Abstract
The mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) are important nutrient- and energy-sensing and signalling proteins in skeletal muscle. AMPK activation decreases muscle protein synthesis by inhibiting mTOR signalling to regulatory proteins associated with translation initiation and elongation. On the other hand, essential amino acids (leucine in particular) and insulin stimulate mTOR signalling and protein synthesis. We hypothesized that anabolic nutrients would be sensed by both AMPK and mTOR, resulting in an acute and potent stimulation of human skeletal muscle protein synthesis via enhanced translation initiation and elongation.
We measured muscle protein synthesis and mTOR-associated upstream and downstream signalling proteins in young male subjects (n= 14) using stable isotopic and immunoblotting techniques. Following a first muscle biopsy, subjects in the ‘Nutrition’ group ingested a leucine-enriched essential amino acid–carbohydrate mixture (EAC). Subjects in the Control group did not consume nutrients. A second biopsy was obtained 1 h later. Ingestion of EAC significantly increased muscle protein synthesis, modestly reduced AMPK phosphorylation, and increased Akt/PKB (protein kinase B) and mTOR phosphorylation (P < 0.05). mTOR signalling to its downstream effectors (S6 kinase 1 (S6K1) and 4E-binding protein 1 (4E-BP1) phosphorylation status) was also increased (P < 0.05). In addition, eukaryotic elongation factor 2 (eEF2) phosphorylation was significantly reduced (P < 0.05). Protein synthesis and cell signalling (phosphorylation status) was unchanged in the control group (P > 0.05).
We conclude that anabolic nutrients alter the phosphorylation status of both AMPK- and mTOR-associated signalling proteins in human muscle, in association with an increase in protein synthesis not only via enhanced translation initiation but also through signalling promoting translation elongation.
Mammalian cells contain highly conserved nutrient- and energy-sensing pathways which are vital to cell survival and growth. The AMP-activated protein kinase (AMPK) is a major cell energy-sensing protein which, when activated during energetic stress such as hypoxia, glucose starvation, or physical exercise, stimulates catabolic pathways and inhibits anabolic pathways in an effort to supply ATP for cell survival (see Hardie, 2005). We and others have recently shown that a major anabolic pathway, muscle protein synthesis, is inhibited when AMPK is activated during energetic stress (Bolster et al. 2002; Dreyer et al. 2006; Williamson et al. 2006a). This inhibition may be explained, in part, by the ability of AMPK to act as a negative upstream regulator of the mammalian target of rapamycin (mTOR) pathway (Inoki et al. 2003; Williamson et al. 2006b).
There has been a large amount of work in cells and rodents that has examined the effect of insulin and nutrients (primarily amino acids) in the regulation of protein synthesis (see Avruch et al. 2005, 2006; Kimball & Jefferson, 2006; Proud, 2006). Specifically, insulin stimulates protein synthesis by activating the insulin-signalling pathway leading to an increase in phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt/PKB) activity. Akt phosphorylates and inhibits the tuberous sclerosis complex (TSC2), which increases mTOR kinase activity (Avruch et al. 2006; Proud, 2006). mTOR signalling to its downstream effectors ribosomal S6 kinase 1 (S6K1) and 4E-binding protein 1 (4E-BP1) is an important mechanism for stimulating translation initiation (Wang & Proud, 2006). Amino acids apparently do not influence TSC2 or Akt/PKB, and can be sensed by a novel class 3 PI3K within cells, which enhances phosphorylation and activation of mTOR by an unknown mechanism (Byfield et al. 2005; Nobukuni et al. 2005). However, recent work has also shown that S6K1 can phosphorylate eukaryotic elongation factor 2 (eEF2) kinase, leading to a reduced phosphorylation/activation of eEF2 and a stimulation of translation elongation (Wang et al. 2001).
Within the past few years, the work in cells and rodents has been translated into human studies. It has now been shown that a 2 h hyperinsulinaemic–euglycaemic clamp (Hillier et al. 2000; Greiwe et al. 2001) or a 6 h hyperinsulinaemic infusion of insulin (Liu et al. 2004) can increase the phosphorylation status of S6K1. In addition, a 2 h infusion of leucine (Greiwe et al. 2001) and a 6 h infusion of branched-chain amino acids (Liu et al. 2001) or a mixture of essential and non-essential amino acids increases the phosphorylation status of both S6K1 and 4E-BP1 (Liu et al. 2002). However, muscle protein synthesis was not directly measured in the studies mentioned above. More recently, an oral ingestion of 10 g of essential amino acids was found to increase both mTOR and S6K1 phosphorylation in association with an increase in muscle protein synthesis in human subjects (Cuthbertson et al. 2005).
We have recently shown in human subjects that an increase in insulin concentration to prandial levels can directly stimulate muscle protein synthesis as long as blood amino acid concentrations are maintained at the baseline level (Bell et al. 2005; Fujita et al. 2006). In addition, when both glucose and amino acids are provided, the muscle protein synthesis response is increased to a greater extent than with either insulin or amino acids alone (Rasmussen et al. 2000; Volpi et al. 2000; see Rasmussen & Phillips, 2003).
