Abstract
Peripheral administration of bombesin (BN) and the related mammalian peptides, gastrin-releasing peptide (GRP) and neuromedin B (NMB), suppress food intake in rats. To examine whether all BN-like peptides utilize the same neural pathways to reduce feeding, rats were treated on postnatal day 2 with the injection vehicle or capsaicin, a neurotoxin that damages a subset of visceral afferent fibers. When rats reached adulthood, we compared the ability of a dose range of systemically administered BN, GRP18–27 and NMB to reduce intake of a 0.5 kcal/ml glucose solution in a short-term feeding test. Our results demonstrate that capsaicin treatment abolished or attenuated the suppression of glucose intake produced by BN and NMB but had no effect on the ability of GRP to reduce feeding. These results suggest that different neural substrates underlie the anorexic effects of peripherally administered BN-like peptides.
Keywords: Bombesin, Gastrin-releasing Peptide, Neuromedin B, Capsaicin, Food Intake
INTRODUCTION
Bombesin (BN), a tetradecapeptide originally isolated from the skin of the European frog, Bombina bombina (1), belongs to a large family of structurally related amphibian and mammalian peptides. BN has been shown to have potent physiological and behavioral effects in mammals (3, 7, 23) including the ability to suppress food intake after either peripheral or central administration (11, 12, 17).
A role for BN-like peptides in the control of food intake has been supported by experiments demonstrating that exogenous administration of BN reduces feeding in a number of mammalian species and elicits a normal post-prandial sequence of behaviors (9, 11, 13). The peripheral effects of BN on food intake have been proposed to be mediated by both vagal and spinal neural pathways. Gastro- intestinal deafferentation surgically produced by complete subdiaphragmatic vagotomy combined with dorsal rhizotomy (T3–T6) and spinal cord transaction (T6) abolished the inhibition of feeding produced by systemic BN administration (34). Furthermore, BN’s peripheral (34) effects on feeding were attenuated or abolished in rats pretreated systemically with capsaicin, a neurotoxin that selectively damages a subset of primary sensory afferent neurons that include vagal and spinal fibers (18, 25).
Although BN itself is not present in mammals, gastrin-releasing peptide (GRP) and neuromedin B (NMB) are two homologs that have been isolated from mammalian tissue and their receptors cloned and characterized (2, 31, 37). Structural and pharmacological differences have led to the identification of two BN receptor subtypes referred to as BB2 or GRP-preferring (GRP-R) and BB1 or NMB-preferring (NMB-R)(36). The GRP-R subtype preferentially binds GRP but has a low affinity for NMB, whereas the NMB-R subtype has a high affinity for NMB and a lower affinity for GRP. BN binds with equal and high affinity to both receptor subtypes.
Like BN, GRP and NMB also reduce food intake when administered systemically but differ from each other, as well as from BN, in both potency and duration of action (BN>GRP>NMB) (20, 21, 32). As mentioned above, BN binds both GRP and NMB receptors and thus the contribution of each to feeding suppression by BN is not known. Differences in the satiety actions of BN-like peptides present the possibility that they may utilize different neural substrates to suppress feeding, however studies designed to address this question have not yet been conducted. The goal of the present study was to compare the feeding response to systemic administration of BN, GRP and NMB in adult rats that had been treated as neonates with capsaicin. Our results indicate that there was a differential effect of capsaicin treatment on feeding suppression by BN-like peptides.
METHODS
Animals
The experimental subjects were male Sprague-Dawley rats (Charles River, Kingston, NY) kept in a temperature-controlled room on a 12:12 h light:dark cycle. Rats were maintained on standard laboratory rodent chow (Prolab RMH 1000) with ad libitum access to tap water except during behavioral testing as described below. All procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University.
Capsaicin treatment
On postnatal day 2, male Sprague-Dawley rat pups were individually removed from their dam and briefly anesthetized by cold immersion on crushed ice. They were then injected subcutaneously using a 30 gauge needle in the dorsal neck region with a single dose of 50 mg/kg capsaicin (Sigma, St. Louis MO, purity 98%) mixed in a vehicle consisting of 10:10:80 vol/vol/vol of Tween 80:ethanol:saline. They were immediately placed under a heat lamp and returned to their home cage when respiration appeared normal and they had regained mobility. The control group was treated in the identical manner with an equal volume of the vehicle solution.
