Abstract
The ability of the skeletal musculature to use amino acids to build or renew constitutive proteins is gradually lost with age and this is partly due to a decline in skeletal muscle insulin sensitivity. Since long-chain omega-3 polyunsaturated fatty acids (LCn–3PUFA) from fish oil are known to improve insulin-mediated glucose metabolism in insulin-resistant states, their potential role in regulating insulin-mediated protein metabolism was investigated in this study. Experimental data are based on a switchback design composed of three 5 week experimental periods using six growing steers to compare the effect of a continuous abomasal infusion of LCn–3PUFA-rich menhaden oil with an iso-energetic control oil mixture. Clamp and insulin signalling observations were combined with additional data from a second cohort of six steers. We found that enteral LCn–3PUFA potentiate insulin action by increasing the insulin-stimulated whole-body disposal of amino acids from 152 to 308 μmol kg−1 h−1 (P = 0.006). The study further showed that in the fed steady-state, chronic adaptation to LCn–3PUFA induces greater activation (P < 0.05) of the Akt–mTOR–S6K1 signalling pathway. Simultaneously, whole-body total flux of phenylalanine was reduced from 87 to 67 μmol kg−1 h−1 (P = 0.04) and oxidative metabolism was decreased (P = 0.05). We conclude that chronic feeding of menhaden oil provides a novel nutritional mean to enhance insulin-sensitive aspects of protein metabolism.
As mammals progress from the neonate to maturity there is a decline in the sensitivity of muscle protein metabolism to insulin (Wray-Cahen et al. 1997; Davis et al. 1998). This developmental insulin resistance may have an impact on problems encountered during the ageing process, including sarcopaenia, or the ability to resist clinical trauma, such as surgical recovery, sepsis and acquired immunodeficiency syndrome (Vessby et al. 1994; Garlick et al. 1998). Furthermore, the development of new approaches that facilitate the maintenance of muscle mass during adulthood are also relevant to those addressing the obesity epidemic because muscle mass defines, in part, the basal metabolic rate.
In livestock, when such a developmental decline in protein metabolism occurs, it does so with both metabolic and economic consequences. Inefficient use of dietary amino acids for muscle protein deposition leads to high production costs and environmental nitrogen pollution. In this respect, genetic selection has been widely used to increase efficiency in production, but this approach requires that improvements be made over long periods of time. In addition, the use of biotechnology and genetic engineering in the food chain to enhance growth efficiency has intensified concerns and controversies amongst the general public.
Improved understanding of factors that mediate the down-regulation of insulin action on the musculature with age, and that render this tissue less sensitive to interventions that modulate protein metabolism, growth and muscle mass, is of paramount importance to both human health and applied animal science. In this respect, up-regulation of skeletal muscle insulin sensitivity by long-chain n–3 polysunaturated fatty acids (LCn–3PUFA) after their incorporation into muscle membranes has been shown in pathophysiological states, such as obesity, type 2 diabetes and high-fat feeding-induced insulin resistance (Storlien et al. 1987; Simopoulos, 1991; Storlien et al. 1991; Borkman et al. 1993; Liu et al. 1994; Pan et al. 1995). The ability of these fatty acids to improve glucose utilization in response to insulin stimulation is exerted via increases in both the abundance of muscle insulin receptors and the insulin binding capacity (Liu et al. 1994). Previous work has focused on the ability of LCn–3PUFA to enhance muscle insulin sensitivity to glucose in the context of certain pathological states, but the capacity of LCn–3PUFA to act as regulators of protein metabolism in healthy models has not yet been investigated. Here, we postulate that enteral LCn–3PUFA, once incorporated into muscle membrane phospholipids of steers, enhance the sensitivity of muscle to insulin action, thereby promoting protein anabolism by reducing catabolic pathways and enhancing the activation of the insulin signalling pathway.
Methods
Animals, feeding and treatments
Six Red Angus × Simmental crossbred steers were divided into two groups based on body weight that were used to compare two treatments over three experimental periods of 35 days according to a double switchback design. The animals were not implanted with growth promoters and they weighed 291 kg at the beginning of the experiment. The two iso-energetic treatments (Table 1) consisted of: (1) a control oil mixture based on 60% cotton seed: 40% extra virgin olive oils (0% menhaden oil), having a similar fatty acid profile to beef tallow but less saturated in order to assist solubility for infusion; and (2) 4% menhaden oil, providing a high amount of LCn–3PUFA. Cotton seed oil was from Cedar Vale Natural Health Products, Cedar Vale, KS, USA; extra virgin olive oil was the first-cold press from Olivia, Imperial Foods Inc. Québec, QC, Canada; menhaden oil was alkali refined bleached and pressed, 500 p.p.m. ethoxyquin, from Omega Protein Inc., Reedville, VA, USA.
Table 1.
Fatty acid composition of experimental oils expressed as percentage of total
| Control oil | Menhaden oil | |
|---|---|---|
| C14: 0 | 0.4 | 10.7 |
| C14: 1 n–5 | nd | 0.4 |
| C16: 0 | 18.1 | 17.5 |
| C16: 1 n–7 | 0.8 | 13.4 |
| C18: 0 | 2.9 | 3.3 |
| C18: 1 | 40.6 | 10.3 |
| C18: 2 n–6 | 35.7 | 1.5 |
| C18: 3 n–6 | 0.1 | 0.5 |
| C18: 3 n–3 | 0.6 | 1.6 |
| C18: 4 n–3 | nd | 4.2 |
| C20: 0 | 0.4 | 0.2 |
| C20: 1 | 0.3 | 1.0 |
| C20: 2 n–6 | nd | 0.3 |
| C20: 3 n–6 | nd | 0.3 |
| C20: 3 n–3 | nd | 0.3 |
| C20: 4 n–6 | nd | 0.8 |
| C20: 4 n–3 | nd | 2.0 |
| C20: 5 n–3 | nd | 13.5 |
| C22: 0 | 0.2 | 0.1 |
| C22: 1 n–9 | nd | 0.3 |
| C22: 4 n–6 | nd | 0.1 |
| C22: 5 n–6 | nd | 0.9 |
| C22: 5 n–3 | nd | 2.3 |
| C22: 6 n–3 | nd | 14.4 |
| C24: 1 n–9 | nd | 0.4 |
| SAT | 21.9 | 31.7 |
| PUFA | 36.4 | 42.6 |
| P/S | 1.7 | 1.4 |
| n–3 | 0.6 | 38.2 |
| n–6 | 35.8 | 4.4 |
| n–3/n–6 | 0.02 | 8.8 |
| LCn–3 | nd | 32.5 |
| LCn–6 | nd | 2.4 |
SAT, total saturated fatty acids; PUFA, total polyunsaturated fatty acids; P/S, polyunsaturated/saturated fatty acid ratio; n–3, total n–3 fatty acids; n–6, total n–6 fatty acids; n–3/n–6, ratio of total n–3 fatty acids/total n–6 fatty acids; LCn–3, 20: 5n–3 + 22: 5n–3 + 22: 6n–3; LCn–6, 20: 3n–6 + 20: 4n–6; nd, not detected.
