (Proske et al. 2000), maintenance of postural stability (Lord & Ward, 1994), and control of posture and balance during the swing phase of locomotion (Sorensen et al. 2002). For that reason, it is probable that age-related changes of muscle spindles engender a decline in postural and locomotion control among elderly people. A previous physiological study (Miwa et al. 1995) revealed that the dynamic and static length sensitivities of muscle spindle primary endings in response to ramp stretch were decreased in aged rats. Furthermore, recent morphological studies have revealed that sensory nerve endings which are immunoreactive to PGP9.5 showed degeneration with ageing (Yamamoto et al. 2003; Winarakwong et al. 2004). However, little is known about actual effects of ageing on the morphology-function relationship in muscle spindles. Functional and structural changes reportedly occur in peripheral nerves of old subjects: a decline in nerve conduction velocity (Lafratta & Canestrari, 1966; Dorfman & Bosley, 1979; Chase et al. 1992), axonal atrophy (Ochoa & Mair, 1969; Knox et al. 1989; Chase et al. 1992), a decline in internodal length (Lascelles & Thomas, 1966), and demyelination (Knox et al. 1989; Adinolfi et al. 1991). Age-related function and structural alterations at the neuromuscular junction and in spinal cord motor neurons have also been investigated: skeletal muscle denervation and re-innervation, and motor unit remodelling or loss in ageing rats or humans (Hashizume et al. 1988; Kanda & Hashizume, 1989, 1992; Einsiedel & Luff, 1992; Doherty et al. 1993). Functional deficits might be the consequence of structural changes that engender a slowly progressive loss of neurons and nerve fibres (Verdu et al. 2000). The present study was intended to extend the knowledge of age-related changes in physiological properties and structure in muscle spindles.
Muscle spindles have two kinds of endings, which are supplied, respectively, by Ia and II fibres: primary and secondary endings. Morphologically, primary endings consist of spiral or annular terminations on each intrafusal fibre in the equatorial region, whereas secondary endings have extensive irregular spiral or annular terminations on chain fibres and some spray-like terminations on bag fibres in the juxta-equatorial region (Ruffini, 1898; Boyd, 1962, Boyd, 1985; Banks et al. 1982; Hunt, 1990). Electrophysiological experiments have classified primary and secondary endings, usually based on their differences in conduction velocity (CV) and response to ramp-and-hold or sinusoidal stretches. Primary endings show faster CV, higher dynamic response, and a narrower linear range on sinusoidal stretch. In contrast, secondary endings show slower CV, lower dynamic response, and a broader linear range on sinusoidal stretch (Matthews, 1963; Matthews & Stein, 1969; Hunt & Ottoson, 1975; Cheney & Preston, 1976; Barker et al. 1986; Wei et al. 1986; Scott, 1990; De-Doncker et al. 2003). Miwa et al. (1995) assumed that muscle spindles showing abrupt cessation of firing during the release phase were primary endings and that those showing slowing of firing were secondary endings. However, that study examined few samples. Moreover, different from the case of young rats, it was difficult to use the CV and dynamic index in old rats because no clear transition point was found. The possibility remains that the sample of Miwa and colleagues was biased in preference to select in favour of secondary endings. Whether or not the current criteria are generally available for classification of primary and secondary endings in muscle spindles of old rats remains to be clarified. The first aim of the present study is to examine whether or not electrophysiological criteria, e.g. CV, response to ramp-and-hold and response to sinusoidal stretches, or their combination, are useful for classifying muscle spindles of old rats into primary and secondary endings. The muscle depolarizing drug succinylcholine (SCh) has been used for distinguishing the afferents of primary and secondary endings: SCh greatly increases the dynamic response of primary endings to muscle stretch relative to that of secondary endings (Rack & Westbury, 1966; Cody et al. 1972; Inoue et al. 1981; Gregory & Proske, 1987; Kishimoto et al. 1998). In addition, we attempted to classify spindle endings by their dynamic response change following injection of SCh. The second aim of this study is the investigation of age-related morphological changes in sensory endings by teased silver-impregnated muscle spindles. Through addition of morphological quantitative studies of spindle afferents, we re-examined age-related changes of muscle spindles in old rats.
Methods
Animals
Experiments used male Fischer 344/DuCrj rats of three age groups: young (4–13.5 months of age; body weight, 412.2 ± 41.3 g; 36 rats), middle-aged (20–22 months of age; body weight, 434.2 ± 21.5 g; 13 rats), and old (28–31 months of age; body weight, 388.9 ± 37.9 g; 19 rats). They had been raised under SPF conditions with ad libitum access to food. The mean survival time of this strain in the animal facility is about 28 months. All procedures were approved by the Committee of Animal Care of the Tokyo Metropolitan Institute of Gerontology.
Surgical and experimental procedures
Rats were anaesthetized using pentobarbital sodium (young, middle-aged, 50 mg kg−1i.p.; old, 25 mg kg−1i.p.). Adequate depth of anaesthesia was monitored frequently by checking the pupil size and the flexion reflex to paw pinch. Especially, before giving the muscle relaxant (SCh), it was confirmed that anaesthesia was sufficiently deep to suppress the flexion reflex to paw pinch. Supplementary injections (10 mg kg−1) were administered when necessary. After the experiment, each rat was killed using an overdose of pentobarbital sodium (100 mg kg−1).
The trachea, a common carotid artery and an external jugular vein were cannulated. The rats were ventilated artificially and their arterial blood pressure was monitored. The left gastrocnemius muscle was isolated from the surrounding tissue. Except for the nerve to the gastrocnemius muscle, the left hip and hindlimb muscles were denervated. The calcaneal tendon was removed from the calcaneum and was attached to a stretcher using a suture hook. Before detaching the tendon from the calcaneum, the gastrocnemius muscle length was measured. The leg and lumbosacral spine were immobilized in a metal frame using pins and clamps. The lumbosacral spinal cord was exposed by a laminectomy and both ventral and dorsal roots L4–S1 were severed near the entry to the spinal cord. The exposed area of the spinal cord and limb were covered with pools of mineral oil warmed to 35–38°C. Rectal temperature was monitored and controlled to 36–38°C using a heating blanket and infrared radiation. In some experiments, lactated Ringer solution (Otsuka Pharmaceutical Co. Ltd) or 4% Ficoll (Pharmacia Fine Chemicals) solution was infused (0.69–1.3 ml h−1) to maintain adequate blood pressure. Single afferent discharges of the muscle spindles in the gastrocnemius muscle were recorded from a finely split dorsal root (L4–L6). Muscle spindles were distinguished from Golgi tendon organs by their different discharge behaviour during a twitch. The Golgi tendon organs are situated in series with the extrafusal muscle fibres and were thus excited by the twitch. The muscle spindles, however, lie in parallel with the extrafusal muscle fibres and were therefore paused by the twitch.