When rats reached an average body weight of 300 g, the efficacy of the capsaicin treatment was evaluated by examining the number of eye wipes in response to a mild irritant, ammonium hydroxide (NH4OH). This test is based on the finding that systemic capsaicin treatment damages afferent trigeminal innervation of the cornea resulting in long-term desensitization of the eye to chemical stimuli (35) and is a widely accepted method to verify the effectiveness of capsaicin treatment (4, 6, 10, 18, 22, 29). For this test, each rat received one drop of a 1% NH4OH solution into the right eye and the number of wiping motions was counted for a 10 sec period. Capsaicin treated rats that displayed ≤ 2 eye wipes in 10 sec were used in the behavioral experiments. Vehicle treated rats wiped ≥8 times in response to this stimulus.
Behavioral testing
At weaning, rats were housed in individual hanging wire mesh cages in a temperature-controlled room on a 12:12 h light:dark cycle with tap water always available. Pelleted rat chow was fed ad libitum except during behavioral tests. All behavioral testing was conducted in the rats’ home cage during the light portion of the light:dark cycle.
Behavioral tests were performed on rats that were adapted to a feeding schedule in which they had access to chow from 1700 h to 900 h. After 5h daytime food deprivation (1400 h) they were trained to drink a solution of 0.5 kcal/ml glucose for 30 min. When stable 30 min glucose intake was established, glucose consumption was assessed following intraperitoneal (ip) administration of either 0.9% physiological saline (control injection) or BN (1, 3.2 and 10 nmol/kg), the biologically active portion of GRP, GRP18-27 (3.2, 10 and 32 nmol/kg) and NMB (32, 100 and 178 nmol/kg). Injections were given 5 min before presentation of the glucose solution and glucose intake was measured 15 and 30 min after presentation. Saline and peptide injections were given in counterbalanced order with at least 48 h between peptide injections. The order for administration of each peptide was from the lowest to the highest dose and intake for each dose was compared to its corresponding baseline.
Data from two separate groups of animals were pooled with the order of peptide administration randomized between the groups (Group 1= BN, GRP18-27, NMB and Group 2= GRP18-27, NMB, BN). There was no significant difference in body weight between capsaicin and vehicle treated animals in either group. Within each treatment group, data were analyzed at the 15 min time point using a 2 (saline, peptide)× 3 (dose) repeated measures ANOVA comparing intake after saline injection with intake after peptide injection at the three doses. Mean differences were evaluated by planned t comparisons using the overall error term from the analysis of variance. The 15 min time point was selected because the majority of suppression occurs across all peptides during this time but begins to diminish for GRP and NMB by the 30 min time-point (21). Differences between treatment groups were compared by converting data for each animal to percent suppression of food intake using the formula [(intake after saline - intake after peptide) / (intake after saline)]× 100. Data were then subjected to ANOVA (2× 3) to examine the effect of treatment (vehicle vs. capsaicin) repeated over dose followed by Tukey’s test for pairwise multiple comparisons.
RESULTS
Four rats in the capsaicin group were excluded from the study, three for failure to meet the criteria for the eye wipe response test and one for failure to consume glucose. An additional rat in this group died during the final stages of the NMB testing and was eliminated from the study.
The effect of systemic neonatal capsaicin treatment on the suppression of glucose intake by peripherally administered BN-like peptides relative to intake after saline injection is shown in Figs. 1, 2 and 3. In agreement with our previous study (21), we found that the rank order of potency to suppress glucose intake in vehicle treated control rats was BN>GRP18-27>NMB (21).
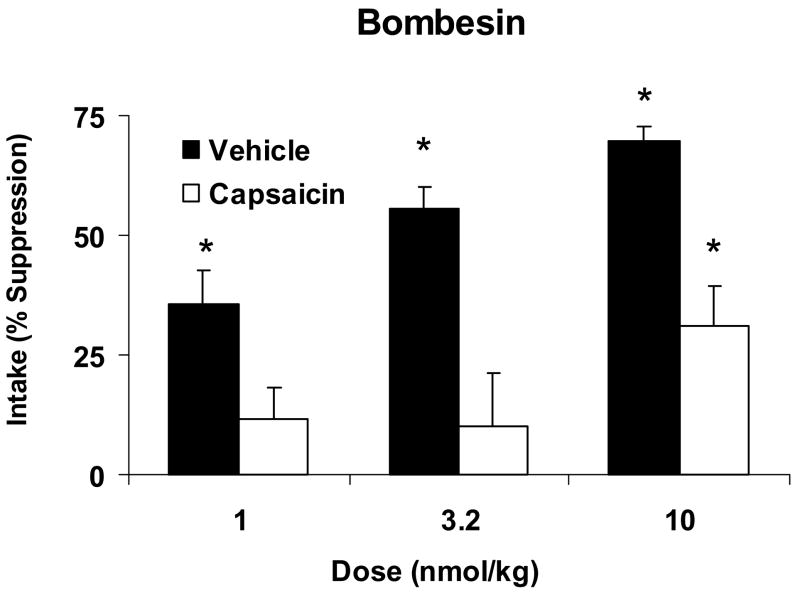
Fig. 1.
