Abstract
The purpose of this study was to quantitatively assess the role of Rho kinase in modulating the pattern and amount of local cell-induced collagen matrix remodeling.
Human corneal fibroblasts were plated inside 100 μm thick fibrillar collagen matrices and cultured for 24 hours in media with or without the Rho kinase inhibitor Y-27632. Cells were then fixed and stained with phalloidin. Fluorescent (for f-actin) and reflected light (for collagen fibrils) 3-D optical section images were acquired using laser confocal microscopy. Fourier transform analysis was used to assess collagen fibril alignment, and 3-D cell morphology and local collagen density were measured using MetaMorph.
Culture in serum-containing media induced significant global matrix contraction, which was inhibited by blocking Rho kinase (p < 0.001). Fibroblasts generally had a bipolar morphology and intracellular stress fibers. Collagen fibrils were compacted and aligned parallel to stress fibers and pseudopodia. When Rho kinase was inhibited, cells had a more cortical f-actin distribution and dendritic morphology. Both local collagen fibril density and alignment were significantly reduced (p<0.01).
Overall, the data suggests that Rho kinase dependent contractile force generation leads to co-alignment of cells and collagen fibrils along the plane of greatest resistance, and that this process contributes to global matrix contraction.
Keywords: Collagen matrices, Rho kinase, Corneal fibroblasts, Cell mechanics, Confocal microscopy, Cytoskeleton, Actin, Myosin, Fourier Transform
INTRODUCTION
The organization of extracellular matrices by cells through the exertion of mechanical forces drives fundamental processes such as developmental morphogenesis, wound healing, and the organization of bioengineered tissues. Most previous studies of cell mechanical behavior have used planar elastic substrates. In early studies, silicon substrates were used to estimate the forces generated by migrating cells [1-3]. More recently, Wang and coworkers have used flexible polyacrylamide sheets embedded with fluorescent microspheres to study various aspects of cell motility [4, 5]. Although these elegant studies have provided important insights into cell mechanical behavior, cellular interactions with a collagen-coated 2-D substrate is different than what occurs in vivo; since bound, non-fibrillar collagen cannot undergo cell-induced reorganization and alignment. Furthermore, cells reside within 3-D extracellular matrices in vivo, and ECM geometry has been shown to effect cell morphology, adhesion organization and mechanical behavior [6-12].
An alternative to planar elastic substrates is the fibroblast populated collagen matrix model, in which cells are plated inside a 3-D fibrillar collagen matrix [13-16]. In this model, matrix contraction occurs predominantly by rearrangement of existing collagen fibrils through the application of mechanical forces. 3-D collagen matrices are a standard in vitro model for studying the mechanisms regulating cell-mediated matrix reorganization and wound contraction, and measuring overall collagen matrix contraction and/or force generation by fibroblasts using this model has provided valuable insights into the signaling pathways involved in various aspects of cell-matrix mechanical interactions [17]. However, these global measurements do not provide a detailed understanding of the underlying pattern of force generation and matrix reorganization at the cellular and sub-cellular level. More specifically, it is not known how the local pattern of ECM remodeling (i.e. compaction and alignment of collagen fibrils) is modulated by specific growth factors or cytokines.
Previous studies have established that the Rho-family of small GTPases such as Rho, Rac, and Cdc42 play a central role in regulating the cytoskeletal changes associated with cell mechanical activity in a variety of cell types. In fibroblasts on rigid 2-D substrates, activated Rho stimulates the formation of stress fibers and the development of focal contacts [18-22], and these cytoskeletal changes are dependent on actomyosin contraction [21, 23]. Activated Rho binds to and activates Rho kinase (ROCK), which inhibits myosin light chain (MLC) phosphatase, resulting in elevated MLC phosphorylation [19, 24-27]. In 3-D culture, activation of Rho by lysophosphatidic acid (LPA) stimulates global collagen matrix contraction [28-31], and this response appears to be mediated by Rho kinase [19, 32]. However, the role of Rho kinase in modulating the pattern and amount of local cell-induced collagen matrix reorganization has not been established.
In this study, we use quantitative confocal imaging to assess 3-D cell morphology, f-actin organization, and both the pattern and amount of local cell-induced collagen matrix reorganization by human corneal fibroblasts in 3-D collagen matrices, and investigate the role of Rho kinase in modulating these cell-matrix mechanical interactions. The data suggests that Rho kinase dependent actomyosin contraction by corneal fibroblasts leads to co-alignment of cells and collagen fibrils along the plane of greatest mechanical resistance, and that this process plays a major role in global matrix contraction and reorganization. Activation of Rho kinase in vivo may be critically involved in the transformation of quiescent dendritic corneal keratocytes to activated fibroblasts, which mediate corneal wound healing and subsequent matrix remodeling.
MATERIALS AND METHODS
Cells
Studies were performed using a previously characterized telomerase-infected, extended lifespan human corneal fibroblast cell line, HTK [33]. HTK cells were cultured in serum-containing (S+) medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM) (GIBCO Invitrogen Cell Culture, Carlsbad, CA) supplemented with 1% penicillin, 1% streptomycin and 1% Fungizone (Biowhittaker, Inc., Wakersville, MD) and 10% fetal bovine serum (Sigma Chemical Corp., St. Louis, MO).
Collagen Matrices
Hydrated collagen matrices were prepared by mixing neutralized bovine dermal collagen (PureCol, Inamed Corp, Fremont, CA) with 10X DMEM to achieve a final collagen concentration of 2.48 mg/ml [34]. For plating cells inside the matrix, a 50μL of suspension of HTK cells was mixed with 500μL of collagen solution. The pH of the collagen solution was adjusted to 7.2-7.4 by addition of 0.1 N NaOH or 0.1 N HCL. The cell/collagen mixture was preincubated at 37°C for 5 minutes, and 30-μL aliquots (containing approximately 2,000 cells) were then poured onto culture dishes (Delta T; Bioptechs, Inc., Butler, PA). These dishes have glass bottoms of #1 cover slip thickness; this increases the free working distance as compared to plastic. Each aliquot was spread over a central 12-mm diameter circular region on the dish and was approximately 100μm thick. The dish was then placed in a humidified incubator (37°C, 5% CO2) for 60 minutes for polymerization and overlaid with 1.5ml of either S+ media or S+ with the Rho kinase inhibitor Y-27632 (10μM), and incubated 24 hrs prior to imaging.
