Abstract
The inhibitory neurotransmitter GABA plays a key role in the modulation of paraventricular nucleus (PVN) neuronal excitability and sympathoexcitatory outflow, under both physiological and pathological conditions. In addition to mediating conventional synaptic transmission (phasic inhibition), GABAA receptors of distinct biophysical, molecular and pharmacological properties have been recently found to underlie a slower, persistent form of inhibition (tonic inhibition). Whether the ‘tonic’ inhibitory modality is present in presympathetic PVN neurons, and what its role is in modulating their activity is at present unknown. Here, we combined tract-tracing techniques with patch-clamp electrophysiology to address these questions. Recordings obtained from PVN-RVLM (rostral ventrolateral medulla) projecting neurons show that besides blocking GABAA-mediated inhibitory postsynaptic currents (IPSCs, Iphasic), the GABAA receptor blockers bicuculline and picrotoxin caused an outward shift in the holding current (Itonic). Conversely, the high affinity GABAA blocker gabazine blocked Iphasic without affecting Itonic. THIP, a GABAA receptor agonist that preferentially activates δ- over γ-containing receptors, enhanced the magnitude of Itonic. Our results also indicate that during conditions of strong and/or synchronous synaptic activity, Itonic may be activated by spillover of synaptically released GABA. Blockade of Itonic induced membrane depolarization, increased firing activity, and enhanced the input–output function of PVN-RVLM neurons. Altogether, our results support the presence of a persistent GABAA-mediated inhibitory modality in presympathetic PVN neurons, which plays a major role in modulating their excitability and firing activity.
The hypothalamic paraventricular nucleus (PVN) plays an important role in the coordination of autonomic and neuroendocrine homeostatic responses (Swanson & Kuypers, 1980; Guyenet, 2006). PVN influence on autonomic outflow is largely mediated through descending projections to other sympathetic centres, including the rostral ventrolateral medulla (RVLM) and the spinal cord (Swanson & Kuypers, 1980; Shafton et al. 1998; Pyner & Coote, 2000).
The RVLM is a pivotal centre for the control of tonic and reflex sympathetic activity, and functional data support the idea that PVN-RVLM projections are important in cardiovascular control. Activation ofPVN-RVLM neurons elicits sympathoexcitatory and pressor responses (Coote et al. 1998; Yang & Coote, 1998; Tagawa & Dampney, 1999) and PVN-RVLM neurons are activated in response to fluid balance and cardiovascular challenges (Badoer & Merolli, 1998; Horiuchi et al. 1999; Stocker et al. 2006). Moreover, PVN-RVLM projections contribute to enhanced sympathoexcitatory drive during hypertension (Allen, 2002).
Despite the importance of PVN-RVLM neurons in autonomic control in health and disease conditions, the precise cellular mechanisms controlling their excitability are still poorly understood. Accumulating evidence indicates that PVN-RVLM neurons and sympathetic outflow are tonically inhibited by GABA (Zhang & Patel, 1998; Chen et al. 2003; Li et al. 2003; Li & Pan, 2005), and an altered PVN GABAergic function contributes to enhanced sympathoexcitatory drive during hypertension (Martin & Haywood, 1998; Li & Pan, 2006) and heart failure (Zhang et al. 2002).
Most GABA actions within the PVN are mediated by ionotropic GABAA receptors, classically known to mediate a spatially and temporally restricted inhibition (‘phasic’ modality), critical for timing-based signalling (Jonas et al. 2004). Recently, however, GABAA receptors were also found to induce a much slower inhibition (‘tonic’ modality), which is temporally and spatially dissociated from synaptically released GABA, resulting from the persistent activation of GABAA receptors of distinct biophysical and pharmacological properties, and subcellular distribution (Semyanov et al. 2004; Farrant & Nusser, 2005). ‘Tonic’ inhibition plays a critical role in modulating network excitability in various CNS regions, including the cortex and cerebellum (Brickley et al. 1996; Nusser & Mody, 2002; Semyanov et al. 2003).
Here, we provide evidence supporting the presence of this ‘tonic’ inhibitory modality in PVN-RVLM neurons, assess its interaction with phasic synaptic inhibition, and demonstrate a key role of tonic GABAA inhibition in controlling PVN-RVLM excitability and firing activity.
Methods
Male Wistar rats (180–220 g; Harlan Laboratories, Indianapolis, IN, USA) were housed in a 12–12 h light/dark schedule and allowed free access to food and water. All animal experimentation adhered to the policies of the University of Cincinnati regarding the use and care of animals.
Retrograde labelling
PVN-RVLM neurons were retrogradely labelled as previously described (Li et al. 2003). Briefly, rats were anaesthetized by intraperitoneal injection of a ketamine–xylazine mixture (90 mg kg−1 and 5 mg kg−1, respectively). The depth of anaesthesia achieved was monitored using a positive toe and tail pinch, the respiration rate and the degree of muscle relaxation. The head of the rat was placed in a stereotaxic frame, and a 4 mm burr hole was made in the skull. Rhodamine-labelled microspheres (Lumaflor, Naples, FL, USA) were microinjected unilaterally (200 nl) in the RVLM at bregma (B) −11.96, L 2.0, D 8.0. Postoperatively, analgesic treatment was provided (a single local s.c. infusion of 0.1 ml lidocaine) and a heat pad was used to provide supplemental heating until rats fully recovered from the anaesthesia.
Animals were allowed to recover, and electrophysiological experiments were performed 5–10 days following the microinjection procedure. The injection site and extension were confirmed histologically as previously described (Li et al. 2003).