Percent suppression of 15 min. glucose intake produced by systemic administration of BN at 1.0, 3.2 and 10 nmol/kg relative to intake after saline administration in vehicle-treated (Vehicle, open bars) or capsaicin-treated (Capsaicin, solid bars) rats. Data are expressed as means ± SEM. *Indicates a significant within-group BN-induced suppression of intake compared to intake after the corresponding saline injection. Glucose intake was significantly suppressed intake at all doses in vehicle-treated rats but at only the highest dose in capsaicin-treated rats.
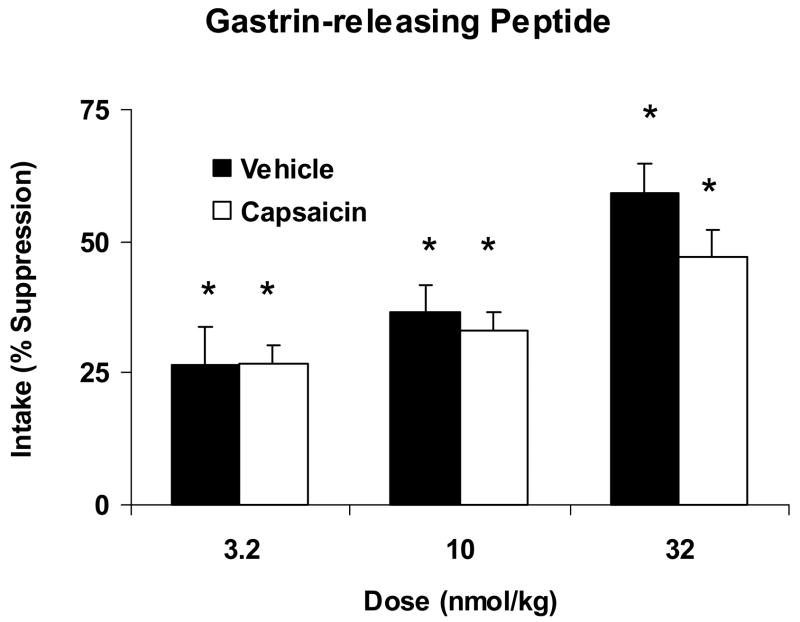
Fig. 2.
Percent suppression of 15 min. glucose intake produced by systemic administration of GRP18-27 at doses of 3.2, 10 and 32 nmol/kg relative to intake after saline administration in vehicle-treated (Vehicle, open bars) or capsaicin-treated (Capsaicin, solid bars) rats. Data are shown as means ± SEM. *Indicates a significant within-group suppression of intake after GRP18-27 compared to intake after the corresponding saline injection. GRP18-27 significantly suppressed intake at all doses in both vehicle and capsaicin treated rats with no differences in suppression between groups.
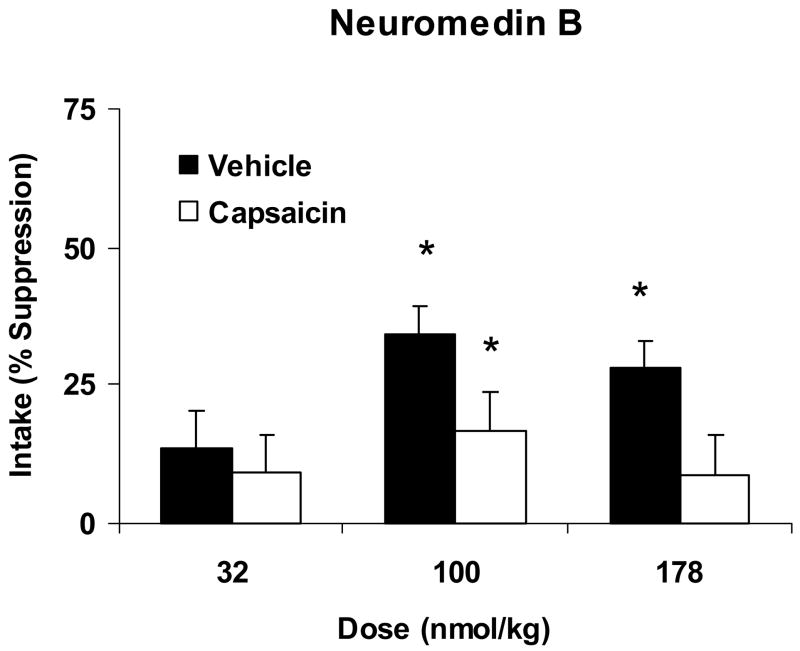
Fig. 3.
