Abstract
Abdominal surgery causes postoperative gastrointestinal dysmotility which can progress to paralytic ileus. Surgery causes inflammatory responses leading to loss of interstitial cells of Cajal (ICC), which generate intestinal pacemaker activity. Here, we demonstrate that a deficiency in or pharmacological inhibition of inducible nitric oxide synthase (iNOS) before surgery protects ICC from postoperative damage. Ileal segments from wild-type, iNOS and cyclooxygenase-2 (COX-2) knockout mice were resected and reconstructions were performed by end-to-end anastomoses. Wild-type animals were exposed to iNOS inhibitors before surgery, and electrical activity and ICC were examined 5 h after surgery. Intestinal surgery on wild-type mice caused a significant reduction in ICC and pacemaking at distances up to 5 cm from the anastomosis site. ICC networks and pacemaking were protected in iNOS−/− mice. In animals treated preoperatively with iNOS inhibitors, pacemaker activity was depressed only at the anastomosis site. COX-2 deficiency also muted postoperative disruption in pacemaker activity. Postoperative surgical damage consists of a local response and a more widespread response in which ICC and pacemaker activity are disrupted. Damage to ICC and pacemaking was greatly attenuated in the absence of NO derived from iNOS. Thus, management of iNOS expression or activity prior to intestinal surgery protects against postsurgical dysmotility and reduces the severity of postoperative ileus.
Interstitial cells of Cajal (ICC) generate and propagate electrical rhythmicity and receive and transduce motor neural and mechanical inputs in gastrointestinal (GI) muscles (Sanders et al. 2006). Therefore, these cells are fundamental to the generation and regulation of motor patterns in the GI tract. Several recent reports have described loss or reduced density of ICC in a variety of genetic, surgical, infectious and idiopathic motility disorders (Yamataka et al. 1998; Hagger et al. 2000; He et al. 2000; Feldstein et al. 2003; Zarate et al. 2003), and these observations have suggested new hypotheses about the aetiology of GI motor dysfunction. For example, in a model of type 1 diabetes, we reported loss of pacemaker ICC in the gastric antrum that resulted in abnormal electrical rhythmicity, complete loss of rhythmicity in specific regions of muscle, defective slow-wave propagation to regions lacking ICC, and reduced rate of gastric emptying (Ördög et al. 2000). Studies of human diabetics have also reported reduction in ICC in the stomach, small bowel and colon (He et al. 2001; Nakahara et al. 2002; Forster et al. 2005). More recent studies have suggested that the loss of ICC in diabetes may result from a general myopathy, and specifically loss of stem cell factor, the ligand for Kit, that accompanies loss of insulin and insulin-like growth factor-1 (IGF-1) signalling in diabetic animals (Horvath et al. 2006). Others have shown effects ranging from ultrastructural abnormalities to frank loss of cells in infectious models of inflammatory bowel disease, and speculated that changes in ICC networks might contribute to motility dysfunction accompanying these diseases.
We have previously studied the loss of ICC in partial mechanical obstruction and in surgical resection (Chang et al. 2001; Yanagida et al. 2004). Partial obstruction leads to hypertrophy of the bowel that develops over a period of days. ICC are reduced in a spatial and temporal manner such that just proximal to the site of obstruction there are few ICC and major defects in the ability to generate or propagate slow waves and to respond to excitatory and inhibitory neural inputs (Chang et al. 2001). The lesion, along with the hypertrophy, decreases as a function of distance proximal to the site of obstruction. A recent study has proposed an inflammatory link in the loss of ICC proximal to intestinal obstructions, showing a significant increase in the number of ED2-positive macrophages and expression of the pro-inflammatory cytokine tumour-necrosis factor-α (TNF-α) (Won et al. 2006). Surgical resection leads to changes in ICC that are at least an order of magnitude more rapid than observed in obstruction models. We have previously noted dramatic reduction of ICC in the small intestine within 5 h of resection, and suggested that deleterious effects on ICC could be related to the common occurrence of postsurgical motility defects (Yanagida et al. 2004). Infiltration of the resection site by leucocytes (Yanagida et al. 2004), and the link between loss of ICC and inflammatory processes (Won et al. 2006), suggest the possibility that surgery may promote the loss of ICC via an inflammatory pathway. Previous studies have shown that manipulation of the bowel and trauma lead to upregulation of iNOS and cyclooxygenase-2 (COX-2) expression (Kalff et al. 2000, 2003). Here, we have performed small bowel resection on wild-type, iNOS and COX-2 mutant mice, and compared ICC loss and functional defects in these animals in response to surgery. Our results suggest the exciting possibility that preoperative inhibition of iNOS might protect ICC networks and electrical rhythmicity from the deleterious effects of intestinal surgery.
Methods
Animal surgery
Adult BALB/c mice (40–60 days and body weights of 25–30 g) were obtained from breeder pairs purchased from Charles River Laboratories (Wilmington, MA, USA). iNOS and COX-2 mutant mice (iNOS−/− and COX-2−/−) and their age-matched wild-type controls (C57BL/6 and B6129SF2/J, respectively), obtained from The Jackson Laboratory (Bar Harbour, ME, USA), were also utilized for functional and morphological studies. All surgical manipulations were performed under anaesthesia as previously described (Yanagida et al. 2004). Briefly, mice were anaesthetized with a single subcutaneous dose of pentobarbital (Nembutal 80 μg g−1; Abbott Laboratory, Chicago IL, USA). When the absence of a hind limb pinch-withdrawal reflex was verified, a medial laparotomy was performed. A segment of ileum (approximately 20 mm in length, 50–60 mm from the ileocaecal sphincter was resected after a single branch of mesenteric artery supplying the piece of resected intestine was ligated. Ileal reconstruction was performed by an end-to-end anastomosis using fine suture thread (7-0 Ethilon; Ethicon, Somerville, NJ, USA) without disrupting the blood supply to the intestine. The intestine was replaced into the peritoneal cavity, and the abdomen closed with 5-0 silk suture thread. During surgery, and postoperatively during recovery, body temperature was maintained at 36–38°C using a heating pad. Throughout the surgery and recovery, the animals were intermittently oxygenated with a 97% O2–3% CO2 mixture using a fine polyethylene tube placed over the nasal passage. A group of sham-operated animals was also prepared as controls. These animals underwent the laparotomy but did not undergo intestinal resection. After 5 h, mice had recovered from surgery and anaesthesia and moved freely about their cages. In some experiments wild-type control mice were injected subcutaneously with aminoguanidine (20 mg kg−1) or N-(3-(aminomethyl) benzyl)acetamidine (1400W; 2 mg kg−1) 30 min prior to surgery to examine the effects of iNOS inhibition on recovery from surgery.