Dietary oils were continuously infused into the abomasum at 4% of dry matter intake using peristaltic pumps (Patrol enteral pump; Abbott Laboratories, Chicago, IL, USA) over the 35 consecutive days of each experimental period. In the switchback design, the two different treatment sequences, once adequately allocated in each block, allowed testing equally each treatment as follows: for three steers the order was Control→LCn–3PUFA→Control while for the other three the order was LCn–3PUFA→Control→LCn–3PUFA. In total, n = 9 for LCn–3PUFA treatment, and n = 9 for control treatment. The measurements made on each steer are shown in Fig. 1.
Figure 1. Graphic scheme of measurements made on steers during each experimental period of the study.
Each experimental period was allocated days 1–14 for the modification of the fatty acid profile of muscle phospholipids. Performances were evaluated on days 15–25, and the physiological measurements were carried out between days 25 and 35. Order of phenylalanine or bicarbonate infusions were randomised in each period, with a 48 h minimal internal. Additional data from a 2nd cohort of 6 steers originating from a Latin square design were used to analyse insulin-stimulated glucose and amino acid disposal and fed steady-state muscle insulin signalling.
Menhaden and control oils were kept in N2-flushed amber bottles. Peroxide indexes were determined weekly to control the quality of both oils (Chapman et al. 1949) and they were kept lower than 10 mEq kg−1 during the experiment. The experimental proposal and procedures were approved by the Animal Care and Use Committee of Université Laval, and were conducted in accordance with the guidelines of the Canadian Council on Animal Care (Canadian Council on Animal Care, 1993).
At the onset of the experiment, the oil infusion rate was set at 6% of dry matter intake, but this caused reduced intake and so was readjusted to 4% for the remainder of the trial. The oil infusion rate was adjusted based on the weights of the steers at days 1, 15, 28 and 35 of each experimental period. If an animal ate more than its predicted requirement (National Research Council, 2000), in order to minimize fattening, this adjustment was set according to the predicted rather than actual dry matter intake.
Steers were maintained in air-conditioned rooms at 16°C, in tie stalls equipped with rubber mats and bedded with wood shavings. They were fed a total mixed ration typical for the growing stage, programmed for 1.33 kg day−1 of body weight gain (Table 2). Steers were fed ad libitum twice daily, allowing a minimum of 10% refusals. Refusals were weighed daily and sampled twice a week throughout the experiment. A constant ratio of ingredients was maintained by determining weekly dry matter of silages and pelleted concentrates. Crude protein was deliberately fed 15% over requirements to avoid protein limitation in the response to treatments (Table 2). From days 19 to 35 of each experimental period, the animals were restricted to 98% of the previous average 7 day ad libitum intake and fed every 2 h with automated feeders (Fig. 1). Steers were equipped with chronic catheters implanted into the abomasum for oil infusions and into a mesenteric artery for sampling as previously described (Thivierge et al. 2002a). The patency of the abomasal catheter failed for two steers during the experiment. Those animals were then fitted with a rumen fistula. The oil infusions were then performed into the abomasum through the sulcus omasi. A 3–4 week period of postsurgical recovery was allowed before the onset of the experiment.
Table 2.
Feed and chaemical composition of the basal diet on dry matter basis
| Ingredient | % |
|---|---|
| Corn silage | 31 |
| Grass silage | 13 |
| Pelleted concentrates | 56 |
| Wheat | 35 |
| Cracked corn | 32 |
| Soybean meal | 14 |
| Soybean hulls | 13 |
| Dried molasses | 0.94 |
| Urea | 0.87 |
| Salt | 0.75 |
| Calcium oxide | 1.50 |
| Calcium sulphate | 0.20 |
| Dicalcium phosphate | 0.32 |
| Vitamin premix | 0.09 |
| Lignosol | 0.57 |
| Rumensin®1, p.p.m. | 28 |
| Chaemical composition | % |
| Dry matter | 55 |
| Crude protein2 | 15 |
| Acid detergent fibres | 17 |
| Neutral detergent fibres | 28 |
The basal diet was initially formulated to meet 104% of energy and 114% of crude protein requirements (National Research Council, 2000); based on experimental average characteristics of steers and feed analyses it met instead 96% and 112% of net energy for gain and crude protein requirements, respectively. The basal diet supplied more than 97% of daily requirements of essential amino acids with the exception of histidine (92% of requirements) (Fox et al. 1992). To meet this total requirement, 2 g of histidine (feeding grade; ACP Chemicals Inc., Quebec, Canada) were injected daily into the abomasum throughout the experiment; 1Elanco Animal Health Division, Eli Lilly Canada Inc.; 266% rumen-degradable and 34% rumen-undegradable proteins.
The parameters presented in this manuscript such as performances (intake, feed conversion, body weight gain), phenylalanine kinetics measured during the labelled phenylalanine infusion study (plasma and breath gas isotopic enrichments, phenylalanine whole-body flux, and plasma amino acid concentrations), and CO2 production with breath isotopic enrichments measured during the labelled bicarbonate infusion were analysed according to the switchback design as originally planned. The hyperinsulinaemic–euglycaemic–euaminoacidaemic clamps were analysed only for experimental periods 2 and 3 because chronic adaptation to menhaden oil feeding induced unexpectedly high amino acid disposal rates such that the concentration of the amino acid solution was insufficient to maintain the amino acid clamp during experimental period 1. As a result, for the next two experimental periods, the amino acid solution was concentrated by a factor of 2.7 and the length of infusion lines were shortened to 2.44 m to reduce resistance to flow. This provided only six observations per treatment. To increase the number of observations, additional data from a second study were combined with data from the first study to increase the number of observations from 6 to 12 per treatment (Fig. 1). Signalling data from the second study were also combined with data from the first study to increase the number of observations from 9 to 14 per treatment. These additional data were obtained from a dose–response study involving six similar Red Angus × Simmental crossbred steers that were infused graded amounts of menhaden oil over 42 day experimental periods using a double 3 × 3 Latin square design, but whose care and feeding were otherwise similar to that in the first study. Only data from the 0 and 4% menhaden oil treatment were included herein. According to the Latin square design, the six observations per treatment were measured on two steers at each of the three experimental periods (Fig. 1). The steers were about 6 weeks younger and weighed 275 kg on average at the onset of the experiment. Data for glucose and amino acid disposal monitored during clamps were normalized for body weight as outlined previously.