Percent suppression of 15 min. glucose intake produced by systemic administration of 32, 100 and 178 nmol/kg of NMB relative to intake after saline administration in vehicle-treated (Vehicle, open bars) or capsaicin-treated (Capsaicin, solid bars) rats. Data are expressed as means ± SEM. *Indicates a significant within-group suppression of intake by NMB relative to intake after the corresponding saline injection. NMB significantly reduced glucose intake in vehicle treated rats at 100 and 178 nmol/kg. A significant suppression of intake in capsaicin treated rats was only observed at the 100 nmol/kg dose.
A comparison between vehicle and capsaicin treated rats revealed a significant effect of treatment on the ability of BN to reduce food intake [F(1,32)=35.934, P < 0.001] (Fig. 1). Analysis of variance on the individual group responses demonstrated that in vehicle treated control rats there was a significant effect of BN on glucose intake [F(1, 17)=72.193, P<0.001]. There was also a significant effect of dose [F(2,17)=7.587, P<0.002]. Post hoc analysis indicated vehicle treated rats significantly suppressed their glucose intake relative to consumption after the saline control injection at 1.0, 3.2 and 10 nmol kg by 35.4 ± 7.3, 55.7 ± 4.6 and 69.7 ± 2.9 percent, respectively.
In capsaicin treated rats, BN also produced a significant effect on glucose intake [F(1, 15)=14.38, P=0.002]. However, in these animals, the suppression of glucose intake by BN only occurred at the 10 nmol/kg dose and the response was attenuated compared to that of vehicle-treated rats (% suppression= 30.9 ± 8.6 in capsaicin treated vs. 69.7 ± 2.9 in vehicle treated, P<0.01).
The effect of GRP18-27 on glucose intake in capsaicin treated and their respective vehicle treated controls is shown in Fig. 2. In contrast to the results with BN, there was no significant effect of treatment (vehicle vs. capsaicin) on the ability of GRP18-27 to reduce glucose intake [F(1,32)=1.187, P=0.284]. In vehicle treated rats, there was a significant effect of GRP18-27 to reduce glucose intake compared to intake after the saline injection [F(1,17)=60.09, P<0.001]. Planned t comparisons indicated that intake was significantly suppressed at all three doses of GRP18-27 (P<0.01). In rats treated with capsaicin, GRP18-27 also significantly affected glucose intake relative to intake after saline injection [F(1,15)=88.15, P<0.001]. Similar to vehicle treated rats, post hoc tests revealed that suppression of intake occurred at all three doses of GRP18-27 (P<0.05). The suppression of glucose intake by capsaicin treated rats did not differ significantly from vehicle treated rats (P>0.05).
The suppression of glucose intake in response to ip administration of NMB in vehicle and capsaicin treated rats is demonstrated in Fig. 3. Similar to the results with BN, there was a significant effect of treatment (vehicle vs. capsaicin) on NMB-induced suppression of intake [F(1,32)=4.762, P=0.037). In vehicle treated rats, glucose intake was significantly reduced by NMB relative to intake after the control injection [F(1,17)=53.015, P<0.001). Post-hoc analyses indicated that vehicle treated rats exhibited a significant suppression of glucose intake at doses of 100 (p<0.01) and 178 nmol/kg (p<0.05). In rats treated with capsaicin, there was also a significant effect of NMB on glucose intake compared to intake after saline injection [F(1,15)=7.937, P=0.013]. Planned t comparisons indicated that the suppression of intake was significant only at the 100 nmol/kg NMB dose (p<0.01).
