Abstract
Studies in behaving animals suggest that neurones located in the perifornical (PF) region of the posterior hypothalamus promote wakefulness and suppress sleep. Among such cells are those that synthesize the excitatory peptides, orexins (ORX). Lack of ORX, or their receptors, is associated with narcolepsy/cataplexy, a disorder characterized by an increased pressure for rapid eye movement (REM) sleep. We used anaesthetized rats in which pontine microinjections of a cholinergic agonist, carbachol, can repeatedly elicit REM sleep-like episodes to test whether activation of PF cells induced by antagonism of endogenous, GABAA receptor-mediated, inhibition suppresses the ability of the brainstem to generate REM sleep-like state. Microinjections of the GABAA receptor antagonist, bicuculline (20 nl, 1 mm), into the PF region elicited cortical and hippocampal activation, increased the respiratory rate and hypoglossal nerve activity, induced c-fos expression in ORX and other PF neurones, and increased c-fos expression in pontine A7 and other noradrenergic neurones. The ability of pontine carbachol to elicit any cortical, hippocampal or brainstem component of the REM sleep-like response was abolished during the period of bicuculline-induced activation. The activating and REM sleep-suppressing effect of PF bicuculline was not attenuated by systemic administration of the ORX type 1 receptor antagonist, SB334867. Thus, activation of PF neurones that are endogenously inhibited by GABAA receptors is sufficient to turn off the brainstem REM sleep-generating network; the effect is, at least in part, due to activation of pontine noradrenergic neurones, but is not mediated by ORX type 1 receptors. A malfunction of the pathway that originates in GABAA receptor-expressing PF neurones may cause narcolepsy/cataplexy.
Correlations of the location of forebrain lesions with behavioural symptoms in patients with lethargic encephalitis (von Economo, 1930) and animal experiments with local suppression of cell activity (Sallanon et al. 1989) demonstrated that the posterior hypothalamus plays an important role in the promotion and maintenance of wakefulness. This role was further underscored and localized to the perifornical (PF) region of the posterior hypothalamus by the discovery that the region contains cells that synthesize the excitatory peptides, orexins (ORX, also known as hypocretins). The absence of ORX, or their receptors, is associated with narcolepsy/cataplexy, a disorder manifested by an uncontrollable occurrence of rapid eye movement (REM) sleep or some of its phenomena, including sudden spells of postural atonia (Chemelli et al. 1999; Lin et al. 1999). These findings provided the basis for the concept that activating influences that originate in the PF region and are mediated by ORX neurones suppress sleep and, in particular, oppose generation of REM sleep (Kilduff & Peyron, 2000; Bourgin et al. 2000; Mignot et al. 2002; Saper et al. 2005), a state principally orchestrated by neurones of the brainstem reticular formation (Jouvet, 1962, 1994).
The nature of sleep-modulating effects that emanate from the PF region is difficult to assess in behaving animals because the region is reciprocally interconnected with multiple brain regions important for both the control of sleep–wake states and numerous wake-associated behaviours (Allen & Cechetto, 1992, 1993; Bittencourt & Elias, 1993; Luppi et al. 1995; Oldfield et al. 2002; Berthoud, 2002; Jones, 2005; Saper et al. 2005). Here, we used a reduced animal model to test whether activation of PF cells, including those containing ORX, prevents generation of REM sleep-like state pharmacologically triggered from the dorsomedial pons. Specifically, we used anaesthetized rats in which pontine microinjections of a cholinergic agonist, carbachol, can elicit repeatedly REM sleep-like episodes comprising cortical and hippocampal activation, silencing of brainstem aminergic cells and suppression of motoneuronal activity (Woch et al. 1996; Kubin, 2001; Fenik et al. 2005). In the same model, microinjections of the GABAA receptor antagonist, bicuculline, into the PF region cause autonomic and electrocortical activation (DiMicco et al. 1987; de Novelis et al. 1995; Marchenko et al. 2002) and, in behaving animals, reduce sleep and elicit behavioural arousal (DiScala et al. 1984; Alam et al. 2005; Goutagny et al. 2005). We found that the ability of pontine carbachol to elicit REM sleep-like episodes was reversibly blocked when PF bicuculline exerted its activating effects. The suppressant effect of bicuculline on REM sleep-like state was associated with activation of ORX and other neurones in the PF region, as well as pontine noradrenergic cells, and was not prevented by the ORX type 1 receptor antagonist. Thus, an increased activity of ORX-containing and other PF neurones can powerfully inhibit the brainstem REM sleep-generating network. Preliminary results have been published (Lu et al. 2006).
Methods
Experiments were performed on 16 adult, male Sprague–Dawley rats obtained from Charles River Laboratories (Wilmington, MA, USA). All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Animal preparation
The rats were pre-anaesthetized with isoflurane followed by urethane (1.0 g kg−1, i.p., supplemented by 50 mg i.v. injections as needed), and prepared as previously described (Fenik et al. 2005). The trachea was cannulated and catheters inserted into the femoral artery and vein for arterial blood pressure monitoring and fluid injections. To monitor both the central respiratory rhythm and motor activity, the hypoglossal (XII) nerve was dissected and prepared for recording (Fenik et al. 2001). Spontaneous XII nerve activity in anaesthetized rats reflects rhythmic excitatory drive from central inspiratory neurones (e.g. Woch et al. 2000). Under steady ventilatory conditions, the magnitude of these rhythmic bursts is also modulated by supraspinal drives that determine the level of motor activation, thus making XII nerve activity suitable for monitoring of both the central motor drive and central respiratory rhythm. To enhance baseline XII nerve activity and make it independent of lung volume feedback, both cervical vagi were cut.
The animal's head was placed in a stereotaxic head holder and two openings were made for inserting drug-containing pipettes, one into the dorsomedial pontine reticular formation on the right side and another into the posterior hypothalamus on the left side. The cortical EEG, hippocampal and XII nerve signals were conditioned as previously described (Fenik et al. 2005), and continuously monitored together with end-expiratory CO2, blood pressure and core body temperature. After the preparatory procedures, the animals were neuromuscularly blocked (pancuronium bromide, 2 mg kg−1i.v., supplemented with additional injections as needed) and artificially ventilated with an air–oxygen mixture (30–60% O2). End-expiratory CO2 was kept constant at ∼5% throughout each study (Columbus Instruments capnograph, Columbus, OH, USA). Following neuromuscular blockade, the adequacy of anaesthesia was assessed based on stable rhythmic bursts of central inspiratory activity in the XII nerve, steady blood pressure and stable and slow cortical EEG. The rectal temperature was maintained at 35.5–36.5°C.