Experimental animals and sham-operated controls were killed for electrophysiological and immunohistochemical studies at 5 h post surgery by applying anaesthesia with isoflurane followed by exsanguination and decapitation. After each animal was killed, the entire gastrointestinal tract, from 5 mm oral to the lower oesophageal sphincter to 10 mm oral to the internal anal sphincter, was removed and placed in Krebs–Ringer buffer (KRB). The reconstructed intestines from operated and sham procedures did not appear ischaemic and were not obstructed.
The animals were maintained and the experiments performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all procedures used were approved by the Institutional Animal Use and Care Committee at the University of Nevada.
Electrophysiological experiments
A region of small intestine 100 mm in length, from 50 mm oral to the site of anastomosis to 50 mm anal to the site was isolated. The intestine was opened along the mesenteric border, and lumenal contents were washed away with KRB. Segments of the bowel were pinned to the base of a Sylgard silicone elastomer (Dow Corning Corp., Midland, MI, USA) dish and the mucosa was removed by sharp dissection. The electrical activity of the small intestinal muscles was examined using intracellular microelectrode recording techniques. For the intracellular recordings, strips of muscle (10 mm × 5 mm) were cut from sites oral to the anastomosis. The specific sites from which recordings were made are designated in the text and figures. The muscles were placed in a recording chamber with the submucosal aspect of the muscle facing upward. Impalements of cells were made with glass microelectrodes having resistances of 50–90 MΩ and transmembrane potentials were amplified using Axoprobe-1A amplifier (Axon Instruments, Union City, CA, USA). Data were acquired on the computer and slow waves were analysed and final figures produced using Microcal Origin (Microcal Software Inc.) and Power Point software (Microsoft Inc.). Experiments were performed in the presence of nifedipine (1 μm) in the perfusion solution to reduce contractions and facilitate impalements of cells for extended periods. We have previously shown that slow waves are not affected by nifedipine (Ward et al. 1994).
Solutions and drugs
The bath chambers were constantly perfused with oxygenated KRB of the following composition (mm): NaCl 120.35; KCl 5.9; MgCl2 1.2; NaHCO3 15.5; NaH2PO4 1.2; dextrose 11.5; CaCl2 2.5. The pH of the KRB was 7.3–7.4 when bubbled with 97% O2–3% CO2 at 37 ± 0.5°C. After pinning, the muscles were left to equilibrate for at least 1 h before experiments were begun. Nifedipine, aminoguanidine and 1400W were obtained from Sigma (St Louis, MO, USA).
Analysis of electrophysiological data
Data are expressed as means ± standard errors of the mean. Differences in the data were evaluated by one-way ANOVA followed by all-pairwise multiple comparisons against the control (Tukey's test) for statistical comparisons. A probability value of P < 0.05 was used as a cut-off for statistical significance in all procedures. P values quoted for ANOVA are the values for the individual post hoc test. The ‘n’ values reported in the text refer to the number of muscle strips from which recordings were performed. Each muscle strip used in an ‘n’ value was taken from a separate animal. Several electrical parameters were analysed: (i) resting membrane potential (RMP), (ii) slow-wave amplitude, and (iii) frequency.
Immunohistochemistry
Immunohistochemical analysis was performed on pieces of muscles at the same distances from the site of anastomosis and directly adjacent to the regions of tissue used for electrophysiological studies. Tissues from approximately the same region of intestine were also prepared from sham-operated animals. Whole-mount preparations were prepared by removing the mucosa by sharp dissection. The remaining strips of tunica muscularis were pinned to the base of a dish filled with Sylgard elastomer (Dow Corning Corp.) with the mucosal side of the circular muscle layer facing upward. Tissues were fixed in acetone (4°C for 10 min). Following fixation, preparations were washed for 60 min in phosphate-buffered saline (PBS; 0.01 m, pH 7.4). Incubation of tissues in 1% bovine serum albumin for 1 h at room temperature containing 0.3% Triton X-100 was used to reduce non-specific antibody binding. For examination of ICC, tissues were incubated overnight at 4°C with a rat monoclonal antibody raised against Kit protein (ACK2; 5 μg ml−1 in PBS; Gibco-BRL, Gaithersburg, MD, USA). Immunoreactivity was detected using fluorescein-isothiocyanate-conjugated secondary antibody (FITC-anti rat; Vector Laboratories, Burlingame, CA, USA; 1:100 in PBS, 1 h, room temperature). Control tissues were prepared in a similar manner, omitting ACK2 from the incubation solution. Tissues were examined with a Zeiss LSM 510 Meta confocal microscope with an excitation wavelength appropriate for FITC (488 nm). Confocal micrographs are digital composites of Z-series scans of 10–30 optical sections through a depth of 10–30 μm. Final images were constructed with Zeiss software.