Electrophysiological recordings
Patch-clamp recordings from identified PVN-RVLM neurons were obtained in hypothalamic slices (200 μm) as previously described (Li et al. 2003). Briefly, rats were anaesthetized with pentobarbital sodium (50 mg kg−1i.p.), decapitated and brains rapidly extracted. Slices were perfused with artificial cerebrospinal fluid (aCSF) (mm): NaCl 126; KCl 2.5; MgSO4 1; NaHCO3 26; NaH2PO4 1.25; glucose 20; ascorbic acid 0.4; CaCl2 1; pyruvic acid 2; pH was 7.3–7.4, saturated with 95% O2–5% CO2. Recordings were obtained either at room temperature or at 35°C, as indicated, using a Multiclamp 700A amplifier (Axon Instruments, Foster City, CA, USA). Current and voltage output were filtered at 2 kHz and digitized at 10 kHz (Digidata 1322A, PClamp 9 software, Axon Instruments). For voltage-clamp experiments, patch pipettes were filled with a high Cl−-containing solution (mm): 140 KCl; 10 Hepes; 0.9 Mg2+ATP; 20 phosphocreatine (Na+); 0.3 Na+GTP and 10 EGTA. For current-clamp experiments, 140 KCl was replaced with 130 potassium gluconate + 10 KCl.
Spontaneous inhibitory postsynaptic currents (sIPSCs, recorded at −70 mV) were detected and analysed using Minianalysis (Synaptosoft). A detection threshold was set at −20 pA and 150 pA ms for IPSC peak and area, respectively, to extract IPSCs without contamination with glutamate-mediated EPSCs (Park et al. 2006). GABA IPSCs were evoked using extracellular stimulation (100–350 μA, 0.1 ms, 10 pulses at 20 Hz) applied dorsolaterally to the PVN, in the presence of the glutamate receptor antagonist kynurenate (1 mm). IPSCs rise time, peak amplitude and decay time constants were calculated. The mean synaptic current (Iphasic) was calculated as the charge transfer of the averaged IPSC (integrated area under sIPSC) × mean sIPSC frequency (Park et al. 2006).
The holding current (Iholding) and root mean square (RMS) noise were measured in 50 ms epochs of traces lacking PSCs, separated by ∼800 ms, in periods of control ACSF and in the presence of GABAA blockers (n= 40 epochs in each case). The mean Iholding within the control and treated period was calculated as the mean of peak-to-peak amplitude of individual points within each period, and the GABAA receptor-mediated tonic current (Itonic) was defined as the difference in Iholding before and after application of GABAA receptor blockers. RMS noise was measured in the same epochs using algorithms provided by MiniAnalysis software. To assess drug effects on RMS within individual neurons, cumulative histograms of the measured RMS noise within individual epochs of control and treated periods were built and compared using Kolmogorov–Smirnov tests. To compare data from a group of neurons, a mean RMS value from the various epochs analysed from the control and treated periods within a neuron was obtained. The charge transfer (Qtonic) associated with Itonic current was calculated using the equation Qtonic= (Icon−Idrug) ×T, where Icon and Idrug are the holding current (pA) during control and drug conditions, respectively, and T is recorded time. To estimate Itonic density, its measured amplitude was normalized to the cell membrane capacitance, obtained by integrating the area under the transient capacitive phase of a 5 mV depolarizing step pulse, in voltage-clamp mode (Park et al. 2006).
Mean values of membrane potential (Vm) were obtained from averaging various recording segments lacking action potentials. Firing rate (number of action potentials in 10 s bins) was calculated during an ∼3 min period before and after bath application of GABAA receptor blockers, and interspike interval was calculated using Minianalysis software. Input–output relationships were built by plotting the number of evoked spikes versus injected current in response to depolarizing pulses of varying amplitudes (0–60 pA, 1 s duration), and fitted by a second order polynomial function (mean R2= 0.98 ± 0.01). The mean of the first term of the function was used to obtain the steepness of the relationship (Park et al. 2006).
Statistical analysis
Numerical data are presented as means ±s.e.m. Student's paired t tests were used to compare the effects of a drug treatment. Analysis of variance repeated measures (ANOVA-RM), followed by Tukey's post hoc tests, were used as needed. Cumulative histograms were compared using Kolmogorov–Smirnov tests.
Results
Two GABAA-mediated inhibitory modalities are observed in PVN-RVLM neurons
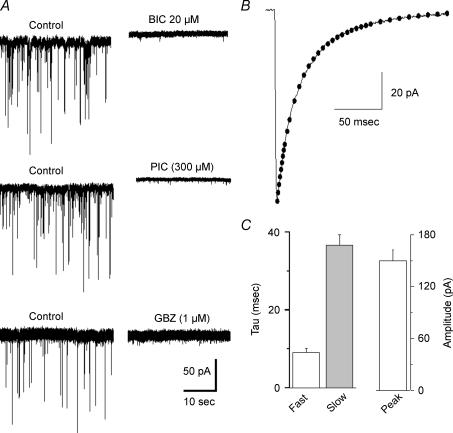
Patch-clamp recordings were obtained from a total of 77 retrogradely labelled PVN-RVLM neurons. A detailed analysis of spontaneous GABAA-mediated inhibitory postsynaptic currents (IPSCs) was obtained from 19 PVN-RVLM neurons, and results are summarized in Fig. 1. In our recording conditions, GABAA IPSCs occurred at a mean frequency of 1.63 ± 0.34 Hz, had a mean amplitude of 97.21 ± 8.07 pA, displayed a rapid onset (0.97 ± 0.07 ms), and decayed with a time course best fitted by a biexponential function (τfast: 9.24 ± 0.98 ms, τslow: 37.36 ± 2.62 ms). The calculated IPSC charge transfer and mean current were 0.22 ± 0.03 pC and 3.86 ± 0.77 pA, respectively (n= 19). IPSCs were completely blocked by the GABAA receptor antagonists bicucculline (BIC, 20 μm), gabazine (GBZ, 1–100 μm) or picrotoxin (PIC, 300 μm) (Fig. 1).
Figure 1. Spontaneous GABAA receptor-mediated IPSCS (phasic activity) in PVN-RVLM neurons.