Drugs and microinjections
Carbamylcholine chloride (carbachol, cholinergic receptor agonist) and (−)-bicuculline methiodide (GABAA receptor antagonist) were obtained from Sigma (St Louis, MI, USA). The solutions for brain microinjections were prepared in 0.9% NaCl and, to mark the injection sites, 2% Pontamine sky blue dye (ICN Biomedicals, Aurora, OH, USA) was added. Microinjections were made from glass pipettes (A-M Systems, Carlsborg, WA, USA) having tip diameters of 25–30 μm and filled with either carbachol (10 mm) or bicuculline (1 mm). The carbachol pipette was inserted into a site in the dorsomedial pontine reticular formation that we previously identified as effective for eliciting REM sleep-like effects in anaesthetized rats (Kubin, 2001; Fenik et al. 2002, 2005), and the bicuculline pipette was inserted into the PF region of the posterior hypothalamus aiming at the following coordinates: AP −3.1 mm from bregma, lateral 1.3 mm, H 9.1 mm (Paxinos & Watson, 1991). The injections were 10 nl for carbachol and 20 nl for bicuculline and were made over 10–20 s by applying pressure to the fluid in the pipette while monitoring the movement of the meniscus with a calibrated microscope. In chronically instrumented, behaving animals, PF microinjections of the same dose of bicuculline block natural REM sleep for about 50 min and prevent the occurrence of slow-wave sleep for about 30 min post-injection, whereas a 10 times lower dose has insignificant effects (L. Kubin and G. L. Mann, unpublished observations). SB334867A (1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthydrin-4-yl urea hydrochloride, a non-peptide selective ORX type 1 receptor antagonist; Tocris, Ellisville, MO, USA) (Smart et al. 2001) was suspended in 10% encapsin and 4% DMSO in distilled water and administered intraperitoneally (30 mg kg−1), as previously described (Harris et al. 2005). This dose was selected because it has been well documented to fully and selectively block various behavioural effects of exogenously administered ORX in rats (Rodgers et al. 2001; Smith et al. 2003; Yamada et al. 2005; Harris et al. 2005).
Experimental protocol and measurements
In the main protocol, at least two pontine carbachol microinjections were made at the beginning of each experiment to verify the repeatability of the REM sleep-like pattern of the response. The responses to carbachol can be elicited repeatedly, without adaptation, at intervals at least as short as 30 min and typically last 2–4 min (Fenik et al. 2002, 2005). In contrast, the effects elicited by PF bicuculline often last more than 30 min. This difference in duration of the effects allowed us to conduct, in some animals, two tests with carbachol injections during the time when bicuculline was effective. Following bicuculline injection into the PF region, a minimum of four carbachol injections were made at intervals of at least 30 min; one or two when the effects of bicuculline were present and additional ones after the disappearance of the effects of bicuculline.
For spectral analysis of cortical and hippocampal activity, the signals were digitized (100 Hz; Spike-2, CED, Cambridge, UK). Power spectra of the signals were determined in the 0.5–20 Hz range over 10 min periods prior to and at the peak of the response to bicuculline using a sleep scoring software (Somnologica, MedCare, Buffalo, NY, USA). The power of the hippocampal signal in the theta-like frequency range (3–5 Hz) and EEG power in the 6–12 Hz range were also determined in successive 15 s intervals across the entire duration of the recording; carbachol and/or bicuculline injections elicit characteristic changes in these bands. The magnitude of XII nerve activity was measured from the moving average of the signal as the difference between the peak activity during the inspiratory phase of the central respiratory cycle and the level of the signal during the central expiration (no activity). The durations of the responses to the drugs were measured as the time during which XII nerve activity was reduced (carbachol) or increased (bicuculline) compared with the period before the injections. The central respiratory rate was determined from XII nerve activity. The magnitudes of the effects elicited by carbachol in all outputs (cortical and hippocampal powers, XII nerve activity, respiratory rate) prior to bicuculline injections were used as a reference; the measures characterizing the responses to carbachol following bicuculline injection were expressed within each experiment as a percentage of their pre-bicuculline values.
Histological and immunohistochemical procedures
At the conclusion of the experiments, an additional high dose of urethane was injected (2 g kg−1) and the animal was perfused with 4% phosphate-buffered formalin (for immunohistochemistry) or 10% formalin (for recovery of injection sites only). The brain was removed, post-fixed in the same fixative, cryoprotected in 30% sucrose and cut into five series of 35 μm coronal sections. Selected sections containing the blue dye were mounted and stained with neutral red to localize the injection sites. Hypothalamic sections were immunohistochemically processed for c-fos (1: 100 000; Oncogene, Temecula, CA, USA), as a marker of cell activation, and prepro-ORX (1: 500; Chemicon). Using avidin–biotin–horseradish peroxidase histochemistry (Vector, Burlinghame, CA, USA), with and then without heavy metal intensification, c-fos expressed in neuronal nuclei was stained black, and prepro-ORX-expressing cells, brown. The same approach was applied to brainstem sections to visualize c-fos in pontomedullary catecholaminergic neurones labelled for tyrosine hydroxylase (1: 35 000; Sigma; Rukhadze & Kubin, 2007a) and pontine cholinergic neurones immunohistochemically labelled for nitric oxide synthase (1: 5000; Sigma) (Vincent & Kimura, 1992; Rukhadze & Kubin, 2007b).
Cell counting and statistical analysis
For cell counting, sections were observed using Leica DML microscope at 200× magnification. All catecholaminergic cells, with and without c-fos-positive nuclei, were counted in every fifth section in the medullary A1/C1 and pontine A5, A7 and subcoeruleus (Sub-C) regions. Cells were separately counted on each side, but since no systematic differences between the two sides were noted, the counts from both sides were combined. The percentage of TH-positive cells expressing c-fos was then determined relative to all catecholaminergic cells in each group. In the hypothalamus, ORX cells with and without c-fos-positive nuclei and c-fos nuclei of other cells were counted in four sections adjacent to the injection site using two 500 μm × 500 μm counting boxes anchored medial and lateral to the ventral tip of the fornix (see Fig. 5A). The counting boxes were further divided into a 125 μm × 125 μm grid, which limited the highest number of cells or nuclei counted within a single subfield under any experimental conditions to less than 30. The counts on the side injected with bicuculline were compared with those obtained from identically anchored counting boxes on the opposite side of each section, and also between the two counting boxes on the injected side of which one did and the other did not contain the centre of the injection site.
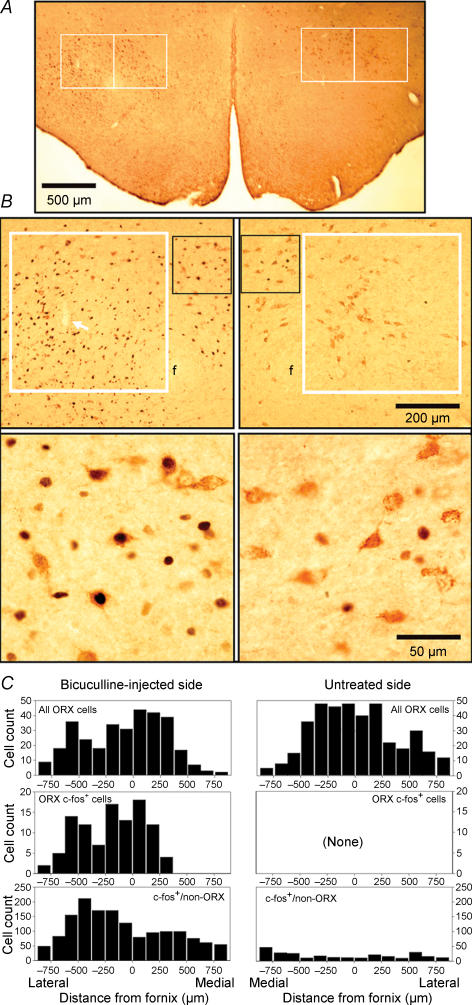
Figure 5. Effect of PF bicuculline injections on c-fos expression in ORX and other neurones near the injection site.