F-actin Staining
After 24hrs of incubation, cells were fixed using 3% paraformaldehyde in phosphate buffer for 3 min, and permeabilized with 0.5% Triton X-100 in phosphate buffer for 3 min. Cells were then incubated in Alexa Fluor 546 Phalloidin (1:20, Molecular Probes, Eugene, OR) for 60 minutes.
Laser Confocal Microscopy
After labeling with F-actin, fluorescent (for f-actin) and reflected light (for collagen fibrils) 3-D optical section images were acquired simultaneously using laser confocal microscopy (Leica SP2, Heidelberg, Germany). A HeNe laser (633nm) was used for reflected light and GreNe (543nm) laser was used for fluorescent images of f-actin. A stack of optical sections (z-series) was acquired for each cell imaged by changing the position of the focal plane in 0.5 - 1 μm steps using a 63X water immersion objective (1.2 NA, 220 μm free working distance).
Global Matrix Remodeling
An inverted microscope with DIC imaging modules (TE300; Nikon, Tokyo, Japan) was used to measure global matrix contraction. Since the bottom of the matrices remain attached to the dish, cell-induced contraction resulted in a decrease in matrix height [17]. Height was measured by focusing on the top and bottom of each matrix at 6 different locations, at 1 hour and 24 hours after plating. Measurements were performed in triplicate for each condition (S+, S+ with Y-27632, and control matrices without cells).
Estimates of the expected increase in overall collagen density induced by global matrix contraction were made using the equation:Estimated % Increase in collagen density = 100 × { 100/(100 - %GlobalMatrixContraction) - 1}
This calculation is based on the assumption that the percentage change in matrix height results in an equal percentage change in matrix volume (since the diameter is constant), and that the total amount of collagen in the matrix does not change.
Image Processing and Analysis
Local collagen fibril orientation
In order to evaluate collagen fibril orientation in the matrices, we applied a Fourier Transform (FT) analysis procedure previously used in other tissues [35-37]. This approach is based on the fact that the relative strength of different angle bands within the FT spectrum is a global indicator of the relative number and magnitude of fibers oriented at 90 degrees to that angle in the original image. A custom program written in Matlab was used for performing the FT analysis. Contrast was normalized in each image so that the minimum gray level was 0 and the maximum gray level was 255. A Welch window was then applied to eliminate edge effects in the discrete FT. The FT Power Spectrum of the windowed image was then calculated, and rotated 90 degrees to align the data with the original image. Using polar coordinates (r,φ), line averages (I(φ)) from the center to the periphery of the rotated FT power spectrum were calculated along the radial direction at 1 degree intervals. Points close to the center (r < 3) were excluded from the line averages, since they represent low-frequency information (such as shading), which is not of interest.
An “orientation index” (OI) was then used to quantify the degree of orientation of the collagen fibrils along a given angle, using the equation:
Where,
and θ=0° is the x axis in the original image. The OI has the value of 100% for fibers totally oriented parallel to a given θ, -100% for an orientation perpendicular to a given θ, and 0% for a completely random distribution of fibers. Because the OI is based on the dimensionless cos2 value, it is dependent on the relative magnitude of the line averages, not the absolute magnitudes [35].
Cell morphology
Changes in cell morphology were measured using MetaMorph (Universal Imaging Corp., Downingtown, PA., USA). Projected cell length was calculated by outlining the maximum intensity projection image of a cell (generated from the f-actin z-series), thresholding, and applying the Integrated Morphometry Analysis (IMA) routine. The length is calculated by IMA as the longest distance between any two points in the cell. The height of cells was calculated by measuring the distance between the first and last planes in the z-series in which a portion of the cell was visible.
Collagen density
The percent of reflected light confocal images occupied by collagen fibrils was used as an indicator of collagen fibril density. Using Metamorph, images were thresholded manually to segment out the collagen fibrils, and binarized. The number of segmented pixels was then measured from the binary image using IMA, and expressed as a percent of the total number of pixels in the original image. Due to the subjective nature of interactive thresholding, this procedure was performed blinded (without knowledge of the treatment group from which the image originated).
Color Overlays and Movies
Color overlays of corresponding reflected light and fluorescent optical section images were made for each confocal z-series, using the “Overlay Images” feature in Metamorph (with f-actin in green and collagen in red). Maximum intensity projections of the overlay z-series were then generated over a range of projection angles using the “3-D Reconstruction” function in Metamorph. The resulting projections were then saved as a Movie file to allow visualization of the 3-D interactions between cells and collagen fibrils.
Statistics
All statistical analyses were performed using SigmaStat version 3.11 (Systat Software, Inc., Point Richmond, CA). For experiments with only two groups, means were compared using a Student t-test. For experiments with more than two groups, means were compared using ANOVA with the Holm-Sidak post-hoc multiple comparison method.
RESULTS
F-actin organization and cell morphology
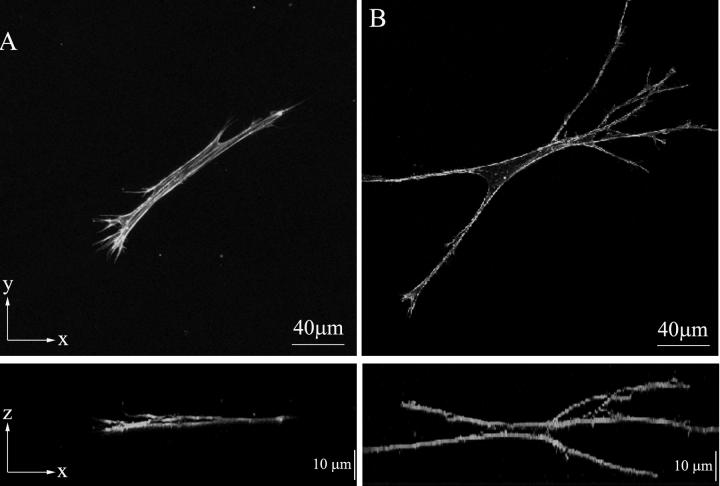
Human corneal fibroblasts incubated in S+ medium generally had a bipolar or stellate morphology with pseudopodial processes (Fig. 1A, top panel), and did not form broad lamellipodia as is observed in cells plated on top of fibrillar collagen matrices [38], or on planar substrates [5]. This is consistent with previous observations of corneal fibroblasts within 3-D collagen matrices [34, 39, 40]. Parallel arrays of microfilament bundles (stress fibers) were often observed within the cell body and pseudopodial processes. Cells were always aligned nearly parallel to the bottom of the culture dish on which the collagen matrix was plated (Fig. 1A, bottom panel).