The initial muscle length was set at a length that generates muscle tension of about 50 g. The muscle was stretched by 2.0 mm at 2, 4, 10 and 20 mm s−1, followed by a 5 s plateau and a 1 s release phase. In addition, the muscle was stretched using a sinusoidal wave (amplitude, 0.02–2.0 mm; frequency, 0.5–2.0 Hz). Each series of stretches was repeated six times every 15 s. In some experiments, the effects of SCh on the stretch responses were tested by i.v. injection of SCh (200 μg kg−1) during ramp-and-hold stretches (2 mm at 10 mm s−1). Muscle spindles' afferent spikes were recorded using a digital tape recorder (Instrumentation Cassette Recorder PC-108; Sony Corp., Japan) for storage. The CV of each afferent fibre was calculated from latency, which was determined by backward spike-triggered averaging from the gastrocnemius nerve and the conduction distance, as measured at the end of the experiment. After electrophysiological experimentation, the medial gastrocnemius muscle was dissected and immersed immediately in freshly prepared fixing solution (chloral hydrate, 1 g; 95% alcohol, 45 ml; distilled water, 50 ml; conc. nitric acid, 1 ml). The silver impregnation staining method was used to carry out morphological observation (Barker & Ip, 1963).
Electrophysiological analysis of muscle spindle afferents
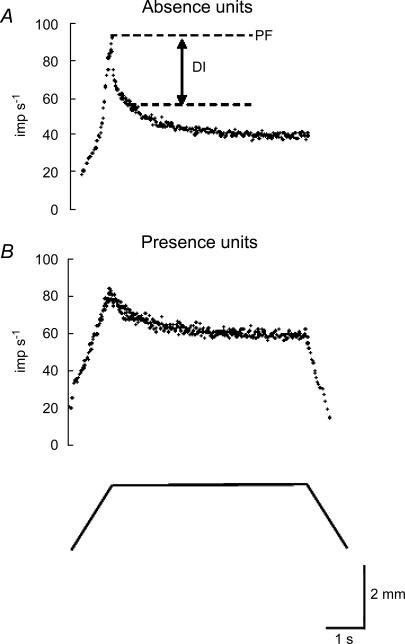
The recorded afferent discharges were treated as cluster cuts using Discovery software (DataWave Technologies Corp., Longmont, CO, USA) to classify spike waveforms, if not single units. The instantaneous discharge frequency was calculated as the inverse of the interspike interval. Typical responses of muscle spindle afferents to ramp-and-hold stretch at 2 mm s−1 are illustrated in Fig. 1. The peak frequency (PF) was the maximum frequency at the end of the dynamic stretch. The dynamic index (DI) represents differences between the PF and the frequency at 0.5 s after completion of the stretch (Crowe & Matthews, 1964). We classified the recorded muscle spindle afferents into Absence (Fig. 1A) and Presence units (Fig. 1B) according to their responses during the release phase, which showed either absence or presence of the slowing discharge (Hunt & Ottoson, 1975; Hunt, 1990; De-Doncker et al. 2003). We performed preliminary tests at different velocities of release as 0.5, 1, 2, 4, 8 and 10 mm s−1 to determine the most effective separation of the ending types. Responses of the release phase were apparently dependent on the velocity of release. All showed slowing discharge at slower velocity (0.5, 1 mm s−1), and a paused discharge at faster velocity (4, 8, 10 mm s−1). However, at 2 mm s−1, most afferents of slow conduction velocity units showed slowing discharge, and most afferents of fast conduction velocity units showed an interruption of discharge. Therefore, we used 2 mm s−1 as the velocity of release in these experiments. The units were not included in the sample when there were afferents with intermediate properties which were classifiable as neither Presence nor Absence type at this release velocity.
Figure 1. Instantaneous discharge of muscle spindle afferents to ramp-and-hold stretch at 2 mm s−1.
A, discharge ceases during the release phase (Absence units). B, discharge continues during the release phase (Presence units). The lower trace shows the muscle displacement. PF, peak frequency; DI, dynamic index.
In addition, muscle spindle afferents were characterized using the following criteria: CV, dynamic sensitivity to ramp-and-hold stretches, effect of SCh during ramp-and-hold stretches, and the linear range in response to sinusoidal stretches. Dynamic sensitivity was expressed as the relationship between DI and stretch velocities. We estimated the slope of the regression lines (exponent coefficient) on a double-logarithmic coordinate system. The effect of SCh during ramp-and-hold stretches was estimated by comparing DI before and after i.v. injection of SCh (200 μg kg−1). For sinusoidal stretch, the sine curve was fitted to the discharge rate using the least mean square method, as used in previous studies (Matthews & Stein, 1969; Kakuda, 2000), and the mean level and response amplitude (half-peak-to-peak amplitude) were measured (Fig. 2A). We estimated the linear range within which the response amplitude of afferents to the amplitude of stretch is highly linear. Figure 2B shows that, at a fixed frequency (0.5 Hz), the linear response of Absence units was limited to the low amplitude of stretch, but the linear response of Presence units to stretch continued to large amplitudes of stretch (Matthews & Stein, 1969; Hasan & Houk, 1975; Hulliger et al. 1977; Kakuda, 2000).
Figure 2. Measurement of the response of muscle spindle afferents to sinusoidal stretch.
A, instantaneous discharge of muscle spindle afferents (^) and the fitted sine curve (thick line). B, respective relations between the amplitudes of stretch and the response of Presence and Absence units.
Morphological observation
The muscle spindles were isolated by teasing with fine needles and then placed on a glass slide. Teased preparations of muscle spindles were examined using a light microscope (Eclipse E800; Nikon Corp., Tokyo, Japan) and were photographed (C5810; Hamamatsu Photonics KK, Shizuoka, Japan). Primary and secondary endings were identified based on their different locations, their axon diameters, and appearances of the muscle spindles. For old rats, differences in their location were used in most instances because their appearance and axon diameters apparently changed with age. We observed their morphological appearance and quantitatively assessed measured morphometrics including the ending length (longitudinal extent of unmyelinated endings that lie along fibres), number of bands for each intrafusal fibre, and axon diameters of spindle afferents using image processing software (ImageJ 1.34s; National Institutes of Health, USA). The diameters of Ia and II afferents were measured near the muscle spindle entry.
Data analyses
The electrophysiological data of muscle spindle afferents were pooled in each age group. For data of morphometrics of primary and secondary endings, the mean value was calculated for each muscle spindle; then the mean value for each age group was obtained. All means are given along with the standard deviation (s.d.). The effects of ageing and differences between muscle spindle ending types were evaluated using one-way analysis of variance with Sheffe's post hoc analysis. The criterion for accepting statistical significance was P < 0.05.
Results
In all, 197 spindle afferents were analysed: those of 82 young rats, 44 middle-aged rats, and 71 old rats. Two types of response (presence or absence of slowing discharge) during the release phase under ramp-and-hold stretch were observed in all age groups. The Presence units were recorded for 18 afferents in young rats, 20 afferents in middle-aged rats, and 60 afferents in old rats. Absence units were recorded in 64 afferents in young rats, 24 afferents in middle-aged rats, and 11 afferents in old rats.