A, a low-magnification image of hypothalamic section at a level adjacent to the site of bicuculline injection. The section is double-labelled for c-fos (black) and prepro-ORX (brown). The white frames show the size and position of the two 500 μm × 500 μm boxes used to count cells on the injected side (left) and symmetrically located regions on the opposite side. The boxes were anchored at the mid-point of the ventral edge of the fornix (f). B, enlargements of the regions enclosed within, and medial to, the lateral counting boxes shown in A. The lower panels show further enlarged details enclosed in the black frames in the corresponding top panels. High density of c-fos-positive nuclei on the injected side (left) contrasts with nearly absent c-fos staining on the opposite side. On the injected side, many ORX neurones express c-fos, whereas on the opposite side c-fos is absent from ORX neurones. The white arrow inside the white frame in the upper left panel points to the track of the microinjection pipette. C, cell counts obtained in this experiment from four hypothalamic sections spaced at 175 μm intervals. The bars show cells counted in 125-μm-wide rectangles contained within the counting boxes placed as in A and in additional rectangles extended an additional 375 μm medially and laterally from the counting boxes. Similar counts of ORX cells were obtained from both sides (top graphs), but only on the injected side some ORX cells were c-fos-positive (c-fos+; middle graphs). The counts of c-fos-positive nuclei in non-ORX cells were of the order of 150–200 in the bins located near the bicuculline injection site, whereas on the opposite side most bins contained less than 15 c-fos-positive nuclei (bottom graphs).
Following verification that the measures were normally distributed, paired, two-tailed Student's t tests were used for statistical analysis (SigmaPlot, Jandel, San Rafael, CA, USA). Differences were considered significant when P was less than 0.05. The variability of the means is characterized by the standard error (s.e.m.) throughout the report.
Results
Properties of pontine carbachol-induced REM sleep-like episodes
The properties of the REM sleep-like episodes elicited in urethane-anaesthetized rats by carbachol injections into a discrete region of the dorsomedial pontine tegmentum have been previously described (Kubin, 2001; Fenik et al. 2002, 2005). They include activation of the cortical EEG, appearance of hippocampal theta-like activity (2.8–4.0 Hz in anaesthetized rats (Vertes et al. 1993)), depression of XII nerve activity and slowing of the central respiratory rhythm (Fig. 1A). When elicited from an optimal pontine site with small volumes of carbachol (10 nl), the effect occurs within seconds, lasts 2–4 min, and can be triggered repeatedly by additional carbachol injections placed at the same site at intervals at least as short as 30 min. Figure 1B shows the distribution of the pontine sites from which REM sleep-like episodes were elicited in this study.
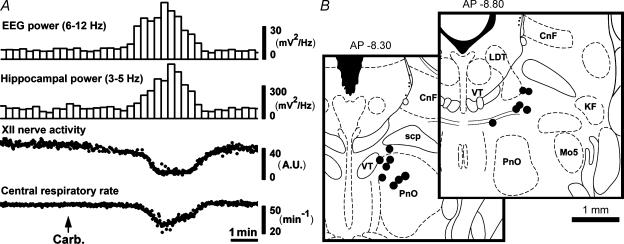
Figure 1. Carbachol-elicited REM sleep-like episodes.
A, a typical REM sleep-like episode elicited by pontine carbachol (10 nl) injection into the dorsomedial pontine tegmentum. The episodes last 2–4 min and include activation of the cortical EEG, the appearance of theta-like activity in the hippocampus, suppression of XII nerve activity and slowing of the central respiratory rhythm. The two bottom traces are composed of discrete points that represent the peaks of the moving average of XII nerve activity and instantaneous respiratory rate, respectively, measured in successive respiratory cycles. B, distribution of the sites of carbachol injections made in this study. The centres of the injection sites are superimposed on the closest standard cross-sections from a rat brain atlas (Paxinos & Watson, 1991). Abbreviations: CnF, cuneiform nucleus; KF, Kölliker–Fuse nucleus; LDT, laterodorsal tegmental nucleus; Mo5, trigeminal motor nucleus; PnO, nucleus pontis oralis; scp, superior cerebellar peduncle; VT, ventral tegmental nucleus.
In the six rats used in the first part of the study, carbachol injections conducted at the beginning of the experiment depressed XII nerve activity by 74 ± 4.9% (P < 0.001; paired t test) and decreased the central respiratory rate from 45.1 ± 1.8 min−1 to 35.8 ± 1.5 min−1 (P < 0.01). The power of cortical EEG in the 6–12 Hz range increased from 7.6 ± 0.9 mV2 Hz−1 to 33 ± 6.0 mV2 Hz−1 (P < 0.01) and the power of hippocampal theta-like activity increased from 84 ± 11 mV2 Hz−1 to 336 ± 64 mV2 Hz−1 (P < 0.01).
Activating effects of unilateral injections of bicuculline into the hypothalamic PF region
Microinjections of bicuculline (20 nl, 1 mm), into the PF region activated the cortical EEG and hippocampal activity, as indicated by a rightward shift of the power spectra of both signals (Fig. 2A). At the time of maximal response to bicuculline, the peak of the cortical spectrum was shifted from 0.9 ± 0.1 Hz to 1.4 ± 0.1 Hz (P < 0.01) and the peak of the hippocampal power shifted from 1.00 ± 0.04 Hz to 1.8 ± 0.2 Hz (P < 0.01) and a secondary peak appeared at 2.5–3.5 Hz (theta-like rhythm). The mean latency of these excitatory effects was 1.2 ± 0.3 min, and the mean duration 33.5 ± 2.6 min (range: 26–45 min) for the six experiments described in the next section.
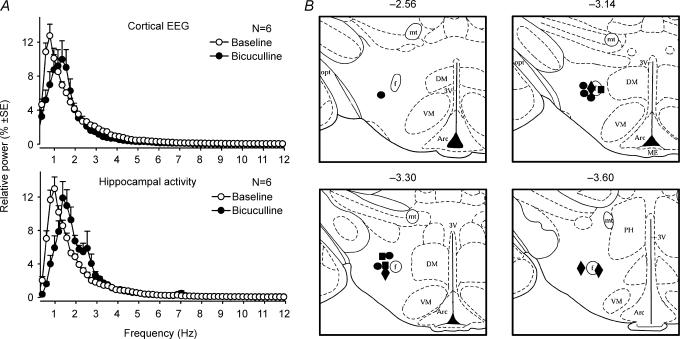
Figure 2. Cortical and hippocampal activation elicited by bicuculline injections into the hypothalamic PF region.