Figure 1.
Maximum intensity projections of f-actin organization along the z-axis (top) or y-axis (bottom) in cells cultured in S+ media (A) or S+ containing Y-27632 (B). A. Cells in complete media have a bipolar morphology with thin pseudopodial processes (top). Microfilament bundles (stress fibers) are often observed. Cells and cellular processes are oriented nearly parallel to the bottom of the culture dish (bottom). B. Inhibition of Rho kinase results in dramatic cell elongation, a more stellate morphology, thinner dendritic processes, and a more 3-dimensional structure (extension of processed in the z-axis, bottom). F-actin is more cortical, and stress fibers are rarely observed.
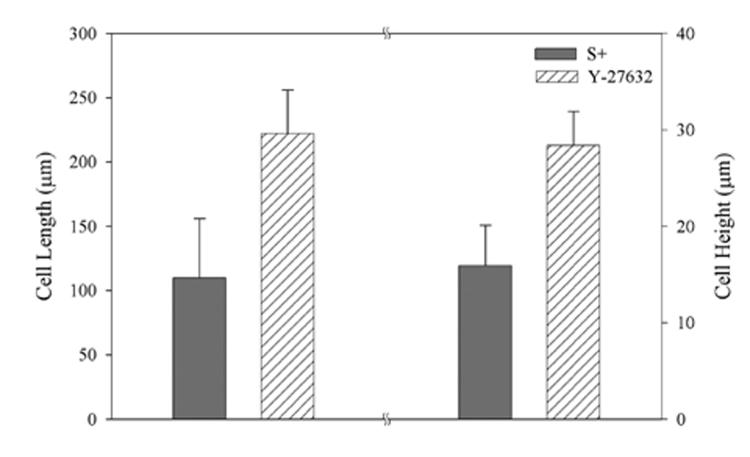
Cells treated with the Rho kinase inhibitor Y-27632 appeared more elongated, and had a stellate morphology with numerous dendritic processes (Fig. 1B, top panel). F-actin staining was generally limited to the cell cortex. When present, microfilament bundles exhibited much weaker staining than those observed after culture in S+. Quantitative analysis (Fig. 2) demonstrated that blocking Rho kinase resulted in a statistically significant increase in both projected cell length (148 ± 58 μm vs. 209 ± 39 μm, p < 0.001) and height (16 ± 4 μm vs. 28 ± 10 μm, p < 0.001). The increased height is best appreciated by comparing maximum intensity projections generated along the x-axis (Fig. 1, bottom panels). Overall, cells were elongated, had fewer stress fibers, and had more branches and 3-D extensions when Rho kinase was inhibited.
Figure 2.
Quantitative analysis of cell morphology. Both cell length and cell height were significantly increased when Rho kinase was inhibited (p < 0.001).
Global collagen matrix reorganization
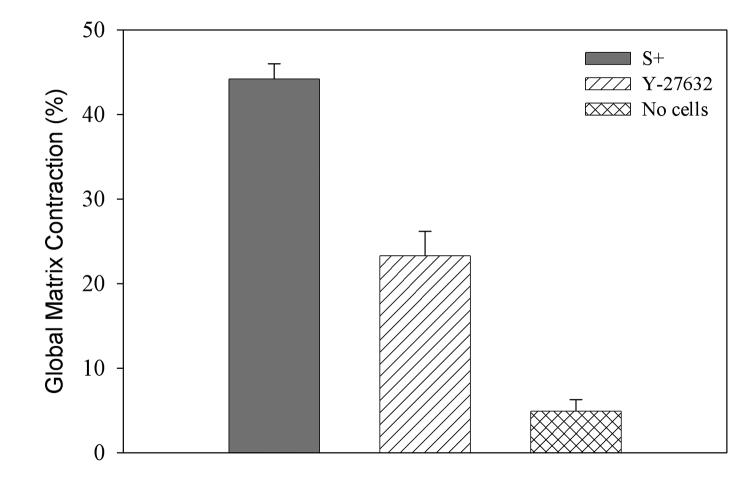
Collagen matrices with or without cells were prepared and incubated for 24 hrs in S+, or in S+ with Y-27632. Global matrix reorganization was measured as a decrease in matrix height, using 1 hour measurements as a reference (Fig. 3). Significant matrix contraction was observed following culture in both S+ (44.2 ± 1.8%, p < 0.001) and S+ containing Y-27632 (23.3 ± 2.9%, p <0.001), as compared to control matrices without cells (4.9 ± 1.4%). As compared to S+, the amount of contraction was significantly less when Rho kinase was inhibited (p < 0.001). These results are consistent with previous results showing both Rho kinase dependent and Rho kinase independent contributions to global matrix contraction by dermal fibroblasts [32].
Figure 3.
Assessment of global matrix contraction over a 24 hour time period. Rho kinase inhibition significantly reduced the amount of cell-induced global matrix contraction (p < 0.001).