A, sIPSCs in PVN-RVLM neurons were blocked by the GABAA receptor blockers bicuculline (BIC, 20 μm), picrotoxin (PIC, 300 μm) and gabazine (GBZ, 1 μm). Note that BIC and PIC induced, in addition, an outward shift in Iholding. B, representative example of an averaged sIPSC (n= 84) from a PVN-RVLM neuron. The decay time course was best fitted by a bi-exponential function as shown by the dotted line. C, summary of main IPSCs properties in PVN-RVLM neurons (n= 19).
Despite the fact that we were able to differentiate GABAA IPSCs from glutamate-mediated EPSCs based on selection criteria (see Methods), we performed a subset of experiments in which GBZ (1 μm) was tested in the presence of the AMPA and NMDA receptor antagonists CNQX (10 μm) and AP5 (100 μm). In all cases (n= 4), PSCs were completely blocked by 1 μm GBZ (not shown). On the other hand, a partial inhibition of GABAA IPSCs was observed when a lower concentration of GBZ (0.1 μm, n= 4) or BIC (2 μm, n= 5) were tested (58.9 ± 8.4% and 72.9 ± 12.4% decreased IPCS frequency, for GBZ and BIC, respectively).
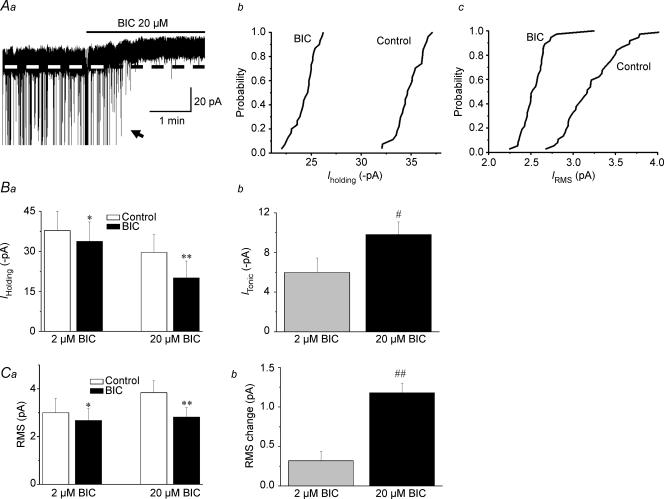
In addition to blocking synaptic transmission (IPSCs, Iphasic), BIC (20 μm) shifted outwardly the holding current (Iholding) (∼10 pA), and reduced RMS (n= 19, P < 0.01 in both cases, Fig. 2), supporting the presence of a sustained, GABAA receptor-mediated tonic current (Itonic) in PVN-RVLM neurons. At a lower concentration of 2 μm (n= 5), BIC still induced a significant shift in Iholding (4.19 ± 2.18 pA, n= 5, P < 0.05), and reduced RMS (P < 0.05). In both cases, however, the effects were significantly smaller than those observed with 20 μm BIC (see Fig. 1B and C). The BIC-sensitive Itonic was not affected when recordings were obtained in the presence of the AMPA and NMDA glutamate receptor antagonists CNQX (10 μm) and AP5 (100 μm), respectively (8.87 ± 2.33 pA, n= 4, P > 0.5 compared with 9.30 ± 1.17 pA in control).
Figure 2. GABAA receptor-mediated tonic (Itonic) currents in PVN-RVLM neurons are sensitive to the GABAA receptor antagonist bicucculline (BIC).
A, representative example showing that in addition to blocking fast IPSCs, BIC (20 μm) induced an outward shift in Iholding and reduced RMS (Aa). IPSCs shown in this trace were truncated, to better depict changes in holding current. Cummulative distribution histograms of Iholding and RMS before and after BIC, obtained from the same cell as in Aa, are shown in Ab and Ac, respectively. In both cases, BIC significantly shifted the curves to the left (P < 0.0001, Kolmogorov–Smirnov test). Mean Iholding and RMS before and after application of 2 and 20 μm BIC are summarized in Ba and Ca (n= 19), respectively. Summary of mean changes in Iholding (e.g. Itonic) and RMS induced by BIC are shown in Bb and Cb, respectively. *P < 0.05 and **P < 0.01 versus control; #P < 0.05 and ##P < 0.001, compared with 2 μm BIC.
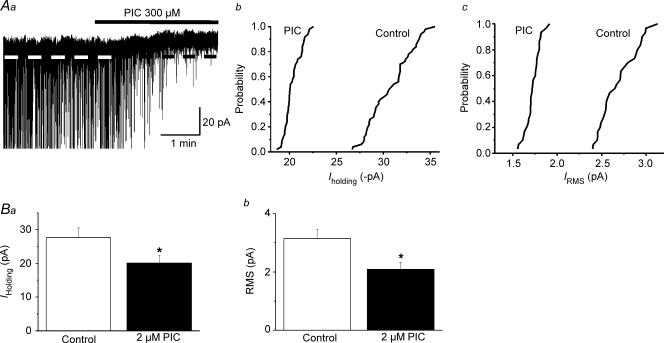
Similar effects on GABAAItonic and RMS were observed when the Cl− channel blocker PIC was tested (300 μm, n= 5, Fig. 3).
Figure 3. Itonic in PVN-RVLM neurons is blocked by the GABAA channel blocker picrotoxin (PIC).
Aa, representative current trace showing an outward shift in Iholding in the presence of PIC (300 μm). IPSCs were truncated, to better depict changes in holding current Similar to BIC, PIC significantly shifted to the left the cumulative distribution histograms of Iholding (Ab) and RMS (Ac) (P < 0.0001, Kolmogorov–Smirnov test). Mean Iholding and RMS values before and after application of PIC are shown in Ba and Bb (n= 5), respectively. *P < 0.01, compared with control.