DISCUSSION
The results of this study demonstrate that the suppression of food intake produced by peripheral administration of BN-like peptides in adult rats is differentially affected by neonatal capsaicin treatment. Capsaicin treatment abolished or attenuated the reduction of short-term food intake by BN and NMB but had no significant effect on suppression produced by GRP18-27 suggesting that different neural substrates underlie the ability of BN-like peptides to reduce food intake.
A role for BN-like peptides in the control of food intake has been proposed based on studies showing that peripheral administration reduced food intake in a number of different species and experimental conditions without causing sickness or malaise (9, 11, 13). In the present study, we found that the rank order of potency to suppress food intake in vehicle treated control rats was BN>GRP18-27>NMB. These results are in agreement with our previous study in normal rats comparing the effects of BN-like peptides on glucose intake using a between-group design and completely randomized doses (21), and with studies where BN was compared individually to GRP, GRP18-27 and NMB (13, 32, 33).
Although the effects of BN on food intake are well-documented, the mechanism and site of action are not completely determined. Results from surgical transaction studies have shown the necessity of both vagal and spinal pathways in mediating BN satiety (34). It is presumed that the critical population of neurons that are destroyed by capsaicin treatment, and eliminate BN’s feeding effects, comprise a subset of vagal and spinal afferent fibers. In electrophysiological studies by Ewart et al. (8), it was demonstrated that close arterial injection of BN locally to the stomach changed the excitability of dorsal vagal complex neurons that were responsive to gastric mechanoreceptor activation. While this response was completely abolished by vagotomy it was unchanged by systemic capsaicin treatment. Although these results support a role for the vagus nerve in the brain stem response to BN and gastric distension, our finding that capsaicin treated rats fail to suppress feeding after peripheral administration of low BN doses suggests that mechanoreceptor activation does not contribute to BN satiety at these dosages.
Previous studies have demonstrated that the effect of capsaicin treatment on suppression of food intake by systemically administered BN was attenuated, but not completely abolished, in rats treated in adulthood. In a subsequent study by Michaud et al. (25), it was reported that rats treated with capsaicin as neonates exhibited a complete abolition of the feeding suppressant effects of BN. The present experiment replicated those results at the two lower BN doses but not at the highest dose. Although there were several variations in our experimental design, our highest BN dose produced a greater suppression of intake in vehicle treated rats and thus, it is possible that, the degree of stimulation of critical receptor populations was not equivalent between the two studies. Nevertheless, both studies support the conclusion that the neural circuitry necessary for systemically administered BN to reduce food intake originates in the periphery in neurons that are vulnerable to capsaicin-induced neurotoxicity. However, our data showing that GRP’s ability to reduce food intake was not compromised by neonatal capsaicin treatment suggests that GRP’s effects on feeding are either dependent upon peripheral neural pathways that are not capsaicin-sensitive or involve direct activation of GRP receptors in the central nervous system.
We do not know whether the differential effect of capsaicin treatment on the ability of BN, GRP and NMB to inhibit food intake results from differences in the pharmacological properties or bioavailability of each peptide. Because BN binds with equal affinity to GRP and NMB receptors its behavioral effects could result from activation of either or both receptor subtypes. Studies designed to assess the relative contribution of each receptor subtype to BN satiety have produced equivocal results. For example, co-administration of GRP and NMB mimic the satiating and microstructural profile of licking produced by BN leading to the interpretation that the effects of BN on food intake result from its interaction with both receptor subtypes (21, 33). In contrast, pharmacological experiments have demonstrated that blockade of GRP receptors with specific GRP-R antagonists completely blocked ip BN’s ability to reduce food intake suggesting that BN acts solely through the GRP receptor. Consistent with our data, experiments comparing the effects of BN and GRP on sham feeding (a paradigm where food is not allowed to accumulate in the stomach) demonstrated potent inhibition of sham feeding by BN but no effect on GRP’s ability to suppress sham intake suggesting that different mechanisms underlie the ability of BN and GRP to reduce food intake (30). Alternatively, because the duration of action for BN to reduce food intake is longer than that of the mammalian peptides (21), it is possible that increased bioavailability may permit greater access to key receptor populations that contribute to BN’s feeding effects.