In vivo assays
Hyperinsulinaemic–euglycaemic–euaminoacidaemic clamp procedure
Clamps were performed during steady feeding conditions achieved through 98% restricted 2 h feeding (Lapierre et al. 1999; Thivierge et al. 2002b). Hyperinsulinaemic – euglycaemic – euaminoacidaemic clamps were conducted according to the procedures described by Wray-Cahen et al. (1997). Insulin stock solution (1 mg ml−1) was freshly prepared daily by dissolving bovine lyophilized insulin (Sigma I-5500, 27 IU mg−1, Sigma Chaemical, St Louis, MO, USA) in 0.01 n HCl and then mixing with sterile physiological saline containing 4% filter-sterilized bovine plasma. The insulin infusates were individually prepared by diluting the appropriate stock amount with physiological saline, according to individual weight of the steers. Insulin sensitivity was assessed by conducting a 40 mU kg−1 h−1 insulin clamp. To establish basal concentrations of glucose and amino acids, using branched-chain amino acids as an index (BCAA), four blood samples were acquired every 10 min and immediately analysed. Blood glucose was quantified by peroxidase reaction (YSI 2300 STAT Plus analyser; Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma concentrations of total BCAA were measured by analysis of leucine, isoleucine and valine deamination by leucine dehydrogenase with stoichiometric reduction of NAD measured by spectrophotometry (Beckett et al. 1996). Once glucose and amino acids baselines were established, the clamp was initiated. During clamps, plasma samples were taken every 10 min and they were immediately analysed for glucose and branched-chain amino acid concentration. Dextrose (50% sterile; CDMV, St Hyacinthe, Quebec, Canada) and a complete sterile solution of l-amino acids with a composition similar to that of the bovine muscle mixed proteins (Table 3) were infused i.v. into a jugular vein (Plum Lifecare Pumps, series 1.6; Abbott Laboratories, Chicago, IL, USA) to maintain circulating glucose and branched-chain amino acid concentrations at ± 10% baseline values. Clamps were maintained over a 180 min period on average; a period of 120 min was required to reach steady state glucose and amino acid utilization rates, and steady state disposal of glucose and amino acids was monitored during an additional 60 min. Disposals are presented following normalization for body weight. During the last 60 min of each clamp (steady state period), four blood samples were collected at 20 min intervals, analysed for glucose, centrifuged and the plasma frozen at −20°C until later determination of insulin and amino acid concentrations. Limited substitutions of tyrosine by phenylalanine and cysteine by methionine in the auxiliary amino acid solution were carried out to avoid oversupply of phenylalanine and methionine during clamps due to their low whole body turnover rate (Table 3).
Table 3.
Composition of the l-amino acid mixture with a similar composition to the bovine muscle mixed proteins used during the 40 mU kg−1 h−1 hyperinsulinaemic–euglycaemic–euaminoacidaemic clamps
| l-Amino acid | g l−1 |
|---|---|
| Alanine | 11.1 |
| Aspartic acid | 2.8 |
| Arginine | 12.2 |
| Glutamic acid | 4.5 |
| Glycine | 9.8 |
| Histidine | 6.8 |
| Isoleucine | 9.3 |
| Leucine | 15.1 |
| Lysine | 16.8 |
| Phenylalanine1 | 11.2 |
| Proline | 8.9 |
| Methionine2 | 5.3 |
| Serine | 7.3 |
| Threonine | 8.1 |
| Tryptophane | 2.3 |
| Tyrosine1 | 0.2 |
| Valine | 9.9 |
l-Amino acids medical grade (Sigma-Aldrich, ON, Canada) were used with solution pH adjusted to pH 7.40 using NaOH and osmolarity to 300 mosmol l−1 (150 mm) using NaCl; solutions sterilized through vacuum 0.22 μm pore size filters. Composition of the bovine muscle mixed protein solution established by averaging data from two publications (McCance et al. 1978; Schweigert, 1987). Glutamine and asparagine were not added to the solution due to solubility and stability problems. 1Tyrosine content was limited due to its low solubility and additional phenylalanine was added supplying 50% of tyrosine requirement. 2Cysteine was partly replaced by methionine that represented 25% of cysteine requirement.
Fed steady-state whole-body amino acid tracer kinetics followed by muscle biopsies
l-[1-13C]Phenylalanine kinetics were conducted during steady nutritional intake of nutrients achieved through 98% restricted 2 h feedings (Lapierre et al. 1999). The kinetics data are independent from those of the clamp and expand the response to LCn–3PUFA to a feeding-induced stimulation. A minimum of 48 h separated the amino acid tracer and the bicarbonate infusion studies enabling the measure of both whole-body flux and oxidation using stable isotopes in large animals (Lapierre et al. 1999). Each measurement was conducted either before or after the other, always respecting the 48 h minimal delay. 13C natural abundance in breath gas during the bicarbonate and the phenylalanine studies represented 1.08519 ± 0.0025889 and 1.08769 ± 0.0022260 atom percent, respectively. The onset of the tracer infusion was preceded by four background samplings of blood and breath gas to determine the isotope natural abundance. Breath gases were sampled using a face mask equipped with a one-way valve bag and were directly transferred into sterile vacutainers in triplicates. Whole body irreversible loss rate of phenylalanine was measured using a continuous 8 h infusion of l-[1-13C]phenylalanine (1.67 μmol kg−1 h−1; 97.9% mol percent excess, Cambridge Isotopes Laboratories, Andover, MA, USA), preceded by a pulse dose injection (1.67 μmol kg−1). During hours 6–8 of the tracer infusion period, five blood and five breath gas samples were taken at 30 min intervals. Blood samples were centrifuged at 4°C and the plasma kept frozen at −20°C until further analyses for amino acids and phenylalanine isotopic enrichments. Breath samples were transferred into sterile vacutainers and analysed in triplicate using an isotopic ratio mass spectrometer. Between 8 and 9 h of the tracer infusion, steers were sedated with a mixture of acepromazine (Atravet, 0.1 mg kg−1, Ayerst Veterinary Laboratories, Guelph, ON, Canada) and Butorphanol (Torbugesic, 0.05 mg kg−1, Ayerst Veterinary Laboratories, Guelph, ON, Canada). Thirty minutes later, a biopsy in longissimus dorsi was performed under local anaesthesia (8 ml lidocaine-HCl 5%, Bioniche Pharma Canada Ltd, Belleville, ON, Canada). Muscle samples were rapidly weighed and immediately frozen in liquid N2. They were kept at −80°C until analysed for membrane phospholipid fatty acid profiles as well as insulin signalling. Signalling data were independent from the clamp data. Sedatives used were chosen to minimize hyperglycaemia and alteration of metabolism after their administration (Adams, 2001). Every steer was sedated similarly between experimental periods in accordance to the protocol outlined above based on their live weight. Unfortunately, 13CO2 isotopic enrichment in breath measured during the l-[1-13C]phenylalanine infusion was barely detectable in most of the steers receiving menhaden oil. Mean treatment effects on whole-body phenylalanine oxidation rate were then approximated assuming nil oxidation rates when the isotopic enrichments of expired CO2 equated to zero; however, we are aware that such an occurrence is not physiologically plausible. This assumption enabled the estimate of whole-body protein synthesis.
