SUMMARY
The universal bacterial transcription elongation factor NusA mediates elongation activities of RNA polymerase. By itself, NusA induces transcription pausing and facilitates intrinsic termination, but NusA also is a cofactor of antiterminators that antagonize pausing and prevent termination. We show that NusA is required for λ-related phage 82 antiterminator Q82 to construct a stable complex in which RNA-based termination mechanisms have restricted access to the emerging transcript; this result suggests a locale for both Q82 and NusA near the β flap domain of RNAP. Furthermore, since NusA is not required for the antipausing activity of Q82 in vitro, we distinguish two distinct activities of antiterminators, namely antipausing and RNA occlusion, and we discuss their roles in Q82 function.
Keywords: Transcription, elongation, antitermination, pausing
INTRODUCTION
The elongation stage of RNA synthesis is an important focus of regulation of transcription by multisubunit RNA polymerases. Bacterial RNA polymerase (RNAP) is the target of well characterized antiterminators such as phage λ gene Q and N proteins and HK022 put RNA (DeVito and Das, 1994; Greenblatt et al., 1998; Roberts et al., 1998; Sen et al., 2001), and the bacterial RfaH and ribosomal gene antitermination systems (Artsimovitch and Landick, 2002; Condon et al., 1995; Vogel and Jensen, 1997). These regulators not only antiterminate but also inhibit transcription pausing at non-terminator sites, and therefore fundamentally affect the elongation stage of transcription. For example, protein Q accelerates the release of RNAP from a σ70-dependent pause near the transcription start site (Grayhack et al., 1985), and Q, N, and RfaH all inhibit pausing during elongation (Artsimovitch and Landick, 2002; Rees et al., 1997; Yang and Roberts, 1989). In a potentially similar way, eukaryotic PolII undergoes promoter-proximal pausing after making a few tens of nucleotides of RNA (Saunders et al., 2006), and the ability of regulators to antagonize this paused state may be fundamental to control by transcription activators like the HIV regulator TAT (Peterlin and Price, 2006). Universal elongation proteins like NusA and NusG of eubacteria, and Spt4/Spt5 (the complex DSIF), NELF and pTEFb of eukaryotes, mediate both pausing and the function of elongation regulators. Several of these factors bind RNA (NusA, N, NELF, and TAT), and others can act in complexes with RNA, suggesting that the transcript is an important target of regulation.
Atomic structures and models of RNA polymerases provide a framework to understand how regulators of elongation act, particularly by revealing structural features that may be targets of the opposing activities of terminators and antiterminators. In an elongation complex, RNAP binds the emerging transcript, the DNA-RNA hybrid, and the downstream double-stranded DNA (Korzheva et al. 2000), all interactions that must be disrupted in termination. In E. coli, there are three characterized modes of transcription termination. In intrinsic termination, a hairpin structure in the emerging RNA destabilizes a complex containing a ribo-uridine-rich RNA/DNA hybrid (Nudler and Gottesman 2002; Santangelo and Roberts 2004; Yarnell and Roberts 1999). The ATP-dependent termination enzyme Rho (Richardson 2003; Skordalakes and Berger 2003) also targets the emerging transcript, perhaps pulling it from the complex, whereas the ATP-dependent enzyme Mfd translocates on upstream DNA, likely inducing collapse of the transcription bubble (Park et al. 2002). These mechanistic differences may help reveal pathways of termination and antitermination.
The λ (and related) phage gene Q antiterminators provide important models of the mechanism of action of elongation regulatory factors. Despite the lack of significant homology in the sequences of some of the Q proteins from the related phages, several features of their functions are highly conserved. In engaging RNAP, Q binds DNA and interacts with RNAP at a σ70-dependent pause site 16-25 nucleotides (nt) from the initiation site, contacting σ70 (Ko et al., 1998; Marr et al., 2001; Nickels et al., 2006; Nickels et al., 2002) and, presumably, subunits of the RNAP core enzyme (Santangelo et al., 2003), although no defined core binding site has been characterized. Q becomes a stable subunit of the complex (Yarnell and Roberts, 1999, Deighan and Hochschild, 2007), causing it to transcribe past both intrinsic and Rho-dependent termination signals and leading to expression of the phage late genes (Roberts et al., 1998; Yarnell and Roberts, 1992). Static Q-modified complexes are stabilized against dissociation by an intrinsic terminator (Yarnell and Roberts, 1999), an activity that can explain antitermination. In addition, however, the antipausing activity of Q suggests its potential to act by speeding the enzyme through limiting steps in termination, a mechanism that has been considered also for the N protein. The nature of these two activities, and their relation to the function of Q and other antiterminators, has been obscure.
The highly conserved and essential bacterial protein NusA, a 55kd (in E. coli) monomeric protein with S1 and KH RNA binding domains (Gopal et al., 2001), is a cofactor of Q-mediated antitermination. Despite the identification of various roles of NusA in transcription, its exact mechanism in regulating termination and antitermination has been mysterious. NusA was discovered through its role in phage λ protein N-mediated antitermination (Friedman and Baron, 1974), where it engages in a multiprotein complex that interacts with the RNA (nut site) and modifies RNAP to transcribe past termination signals (Das et al., 1996; Horwitz et al., 1987). In an analogous activity, NusA is also required for antitermination at the bacterial ribosomal RNA operons (Squires et al., 1993). During transcription in vitro, NusA enhances pausing and intrinsic termination, but, contrarily, inhibits Rho-dependent termination (Lau et al., 1983; Sigmund and Morgan, 1988). The lethality of a deletion of E. coli nusA is suppressed by a compensatory mutation in rho (Zheng and Friedman, 1994), suggesting that the essential cellular function of NusA is to prevent inappropriate Rho-dependent termination. The primary activity of NusA may be to bind RNA near the transcript exit site of RNAP, where it could mediate RNA interactions with RNAP and other factors, for example in termination, and could act in conjunction with the N antiterminator to interfere with intrinsic terminator hairpin formation (Gusarov and Nudler, 2001; Liu and Hanna, 1995).
In standard conditions in vitro, NusA stimulates function of the antiterminator Q from phage λ (Qλ) substantially (Grayhack et al., 1985) and that of Q from related λ-like phage 82 (Q82) slightly (Yang et al., 1989); this variable requirement has left the role of NusA in Q function obscure. Here we show that NusA is responsible for a Q82-mediated structural modification of the transcription complex that physically protects the emerging transcript and prevents it from engaging in interactions required for termination; this function of NusA is essential for the protection of static complexes against dissociation by RNA based termination mechanisms and for Q82-mediated antitermination during inefficient transcription elongation in vitro. These results define a physical basis for an antitermination activity and provide insights into the role of NusA function in cellular transcription. Furthermore, they suggest a locale for both NusA and Q82 in the transcription complex. The kinetic activity of Q82 (namely antipausing) does not require NusA in vitro and is distinct from the NusA-dependent protection of static complexes via occlusion of the RNA. Although the NusA-independent kinetic effect of Q82 may suffice for its antitermination activity when bacterial cells are growing under optimal conditions, we suggest that the NusA- and Q82 - dependent occlusion of the transcript is responsible for antitermination in vivo under conditions of stress and dormancy, when transcription elongation is inefficient.