Local collagen matrix reorganization
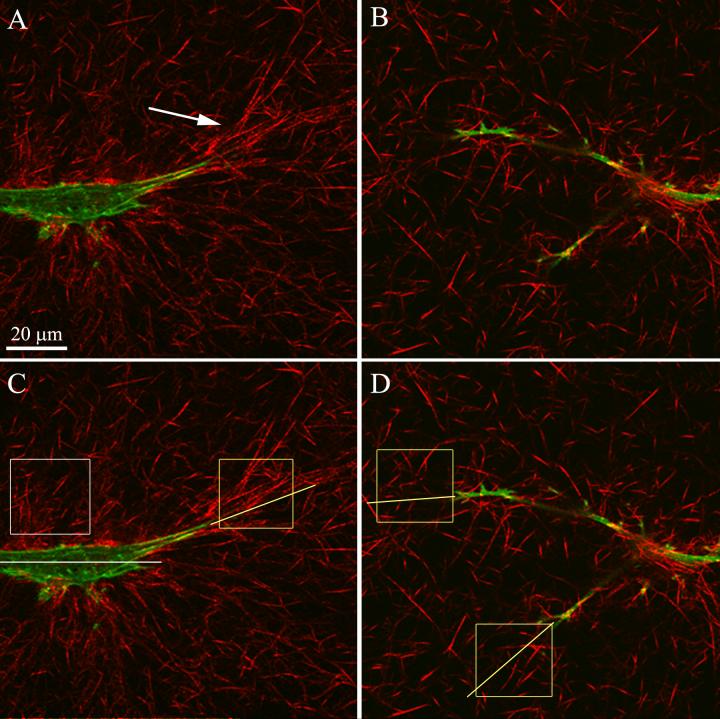
In order to assess the pattern of local cell-induced matrix reorganization which underlies global matrix contraction, reflected light confocal microscopy was performed. This technique allows detailed visualization of the cells and the fibrillar collagen surrounding them [41-43]. By overlaying the corresponding reflected light and fluorescent optical section images, cell-matrix interactions could be directly visualized (Fig. 4). Following culture in S+, collagen at the ends of cells appeared to be compacted and aligned parallel to the long axis of pseudopodia (Fig. 4A, arrow). In contrast, collagen fibrils adjacent to the cell body were either randomly distributed, or compacted and aligned nearly perpendicular to the long axis of the cell. Since entire cells were not visible in a single plane, 3-D reconstructions (maximum intensity projections) of z-series were generated. Cell-induced matrix reorganization is best appreciated in movies showing these maximum intensity projection overlays over a range of projection angles (Movies 1-3). Note that collagen fibrils are oriented nearly parallel to the direction of stress fiber alignment at the ends of the pseudopodia.
Figure 4.
Color overlays of f-actin (green) and collagen fibils (red) allows interactions between cells and the extracellular matrix to be directly visualized. Single optical sections are shown. A. Collagen fibrils are compacted and aligned nearly parallel to the pseudopodial tips at the end of cells in S+ (arrow). In contrast, collagen is sometimes aligned more perpendicular to the cell axis along the cell body. B. Less compaction and alignment of extracellular matrix is observed in the presence of Y-27632. C, D. Boxes indicate sub-regions used for quantitative analysis of local collagen density and alignment at the ends of pseudopodia (yellow boxes) or adjacent to the cell body (white box).Yellow lines indicate axis of pseudopodia, and white line indicates cell body axis.
Following culture in media containing Y-27632, there appeared to be much less compaction and alignment of collagen fibrils surrounding the cell (Fig. 4B, Movies 4 and 5). In some cases, fibrils were aligned somewhat parallel to the ends of pseudopodia, but overall the fibrils remained more randomly distributed. The groups of collagen fibrils oriented perpendicular to the cell body that were sometimes observed following culture in S+ alone were rarely detected when Rho kinase was inhibited. We also qualitatively assessed a few matrices at 4 hours, and although cells in both culture conditions appeared smaller than they are at 24 hours, cells treated with Y-27632 had already developed a more dendritic morphology than those in S+ alone (Movies 6 and 7). Most HTK cells in S+ do not have stress fibers at this time point, and only a small amount of matrix realignment is observed.
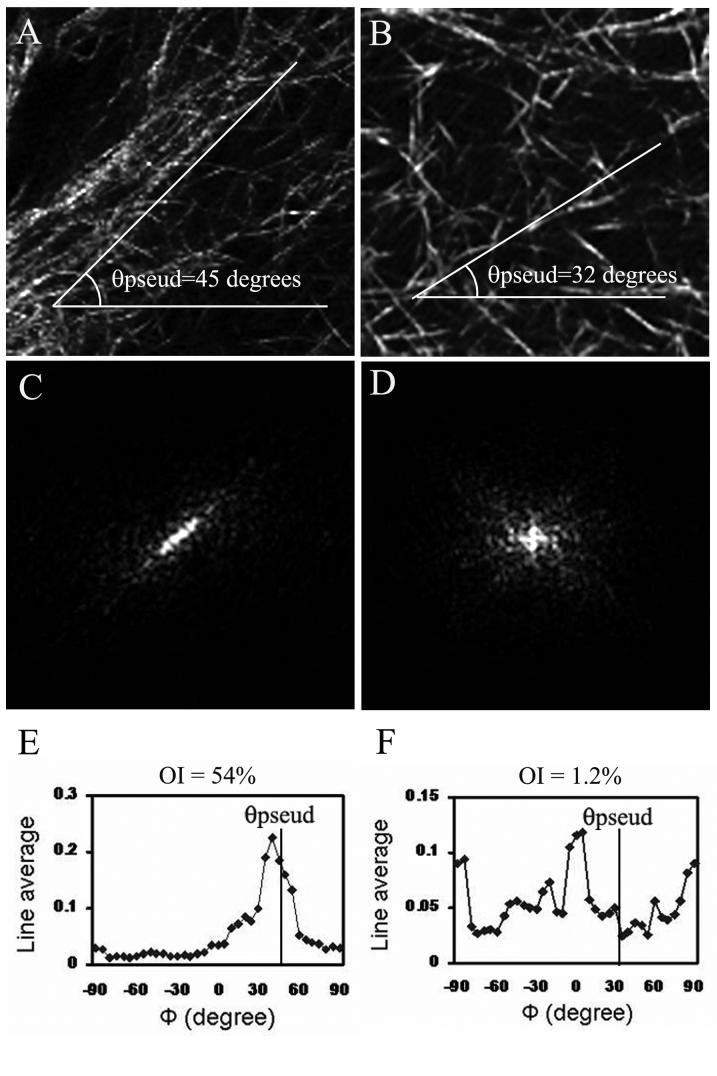
Collagen fibril orientation was quantified using Fourier Transform (FT) analysis of sub-regions from the reflected light images. For each cell, 2-3 sub-regions were taken both adjacent to the cell body (e.g. white box in Fig. 4C) and at the ends of pseudopodia (e.g. yellow boxes in Fig. 4C,D). The procedure for quantitative analysis of collagen fibril orientation is shown in Figure 5. In this example, sub-regions were taken from the ends of pseudopodia in two cells following culture in S+ (left) or S+ containing Y-27632 (right). The pseudopodia tips were located at the bottom left corner of each image (just outside the sub-region boundary); θpseud is the angle of the long axis of each pseudopod. Collagen fibrils at the end of the cell in S+ (Fig. 5A) appear to be compacted and aligned nearly parallel to the pseudopodial axis (θpseud = 45° in this example). After rotating 90°, the FT power spectrum (Fig. 5C) reveals a bright band-like signal aligned with the compacted collagen fibrils. This results in a line average plot with one distinct peak near θpseud (Fig. 5E). The magnitude of the orientation index (OI) at θpseud is 54%, indicating substantial compaction and alignment of collagen parallel to the pseudopodial axis (OI = 100% for collagen fibers totally oriented parallel to a given θ, -100% for an orientation perpendicular to a given θ, and 0% for a completely random distribution).