Therefore, we hypothesized that an increase in essential amino acid (leucine in particular) and glucose/insulin availability would be sensed by both AMPK and mTOR in human muscle, which would enhance Akt/mTOR signalling to key regulators of translation initiation and elongation, and induce a potent and rapid increase in the rate of muscle protein synthesis.
Methods
Subjects
We studied 14 young male subjects (7 Control and 7 Nutrition). All subjects were healthy and physically active, but were not currently engaged in an exercise training programme. All subjects gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch. Screening of subjects was performed with clinical history, physical examination, and laboratory tests including complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance test (OGTT), hepatitis B and C screening, HIV test, thyroid-stimulating hormone (TSH), lipid profile, urinalysis, drug screening, and ECG. The subjects' characteristics are summarized in the Results section. The subjects were randomized to either the Nutrition group or a Control group for the infusion experiment.
Study design
Each subject was admitted to the General Clinical Research Center of the University of Texas Medical Branch the day before the exercise study, and a dual-energy X-ray absorptiometry (DEXA) scan (Hologic QDR 4500W, Bedford, MA, USA) was performed to measure body composition and lean mass. The subjects were then fed a standard dinner, and a snack was given at 2200 h. The subjects were studied following an overnight fast under basal conditions, and refrained from exercise for 24 h prior to study participation. The morning of the study, polyethylene catheters were inserted into a forearm vein for tracer infusion, in the contralateral hand vein which was heated for arterialized blood sampling, and in the femoral artery and vein (retrograde placement) of the leg for blood sampling. The femoral lines were placed in the same leg from which muscle biopsies were obtained. The arterial catheter was also used for the infusion of indocyanine green (ICG, Akorn, Inc., Buffalo Grove, IL, USA) to determine blood flow.
After drawing a background blood sample, a primed continuous infusion of l-[ring-2H5] phenylalanine (Cambridge Isotope Laboratories, Andover, MA, USA) was begun (time = 0 h at 0800 h) and maintained at a constant rate until the end of the experiment (Fig. 1). The priming dose for the labelled phenylalanine was 2 μmol kg−1 and the infusion rates was 0.05 μmol kg−1 min−1. All subjects were studied at the exact same time (i.e. between 0800 h and 1100 h).
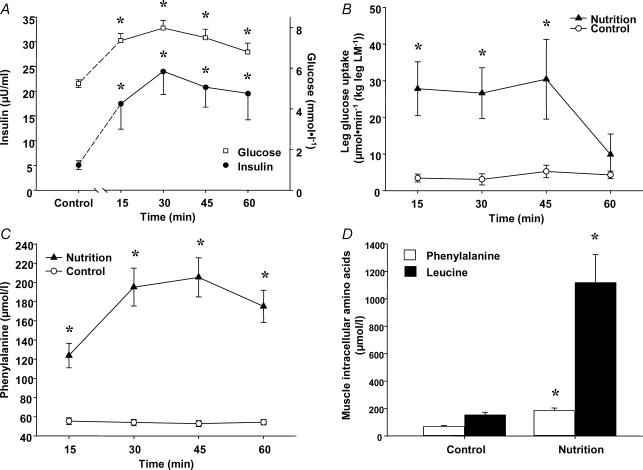
Figure 1. Substrate and hormone kinetics following nutrient intake.
Control data are from fasting subjects. Nutrition data are from subjects following an ingestion of essential amino acids and carbohydrate. Following nutrient ingestion: A, arterial glucose and insulin concentrations; B, glucose uptake across the leg; C, arterial phenylalanine concentration; D, muscle free phenylalanine and leucine concentrations. *P < 0.05 versus Control.
Two hours following the initiation of the tracer infusion, the first muscle biopsy was obtained from the lateral portion of the vastus lateralis of the leg, with the biopsy site between 15 and 25 cm from the mid-patella. The biopsy was performed using a 5 mm Bergström biopsy needle, under sterile procedure and local anaesthesia (1% lidocaine). Muscle tissue was immediately blotted and frozen in liquid nitrogen (within seconds) and stored at −80°C until analysis. Immediately after the first biopsy, the Nutrition group ingested a solution (500 ml) which contained essential amino acids (0.35 g (kg FFM)−1 (fat-free mass), 19.4 ± 0.7 g) and carbohydrate (0.5 g (kg FFM)−1, 27.3 ± 1.1 g), while the Control group did not consume any nutrient. The rationale for having a Control group was to ensure that the muscle biopsy procedure did not alter phosphorylation status of the various signalling proteins. The composition of the essential amino acid mixture was as follows: histidine (8%), isoleucine (8%), leucine (35%), lysine (12%), methionine (3%), phenylalanine (14%), threonine (10%), and valine (10%). Therefore, our essential amino acid mixture was highly enriched in leucine. To minimize changes in blood phenylalanine enrichment we added a small amount of l-[ring-2H5] phenylalanine to the essential amino acid mixture in order to increase the mixture's l-[ring-2H5] phenylalanine enrichment to the predicted blood l-[ring-2H5] phenylalanine enrichment (i.e. 6.5%). The total caloric content of the nutrient solution was 783 ± 33 kJ (14.7 kJ (kg FFM)−1). Immediately after the solution was ingested, a continuous infusion of ICG was started in the femoral artery (0.5 mg min−1). Fifteen minutes after ICG infusion was started, blood samples were collected from the femoral vein and the arterialized hand vein to measure ICG concentration, blood glucose, insulin, and phenylalanine concentrations. Three more sets of blood samples were taken at 15 min intervals, and a second biopsy was obtained at 3 h post-tracer infusion (i.e. 1 h after the first biopsy and the ingestion of the nutrients).