A, the average cortical and hippocampal power spectra obtained from 6 rats under control conditions and following bicuculline microinjections (20 nl, 1 mm) into the PF region of the posterior hypothalamus; a rightward shift of the peaks indicates activation, and the secondary peak that appears in the hippocampal spectrum following bicuculline represents theta-like activity in anaesthetized rats (Vertes et al. 1993). B, distribution of hypothalamic bicuculline injection sites. •, injection sites in the 6 rats in which we studied the effect of PF bicuculline on REM sleep-like effects elicited by pontine carbachol. ▪, injection sites in the 3 rats used in the experiments with c-fos immunohistochemistry. ♦, injection sites in the 4 rats in which PF bicuculline injections were preceded by administration of type 1 ORX receptor antagonist. In all three groups, bicuculline was injected at similar sites and produced similar activating effects. Abbreviations: Arc, arcuate nucleus; DM, dorsomedial hypothalamic nucleus; f, fornix; mt, mammillothalamic tract; opt, optic tract; PH, posterior hypothalamic area; VM, ventromedial hypothalamic nucleus; 3V, third ventricle.
Parallel to cortical and hippocampal activation, the magnitude of respiratory modulation of XII nerve activity was doubled (increased by 100 ± 24%, n= 6; P < 0.05, paired t test), and the central respiratory rate accelerated from 45.1 ± 1.9 min to 47.8 ± 2.1 min (n= 6; P < 0.01). Consistent with previous reports (DiMicco et al. 1987; de Novelis et al. 1995), bicuculline also caused a moderate increase of arterial blood pressure (not shown). Figure 2B shows the distribution of PF bicuculline injection sites (filled circles for the 6 rats further described in the next section; other symbols for additional experiments described in subsequent sections). All injections were located near the fornix at AP levels from −2.5 to −3.6 mm from bregma (Paxinos & Watson, 1991). Figure 3 shows a typical response to PF bicuculline (together with responses to pontine carbachol elicited at successive phases of the main experimental protocol).
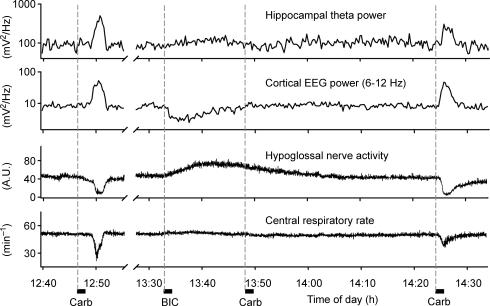
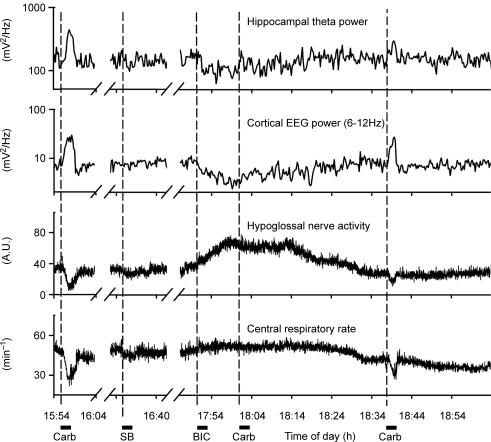
Figure 3. Bicuculline microinjections into the posterior hypothalamus reversibly block the ability of pontine carbachol to elicit REM sleep-like episodes.
Records from an experiment with pontine carbachol injections made before, during and after a PF bicuculline injection (‘BIC’). REM sleep-like episodes could be elicited by pontine carbachol before bicuculline (‘Carb’ at 12.47 h), no component of the response to carbachol could be triggered when the drug was injected 15 min after the onset of the response to bicuculline (‘Carb’ at 13.48 h), but then carbachol was effective again when the effects of PF bicuculline had dissipated (‘Carb’ at 14.24 h). Note that all the effects of carbachol (the appearance of hippocampal theta-like activity, activation of the cortical EEG, depression of XII nerve activity and slowing of the central respiratory rate) were blocked during the response to bicuculline even though the effects of bicuculline itself on the hippocampal and cortical activities and respiratory rate were relatively small at the time of the carbachol injection made at 13.48 h. Dashed vertical lines mark the start times of successive microinjections of carbachol and bicuculline.
Bicuculline injections into the PF hypothalamus block the ability of pontine carbachol to trigger REM sleep-like episodes
In six rats, the effectiveness of pontine carbachol was first verified. Then, bicuculline was injected into the hypothalamic PF region, followed by one or two pontine carbachol injections during the period when the activating effects of PF bicuculline were present. Additional carbachol injections were then made after the recovery from the effects of bicuculline. During the period of bicuculline-induced activation, the ability of pontine carbachol to produce REM sleep-like episodes was entirely blocked in four rats and strongly suppressed in the remaining two animals. Figure 3 shows an example of an experiment in which pontine carbachol injection could not trigger a REM sleep-like episode when it was made 15 min after the onset of the response to bicuculline. In the subsequent test with carbachol, conducted 37 min later (22 min after XII nerve activity and all other signals had returned to their pre-bicuculline levels), all REM sleep-like effects were elicited again.
Figure 4 summarizes the results from six rats in which multiple pontine carbachol injections were made before, during and after hypothalamic bicuculline injection according to the experimental protocol illustrated in Fig. 3. The magnitudes of the responses to pontine carbachol elicited in distinct outputs are shown as the percentage of the corresponding responses elicited in the same animal following carbachol injections made prior to PF bicuculline. The ability of pontine carbachol to trigger REM sleep-like episodes was blocked in all those tests in which carbachol was injected during the initial 11.7–54.1% of the period of bicuculline-induced activation. In the two tests when the effects of carbachol were only partially suppressed, carbachol injections were made just after the onset of the response to bicuculline (at the time corresponding to the first 5.9 or 7.6% of the duration of the response), therefore during the period when the activating effects of PF bicuculline were not yet fully developed.
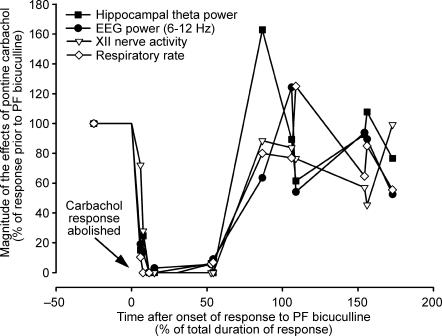
Figure 4. Disinhibition of hypothalamic PF neurones results in a parallel suppression of all components of the REM sleep-like response elicited by pontine carbachol.
The graph shows the results from all pontine carbachol injections made in 6 experiments conducted according to the protocol illustrated in Fig. 3. The magnitudes of the different components of the response to carbachol are expressed as a percentage of the corresponding effect elicited in each animal by pontine carbachol prior to hypothalamic injection of bicuculline. To reflect the sequential nature of these experiments, the abscissa is normalized to the duration of bicuculline-induced activation (which varied among the animals; see text). Different symbols correspond to distinct output parameters, with each symbol of the same type representing one test with pontine carbachol. Carbachol was ineffective (corresponding to 0% on the ordinate) in all tests conducted within 12–54% of the duration of the response to bicuculline. All components of the response to pontine carbachol recovered around the time of termination of the activating effect of bicuculline (over ∼88% on the abscissa).