Figure 5.
Example of Fourier Transform approach to quantifying local collagen fibril alignement. Sub-regions were taken from the ends of pseudopodia following culture in S+ (left) and Y-27632 (right). The pseudopodia tips were located at the bottom left corner of each image (just outside the sub-region boundary). A. Collagen fibrils at the end of a cell are aligned nearly parallel to the pseudopodial tip in complete media. C. After rotating 90°, the power spectrum reveals a bright band-like signal aligned nearly parallel to the group of collagen fibrils. E. The line average plot has one distinct peak near θpseud. The magnitude of the orientation index at θpseud is 54%, indicating substantial compaction and alignment of collagen parallel to the pseudopodial axis. B. Collagen is more randomly oriented in Y-27632, and the rotated FT power spectrum (D) is more dispersed. F. The corresponding line average plot has a more uniform distribution without a single dominant peak. The magnitude of the OI at θpseud is only 1.2%, indicating little compaction and alignment of collagen parallel to the pseudopodial axis.
Collagen fibrils appeared to be more randomly oriented when Rho kinase was inhibited (Fig. 5B). The FT power spectrum is more dispersed without predominant alignment along any particular axis (Fig. 5D). The corresponding line average plot has a more uniform distribution without a single dominant peak (Fig. 5F). The magnitude of the OI at θpseud is only 1.2% in this example, indicating little compaction and alignment of collagen parallel to the pseudopodial axis.
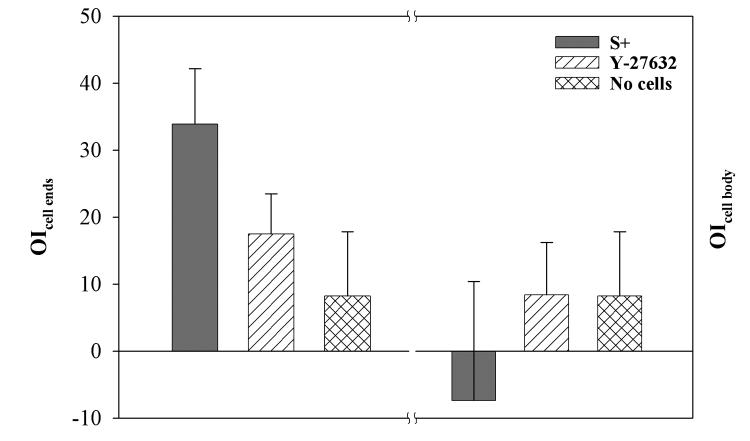
The OI measurements from the FT analysis are summarized in Fig. 6. Sub-regions surrounding 7 cells were analyzed for each condition, using matrices without cells as controls (for controls θpseud and θcell body were assumed to be 0°). For each sub-region, the OI was averaged for 3 optical sections: one at the level of the pseudopodial tip, one 1 micron above this plane, and one 1 micron below this plane. Significant alignment of collagen was observed at the ends of cells after culture in both S+ (33.9 ± 8.3%, p < 0.001) and S+ containing Y-27632 (17.5 ± 6.0%, p < 0.001), but not in control matrices (8.2 ± 10.4%). As compared to S+, the overall degree of alignment was reduced in the presence of Y-27632 (p < 0.01). Adjacent to the cell body, preferential alignment of collagen was not detected under either condition evaluated (OI was not significantly different than 0). The small negative value of the OI along the cell body following culture in S+ media reflects the fact that although regions of fibril alignment perpendicular to the cell body were often observed, regions with randomly aligned fibrils were present as well.
Figure 6.
Summary of OI results from FT analysis. Significant alignment of collagen was observed at the ends of pseudopodia after culture in both S+ (p < 0.001) and S+ containing Y-27632 (p < 0.001), but not in control matrices. As compared to S+, the overall degree of alignment was reduced in the presence of Y-27632 (p < 0.01). Adjacent to the cell body, preferential alignment of collagen was not detected under either condition evaluated.
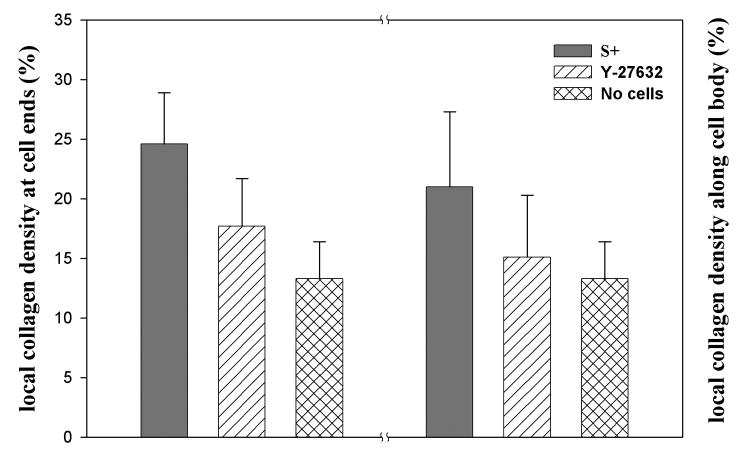
We also assessed the relative collagen fibril density in the image sub-regions surrounding cells, by calculating the area occupied by collagen fibrils (Fig. 7). The overall collagen density surrounding cells (average of ends and cell body) was significantly greater following culture in S+ (22.9 ± 5.4%), as compared to S+ containing Y-27632 (16.4 ± 4.6%, p < 0.01) or control matrices without cells (13.3 ± 3.6%, p < 0.01). Collagen density surrounding cells following culture in S+ was 72.1% greater than control matrices without cells. Following culture in S+ containing Y-27632, collagen density was 23.3% greater than controls. Based on the results of our global matrix contraction measurements, overall collagen density was expected to be 64% and 22.5% greater than control matrices following culture in S+, and S+ containing Y-27632, respectively.