Axonal conduction velocity
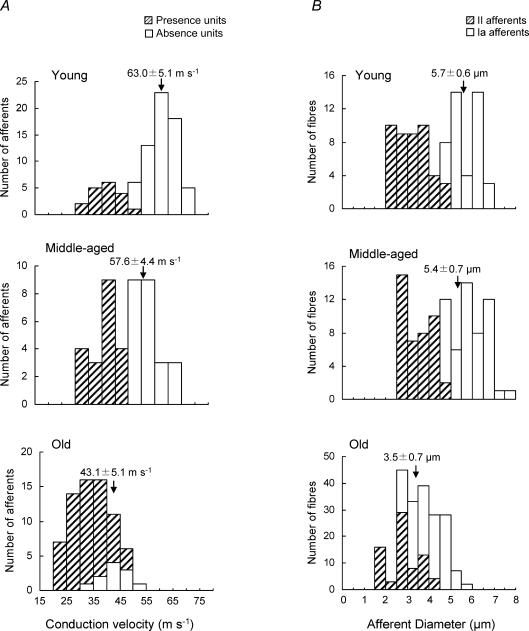
Figure 3A shows histograms of the CV distributions for afferents in three age groups. The respective CVs of Presence units in young, middle-aged and old rats were 34–53 m s−1, 33–50 m s−1, and 21–50 m s−1. Those of Absence units in young, middle-aged and old rats were 52–74 m s−1, 53–66 m s−1, and 35–50 m s−1, respectively. The CV distribution showed two peaks in young rats, although they overlapped slightly; also, a dividing point at 50 m s−1 was apparent for middle-aged rats. In contrast, Presence and Absence units were widely overlapped in old rats. The CVs of both Presence and Absence units in old rats were significantly lower than those in young and middle-aged rats, but those of Absence units differed to a greater degree. Figure 3B shows that the mean diameters of both Ia and II afferents in old rats (3.5 ± 0.7 and 2.3 ± 0.7 μm, respectively) were significantly thinner than those of young (5.7 ± 0.6 and 3.2 ± 0.7 μm) and middle-aged rats (5.4 ± 0.7 μm and 3.0 ± 0.6 μm).
Figure 3. Distribution histogram of conduction velocity of muscle spindle afferents (A) and of the diameters of Ia and II afferents near muscle spindle entry (B) in young, middle-aged and old rats.
A, the distribution of CV in young and middle-aged rats shows two peaks, although the CVs of Presence and Absence units in old rats are mostly overlapped. B, the distribution of diameters of 40 spindle Ia afferents and 45 II spindle afferents in young rats, 52 spindle Ia afferents and 43 II spindle afferents in middle-aged rats, and 128 spindle Ia afferents and 73 II spindle afferents in old rats. The mean diameter of Ia afferents in old rats was much less than those of young and middle-aged rats. These morphological data suggest the decrease in CV of old rats. The values of CV and diameters (means ±s.d.) of Absence units are indicated in the figure.
Dynamic response during ramp-and-hold stretch
The DI increased concomitant with the increased stretch velocity. The plots of DI (log) versus stretch velocity (log) were well fitted by straight lines (Fig. 4A). The slopes of the regression lines (exponent coefficients), which indicated dynamic sensitivity, for Absence units of muscle spindles in old rats (0.45 ± 0.07 imp s−1 per mm s−1) were significantly smaller than those for muscle spindles in young and middle-aged rats (0.53 ± 0.05 and 0.51 ± 0.05 imp s−1 per mm s−1, respectively), but those of Presence units did not differ (Fig. 4B–D). The exponent coefficients of Presence and Absence units in old rats were overlapped over a wider range (0.3–0.5 imp s−1 per mm s−1) than for young and middle-aged rats (0.45–0.5 imp s−1 per mm s−1).
Figure 4. The representative relationship between DI (log) and stretch velocities (log), indicating the dynamic sensitivity (A) and the distribution of the slopes of the regression lines (exponent coefficients) indicating the dynamic sensitivity for Absence units and Presence units of muscle spindles in young (B), middle-aged (C), and old (D) rats.
The distribution for Absence units in old rats is shifted markedly to the left.
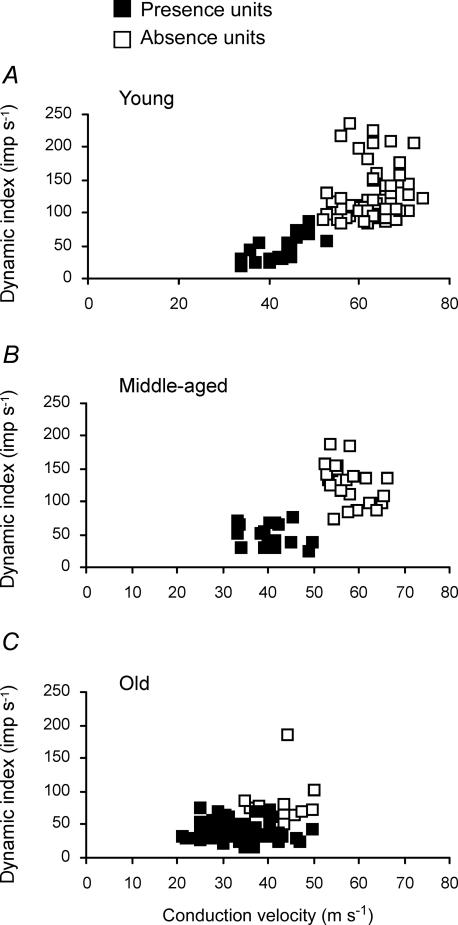
Figure 5 shows DI at 20 mm s−1 plotted against CV for the three age groups. The Presence units of young and middle-aged rats were distributed around lower DI (45.3 ± 18.9 imp s−1 and 46.9 ± 17.3 imp s−1, respectively) and slower CV (42.5 ± 5.3 m s−1 and 40.5 ± 4.6 m s−1, respectively). The Absence units of young and middle-aged rats were distributed around higher DI (125.8 ± 39.5 imp s−1 and 128.5 ± 30.6 imp s−1, respectively) and faster CV (63.0 ± 5.1 m s−1 and 57.6 ± 4.4 m s−1, respectively). In contrast, as shown in Fig. 5C, both Presence and Absence units in old rats were distributed around lower DI (39.4 ± 13.8 imp s−1 and 85.3 ± 53.7 imp s−1, respectively) and slower CV (33.6 ± 6.3 m s−1 and 43.1 ± 5.3 m s−1, respectively). The DI values of Absence units in old rats were significantly lower than those of young and middle-aged rats. Consequently, the exact point of difference between Presence and Absence units was indistinct despite the significant difference between the means of the Absence and Presence units in the old rats. Apparently, the DIs and CVs of Absence units were shifted toward those of Presence units. The decrease of DI in old rats was mainly attributable to the low peak frequency (PF). The discharge frequency of muscle spindle afferents to ramp stretch rose abruptly to a peak value at the end of stretch in young rats (PF, 196.8 ± 71.1 imp s−1 at 20 mm s−1), although they rose slowly in old rats (PF, 138.6 ± 45.6 imp s−1). The discharge frequency of muscle spindle afferents at 0.5 s after completion of ramp stretch was similar for young (92.3 ± 32.8 imp s−1) and old rats (91.1 ± 29.6 imp s−1).
Figure 5. Dynamic index at 20 mm s−1 against conduction velocity for young (A), middle-aged (B), and old (C) rats.
A and B, distinct populations of Presence and Absence units. C, both Presence and Absence units are distributed around the lower dynamic index and slower conduction velocity. Absence units are shifted toward Presence units in old rats.