Production of CO2 was measured using a similar procedure as described for the phenylalanine kinetics with a 48 h period between isotope studies (Lapierre et al. 1999). A primed (0.252 mmol) continuous (0.180 mmol h−1) infusion of NaH13CO3 (99 atom percent excess; Cambridge Isotope Laboratories, Andover, MA, USA) into a jugular vein was conducted over a 3 h period. Using a face-mask, four background samples were taken at 10 min intervals and then five samples were taken at 30 min intervals during the last 2 h of the labelled bicarbonate infusion. These were all analysed in triplicate for quantification of carbon isotope ratios.
Laboratory assays
Feeds were analysed for total N, acid detergent fibres, and neutral detergent fibres according to the Association of Official Analytical Chemists (1990). Plasma insulin was analysed by radioimmuno assay (Lapierre et al. 1992; Thivierge et al. 2005) using 125I-labelled porcine insulin and a guinea pig anti-bovine insulin serum (intra-assay c.v. 9%; interassay c.v. 9%). Plasma amino acid concentrations were determined by HPLC (Waters, Alliance system) with precolumn derivatization according to the Pico-Tag procedure as previously detailed (Thivierge et al. 2005).
Isotopic enrichments of phenylalanine in plasma were determined after the conversion of phenylalanine into the n-propyl ester heptafluorobutyramide derivative (Thivierge et al. 2005), and analysed by gas chromatography–mass spectrometry (HP 6890 gas chromatograph; Hewlett Packard Co., Palo Alto, CA, USA) coupled to a quadrupole mass spectrometer model 5973 with mass selective detector operating in the negative chaemical ionization mode. Selective ion monitoring was carried out at m/z 383, 384. The 13C/12C isotopic ratio of the breath CO2 was measured in triplicates on an isotope ratio mass spectrometer (IsoPrime, GV Instruments Ltd, Manchester, UK) monitoring for masses 44, 45 and 46 in continuous flow mode. The Isoprime was interfaced to a multifunctional head space analyser (Multiflow Bio, GV Instruments Ltd) configured for breath sample analysis and equipped with a Gilson autosampler (Gilson 222XL). The international standard for carbon was Peedee Belemnite carbonate (PDB).
Fatty acid composition of phospholipid fractions were measured by gas chromatography (Julien, et al. 2006), as were enteral oils (Chouinard et al. 1999). Approximately 150 mg of frozen longissimus dorsi samples were used for the present study. Lipids were extracted along with internal standards (C:15, Avanti Polar Lipids, Alabaster, AL, USA) in a chloroform–methanol (C-M) mixture (2: 1, by volume). Extracted lipids were then weighed and dissolved in a chloroform–methanol mixture (3: 1, by volume). Polar lipids (phospholipids, i.e. phosphatidylcholine, phosphatidyethanolamine, phosphatidylinositol, phosphatidylserine and sphingomyelin) were separated by thin-layer chromatography (TLC; Silica Gel H, 250 μm, Analtech Inc, Newark, DE, USA) using an isopropyl-ether–acetic acid mixture (96: 4, by volume). Fractions were then recovered in individual glass tubes and direct transesterification was performed by adding acetyl chloride (Lepage et al. 1986). Fatty acid methyl esters of enteral oils were prepared by base-catalysed transmethylation (Chouinard et al. 1999). Fatty acid methyl esters of phospholipids were analysed by gas chromatography using Hewlett-Packard 5890, series II (Hewlett-Packard, Toronto, Canada) equipped with a fused silica column (DB23; 30 m, 0.25 mm internal diameter, 0.25 μm film, Agilent Technologies, Mississauga, Canada), helium as carrier gas, a split ratio of 1: 72, a flow of 0.72 ml min−1, and a coupled flame ionization detector (FID). The fatty acid methyl esters were identified by comparison with retention times of a Supelco 37 component FAME mix (Supelco Inc., Bellefonte, PA, USA) and by using one internal standards (C:15, Avanti Polar Lipids, Alabaster, AL, USA), and expressed in mg of fatty acids per 100 g of wet tissue, or as the percentage of total fatty acids. Fatty acid methyl esters of enteral oils were similarly analysed by gas chromatography without using an internal standard.
Western blot analysis was performed essentially as previously described (Tremblay & Marette, 2001). In brief, muscle homogenates (50 μg) were subjected to SDS- PAGE (7.5% gel) and electrophoretically transferred to polyvinyldiene difluoride (PVDF) filter membranes for 2 h. PVDF membranes were then blocked for 1 h at room temperature with buffer I (50 mmol l−1 Tri-HCL, pH 7.4, 150 mmol l−1 NaCl) containing 0.04% NP-40, 0.02% Tween-20, and 5% non-fat milk. This was followed by overnight incubation at 4°C with primary antibodies against GLUT 4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), or Akt S473, mTOR S2448, 4E-BP1 S65, and S6K T389 (all from Cell Signalling Technology Inc., Danvers, MA, USA). The PVDF membranes were then washed for 30 min followed by 1 h incubation in buffer I containing 1% BSA and either anti-mouse or anti-rabbit immunoglobulin G conjugated to horseradish peroxidase. The PVDF membranes were then washed for another 30 min in buffer I and the immunoreactive bands were detected by the enhanced chemiluminescence method. Following this, the PVDF membranes were stripped in β-mercaptoethanol for 45 min at 70°C then probed for total proteins using antibodies against Akt1/2 (Santa Cruz Biotechnology), or mTOR, S6K and 4E-BP1 (all from Cell Signalling Technology) in the same manner as outlined above.
Calculations
Whole-body irreversible loss rate of phenylalanine
Whole body (WB) irreversible loss rate (ILR) was calculated by the isotopic dilution of the tracer corrected for the tracer infusion rate:
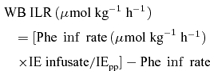 |
where IEpp represents phenylalanine isotopic enrichment (IE) in the arterial plasma precursor pool. Isotopic enrichments are presented as mol percent excess (MPE) and were calculated according to Campbell (1974).
 |
where IE in breath CO2 are presented as atom percent excess (APE). The partial recovery of labelled CO2, which is consecutive to tissue sequestration, represented on average 0.796 ± 0.001215 of the labelled bicarbonate infused in the current study, similar to values in sheep (Ram et al. 1999).