Figure 7.
Summary of local collagen density measurements. Both at the ends of cells and along the cell body, collagen density was significantly greater following culture in S+ (p < 0.01), as compared to S+ containing Y-27632 or control matrices without cells.
DISCUSSION
Corneal wound healing following lacerating injury, penetrating keratoplasty or refractive surgery involves an ordered sequence of cell-matrix mechanical interactions. In the corneal stroma, quiescent keratocytes normally have a dendritic morphology and a cortical distribution of f-actin [44]. Following injury or surgery, keratocytes surrounding the wound transform to an activated, fibroblast phenotype characterized by a more bipolar morphology and prominent intracellular stress fibers [45, 46]. Corneal fibroblasts migrate into the wound, synthesize new extracellular matrix, and reorganize the ECM through the application of mechanical forces. Wound contraction and matrix remodeling by corneal fibroblasts ultimately determine a patient’s corneal clarity and refractive outcome [47-49]. Understanding the underlying cellular and molecular mechanisms that regulate these biophysical processes in corneal fibroblasts could ultimately lead to more effective approaches to modulating the wound healing response in vivo.
Previous in vitro studies on rigid substrates have shown that serum induces transformation of quiescent corneal keratocytes to a wound healing, fibroblast phenotype [23]. In this study, following culture S+ media (in which Rho is activated) corneal fibroblasts in 3-D collagen matrices had a bipolar or stellate morphology, and stress fibers were often observed along the cell body and pseudopodial processes, consistent with the in vivo corneal fibroblast phenotype. In contrast, cells treated with the Rho kinase inhibitor Y-27632 were more elongated, had a dendritic morphology, and f-actin staining that was generally limited to the cell cortex, similar to quiescent keratocyte phenotype in vivo. Consistent with previous studies using dermal fibroblasts, global matrix contraction was also significantly reduced when Rho kinase was inhibited [19, 32, 50]. Overall, the data suggest that activation of Rho kinase may play a key role in mediating the transformation of quiescent corneal keratocytes to a contractile, fibroblast phenotype during corneal wound healing.
Because the attachment plane of the matrices is large compared to the matrix height (1 cm vs. 100 μm) and the top of the matrix is unconstrained, there is more resistance to collagen displacement in the x, y plane than there is along the z-axis. This is why contraction of attached collagen matrices occurs through a decrease in height, not diameter. In order to provide insights into the underlying mechanism of global matrix contraction, quantitative analysis of 3-D cell morphology and both the pattern and amount of local cell-induced collagen matrix reorganization was performed. After 24 hours of culture, cells in S+ were always aligned nearly parallel to the bottom of the culture dish on which the collagen matrix was plated, and collagen fibrils were compacted and aligned parallel to the long axis of stress fibers and pseudopodia. In contrast, cells had longer extensions along the z-axis when Rho kinase was inhibited, and collagen surrounding the cells was less compacted and more randomly organized. Thus Rho kinase dependent contraction by corneal fibroblasts leads to co-alignment of cells and collagen fibrils along the plane of greatest mechanical resistance. Interestingly, these data are consistent with previous studies which demonstrate gradual alignment of stress fibers along the axis of greatest mechanical resistance during in vivo corneal wound healing [37, 51].
Previous investigators have visualized the collagen organization surrounding isolated cells using DIC imaging or electron microscopy. Qualitative analysis of these 2-D images suggests that existing matrix fibrils generally become aligned parallel to the long axis of the cell, consistent with the quantitative results from this study [10, 15, 52, 53]. However, confocal reflection imaging allows the 3-D collagen architecture to be reconstructed, and thus provides better insights into cell-induced matrix reorganization than 2-D DIC or TEM imaging. Friedl and coworkers have used this technique to study local matrix reorganization by different cell types within 3-D collagen lattices [41, 42, 54]. In fibroblasts and MV3 melanoma cells, β1 integrins are clustered predominantly at the leading edge of cells in association with aligned and compacted collagen fibers [55]. In contrast, migrating T-lymphocytes are free of clustered β1 integrins, and local matrix reorganization is not observed. These results suggest that sustained force generation by large, slow moving cells in S+ media leads to increased structural matrix reorganization as compared to the more transient forces produced by migratory cells. The results of this study suggest that in corneal fibroblasts, these sustained forces are Rho kinase dependent.
Although global matrix contraction, and compaction and alignment of pseudopodia were significantly reduced when Rho kinase was inhibited, they were still greater than control matrices without cells. Thus cellular force generation was only partially blocked by inhibiting Rho kinase. In previous studies using time-lapse DIC imaging, we have investigated the dynamic pattern of force generation by corneal fibroblasts within 3-D collagen matrices. These studies demonstrated that in cells cultures in S+, extension of pseudopodial processes generated tractional forces on the ECM as indicated by pulling in of the collagen fibrils in front of the cell. At the same time, regions of ECM compression were produced at the base of pseudopodia. After 24 hours of culture in S+, addition of Y-27632 induced rapid cell body elongation and relaxation of matrix stress along the cell body, as well as pseudopodial and/or filopodial extension [40]. However, smaller forces continued to be generated at the leading edge of extending pseudopodia, as indicated by transient displacement (pulling in) of collagen fibrils. Furthermore, Grinnell and coworkers have demonstrated that LPA stimulated dermal fibroblasts can displace the collagen surrounding them when Rho kinase is inhibited (as indicated by movement of embedded microspheres) [32]. Sheetz and coworkers investigated the relationship between force and focal complex development during spreading of NIH 3T3 fibroblasts on rigid substrates by using an optical laser trap to monitor the force on beads coated with a fragment of fibronectin type III [56]. The development of force was inhibited by dominant-negative Rac and by myosin light chain kinase inhibition, but not by dominant-negative Rho or Rho kinase inhibition. Taken together, these studies suggest that small, transient forces can be generated at the leading edge of extending pseudopodia via Rho kinase-independent myosin light chain phosphorylation. In contrast, larger forces generated along the cell body and at the base of pseudopodia are Rho/Rho kinase-dependent. Thus, the residual matrix reorganization observed following sustained Rho kinase inhibition in the current study likely results from localized tractional forces generated during formation and extension of dendritic cell processes.