Effects of succinylcholine
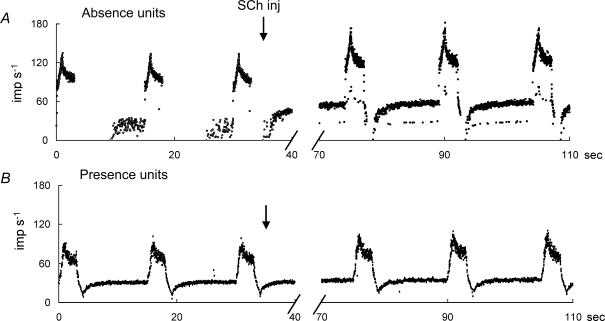
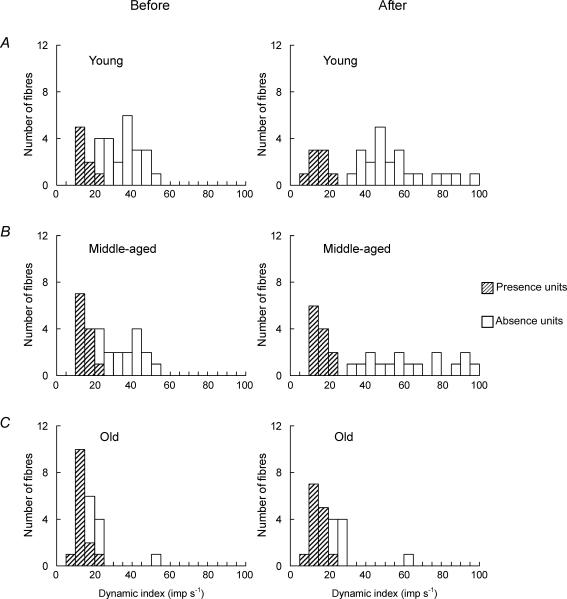
In all, 80 afferents (30 afferents from young rats, 28 afferents from middle-aged rats, and 22 afferents from old rats) were recorded for a ramp-and-hold stretch before and after the i.v. injection of SCh (200 μg kg−1), with the intention of testing for increased dynamic response. Most units indicated an increased discharge at 30 s after the i.v. dose of SCh and reached a peak value at 60–120 s, with full recovery at 1200 s. After i.v. SCh, Absence units (Fig. 6A) showed slightly slowing discharge during the release phase and a great increase in DI (18.9 ± 14.1 imp s−1), whereas Presence units (Fig. 6B) showed a low increase or decrease in DI (2.2 ± 3.9 imp s−1) in young rats. A significant difference was found between the mean values of DI before and after SCh injection for Absence units (P < 0.001) in young and middle-aged rats. Furthermore, SCh injection enables clearer classification of spindle afferents into Absence units and Presence units (Fig. 7A and B). However, in old rats, spindle afferents were not classifiable into Absence units and Presence units because of the small increase of DI after SCh injection (Fig. 7C).
Figure 6. Representative responses to ramp-and-hold stretch before and after SCh injection (200 μg kg−1).
A and B, representative responses of Absence and Presence units, respectively.
Figure 7. Distribution histograms of the values of DI of Absence and Presence units before and after SCh injection in young (A), middle-aged (B), and old (C) rats.
The responses show no distinction in old rats after SCh injection.
Response to sinusoidal stretch
Of the 197 spindle afferents studied previously under ramp-and-hold stretch, 24 were analysed using sinusoidal stretch (5 afferents from young rats, 5 afferents from middle-aged rats, and 14 afferents from old rats). Units with Presence or Absence under ramp-and-hold stretch corresponded to units with a broader linear range or units with a narrow linear range under sinusoidal stretch. The Absence units of all age groups increased nonlinearly to graded amplitude of sinusoidal stretch. That linear increase was limited to amplitudes of less than 0.08 mm (linear range, 0.02–0.08 mm) in all age groups. The Presence units of all age groups increased linearly with amplitude to 2 mm (linear range, 0.02–2 mm).
Morphological observation
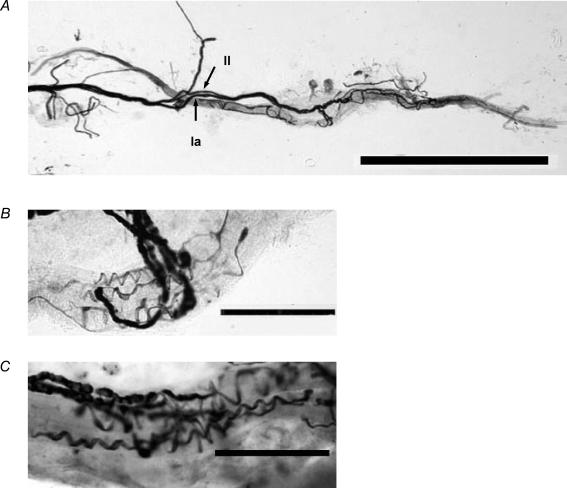
For silver analyses, 121 spindles were teased from 10 medial gastrocnemius muscles that had been used in electrophysiological experiments: 34 spindles from three muscles of young rats, 35 spindles from three muscles of middle-aged rats, and 52 spindles of old rats. Figure 8A shows a representative complete muscle spindle of a young rat under a light microscope. The Ia and II axons were readily distinguishable by their different diameters. Primary and secondary endings were readily distinguishable by their different locations and appearance in the muscle spindle. Terminations of primary endings innervated each intrafusal muscle fibre in the equatorial region; terminations of secondary endings innervated in the juxta-equatorial region. The bag and chain fibres were readily identified by their markedly different diameters. We were unable to determine bag fibre types (bag1, bag2) with certainty, but terminals of primary afferent always supplied to at least one of the bag fibres in all age groups. Therefore, there might not be changes with ageing in the distribution of terminations on the different intrafusal fibres for either primary or secondary endings. The mean numbers of primary and secondary endings for each spindle did not differ among age groups. Most muscle spindles had one primary ending adjacent to one secondary ending (young, 76.5 ± 4.7%; middle-aged, 76.2 ± 15.8%; old, 76.3 ± 9.0%). Some muscle spindles had one primary ending and two secondary endings (young, 14.6 ± 4.9%; middle-aged, 9.2 ± 10.1%; old, 12.6 ± 11.6%) or no secondary ending (young, 8.8 ± 0.4%; middle-aged, 14.6 ± 5.9%; old, 11.1 ± 6.0%).
Figure 8. Example of teased silver-impregnated muscle spindles viewed under a light microscope.
A, all silver-impregnated spindles isolated from the medial gastrocnemius muscles of a young rat. Arrows, respectively, indicate Group I and Group II. B and C, equatorial regions of muscle spindles in a young rat and a middle-aged rat, respectively. The primary endings are spiral. Scale bars indicate 1 mm in A, and 100 μm in B and C.