Bicuculline injections activate ORX and other PF neurones
To assess the extent of neuronal activation produced within the PF hypothalamus by bicuculline and to determine whether ORX cells were activated, we combined immunohistochemistry for prepro-ORX and c-fos, a transcription factor frequently used as a marker of neuronal activation. We used three rats in which bicuculline (20 nl, 1 mm) injections into the PF region elicited cortical and respiratory activation, as described in the preceding section (injection sites marked by filled squares in Fig. 2B). In one of these rats, no carbachol injections were made. Another rat received three pontine carbachol injections, one prior to bicuculline injection, one during the response to bicuculline (ineffective) and one at the end of the response to bicuculline (effective). The third rat had six carbachol injections, three before, two during (ineffective) and one after bicuculline injection. All animals were killed 45–60 min after the end of the response to bicuculline. We then counted ORX cells with and without c-fos-positive nuclei, as well as all other c-fos-stained nuclei, bilaterally in two 500 μm × 500 μm areas, as shown in Fig. 5A. The counts were obtained from four sections closest to the centre of the injection site, thus within approximately ±263 μm anterior and posterior to the injection site.
Figure 5B shows an example of the distribution of c-fos-positive nuclei (black) and ORX cells (brown) on the bicuculline-injected and untreated side in a hypothalamic section from an AP level adjacent to the injection site. In all three experiments, high numbers of c-fos-positive nuclei were present on the injected side but only few in the corresponding area on the opposite side. Many ORX neurones on the injected side were c-fos-positive, whereas on the opposite side we found in 2 of the 3 rats only four ORX neurones weakly stained for c-fos. The average number of c-fos-positive ORX neurones counted in four sections in the counting boxes that contained the injection site was 48 ± 4 per rat. This was more than in the adjacent counting box located at a distance from the injection site (31 ± 6, P < 0.02, n= 3, paired t test). These counts corresponded to 63 ± 18% and 36 ± 9% of all ORX cells counted in the two counting areas.
The numbers of c-fos-positive nuclei belonging to unidentified (non-ORX) cells were much higher than those of c-fos-expressing ORX neurones. There were 759 ± 53 c-fos-positive nuclei per rat in the counting box containing the injection site and 319 ± 27 nuclei in the adjacent counting box (P < 0.02, paired t test). These counts were much higher than the counts of c-fos nuclei in the corresponding counting boxes on the opposite side (68 ± 7 and 70 ± 22; P < 0.005 and P < 0.02, respectively). The average ratios of c-fos-positive nuclei in the counting boxes on the injected side and the corresponding counting boxes on the untreated side were 11 ± 1 and 5.4 ± 1.3. Some non-ORX cells that were activated by bicuculline had relatively large nuclei, as those of ORX neurones, but there were also many c-fos-positive nuclei of about half of that size, suggesting that neurones of several different phenotypes were activated by bicuculline. (Such cells did not include melanin-concentrating hormone-containing neurones because they are not excited by PF bicuculline (Marchenko et al. 2002; Alam et al. 2005; Goutagny et al. 2005).) In the counting box containing the injection site, ORX cells with c-fos represented 6.4 ± 0.5% of all cells expressing c-fos, and in the counting box located further away from the injection site they represented 9.7 ± 2.1% (P > 0.2, not significant).
Figure 5C shows the distribution of c-fos-positive ORX and other cells in one rat in which cell counting was extended by an additional 375 μm medial and lateral from the standard counting boxes to cover the entire mediolateral extent of the lateral hypothalamus. The counts are shown in successive vertical 125 μm-wide sections of the analysed area.
Perifornical bicuculline activates pontine noradrenergic neurones
In urethane-anaesthetized rats, brainstem noradrenergic neurones are spontaneously active (Kubin, 2001; Fenik et al. 2005) and some express c-fos. Since in behaving animals activation of noradrenergic neurones results in suppression of natural REM sleep (e.g. Bourgin et al. 2000), we wished to determine whether noradrenergic cells were activated following PF injections of bicuculline.
We used brainstem sections from the three rats described in the preceding section and from three matched control rats. Of the three control animals, one received only one vehicle (saline with dye) injection into the PF region (that had no effect). The other two rats had three or six pontine carbachol injections timed to match those made in the remaining two rats with PF injections of bicuculline that were used for immunohistochemical procedures. The three control rats were killed and perfused at times after conclusion of the microinjection procedures that matched the timing of microinjections made in the three bicuculline-injected rats.
Figure 6 shows that, in all cell groups analysed (A7, subcoeruleus region, A5 and medullary A1/C1 region), the percentages of catecholaminergic cells with c-fos-positive nuclei tended to be higher in the rats with PF bicuculline injections than in the control animals. The increased percentage of c-fos-positive cells was significant for the A7 group (P < 0.05) alone, close to significant for the A5 group (P= 0.1) and only marginal for the A1/C1 and subcoeruleus regions. Nevertheless, for all four regions combined, the increased percentage of c-fos-positive cells in the bicuculline-injected rats was strongly significant (P < 0.01, d.f. = 11; paired t test).
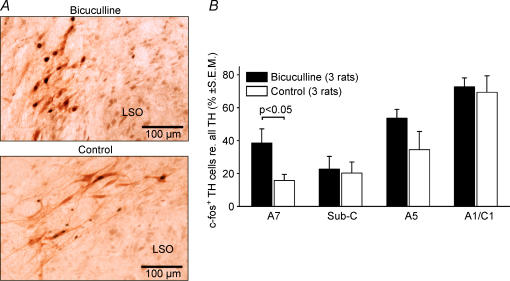
Figure 6. Perifornical (PF) hypothalamic bicuculline increases c-fos expression in pontomedullary catecholaminergic neurones.
A, most noradrenergic A5 neurones have c-fos-positive nuclei in an animal that received PF bicuculline injection (top panel), whereas only a few are c-fos-positive in a control animal (bottom panel). LSO, dorsolateral margin of the lateral superior olive. B, average percentages of c-fos-positive neurones in different catecholaminergic groups in rats with PF bicuculline injections and control animals. In each group, more cells expressed c-fos following PF bicuculline. The difference between the control and bicuculline-injected rats was significant for the A7 group and for all four groups combined.
In the locus coeruleus (LC), a relatively small percentage of neurones (5–40 per section) expressed c-fos under any conditions. There appeared to be a trend towards an increased number of c-fos-positive LC neurones in bicuculline-injected rats, but the high density of noradrenergic neurones in the LC precluded accurate cell counting and determination of the percentages of those that had c-fos-stained nuclei.
In contrast to pontomedullary catecholaminergic neurones, no ventral tegmental dopaminergic neurones (also tyrosine hydroxylase-positive) had c-fos under any experimental conditions. Thus, c-fos immunohistochemistry could not be used to assess whether they were activated in bicuculline-injected rats. In additional series of sections from the same three bicuculline-injected and three control rats, we also used immunohistochemistry for neuronal nitric oxide synthase to visualize pontine cholinergic neurones (Vincent & Kimura, 1992). However, similar to ventral tegmental dopaminergic neurones, pontine cholinergic neurones did not express c-fos in either bicuculline-injected or control rats.
Pre-treatment with type 1 ORX receptor antagonist does not abolish the activating and REM sleep-like state-suppressing effects of PF bicuculline
Given that ORX cells were among those activated by PF bicuculline, we conducted an additional four experiments in which PF bicuculline injections were preceded by a systemic administration of the type 1 ORX receptor antagonist, SB334867. This antagonist was used because prior studies have shown that type 1 ORX receptors are strongly expressed in brainstem wakefulness-related, including noradrenergic, neurones (Bourgin et al. 2000; Marcus et al. 2001). As in earlier studies (Rodgers et al. 2001; Smith et al. 2003; Yamada et al. 2005; Harris et al. 2005), the antagonist was administered 63–87 min prior to PF bicuculline injection, and then carbachol injections were also made to elicit REM sleep-like episodes before, during and following PF bicuculline injections.