Results
Disruption in pacemaking activity and ICC-MY networks after intestinal resection
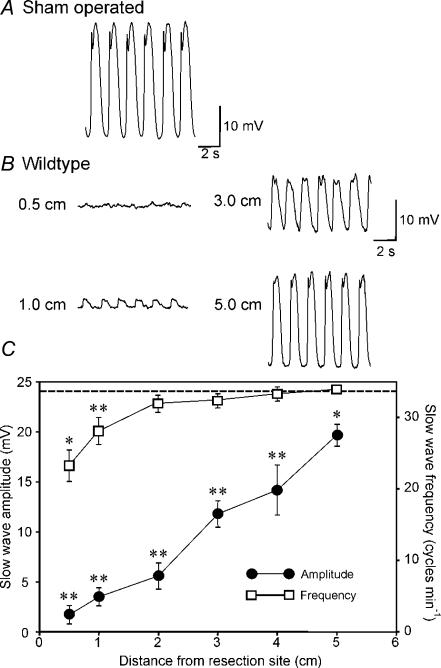
Intracellular electrical recordings were performed on murine intestinal muscles 5 h after intestinal resection and reconstruction surgery. We have previously shown that the pacemaker activity of intestinal muscles was reduced equally oral and aboral to the site of anastomosis (Yanagida et al. 2004). Therefore, in this study we examined electrical activity and ICC populations 0–5 cm oral from the site of anastomosis. RMP, measured with intracellular microelectrodes, averaged −60 ± 2 mV at 1 cm from the site of anastomosis and −62 ± 1 mV at 5 cm (n= 10 and 13, respectively). These values were not statistically different from RMPs of sham-operated mice (−64 ± 2 mV, P > 0.05), and were not significantly different from each other within the group of the control surgery mia (1–5 cm, P > 0.05, by ANOVA). The amplitude of electrical slow waves was substantially reduced however, near the site of anastomosis, averaging 1.7 ± 0.9 and 3.5 ± 0.9 mV at 0.5 and 1 cm, respectively (n= 10), in comparison with slow waves recorded from muscles of sham-operated mice (i.e. 24.1 ± 0.9 mV in amplitude; n= 8; P < 0.01). Slow-wave amplitude increased as a function of distance from the site of anastomosis, but slow waves were still significantly depressed in amplitude at 5 cm from the anastomosis site (i.e. 19.7 ± 1.1 mV; n= 13; P < 0.05; Fig. 1B and C). These values were significantly different from each other within the group of the control operation animals (P < 0.001, by ANOVA). Although amplitude of slow waves was reduced at all recording sites, a significant decrease in slow wave frequency was noted only 0.5–1 cm from the site of anastomosis. For example, at 1.0 cm from the anastomosis, slow-wave frequency averaged 28.1 ± 1.9 cycles min−1 (P < 0.05), and at 5 cm slow-wave frequency was 33.9 ± 0.6 cycles min−1. The frequency of slow waves at 2–5 cm from the anastomosis was not significantly different from the slow-wave frequency in muscles from sham-operated mice (31.9 ± 0.3 cycles min−1; n= 8; P > 0.05). These values were significantly different from each other within the group of the control operation animals (P < 0.001, by ANOVA). Figure 1C shows averaged slow-wave amplitude and frequency as a function of distance from the anastomosis site for eight experiments.
Figure 1. Disruption of pacemaker activity in the small intestine 5 h after intestinal resection.
A and B, typical intracellular electrical activity (slow waves) recorded from the circular muscle of the small intestine of a sham-operated mouse (A) and at various distances (0.5–5 cm) from the site of an intestinal anastomosis in a BALB/c mouse (B). Slow-wave amplitude was dramatically reduced near the site of anastomosis and increased in amplitude as a function of distance from the resection site. C, summary of the changes in slow-wave amplitude (•) and frequency (□) as a function of distance from the site of anastomosis (n= 13 animals). The data are compared to the amplitude of slow waves in sham-operated animals (dashed line; n= 8 animals). Slow-wave amplitude was significantly reduced at all sites tested after surgery compared with sham-operated animals. Frequency was reduced only at 0.5 and 1 cm from the site of the anastomosis (*P < 0.05, **P < 0.01). Values in the amplitude and frequency of slow waves were significantly different from each other within the group of the control operation model (both P < 0.001, by ANOVA).
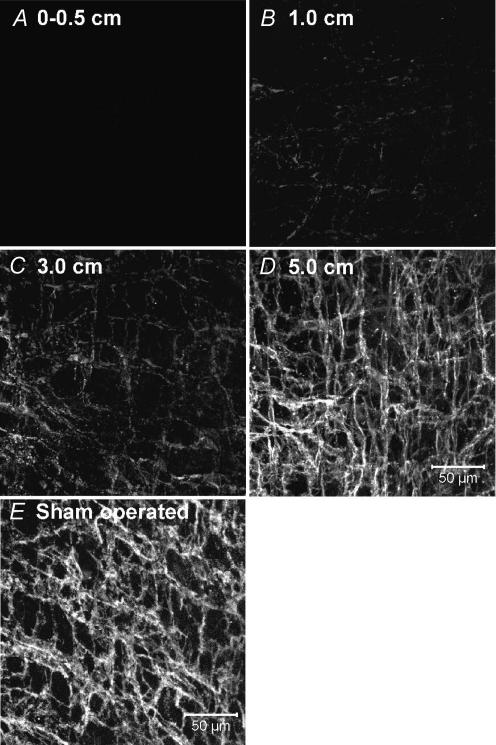
The reduction in pacemaker activity was associated with reduced numbers of Kit-immunopositive cells in the myenteric region (ICC-MY) of the small bowel near the site of anastomosis (Fig. 2A–D). This was previously shown to be due to loss of cells with ICC ultrastructure and not merely reduced Kit expression (Yanagida et al. 2004). The lesion in the ICC-MY network decreased as a function of distance from the anastomosis. The ICC-MY network could not be resolved at the site of anastomosis (Fig. 2A), whereas sham-operated animals display a dense Kit-immunopositive network of ICC-MY (Fig. 2E). At 1 cm above the anastomosis site, only a few dispersed ICC-MY were observed (Fig. 2B), and the density of the ICC-MY network increased with distance until it appeared similar to the ICC-MY in sham-operated animals at approximately 5 cm (Fig. 2C and D).
Figure 2. Intestinal resection damages the ICC-MY network.
A, absence of ICC-MY adjacent to the site of anastomosis (0–0.5 cm). Previous studies have shown that this reduction in ICC-MY results from actual loss of ICC and not just reduced expression of Kit (Yanagida et al. 2004). B, very faint Kit-immunoreactivity in the severely disrupted ICC-MY network, 1 cm from the site of anastomosis. C, ICC-MY at 3 cm were disrupted, but the degree of Kit labelling was improved. At 5 cm from the anastomosis site (D), ICC-MY were similar in appearance to the cells observed in sham operated animals (E). E, scale bar represents 50 μm, and applies to A–E.
Role of iNOS and cyclooxygenase production in the disruption of pacemaking activity and ICC-MY networks following intestinal resection
Even handling of the intestine can upregulate expression of iNOS and COX-2, and these inflammatory mediators may be involved in reducing mechanical activity and contributing to postoperative ileus (Kalff et al. 2000, 2003). We have also shown that following surgical resection the tunica muscularis near the site of anastomosis becomes infiltrated with immune cells (Yanagida et al. 2004). It is possible that production of inflammatory mediators may cause the lesion in ICC and loss of pacemaker activity. Experiments were performed to test the role of nitric oxide and cyclooxygenase products on ICC-MY and pacemaker activity by examining the effects of surgical resection on iNOS−/− and COX-2−/− mice.