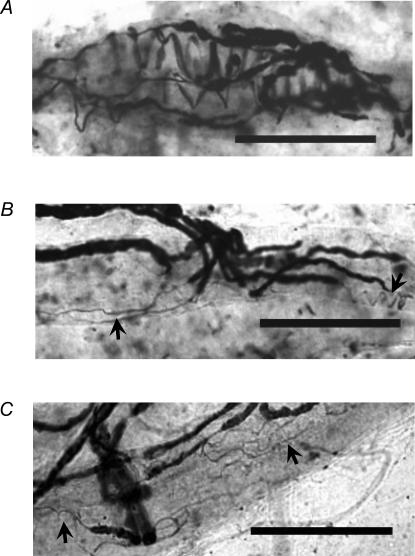
Interestingly, the morphological appearances of primary endings in aged muscle spindles differed from those of the young muscle spindles, despite the similarity of the muscle spindles' overall features. Those of muscle spindles of young and middle-aged rats were spiral (Fig. 8B and C), whereas those of old rats showed tapered and irregular configurations rather than spirals. We defined sensory endings as three types of muscle spindles in old rats: spiral (i.e. annulospiral, as shown in Fig. 9A), less spiral (i.e. flat and spiral, as shown in Fig. 9B), and non-spiral (i.e. not at all spiral, as shown in Fig. 9C). Most muscle spindle primary endings were spiral type endings in young and middle-aged rats. However, in old rats, the less spiral type and non-spiral type accounted, respectively, for 46.3 ± 0.5% and 40.5 ± 9.3% for each muscle (Table 1). Furthermore, the mean length of 45 primary endings (longitudinal extent of unmyelinated endings that lie along fibres) in young rats was 167.1 ± 32.8 μm. That of 34 primary endings in middle-aged rats was 165.9 ± 24.0 μm. In contrast, 81 primary endings of old rats had mean length of 126.8 ± 24.8 μm (Table 2). The primary endings of old rats were significantly shorter than those of young and middle-aged rats. Many less spiral or non-spiral type endings existed in old rats; consequently, the transverse bands for each intrafusal fibre were significantly fewer than for those of young and middle-aged rats (Table 2). Because of the irregular characteristics of secondary endings, it was not possible to make a quantitative assessment of appearances of muscle spindles of old rats, but most appeared to be similar to those supplied to young and middle-aged spindles.
Figure 9. Equatorial regions of muscle spindles in an old rat.
Primary endings are three muscle spindle types in old rat: as spiral (A), less spiral (B), and non-spiral (C). Arrows in B and C indicate tapered and irregularly shaped sensory terminals. See text for type definitions. Scale bars indicate 100 μm in A, B and C.
Table 1.
Numbers of muscle spindle types from medial gastrocnemius muscles of young, middle-aged, and old rats
| Age groups | No. of spindles | Spiral | Less spiral | Non spiral |
|---|---|---|---|---|
| Young no. 1 | 11 | 11 | 0 | 0 |
| Young no. 2 | 12 | 12 | 0 | 0 |
| Young no. 3 | 11 | 11 | 0 | 0 |
| Middle-aged no. 1 | 10 | 10 | 0 | 0 |
| Middle-aged no. 2 | 12 | 11 | 1 | 0 |
| Middle-aged no. 3 | 13 | 13 | 0 | 0 |
| Old no. 1 | 13 | 2 | 6 | 5 |
| Old no. 2 | 11 | 2 | 5 | 4 |
| Old no. 3 | 13 | 0 | 6 | 7 |
| Old no. 4 | 15 | 3 | 7 | 5 |
Most muscle spindle primary endings in young and middle-aged rats are spiral type endings. Many muscle spindles in old rats are of less-spiral and non-spiral types.
Table 2.
Mean length of endings and mean number of transverse terminal bands
| Age groups | No. primary endings analysed | Mean ending length ±s.d. (μm) | Mean no. transverse bands ±s.d. |
|---|---|---|---|
| Young | 45 (0) | 167.1 ± 32.8 | 9.7 ± 2.5 |
| Middle-aged | 34 (1) | 165.9 ± 24.0 | 8.8 ± 2.2 |
| Old | 81 (65) | 126.8 ± 24.8* | 3.4 ± 2.6* |
The mean length of endings and mean number of bands for each intrafusal fibre are significantly less in primary endings of old rats
P< 0.01. Numbers of less-spiral and non-spiral endings are shown in parentheses.
Discussion
We demonstrated that most units sampled from old rats showed properties of secondary muscle spindles that are observed in young rats. Our light microscopic observations revealed that primary endings were changed into less spiral or non-spiral endings in old rats, but they still existed. These findings suggest that primary muscle spindle endings tend to become physiologically similar to secondary endings in old rats.
Classification of primary and secondary endings in old rats
Conduction velocity
The CV has long been used as a criterion for classifying muscle spindle afferents in cat (Matthews, 1963; Wei et al. 1986; Barker et al. 1986; Scott, 1990), rat (De-Doncker et al. 2003), and primate (Cheney & Preston, 1976). The present experiments classified muscle spindle afferents into Absence and Presence units as two peaks in young and middle-aged rats (results presented herein suggest that our Absence units corresponded to primary endings and our Presence units to secondary endings), whereas the CVs in old rats were lower for both Absence units (31.7%) and Presence units (20.9%) than those of young or middle-aged rats. These results were supported by the present morphological data, which show decreased diameters of Ia and II afferents in old rats, and by data obtained by many previous physiological and morphological studies (Chase et al. 1992; Bergman & Ulfhake, 1998; Pannese et al. 1998; Sugiura & Kanda, 2004). Chase et al. (1992) described that an age-dependent decrease in CV was related to decreased axon diameter and thinning of the myelin sheath. Our previous experiments (Sugiura & Kanda, 2004) revealed that the decrease in CV of motoneurons with ageing was greater for CV of motoneurons belonging to the fast motor units than for those of motoneurons belonging to the slow motor units. The difference between these two groups was therefore small. Evidence also indicates loss of the dorsal root ganglion neurons (DRG) and selective cell body atrophy among myelinated primary afferents with ageing (Bergman & Ulfhake, 1998; Pannese et al. 1998).
Dynamic response
The present experiments showed that the dynamic sensitivity of muscle spindle primary endings was decreased in old rats: the peak frequency during the dynamic phase of muscle stretch was much lower than that in young and middle-aged rats. The peak discharge frequency was determined by both ionic and mechanical properties of intrafusal muscle fibres (Boyd & Smith, 1984). Fischer & Schafer (2000) revealed that both the impulse activity and the sensitivity to stretch decreased in a high Ca2+ solution and increased in a low Ca2+ solution. Matthews (1964) suggested that such a pattern of sensory discharge might occur as a result of visco-elastic properties of the spindle receptor. Direct observation of spindles revealed that the bag fibres behave in a visco-elastic manner, whereas the chain fibres appear to be almost elastic (Boyd, 1966; Gladden, 1972; Cooper & Gladden, 1974; Boyd & Ward, 1975). The primary sensory ending is wrapped around both the viscous bag and the elastic chain fibres, whereas the secondary sensory ending lies principally around the elastic chain fibres (Banks et al. 1982; Boyd, 1985). Therefore age-related changes in the ionic and mechanical properties of intrafusal fibres might engender decrease of the dynamic response of the primary endings. The injection of SCh helped clarify the classification of spindle afferents in young and middle-aged rats, but it did not allow two group endings to be distinguished clearly because of the small increase in the dynamic response of Absence units in old rats. These results also suggested that mechanical properties of nuclear bag fibres might change with ageing because the excitation by SCh of the spindle primary endings has been known to cause intrafusal contraction of the two nuclear bag fibres (Gladden, 1976; Dutia, 1980; Carr & Proske, 1996). To our knowledge, however, no study has examined age-related changes in the ionic and mechanical properties of intrafusal fibres. Another reason for the decline of dynamic response in old rats is considered to be the change in the distribution of primary and secondary terminations on the different intrafusal fibres. We also tested a similar parameter described by Taylor et al. (1992) to examine age-related changes in the distribution pattern of units (data not shown), but our data showed no clear distribution in old rats because of the few samples that were used. More details of age-related changes in ionic and mechanical properties of intrafusal fibres, along with details of the distribution of terminations on different intrafusal must be obtained in future studies.