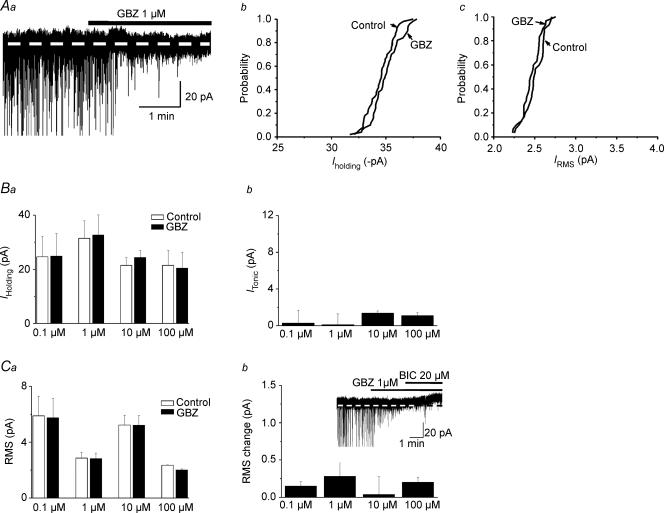
Differently from BIC and PIC, the high affinity GABAA receptor blocker GBZ, at a wide range of concentrations tested (0.1–100 μm), failed to shift Iholding or to change baseline RMS (n= 4, 8, 9 and 4 for 0.1, 1, 10 and 100 μm, respectively, P > 0.1 in all cases, see Fig. 4).
Figure 4. Itonic in PVN-RVLM neurons is insensitive to the high affinity GABAA receptor antagonist gabazine (GBZ).
Aa, representative current trace showing that GBZ (1 μm) blocked fast IPSCs, but failed to induce changes in Iholding or RMS. IPSCs were truncated to better depict changes in holding current. Cumulative distribution histograms of Iholding and RMS before and after GBZ (1 μm), obtained from the same cell as in Aa, are shown in Ab and Ac, respectively. GBZ failed to significantly alter the curves in both cases (P > 0.5, Kolmogorov–Smirnov test). Mean Iholding and RMS before and after application of 0.1–100 μm GBZ are summarized in Ba and Ca, respectively (n= 4–9). Mean changes in Iholding and RMS induced by BIC are summarized in Bb and Cb (n= 3–8), respectively. Note that in all cases, GBZ failed to affect Itonic and RMS. The inset in Cb depicts a representative example showing that in the presence of GBZ, an outward shift in Iholding was still evoked by further addition of BIC.
When 20 μm BIC was applied in the presence of 1 μm GBZ, the outward shift in Iholding was still observed (5.54 ± 1.61 pA, n= 5, Fig. 4C, inset). Altogether, these results indicate that the consecutive use of GBZ (1–100 μm) and BIC (20 μm) enable the distinction of the phasic and tonic GABAA-mediated inhibitory modalities in PVN-RVLM neurons.
In a separate set of experiments, recordings were obtained at more physiological temperatures (∼35°C). As previously reported (Wall & Usowicz, 1997; Semyanov et al. 2003; Park et al. 2006), increasing the recording temperature did not affect Itonic magnitude (room temperature: 9.79 ± 1.28 pA, n= 17; 35°C: 11.38 ± 0.57 pA, n= 5, P > 0.5) or RMS (room temperature: 3.39 ± 0.32 pA, n= 17; 35°C: 3.26 ± 0.43 pA, n= 5, P > 0.5).
Overall, the mean GABAAItonic amplitude and density in PVN-RVLM neurons were 9.79 ± 1.28 pA and 0.30 ± 0.04 pA pF−1, respectively, the former being significantly larger that the mean GABAAIphasic (3.86 ± 0.77 pA, P < 0.01). Thus, most (∼70%) of the total GABAA receptor-mediated current (mean phasic + mean tonic) in PVN-RVLM neurons is carried by the ‘tonic’ modality.
GABAAItonic in PVN-RVLM neurons is enhanced by the δ-subunit selective agonist THIP
Numerous studies indicate that tonic inhibition in various neuronal types is mediated by extrasynaptic GABAA receptors containing the δ-subunit (Brickley et al. 1996; Wall & Usowicz, 1997; Stell & Mody, 2002;
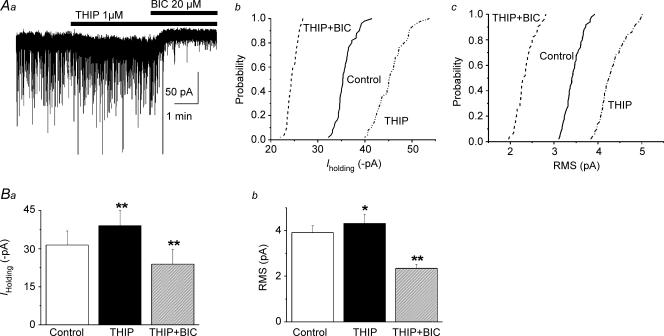
Cope et al. 2005). To determine whether this was also the case in PVN-RVLM neurons, we tested the effects of 4,5,6,7-tetrahydroisothiazolo-[5,4-c]pyridin-3-ol (THIP), a GABAA agonist shown to preferentially activate δ- over γ-containing GABAA receptors (Adkins et al. 2001; Brown et al. 2002; Drasbek et al. 2007). Bath application of 1 μm THIP significantly increased Iholding (control: 31.42 ± 5.54 pA, THIP: 39.00 ± 6.02 pA, P < 0.002, n= 9) and RMS (control: 3.58 ± 0.40 pA, THIP: 3.96 ± 0.47 pA, P < 0.01, n= 9), effects that were reversed by subsequent application of BIC (20 μm) (Fig. 5). On the other hand, the frequency, the amplitude or the decay kinetics of GABAA IPSCs were not affected by THIP (IPSC amplitude: control: 58.42 ± 5.7 pA, THIP: 54.38 ± 4.94 pA; IPSC weighted τ: control: 27.46 ± 2.86 ms, THIP: 27.54 ± 1.84 ms; IPSC frequency: control: 2.41 ± 0.85 Hz, THIP: 2.11 ± 0.68 Hz, P > 0.5, n= 9 in all cases).
