Abstract
This study was undertaken to address the lack of information about tongue functional deformation in relation to jaw movement and muscle activity. Dimensional changes in tongue anterior and base widths, body length and base thickness were measured using six ultrasonic crystals implanted into the tongue in eight Yucatan minipigs. Jaw movements were captured on videotape and digitized, and electromyography (EMG) of tongue intrinsic (verticalis/transversus [V/T], superior and inferior longitudinalis [SL, IL]), extrinsic (genioglossus and styloglossus [GG, SG) and jaw (masseter and digastricus [MA, DI]) muscles were recorded. Signals from these three sources were synchronized. Tongue dimensions showed stereotyped and rhythmic changes during chewing cycles, with the largest changes in the body length and base thickness of the contralateral (non-working) side. The anterior tongue widened during jaw opening while the tongue base widened and thickened during jaw closing. The body lengthening accompanied base widening and ended at early power stroke, while base thickening lasted through most of the power stroke. Significant associations were found between changes of anterior width, body length and base thickness and integrated EMGs of VT, SL, SG, GG, MA and DI, but not IL. Thus, majority of tongue dimensional expansions occur during jaw closing. Intrinsic tongue muscle activities are not correlated more with tongue dimensional changes than are extrinsic tongue and jaw muscle activities.
Keywords: Tongue deformation, Jaw movement, EMG, Ultrasound, Mastication
Introduction
As an incompressible muscular organ with the nature of a hydrostat,1 the tongue movement involves complex changes of shape in three dimensions (3D) including simultaneous lengthening and shortening of different regions and a great variety of nonlinear movements.2,3 Few studies have related tongue 3D changes to jaw movement or muscle activity, and no study has actually measured internal 3D changes during function. The present study hypothesized that: (1) tongue dimensional changes would show chewing-side differences in timing and amplitude; (2) tongue dimensional changes would be less stereotyped than jaw movements and tongue displacement; (3) tongue dimensional changes would be more correlated with intrinsic than extrinsic tongue and jaw muscle activities.
Materials and methods
Data were collected from eight 12-week-old Yucatan miniature pigs (four of each gender) during naturally chewing of pig chow. The animal use protocol was approved by UW Animal Welfare Office.
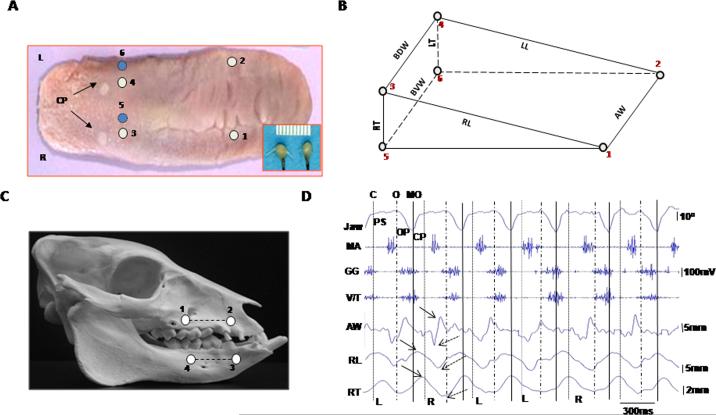
Six piezo ultrasonic crystals were implanted into the tongue to circumscribe a wedge-shaped volume inside the anterior 2/3 of the tongue (Fig. 1A,B). These crystals functioned as tiny ultrasonic transducers that reported the real-time distance changes (in mm) between pairs of crystals with a claimed resolution of 0.02 mm (Sonometric Co. London, Canada. See Fig. 1A, inset). Muscle activities were acquired by inserting pairs of 0.05 mm nickel–chromium wire electrodes into tongue intrinsic (V/T, SL and IL), extrinsic (GG and SG), and jaw (MA and DI) muscles and by recording using a Biopac system (Biopac, Co. Goleta, CA). The accuracy of electrode placement was verified by back stimulations and postmortem dissection. Jaw movements were captured by a digital video camera and digitized using four fluorescent markers glued to upper and lower lips (Fig. 1C). Gape was calculated as segment angulation using a Motus system (Vicon/Peak Co. Centennial, CO). Signals from these three sources were synchronized through an Event & Video Control and Analog to Digital Interface pertaining to the Motus system. Jaw movements were recorded before as well as after the implantations of ultrasonic transducers and EMG electrodes.