Response to sinusoidal stretch
The linear range of Presence units was broader than that of Absence units for all age groups. These results accord with previous studies, in which secondary endings showed a broader linear range than primary endings in rat (De-Doncker et al. 2003), cat (Matthews & Stein, 1969; Hasan & Houk, 1975; Hulliger et al. 1977), and human (Kakuda, 2000). Units with Presence or Absence under ramp-and-hold stretch corresponded to units with a broader linear range or units with a narrower linear range under sinusoidal stretch, suggesting that our Absence units corresponded to primary endings and that our Presence units to secondary endings, as previously described. Therefore, a linear range during sinusoidal stretch and two types of response (presence or absence of slowing discharge) during the release phase under ramp-and-hold stretch might be the only criteria for classification of primary and secondary endings, even in the aged rat model. On the other hand, the population of Absence units was far smaller among old rats (11/71) than among young (64/82) or middle-aged rats (24/44), thereby suggesting one of two possibilities. Primary endings disappear preferentially, or primary endings change to show slowing discharges similarly to secondary endings during the release phase under ramp-and-hold stretch in old rats. Therefore, such criteria as the linear range during sinusoidal stretch and two types of response (presence or absence of slowing discharge) during the release phase under ramp-and-hold stretch are not useful for classifying primary and secondary endings in muscle spindles of old rats.
Age-related morphological changes of sensory nerve endings
One study reported that there were 28 spindles in rat medial gastrocnemius muscle (Arendt & Asmussen, 1974). However, 17 spindles were identified in the rat medial gastrocnemius muscle that we counted by serial section (data not shown). A study of the number of spindles in certain cat limb muscles (Barker & Chin, 1960) revealed considerable interindividual variation. Therefore, we infer that the morphological differences found in the present experiments reflect general changes that occur in old rats.
Age-related morphological changes in peripheral nerves have been reported extensively: axonal atrophy (Ochoa & Mair, 1969; Knox et al. 1989; Chase et al. 1992), decreased internodal length (Lascelles & Thomas, 1966), demyelination (Knox et al. 1989; Adinolfi et al. 1991), and decline in motor nerve terminal branches and acetylcholine receptor rich areas of the neuromuscular junction (Oda, 1984; Balice-Gordon, 1997). The present study provides evidence that degenerative changes also exist in sensory nerve endings in old rats. Immunohistochemical and fine structural studies revealed drastic changes in the structures, not only of intrafusal muscle fibres but also of nerve endings in aged rat jaw muscle spindles (Winarakwong et al. 2004). Electron microscopic observations of them showed that closely packed myofibrils become attenuated; some sensory terminal profiles of bag fibres enlarge, and occasionally parts of the equatorial region of intrafusal fibres were devoid of nerve terminals. Moreover, the morphology of immunoreactive sensory terminals of old animals also displayed somewhat irregular and less crowded sensory endings. The present morphology results for primary endings of old rats are consistent with those results. Other light microscopic observations indicated degenerative changes in aged human intrafusal muscle fibres and their motor nerve endings (Swash & Fox, 1972; but see also Winarakwong et al. 2004).
Several physiological and morphological studies of regenerated muscle spindles after nerve injury have shown that the responses of the regenerated spindle afferents to stretch were restored gradually to normal spindles during recovery, although all their endings were abnormal, showing tapered or irregular configurations rather than a spiral form (Quick & Rogers, 1983; Barker et al. 1986; Banks & Barker, 1989; Barker & Scott, 1990). However, the mechanism of that recovery in the response of muscle spindle afferents to stretch is less clear. Hyde & Scott (1983) suggested that the return to normality is probably the result of the pacemaker thresholds of the afferents gradually regaining their normal levels. Nevertheless, the present study showed that age-related changes of muscle spindle physiological properties are attributable to morphological changes of the terminals in old rats. The physiological findings merely suggested two possibilities: preferred dropout of the primary endings occurred in muscle spindles of old rats; alternatively, their physiological properties change to secondary-ending-like response. Additional morphological findings revealed that the primary endings exist in muscle spindles of old rats, therefore supporting the latter possibility. The innervated extent of sensory endings on intrafusal fibres reflects a capacity for depolarization of sensory endings because the stretch of intrafusal fibres mechanically deforms the sensory terminals, thereby altering the ionic permeability of the sensory terminal, which in turn depolarizes the terminal (Hunt et al. 1978). Consequently, it is strongly suggested that the present morphological results, which lack longitudinal innervated length and transverse banding, are related to reduction of dynamic responsibilities of the primary endings.
McCloskey (1978) reported that the discharges of primary endings are dependent on information about changes in muscle length and velocity, whereas the discharges of secondary endings are mainly dependent on information about changes in muscle length. For that reason, results of our study support the observation that the difference between young and old people in velocity of sway is greater with dynamic posturography than with static posturography (Baloh et al. 1994). Alteration of ionic conditions of peri-sensory nerve endings should also be investigated. Such studies will further elucidate age-related motor changes.
Acknowledgments
We are grateful to Dr S. Nomoto for critical reading of the manuscript.