Insulin, blood flow, and glucose uptake
Plasma insulin concentrations were determined by ELISA (Linco Research, St. Charles, MO, USA). Serum ICG concentration for the determination of leg blood flow was measured spectrophotometrically (Beckman Coulter, Fullerton, CA, USA) at λ= 805 nm (Jorfeldt & Wahren, 1971). Plasma glucose concentration was measured using an automated glucose and lactate analyser (YSI, Yellow Springs, OH, USA). Leg glucose utilization was calculated as net glucose uptake across the leg:
where CA and CV are the blood glucose concentrations in the femoral artery and vein, respectively, and were expressed as micromoles of glucose utilized per minute per kilogram of fat-free mass (FFM) of the leg (μmol min−1 (kg leg FFM)−1).
SDS PAGE and Western blot analysis
Details of the immunoblotting procedures have been previously published (Dreyer et al. 2006). Briefly, ∼30–50 mg of frozen tissue was homogenized (1: 9, w/v) and centrifuged for 10 min at 4°C and 6000g, followed by removal of the supernatant. Total protein concentrations were determined by using the Bradford assay (Bio-Rad, Smartspec plus spectrophotometer). The supernatant was diluted (1: 1) in a sample buffer mixture and then boiled for 3 min at 100°C. 50–100 μg of total protein were loaded onto each lane and the samples were separated by electrophoresis (100 V for 60 min) on a 7.5% polyacrylamide gel (Bio-Rad, Criterion), except for 4E-BP1 which was run on a 15% gel. A molecular weight ladder (Bio-Rad, Precision Plus protein standard) and a rodent internal loading control were also included on each gel. Following electrophoresis, protein was transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA) at 50 V for 60 min. We confirmed equal protein loading on each gel and that an equivalent amount of protein was transferred to the membrane, by Coomassie and/or Ponceau S staining. Blots were incubated in a single primary antibody overnight at 4°C (antibody concentrations are described below). The next morning, blots were incubated in secondary antibody for 1 h at room temperature. Chemiluminescent solution (ECL plus, Amersham BioSciences, Piscataway, NJ, USA) was applied to each blot. After a 5 min incubation, optical density measurements were obtained with a phosphoimager (Bio-Rad, Hercules) and densitometric analysis was performed using Quantity One software (Version 4.5.2) (Bio-Rad, Hercules). Following quantification of each phosphorylated protein, the membrane was stripped and incubated with a specific antibody to determine total protein content of each protein (Cell Signaling, Beverly, MA, USA). The immunoblotting data are expressed as protein phosphorylation status (in arbitrary units), because total protein abundance did not change during the 1 h between muscle biopsies.
Antibodies
The primary phospho-antibodies used were all purchased from Cell Signalling: phospho-mTOR (Ser 2448; 1: 1000); phospho-p70 S6K1 (Thr 389; 1: 500); phospho-Akt (Ser 473; 1: 500); phospho-Tuberin/TSC2 (Thr 1462; 1: 500); phospho-4E-BP1 (Thr 37/46; 1: 500); phospho-eEF2 (Thr 56; 1: 1000); phospho-AMPKα (Thr 172; 1: 1000); phospho-IRS-1 (Ser 312; 1: 500). Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from Amersham Bioscience (1: 2000).
Amino acid kinetics and muscle protein synthesis
The kinetics of muscle phenylalanine were calculated using a three-pool model (Biolo et al. 1992) and the detailed calculations have been reported previously (Bell et al. 2005; Rasmussen et al. 2006). With this model, the free (unbound) amino acid enrichments and concentrations in the femoral artery and vein, and in the muscle tissue are used to calculate the rates of phenylalanine transport from the artery into the tissue, and from the tissue into the venous blood, utilization for muscle protein synthesis, release from muscle protein breakdown, and net balance. Leg blood flow is calculated from the steady-state ICG concentration values in the femoral and wrist veins, as previously described (Jorfeldt & Wahren, 1971). Data are expressed per 100 ml of leg volume.
We also calculated the fractional synthetic rate (FSR) of mixed muscle proteins by directly measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEP/t), and using the precursor-product model to calculate the synthesis rate as follows (Calder et al. 1992; Toffolo et al. 1993).
ΔEp is the increment in protein-bound phenylalanine enrichment between the two biopsies, t is the time between the two biopsies, EM(1) and EM(2) are the phenylalanine enrichments in the free intracellular pool in the two biopsies. Data are expressed as percentages per hour.