RESULTS
Preliminary characterization: A defined Q82-modified RNAP elongation complex as a model of transcript release mechanisms
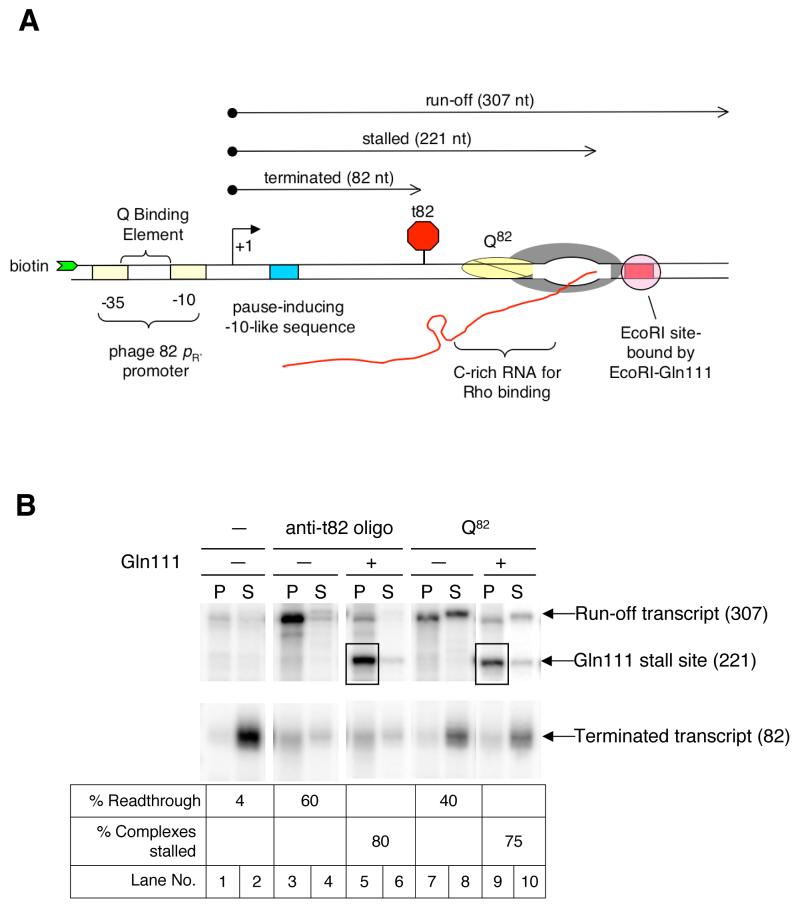
We first describe a defined, stalled substrate constructed to characterize biochemically the effect of Q82 on RNAP. Since Q is active in purified systems, we used only RNAP, Q82, and NusA protein, along with template DNA and small molecules. A homogenous population of complexes modified by Q82 was obtained by transcription through an efficient intrinsic terminator (t82) to remove unmodified complexes. These complexes were stalled at a downstream EcoR1 site by the roadblock protein EcoRI-Gln111, a cleavage deficient mutant of the endonuclease EcoR1, such that the 3′ end of the RNA in the stalled complex is 14 nt upstream of the EcoR1 recognition sequence (Pavco and Steege, 1990) (Figure 1A & 1B). To obtain equivalent complexes unmodified by Q82, we added an oligonucleotide (anti-t82) complementary to the upstream stem of the t82 hairpin that prevents hairpin formation, allowing unmodified elongation complexes to skip the terminator (Figure 1B). We used biotinylated DNA and performed the transcription on streptavidin-coated magnetic beads, so that magnetic partitioning could be used to detect termination, which is accompanied by RNA release into the supernatant (also see Supplemental Information).
Figure 1.
- Schematic of the experimental method used to construct defined populations of Q82-modified complexes. The template was obtained from pSS100 and contained the phage 82 late gene promoter, the intrinsic terminator t82, a C-rich region for optimal Rho binding to the RNA, and an EcoR1 site. Distances are indicated from the transcription start site.
- Gel resolution of elongation complexes filtered past t82 using either the oligo anti-t82 or the antiterminator Q82, and stalled at a downstream site using the roadblock protein EcoR1-Gln111. After incubation with the indicated components, reaction mixtures were separated into magnetic bead-bound pellet (P) and supernatant (S) fractions. Numbers in parentheses indicate lengths of the RNA transcripts.
RNA release from stalled complexes by an oligonucleotide or Rho mimics termination pathways and their response to NusA
Before considering the effect of Q, we show in this section that the stalled complex matches elongating complexes in its response to terminators and to NusA, and is thus an appropriate model system for antitermination. We use Oligonucleotide-Mediated Release (OMR) (Yarnell and Roberts, 1999) to simulate intrinsic termination on stalled elongation complexes lacking terminator hairpins, and to allow the release step of the reaction to be manipulated. Briefly, in OMR, an oligonucleotide (oligo) complementary to the RNA emerging from the elongation complex is provided in trans; this oligo mimics the upstream stem of the terminator hairpin, base pairing to the RNA and extracting it from the elongation complex.
We tested a series of GC-rich oligos of length 15 (15mers) that anneal at different distances from the RNA 3′ end such that their 5′ ends span the interval from -9 to -14 from the 3′ end of the RNA (Figure 2A). In a standard reaction containing NusA, about 60% of the RNA is released when the oligo extends to -9 or -10, whereas there is no release at all when the oligo extends to -12. Therefore, the 5′ end of the oligo has to anneal to at least position -11 of the RNA in order to destabilize the elongation complex and release RNA, a result that defines precisely the boundary of oligo release activity, and is overall comparable to the extent of base pairing in terminator hairpins (Wilson and von Hippel, 1994) and with previous results for OMR (Yarnell and Roberts, 1999).
Figure 2.
Stalled elongation complexes as models for transcription termination mechanisms and effect of NusA on Q82-mediated stabilization of stalled elongation complexes on a template from pSS100
A. Stalled unmodified complexes, made and maintained in the presence of NusA, were incubated at 37 °C for 10′ with oligos that annealed at different distances from the RNA 3′ end, as shown in the schematic (the stalled complexes may include some backtracked complexes not shown in the schematic), and transcript release efficiency was measured. Quantification of the data in the gel (left) is shown in the graph (right).
B. Stalled Q82-modified or unmodified complexes, made and maintained in the presence or absence of NusA, were incubated at 37°C for 10′ with 1 μM each of a set of oligos of varying lengths, all annealing at their 5′ end to -9 of the transcript.