References
- Adinolfi AM, Yamuy J, Morales FR, Chase MH. Segmental demyelination in peripheral nerves of old cats. Neurobiol Aging. 1991;12:175–179. doi: 10.1016/0197-4580(91)90058-r. [DOI] [PubMed] [Google Scholar]
- Alexander NB. Postural control in older adults. J Am Geriatr Soc. 1994;42:93–108. doi: 10.1111/j.1532-5415.1994.tb06081.x. [DOI] [PubMed] [Google Scholar]
- Arendt KW, Asmussen G. Number and distribution of muscular spindles in the triceps surae muscle of the rat. Anat Anz. 1974;136:207–216. [PubMed] [Google Scholar]
- Balice-Gordon RJ. Age-related changes in neuromuscular innervation. Muscle Nerve. 1997;5(Suppl):S83–S87. doi: 10.1002/(sici)1097-4598(1997)5+<83::aid-mus20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Fife TD, Zwerling L, Socotch T, Jacobson K, Bell T, Beykirch K. Comparison of static and dynamic posturography in young and older normal people. J Am Geriatr Soc. 1994;42:405–412. doi: 10.1111/j.1532-5415.1994.tb07489.x. [DOI] [PubMed] [Google Scholar]
- Banks RW, Barker D. Specificities of afferents reinnervating cat muscle spindles after nerve section. J Physiol. 1989;408:345–372. doi: 10.1113/jphysiol.1989.sp017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW, Barker D, Stacey MJ. Form and distribution of sensory terminals in cat hindlimb muscle spindles. Philos Trans R Soc Lond B Biol Sci. 1982;299:329–364. doi: 10.1098/rstb.1982.0136. [DOI] [PubMed] [Google Scholar]
- Barker D, Chin NK. The number and distribution of muscle-spindles in certain muscles of the cat. J Anat. 1960;94:473–486. [PMC free article] [PubMed] [Google Scholar]
- Barker D, Ip MC. A silver method for demonstrating the innervation of mammalian muscle in teased preparations. J Physiol. 1963;169(Suppl):73P–74P. [Google Scholar]
- Barker D, Scott JJ. Regeneration and recovery of cat muscle spindles after devascularization. J Physiol. 1990;424:27–39. doi: 10.1113/jphysiol.1990.sp018053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D, Scott JJ, Stacey MJ. Reinnervation and recovery of cat muscle receptors after long-term denervation. Exp Neurol. 1986;94:184–202. doi: 10.1016/0014-4886(86)90282-7. [DOI] [PubMed] [Google Scholar]
- Bergman E, Ulfhake B. Loss of primary sensory neurons in the very old rat: neuron number estimates using the disector method and confocal optical sectioning. J Comp Neurol. 1998;396:211–222. [PubMed] [Google Scholar]
- Boyd IA. The structure and innervation of the nuclear bag muscle fiber system and the nuclear chain muscle fiber system in mammalian muscle spindles. Philos Trans R Soc Lond B Biol Sci. 1962;245:81–136. [Google Scholar]
- Boyd IA. The mechanical properties of mammalian intrafusal muscle fibres. J Physiol. 1966;187(Suppl):10–12. P. [Google Scholar]
- Boyd IA. Muscle spindles and stretch reflexes. In: Swash M, Kennard C, editors. Scientific Basis of Clinical Neurology. London: Livingstone; 1985. pp. 74–96. [Google Scholar]
- Boyd IA, Smith RS. The muscle spindle. In: Dyck PJ, Thomas PK, Lombert EH, Bunge R, editors. Peripheral Neuropathy. London: W.B. Saunders; 1984. pp. 171–202. [Google Scholar]
- Boyd IA, Ward J. Motor control of nuclear bag and nuclear chain intrafusal fibres in isolated living muscle spindles from the cat. J Physiol. 1975;244:83–112. doi: 10.1113/jphysiol.1975.sp010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RW, Proske U. Action of cholinesters on sensory nerve endings in skin and muscle. Clin Exp Pharmacol Physiol. 1996;23:355–362. doi: 10.1111/j.1440-1681.1996.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Chase MH, Engelhardt JK, Adinolfi AM, Chirwa SS. Age-dependent changes in cat masseter nerve: an electrophysiological and morphological study. Brain Res. 1992;586:279–288. doi: 10.1016/0006-8993(92)91637-t. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Preston JB. Classification and response characteristics of muscle spindle afferents in the primate. J Neurophysiol. 1976;39:1–8. doi: 10.1152/jn.1976.39.1.1. [DOI] [PubMed] [Google Scholar]
- Cody FW, Lee RW, Taylor A. A functional analysis of the components of the mesencephalic nucleus of the fifth nerve in the cat. J Physiol. 1972;226:249–261. doi: 10.1113/jphysiol.1972.sp009983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Gladden MH. Elastic fibres and reticulin of mammalian muscle spindles and their functional significance. Q J Exp Physiol Cogn Med Sci. 1974;59:367–385. doi: 10.1113/expphysiol.1974.sp002280. [DOI] [PubMed] [Google Scholar]
- Crowe A, Matthews PB. The effects of stimulation of static and dynamic fusimotor fibres on the response to stretching of the primary endings of muscle spindles. J Physiol. 1964;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Doncker L, Picquet F, Petit J, Falempin M. Characterization of spindle afferents in rat soleus muscle using ramp-and-hold and sinusoidal stretches. J Neurophysiol. 2003;89:442–449. doi: 10.1152/jn.00153.2002. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74:868–874. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- Dorfman LJ, Bosley TM. Age-related changes in peripheral and central nerve conduction in man. Neurology. 1979;29:38–44. doi: 10.1212/wnl.29.1.38. [DOI] [PubMed] [Google Scholar]
- Dutia MB. Activation of cat muscle spindle primary, secondary and intermediate sensory endings by suxamethonium. J Physiol. 1980;304:315–330. doi: 10.1113/jphysiol.1980.sp013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsiedel LJ, Luff AR. Effect of partial denervation on motor units in the ageing rat medial gastrocnemius. J Neurol Sci. 1992;112:178–184. doi: 10.1016/0022-510x(92)90148-e. [DOI] [PubMed] [Google Scholar]
- Fischer M, Schafer SS. Effects of calcium on the discharge pattern of primary and secondary endings of isolated cat muscle spindles recorded under a ramp-and-hold stretch. Brain Res. 2000;875:78–88. doi: 10.1016/s0006-8993(00)02577-4. [DOI] [PubMed] [Google Scholar]
- Gladden MH. Elastic fibres in muscle spindles of the cat. J Physiol. 1972;227(Suppl):45–46. P. [PubMed] [Google Scholar]
- Gladden MH. Structural features relative to the function of intrafusal muscle fibres in the cat. Prog Brain Res. 1976;44:51–59. doi: 10.1016/S0079-6123(08)60722-0. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Proske U. Responses of muscle receptors in the kitten to succinyl choline. Exp Brain Res. 1987;66:167–174. doi: 10.1007/BF00236212. [DOI] [PubMed] [Google Scholar]
- Hasan Z, Houk JC. Transition in sensitivity of spindle receptors that occurs when muscle is stretched more than a fraction of a millimeter. J Neurophysiol. 1975;38:673–689. doi: 10.1152/jn.1975.38.3.673. [DOI] [PubMed] [Google Scholar]
- Hashizume K, Kanda K, Burke RE. Medial gastrocnemius motor nucleus in the rat: age-related changes in the number and size of motoneurons. J Comp Neurol. 1988;269:425–430. doi: 10.1002/cne.902690309. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, Goldberger AL. Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington's disease. J Appl Physiol. 1997;82:262–269. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Matthews PB, Noth J. Static and dynamic fusimotor action on the response of Ia fibres to low frequency sinusoidal stretching of widely ranging amplitude. J Physiol. 1977;267:811–838. doi: 10.1113/jphysiol.1977.sp011839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC. Mammalian muscle spindle: peripheral mechanisms. Physiol Rev. 1990;70:643–663. doi: 10.1152/physrev.1990.70.3.643. [DOI] [PubMed] [Google Scholar]