In all four rats, carbachol injections were effective at the beginning of the study. The subsequent administration of SB334867 did not have any consistent effects on XII nerve activity, central respiratory rhythm, the power spectra of the cortical or hippocampal activity, or the ability of carbachol to elicit REM sleep-like effects when measured 26–32 min after SB334867 administration. Hypothalamic bicuculline injections (diamonds in Fig. 2B) made after SB334867 increased XII nerve activity, accelerated the central respiratory rhythm, and caused cortical and hippocampal activation. At the peak of the responses, which occurred 67–97 min after SB334867, XII nerve activity was increased by 121 ± 34%, the respiratory rate increased from 45.1 ± 1.6 min−1 to 49 ± 1.2 min−1 (P < 0.05, paired t test), the peak of the cortical power spectrum shifted rightwards from 0.83 ± 0.05 Hz to 1.5 ± 0.22 Hz (P < 0.05), and hippocampal power spectrum from 0.97 ± 0.19 Hz to 1.86 ± 0.45 Hz. None of these effects was different from those obtained without pre-treatment with SB334867. Also, similar to the experiments without SB334867, the REM sleep-like responses to pontine carbachol were blocked during the period of bicuculline-induced activation and then could be elicited again when the activating effect of bicuculline dissipated. Figure 7 shows the records from one of the experiments with SB334867 using the same format as in Fig. 3.
Figure 7. Posterior hypothalamic injections of bicuculline have activating effects and block REM sleep-like episodes triggered by pontine carbachol in rats pre-treated with type 1 ORX receptor antagonist.
Records from 1 of the 4 rats treated with SB334867 (SB; 30 mg kg−1, i.p.) about 80 min prior to PF bicuculline injection, shown in the same format as in Fig. 3. As without SB334867 (Fig. 3), PF bicuculline had activating effect and blocked the ability of pontine carbachol to elicit a REM sleep-like episode (‘Carb’ at 18.01 h). Carbachol was then effective again after termination of bicuculline effects (at 18.37 h).
Discussion
We hypothesized that activation of PF cells with bicuculline would alter the magnitude of the effects of pontine carbachol, with possibly a differential action on distinct aspects of the REM sleep-like response. Contrary to our expectation, we found that microinjections of bicuculline into the hypothalamic PF region entirely blocked the ability of pontine carbachol to elicit any REM sleep-like effects. Importantly, the components of the response that were eliminated had different principal sites of origin, such as the brainstem for suppression of XII motoneuronal activity and decrease of respiratory rate (Kimura et al. 1990), the ponto-septo-hippocampal system for the theta-like rhythm (Vertes & Kocsis, 1997), and the basal forebrain and cortex for cortical activation (e.g. Fadel et al. 2005; Jones, 2005). Thus, it appears that activation of GABAA receptor-expressing PF neurones caused a concerted activation of those hypothalamic descending pathways that block the occurrence of REM sleep.
Possible mechanisms of REM sleep suppression by activation of hypothalamic PF neurones
We based our experimental design on the findings that GABAergic sleep-active anterior hypothalamic neurones project to the PF hypothalamus (Saper et al. 2001, 2005; Uschakov et al. 2006) and GABA release is increased in the PF region during sleep (Nitz & Siegel, 1996). Conversely, wakefulness and arousal are associated with increased activity of PF neurones (Estabrooke et al. 2001; Alam et al. 2002; España et al. 2003; Koyama et al. 2003; Mileykovskiy et al. 2005). In the dorsomedial pons, acetylcholine levels are increased during REM sleep (Kodama et al. 1990), and cholinergic receptor agonists injected into this region trigger, or enhance, the electrophysiological phenomena of REM sleep (reviewed by Kubin, 2001). Thus, we reasoned that the effect of disinhibition of PF neurones on cholinergic triggering of the REM sleep-like state from the pons may mimic the hypothalamo–pontine interactions that occur during the natural control of sleep and wakefulness. It needs to be noted that, although carbachol models of REM sleep adequately mimic many cellular and electrophysiological phenomena of this state, they do not exhibit certain phasic phenomena, such as muscle twitches or transient accelerations of the respiratory rate (Kimura et al. 1990; Kubin, 2001). Therefore, we do not know whether such phasic changes, not present in our model, also would be affected by disinhibition of PF neurones. Nevertheless, our results demonstrate that PF neurones, and specifically those that express GABAA receptors, can prevent generation of multiple REM sleep-like electrophysiological events that are normally initiated and orchestrated by a network of pontomedullary neurones.
It is unlikely that the abolition of the effects of carbachol was due to an occlusion, or saturation, of the systems involved because all aspects of the response to carbachol were eliminated and then re-appeared simultaneously and the effects of bicuculline only on the outputs studied were relatively modest when compared with either the effects of carbachol only or to other experimental conditions in which altering the levels of XII nerve activity or respiratory rate does not interfere with the ability of carbachol to trigger REM sleep-like effects. For example, carbachol can still trigger REM sleep-like episodes in anaesthetized rats when XII nerve activity and respiratory rate are increased by increasing the chemical drive for breathing or when XII nerve activity is increased by microinjections of excitatory compounds directly into the XII nucleus. Therefore, it appears that disinhibition of PF cells sets the pontine REM sleep-generating network in a mode in which it cannot be activated even by carbachol injections that are otherwise extremely effective in anaesthetized rats. This interpretation is consistent with current hypotheses about the role of the PF hypothalamus and ORX neurones in the control of REM sleep (Chemelli et al. 1999; Lin et al. 1999; Kilduff & Peyron, 2000; Mignot et al. 2002), including the concept that neurones of this region stabilize behavioural states and prevent random state transitions (Saper et al. 2001, 2005).
Activation of PF neurones, including those containing ORX, may block the occurrence of REM sleep through activation of multiple wake- and movement-related neurones, such as serotonergic, noradrenergic, dopaminergic and cholinergic (Kilduff & Peyron, 2000; Bourgin et al. 2000; Fadel et al. 2005; Jones, 2005). In agreement with this, we found increased c-fos expression in pontomedullary catecholaminergic neurones. The percentage of those expressing c-fos was substantially increased in the A7 and A5 groups, and possibly also in the LC. Activation of LC neurones by various means prevents REM sleep (e.g. see Bourgin et al. 2000), whereas a contribution of other pontine noradrenergic neuronal groups to the regulation of sleep has not been well defined. However, it is noteworthy that, in anaesthetized rats, silencing of A5 neurones by clonidine can trigger REM sleep-like episodes similar to those elicited by dorsomedial pontine injection of carbachol, whereas silencing of LC neurones does not have such an effect (Fenik et al. 2002). A chemical lesion of LC neurones also has minor effects on the amounts of sleep, including REM sleep (Blanco-Centurion et al. 2004). Thus, our finding that activation of A5 and A7 neurones occurs in association with suppression of the ability of pontine carbachol to trigger REM sleep-like episodes supports the possibility that these noradrenergic groups have a REM sleep-suppressing function. Based on the distribution of efferent projections from the PF region, including those containing ORX, excitatory effects of PF bicuculline on brainstem serotonergic, dopaminergic and cholinergic neurones could also contribute to the abolition of the ability of carbachol to trigger the REM sleep-like state in our experiments (Allen & Cechetto, 1992; Luppi et al. 1995; Marcus et al. 2001; Oldfield et al. 2002; Berthoud, 2002; Samuels et al. 2003). This remains to be investigated and, at least in the case of cholinergic and dopaminergic neurones, may require methods other than c-fos immunohistochemistry because these neurones did not express c-fos under our experimental conditions.