Statistical analysis
All values are expressed as mean ±s.e.m. Comparisons for signalling proteins, leg glucose uptake, plasma and intracellular amino acid concentrations, and blood phenylalanine enrichments were performed using analysis of variance with repeated measures, the effects being subject, group (Control, Nutrition), and time (Pre, Post), using JMP statistical software version. 4.0.5 (SAS Institute). In addition, analysis of covariance was performed if differences existed at baseline between groups. One-way analysis of variance was used to compare plasma insulin and glucose concentrations. Post hoc testing was performed using the Tukey–Kramer test when appropriate. The two-tailed unpaired Student's t test was used to compare subject characteristics, amino acid kinetics, and fractional synthetic rate. Significance was set at P < 0.05.
Results
Subjects
Subjects were matched for age (27 ± 2 versus 25 ± 2 years), height (1.73 ± 0.03 versus 1.71 ± 0.01 m), weight (77 ± 6 versus 70 ± 4 kg), body mass index (25 ± 2 versus 24 ± 1 kg m−2), and total (59 ± 4 versus 55 ± 2 kg) and leg (10 ± 1 versus 9 ± 1 kg) lean muscle mass in the Control (n= 7) and Nutrition (n= 7) groups, respectively.
Insulin, glucose uptake, and amino acids
Arterial insulin concentrations were significantly increased in the Nutrition group at each time point throughout the hour following the ingestion of essential amino acids and carbohydrate (P < 0.05, Fig. 1A). The average leg blood flow tended to be higher in the Nutrition group (3.9 ± 0.5 versus 5.4 ± 1.0 ml min−1 (100 ml leg)−1) following nutrient intake (P= 0.10). The blood flow data were then used to calculate glucose uptake across the leg (see Methods). Glucose uptake in the Nutrition group increased significantly after the nutrient intake and peaked at 30–45 min post-ingestion, and then returned to baseline levels 1 h post-ingestion (P < 0.05, Fig. 1B).
Compared to the Control group, arterial phenylalanine concentration significantly increased after nutrient intake in the Nutrition group (P < 0.05, Fig. 1C). The muscle concentrations of free phenylalanine and leucine also increased significantly in the Nutrition group following nutrient intake (P < 0.05, Fig. 1D).
AMPK and insulin signalling: upstream regulators of mTOR
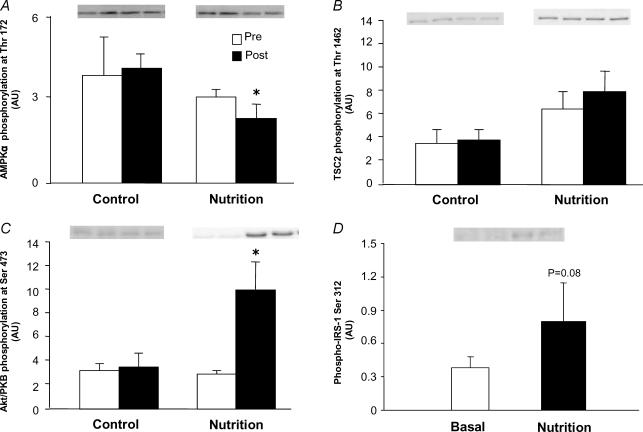
The phosphorylation of AMPKα at Thr 172 (a measure of both the α1 and α2 catalytic subunit phosphorylation status) slightly but significantly decreased in the Nutrition group following nutrient intake (P < 0.05, Fig. 2A). The phosphorylation of TSC2 (a substrate for both AMPK and Akt/PKB) at Thr 1462 was not different between groups. The phosphorylation of Akt/PKB at Ser 473 increased significantly in the Nutrition group following nutrient intake (P < 0.05, Fig. 2C). The phosphorylation of IRS-1 at Ser 312 tended to increase in the Nutrition group (P= 0.08) following nutrient intake (Fig. 2D). No significant changes in AMPKα, TSC2, or Akt/PKB phosphorylation were observed in the Control group.
Figure 2. Phosphorylation status of upstream regulators of mTOR.
Data in A–C are presented as Pre and Post. Pre data are from the first muscle biopsy in both the Control and Nutrition groups and indicate fasting phosphorylation status. Post data are from the second muscle biopsy obtained 1 h later. In the Control group no nutrients were ingested and the second biopsy was able to assess if there was any effect of the biopsy procedure on phosphorylation status. The Post data in the Nutrition group reflect the change in phosphorylation status 1 h after ingestion of essential amino acids and carbohydrate. A, AMPKα phosphorylation at Thr 172; B, TSC2 phosphorylation at Thr 1462; C, Akt/PKB phosphorylation at Ser 473; D, IRS-1 Ser 312 phosphorylation in the Nutrition group at baseline (basal) and 1 h after nutrient ingestion (Nutrition). *P < 0.05 versus Pre.