Previous studies have shown that capsaicin treatment in adult rats can produce transient overconsumption of high fat foods and chronic overconsumption of low concentration sucrose solutions (5, 16). These data present the possibility that the inability of BN to reduce food intake in capsaicin treated rats could be due to an independent increase in intake of the test diet by altering mechanisms related to palatability or reward, rather than by a direct action on neural pathways involved in BN satiety. However, we found that baseline glucose intake in rats treated with capsaicin as neonates did not differ from that of vehicle treated control rats. This finding is consistent with data from Michaud et al. (25) who failed to observe differences in intake of a palatable solid food between rats treated with capsaicin as neonates and vehicle treated rats. Together, these results suggest that changes in the hedonic response to the test diet do not contribute to the failure of BN to reduce food intake in neonatally capsaicin treated rats.
Although experimental evidence supports the notion that BN’s actions to suppress feeding arise from peripheral neural signals, several studies have also addressed the importance of central receptors for BN-like peptides for the peripheral feeding effects of BN. Blockade of central GRP or GRP receptors either by immunoneutralization or selective receptor antagonists eliminated the ability of peripherally administered BN to inhibit feeding (24). Comparable results have also been obtained in an experiment evaluating the necessity of hindbrain GRP receptors in feeding suppression produced by peripheral GRP (19). These studies have led to the proposal that BN and GRP activate peripheral neuronal circuitry leading to a central release of BN-like peptides, rather than by acting directly in the brain through an endocrine mechanism. This hypothesis was further supported by data showing that neonatal capsaicin treatment completely abolished the ability of peripherally administered BN to suppress food intake while having no affect on feeding suppression produced by central BN administration (25).
In the present study, we utilized capsaicin to chemically ablate a more selective population of the same neural pathways (ie, vagal and spinal) that have been shown to be necessary for feeding suppressive effects of systemically administered BN (34). The neurotoxicity produced by capsaicin is generally restricted to small diameter, primary sensory neurons of visceral and somatic origin (14, 15, 35). Many studies have reported degeneration from neonatal or adult capsaicin treatment in areas of the spinal cord and hindbrain that receive afferent projections of sensory neurons, though damage is not strictly confined to these regions (15, 27, 28). Although capsaicin-induced damage to peripheral afferents has been well documented, capsaicin administered systemically readily crosses the blood brain barrier (BBB) presenting the possibility that capsaicin treatment could lead to changes in BBB permeability. Studies evaluating acute effects of capsaicin treatment have shown that BBB permeability is unaffected by concentrations of capsaicin that are known to release vasoactive neuropeptides and increase vascular permeability in peripheral tissues (26). Therefore, it is not likely that changes in permeability of the blood brain barrier would differentially affect the ability of BN-like peptides to cross the BBB and account for the differences in feeding suppression observed following capsaicin treatment. However, capsaicin does cause degeneration of nerve terminals in brain areas that are not known to receive primary sensory innervation, and more extensive damage to central neuronal elements has been reported when animals are treated as preweanlings than when treated as adults (27, 28). Thus, it is possible that the differential effect of capsaicin treatment on feeding suppression by BN-like peptides could be attributed to differences in their reliance on distinct central neural pathways that are damaged by capsaicin.
In summary, this study demonstrates that neonatal capsaicin treatment differentially affects the ability of BN, GRP and NMB to reduce food intake in adult rats. These results suggest that different neural substrates underlie the feeding effects of systemically administered BN-like peptides.