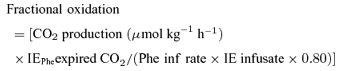 |
 |
Statistical analyses
Experimental data analysed according to the switchback design
Data on fatty acid composition of muscle phospholipid fractions (Tables 4 and 5), performances (Table 6), isotopic enrichments of phenylalanine and breath CO2, whole body flux of phenylalanine (Table 7), and plasma amino acids measured during the phenylalanine kinetic study (Fig. 7) were analysed according to a double switchback design involving six steers divided in two groups, investigating two treatments over three experimental periods using the Mixed procedures of SAS (SAS, 2000). The model included block, period, and treatment as fixed effects and steer (block × sequence) as random effect. Least square means with standard error of the mean (s.e.m.) are presented. When there is no missing value, s.e.m. is similar for both control and menhaden oil treatments; when there is a missing value, as indicated in the footnotes of the tables, the higher s.e.m. value is presented to simplify the presentation of the data. Probabilities were interpreted using type 1 error. One steer in the control treatment had a digestive problem at the time of performance measurements and again during kinetics. This resulted in missing data for performance, kinetics and insulin signalling. Analyses of the membrane phospholipid fractions were conducted on the muscle biopsy of that animal as this parameter does not relate to feed intake. The least square means of isotopic enrichments of breath CO2 measured during the l-[1-13C]phenylalanine kinetic study were estimated from the switchback ANOVA model described above and were further tested for the difference from zero according to Student's t statistic (Table 7).
Table 4.
Sums of fatty acids (% by weight) of longissimus dorsi phospholipid fractions of steers infused for 35 days with control or menhaden oil rich in LCn–3PUFA
| Control oil | Menhaden oil | s.e.m. | P-value | |
|---|---|---|---|---|
| Phosphatidylcholine | ||||
| SAT | 33.4 | 33.8 | 0.7 | ns |
| PUFA | 63.7 | 74.9 | 2.2 | < 0.01 |
| P/S | 1.9 | 2.2 | 0.1 | 0.04 |
| n–3 | 11.5 | 23.8 | 2.0 | < 0.01 |
| n–6 | 44.5 | 32.2 | 1.9 | < 0.01 |
| n–3/n–6 | 0.3 | 0.8 | 0.1 | < 0.01 |
| LCn–3 | 10.8 | 22.6 | 1.9 | < 0.01 |
| LCn–6 | 5.3 | 7.2 | 0.3 | < 0.01 |
| Phosphatidylethanolamine | ||||
| SAT | 26.4 | 28.2 | 0.9 | ns |
| PUFA | 84.3 | 92.4 | 3.0 | 0.06 |
| P/S | 3.2 | 3.3 | 0.2 | ns |
| n–3 | 29.9 | 39.3 | 2.9 | 0.01 |
| n–6 | 35.2 | 26.1 | 2.4 | < 0.01 |
| n–3/n–6 | 1.0 | 1.6 | 0.2 | < 0.01 |
| LCn–3 | 29.9 | 39.3 | 2.9 | 0.01 |
| LCn–6 | 16.3 | 14.9 | 0.9 | ns |
| Phosphatidylinositol | ||||
| SAT | 52 | 50 | 1.1 | ns |
| PUFA | 45.9 | 49.9 | 1.9 | ns |
| P/S | 0.9 | 1.0 | 0.1 | ns |
| n–3 | 13.5 | 18.4 | 1.5 | 0.02 |
| n–6 | 24.7 | 21.8 | 1.4 | 0.02 |
| n–3/n–6 | 0.6 | 1.9 | 0.1 | < 0.01 |
| LCn–3 | 13.5 | 18.4 | 1.5 | 0.02 |
| LCn–6 | 13.1 | 13 | 0.7 | ns |
| Phosphatidylserine | ||||
| SAT | 55.3 | 51.5 | 1.9 | ns |
| PUFA | 29.8 | 37.8 | 2.1 | < 0.01 |
| P/S | 0.6 | 0.7 | 0.1 | 0.03 |
| n–3 | 6.1 | 17.5 | 1.8 | < 0.01 |
| n–6 | 22.8 | 16.4 | 1.6 | 0.01 |
| n–3/n–6 | 0.3 | 1.2 | 0.2 | < 0.01 |
| LCn–3 | 6.1 | 17.5 | 1.8 | < 0.01 |
| LCn–6 | 5.2 | 6.2 | 1.2 | ns |
Least square means ±s.e.m.; n = 9 observations per treatment resulting from testing 2 treatments over 3 experimental periods in 6 steers according to a switchback design; SAT, total saturated fatty acids; PUFA, total polyunsaturated fatty acids; P/S, polyunsaturated/saturated fatty acid ratio; n–3, total n–3 fatty acids; n–6, total n–6 fatty acids; n–3/n–6, ratio of total n–3 fatty acids/total n–6 fatty acids; nd, not detected; ns, not significant.
Table 5.