Downstream effectors of mTOR signalling and translation initiation and elongation
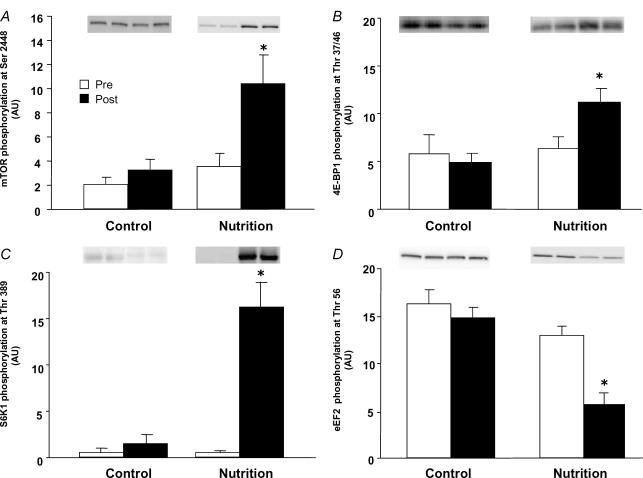
The phosphorylation of mTOR at Ser 2448 increased more than two-fold with nutrient intake in the Nutrition group (P < 0.05, Fig. 3A). The phosphorylation of 4E-BP1 at Thr 37/46 was significantly increased in the Nutrition group following nutrient intake (P < 0.05, Fig. 3B). Similarly, phosphorylation of S6K1 at Thr 389 increased to a large extent following nutrient intake in the Nutrition group (P < 0.05, Fig. 3C). The phosphorylation of eEF2 at Thr 56 decreased significantly in Nutrition group (P < 0.05, Fig. 3D). No significant changes in mTOR, 4E-BP1, S6K1 or eEF2 phosphorylation were observed in the Control group.
Figure 3. Phosphorylation status of downstream components of the mTOR signalling pathway.
Data are presented as Pre and Post. Pre data are from the first muscle biopsy in both the Control and Nutrition groups and indicate fasting phosphorylation status. Post data are from the second muscle biopsy obtained 1 h later. In the Control group no nutrients were ingested and the second biopsy was able to assess if there was any effect of the biopsy procedure on phosphorylation status. The Post data in the Nutrition group reflect the change in phosphorylation status 1 h after ingestion of essential amino acids and carbohydrate. A, mTOR phosphorylation at Ser 2448; B, 4E-BP1 phosphorylation at Thr 37/46; C, S6K1 phosphorylation at Thr 389; D, eEF2 phosphorylation at Thr 56. *P < 0.05 versus Pre.
Phenylalanine kinetics
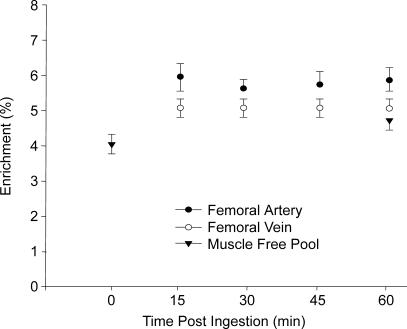
Femoral arterial and venous phenylalanine enrichments were stable throughout the experiment, and enrichments at each time point were not different between groups (P > 0.05, Fig. 4). The average intracellular phenylalanine enrichments (i.e. the muscle-free pool) were not different between groups (P > 0.05), and the intracellular phenylalanine enrichments for the Nutrition group were also similar between biopsies (P > 0.05, Fig. 4). Nutrient intake significantly increased blood and muscle phenylalanine concentrations (as shown in Fig. 2). Leg and muscle phenylalanine kinetics are shown in Table 1. Phenylalanine delivery to the leg and release from the leg were significantly higher in the Nutrition group (P < 0.05). Phenylalanine net balance was negative in the Control group, indicating net protein catabolism, and was positive and significantly higher in the Nutrition group (P < 0.05), indicating net protein anabolism.
Figure 4. Phenylalanine enrichmnts (tracer/tracee) in the femoral artery and vein during the hour following nutrient ingestion.
Blood enrichments were stable throughout the experiment and were not different between time points (P > 0.05). Enrichments were also stable in the Control group (data not shown) and were not different from the Nutrition group (P > 0.05). Intracellular phenylalanine enrichment in the muscle free pool is shown for the biopsy collected immediately before and 1 h following nutrient ingestion. Muscle free pool enrichment was not different between the two time points (P > 0.05).
Table 1.
Phenylalanine kinetics across the leg
| Control | Nutrition | |
|---|---|---|
| NB – net balance across the leg | −16 ± 5 | 73 ± 31* |
| Fin– delivery to the leg | 210 ± 31 | 788 ± 117* |
| Fout– release from the leg | 226 ± 34 | 715 ± 131 |
| FM,A– transport into muscle free pool | 161 ± 27 | 396 ± 89* |
| FV,M– transport from muscle free pool | 176 ± 30 | 323 ± 78* |
| FM,O– release from proteolysis | 63 ± 7 | 115 ± 44 |
| FO,M– utilization for protein synthesis | 47 ± 4 | 188 ± 51* |
| ICav – intracellular availability | 224 ± 33 | 511 ± 122* |
Control data are an hourly average of basal phenylalanine kinetics in the absence of nutrients. Nutrition data are an hourly average of phenylalanine kinetics following nutrient intake of essential amino acids and carbohydrate. Values are expressed in nmol min−1 (100 ml leg volume)−1.
P < 0.05 versus Control.
Phenylalanine transport into the muscle and out of the muscle were both significantly higher in the Nutrition group (P < 0.05) as compared to Control. Phenylalanine release from proteolysis was not significantly higher in the Nutrition group (P > 0.05). Phenylalanine utilization for muscle protein synthesis was significantly higher in the Nutrition group (P < 0.05). The intracellular phenylalanine availability increased by more than two-fold in the Nutrition group (P < 0.05).