Figure 5. The δ subunit-specific GABAA agonist THIP enhanced GABAAItonic.
Aa, representative current trace showing that THIP (1 μm) enhanced Iholding and RMS in a PVN-RVLM neuron. Note that the effect was blocked by BIC. Cummulative distribution histograms of Iholding and RMS before and after THIP, and THIP + BIC, obtained from the same cell as in Aa, are shown in Ab and Ac, respectively. In both cases, THIP significantly shifted the curves to the right (P < 0.0001, Kolmogorov–Smirnov test), while the opposite was observed when BIC was added (THIP + BIC) (P < 0.0001, Kolmogorov–Smirnov test). The effects of THIP and THIP + BIC on mean Iholding and RMS are summarized in Ba and Bb (n= 9), respectively. *P < 0.05 and **P < 0.01 versus control.
GABAAItonic in PVN-RVLM neurons is influenced by synaptic GABA activity
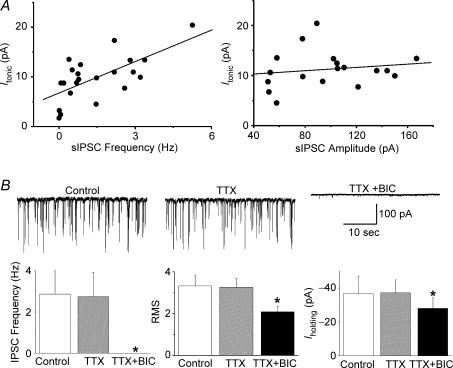
A significant correlation between basal Itonic magnitude and IPSCs frequency (R2= 0.66, n= 19, P < 0.001), but not amplitude (R2= 0.16, n= 19, P > 0.5, Fig. 6A) was found, suggesting that Itonic is influenced by the degree of ongoing synaptically released GABA. This interaction, however, did not involve action potential-dependent synaptic activity: the Na+ channel blocker TTX (0.5 μm) failed to affect IPSC frequency, baseline RMS or Iholding (n= 5, P > 0.5, Fig. 6B and C). Furthermore, BIC in the presence of TTX still outwardly shifted Iholding (Fig. 6B and C), an effect not significantly different from that observed in control conditions (9.79 ± 1.28 pA versus 9.04 ± 2.05 pA, in control and TTX, respectively, P > 0.5).
Figure 6. Itonic is influenced by synaptically released GABA.
A, a significant positive correlation between the magnitude of Itonic and IPSCs frequency (left panel, R2= 0.66) but not IPSC amplitude (right panel, R2= 0.16) was observed. B, representative examples (upper panels) and summary data (lower panels) of the effects of TTX (0.5 μm) and BIC (20 μm) on phasic and tonic GABAA receptor-mediated currents. TTX failed to affect IPSCs frequency, RMS or Iholding. In the presence of TTX, BIC still induced an outward shift in Iholding and decreased RMS. *P < 0.05, compared with control (n= 5).
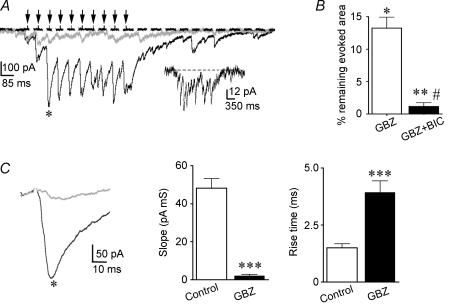
The influence of synaptically released GABA on Itonic was further studied during conditions of high afferent synaptic activity. During repetitive afferent stimulation, IPSCs successively summated, reaching a plateau ‘envelope’ inward current after the third stimulus pulse. While 1 μm GBZ largely reduced the evoked envelope current (∼15% of the evoked current was still observed in GBZ), addition of BIC almost complete inhibited the evoked response (only ∼1% of the evoked current was still observed) (Fig. 7A and B).
Figure 7. Synchronous activation of GABAergic terminals activates Itonic.
A, representative traces showing an ‘envelope’ inward current following evoked GABAA IPSCs using extracellular stimulation (150 μA, 10 pulses at 20 Hz) dorsolaterally to the PVN in control ACSF (black trace), 1 μm GBZ (grey trace) and 1 μm GBZ + 20 μm BIC (dashed trace). Arrows indicate the stimulus delivery times. In the presence of GBZ, the magnitude of the evoked response was robustly, though not completely, blocked. Further addition of BIC completely blocked the evoked response. The GBZ-insensitive current is shown in the inset at an expanded scale. B, summary data showing the effects of GBZ and GBZ + BIC on the area of the evoked response (n= 7) *P < 0.02 and **P < 0.01 when compared with control. #P < 0.05 when compared with GBZ. C, the third IPSCs evoked in the train shown in A (black, control ACSF; grey, GBZ) are displayed at expanded time/amplitude scales (left). Note the slower IPSC rising kinetics in the presence of GBZ. Summary data of the mean evoked IPSC rising slope (middle) and rise time (right) in control ACSF in the presence of GBZ (n= 7). ***P < 0.001 when compared with control.
When the GBZ-insensitive evoked current was observed at an expanded scale, IPSC-like events were still noticeable, although their summation during the whole response was not so evident as in the absence of GBZ. The mean rise time and rising slope of the evoked IPSC-like events in the presence of GBZ were significantly slower than those evoked in control conditions (P < 0.001 in both cases, Fig. 7C). Overall, these results support the activation of GABAAItonic during high frequency, synchronous synaptic GABA release (see Discussion).