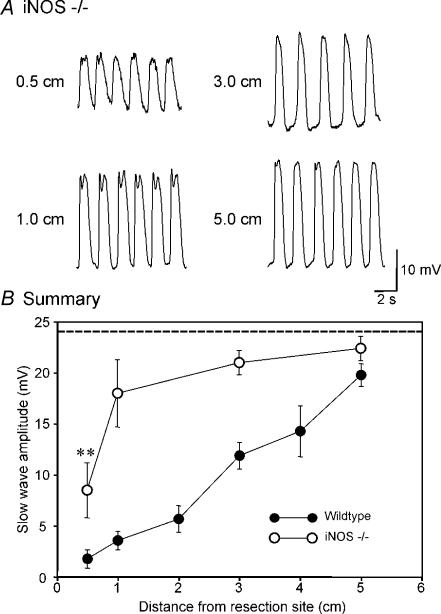
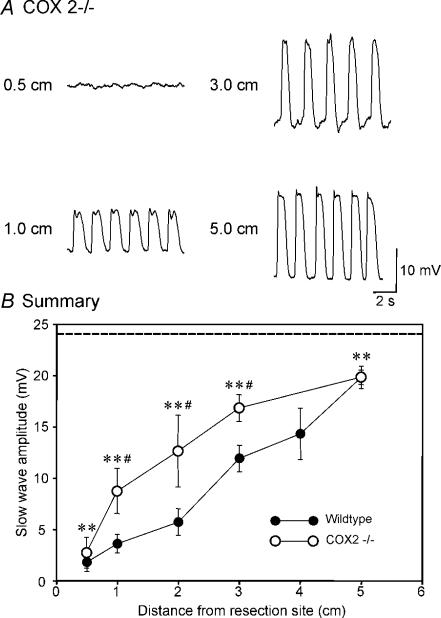
In contrast to the findings from sham-operated mice, there was significant reduction in pacemaker activity only in the region closest to the site of anastomosis from which recordings could be made (0.5 cm) in iNOS−/− mice. Here, the amplitude of slow waves averaged 8.4 ± 2.7 mV while slow waves in muscles of sham-operated mice were 24.1 ± 0.9 mV in amplitude (n= 5, P < 0.01; Fig. 3B). At 1 cm above the site of anastomosis, slow-wave amplitude was 17.9 ± 3.3 mV, and increased with distance at 3 and at 5 cm to 20.9 ± 1.2 and 22.3 ± 1.2 mV, respectively. Slow waves at 1 cm and above were not significantly different from the slow waves in sham-operated muscles (n= 5; P > 0.05; Fig. 3B). Pacemaker frequency was unchanged from control at any site above the anastomosis (i.e. frequency at 0.5 cm was 32.0 ± 2.2 cycles min−1, at 1 cm 32.0 ± 0.3 cycles min−1, at 3 cm was 32.0 ± 0.8 cycles min−1, and at 5 cm was 32.0 ± 0.4 cycles min−1 (n= 5; P > 0.05). Slow-wave amplitudes and the frequency were also not significantly different from each other within the group (1–5 cm, P > 0.05, by ANOVA). A summary of slow-wave amplitude as a function of distance in intestines of iNOS−/− mice following resection is shown in comparison to the same data from wild-type controls in Fig. 3B.
Figure 3. Disruption in pacemaker activity was reduced in iNOS−/− mice after intestinal resection.
A, pacemaker activity at various distances from the site of the intestinal anastomosis (0.5–5 cm). Slow waves were reduced only 0.5 cm from the site of anastomosis. At all other sites (1.0, 3.0 and 5.0 cm) slow-wave amplitude was not statistically different from slow waves in sham-operated animals. B, summary of the changes in slow-wave amplitude (•, wild-type; ^, iNOS−/− mice; n= 5) as a function of distance from the site of anastomosis. The dashed line shows the average slow-wave amplitude in sham-operated mice. **P < 0.01 compared with sham-operated mice by t test. Slow-wave amplitudes were significantly different in 0.5–5 cm (P < 0.05), but not different from each other within the group (1–5 cm, P > 0.05, by ANOVA).
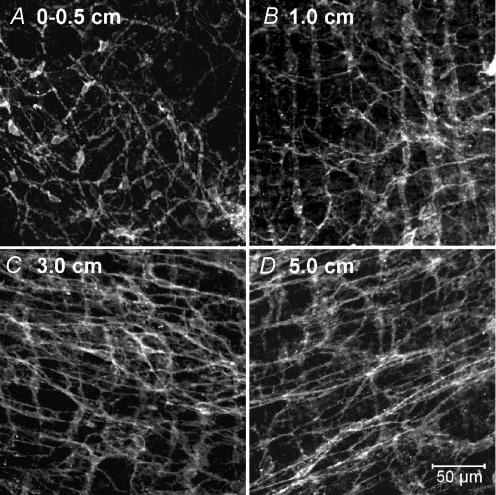
Reduced loss of pacemaker activity near the site of anastomosis in iNOS−/− mice suggests that ICC-MY networks may be protected by inactivating iNOS. Thus, we examined ICC-MY in iNOS−/− mice and the effects of intestinal resection on the ICC-MY network. Kit immunohistochemistry was performed on whole mounts from the iNOS−/− mice at 0–0.5–5 cm from the site of anastomosis. Reduced ICC-MY were observed only in muscles adjacent to the site of anastomosis (i.e. up to 0.5 cm; Fig. 4A), and at 1, 3 and 5 cm from the anastomosis, ICC-MY networks appeared normal (Fig. 4B–D).
Figure 4. Protection of the ICC-MY network after intestinal resection by genetic disruption of iNOS.
A–D, ICC-MY networks adjacent to (0.5 cm) and at 1.0, 3.0 and 5.0 cm from the site of anastomosis. In iNOS−/− mice, the ICC-MY network was slightly damaged only near the site of the anastomosis. ICC-MY appeared normal and similar to cells in sham-operated animals (see Fig. 2E) in all other samples examined. D, scale bar represents 50 μm, and applies to A–D.