The complexity of collagen fibril organization makes quantitative analysis of orientation information in 3-D difficult. Davidson et al. have used a pattern matching method to analyze fibril orientation of composite materials [57]. To define the orientation of a fiber unambiguously in space, two angles are required; in-plane angle φ and out-of-plane angle θ. A number of serial sections of images can be obtained, and the pattern matching method is then used to correlate the fiber images from each section, allowing full 3-D orientation data to be extracted. However, the measurement error at low values of θ can be large, so that smaller angles are cut to attain greater accuracy. This technique is also limited to unidirectional continuous fibril composites and relies on number of other assumptions. The Hough transform [58] is commonly used for the detection of regular curves such as lines, circles, and ellipses but this cannot detect the ends of fibers and can become unreliable for irregular curves like collagen fibers. Thus to assess local matrix remodeling in the present study, we evaluated the alignment of collagen fibrils using 2-D Fourier transform analysis, which avoids the artifacts mentioned above. We have used this approach previously to assess stress fiber orientation during in vivo corneal wound healing [37, 51]. Schwartz and coworkers have used a similar FT approach to assess flow-induced collagen matrix remodeling [59]. In their study, the peak of the FT line averages was used to define an orientation angle, and the degree of alignment at this angle was determined by using a ratio of the sum of frequencies within 20° of that angle against the sum of all angles. We instead used a previously described orientation index, which allows the degree of collagen fibril alignment along a particular angle to be expressed as a single normalized value (%). While the angle of maximum fibril alignment can also be calculated using this approach, we found the use of a single orientation index more useful in the current study. Overall, such quantitative approaches should be valuable for future investigations of the underlying mechanisms of local cell-induced matrix remodeling using confocal reflection imaging.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Drs. Fred Grinnell and William Snell for their helpful comments and suggestions.
Footnotes
This study was supported in part by NIH EY 13322, NIH infrastructure grant EY 16664, and an unrestricted grant and Lew R. Wasserman Merit Award (WMP) from Research to Prevent Blindness, Inc., NY, NY.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Harris AK, Wild P, Stopak D. Silicone rubber substrata: A new wrinkle in the study of cell locomotion. Science. 1980;208:177–189. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Leonard M, Oliver T, Ishihara A, Jacobson K. Traction forces generated by locomoting keratocytes. J Cell Biol. 1994;127:1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001;3:466–72. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 4.Pelham RJ, Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 2001;153:881–8. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bard JBL, Hay ED. The behavior of fibroblasts from the developing avian cornea: Morphology and movement in situ and in vitro. J. Cell Biol. 1975;67:400–418. doi: 10.1083/jcb.67.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 8.Doane KJ, Birk DE. Fibroblasts retain their tissue phenotype when grown in three-dimensional collagen gels. Exp Cell Res. 1991;195:432–442. doi: 10.1016/0014-4827(91)90394-a. [DOI] [PubMed] [Google Scholar]
- 9.Friedl P, Brocker E-B. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasek JJ, Hay ED, Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: Distribution of actin, α-actinin and myosin. Dev Biol. 1982;92:107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- 11.Abbott A. Biology’s new dimension. Nature. 2003;424:870–872. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 12.Beningo KA, Dembo M, Wang Y-L. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. PNAS. 2004;101:18024–18029. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell E, Ivarsson B, Merril C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vivo. Proc. Natl. Acad. Sci. U.S.A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grinnell F, Lamke CR. Reorganization of hydrated collagen lattices by human skin fibroblasts. J. Cell Sci. 1984;66:51–63. doi: 10.1242/jcs.66.1.51. [DOI] [PubMed] [Google Scholar]
- 16.Stopak D, Harris AK. Connective tissue morphogenesis by fibroblast traction. Dev Biol. 1982;90:383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- 17.Grinnell F. Fibroblast-collagen matrix contraction: growth-factor signalling and mechanical loading. Trends. Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- 18.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell biol. 1999;147:1009–1021. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: role of rho, myosin light chain kinase, and myosin light chain phosphotase. Exp Cell Res. 2000;254:210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- 20.Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- 22.Anderson S, DiCesare L, Tan I, Leung T, SundarRaj N. Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblasts and myofibroblasts. Exp Cell Res. 2004;298:574–583. doi: 10.1016/j.yexcr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Jester JV, Chang J-H. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 24.Kimura K, Ito M, Amano M, Chihara K, Fukuta Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of mysoin phosphatase by rho and rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 25.Chrzanowska-Wodnicka C, Burridge K. Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton in response to serum or LPA stimulation. J. Cell Sci. 1994;107:3643–3564. doi: 10.1242/jcs.107.12.3643. [DOI] [PubMed] [Google Scholar]
- 26.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 27.Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 28.Tomasek JJ, McConathy WJ, Checovich WJ, Mosher DF. Lipoproteins promote fibroblast-mediated collagen lattice contraction. Mol. Biol. Cell. 1992;3:234A. [Google Scholar]