Itonic restrains firing activity and modulates the input–output function in PVN-RVLM neurons
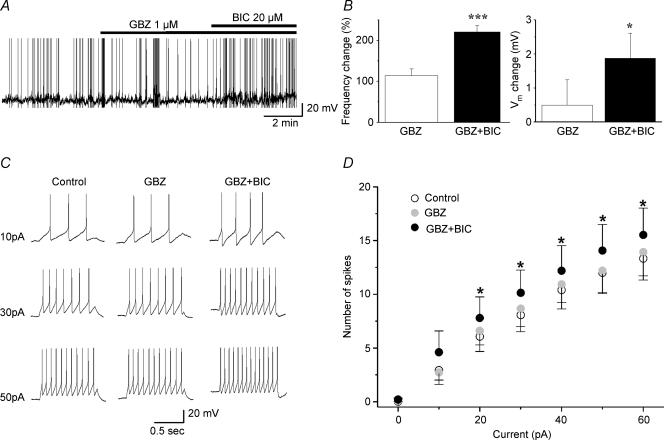
PVN-RVLM neurons recorded in the current-clamp mode (n= 8), were spontaneously active and fired in a continuous irregular mode. The sequential application of GBZ and GBZ + BIC was used to differentiate between the roles of Iphasic and Itonic in modulating these parameters. While blockade of Iphasic (GBZ, 1 μm) failed to change PVN-RVLM Vm or firing rate (P > 0.5), further block of Itonic with BIC depolarized them by ∼3 mV and almost doubled their firing discharge (P < 0.05, Fig. 8A and B).
Figure 8. Blockade of Itonic, but not Iphasic induced membrane depolarization, increased firing activity and enhanced the input–output function in PVN-RVLM neurons.
A, representative example showing that the combined use of GBZ + BIC, but not GBZ alone, increased neuronal firing activity of PVN-RVLM neurons. B, summary data depicting changes in Vm and firing discharge (expressed as percentage change of pre-drug control) induced by GBZ and GBZ + BIC (n= 8) *P < 0.05 and ***P < 0.001, when compared with control or GBZ, respectively. C, representative examples of action potentials evoked by depolarizing pulses of increasing amplitude in control ACSF (left panel), GBZ (1 μm, middle panel) and GBZ + BIC (20 μm, right panel). Membrane potential was held at ∼−55 mV in all conditions. D, mean plot of the number of evoked spikes as a function of depolarizing current amplitude in control ACSF, GBZ and GBZ + BIC. *P < 0.05, compared with control.
Input–output (I–O) functions were built using depolarizing current steps of increasing amplitude (see Methods). Our results showed that the evoked firing was dependent both on current injection and on drug treatment (F= 10.16 and 28.62, respectively, P < 0.0001, ANOVA). Post hoc tests demonstrated that while the evoked firing responses were not affected by GBZ (P > 0.5), they were significantly increased by co-application of GBZ + BIC (P < 0.05, Fig. 8C and D). The steepness of the I–O function was found to be dependent on drug treatment (F= 17.68, P < 0.01, one-way ANOVA-RM), being significantly increased in the presence of GBZ + BIC, but not when GBZ was applied alone (control: 0.33 ± 0.07 spikes pA−1; GBZ: 0.36 ± 0.07 spikes pA−1; GBZ + BIC: 0.41 ± 0.08 spikes pA−1, P < 0.01, GBZ + BIC compared with control and GBZ). Altogether, these results support an important role for GABAAItonic in the regulation of membrane excitability and firing discharge in PVN-RVLM neurons.
Discussion
Results from these studies indicate that in addition to mediating conventional quantal synaptic transmission (Iphasic), GABAA receptors in presympathetic PVN neurons also mediate a slower, persistent form of inhibition (Itonic). This is supported by our findings showing that the GABAA receptor and channel blockers BIC and PIC, respectively, induced an outward shift in the holding current, and diminished background noise levels.
Distinct pharmacological properties of GABAA receptors underlying phasic and tonic inhibition in PVN-RVLM neurons
GABAA receptors underlying Itonic in other CNS neuronal populations have been shown to have distinct biophysical and pharmacological properties, molecular configuration and subcellular distribution (i.e. located peri- and/or extra-synaptically) than those mediating Iphasic (see Semyanov et al. 2004 for review). In general agreement, we found GABAA receptors underlying Iphasic and Itonic in PVN-RVLM neurons to have distinct pharmacological and functional properties. While BIC and PIC blocked both synaptic and tonic GABAA receptor-mediated inhibition, the competitive, high affinity antagonist GBZ (within a wide range of concentrations tested) blocked synaptic inhibition without affecting Itonic. Thus, and as previously shown in hippocampal neurons (Stell & Mody, 2002), the lack of sensitivity of Itonic to GBZ suggests that GABAA receptors mediating Itonic in PVN-RVLM neurons have a higher affinity for GABA than those mediating Iphasic. A similar pharmacological profile between Itonic and Iphasic has been reported in other CNS neuronal types (Semyanov et al. 2003), including our recent studies on hypothalamic oxytocin and vasopressin magnocellular neurosecretory neurons (Park et al. 2006).
In addition, numerous studies support a differential subunit composition of GABAA receptors underlying synaptic and tonic inhibition. While association of the γ subunit with α1, α2 or α3 and β2/3 subunits is the predominant configuration of synaptic GABAA receptors, extrasynaptic GABAA receptors underlying tonic inhibition have been shown to contain predominantly the δ subunit, associated either with α5 and/or α6 subunits (Brickley et al. 1996; Wall & Usowicz, 1997; Stell & Mody, 2002; Cope et al. 2005). Our results showing enhancement of GABAAItonic by 1 μm THIP, a compound known to preferentially activate δ- over γ-containing GABAA receptors (Adkins et al. 2001; Brown et al. 2002; Drasbek et al. 2007), suggest that δ subunit-containing GABAA receptors also mediate tonic inhibition in this presympathetic neuronal population. Further experiments are warranted to determine the specific molecular configuration of GABAA receptors underlying synaptic and tonic inhibition in PVN-RVLM neurons.