Next, we tested whether COX-2 products adversely affect pacemaker activity and ICC-MY by examining the effects of intestinal resection in COX-2−/− mice. Surgery disrupted pacemaker activity in COX-2−/− mice adjacent to and at distances up to 3.0 cm from the anastomosis, although this disruption was not as great as observed in wild-type animals. For example, at 0.5 cm slow waves were 2.6 ± 1.5 mV in amplitude after surgery in COX-2−/− mice (n= 3); this was significantly reduced from the sham-operated muscles but not different from the reduction in slow wave in wild-type animals that underwent surgery (P < 0.001 and P= 0.625, respectively). At 1 cm, slow waves were 8.6 ± 2.2 mV in amplitude (n= 6) which was also significantly reduced from the slow waves in sham-operated mice, but significantly greater than slow-wave amplitude in muscles of resected wild-type animals (i.e. P < 0.001 and P < 0.05, respectively). At 5.0 cm from the site of anastomosis, slow waves averaged 19.7 ± 0.7 mV in COX-2−/− muscles; this was not different from the amplitude of slow waves in wild-type mice (19.7 ± 1.1 mV; P > 0.05), but was different from that of sham-operated mice (i.e. 24.1 ± 0.9 mV; P < 0.01; Fig. 5B). These values were significantly different from each other within the group (P < 0.01, by ANOVA). Pacemaker frequency was not significantly different in COX-2−/− mice at 1, 3 and 5 cm from the anastomosis site compared with sham-operated controls (i.e. 31.2 ± 1.0, 31.7 ± 0.7 and 32.6 ± 1.7 cycles min−1, respectively), and was not significantly different from each other within the group (P > 0.05, by ANOVA). Figure 5B shows a summary of slow-wave amplitudes as a function of distance from the anastomosis site in intestines of COX-2−/− mice.
Figure 5. Pacemaker activity was partially protected after intestinal resection by genetic disruption of COX-2.
A, pacemaker activity adjacent to the site of intestinal anastomosis (0.5 cm) and at 1.0, 3.0 and 5 cm oral to the site of anastomosis. B, summary of the changes in slow-wave amplitude as a function of distance from the site of anastomosis in COX-2−/− mice (^, n= 6). The data are plotted for comparative purposes with slow-wave amplitudes from muscles of wild-type animals (•) and sham-operated animals (dashed line). **P < 0.01 in comparison to slow waves of sham-operated mice. #P < 0.05 in comparison to slow waves in wild-type animals after surgery at each distance by t test. These values in the amplitude were also significantly different from each other within the group (0.5–5 cm, P < 0.001; and 1–5 cm, P < 0.01; by ANOVA).
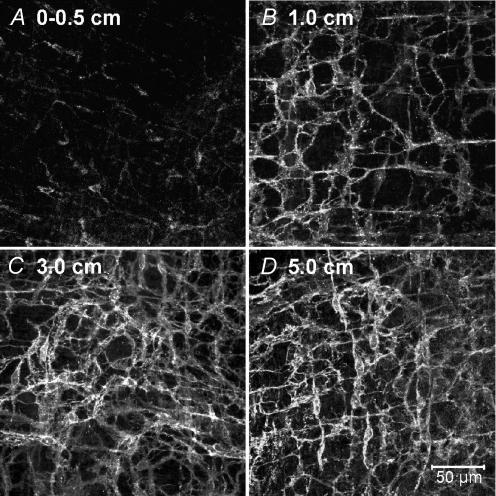
ICC-MY were reduced in COX-2−/− mice in the region adjacent to the site of intestinal anastomosis. However, the extent of damage to ICC-MY networks and the region over which the lesion was observed was less than in wild-type animals after surgery. The damage to ICC-MY was more extensive in COX-2−/− mice, however, than in iNOS−/− mice and in sham-operated mice. Immediately adjacent to the site of anastomosis (0–0.5 cm), Kit immunoreactivity was reduced and there were patches where ICC-MY were absent (Fig. 6A). At 1 cm oral to the anastomosis, the areas missing ICC-MY were reduced in size (Fig. 6B). By 3 and 5 cm, ICC-MY networks appeared relatively normal compared with sham-operated control animals (Fig. 6C and D).
Figure 6. The ICC-MY network was partially protected following intestinal resection by genetic disruption of COX-2.
A–D, the ICC-MY network in muscle samples adjacent to (0–0.5 cm) and at 1.0, 3.0 and 5.0 cm oral to the site of anastomosis. Note that the ICC-MY network was more normal in appearance than in muscles of wild-type animals after intestinal resection (compare with Fig. 2). D, scale bar represents 50 μm, and applies to A–D.
Preoperative treatment with iNOS inhibitors protects against disruption in electrical rhythmicity and damage to ICC-MY networks
Data obtained from iNOS−/− mice suggest the induction of nitric oxide, most likely from resident macrophages and infiltrating neutrophils following intestinal resection, is a major factor causing damage to ICC-MY and disruption in electrical pacemaker activity. We tested whether pretreatment of animals with specific iNOS inhibitors prior to intestinal surgery would protect against loss of ICC-MY and electrical rhythmicity. Such a procedure may provide the basis for therapeutic protection of ICC during abdominal surgery and enhance the recovery of intestinal motility after surgery.
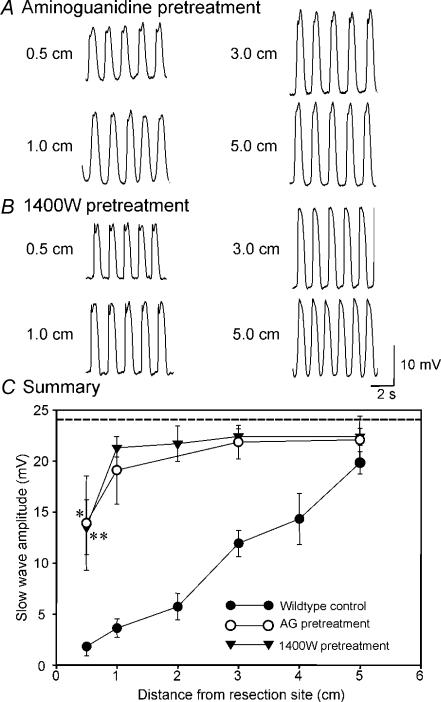
Wild-type animals were treated with a single dose of the iNOS inhibitors, aminoguanidine (20 mg kg−1) or N-(3-(aminomethyl)benzyl)acetamidine (1400W; 2 mg kg−1), respectively, 30 min before surgical procedures were initiated. Pacemaker activity was depressed only at 0.5 cm from the site of anastomosis in animals pretreated with iNOS inhibitors (Fig. 7A and B). At more distant sites, slow-wave amplitudes were not reduced from the amplitudes of slow waves in sham-operated mice (P > 0.05, 1–5 cm). These values were also not significantly different from each other within the group (1–5 cm, P > 0.05, by ANOVA). For example, in animals treated with aminoguanidine (20 mg kg−1) resting membrane potentials were −62 ± 2 mV at 1 cm, −62 ± 1 mV at 3 cm, and −63 ± 2 mV at 5 cm, from the site of anastomosis, and slow waves averaged 19.1 ± 3.3, 21.9 ± 1.7 and 22.0 ± 1.9 mV at the same sites of recording, respectively (n= 6; Fig. 7A). These values were not significantly different from slow waves in sham-operated mice (all P > 0.05). Pacemaking frequency was also unaltered in animals treated with aminoguanidine (i.e. 32 ± 1 cycles min−1 at all sites). Values were also not significantly different from each other within the group (P > 0.05, by ANOVA). Data from these experiments are summarized in Fig. 7C. Pretreatment with 1400W (2 mg kg−1) also protected against reduced pacemaker activity due to surgery. Resting membrane potential was not affected in these animals and averaged −64 ± 1, −63 ± 3 and −63 ± 1 mV, at 1, 3 and 5 cm from the site of anastomosis, respectively, and slow waves averaged 21.3 ± 0.9, 22.5 ± 0.8 and 22.4 ± 0.8 mV at 1, 3 and 5 cm from the site of anastomosis, respectively (Fig. 7B). Pacemaker frequency was also the same all recording sites (i.e. 32.0 ± 1 cycles min−1; P > 0.05; n= 5 or 6). All values were not significantly different from each other within the group of 1400W pretreated surgery mice (1–5 cm, P > 0.05, by ANOVA). These data are summarized in Fig. 7C.