C. Stalled Q82-modified or unmodified complexes, made and maintained in the presence or absence of NusA, were incubated at 37°C for 10′ with varying concentrations of the 15mer oligo that extends from -9 to -23 of the transcript.
D, E. Time course of RNA release from stalled Q82-modified or unmodified complexes, made and maintained in the presence or absence of NusA, and treated with (D) 1 μM of 15mer oligo or (E) 10 nM Rho and 1 mM dATP.
Using a series of oligos annealing with their 5′ ends at -9 of the transcript, we determined the effect of altering oligo length on the efficiency of RNA release. Oligos of length 10, 11 or 12 are unable release RNA from the stalled complexes in the absence of NusA (Figure 2B). However, longer oligos are active and the efficiency of RNA release increases with length, suggesting that release efficiency increases with the strength of interaction between the oligo and the RNA. NusA allows substantial release of RNA from the stalled complex with 10mer, 11mer, and 12mer oligos and stimulates release with oligos of length 13-20 by about 1.5-2-fold (Figure 2B). The effect persists through a roughly linear time course of release as shown using the 15mer (Fig. 2D). In addition, using the 15mer at various concentrations, we find up to four-fold effect of NusA at lower concentrations (Figure 2C). When the oligo concentration is sufficiently high (∼100 μM), the originally reported conditions of the assay (Yarnell and Roberts, 1999), the effect of NusA is slight (data not shown). These effects are consistent with the magnitude of NusA effects on intrinsic termination in vitro. The results suggest that NusA facilitates oligo binding to the RNA, consistent with previous suggestions that NusA interacts with RNA in a paused elongation complex (Toulokhonov et al., 2001) and promotes RNA hairpin formation (Gusarov and Nudler, 2001).
We also find that NusA inhibits RNA release by Rho from the stalled complex (Figure 2E); the effect persists over a roughly linear time course of release. This result is consistent with the effect of NusA on Rho-dependent termination in vitro, and suggests that NusA inhibits access by Rho to the elongation complex, and specifically to the RNA.
Together, these results suggest that the stalled elongation complex is an appropriate model system to study antitermination.
Q82 protects stalled transcription elongation complexes against oligo- and Rho-mediated RNA release in a NusA-dependent manner
We showed previously (Yarnell & Roberts, 1999) that Q82-modified RNAP complexes that are stalled at random sites during elongation are resistant to OMR. Here we show, using the defined stalled complex and the approach described above, that this resistance depends completely upon the presence of NusA (Figure 2 B, C, and D). The stabilizing effects of Q82 are lost in the absence of NusA even if shorter oligos (Figure 2B) or lower concentrations of the 15mer oligo (Figure 2C) are used to weaken oligo binding, indicating that NusA is absolutely required for Q82 to stabilize stalled complexes against oligo-mediated release.
We asked if Q82 protects static elongation complexes against Rho as well. Rho is thought to act in kinetic competition with the elongating RNAP (Jin et al., 1992), and it has been suggested that the antipausing function of Q could explain antitermination at Rho-dependent terminators (Yang and Roberts, 1989). However, we show that Rho is unable to release RNA from stalled Q82-modified complexes (Figures 2E, 4B), indicating that Q82 need not simply work by reducing pausing at Rho-dependent termination sites, but has an additional protective effect against Rho as well. As expected, the protective effect of Q82 against Rho is completely lost in the absence of NusA (Figure 2E), as for OMR.
Figure 4.
- 1 μM of the 15mer release oligo;
- 10 nM Rho + 1 mM dATP as energy source; and
- 50 nM Mfd + 1 mM dATP as energy source.
These results were surprising because, as mentioned above, Q82-mediated antitermination in solution experiments is mostly independent of NusA. Figures 3A and 3B illustrate the significant but modest effect of NusA on Q82-mediated antitermination at both an intrinsic terminator and Rho-dependent terminator during transcription in solution from the phage 82 late gene promoter. Similarly, we showed that Q82-modified elongation complexes are able to transcribe past the filtering intrinsic terminator t82 and reach the EcoR1 stall site in the absence of NusA in the system described above (Figure 2B, C, D and E). Furthermore, quantitative western blot analysis of the stalled complexes reveals that Q82 stably associates with elongation complexes even in the absence of NusA (data not shown), so that engagement of Q82 does not require NusA.
Figure 3.
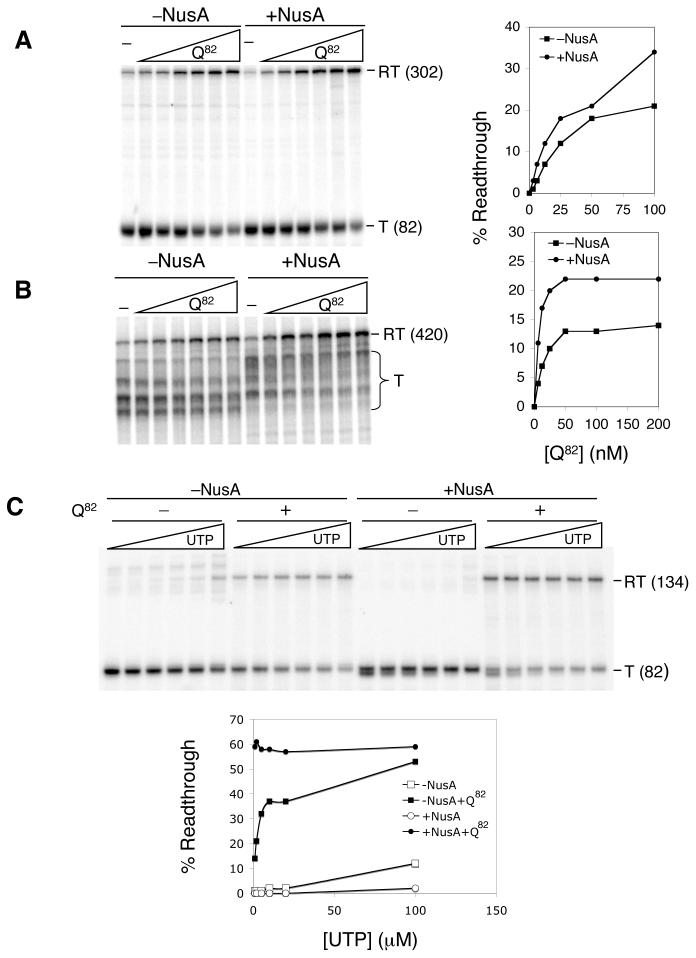
- Template p82a containing the wild type phage 82 late gene promoter and the t82 intrinsic terminator segment was transcribed in the absence or presence of NusA, with 0, 3.1, 6.2, 12.5, 25, 50 and 100 nM Q82. The percent readthrough was calculated as the moles readthrough RNA (RT) divided by the sum of moles RT and moles terminated RNA (T), minus background.