- 29.Sanderson PL, Morris AM, Stanley JK, Fahmy NRM. Lipids and Dupuytren’s disease. J. Bone Joint Surg. 1992;74B:723–927. doi: 10.1302/0301-620X.74B6.1447259. [DOI] [PubMed] [Google Scholar]
- 30.Kolodney MS, Elson EL. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J. Biol. Chem. 1993;268:23850–23855. [PubMed] [Google Scholar]
- 31.Rayan GM, Parizi M, Tomasek JJ. Pharmacologic regulation of Dupuytrens’s fibroblast contraction in vitro. J. Hand Surg. 1996;21A:1065–1070. doi: 10.1016/S0363-5023(96)80317-0. [DOI] [PubMed] [Google Scholar]
- 32.Tamariz E, Grinnel F. Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell. 2002;13:3915–3929. doi: 10.1091/mbc.E02-05-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jester JV, Huang J, Fisher S, Spiekerman J, Chang JH, Wright WE, Shay JW. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life, human corneal fibroblasts. Invest Ophthal Vis Sci. 2003;44:1850–1858. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]
- 34.Petroll WM, Ma L. Direct, dynamic assessment of cell-matrix interactions inside fibrillar collagen lattices. Cell Motil Cytoskeleton. 2003;55:254–264. doi: 10.1002/cm.10126. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhuri S, Nguyen H, Rangayyan RM, Walsh S, Frank CB. A Fourier domain directional filtering method for analysis of collagen alignment in ligaments. IEEE Trans Biomed Eng. 1987;34:509–518. doi: 10.1109/tbme.1987.325980. [DOI] [PubMed] [Google Scholar]
- 36.Kronick PL, Sacks MS. Quantification of vertical-fiber defect in cattle by small angle light scattering. Conn Tiss Res. 1991;27:1–13. doi: 10.3109/03008209109006991. [DOI] [PubMed] [Google Scholar]
- 37.Petroll WM, Cavanagh HD, Barry-Lane P, Andrews P, Jester JV. Quantitative analysis of stress fiber orientation during corneal wound contraction. J. Cell Sci. 1993;104:353–363. doi: 10.1242/jcs.104.2.353. [DOI] [PubMed] [Google Scholar]
- 38.Roy P, Petroll WM, Cavanagh HD, Chuong CJ, Jester JV. An in vitro force measurement assay to study the early mechanical interaction between corneal fibroblasts and collagen matrix. Exp. Cell Res. 1997;232:106–117. doi: 10.1006/excr.1997.3511. [DOI] [PubMed] [Google Scholar]
- 39.Petroll WM, Vishwanath M, Ma L. Corneal fibroblasts respond rapidly to changes in local mechanical stress. Invest Ophthalmol Vis Sci. 2004;45:3466–3474. doi: 10.1167/iovs.04-0361. [DOI] [PubMed] [Google Scholar]
- 40.Vishwanath M, Ma L, Jester JV, Otey CA, Petroll WM. Modulation of corneal fibroblast contractility within fibrillar collagen matrices. Invest Ophthalmol Vis Sci. 2003;44:4724–4735. doi: 10.1167/iovs.03-0513. [DOI] [PubMed] [Google Scholar]
- 41.Friedl P, Maaser K, Klein CE, Niggemann B, Krohne G, Zanker KS. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res. 1997;57:2061–2070. [PubMed] [Google Scholar]
- 42.Friedl P, Noble PB, Zanker KS. T lymphocyte locomotion in a three-dimensional collagen matrix. J Immunol. 1995;154:4973–4985. [PubMed] [Google Scholar]
- 43.Petroll WM, Cavanagh HD, Jester JV. Dynamic three-dimensional visualization of collagen matrix remodeling and cytoskeletal organization in living corneal fibroblasts. Scanning. 2004;26:1–10. doi: 10.1002/sca.4950260102. [DOI] [PubMed] [Google Scholar]
- 44.Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: In situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35:730–743. [PubMed] [Google Scholar]
- 45.Garana RMR, Petroll WM, Herman I, Barry P, Andrews P, Cavanagh HD, Jester JV. Radial Keratotomy: II. Role of the myofibroblast in corneal wound contraction. Invest Ophthal Vis Sci. 1992;33:3271–3282. [PubMed] [Google Scholar]
- 46.Moller-Pedersen T, Li H, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy using in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 1998;39:487–501. [PubMed] [Google Scholar]
- 47.Petroll WM, New K, Sachdev M, Cavanagh HD, Jester JV. Radial Keratotomy III. Relationship between wound gape and corneal curvature in primate eyes. Invest. Ophthal. Vis. Sci. 1992;33:3283–3291. [PubMed] [Google Scholar]
- 48.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of the myofibroblast. Prog Retinal Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 49.Moller-Pedersen T, Vogel MD, Li H, Petroll WM, Cavanagh HD, Jester JV. Quantification of stromal thinning, epithelial thickness, and corneal haze following photorefractive keratectomy using in vivo confocal microscopy. Ophthalmol. 1997;104:360–368. doi: 10.1016/s0161-6420(97)30307-8. [DOI] [PubMed] [Google Scholar]
- 50.Lee DJ, Ho C-H, Grinnell F. LPA-stimulated fibroblast contraction of floating collagen matrices does not require Rho kinase activity or retraction of fibroblast extensions. Exp. Cell Res. 2003;289:86–94. doi: 10.1016/s0014-4827(03)00254-4. [DOI] [PubMed] [Google Scholar]
- 51.Petroll WM, Cavanagh HD, Jester JV. Assessment of stress fiber orientation during healing of radial keratotomy wounds using confocal microscopy. Scanning. 1998;20:74–82. doi: 10.1002/sca.1998.4950200202. [DOI] [PubMed] [Google Scholar]
- 52.Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- 53.Hay ED. Interaction of migrating embryonic cells with extracellular matrix. Exp. Biol. Med. 1985;10:174–193. [Google Scholar]
- 54.Hegerfeldt Y, Tusch M, Brocker E-B, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, b1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–2130. [PubMed] [Google Scholar]
- 55.Friedl P, Zanker K, Brocker E-B. Cell migration strategies in 3-D extracellular matrix: Differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech. 1998;43:369–378. doi: 10.1002/(SICI)1097-0029(19981201)43:5<369::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 56.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson NC, Clarke AR, Archenhold G. Large-area, high resolution image analysis of composite materials. J Microsc. 1997;185 [Google Scholar]
- 58.Davidson NC, Clarke AR. Extending the dynamic range of fibre length and fibre aspect ratios by automated image analysis. J Microsc. 1999;196:266–272. doi: 10.1046/j.1365-2818.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 59.Ng CP, Swartz MA. Mechanisms of interstitual flow-induced remodeling of fibroblast-collagen cultures. Ann Biomed Eng. 2006;34:446–454. doi: 10.1007/s10439-005-9067-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.