Acknowledgments
This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grant DK-46448.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971;27:166–7. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- 2.Battey J, Wada E. Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci. 1991;14:524–8. doi: 10.1016/0166-2236(91)90005-f. [DOI] [PubMed] [Google Scholar]
- 3.Bertaccini G, Erspamer V, Impicciatore M. The actions of bombesin on gastric secretion of the dog and the rat. Br J Pharmacol. 1973;49:437–44. doi: 10.1111/j.1476-5381.1973.tb17254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthoud HR, Patterson LM, Willing AE, Mueller K, Neuhuber WL. Capsaicin-resistant vagal afferent fibers in the rat gastrointestinal tract: anatomical identification and functional integrity. Brain Res. 1997;746:195–206. doi: 10.1016/s0006-8993(96)01222-x. [DOI] [PubMed] [Google Scholar]
- 5.Chavez M, Kelly L, York DA, Berthoud HR. Chemical lesion of visceral afferents causes transient overconsumption of unfamiliar high-fat diets in rats. Am J Physiol. 1997;272:R1657–63. doi: 10.1152/ajpregu.1997.272.5.R1657. [DOI] [PubMed] [Google Scholar]
- 6.Donnerer J, Lembeck F. Neonatal capsaicin treatment of rats reduces ACTH secretion in response to peripheral neuronal stimuli but not to centrally acting stressors. Br J Pharmacol. 1988;94:647–52. doi: 10.1111/j.1476-5381.1988.tb11571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erspamer V, Erpamer GF, Inselvini M. Some pharmacological actions of alytesin and bombesin. J Pharm Pharmacol. 1970;22:875–6. doi: 10.1111/j.2042-7158.1970.tb08465.x. [DOI] [PubMed] [Google Scholar]
- 8.Ewart WR, Jones MV, Primi MP. Bombesin changes excitability of rat brain stem neurons sensitive to gastric distension. Am J Physiol. 1990;258:G841–7. doi: 10.1152/ajpgi.1990.258.6.G841. [DOI] [PubMed] [Google Scholar]
- 9.Flynn FW. Bombesin-like peptides in the regulation of ingestive behavior. Ann N Y Acad Sci. 1994;739:120–34. doi: 10.1111/j.1749-6632.1994.tb19814.x. [DOI] [PubMed] [Google Scholar]
- 10.Gamse R. Capsaicin and nociception in the rat and mouse. Possible role of substance P. Naunyn Schmiedebergs Arch Pharmacol. 1982;320:205–16. doi: 10.1007/BF00510129. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs J, Fauser DJ, Rowe EA, Rolls BJ, Rolls ET, Maddison SP. Bombesin suppresses feeding in rats. Nature. 1979;282:208–10. doi: 10.1038/282208a0. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs J, Kulkosky PJ, Smith GP. Effects of peripheral and central bombesin on feeding behavior of rats. Peptides. 1981;2(Suppl 2):179–83. doi: 10.1016/0196-9781(81)90028-0. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs J, Smith GP. The actions of bombesin-like peptides on food intake. Ann N Y Acad Sci. 1988;547:210–6. doi: 10.1111/j.1749-6632.1988.tb23889.x. [DOI] [PubMed] [Google Scholar]
- 14.Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced rselective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–3. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- 15.Jancso G, Kiraly E, Joo F, Such G, Nagy A. Selective degeneration by capsaicin of a subpopulation of primary sensory neurons in the adult rat. Neurosci Lett. 1985;59:209–14. doi: 10.1016/0304-3940(85)90201-0. [DOI] [PubMed] [Google Scholar]
- 16.Kelly LA, Chavez M, Berthoud HR. Transient overconsumption of novel foods by deafferentated rats: effects of novel diet composition. Physiol Behav. 1999;65:793–800. doi: 10.1016/s0031-9384(98)00237-6. [DOI] [PubMed] [Google Scholar]
- 17.Kulkosky PJ, Gibbs J, Smith GP. Feeding suppression and grooming repeatedly elicited by intraventricular bombesin. Brain Res. 1982;242:194–6. doi: 10.1016/0006-8993(82)90511-x. [DOI] [PubMed] [Google Scholar]
- 18.Ladenheim EE, Ritter RC. Capsaicin attenuates bombesin-induced suppression of food intake. Am J Physiol. 1991;260:R263–6. doi: 10.1152/ajpregu.1991.260.2.R263. [DOI] [PubMed] [Google Scholar]
- 19.Ladenheim EE, Taylor JE, Coy DH, Moore KA, Moran TH. Hindbrain GRP receptor blockade antagonizes feeding suppression by peripherally administered GRP. Am J Physiol. 1996;271:R180–4. doi: 10.1152/ajpregu.1996.271.1.R180. [DOI] [PubMed] [Google Scholar]