- Template pXY312 containing wild type phage 82 late gene promoter upstream of the λ tR1 Rho-dependent termination sites was transcribed as in (A), except in the presence of 54 nM Rho, with 0, 6.2, 12.5, 25, 50, 100 and 200 nM Q82.
- Complexes were synthesized to +29 by CTP deprivation on template pAH100 in the presence or absence of NusA and Q82 as described. Transcription buffer was replaced and synthesis was continued in the presence or absence of NusA and Q82 as indicated, with 100μM CTP, ATP, and GTP, and with 1, 2, 5, 10, 20 or 100 μM UTP.
Q82 activity depends upon substrate NTP concentration in the absence of NusA
If NusA is required with Q82 to construct a barrier to terminators that acts even on static transcription complexes, how does Q82 antiterminate in the absence of NusA? An important clue is that the antipausing activity of Q82, i.e. its ability to reduce transcriptional pausing, does not require NusA (Yang and Roberts, 1989). Taken together, these results thus suggest two distinct activities of Q82: a protective effect on static complexes that is clearly NusA-dependent, and a NusA-independent activity that we suggest is antipausing, and thus has a kinetic basis. Antipausing could represent an enzymatic change that increases the rate of elongation at sites where termination is in competition with elongation (Yang and Roberts, 1989; Rees et al. 1997). However great this acceleration might be, it should be possible to slow elongation sufficiently by reducing the substrate NTP concentration to allow a terminator to function even in the presence of the Q82 antipausing activity.
To test this, we measured the antitermination activity of Q82 in the presence and absence of NusA in UTP concentrations between 1μM and 100μM; UTP was chosen because it is the next nucleotide to be inserted at the site of release of the terminator used (t82). We used a modified template on which Q82 -modified or unmodified magnetic bead-bound complexes could be stopped at +29, beyond the modification site, by withholding CTP; continued synthesis at varying [UTP] in the presence and absence of NusA then revealed the effect of [UTP] on antitermination. Figure 3C shows that whereas at 100μM UTP the antitermination efficiency is similar in the presence and absence of NusA, at 1μM UTP antitermination is about 5-fold deficient in the absence of NusA. We conclude that a primary effect of Q82 in vitro in the absence of NusA is kinetic, i.e. antipausing.
Q82 does not stabilize stalled elongation complexes against Mfd-mediated RNA release
Like Rho, the release factor Mfd is an enzyme that utilizes ATP energy to dissociate elongation complexes, although it has a different mechanism of action—binding DNA upstream of the complex and inducing forward translocation of RNAP without transcript elongation (Park et al., 2002). In order to test whether Q82-modification inhibits Mfd activity, as might be true if the elongation complex is stabilized by Q82 in some general way, we treated the stalled complexes with Mfd. In parallel experiments, we treated the complexes with either the 15mer release oligo or Rho. Figure 4C shows that Q82 has no detectable effect of stabilizing complexes against the activity of Mfd, even in the presence of NusA. This result argues that Q82 does not stabilize the complexes in a general way, e.g. by strengthening the DNA-RNA hybrid.
Our understanding of both the structure of elongation complexes and the three mechanisms of termination suggests an interpretation of this result and a likely location for Q82 and NusA. Both the release oligo (or the upstream part of an intrinsic terminator stem) and Rho factor require access to emerging RNA in order to terminate transcription. On the other hand, Mfd binds a distinct region of RNAP and acts on DNA (Park et al., 2002). The common requirement for the emerging transcript in intrinsic and Rho-dependent termination, along with a requirement for the RNA binding protein NusA in Q82-mediated stabilization of complexes, suggests that Q82 and NusA interact with RNAP near the RNA exit channel to restrict access to the emerging transcript.
Q82 and NusA restrict access to about 10 nt of the RNA transcript once it has emerged from the transcription elongation complex
To test the model that Q82 and NusA restrict access to the RNA, we probed the emerging transcript by performing RNA footprinting assays of Q82-modified and unmodified stalled elongation complexes using RNase H and RNase A. In particular, we tested the accessibility of RNA in the region upstream of 14 nucleotides from the growing end—a region accessible in unmodified transcription complexes (Komissarova and Kashlev, 1998), and the site where the RNA hairpin of intrinsic terminators forms (Wilson and von Hippel, 1994). As an important control for both of these RNA accessibility studies, we added Q82 to the originally unmodified complexes after stalling. Since Q82 can productively engage a transcription complex only at the promoter proximal pause site and is inactive if added later (Roberts et al., 1998), this controls for any effects of non-specific binding of Q82 to the RNA. As a further control to ensure that both complexes have an identical composition, we also added back the anti-t82 oligo to Q82-modified complexes after they were stalled.
In the first approach, we added a DNA oligonucleotide complementary to this emerging RNA, and determined if an RNase H-sensitive DNA/RNA hybrid can form. RNase H is an enzyme that recognizes RNA-DNA hybrids, makes an endonucleolytic cut in the 3′ region of the RNA, and specifically degrades the RNA component within the hybrid (Yazbeck et al., 2002). We first chose a 15mer oligo that anneals to RNA from -14 to -28, since we expect the RNA to have completely emerged from the elongation complex by about -14 (Komissarova and Kashlev, 1998). This probe cannot cause OMR, which requires that the oligo anneal to at least -11 (Figure 2A), so that it is appropriate for measuring RNA access.
In the presence of NusA, Q82-modification strongly reduces RNA cleavage by RNase H when the 15mer oligo annealing to -14 is used as probe (Figure 5B), by about five-fold. The kinetics of digestion suggest that about 20% of complexes are unmodified and sensitive to RNase H, possibly due to loss of Q82 or to other factors during experimental manipulations, and the remainder are highly resistant to digestion. From this result, we conclude that when Q82 is a stable part of the elongation complex, and NusA is also present, access to RNA emerging from the complex is restricted.
Figure 5.
- Schematic of the set of nested oligos that were used as probes for RNA accessibility studies with RNase H.
- A time course of digestion is shown using the oligo that anneals to -14 of the transcript.
- Effect of oligo position on RNA protection. Complexes were incubated for 10′ at 37°C with 1 μM of each oligo in the presence of RNase H as described.
To determine the extent of this Q82-mediated protection of the RNA, we designed a series of 13 nested oligos complementary to a region of sequentially overlapping sites on the RNA emerging from the elongation complex (Figure 5A), with their 5′ ends extending from -13 to -25. As expected, RNase H-mediated cutting was efficient in Q82-unmodified complexes with all oligos tested (Figure 5C). Q82 interfered with RNase H cutting when the oligo 5′ ends were complementary to a region from -13 through -21 of the RNA (Figure 5C); the RNA becomes equivalently accessible in modified and unmodified complexes to oligos annealing further upstream, suggesting a boundary of Q82-mediated protection that extends to about 10 nt of the RNA after it exits the elongation complex.