Fatty acid composition (% by weight) of longissimus dorsi phospholipid fractions of steers infused for 35 days with control or menhaden oil rich in LCn–3PUFA
| Control oil | Menhaden oil | s.e.m. | P-value | |
|---|---|---|---|---|
| Phosphatidylcholine | ||||
| 14: 0 | 0.5 | 0.4 | 0.2 | ns |
| 16: 0 | 27.3 | 27.7 | 0.5 | ns |
| 16: 1 n–7 | 0.4 | 0.9 | 0.2 | 0.04 |
| 18: 0 | 5.6 | 5.7 | 0.4 | ns |
| 18: 1 | 10.3 | 9.3 | 0.6 | ns |
| 18: 2 n–6 | 39.4 | 24.9 | 1.9 | < 0.01 |
| 18: 3 n–3 | 0.7 | 1.2 | 0.1 | < 0.01 |
| 20: 3 n–6 | 1.1 | 1.4 | 0.1 | 0.03 |
| 20: 4 n–6 | 4.2 | 5.8 | 0.5 | < 0.01 |
| 20: 5 n–3 | 7.6 | 17.7 | 1.6 | < 0.01 |
| 22: 5 n–3 | 1.7 | 1.8 | 0.2 | ns |
| 22: 6 n–3 | 1.2 | 3.3 | 0.2 | < 0.01 |
| Phosphatidylethanolamine | ||||
| 14: 0 | nd | nd | ||
| 16: 0 | 3.7 | 3.6 | 0.5 | ns |
| 16: 1 n–7 | nd | nd | ||
| 18: 0 | 22.7 | 24.6 | 0.8 | ns |
| 18: 1 | 8.6 | 6.3 | 0.6 | 0.01 |
| 18: 2 n–6 | 19.1 | 11.1 | 1.7 | < 0.01 |
| 18: 3 n–3 | nd | nd | ||
| 20: 3 n–6 | 1.4 | 0.8 | 0.3 | 0.04 |
| 20: 4 n–6 | 14.9 | 14.1 | 0.7 | ns |
| 20: 5 n–3 | 19.9 | 26.1 | 2.9 | 0.07 |
| 22: 5 n–3 | 6.2 | 4.4 | 0.4 | 0.02 |
| 22: 6 n–3 | 4.1 | 8.5 | 0.6 | 0.01 |
| Phosphatidylinositol | ||||
| 14: 0 | nd | nd | ||
| 16: 0 | 4.4 | 6.6 | 0.6 | 0.02 |
| 16: 1 n–7 | nd | nd | ||
| 18: 0 | 47.5 | 43.4 | 1.3 | 0.05 |
| 18: 1 | 9.7 | 9.9 | 0.6 | ns |
| 18: 2 n–6 | 11.9 | 8.5 | 0.9 | 0.03 |
| 18: 3 n–3 | nd | nd | ||
| 20: 3 n–6 | 2.9 | 3.0 | 0.4 | ns |
| 20: 4 n–6 | 10.2 | 9.9 | 0.4 | ns |
| 20: 5 n–3 | 7.7 | 9.6 | 1.1 | 0.09 |
| 22: 5 n–3 | 4.0 | 5.4 | 0.4 | 0.03 |
| 22: 6 n–3 | 1.9 | 3.6 | 0.3 | < 0.01 |
| Phosphatidylserine | ||||
| 14: 0 | nd | nd | ||
| 16: 0 | 8.1 | 5.7 | 1.0 | ns |
| 16: 1 n–7 | nd | nd | ||
| 18: 0 | 47.3 | 45.8 | 1.9 | ns |
| 18: 1 | 15.9 | 14.3 | 1.6 | ns |
| 18: 2 n–6 | 17.8 | 10.1 | 1.1 | < 0.01 |
| 18: 3 n–3 | nd | nd | ||
| 20: 3 n–6 | 1.7 | 2.1 | 0.6 | ns |
| 20: 4 n–6 | 3.5 | 4.1 | 0.9 | ns |
| 20: 5 n–3 | 1.1 | 3.7 | 0.6 | 0.01 |
| 22: 5 n–3 | 2.7 | 6.2 | 0.6 | < 0.01 |
| 22: 6 n–3 | 1.9 | 8.0 | 0.6 | < 0.01 |
Least square means ±s.e.m.; n = 9 observations per treatment resulting from testing 2 treatments over 3 experimental periods in 6 steers according to a switchback design; nd = not detected; ns, not significant.
Table 6.
Performance of growing steers infused for 35 d with control or menhaden oil rich in LCn–3PUFA
| Control oil | Menhaden oil | s.e.m. | P-value | |
|---|---|---|---|---|
| Weight (kg) | 420 | 416 | 17 | ns |
| Body weight gain (kg d−1) | 1.32 | 1.29 | 0.12 | ns |
| Dry matter intake (kg d−1) | 7.7 | 7.0 | 0.2 | 0.05 |
| Feed intake to gain ratio | 6.57 | 5.40 | 0.75 | 0.17 |
Least square means ±s.e.m.; n = 9 observations per treatment resulting from testing 2 treatments over 3 experimental periods in 6 steers according to a switchback design. One missing value for the control treatment (n = 8), the higher s.e.m. value is presented.
Table 7.
Arterial plasma concentrations, isotopic enrichments (IE), kinetic parameters of whole-body irreversible loss rate (ILR) of phenylalanine and isotopic enrichments of breath CO2 in steers infused for 35 days with control or menhaden oil rich in LCn–3PUFA
| Control oil | Menhaden oil | s.e.m. | P-value | |
|---|---|---|---|---|
| Phenylalanine concentration (μm) | 61.2 | 66.0 | 2.8 | ns |
| Phenylalanine IE MPE1 | 1.71 | 2.47 | 0.21 | 0.03 |
| Whole-body ILR (μmol kg−1 h−1) | 84.8 | 65.0 | 6.6 | 0.04 |
| Breath CO2 IEPhe2 APE1 | 0.001888* | 0.000475†,‡ | 0.00044 | 0.05 |
| Fractional oxidation | 0.134 | 0.034 | 0.040 | 0.10 |
| Oxidation (μmol kg−1 h−1) | 12.4 | 2.2 | 3.3 | 0.05 |
| Whole-body protein synthesis (μmol kg−1 h−1) | 74.3 | 62.8 | 7.4 | ns |
| CO2 production (μmol kg−1 h−1) | 8662 | 7948 | 1061 | ns |
| Breath CO2 IEbic3 APE | 0.004494 | 0.004692 | 0.000519 | ns |
Least square means ±s.e.m.; n = 9 observations per treatment resulting from testing 2 treatments over 3 experimental periods in 6 steers according to a switchback design. One missing value for the control treatment (n = 8), the higher s.e.m. value is presented.
Mol percent excess or atom percent excess.
Isotopic enrichments of breath CO2 measured on samples taken during the l-[1-13C]phenyalalanine kinetic study.
Isotopic enrichments of breath CO2 measured on samples taken during the NaH13CO3 kinetic study.
Isotopic enrichment in control steers is different from zero according to a P = 0.002.
isotopic enrichments of menhaden oil-treated steers are not different from zero according to a P = 0.31.
Close to the precision of the isotopic ratio mass spectrometer.
Figure 7. Plasma concentrations of amino acids measured during whole-body phenylalanine kinetics in steers infused with control or menhaden oil for 35 days.
Total, the sum of total amino acids; EAA, the sum of essential amino acids, i.e. Arg, His, Ile, Leu, Lys, Met, Phe, Thr, Val; NEAA, the sum of non-essential amino acids, i.e. Ala, Asp, Glu, Gly, Pro, Ser, Tyr; BCAA, the sum of branched-chain amino acids, i.e. Ile, Leu, Val; Not OX, the sum of amino acids the most preserved from oxidation in the bovine, i.e. His, Met, Phe; N shuttles, amino acids shuttling N between tissues of the body, i.e. Ala, Asp, Glu. *P≤ 0.05; †P≤ 0.10; n = 9 observations per treatment resulting from testing 2 treatments over 3 experimental periods in 6 steers according to a switchback design; n = 8 for the control treatment.
Regression analysis
Regression between feed conversion (Fig. 2), designated as the dependent variable, and LCn–3PUFA percentage in the phosphatidylcholine fraction, designated as the independent variable, was conducted using the regression procedure of SAS according to a second degree polynomial model.
Figure 2. Regression between feed conversion and the content of LCn–3PUFA in phosphatidylcholine of longissimus dorsi muscle.