Synaptic GABA activity influences GABAAItonic in PVN-RVLM neurons
The precise mechanisms that underlie and modulate GABAAItonic inhibition in the CNS are in general poorly understood. Both vesicular (i.e. spillover of transmitter released from neighbouring synapses (Rossi & Hamann, 1998), as well as non-vesicular mechanisms (i.e. ambient GABA levels modulated by glial GABA transporters) (Wall & Usowicz, 1997; Semyanov et al. 2003) have been shown to act as sources of GABA that activate Itonic. In the present studies, we found a positive correlation between basal IPSC frequency and Itonic magnitude, suggesting that asynchronous, synaptically released GABA under basal conditions may contribute to Itonic. Since neither IPSCs nor Itonic were dependent on action potential-evoked GABA release, our results suggest that spontaneous release of GABA (miniature events) may be sufficient to contribute to Itonic in PVN-RVLM neurons. It is worth noting, however, that synaptic GABA activity in the intact animal is controlled in an activity-dependent manner, driven in part by vagal afferent activity (Coote, 2005). Thus, due to the absence of peripheral inputs actively driving GABAergic activity in our slice preparation, the degree of GABAA-mediated phasic/tonic interactions reported here may be underestimated.
In clear contrast to these finding in PVN-RLVM neurons, we recently reported Itonic in magnocellular neurosecretory neurons to be independent on the degree of ongoing synaptic activity (Park et al. 2006). While the mechanisms underlying such disparity in the relationship between basal phasic and tonic GABAA activity in these two hypothalamic neuronal populations are at present unknown, it is tempting to speculate that distinct neuronal–glial microenvironments could constitute a contributing factor. In this sense, magnocellular neurosecretory neurons and their synapses are characterized by being tightly enwrapped by thin glial processes (Theodosis & Poulain, 1984; Tweedle & Hatton, 1984). This unique neuronal–glial structural arrangement would act as a diffusion barrier (Piet et al. 2004), limiting the ability of synaptically released GABA to spillover from the synaptic cleft (Isaacson et al. 1993) to activate extrasynaptic GABAA receptors mediating Itonic. Furthermore, this neuronal–glial arrangement favours a strong and efficient removal of GABA from the cleft through active uptake mechanisms (Conti et al. 2004), further limiting GABA spillover under basal conditions. This is, in fact, supported by our recent study showing that high affinity glial GABA transporters efficiently modulate GABAAItonic in the magnocellular supraoptic nucleus (SON) neurons (Park et al. 2006). A detailed ultrastructural characterization of the microanatomical inter-relationship among PVN-RVLM neurons, their synapses and associated glial processes (i.e. the ‘tripartite synapse’) (Araque et al. 1999) would be needed to determine in fact whether a ‘leaky’ neuronal–glial microenvironment may facilitate spillover of GABA and activation of Itonic under basal condition in this neuronal population.
In addition, while a vesicular source of GABA seems to contribute to Itonic in PVN-RVLM neurons, a role for glial GABA transporters cannot be ruled out at present. Future studies will be needed to address the role of non-vesicular sources in the control of Itonic in PVN-RVLM neurons.
In contrast to cell-type differences in the relationship between spontaneous synaptic GABA release and Itonic, we found Itonic in both magnocellular neurosecretory (Park et al. 2006) and presympathetic PVN neurons (this study) to be similarly influenced by GABA released during high frequency, synchronous activation of GABAergic terminals. This is supported by our results showing a GBZ-insensitive, BIC-sensitive component on the evoked GABAA response, and by the presence of smaller and slower rising IPSC-like events in the presence of GBZ. Since the kinetics of activation of GABAA receptors depend on agonist concentration (Maconochie et al. 1994; Jones & Westbrook, 1995; Galarreta & Hestrin, 1997), the observed slower IPSC-like events could reflect spillover and diffusion of GABA out of the cleft, resulting in lower GABA concentration reaching extrasynaptically located GABAA receptors (Rossi & Hamann, 1998). This is in agreement with work by Rossi & Hamann (1998) showing that single evoked IPSCs in cerebellar granule cells display fast and slow rising components, the latter mediated by activation of extrasynaptic GABAA receptors (Rossi & Hamann, 1998). Alternatively, the IPSC-like events recorded in the presence of GBZ could result from the activation of a subpopulation of GABAA receptors located at distal synaptic sites, which are insensitive to GBZ and whose slower kinetics resulted from dendritic filtration. This is, however, unlikely, because all GABAA IPSCs, at least those occurring spontaneously, were blocked by GBZ.
Altogether, our results suggest that the sources contributing to Itonic in the hypothalamus may vary in a cell-type-dependent manner, being influenced in part by differences in the degree of ongoing synaptic activity and/or differences in the microanatomy of the neuronal/synaptic–glial microenvironment.
GABAAItonic inhibits PVN-RVLM excitability and firing activity
In agreement with the fact that Itonic accounted for ∼70% of the total GABAA-receptor mediated current in PVN-RVLM neurons, we found this inhibitory modality to influence membrane potential and repetitive firing activity in this neuronal population. Thus, pharmacological blockade of Itonic induced membrane depolarization, increased firing discharge, and enhanced the gain of the input–output function in PVN-RVLM neurons. Blockade of Iphasic, on the other hand, failed to induce significant changes in any of these parameters. Similar responses were reported in other CNS neuronal types, including SON magnocellular neurosecretory neurons (Park et al. 2006), thalamic neurons (Cope et al. 2005) and cerebellar granule cells (Mitchell & Silver, 2003). Thus, as previously proposed (Semyanov et al. 2004), it is likely that these two inhibitory modalities contribute differently to information processing in PVN-RVLM neurons, with Iphasic mediating specific point-to-point inhibition and/or fine-tuning spike timing (Bevan et al. 2002; Kononenko & Dudek, 2004), and Itonic influencing overall neuronal excitability level.