To confirm the result, we tested RNA accessibility with a more direct footprinting assay, using the single-strand specific RNase A, which cuts to the 3′ side of cytidine and uridine; the RNA was labeled at the 3′ penultimate nucleotide (G). In the RNase A footprint of Figure 6A, we show that in the presence of NusA and Q82, cleavage of RNA is significantly reduced at sites -19, -21, -24, -25 and -27 with respect to the 3′ end of RNA. In agreement with the RNase H results, this footprint indicates that Q82 protects the RNA for about 10 nt from the point of its exit from the elongation complex.
Figure 6.
- RNase A footprint time course of RNA labeled at the 3′ penultimate position in Q82-modified and unmodified stalled complexes made in the presence of NusA on a template from pSS100.
- RNase I footprint time course of 5′ end-labeled RNA in Q82-modified and unmodified complexes made in the presence of NusA, stalled artificially at position 25 in the absence of CTP, and then advanced to position 76 in the absence of UTP, on a template from pSS418.
Q82 initially engages the transcription complex at a σ70-dependent pause site, 25 nt from the start site, where it binds both the Q82 -binding element (QBE) on DNA and RNAP. We asked if the RNA is also protected when Q82 binds this early elongation complex. Using a template that contains a modified phage 82 late gene promoter, we elongated complexes to and stalled them at the pause site by nucleotide deprivation in the presence of NusA, added Q82 (or not in the control), and elongated them to a downstream position. This procedure provided a mix of complexes still stalled at the pause site (25 nt) and at the downstream site (76 nt); the RNA was labeled at its 5′ end for this assay. We then produced a footprint by digestion with RNase I, which cuts 3′ of every nucleotide. In general, the 25 nt paused RNA is more protected than the 76 nt RNA (Figure 6B), presumably because only 12-13 nt of the RNA would have emerged from the elongation complex, and even less might be available if the complexes are backtracked. The 25 nt RNA is substantially protected by the addition of Q82, suggesting that Q82 is interacting with the RNA even in the early elongation complexes. We do not know the origin of the doublet of about 30nt, although it could arise from the backtracked Q82-dependent pause at +35 by 3′-terminal cleavage.
NusA interacts with Q82 at the promoter-proximal pause site and constructs a stable complex with restricted RNA access
We now show that the Q82-dependent protection of emerging RNA against RNase H digestion depends upon NusA, just as does the protection against oligo- and Rho-mediated RNA release. For the experiment of Figure 7A, we either added or omitted NusA at the beginning of the reaction, and then either included or excluded NusA from the final suspension buffer after washing the magnetic bead-bound transcription complexes (also see Supplemental Information). We then assayed for RNase H protection using the 15mer oligo probe that extends to -14. The experiment shows that NusA must be added initially, presumably as the Q82-bound initial paused complex is formed, in order for the protection to occur; no protection occurs if NusA is added at the end (Figure 7A, lanes 1-4). Consistently, NusA also is necessary during initial elongation in order for Q82 to prevent OMR from stalled complexes (data not shown). Note that Q82 had to be present in complexes formed in the absence of NusA, because its activity was required to move the complexes through the filtering terminator. Furthermore, we have found by western blot analysis that Q82 is present in stopped and washed complexes formed in the absence of NusA (data not shown). Although NusA is thought to interact reversibly with unmodified elongation complexes (Schmidt and Chamberlin, 1984), one interpretation of our results is that a molecule of NusA interacts irreversibly with and becomes a stable part of the Q82-modified complex during early elongation, presumably during the engagement step at the promoter-proximal pause; more detailed investigation of the modified complexes is required to confirm this. NusA also must be available in solution at the time of the RNase H digestion for the full protection effect (Figure 7A, compare lanes 5,6 to lanes 7,8), suggesting that an additional molecule or molecules are required to stabilize the complex, reminiscent of models for the function of the N antiterminator complex that contain two NusA molecules (Gusarov and Nudler, 2001; Horwitz et al., 1987).
Figure 7.
- Q82-modified and unmodified complexes were elongated to the EcoR1 stall site of the template pSS100 in the absence or presence of NusA, and NusA was either excluded from or included in the resuspension buffer. The complexes were treated with RNase H in the absence or presence of the oligo probe that anneals to -14 of the transcript.
- Q82-modified and unmodified complexes, containing either wild type RNAP holoenzyme or RNAP-αΔCTD, were elongated to the EcoR1 stall site of the template pSS100 in the presence of NusA. The complexes were washed in the presence of NusA and treated with RNase H in the absence or presence of the oligonucleotide probe that anneals to -14 of the transcript.
- A model suggesting a location of NusA and Q82 near the RNAP β-flap and the RNAP α-CTD, all the protein components interacting directly or indirectly with the transcript.
The C-terminal domain of the α subunit of RNAP (RNAP-αCTD) is required for NusA to function in Q-mediated antitermination and in termination (Liu et al., 1996); the RNAP-αCTD is believed to bind NusA and potentiate the NusA RNA-binding activity (Liu et al., 1996; Mah et al., 2000). We show here that in complexes made of RNAP lacking the α-CTD (RNAP-αΔCTD), Q82 modification does not protect the RNA from RNase H digestion even in complexes made and maintained in the presence of NusA (Figure 7B). This result demonstrates that the actual interactions of NusA with the RNA and with the RNAP are responsible for the Q82-mediated structural modification of the elongation complex. Again, we note that the complexes containing RNAP-αΔCTD were able to antiterminate past the filtration intrinsic terminator t82 in the presence of Q82, confirming a distinct NusA-independent activity of Q82.
DISCUSSION
We have described the physical basis of an activity of a transcription antiterminator, Q82, and we have provided evidence for a discrete biochemical function of the universal bacterial transcription elongation factor NusA. When it engages RNAP, Q82 constructs a stable NusA-dependent barrier that shields the emerging transcript from the activity of RNA-based termination mechanisms for a span of approximately ten nucleotides, thus explaining the stability of static Q82-modified transcription elongation complexes against intrinsic and Rho-dependent termination and providing strong evidence for the location of NusA and Q82 near the RNAP β flap. This finding suggests that Q82 does not act by providing a general stabilization of transcription complexes - e.g. by stabilizing the RNA/DNA hybrid - as also evidenced by its lack of protection against Mfd-mediated RNA release.
Despite this NusA dependence of Q82-mediated transcript protection, antitermination at intrinsic and Rho-dependent terminators by Q82 is largely NusA-independent in vitro, implying that a distinct activity of Q82 is involved. We provide evidence that this is the antipausing activity common to antiterminators, which accelerates modified RNAP and likely acts by rushing the enzyme past sites where terminator function competes with elongation. The possibility that antitermination results from antipausing by the λ N protein at the site of RNA release of intrinsic terminators, or at the critical pauses of Rho-dependent terminators, has been considered previously, and disfavored based on experiments with model systems (Gusarov and Nudler, 2001; Rees et al., 1997). However, the inferences involved were indirect, because the kinetic properties of an elongation complex at the release site of an active terminator - the only site that matters - is very difficult to measure; therefore, previous experiments do not rule out antipausing as an antitermination mechanism. Because the protective effect of Q82 on RNA in complexes requires NusA, we suggest that the NusA-independent antitermination is likely to be entirely a kinetic effect. However, there could be a weak protective effect of Q alone that we did not detect, and which is important for antitermination in the absence of NusA.