Figure 7. Preoperative treatment with iNOS inhibitors protects against disruption in pacemaker activity after surgery.
A, reduced slow-wave amplitude only in cells immediately adjacent to (i.e. 0.5 cm) the site of anastomosis following presurgical administration of aminoguanidine (AG; 20 mg kg−1) or N-(3-(aminomethyl) benzyl)acetamidine (1400W; 2 mg kg−1; B). Sites above 0.5 cm displayed slow-wave activity of that was not significantly different from normal. These results are similar to data obtained from animals with genetically disrupted iNOS (see Fig. 3). C, slow-wave amplitude as a function of distance from the site of anastomosis in untreated wild-type animals (•), and animals treated preoperatively with AG (^) or 1400 W (▾). *P < 0.05, **P < 0.01 as compared with sham-operated mice by t test (dashed line). These values were not significantly different from each other within the group between the distance of 1–5 cm (AG, P > 0.05; and 1400W, P > 0.05; by ANOVA).
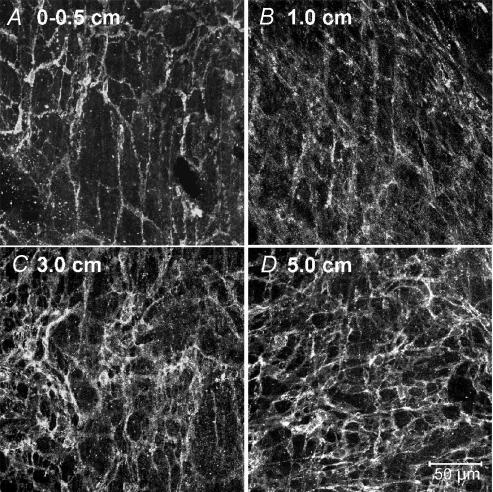
ICC-MY networks were also protected in animals pretreated with iNOS inhibitors, except a slight reduction was noted at the point closest to the anastomosis (0.5 cm; Fig. 8A). The ICC-MY network appeared normal at 1–5 cm from the site of anastomosis (Fig. 8B and D). The same protective effects on ICC-MY were observed with 1400W (data not shown). The images after pretreatment with iNOS inhibitors were similar to the images of ICC-MY in iNOS−/− mice following surgery.
Figure 8. Preoperative treatment with iNOS inhibitors protects the ICC-MY network from damage after intestinal resection.
A–D, preoperative treatment with iNOS inhibitors (aminoguanidine; 20 mg kg−1) protected the ICC-MY network from damage after intestinal resection. ICC-MY networks adjacent to the site of anastomosis appeared relatively normal compared with animals that were untreated (see Fig. 2). D, scale bar represents 50 μm, and applies to A–D.
Discussion
In this study we performed intestinal resections and reconstructions in mice and assayed ICC populations and electrical activity 5 h after surgery. As previously documented, surgical resection causes dramatic reduction in ICC-MY networks and reduced pacemaker function and contractility in a spatially specific manner within several hours after surgery (Yanagida et al. 2004). In spite of the severe changes in electrical rhythmicity after surgery, there was no significant effect on the resting membrane potentials of smooth muscle cells. This suggests that the loss of slow waves was not caused by a general effect of inflammatory mediators on the resting conductances of muscle cells. Reduced slow-wave amplitude is likely to be a consequence of the reduction in ICC-MY, since these are the pacemaker cells in the small bowel (Ward et al. 1994; Huizinga et al. 1995). Previous studies have demonstrated that an inflammatory cascade involving upregulation of cytokines and inflammatory mediators, such as iNOS and COX-2, occurs in response to intestinal surgery (Kalff et al. 1998). Treatment of wild-type animals with inhibitors of iNOS or performing intestinal resections on iNOS knockout mice protected muscles against loss of ICC-MY and slow-wave activity. There was also general enhancement in slow-wave amplitude when animals were pretreated with iNOS inhibitors, and reduction in the spatial loss of ICC-MY and slow waves. These data suggest that disruption of the ICC-MY network may contribute to motility changes that accompany postsurgical ileus. Prophylactic inhibition of iNOS reduces damage to the ICC-MY network and preserves generation and propagation of intestinal slow waves.
Electrical slow waves are periodic depolarizations that bring membrane potentials of smooth muscle cells to the threshold for Ca2+ action potentials. Ca2+ entry into intestinal smooth muscle cells during action potentials is the major signal linking excitation to contraction (Sanders et al. 2006). Thus, reduced slow-wave amplitude translates into loss of phasic contractile activity and impairment of segmentation motility patterns. In the small bowel, the action potential threshold is more than 20 mV positive to the resting membrane potential (i.e. the most negative potential between slow waves). Thus, slow waves up to 5 cm from a site of anastomosis would tend to fall below the threshold for action potentials, and the muscle cells in this region would tend to be mechanically compromised. This would result in a paralytic pseudo-obstruction-like condition within this region of bowel.