It was recently shown that sympathoexcitation induced by blockade of GABAA receptors in the PVN of the intact animal was dependent on ongoing glutamatergic activity (Chen et al. 2003; Li et al. 2006). In addition, we recently reported an increase in glutamate EPSCs in PVN-RVLM neurons in the presence of BIC (Li et al. 2006). Whether increased glutamate activity contributes to the enhanced firing activity in PVN-RVLM neurons following blockade of GABAAItonic remains to be determined. This was not the case, however, in SON magnocellular neurosecretory neurons, in which the BIC-induced depolarization and increased firing rate was shown not to be dependent on ongoing glutamatergic activity (Park et al. 2006).
In summary, our results indicate that GABAA receptors of distinct pharmacological (and probably molecular) properties mediate ‘phasic’ and ‘tonic’ inhibitory modalities in PVN-RVLM neurons. These findings represent, to the best of our knowledge, the first demonstration of a functionally relevant GABAA-mediated persistent, tonic inhibition in CNS presympathetic neurons. Importantly, numerous studies using local PVN microinjections of the GABAA receptor blocker bicuculline support an important role for GABA in the control of PVN sympathetic outflow (Martin & Haywood, 1998; Kenney et al. 2001; Zhang et al. 2002; Chen et al. 2003; LaGrange et al. 2003; Yang & Coote, 2003; Li et al. 2006). Based on our present findings, however, those studies do not differentiate between the involvement of Iphasic and Itonic. Thus, it will be important in the future to determine the relative contribution of these two inhibitory modalities to the control of PVN sympathetic output, both under physiological and pathological conditions.
Acknowledgments
This work was supported by NIH grant RO1 HL68725 (J.E.S.) and research fund of Chungnam National University in 2006 (J.B.P.).
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Badoer E, Merolli J. Neurons in the hypothalamic paraventricular nucleus that project to the rostral ventrolateral medulla are activated by haemorrhage. Brain Res. 1998;791:317–320. doi: 10.1016/s0006-8993(98)00140-1. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Hallworth NE, Bolam JP, Wilson CJ. Regulation of the timing and pattern of action potential generation in rat subthalamic neurons in vitro by GABA-A IPSPs. J Neurophysiol. 2002;87:1348–1362. doi: 10.1152/jn.00582.2001. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension. 2003;42:725–731. doi: 10.1161/01.HYP.0000085197.20043.44. [DOI] [PubMed] [Google Scholar]
- Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res Brain Res Rev. 2004;45:196–212. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol. 2005;90:169–173. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol. 1998;25:461–463. doi: 10.1111/j.1440-1681.1998.tb02235.x. [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. J Neurophysiol. 2007;97:2293–2300. doi: 10.1152/jn.00651.2006. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Properties of GABAA receptors underlying inhibitory synaptic currents in neocortical pyramidal neurons. J Neurosci. 1997;17:7220–7227. doi: 10.1523/JNEUROSCI.17-19-07220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Potts PD, Polson JW, Dampney RA. Distribution of neurons projecting to the rostral ventrolateral medullary pressor region that are activated by sustained hypotension. Neuroscience. 1999;89:1319–1329. doi: 10.1016/s0306-4522(98)00399-6. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron Diversity series: fast in, fast out – temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Weiss ML, Patel KP, Wang Y, Fels RJ. Paraventricular nucleus bicuculline alters frequency components of sympathetic nerve discharge bursts. Am J Physiol Heart Circ Physiol. 2001;281:H1233–H1241. doi: 10.1152/ajpheart.2001.281.3.H1233. [DOI] [PubMed] [Google Scholar]
- Kononenko NI, Dudek FE. Mechanism of irregular firing of suprachiasmatic nucleus neurons in rat hypothalamic slices. J Neurophysiol. 2004;91:267–273. doi: 10.1152/jn.00314.2003. [DOI] [PubMed] [Google Scholar]
- LaGrange LP, Toney GM, Bishop VS. Effect of intravenous angiotensin II infusion on responses to hypothalamic PVN injection of bicuculline. Hypertension. 2003;42:1124–1129. doi: 10.1161/01.HYP.0000102181.83892.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther. 2005;313:1035–1045. doi: 10.1124/jpet.104.082495. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1110–H1119. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience. 2003;118:585–601. doi: 10.1016/s0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol. 2006;291:H2847–H2856. doi: 10.1152/ajpheart.00625.2005. [DOI] [PubMed] [Google Scholar]
- Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open? Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Martin DS, Haywood JR. Reduced GABA inhibition of sympathetic function in renal-wrapped hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1523–R1529. doi: 10.1152/ajpregu.1998.275.5.R1523. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Park JB, Skalska S, Stern JE. Characterization of a novel tonic γaminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006;147:3746–3760. doi: 10.1210/en.2006-0218. [DOI] [PubMed] [Google Scholar]
- Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101:2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAA receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801:239–243. doi: 10.1016/s0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Dampney RA. AT1 receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience. 1984;11:183–193. doi: 10.1016/0306-4522(84)90222-7. [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI. Synapse formation and disappearance in adult rat supraoptic nucleus during different hydration states. Brain Res. 1984;309:373–376. doi: 10.1016/0006-8993(84)90607-3. [DOI] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci. 1997;9:533–548. doi: 10.1111/j.1460-9568.1997.tb01630.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J Physiol. 1998;513:521–530. doi: 10.1111/j.1469-7793.1998.521bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Coote JH. Role of GABA and NO in the paraventricular nucleus-mediated reflex inhibition of renal sympathetic nerve activity following stimulation of right atrial receptors in the rat. Exp Physiol. 2003;88:335–342. doi: 10.1113/eph8802561. [DOI] [PubMed] [Google Scholar]
- Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1006–R1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol. 1998;275:R728–R734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]