Studies of N-mediated antitermination provide a detailed view of the incorporation of NusA into a multiprotein regulatory complex entwined with an RNA site (nut) (Das et al., 1996; Horwitz et al., 1987), which, like the Q-modified complex, antiterminates and antipauses. Although the N complex undoubtedly contacts RNAP, its mechanism and site of action are not well defined. One model system for N that lacks the nut site and proteins other than NusA, and uses N added in excess from solution rather than through the natural multiprotein complex, reveals that N and NusA are able to crosslink to derivatized RNA in the upstream stem-half of an inefficient, modified intrinsic terminator, and to slow the function of this terminator modestly (Gusarov and Nudler, 2001).
The Q system, in contrast, uses the natural engagement mechanism, in which Q interacts with σ70 and core to construct a stable, stoichiometric complex of one or two Q molecules per core that persists during elongation downstream. We presume that Q and NusA interact with and restrict access to the emerging transcript near the RNAP β flap, a region that is also critical for interactions with σ70. The requirement we find for NusA at the initial engagement implies that Q constructs a complex with NusA that cannot be assembled during elongation - although Q itself is stably incorporated into the complex in the absence of NusA. This site of action suggests a possible role for σ70 in the construction of a stable termination-resistant elongation complex (Nickels et al, 2006). The RNAP-α-CTD also plays a key role in the interaction of NusA with the RNA and RNAP, leading to a model of the Q-modified elongation complex that involves complex interactions between the various protein components- β flap, NusA, Q, α-CTD- and the RNA (Figure 7C).
It seems likely that the paradoxical effects of NusA, namely to promote pausing by itself, but also to collaborate with an antiterminator that mediates antipausing, reflect its binding to a single region of RNAP-likely near the β flap-where it interacts with the RNA in different ways (Liu and Hanna, 1995; Mah et al., 2000). Note that Q82 acting without NusA antipauses, and, furthermore, that Q82 completely overcomes pause enhancement by NusA (Yang and Roberts, 1989). Thus, the antipausing configuration is dominant. This could be understood if NusA alone binds RNA so as to enhance pausing, but is reconfigured by Q to bind in a distinct way that protects RNA from being accessed by termination mechanisms. There is evidence that emerging RNA is an agent of pausing at non-terminator sites, through interactions with RNAP: an RNA hairpin stabilizes a pause by binding the β flap and inhibits the ability of the active center to incorporate the next NTP, presumably via long-range conformational changes (Toulokhonov et al., 2001). Furthermore, NusA stimulates this pause. If the emerging transcript generally influences pausing during elongation, then Q could prevent this pause-inducing RNA interaction and the NusA molecule(s) in the Q-NusA complex would be configured to protect the RNA. However, it is also possible that the antipausing activity of Q is mediated by protein-protein interactions that affect the activity of the active center.
Why might there be two different modes of antitermination? One possibility is that one (e.g. antipausing) is more primitive, and that incorporation of NusA—and a set of other proteins in the case of the N protein antiterminator—is a refinement. Such protection against termination may be necessary for the 26 Kb span of the Q-regulated late gene operon. Furthermore, the NusA-dependent protection could act in conditions of distress or dormancy, e.g. an energy deficit where NTPs are not available for efficient synthesis, and preserve modified complexes for better times, ensuring phage growth (for λ) or efficient synthesis of ribosomal RNA when the cell begins to grow.
EXPERIMENTAL PROCEDURES
DNA templates, plasmids, and protein purifications are described in Supplemental Information.
In vitro transcription
For all experiments except where noted, 20 nM RNAP holoenzyme was pre-incubated for 10′ at 37 °C with 2 nM DNA template, in the presence of buffer TB1 (20 mM Tris-Cl pH 8.0, 10% glycerol, 50 mM KCl, 1 mM DTT, 0.1 mM EDTA, 100 μg/ml BSA), 200 μM each of ATP, CTP, GTP, 50μM UTP and 5-10 μCi of α-32P-UTP, in a 25 μl reaction. When present, 150 nM NusA and/or 10 nM Rho were also included during this pre-incubation. Single-round transcription was initiated with a mix of 5 mM MgCl2 and 10 μg/ml rifampicin. When present, Q82 was added to 100 nM 30″ before the initiation of transcription. For the experiments of Figure 3A and B, reactions were stopped by the addition of 125 μl of TB2 (0.6M Tris-Cl pH 8.0, 12 mM EDTA, and 160 μg/ml tRNA), the RNA products were extracted and resolved on a denaturing gel, and RNA was quantified with a PhosphorImager.
For the experiment in Figure 3C, transcription was carried out on template from pAH100 bound to magnetic beads. Open complexes were formed in the presence of 25 μM each of ATP and GTP, 6.25 μM UTP, and 10 μCi of α-32P-UTP (CTP starvation), as described above except that buffer TB3 was used (same as TB1 but 200 mM potassium glutamate replaced 50 mM KCl) and GreB was included to 20 nM; portions were supplemented with 200 nM Q82 and/or 150 nM NusA as indicated. Single round synthesis to +29 was initiated with 4 mM MgCl2 and 10 μg/ml rifampicin and continued for 5′ at 37°. Supernatant was removed and bead-bound complexes were resuspended in TB3 containing 5 mM MgCl2 and 200 nM Q82 and/or 150 nM NusA where present. Synthesis proceeded for 20 min at 37° in the presence of 10 μg/ml rifampicin, 100 μM ATP, CTP, and GTP, and UTP as indicated. The reactions were stopped and RNA was processed as described above.
Transcript release assays
Transcription was performed as described above, on streptavidin coated magnetic beads, using DNA templates from pSS100 labeled with biotin at the 5′ end of the non-template strand. 100 nM of the protein EcoR1-Gln111 was included in the initial pre-incubation to enable stalling of the elongation complexes. Either 5 μM of the anti-t82 oligo or 100 nM of Q82 was added for 30” prior to transcription initiation with the MgCl2/Rifampicin mix; the reaction was allowed to proceed for 15′ at 37 °C. After the specific manipulations described below for the different experiments, magnetic partitioning was used to separate the pellet and supernatant fractions for each reaction; reactions were stopped by the addition of 125 μl of TB2 to the supernatant fraction and 150 μl of TB2* (0.5M Tris-Cl pH 8.0, 10 mM EDTA, and 133 μg/ml tRNA) to the pellet fraction. The two fractions from each reaction were loaded alongside each other on the denaturing gel and the transcripts were analyzed as described above.