- Hunt CC, Ottoson D. Impulse activity and receptor potential of primary and secondary endings of isolated mammalian muscle spindles. J Physiol. 1975;252:259–281. doi: 10.1113/jphysiol.1975.sp011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC, Wilkinson RS, Fukami Y. Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. J Gen Physiol. 1978;71:683–698. doi: 10.1085/jgp.71.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MV, Rees J, Newham DJ. Quadriceps function, proprioceptive acuity and functional performance in healthy young, middle-aged and elderly subjects. Age Ageing. 1998;27:55–62. doi: 10.1093/ageing/27.1.55. [DOI] [PubMed] [Google Scholar]
- Hyde D, Scott JJ. Responses of cat peroneus brevis muscle spindle afferents during recovery from nerve-crush injury. J Neurophysiol. 1983;50:344–357. doi: 10.1152/jn.1983.50.2.344. [DOI] [PubMed] [Google Scholar]
- Inoue H, Morimoto T, Kawamura Y. Response characteristics an classification of muscle spindles of the masseter muscle in the cat. Exp Neurol. 1981;74:548–560. doi: 10.1016/0014-4886(81)90190-4. [DOI] [PubMed] [Google Scholar]
- Kakuda N. Response of human muscle spindle afferents to sinusoidal stretching with a wide range of amplitudes. J Physiol. 2000;527:397–404. doi: 10.1111/j.1469-7793.2000.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K, Hashizume K. Changes in properties of the medial gastrocnemius motor units in aging rats. J Neurophysiol. 1989;61:737–746. doi: 10.1152/jn.1989.61.4.737. [DOI] [PubMed] [Google Scholar]
- Kanda K, Hashizume K. Factors causing difference in force output among motor units in the rat medial gastrocnemius muscle. J Physiol. 1992;448:677–695. doi: 10.1113/jphysiol.1992.sp019064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto H, Bae YC, Yoshida A, Moritani M, Takemura M, Nakagawa S, Nagase Y, Wada T, Sessle BJ, Shigenaga Y. Central distribution of synaptic contacts of primary and secondary jaw muscle spindle afferents in the trigeminal motor nucleus of the cat. J Comp Neurol. 1998;391:50–63. [PubMed] [Google Scholar]
- Knox CA, Kokmen E, Dyck PJ. Morphometric alteration of rat myelinated fibers with aging. J Neuropathol Exp Neurol. 1989;48:119–139. doi: 10.1097/00005072-198903000-00001. [DOI] [PubMed] [Google Scholar]
- Lafratta CW, Canestrari R. A comparison of sensory and motor nerve conduction velocities as related to age. Arch Phys Med Rehabil. 1966;47:286–290. [PubMed] [Google Scholar]
- Lascelles RG, Thomas PK. Changes due to age in internodal length in the sural nerve in man. J Neurol Neurosurg Psychiatry. 1966;29:40–44. doi: 10.1136/jnnp.29.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Ward JA. Age-associated differences in sensori-motor function and balance in community dwelling women. Age Ageing. 1994;23:452–460. [PubMed] [Google Scholar]
- Matthews PB. The response of de-efferented muscle spindle receptors to stretching at different velocities. J Physiol. 1963;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Muscle spindles and their motor control. Physiol Rev. 1964;44:219–288. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- Matthews PB, Stein RB. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969;200:723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI. Kinesthetic sensibility. Physiol Rev. 1978;58:763–820. doi: 10.1152/physrev.1978.58.4.763. [DOI] [PubMed] [Google Scholar]
- Miwa T, Miwa Y, Kanda K. Dynamic and static sensitivities of muscle spindle primary endings in aged rats to ramp stretch. Neurosci Lett. 1995;201:179–182. doi: 10.1016/0304-3940(95)12165-x. [DOI] [PubMed] [Google Scholar]
- Ochoa J, Mair WG. The normal sural nerve in man. II. Changes in the axons and Schwann cells due to ageing. Acta Neuropathol (Berl) 1969;13:217–239. doi: 10.1007/BF00690643. [DOI] [PubMed] [Google Scholar]
- Oda K. Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. J Neurol Sci. 1984;66:327–338. doi: 10.1016/0022-510x(84)90021-2. [DOI] [PubMed] [Google Scholar]
- Overstall PW, Exton-Smith AN, Imms FJ, Johnson AL. Falls in the elderly related to postural imbalance. Br Med J. 1977;1:261–264. doi: 10.1136/bmj.1.6056.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannese E, Sartori P, Martinelli C, Ledda M. Age-related decrease in the overall extent of perikaryal projections in rabbit spinal ganglion neurons. Neurosci Lett. 1998;254:177–179. doi: 10.1016/s0304-3940(98)00591-6. [DOI] [PubMed] [Google Scholar]
- Proske U, Wise AK, Gregory JE. The role of muscle receptors in the detection of movements. Prog Neurobiol. 2000;60:85–96. doi: 10.1016/s0301-0082(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Quick DC, Rogers SL. Stretch receptors in regenerated rat muscle. Neuroscience. 1983;10:851–859. doi: 10.1016/0306-4522(83)90222-1. [DOI] [PubMed] [Google Scholar]
- Rack PM, Westbury DR. The effects of suxamethonium and acetylcholine on the behaviour of cat muscle spindles during dynamics stretching, and during fusimotor stimulation. J Physiol. 1966;186:698–713. doi: 10.1113/jphysiol.1966.sp008063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini A. On minute anatomy of neuromuscular spindles of the cat, and on their physiological significance. J Physiol. 1898;23:190–208. doi: 10.1113/jphysiol.1898.sp000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JJ. Classification of muscle spindle afferents in the peroneus brevis muscle of the cat. Brain Res. 1990;509:62–70. doi: 10.1016/0006-8993(90)90309-y. [DOI] [PubMed] [Google Scholar]
- Sorensen KL, Hollands MA, Patla E. The effects of human ankle muscle vibration on posture and balance during adaptive locomotion. Exp Brain Res. 2002;143:24–34. doi: 10.1007/s00221-001-0962-z. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kanda K. Progress of age-related changes in properties of motor units in the gastrocnemius muscle of rats. J Neurophysiol. 2004;92:1357–1365. doi: 10.1152/jn.00947.2003. [DOI] [PubMed] [Google Scholar]
- Swash M, Fox KP. The effect of age on human skeletal muscle. Studies of the morphology and innervation of muscle spindles. J Neurol Sci. 1972;16:417–432. doi: 10.1016/0022-510x(72)90048-2. [DOI] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Rodgers JF. The classification of afferents from muscle spindles of the jaw-closing muscles of the cat. J Physiol. 1992;456:609–628. doi: 10.1113/jphysiol.1992.sp019356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- Wei JY, Kripke BR, Burgess PR. Classification of muscle spindle receptors. Brain Res. 1986;370:119–126. doi: 10.1016/0006-8993(86)91111-x. [DOI] [PubMed] [Google Scholar]
- Winarakwong L, Muramoto T, Soma K, Takano Y. Age-related changes and the possible adaptability of rat jaw muscle spindles: immunohistochemical and fine structural studies. Arch Histol Cytol. 2004;67:227–240. doi: 10.1679/aohc.67.227. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Tanaka S, Tsubone H, Atoji Y, Suzuki Y. Age-related changes in sensory and secretomotor nerve endings in the larynx of F344/N rat. Arch Gerontol Geriatr. 2003;36:173–183. doi: 10.1016/s0167-4943(02)00165-6. [DOI] [PubMed] [Google Scholar]