Regression between feed conversion is expressed as kg of dry matter intake per day required to gain a kg of body weight, and the content of LCn–3PUFA as a percentage. Y= 0.0165x2− 0.6993x+ 12.081; R2= 0.62; P = 0.02.
Experimental plus additional data – (insulin clamp and insulin signalling parameters)
The absolute disposal rate of glucose and amino acids in response to 40 mU kg−1 h−1 insulin clamp (Fig. 5), with baseline and clamped insulin concentrations, were analysed according to a complete randomized block design testing 12 steers in total per treatment (comprising two cohorts of 6 steers for each 0 and 4% menhaden oil treatment), using data from this experiment and a second experiment as outlined in Methods. This analysis was conducted using the mixed procedures of SAS and the model included treatment as fixed effect and steer (block) as random effect. Probabilities were interpreted using type 1 error.
Figure 5. Absolute net disposal rate of amino acids and glucose in response to 40 mU kg−1 h−1 insulin clamp in steers enterally infused with control or menhaden oil.
Error bars represent 10% baseline error within which euglycaemia and euaminoacidaemia were maintained. These plots combine glucose and amino acid disposal monitored during the clamp conducted in experimental periods 2 and 3 of the switchback design (providing n = 6 per treatment in total) with additional data from a 2nd cohort of 6 steers originating from a Latin square design that also provide n = 6 per treatment to yield n = 12 per treatment for a total of 24 observations. Details are provided in Methods and Fig. 1.
Data on plasma glucose, branched-chain amino acids, and physiological amino acids arising from the insulin-clamp protocol comparing the baseline with the clamped period concentrations (Figs 3 and 4) were analysed for the difference from zero using Student's paired t statistic (SAS, 2000). The comparison between the control and the menhaden oil feeding for the clamped plasma amino acid concentrations, during the steady hour of the clamp (Fig. 4), were also analysed for the difference from zero using Student's paired t test.
Figure 3. Plasma concentrations of branched-chain amino acids (A) and glucose (B) during the baseline and the steady hour of the 40 mU kg−1 h−1 insulin clamp in steers infused with control or menhaden oil for 35 days.
These plots combine glucose and branched-chain amino acid concentrations measured during clamps conducted in experimental periods 2 and 3 of the switchback design (providing n = 6 per treatment in total) with additional data from a 2nd cohort of 6 steers originating from a Latin square design that also provide n = 6 per treatment to yield n = 12 per treatment for a total of 24 observations. Details are provided in Methods and Fig. 1.
Figure 4. Plasma concentrations of individual amino acids during the baseline and the steady-state hour of the 40 mU kg−1 h−1 insulin clamp in steers infused with control or menhaden oil for 35 days.
†Difference with a P≤ 0.05 between baseline and clamped amino acid concentrations; *difference with a P≤ 0.05 for control versus menhaden oil treated steers between amino acid concentrations measured during the steady-state hour of the clamp. These plots combine amino acid concentrations measured during the clamp conducted in experimental periods 2 and 3 of the switchback design (providing n = 6 per treatment in total) with additional data from a 2nd cohort of 6 steers originating from a Latin square design that also provide n = 6 per treatment to yield n = 12 per treatment for a total of 24 observations. Details are provided in Methods and Fig. 1.
The mean value of fold change in the phosphorylation of insulin signalling intermediates and in the protein amount of GLUT4 transporter (Fig. 8) in menhaden oil fed steers was compared to the mean control value using a one-sample t test (Montgomery, 2001).
Figure 8. Representative gels of GLUT4 protein and insulin signalling intermediates of longissimus dorsi muscle for the oil treatment sequence of the switchback design ‘control–LCn–3PUFA–control’.
A, phosphorylation state with protein amount of PKB on its Ser473 residue (P = 0.03); B, phosphorylation state with protein amount of mTOR on its Ser2448 residue (P = 0.06), S6K1 on its Thr389 residue (P = 0.04), and 4E-BP1 on its Ser65 residue (P = 0.10), respectively; C, GLUT4 protein abundance (P = 0.08). This data set combines signalling data from muscle biopsies carried out in experimental periods 1, 2 and 3 of the switchback design (providing n = 9 per treatment in total with n = 8 for the control treatment) with additional signalling data from a 2nd cohort of 6 steers originating from a Latin square design that provides n = 6 per treatment. This yielded n = 13 and 14 observations per treatment for a total of 27 and 28 for control and menhaden oil treatment, respectively. Details are provided in Methods.
Results
Total muscle membrane phospholipid fractions
Membranes of eukaryotes exhibit notable similarities in their composition in lipid fractions (Voelker, 1991). The bovine total muscle membrane phospholipids were not an exception to this as they comprised 68% phosphatidylcholine, 10% phosphatidylethanolamine, 9% phosphatidylinositol, 5% phosphatidylserine and 8% sphingomyelin. However, their fatty acid composition was markedly different among fractions, and was sensitive to lipid dietary intake, in agreement with previous studies using rats (Tables 4 and 5) (Borkman et al. 1993; Liu et al. 1994).
Phosphatidylcholine and phosphatidylethanolamine were, respectively, composed of 69 and 88% of polyunsaturated fatty acids on average. Their C20: 4 n–6 content was low compared with other species (Storlien et al. 1991; Liu et al. 1994). Phosphatidylethanolamine was rich in long-chain n–3 series, and contained substantial amounts of C20: 4 fatty acid compared with other fractions of phospholipids. Menhaden oil feeding increased (P = 0.01) the content of total n–3 fatty acids in these two phospholipid fractions. At the same time, the total amount of n–6 fatty acids was reduced (P < 0.01) by 28%, with C18: 2 n–6 the most affected (−39%, P < 0.01).
Phosphatidylinositol and phosphatidylserine behaved similarly to phosphatidylcholine and phosphatidylethanolamine but the changes in their fatty acid composition occurred to different extents. The fatty acid composition of phosphatidylinositol was the least altered by the enteral fatty acid infusions. The degree of saturation for membrane phospholipids was not sensitive to dietary oils, consistent with observations for non-muscle tissues in other species (MacDonald et al. 1991; Simopoulos, 1991; Connor, 2000).
Growth performance
The animals gained weight at a similar rate (Table 6) but feed intake was reduced by 9% on average (P = 0.05) with enteral menhaden oil. The resulting feed conversion, kg feed intake: kg body weight gain, was not statistically different but did reduce numerically from 6.57 in controls to 5.40 in menhaden oil fed steers. The current study design was not planned to assess performance that would require a larger number of animals. However a second degree polynomial regression, in agreement with the known saturable kinetics associated with feed conversion (Baldwin et al. 1994), suggests that intake required to gain a kilo of weight tends to be reduced when LCn–3PUFA content is increased in the major muscle membrane phospholipid fraction (r2 = 0.63, P = 0.02) (Fig. 2).