For the experiment in Figure 1B, the supernatant for each reaction was separated at the end of the 15′ incubation that generates stalled complexes, without any further manipulation. For the experiments in Figures 2 and 4, once elongation complexes were stalled, the supernatant was removed and the beads resuspended in an equal volume of TB4 (TB1 containing 5 mM MgCl2) and 100 nM NusA when present. They were then treated with either 1 μM of a release oligo (unless a different concentration is specified) (2A, 2B, 2C, 2D and 4A) or 50 nM Mfd + 1mM dATP (Figure 4C) for the indicated times. For the experiments in figures 2E and 4B (involving protein Rho), antiterminator oligo anti-t82 was added to the Q82-modified complexes, following which all reactions were treated with 0.25 U of RNase H, in order to cut the RNA in the region of t82 and reduce secondary structure (which is known to inhibit Rho activity). The supernatant was removed and the beads were resuspended in fresh buffer, and then treated with 10 nM Rho + 1 mM dATP for the indicated times.
RNA accessibility assays
The assays were performed as described in the Supplemental data.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Tom Santangelo and Joo-Seop Park for initial experiments using stalled elongation complexes, Irina Artsimovitch for a gift of GreB protein, and members of the laboratory, Ann Hochschild and Bryce Nickels for criticism of the manuscript. This work was supported by NIH grant 21941 to JWR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Artsimovitch I, Landick R. The transcriptional regulator RfaH stimulates RNA chain synthesis after recruitment to elongation complexes by the exposed nontemplate DNA strand. Cell. 2002;109:193–203. doi: 10.1016/s0092-8674(02)00724-9. [DOI] [PubMed] [Google Scholar]
- Condon C, Squires C, Squires CL. Control of rRNA transcription in Escherichia coli. Microbiological reviews. 1995;59:623–645. doi: 10.1128/mr.59.4.623-645.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Pal M, Mena JG, Whalen W, Wolska K, Crossley R, Rees W, von Hippel PH, Costantino N, Court D, et al. Components of multiprotein-RNA complex that controls transcription elongation in Escherichia coli phage lambda. Methods in enzymology. 1996;274:374–402. doi: 10.1016/s0076-6879(96)74032-6. [DOI] [PubMed] [Google Scholar]
- Deighan P, Hochschild A. The bacteriophage lambdaQ anti-terminator protein regulates late gene expression as a stable component of the transcription elongation complex. Molecular microbiology. 2007;63:911–920. doi: 10.1111/j.1365-2958.2006.05563.x. [DOI] [PubMed] [Google Scholar]
- DeVito J, Das A. Control of transcription processivity in phage lambda: Nus factors strengthen the termination-resistant state of RNA polymerase induced by N antiterminator. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8660–8664. doi: 10.1073/pnas.91.18.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DI, Baron LS. Genetic characterization of a bacterial locus involved in the activity of the N function of phage lambda. Virology. 1974;58:141–148. doi: 10.1016/0042-6822(74)90149-4. [DOI] [PubMed] [Google Scholar]
- Gopal B, Haire LF, Gamblin SJ, Dodson EJ, Lane AN, Papavinasasundaram KG, Colston MJ, Dodson G. Crystal structure of the transcription elongation/anti-termination factor NusA from Mycobacterium tuberculosis at 1.7 A resolution. Journal of molecular biology. 2001;314:1087–1095. doi: 10.1006/jmbi.2000.5144. [DOI] [PubMed] [Google Scholar]
- Grayhack EJ, Yang XJ, Lau LF, Roberts JW. Phage lambda gene Q antiterminator recognizes RNA polymerase near the promoter and accelerates it through a pause site. Cell. 1985;42:259–269. doi: 10.1016/s0092-8674(85)80121-5. [DOI] [PubMed] [Google Scholar]
- Greenblatt J, Mah TF, Legault P, Mogridge J, Li J, Kay LE. Structure and mechanism in transcriptional antitermination by the bacteriophage lambda N protein. Cold Spring Harbor symposia on quantitative biology. 1998;63:327–336. doi: 10.1101/sqb.1998.63.327. [DOI] [PubMed] [Google Scholar]
- Gusarov I, Nudler E. Control of intrinsic transcription termination by N and NusA: the basic mechanisms. Cell. 2001;107:437–449. doi: 10.1016/s0092-8674(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Hager DA, Jin DJ, Burgess RR. Use of Mono Q high-resolution ion-exchange chromatography to obtain highly pure and active Escherichia coli RNA polymerase. Biochemistry. 1990;29:7890–7894. doi: 10.1021/bi00486a016. [DOI] [PubMed] [Google Scholar]
- Horwitz RJ, Li J, Greenblatt J. An elongation control particle containing the N gene transcriptional antitermination protein of bacteriophage lambda. Cell. 1987;51:631–641. doi: 10.1016/0092-8674(87)90132-2. [DOI] [PubMed] [Google Scholar]
- Jin DJ, Burgess RR, Richardson JP, Gross CA. Termination efficiency at rho-dependent terminators depends on kinetic coupling between RNA polymerase and rho. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1453–1457. doi: 10.1073/pnas.89.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko DC, Marr MT, Guo J, Roberts JW. A surface of Escherichia coli sigma 70 required for promoter function and antitermination by phage lambda Q protein. Genes & development. 1998;12:3276–3285. doi: 10.1101/gad.12.20.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komissarova N, Kashlev M. Functional topography of nascent RNA in elongation intermediates of RNA polymerase. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14699–14704. doi: 10.1073/pnas.95.25.14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzheva N, Mustaev A, Kozlov M, Malhotra A, Nikiforov V, Goldfarb A, Darst SA. A structural model of transcription elongation. Science. 2000;289:619–625. doi: 10.1126/science.289.5479.619. [DOI] [PubMed] [Google Scholar]
- Lau LF, Roberts JW, Wu R. RNA polymerase pausing and transcript release at the lambda tR1 terminator in vitro. The Journal of biological chemistry. 1983;258:9391–9397. [PubMed] [Google Scholar]
- Liu K, Hanna MM. NusA contacts nascent RNA in Escherichia coli transcription complexes. Journal of molecular biology. 1995;247:547–558. doi: 10.1006/jmbi.1994.0161. [DOI] [PubMed] [Google Scholar]
- Liu K, Zhang Y, Severinov K, Das A, Hanna MM. Role of Escherichia coli RNA polymerase alpha subunit in modulation of pausing, termination and anti-termination by the transcription elongation factor NusA. The EMBO journal. 1996;15:150–161. [PMC free article] [PubMed] [Google Scholar]