Muscle protein synthesis
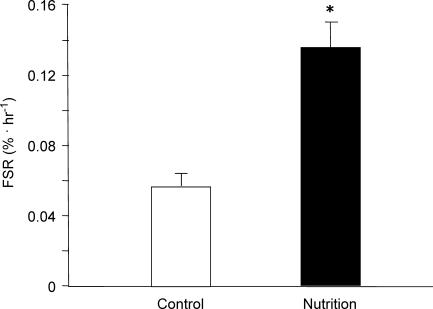
Whereas the signalling proteins are indicators of enhanced translation initiation and elongation, we also measured muscle protein synthesis by direct tracer incorporation into the muscle proteins, i.e. the end product of translation initiation and elongation. Basal protein-bound phenylalanine enrichments were not different between groups (P > 0.05). The average protein-bound phenylalanine enrichment in the Nutrition group at baseline was 0.0152 ± 0.006%, and increased to 0.0230 ± 0.006% 1 h following nutrient ingestion, an increment of 51% (i.e. ΔEp). We found that the mixed muscle protein fractional synthetic rate was increased to a large extent in the Nutrition group following nutrient intake, which was also significantly higher than in the Control group (P < 0.05, Fig. 5).
Figure 5. Muscle protein synthesis (FSR).
The basal FSR in the Control group is the synthesis rate in the absence of nutrients. Nutrition data reflect the large increase in muscle protein synthesis following an ingestion of essential amino acids and carbohydrate. *P < 0.05 versus Control.
Discussion
The primary and novel finding from our study is that an acute increase in nutrient and insulin availability stimulates muscle mTOR signalling to key regulators of not only translation initiation (S6K1 and 4E-BP1) but also translation elongation (eEF2), which potently and rapidly stimulates muscle protein synthesis (i.e. within 1 h). Although we also report a decrease in AMPKα phosphorylation in response to increased nutrient availability (which should enhance mTOR activation), it is readily apparent that the combination of enhanced essential amino acid (most likely due to leucine) and insulin availability increases muscle protein synthesis by enhancing phosphorylation of Akt/PKB, mTOR, 4E-BP1, and especially S6K1. Also of interest was the finding that eEF2 phosphorylation was significantly reduced, indicating that nutrients and insulin also promote translation elongation concurrently with translation initiation.
Our data support the notion that an increase in amino acid availability (leucine in particular) within the muscle, rather than insulin, is the major regulator of muscle protein synthesis. This is because although Akt/PKB phosphorylation was significantly increased following the increase in insulin availability, TSC2 phosphorylation was unchanged. Upstream regulation of mTOR by insulin via Akt/PKB phosphorylation, and inhibition of TSC1–TSC2 has been proposed as an important mechanism for the effect of insulin on increasing mTOR signalling. Insulin activation of mTOR also promotes the phosphorylation of S6K1, an important player in the regulation of translation initiation and cell growth (Baar & Esser, 1999). Since we did not see a significant increase in TSC2 phosphorylation at its Akt/PKB phosphorylation site (Thr 1462), and since the effect of insulin on the promotion of translation initiation appears to be mTOR dependent (i.e. Akt/PKB phosphorylation of TSC2 upstream of mTOR), our data suggest that insulin is playing a supportive and permissive role in the regulation of human muscle protein synthesis. However, we cannot rule out the possibility that Akt/PKB directly phosphorylated mTOR in our study, and future studies need to examine whether the anabolic effect of insulin on human muscle protein synthesis occurs independently of Akt/PKB signalling to TSC2. In addition to promoting translation initiation, insulin can also promote translation elongation by inhibiting eEF2 kinase activity (Redpath et al. 1996). More recently it has also been shown that S6K1 can phosphorylate and inactivate eEF2 kinase in order to promote elongation (Wang et al. 2001). Other phosphorylation sites on eEF2 kinase have also been found to be dependent on mTOR; however, it is not known which kinases, besides S6K1, are responsible for the mTOR-dependent inhibition of eEF2 kinase (Knebel et al. 2001; Browne & Proud, 2004). We also found that eEF2 phosphorylation was reduced when the muscle cells were exposed to essential amino acids, glucose, and insulin (indicating that elongation was also increased). Interestingly, an increase in IRS-1 serine phosphorylation by either mTOR or S6K1 is associated with an inhibition of insulin signalling and can serve as a negative feedback mechanism in muscle cells (Um et al. 2006). However, the modest increase in IRS-1 serine phosphorylation in the Nutrition group appears to have been insufficient for preventing Akt phosphorylation. Although glucose uptake decreased at 60 min following nutrient ingestion in association with the modest (P= 0.08) increase in IRS-1 serine phosphorylation, our data cannot directly determine whether the reduction in glucose uptake was due to an increase in IRS-1 serine phosphorylation.