Respiratory activating effects of PF bicuculline
Prior studies demonstrated that activation of hypothalamic PF neurones increases heart rate and arterial blood pressure (DiMicco et al. 1987; Allen & Cechetto, 1992; de Novelis et al. 1995), thermogenesis (Zaretskaia et al. 2002), exploratory behaviour and food intake (Luiten et al. 1987; Rodgers et al. 2001; Harris et al. 2005), and locomotor activity (DiScala et al. 1984), thus elicits effects typically associated with a high level of arousal and/or motivation. Here, we found that PF bicuculline also increased central respiratory activity. Both the magnitude of respiratory modulation of XII nerve activity and respiratory rate were increased parallel to cortical and hippocampal activation.
These activating effects on XII motoneurones could be mediated by direct projections of hypothalamic ORX neurones to the XII motor nucleus (Kilduff & Peyron, 2000; Marcus et al. 2001; Volgin et al. 2002) and indirectly by pontine noradrenergic neurones (Fenik et al. 2005; Rukhadze & Kubin, 2007a). They could also be secondary to activation of the mesencephalic locomotor region which is likely to occur in response to PF bicuculline (Takakusaki et al. 2005). The increased respiratory rate could occur through an increased excitatory serotonergic drive that facilitates medullary respiratory rhythm-generating neurones (Pena & Ramirez, 2002). Thus, our finding that PF disinhibition increases respiratory activity further expands the concept that neurones of the PF region can synchronously switch multiple neuronal systems into a state characteristic of various conditions associated with a high level of behavioural arousal.
Role of ORX neurones in mediation of the REM sleep-like state-suppressing effect of PF bicuculline
This and earlier studies have shown that PF bicuculline injections activate ORX-containing as well as many non-ORX hypothalamic neurones (Marchenko et al. 2002; Alam et al. 2005; Goutagny et al. 2005). This outcome is similar to the observations that ORX-containing neurones are but one cell type activated in the PF region in association with wakefulness and arousal (Estabrooke et al. 2001; Alam et al. 2002, 2005; España et al. 2003; Koyama et al. 2003). Therefore, several PF cell types may contribute to the abolition of the effectiveness of pontine carbachol.
To begin dissecting the contributions of different PF cells to the ability of bicuculline to produce its activating effects and prevent pontine carbachol from inducing REM sleep-like episodes, we pre-treated rats with a type 1 ORX receptor antagonist. The antagonist that we used has been previously shown to pass the blood–brain barrier (Rodgers et al. 2001). At the dose used, its effects were significant at 20 min after administration and reached a maximum after about 60 min (Rodgers et al. 2001). SB334867 has been repeatedly shown to block the effects of centrally administered ORX (Smith et al. 2003; Yamada et al. 2005) and, in some studies, it antagonized endogenous behavioural effects ascribed to activation of ORX neurones (Rodgers et al. 2001; Harris et al. 2005). In our and other studies (Smith et al. 2003; Yamada et al. 2005), the drug had no effects on the baseline cortical, hippocampal or XII nerve activity, presumably because ORX cells are silent under anaesthesia, as they are in quietly awake and drowsy animals (Mileykovskiy et al. 2005).
We found that neither the ability of PF bicuculline to produce activation nor its ability to block carbachol-induced REM sleep-like effects was altered by pre-treatment with SB334867. In view of the prior evidence for satisfactory efficacy and selectivity for ORX type 1 receptors of the antagonist at the same dose as that used in our study (Rodgers et al. 2001; Smith et al. 2003; Yamada et al. 2005; Harris et al. 2005), this result suggests that the effects of PF bicuculline that we observed were not mediated by type 1 ORX receptors. This, in turn, would point to a minimal role of a direct stimulation of LC by ORX in those effects, as noradrenergic LC neurones express type 1 ORX receptors only (Bourgin et al. 2000; Hervieu et al. 2001; Marcus et al. 2001). On the other hand, considering that PF bicuculline induced c-fos in many ORX neurones, it is possible that the activating effects it induced were mediated, at least in part, by type 2 ORX receptor. This receptor, for which effective antagonists are still being developed, plays a key role in canine narcolepsy (Lin et al. 1999). In rats, chemical lesions of posterior hypothalamic cells expressing this receptor produced narcolepsy-like behaviour (Gerashchenko et al. 2001), and destruction of pontine cells that had this receptor diminished REM sleep-related motor suppression (Blanco-Centurion et al. 2004). However, another study in knock-out mice compelled the authors to conclude that ‘the profound dysregulation of REM sleep control unique to the narcolepsy–cataplexy syndrome emerges from loss of signalling through both OX2R [orexin type 2 receptor]-dependent and OX2R-independent pathways’ (Willie et al. 2003). Therefore, while a contribution of the type 2 ORX receptors needs to be considered, it is important to note that, in our study, many more non-ORX than ORX PF neurones were activated by bicuculline.
Earlier c-fos expression and cell recording studies suggested that non-ORX neurones of the PF region may play an important role in promoting active behaviours associated with wakefulness and would be expected to have actions that oppose sleep (Estabrooke et al. 2001; Alam et al. 2002, 2005; España et al. 2003; Koyama et al. 2003). Some of these cells may have projections to other arousal-related brain regions (Allen & Cechetto, 1992; Bittencourt & Elias, 1993; Luppi et al. 1995; Oldfield et al. 2002; Berthoud, 2002; Saper et al. 2005). Thus, there is need to further dissect the contribution of non-ORX, wake-related PF neurones to the activating and sleep-opposing functions of this hypothalamic region.
Conclusions
We found that unilateral activation of GABAA receptor-expressing cells in the posterior hypothalamic PF region entirely blocks the ability of pontine carbachol to elicit REM sleep-like effects. This effect is probably due to a stereotyped activation of hypothalamic descending pathways that are designed to oppose the occurrence of sleep. At the level of the PF hypothalamus, the effect is, at least in part, mediated by activation of ORX neurones, and at the brainstem level, by noradrenergic neurones. However, other arousal-related hypothalamic and brainstem neurones are also likely to be involved, and the role of type 1 ORX receptors appears to be minimal. Narcolepsy/cataplexy, a disorder of REM sleep characterized by excessive sleepiness and unchecked occurrence of REM sleep-like postural atonia, may result from malfunction of the hypothalamic pathways activated by bicuculline in this study.
Acknowledgments
The study was supported by grant HL-071097 from the National Institutes of Health.