- Mah TF, Kuznedelov K, Mushegian A, Severinov K, Greenblatt J. The alpha subunit of E. coli RNA polymerase activates RNA binding by NusA. Genes & development. 2000;14:2664–2675. doi: 10.1101/gad.822900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr MT, Datwyler SA, Meares CF, Roberts JW. Restructuring of an RNA polymerase holoenzyme elongation complex by lambdoid phage Q proteins. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8972–8978. doi: 10.1073/pnas.161253298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels BE, Roberts CW, Roberts JW, Hochschild A. RNA-mediated destabilization of the sigma(70) region 4/beta flap interaction facilitates engagement of RNA polymerase by the Q antiterminator. Molecular cell. 2006;24:457–468. doi: 10.1016/j.molcel.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels BE, Roberts CW, Sun H, Roberts JW, Hochschild A. The sigma(70) subunit of RNA polymerase is contacted by the (lambda)Q antiterminator during early elongation. Molecular cell. 2002;10:611–622. doi: 10.1016/s1097-2765(02)00648-2. [DOI] [PubMed] [Google Scholar]
- Nudler E, Gottesman ME. Transcription termination and anti-termination in E. coli. Genes Cells. 2002;7:755–768. doi: 10.1046/j.1365-2443.2002.00563.x. [DOI] [PubMed] [Google Scholar]
- Park JS, Marr MT, Roberts JW. E. coli Transcription repair coupling factor (Mfd protein) rescues arrested complexes by promoting forward translocation. Cell. 2002;109:757–767. doi: 10.1016/s0092-8674(02)00769-9. [DOI] [PubMed] [Google Scholar]
- Pavco PA, Steege DA. Elongation by Escherichia coli RNA polymerase is blocked in vitro by a site-specific DNA binding protein. The Journal of biological chemistry. 1990;265:9960–9969. [PubMed] [Google Scholar]
- Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Molecular cell. 2006;23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Rees WA, Weitzel SE, Das A, von Hippel PH. Regulation of the elongation-termination decision at intrinsic terminators by antitermination protein N of phage lambda. Journal of molecular biology. 1997;273:797–813. doi: 10.1006/jmbi.1997.1327. [DOI] [PubMed] [Google Scholar]
- Richardson JP. Loading Rho to terminate transcription. Cell. 2003;114:157–159. doi: 10.1016/s0092-8674(03)00554-3. [DOI] [PubMed] [Google Scholar]
- Roberts JW, Yarnell W, Bartlett E, Guo J, Marr M, Ko DC, Sun H, Roberts CW. Antitermination by bacteriophage lambda Q protein. Cold Spring Harb Symp Quant Biol. 1998;63:319–325. doi: 10.1101/sqb.1998.63.319. [DOI] [PubMed] [Google Scholar]
- Santangelo TJ, Mooney RA, Landick R, Roberts JW. RNA polymerase mutations that impair conversion to a termination-resistant complex by Q antiterminator proteins. Genes & development. 2003;17:1281–1292. doi: 10.1101/gad.1082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo TJ, Roberts JW. Forward translocation is the natural pathway of RNA release at an intrinsic terminator. Molecular cell. 2004;14:117–126. doi: 10.1016/s1097-2765(04)00154-6. [DOI] [PubMed] [Google Scholar]
- Saunders A, Core LJ, Lis JT. Breaking barriers to transcription elongation. Nature reviews. 2006;7:557–567. doi: 10.1038/nrm1981. [DOI] [PubMed] [Google Scholar]
- Schmidt MC, Chamberlin MJ. Amplification and isolation of Escherichia coli nusA protein and studies of its effects on in vitro RNA chain elongation. Biochemistry. 1984;23:197–203. doi: 10.1021/bi00297a004. [DOI] [PubMed] [Google Scholar]
- Selby CP, Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993;260:53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- Sen R, King RA, Weisberg RA. Modification of the properties of elongating RNA polymerase by persistent association with nascent antiterminator RNA. Molecular cell. 2001;7:993–1001. doi: 10.1016/s1097-2765(01)00243-x. [DOI] [PubMed] [Google Scholar]
- Sigmund CD, Morgan EA. Nus A protein affects transcriptional pausing and termination in vitro by binding to different sites on the transcription complex. Biochemistry. 1988;27:5622–5627. doi: 10.1021/bi00415a034. [DOI] [PubMed] [Google Scholar]
- Skordalakes E, Berger JM. Structure of the Rho transcription terminator: mechanism of mRNA recognition and helicase loading. Cell. 2003;114:135–146. doi: 10.1016/s0092-8674(03)00512-9. [DOI] [PubMed] [Google Scholar]
- Squires CL, Greenblatt J, Li J, Condon C, Squires CL. Ribosomal RNA antitermination in vitro: requirement for Nus factors and one or more unidentified cellular components. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:970–974. doi: 10.1073/pnas.90.3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulokhonov I, Artsimovitch I, Landick R. Allosteric control of RNA polymerase by a site that contacts nascent RNA hairpins. Science. 2001;292:730–733. doi: 10.1126/science.1057738. [DOI] [PubMed] [Google Scholar]
- Vogel U, Jensen KF. NusA is required for ribosomal antitermination and for modulation of the transcription elongation rate of both antiterminated RNA and mRNA. The Journal of biological chemistry. 1997;272:12265–12271. doi: 10.1074/jbc.272.19.12265. [DOI] [PubMed] [Google Scholar]
- Wilson KS, von Hippel PH. Stability of Escherichia coli transcription complexes near an intrinsic terminator. Journal of Molecular Biology. 1994;244:36–51. doi: 10.1006/jmbi.1994.1702. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Goliger JA, Roberts JW. Specificity and Mechanism of Antitermination by Q proteins of Bacteiophages λ and 82. Journal of Molecular Biology. 1989;210:453–460. doi: 10.1016/0022-2836(89)90122-8. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Roberts JW. Gene Q antiterminator proteins of Escherichia coli phages 82 and lambda suppress pausing by RNA polymerase at a rho-dependent terminator and at other sites. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:5301–5305. doi: 10.1073/pnas.86.14.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell WS, Roberts JW. The phage lambda gene Q transcription antiterminator binds DNA in the late gene promoter as it modifies RNA polymerase. Cell. 1992;69:1181–1189. doi: 10.1016/0092-8674(92)90639-t. [DOI] [PubMed] [Google Scholar]
- Yarnell WS, Roberts JW. Mechanism of intrinsic transcription termination and antitermination. Science. 1999;284:611–615. doi: 10.1126/science.284.5414.611. [DOI] [PubMed] [Google Scholar]
- Yazbeck DR, Min KL, Damha MJ. Molecular requirements for degradation of a modified sense RNA strand by Escherichia coli ribonuclease H1. Nucleic acids research. 2002;30:3015–3025. doi: 10.1093/nar/gkf429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Friedman DI. Reduced Rho-dependent transcription termination permits NusA-independent growth of Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7543–7547. doi: 10.1073/pnas.91.16.7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.