We do not fully understand the mechanism of reduced slow-wave amplitude in regions near a site of resection. It is possible that there is a generalized rundown in the pacemaker current generated by each ICC as the number of these cells is reduced. It is likely that this would lead to reduced slow-wave frequency; however, this was not generally observed except at sites immediately adjacent to the anastomosis. We have shown previously that most cells, if not all, within ICC-MY networks in the murine small bowel are spontaneously active (Park et al. 2006), and coordinated pacemaking results from coupling of many intrinsically active cells forming a network. Slow waves conduct to smooth muscle cells and are not actively regenerated by smooth muscle (Cousins et al. 2003; Sanders et al. 2006). Thus, the amplitude of slow waves depends upon the magnitude of pacemaker current generated by the ICC-MY network and the coupling between the ICC-MY network and the smooth muscle syncytium. When the number of ICC is reduced near a site of resection, it is likely that the current density generated by remaining ICC would decrease, and this would tend to drive smaller amplitude slow waves in surrounding smooth muscle cells. It is also possible that electrical coupling between ICC-MY and smooth muscle cells (which is already relatively poor; see Cousins et al. 2003) might decrease as ICC are reduced in number and fewer cells are in contact with smooth muscle cells, making pacemaker currents in ICC less capable of depolarizing neighbouring smooth muscle cells.
A previous study showed that muscles displayed greatly reduced spontaneous phasic contractions and lost responsiveness to stimulation by the muscarinic agonist, carbachol in regions above and below a site of resection (Yanagida et al. 2004). Thus, the muscles are also rendered insensitive to a major excitatory neurotransmitter released from enteric motor neurons. Although the resting membrane potentials of smooth muscle cells were not significantly affected near sites of resection, loss of responsiveness to carbachol suggests that smooth muscle cells are affected by inflammatory processes. Reduced phasic contractions and responsiveness to muscarinic agonists suggest that resection would delay intestinal transit. Such studies were not performed in the present series of experiments; however, others have previously shown that induction of iNOS resulting from surgical manipulation of the intestine delayed intestinal transit (Turler et al. 2006).
In the present study, we found that intestinal muscles were protected from loss of ICC and reduction in slow-wave amplitude after surgery when animals were treated with iNOS inhibitors or in animals with iNOS genetically inactivated. These findings support the idea that inhibition of iNOS might reduce the severity of dysmotility in postoperative ileus (Kalff et al. 2000). There was additional protection rendered by genetic inactivation of COX-2, and the spatial loss of ICC-MY was somewhat reduced in these animals. Thus, products of iNOS and COX-2 may have additive effects in postsurgical motility dysfunction.
A network of resident macrophages exists in the tunica muscularis of the small intestine (Mikkelsen & Rumessen, 1992; Mikkelsen, 1995) that is activated during handling and abdominal surgery that initiates a cascade of inflammatory mediators, including iNOS and COX-2 upregulation, as well as upregulation of a variety of other cytokines and chemokines (Kalff et al. 1998, 2000, 2003). These conclusions, deduced from studies on rodents, have also been confirmed in muscles of human patients where it was shown that surgery resulted in activation of resident intestinal macrophages in the tunica muscularis, upregulation of cytokine expression, induction of mediators with inhibitory effects on contractions, and reduction in contractile activity (Kalff et al. 2003). These changes occurred within 3 h of surgery, and messages for both iNOS and COX-2 were elevated and peaked within 3–4 h. This time frame is well within the window of time used for postsurgical observations in our study. In rodents and humans, cytokines are expressed after surgery by resident muscularis macrophages which lie in very close proximity to ICC-MY. Recent experiments have shown that infiltrating leucocytes also greatly increase iNOS expression in the small intestine, and NO from these cells is likely to produce the changes in motility following manipulation of the gut (Turler et al. 2006). Thus, increased production of NO by resident macrophages (Kalff et al. 2000) and infiltrating leucocytes (Turler et al. 2006) may generate relatively high concentrations of NO in the vicinity of the ICC-MY network (Billiar, 1995). NO is known to have both cytotoxic effects via production of nitrotyrosine or peroxynitrite and trophic effects on cells (Kubes & McCafferty, 2000; Hanafy et al. 2001; Witte & Barbul, 2002). Thus, it is possible that NO is a major factor in producing deleterious effects on the ICC-MY network. The pathway manifest in the gut after surgery, whether cytotoxic or trophic, has not been determined. However, a previous study showed that the ICC network is restored to normal function after 24 h in mice, suggesting that whether cell phenotype is altered or cell death occurs in response to elevated NO, the ICC population can turn over very rapidly. With the variety of inflammatory mediators that might be unleashed by intestinal surgery, there are other potential factors that might contribute to the effects of surgery on ICC and pacemaker activity. For example, our data suggest that a prostaglandin or a complement of eicosanoids made by COX-2 might also influence the ICC-MY phenotype. It should be stressed, however, that the deleterious effects of surgery on ICC and pacemaker activity were dramatically reduced in both genetically inactivated iNOS animals and in animals treated with iNOS inhibitors. Thus, NO, or downstream events induced by NO, may be the major factor directing the loss of pacemaker activity and inhibition of contractility after surgery.
The time at which iNOS inhibitors are applied is an important consideration for potential therapeutic use of these compounds in protecting ICC-MY and preventing ileus. Studies on human small intestinal segments removed at surgery showed a substantial enhancement of muscle contractility in response to the COX-2 inhibitor DFU, but little effect was noted using the iNOS inhibitor GW274150C (Kalff et al. 2003). The muscles were exposed to these drugs in organ baths several hours after surgery, at a time when the postsurgical inflammatory processes described above would be already well-established. Thus, after ileus develops, NO has little further impact on contractility. Our data support this idea because NO has hyperpolarizing actions on murine intestinal muscles, and we did not note any differences in resting membrane potentials between sham-operated and resected muscles. Inhibition of prostaglandin synthesis, on the other hand, generally enhances mechanical activity in the small intestine (Sanders, 1978, 1984; Thor et al. 1985), and this may explain the effects of COX-2 inhibitors on postsurgical muscles (Kalff et al. 2003). In the current study, the COX-2 inactivation caused a parallel increase in slow-wave amplitude in muscles above the site of anastomosis, but loss of COX-2 function did not protect against the spatial inhibition of slow waves beginning from the site of anastomosis. In our study, the iNOS inhibitor was given 30 min prior to surgery, and, of course in iNOS−/− animals, this enzyme was never active. These data suggest that iNOS must be inhibited, or enhanced expression must be blocked, before lesions in the ICC-MY network develop in order to obtain a beneficial postsurgical effect.