References
- Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep–wake discharge patterns of neurones recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone immunoreactive neurones during sleep in rats. J Physiol. 2005;563:72–82. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area. I. Descending projections. J Comp Neurol. 1992;315:313–332. doi: 10.1002/cne.903150307. [DOI] [PubMed] [Google Scholar]
- Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area. II. Ascending projections. J Comp Neurol. 1993;330:421–438. doi: 10.1002/cne.903300310. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Elias CF. Diencephalic origins of melanin-concentrating hormone immunoreactive projections to medial septum/diagonal band complex and spinal cord using two retrograde fluorescent tracers. Ann N Y Acad Sci. 1993;680:462–465. doi: 10.1111/j.1749-6632.1993.tb19708.x. [DOI] [PubMed] [Google Scholar]
- Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. Effects of hypocretin2-saporin and antidopamine-β-hydroxylase-saporin neurotoxic lesions of the dorsolateral pons on sleep and muscle tone. Eur J Neurosci. 2004;19:2741–2752. doi: 10.1111/j.1460-9568.2004.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin P, Huitrón-Reséndiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- de Novelis V, Stotz-Potter EH, Morin SM, Rossi F, DiMicco JA. Hypothalamic sites mediating cardiovascular effects of microinjected bicuculline and EAAs in rats. Am J Physiol Regul Integr Comp Physiol. 1995;269:R131–R140. doi: 10.1152/ajpregu.1995.269.1.R131. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Abshire VM, Shekhar A, Wilbe JH., Jr Role of GABAergic mechanisms in the central regulation of arterial pressure. Eur Heart J. 1987;8(Suppl. B):133–138. doi: 10.1093/eurheartj/8.suppl_b.133. [DOI] [PubMed] [Google Scholar]
- DiScala G, Schmitt P, Karli P. Flight induced by infusion of bicuculline methiodide into periventricular structures. Brain Res. 1984;309:199–208. [PubMed] [Google Scholar]
- España RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou T, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neuroscience. 2005;130:541–547. doi: 10.1016/j.neuroscience.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik V, Fenik P, Kubin L. A simple cuff electrode for nerve recording and stimulation in acute experiments on small animals. J Neurosci Meth. 2001;116:147–150. doi: 10.1016/s0165-0270(01)00340-5. [DOI] [PubMed] [Google Scholar]
- Fenik V, Marchenko V, Janssen P, Davies RO, Kubin L. A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol. J Appl Physiol. 2002;93:1448–1456. doi: 10.1152/japplphysiol.00225.2002. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Kohls MD, Greco MA, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutagny R, Luppi PH, Salvert D, Gervasoni D, Fort P. GABAergic control of hypothalamic melanin-concentrating hormone-containing neurons across the sleep-waking cycle. Neuroreport. 2005;16:1069–1073. doi: 10.1097/00001756-200507130-00008. [DOI] [PubMed] [Google Scholar]
- Harris G, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hervieu GL, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Recherches sur les structures nerveuses et les mécanismes responsables des différentes phases du sommeil physiologique. Arch Ital Biol. 1962;100:125–206. [PubMed] [Google Scholar]
- Jouvet M. Paradoxical sleep mechanisms. Sleep. 1994;17:S77–S83. doi: 10.1093/sleep/17.suppl_8.s77. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- Kimura H, Kubin L, Davies RO, Pack AI. Cholinergic stimulation of the pons depresses respiration in decerebrate cats. J Appl Physiol. 1990;69:2280–2289. doi: 10.1152/jappl.1990.69.6.2280. [DOI] [PubMed] [Google Scholar]
- Kodama T, Takahashi Y, Honda Y. Enhancement of acetylcholine release during paradoxical sleep in the dorsal tegmental field of the cat brain stem. Neurosci Lett. 1990;114:277–282. doi: 10.1016/0304-3940(90)90576-u. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–1219. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lu JW, Mann GL, Fenik VB, Kubin L. Disinhibition of perifornical hypothalamic neurons blocks the ability of pontine carbachol to produce REM sleep-like state in anesthetized rats. Sleep. 2006;29(suppl):A22. [Google Scholar]
- Luiten PG, Ter Horst GJ, Steffens AB. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol. 1987;28:1–54. doi: 10.1016/0301-0082(87)90004-9. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- Marchenko V, Swan J, Kubin L. Antagonism of hypothalamic GABAA receptors activates cortical EEG, elicits hippocampal theta and stimulates cardiorespiratory outputs in urethane-anesthetized rats. Abstr Soc Neurosci. 2002;28:870.13. [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5:1071–1075. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, Siegel JM. GABA release in posterior hypothalamus across sleep-wake cycle. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1707–R1712. doi: 10.1152/ajpregu.1996.271.6.R1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. San Diego: Academic Press; 1991. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JCG, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JRS, Upton N, Porter RA, Johns A, Blundell JE. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Kubin L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J Chem Neuroanat. 2007a;33:23–33. doi: 10.1016/j.jchemneu.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Rukhadze I, Kubin L. Mesopontine cholinergic projections to the hypoglossal motor nucleus. Neurosci Lett. 2007b;413:121–125. doi: 10.1016/j.neulet.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, Jouvet M. Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience. 1989;32:669–683. doi: 10.1016/0306-4522(89)90289-3. [DOI] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol. 2003;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol. 2005;493:92–98. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou T, Scammell TE. The sleep switch: hypothalamic regulation of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC. SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MI, Piper DC, Duxon MS, Upton N. Evidence implicating a role for orexin-1 receptor modulation of paradoxical sleep in the rat. Neurosci Lett. 2003;341:256–258. doi: 10.1016/s0304-3940(03)00066-1. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Takahashi K, Saitoh K, Harada H, Okumura T, Kayama Y, Koyama Y. Orexinergic projections to the cat midbrain mediate alternation of emotional behavioural states from locomotion to cataplexy. J Physiol. 2005;568:1003–1020. doi: 10.1113/jphysiol.2005.085829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uschakov A, Gong H, McGinty D, Szymusiak R. Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and perifornical lateral hypothalamus. Eur J Neurosci. 2006;23:3284–3296. doi: 10.1111/j.1460-9568.2006.04860.x. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Colom LV, Fortin WJ, Bland BH. Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Exp Brain Res. 1993;96:419–429. doi: 10.1007/BF00234110. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Volgin D, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. Neuroreport. 2002;13:433–436. doi: 10.1097/00001756-200203250-00014. [DOI] [PubMed] [Google Scholar]
- von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–259. [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of non REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Woch G, Davies RO, Pack AI, Kubin L. Behaviour of raphe cells projecting to the dorsomedial medulla during carbachol-induced atonia in the cat. J Physiol. 1996;490:745–758. doi: 10.1113/jphysiol.1996.sp021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woch G, Ogawa H, Davies RO, Kubin L. Behavior of hypoglossal inspiratory premotor neurons during the carbachol-induced, REM sleep-like suppression of upper airway motoneurons. Exp Brain Res. 2000;130:508–520. doi: 10.1007/s002219900244. [DOI] [PubMed] [Google Scholar]
- Yamada H, Takahashi N, Tanno S, Nagamine M, Takakusaki K, Okumura T. A selective orexin-1 receptor antagonist, SB334867, blocks 2-DG-induced gastric acid secretion in rats. Neurosci Lett. 2005;376:137–142. doi: 10.1016/j.neulet.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]