Fig. 1.
– Somomicrometric configuration. (A): crystal implantation. Numbers indicate crystal sites (circles). CP: circumvallate papillae; R and L: right and left. Inset: a pair of piezo ultrasonic crystals. (B): wedge-shaped volume circumscribed by six crystals. AW: anterior width (1–2); BDW and BVW: base dorsal and ventral widths (3–4 and 5–6); RL and LL: right and left body lengths (1–3 and 2–4); RT and LT: right and left base thicknesses (3–5 and 4–6). (C): fluorescent markers (4 dots) and segments (2 lines). (D): synchronized raw recordings of jaw movement (top Ch.), EMG activity (middle 3 Chs.) and tongue deformation (bottom 3 Chs.). Dotted, dashed and solid vertical lines indicate the end of closing (C), start of opening (O) and maximal opening (MO). PS, OP and CP present power stroke, opening and closing phases of jaw movement. L and R indicate chewing side. Solid and dotted arrows indicate peak and valley, respectively, of tongue dimensional changes. Jaw: jaw movement; MA: masseter, GG: genioglossus; V/T verticalis/transversus; AW: anterior width; RL: right body length; RT: right base thickness.
Sonomicrometric signals from 5 to 15 successive chewing cycles in each pig were low-pass filtered at 30 Hz. Because there was no baseline during chewing, the amplitudes of these signals were calculated by measuring the difference between the peak (maximum) and valley (minimum) from each selected crystal pair (Fig. 1D) and relating this difference to the initial length measured at implantation. The time durations between peaks and valleys were also calculated. EMG signals were band-pass filtered (60–250 Hz). Chewing side was identified by looking at the timing and amplitude differences between bilateral masseter muscles, higher amplitude and delayed onset identified the ipsilateral (working) side.4 Onsets, durations and integrated EMG (IEMG) of each muscle from each chewing cycle were calculated, and values of onsets and durations were further converted to % of the same chewing cycle length measured from jaw movement. From the video, the start of jaw closing, opening and power stroke in each chewing cycle were marked. Since the data were distributed asymmetrically, Kruskall-Wallis H tests were performed to detect differences in onsets and durations of tongue dimensional changes, followed by Mann–Whitney U tests for pair-wise comparisons. Side differences were examined by Mann–Whitney U tests. Spearman's correlations were calculated to detect associations between tongue dimensional changes and IEMG. The significance level was set as p < 0.05.
Results
The maximal jaw opening during chewing was 25°–30° without chewing-side difference. Chewing frequency was 0.44 ± 0.08 Hz, in which jaw opening, closing and power stroke took up 34.2% ± 9.7, 23.5% ± 5.5, and 42.3% ± 7.0, respectively. There was no noticeable difference in jaw movement before and after implantation.
The tongue body length and base thickness changes were stereotyped in accordance with rhythmic jaw movements and EMG bursts, but less regularity was seen in the width changes.
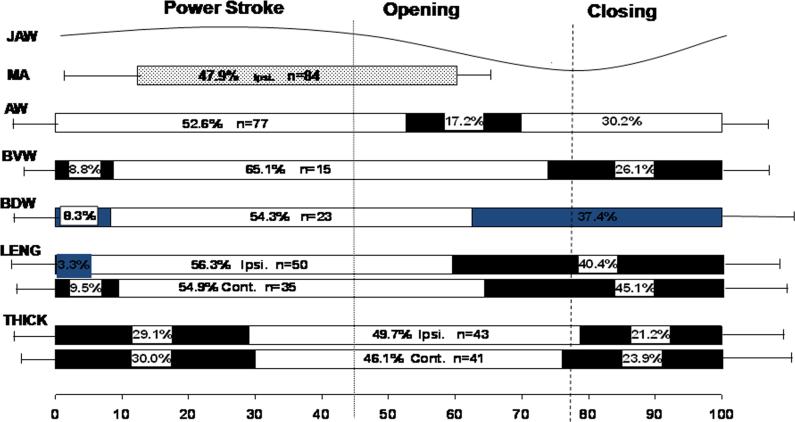
As summarized in Fig. 2, anterior width began increasing in the early jaw opening phase, significantly preceding other dimensions (p < 0.01). The duration of this increase was significantly shorter (17% of chewing cycle length) than those of the length, thickness and posterior width (p < 0.01). Base widths (dorsal and ventral) began increasing later during jaw opening, and continued until early power stroke, similar to body length increase. Base thickness started to increase last at about maximal jaw opening, and lasted until late in the power stroke (>50% of chewing cycle length), significantly longer than body length and all width changes. No chewing-side difference was found in these timings.