On the other hand, our data strongly suggest that amino acid availability is the primary regulator of mTOR signalling and muscle protein synthesis in human skeletal muscle. The present findings are in agreement with our recent work showing that insulin is unable to stimulate muscle protein synthesis in human subjects when amino acid availability is reduced (Bell et al. 2005; Fujita et al. 2006). Furthermore, our data are also supported by previous work indicating that amino acid signalling to mTOR is not dependent on TSC2 (Byfield et al. 2005; Nobukuni et al. 2005). The phosphorylation of mTOR at Ser 2448, 4E-BP1 at Thr 37/46 and S6K1 at Thr 389 increased within 1 h following ingestion of essential amino acids and glucose. The largest increases in phosphorylation occurred with mTOR and S6K1, with the increase in S6K1 phosphorylation being extraordinarily large. Therefore, amino acid (probably leucine) stimulation of mTOR signalling to S6K1 and 4E-BP1 appears to be an important regulator of translation initiation and protein synthesis in human muscle. Elongation also appears to be regulated by amino acid stimulation of mTOR signalling, since we also report a very significant reduction in eEF2 phosphorylation at Thr 56. Although we did not directly measure eEF2 kinase activity or phosphorylation, it is likely that the inhibition of eEF2 activity, and thus decreased eEF2 phosphorylation, in our study occurred because of the large increase in S6K1 phosphorylation (and presumably a large increase in kinase activity). However, we cannot rule out that the decrease in eEF2 phosphorylation was, in part, attributable to the reduction in AMPK activity (Horman et al. 2002).
Our data also support the notion that the combination of amino acids, nutrient energy, and insulin enable a large (if not maximal) protein synthetic response (Anthony et al. 2002). Our previous work has shown that when glucose is added to an amino acid mixture, the stimulation of muscle protein synthesis is larger than with amino acids alone (see Rasmussen & Phillips, 2003). More recently, we have confirmed that insulin can indeed increase muscle protein synthesis in humans (Fujita et al. 2006; Rasmussen et al. 2006); however, the increase in muscle protein synthesis is much smaller than what is seen when amino acid availability is increased (Volpi et al. 2000). Furthermore, if amino acid availability is reduced, the ability of insulin to stimulate protein synthesis is inhibited in human skeletal muscle (Bell et al. 2005). This suggests that insulin is playing an important role in the regulation of human muscle protein synthesis, although amino acid availability is obviously a much more potent stimulator of protein synthesis.
The role of nutrient energy status within the muscle cell must also be considered, since the cellular processes of translation initiation and elongation are energetically expensive. A major cellular energy sensor within human muscle cells is AMPK (see Hardie, 2005). We and others have shown that muscle protein synthesis is inhibited in association with reduced mTOR signalling when AMPK activity and/or energy expenditure is increased (Bolster et al. 2002; Dreyer et al. 2006; Williamson et al. 2006a, 2006b). On the other hand, an increase in nutrient availability has also been shown to reduce AMPK activity (Kraegen et al. 2005), and an increase in ATP availability within cells appears to be sensed by mTOR (Dennis et al. 2001). Our data show that phosphorylation of the catalytic α subunit of AMPK is modestly reduced following the ingestion of essential amino acids and glucose. Therefore, the reduction in AMPK activity during nutrient ingestion may help to enhance protein synthesis by removing the inhibition of TSC2 on mTOR and/or by removing the negative regulation of eEF2. AMPK can phosphorylate TSC2 at Ser 1345 and Thr 1227, which inhibits mTOR activity (Inoki et al. 2003). However, we were unable to obtain the antibodies for the AMPK phosphorylation sites on TSC2 to confirm this. Therefore, future studies are needed to determine the role of both AMPK and insulin in the regulation of TSC2 in human muscle.
We also measured the overall rate of muscle protein synthesis via the direct incorporation of labelled phenylalanine into skeletal muscle over time. Our data show that the ingestion of a relatively small amount of amino acids enriched in leucine in combination with glucose causes a rapid and very large increase in muscle protein synthesis within 1 h. This increase in protein synthesis coincided with the increase in phosphorylation status of Akt/PKB, mTOR, 4E-BP1, and S6K1. In addition, we also report a decrease in the phosphorylation status of AMPK and eEF2. Therefore, it appears that the acute increase in muscle protein synthesis induced by the ingestion of nutrients and insulin was due to enhanced translation initiation and elongation resulting from the activation of mTOR signalling. Using compartmental modelling, we also determined the kinetics of phenylalanine during the experiment. As shown in Table 1, the net balance of phenylalanine across the leg became positive (an index of net muscle anabolism). Furthermore, the overall rates of amino acid transport into and out of the muscle tissue, intracellular amino acid availability, and utilization for muscle protein synthesis significantly increased. These amino acid and protein kinetics findings, interpreted in light of the concomitant cell signalling results, provide strong evidence of a prominent role of mTOR signalling in stimulating muscle protein synthesis in response to nutrients via enhanced translation initiation and elongation in human muscle. However, future work is still required (at both the basic and translational level) to determine the cellular mechanisms for how amino acids activate mTOR, how mTOR regulates different downstream targets, the role of muscle protein breakdown, and to determine how each component of the mTOR- and insulin-signalling pathways contribute to enhancing muscle protein synthesis.