- 20.Ladenheim EE, Taylor JE, Coy DH, Moran TH. Blockade of feeding inhibition by neuromedin B using a selective receptor antagonist. Eur J Pharmacol. 1994;271:R7–9. doi: 10.1016/0014-2999(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 21.Ladenheim EE, Wirth KE, Moran TH. Receptor subtype mediation of feeding suppression by bombesin-like peptides. Pharmacol Biochem Behav. 1996;54:705–11. doi: 10.1016/0091-3057(96)00023-8. [DOI] [PubMed] [Google Scholar]
- 22.Lutz TA, Althaus J, Rossi R, Scharrer E. Anorectic effect of amylin is not transmitted by capsaicin-sensitive nerve fibers. Am J Physiol. 1998;274:R1777–82. doi: 10.1152/ajpregu.1998.274.6.R1777. [DOI] [PubMed] [Google Scholar]
- 23.Marki W, Brown M, Rivier JE. Bombesin analogs: effects on thermoregulation and glucose metabolism. Peptides. 1981;2(Suppl 2):169–77. doi: 10.1016/0196-9781(81)90027-9. [DOI] [PubMed] [Google Scholar]
- 24.Merali ZMT, Kateb P, Piggins H. Antagonism of satiety and groming effect of bombesin by antiserum to bombesin and by [Tyr4, D-Phe12]bombesin: central versus peripheral effect. Ann NY Acad Sci. 1988;547:489–92. [Google Scholar]
- 25.Michaud D, Anisman H, Merali Z. Capsaicin-sensitive fibers are required for the anorexic action of systemic but not central bombesin. Am J Physiol. 1999;276:R1617–22. doi: 10.1152/ajpregu.1999.276.6.R1617. [DOI] [PubMed] [Google Scholar]
- 26.Reid J, McCulloch J. Capsaicin and blood-brain barrier permeability. Neurosci Lett. 1987;81:165–70. doi: 10.1016/0304-3940(87)90359-4. [DOI] [PubMed] [Google Scholar]
- 27.Ritter S, Dinh TT. Capsaicin-induced neuronal degeneration: silver impregnation of cell bodies, axons, and terminals in the central nervous system of the adult rat. J Comp Neurol. 1988;271:79–90. doi: 10.1002/cne.902710109. [DOI] [PubMed] [Google Scholar]
- 28.Ritter S, Dinh TT. Capsaicin-induced neuronal degeneration in the brain and retina of preweanling rats. J Comp Neurol. 1990;296:447–61. doi: 10.1002/cne.902960310. [DOI] [PubMed] [Google Scholar]
- 29.Ritter S, Taylor JS. Capsaicin abolishes lipoprivic but not glucoprivic feeding in rats. Am J Physiol. 1989;256:R1232–9. doi: 10.1152/ajpregu.1989.256.6.R1232. [DOI] [PubMed] [Google Scholar]
- 30.Smith J, Perez S, Rushing PA, Smith GP, Gibbs J. Gastrin-releasing peptide1-27, unlike bombesin, does not reduce sham feeding in rats. Peptides. 1997;18:1465–7. doi: 10.1016/s0196-9781(97)00195-2. [DOI] [PubMed] [Google Scholar]
- 31.Spindel ER, Giladi E, Brehm P, Goodman RH, Segerson TP. Cloning and functional characterization of a complementary DNA encoding the murine fibroblast bombesin/gastrin-releasing peptide receptor. Mol Endocrinol. 1990;4:1956–63. doi: 10.1210/mend-4-12-1956. [DOI] [PubMed] [Google Scholar]
- 32.Stein LJ, Woods SC. Gastrin releasing peptide reduces meal size in rats. Peptides. 1982;3:833–5. doi: 10.1016/0196-9781(82)90023-7. [DOI] [PubMed] [Google Scholar]
- 33.Stratford TR, Gibbs J, Smith GP. Simultaneous administration of neuromedin B-10 and gastrin-releasing peptide(1-27) reproduces the satiating and microstructural effects of bombesin. Peptides. 1996;17:107–10. doi: 10.1016/0196-9781(95)02086-1. [DOI] [PubMed] [Google Scholar]
- 34.Stuckey JA, Gibbs J, Smith GP. Neural disconnection of gut from brain blocks bombesin-induced satiety. Peptides. 1985;6:1249–52. doi: 10.1016/0196-9781(85)90458-9. [DOI] [PubMed] [Google Scholar]
- 35.Szolcsanyi J, Jancso-Gabor A, Joo F. Functional and fine structural characteristics of the sensory neuron blocking effect of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1975;287:157–69. doi: 10.1007/BF00510447. [DOI] [PubMed] [Google Scholar]
- 36.Von Schrenck T, Heinz-Erian P, Moran T, Mantey SA, Gardner JD, Jensen RT. Neuromedin B receptor in esophagus: evidence for subtypes of bombesin receptors. Am J Physiol. 1989;256:G747–58. doi: 10.1152/ajpgi.1989.256.4.G747. [DOI] [PubMed] [Google Scholar]
- 37.Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden AM, et al. cDNA cloning, characterization, and brain region-specific expression of a neuromedin-B-preferring bombesin receptor. Neuron. 1991;6:421–30. doi: 10.1016/0896-6273(91)90250-4. [DOI] [PubMed] [Google Scholar]