In summary, pacemaker activity, with an associated loss of ICC, is acutely disrupted following intestinal resection, resulting in postoperative dysmotility. In iNOS−/− mice or in animals pretreated with an iNOS inhibitor, pacemaker activity and ICC populations were not changed significantly by surgery (except for within a highly localized area at the site of anastomosis that was still less severe than in control mice). The effects of surgery were also less pronounced in COX-2−/− mice, but the effects of COX-2 inactivation were less marked than the effects of loss or inhibition of iNOS. Our observations suggest that postoperative damage consists of two components: a direct local component where cells are damaged by surgical procedures (the site of anastomosis), and a second more extensive component where cells, in particular ICC, are negatively affected by inflammatory responses (e.g. 1–5 cm). When contributions to the inflammatory mediators, particularly from iNOS are blocked, then the widespread loss of ICC is greatly attenuated and pacemaker activity is preserved. Thus, although it has been suggested that iNOS inhibition has some deleterious effects on wound healing, temporal inhibition of iNOS during surgery may be beneficial to recovery from postoperative dysmotility. Our observations suggest that postoperative dysmotility may be lessened by inhibiting iNOS production prior to surgery. This may provide a therapeutic advantage to patients undergoing GI surgery and lessen the severity of postoperative ileus.
Acknowledgments
This work was supported by grants NIH P01 DK41315 and NCRR 1S10RR16871.We would like to thank G. D. S. Hirst and F. R. Edwards for their technical advice and comments on this manuscript.
References
- Billiar TR. Nitric oxide. Novel biology with clinical relevance. Ann Surg. 1995;221:339–349. doi: 10.1097/00000658-199504000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536:555–568. doi: 10.1111/j.1469-7793.2001.0555c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003;550:829–844. doi: 10.1113/jphysiol.2003.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein AE, Miller SM, El-Youssef M, Rodeberg D, Lindor NM, Burgart LJ, Szurszewski JH, Farrugia G. Chronic intestinal pseudoobstruction associated with altered interstitial cells of Cajal networks. J Pediatr Gastroenterol Nutr. 2003;36:492–497. doi: 10.1097/00005176-200304000-00016. [DOI] [PubMed] [Google Scholar]
- Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–108. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Hagger R, Finlayson C, Kahn F, De Oliveira R, Chimelli L, Kumar D. A deficiency of interstitial cells of Cajal in Chagasic megacolon. J Auton Nerv Syst. 2000;80:108–111. doi: 10.1016/s0165-1838(00)00076-x. [DOI] [PubMed] [Google Scholar]
- Hanafy KA, Krumenacker JS, Murad F. NO, nitrotyrosine, and cyclic GMP in signal transduction. Med Sci Monit. 2001;7:801–819. [PubMed] [Google Scholar]
- He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of Cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14–21. doi: 10.1016/s0016-5085(00)70409-4. [DOI] [PubMed] [Google Scholar]
- He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–434. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- Horvath VJ, Vittal H, Lorincz A, Chen H, Almeida-Porada G, Redelman D, Ordog T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of Cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Kalff JC, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 2000;118:316–327. doi: 10.1016/s0016-5085(00)70214-9. [DOI] [PubMed] [Google Scholar]
- Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in paralytic ileus. Ann Surg. 1998;228:652–663. doi: 10.1097/00000658-199811000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalff JC, Turler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ, Billiar TR, Simmons RL, Bauer AJ. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg. 2003;237:301–315. doi: 10.1097/01.SLA.0000055742.79045.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–158. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- Mikkelsen HB. Macrophages in the external muscle layers of mammalian intestines. Histol Histopathol. 1995;10:719–736. [PubMed] [Google Scholar]
- Mikkelsen HB, Rumessen JJ. Characterization of macrophage-like cells in the external layers of human small and large intestine. Cell Tissue Res. 1992;270:273–279. doi: 10.1007/BF00328013. [DOI] [PubMed] [Google Scholar]
- Nakahara M, Isozaki K, Hirota S, Vanderwinden JM, Takakura R, Kinoshita K, Miyagawa J, Chen H, Miyazaki Y, Kiyohara T, Shinomura Y, Matsuzawa Y. Deficiency of Kit-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666–670. doi: 10.1046/j.1440-1746.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- Ördög T, Takayama I, Cheung WKT, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006;290:C1411–C1427. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- Sanders KM. Endogenous prostaglandin E and contractile activity of isolated ileal smooth muscle. Am J Physiol Endocrinol Metab. 1978;234:E209–E212. doi: 10.1152/ajpendo.1978.234.2.E209. [DOI] [PubMed] [Google Scholar]
- Sanders KM. Evidence that prostaglandins are local regulatory agents in canine ileal circular muscle. Am J Physiol Gastrointest Liver Physiol. 1984;246:G361–G371. doi: 10.1152/ajpgi.1984.246.4.G361. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- Thor P, Konturek JW, Konturek SJ, Anderson JH. Role of prostaglandins in control of intestinal motility. Am J Physiol Gastrointest Liver Physiol. 1985;248:G353–G359. doi: 10.1152/ajpgi.1985.248.3.G353. [DOI] [PubMed] [Google Scholar]
- Turler A, Kalff JC, Moore BA, Hoffman RA, Billiar TR, Simmons RL, Bauer AJ. Leukocyte-derived inducible nitric oxide synthase mediates murine postoperative ileus. Ann Surg. 2006;244:220–229. doi: 10.1097/01.sla.0000229963.37544.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg. 2002;183:406–412. doi: 10.1016/s0002-9610(02)00815-2. [DOI] [PubMed] [Google Scholar]
- Won KJ, Suzuki T, Hori M, Ozaki H. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil. 2006;18:53–61. doi: 10.1111/j.1365-2982.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- Yamataka A, Ohshiro K, Kobayashi H, Lane GJ, Yamataka T, Fujiwara T, Sunagawa M, Miyano T. Abnormal distribution of intestinal pacemaker (C-Kit-positive) cells in an infant with chronic idiopathic intestinal pseudoobstruction. J Pediatr Surg. 1998;33:859–862. doi: 10.1016/s0022-3468(98)90660-1. [DOI] [PubMed] [Google Scholar]
- Yanagida H, Yanase H, Sanders KM, Ward SM. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology. 2004;127:1748–1759. doi: 10.1053/j.gastro.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Zarate N, Mearin F, Wang XY, Hewlett B, Huizinga JD, Malagelada JR. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: pathological findings and management. Gut. 2003;52:966–970. doi: 10.1136/gut.52.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]