We conclude that nutrients (essential amino acids and glucose) inhibit AMPK and activate mTOR signalling in human skeletal muscle in association with an increase in protein synthesis. The increase in protein synthesis appears to be due to not only enhanced translation initiation but also signalling promoting translation elongation. Future studies are required to determine if the provision of highly anabolic nutrients (such as those utilized in this experiment) may be useful in counteracting a variety of human muscle-wasting conditions such as those induced by cancer, ageing, AIDS, physical inactivity, muscular dystrophy, bed rest, and stroke.
Acknowledgments
We wish to thank the nurses and personnel of the General Clinical Research Center of the University of Texas Medical Branch for their help with the conduct of the clinical portion of this study, Dr David L. Chinkes for advice and statistical analysis of the enrichment data, and Jessica Lee, Ming Zheng, and Shelley Medina for technical assistance.
This study was supported by grant R01 AR049877 from the National Institute for Arthritis and Musculoskeletal and Skin Diseases, grant S10 RR16650 from the Shared Instrumentation Grant Program, grant P30 AG024832 from the National Institute of Ageing, and grant M01 RR00073 from the General Clinical Research Branch, National Center for Research Resources. H.C.D. was supported by grant H133P040003 from the National Institute of Disability and Rehabilitation Research, Department of Education.
References
- Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282:E1092–E1101. doi: 10.1152/ajpendo.00208.2001. [DOI] [PubMed] [Google Scholar]
- Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care. 2005;8:67–72. doi: 10.1097/00075197-200501000-00010. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70 (S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Bell JA, Fujita S, Volpi E, Cadenas JG, Rasmussen BB. Short-term insulin and nutritional energy provision do not stimulate muscle protein synthesis if blood amino acid availability decreases. Am J Physiol Endocrinol Metab. 2005;289:E999–E1006. doi: 10.1152/ajpendo.00170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolo G, Chinkes D, Zhang XJ, Wolfe RR. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. J Parenter Enteral Nutr. 1992;16:305–315. doi: 10.1177/0148607192016004305. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol Cell Biol. 2004;24:2986–2997. doi: 10.1128/MCB.24.7.2986-2997.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70, S6 kinase. J Biol Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745–E754. doi: 10.1152/ajpendo.00271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiwe JS, Kwon G, McDaniel ML, Semenkovich CF. Leucine and insulin activate p70, S6 kinase through different pathways in human skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281:E466–E471. doi: 10.1152/ajpendo.2001.281.3.E466. [DOI] [PubMed] [Google Scholar]
- Hardie DG. New roles for the LKB1 → AMPK pathway. Curr Opin Cell Biol. 2005;17:167–173. doi: 10.1016/j.ceb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hillier T, Long W, Jahn L, Wei L, Barrett EJ. Physiological hyperinsulinemia stimulates p70 (S6k) phosphorylation in human skeletal muscle. J Clin Endocrinol Metab. 2000;85:4900–4904. doi: 10.1210/jcem.85.12.7036. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136(Suppl. 1):227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- Knebel A, Morrice N, Cohen P. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38delta. EMBO J. 2001;20:4360–4369. doi: 10.1093/emboj/20.16.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, Ruderman NB. Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab. 2005;290:E471–E479. doi: 10.1152/ajpendo.00316.2005. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jahn LA, Long W, Fryburg DA, Wei L, Barrett EJ. Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab. 2001;86:2136–2143. doi: 10.1210/jcem.86.5.7481. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jahn LA, Wei L, Long W, Barrett EJ. Amino acids stimulate translation initiation and protein synthesis through an Akt-independent pathway in human skeletal muscle. J Clin Endocrinol Metab. 2002;87:5553–5558. doi: 10.1210/jc.2002-020424. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wu Y, Nicklas EW, Jahn LA, Price WJ, Barrett EJ. Unlike insulin, amino acids stimulate p70S6K but not GSK-3 or glycogen synthase in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E523–E528. doi: 10.1152/ajpendo.00146.2003. [DOI] [PubMed] [Google Scholar]
- Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans. 2006;34:213–216. doi: 10.1042/BST20060213. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BB, Phillips SM. Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev. 2003;31:127–131. doi: 10.1097/00003677-200307000-00005. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- Redpath NT, Foulstone EJ, Proud CG. Regulation of translation elongation factor-2 by insulin via a rapamycin-sensitive signalling pathway. EMBO J. 1996;15:2291–2297. [PMC free article] [PubMed] [Google Scholar]
- Toffolo G, Foster DM, Cobelli C. Estimation of protein fractional synthetic rate from tracer data. Am J Physiol Endocrinol Metab. 1993;264:E128–E135. doi: 10.1152/ajpendo.1993.264.1.E128. [DOI] [PubMed] [Google Scholar]
- Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metabolism. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90RSK1 and p70, S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology. 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- Williamson DL, Kubica N, Kimball SR, Jefferson LS. Exercise-induced alterations in extracellular signal-regulated kinase 1/2 and mammalian target of rapamycin (mTOR) signalling to regulatory mechanisms of mRNA translation in mouse muscle. J Physiol. 2006a;573:497–510. doi: 10.1113/jphysiol.2005.103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DL, Bolster DR, Kimball SR, Jefferson LS. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am J Physiol Endocrinol Metab. 2006b;291:E80–E89. doi: 10.1152/ajpendo.00566.2005. [DOI] [PubMed] [Google Scholar]