Fig. 2.
– Timing comparisons for tongue dimensional changes in relation to jaw movement and ipsilateral masseter activity. Values are represented as a percentage of the same chewing cycle length defined by the horizontal scale line at the bottom (100%). Dotted and dashed vertical lines indicate start of jaw opening and maximal opening, respectively. Bar lengths indicate the mean duration of increasing (solid bars) and decreasing (empty bars) of dimensional changes. The horizontal lines to the left and right of bars indicate 1 S.D. of onset and duration, respectively; n: numbers of chewing cycles analyzed. Ipsi.: ipsilateral side; Cont.: contralateral side. Jaw: jaw movement; MA: masseter; AW: anterior width; BVW: base ventral width; BDW: base dorsal width, LENG: bilateral body lengths; THICK: bilateral base thicknesses.
Peak values of dimensional changes revealed that amplitudes of base thickness and body lengths were significantly larger in the contralateral than the ipsilateral sides (p < 0.05, data not shown). Most significant correlations between the width amplitudes and muscle activities (IEMGs) were negative (r = –0.37 to –0.69, p < 0.05–0.01), particularly for anterior width. The body length amplitudes of both sides were positively associated with IEMG of GG (r = 0.46 to 0.80, p < 0.01) and negatively associated with SL (r = –0.55 to –0.56, p < 0.01). The contralateral thickness amplitudes were positively correlated with IEMGs of MA and V/T (r = 0.51–079, p < 0.01). Thus, deformational amplitudes did not show more or stronger correlations with IEMGs of tongue intrinsic than extrinsic and jaw muscles.
Discussion
A number of studies have indicated a strong linkage between the tongue and jaw movements during chewing, but few studies have addressed actual tongue deformation. The present study revealed that, except for anterior width, the expansion of tongue dimensions during chewing mainly occurs during jaw closing. During the power stroke, the major dimensional expansion is the posterior thickening while the width and length decrease. Patterns of tongue functional deformation not only show a constant time sequence in relation to the jaw movement and muscle activity, but also present unique features which include differences in anterior and posterior width changes, simultaneous body lengthening and base dorsal widening during jaw closing, and larger changes in the contralateral side.
The present results support the first hypothesis in showing amplitude (but not timing) difference between the ipsilateral and contralateral sides, and positive associations of base thickness change with muscle activity in the contralateral side. However, the second hypothesis was not supported because the stereotyped nature of most dimensional changes existed. Lastly, there was no evidence to support the third hypothesis because tongue dimensional changes were similarly associated with tongue intrinsic muscles and with tongue extrinsic and jaw muscles.
Acknowledgments
The authors would like to thank Dr. Susan Herring for her invaluable helps and comments. This study was supported by grant R01DE15659 from NIDCR (Z.J.L.).
REFERENCES
- 1.Kier WM, Smith KK. Tongues, tentacles and trunks: the biomechanics of movement in muscular-hydrostats. Zool J Linn Soc. 1985;83:307–24. [Google Scholar]
- 2.Hiiemae KM, Hayenga SM, Reese A. Patterns of tongue and jaw movement in a cinefluorographic study of feeding in the macaque. Arch Oral Biol. 1995;40:229–46. doi: 10.1016/0003-9969(95)98812-d. [DOI] [PubMed] [Google Scholar]
- 3.Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Intramural mechanics of the human tongue in association with physiological deformations. J Biomech. 1999;32:1–12. doi: 10.1016/s0021-9290(98)00109-2. [DOI] [PubMed] [Google Scholar]
- 4.Liu ZJ, Herring SW. Masticatory strains on osseous and ligamentous components of the temporomandibular joint in miniature pigs. J Orofac Pain. 2000;14:265–78. [PubMed] [Google Scholar]