Hyperinsulinaemic–euglycaemic–euaminoacidaemic clamps
To assess in vivo insulin sensitivity, euglycaemic–euaminoacidaemic clamps were performed using an insulin infusion rate of 40 mU kg−1 h−1. Branched-chain amino acid concentrations, used as an index of essential amino acids, were maintained at preinfusion levels (Fig. 3), as were the other amino acids (Fig. 4). Glucose concentrations were also maintained at preinfusion levels during the clamp procedure. Corresponding steady-state plasma insulin levels attained during clamps were similar in both groups (158 versus 157 ± 7 μU ml−1), but baseline insulin was lower with menhaden oil feeding as it reached 56 for controls versus 35 ± 7 μU ml−1 for menhaden oil treated steers (P = 0.02). This leaded to a higher increase above baseline of plasma insulin in steers fed enteral menhaden oil as compared to controls. Menhaden oil feeding increased by 108% the amino acid disposal rate during clamps compared with controls (from 152 to 308 μmol kg−1 h−1, P = 0.006; Fig. 5). Insulin-mediated glucose disposal was increased by 37% (P = 0.02; Fig. 5) with menhaden oil feeding, from 643 to 882 μmol kg−1 h−1.
Whole-body amino acid kinetics and plasma amino acids
Kinetics were measured by continuous infusion of labelled phenylalanine when the steers were fed every 2 h. Both plasma phenylalanine isotopic enrichments and concentrations were at steady state during the study (Fig. 6). Phenylalanine enrichments were increased (P = 0.03) from 1.71 to 2.47 MPE with the enteral menhaden oil (Table 7), and whole-body total flux of phenylalanine was reduced from 85 to 65 μmol kg−1 h−1 (P = 0.04). Menhaden oil feeding decreased oxidation from 12.4 to 2.2 μmol kg−1 h−1 (P = 0.05). In control steers, the enrichment of breath 13CO2 derived from the labelled phenylalanine reached 0.001888 APE, but it was 74% lowered to 0.000475 APE with menhaden oil feeding. This latter isotopic enrichment was close to the precision of the isotope ratio mass spectrometer; it was not different from zero (P = 0.31; Table 7), whereas the values for control steers were different from zero (P = 0.002). The total CO2 production was similar for the steers on both enteral oils (Table 7). The resulting protein synthesis remained unchanged to 69 μmol kg−1 h−1 on average.
Figure 6. Isotopic enrichments of arterial plasma phenylalanine between times 380 and 480 min of the phenylalanine kinetic study with plasma phenylalanine concentrations during the same period of time.
Each symbol represents mean values of n = 6–9 steers per treatment ± standard deviation, due to catheter patency. Missing symbols occur when the average data point is unequally represented, such as by only one or two steers of a maximum of 9 per treatment.
Under steady-state feeding, most essential amino acids, non-essential amino acids, branched-chain amino acids and those involved in interorgan nitrogen movements were not different between enteral oils (Fig. 7). However, amino acids with a lower extent of metabolism and oxidized to low rates in cows fed to their requirements, such as phenylalanine, histidine and methionine (Black et al. 1990), were increased (P < 0.05) in arterial plasma with menhaden oil feeding. Augmented concentrations (P < 0.05) of glutamate, proline and arginine were also observed, despite the decline in feed intake. Only valine concentration was reduced (P < 0.05) in menhaden oil treated steers in line with the lowered feed intake.
Cellular insulin signalling
To determine the effect of menhaden oil feeding on postprandial activation of insulin signalling intermediates, especially those involved in the translational control of protein synthesis, Western blot analysis was performed on muscle biopsies taken during the 2 h feed regime. PKB phosphorylation on Ser473, which reflects activation by PI 3-kinase, was significantly increased (1.41 ± 0.17-fold above control, P = 0.03) in muscle of steers fed menhaden oil (Fig. 8A). Similarly, mTOR phosphorylation on Ser2448 tended to be increased (4.74 ± 1.74-fold above control, P = 0.06). This was associated with augmented S6K1 activity, as reflected by increased phosphorylation on Thr389 (2.09 ± 0.46-fold above control, P = 0.04) and a tendency for enhanced 4E-BP1 Ser65 phosphorylation (1.30 ± 0.20-fold above control, P = 0.10) (Fig. 8B). Furthermore, GLUT4 glucose transporter expression also tended to be increased (1.33 ± 0.17-fold above control, P = 0.08) by chronic menhaden oil feeding (Fig. 8C). The results suggest that a higher activation of the Akt–mTOR–S6K pathway underlies, at least in part, the metabolic effects of menhaden oil feeding.
Discussion
Here we demonstrate that long-term enteral menhaden oil infusion potentiates insulin action on protein metabolism by increasing the LCn–3PUFA content in muscle membranes. The beneficial effects of LCn–3PUFA were reflected by increased insulin-stimulated whole body amino acid and glucose disposal, by a reduction in fed steady-state whole-body phenylalanine flux and oxidation, and by promoted insulin signalling pathways in muscle. The use of healthy growing steers in this study provided a model to examine if LCn–3PUFA would up-regulate protein metabolism during a period of otherwise continued decline in muscle insulin sensitivity (Eisemann et al. 1997).
The structure of the membrane phospholipid bilayers is known to be dynamic, with continuous turnover through deacylation and reacylation of fatty acids (MacDonald et al. 1991; Voelker, 1991). The saturation degree of fatty acids within incorporated into the plasma membrane alters membrane fluidity of different tissues (Kamada et al. 1986; Muriana et al. 1992; Daveloose et al. 1993; Liu et al. 1994; Abel et al. 1997). The dietary modulation of membrane fluidity can then have an impact on transmembrane proteins and membrane-bound enzyme activity (Muriana et al. 1992; Liu et al. 1994), and ultimately influence cell metabolism (Liu et al. 1994; Else & Hulbert, 2003). In this study, different fractions of total muscle membrane phospholipids were sensitive to dietary changes, due to the greater affinity of the phospholipid bilayers for n–3 rather than n–6 fatty acids (MacDonald et al. 1991), and the resultant increase in the n–3 fatty acid enrichment (MacDonald et al. 1991; Borkman et al. 1993; Liu et al. 1994). In this context, menhaden oil feeding markedly improved the n–3/n–6 ratio (from 60% to 300% across phospholipid fractions). This n–3 fatty acid enrichment also enhanced the degree of unsaturation (polyunsaturated/saturated ratio) of membrane phospholipids, considered to be an indicator of membrane fluidity (Liu et al. 1994). These changes in composition were reversible within 5 week periods, as has been previously reported for phospholipids in heart mitochondrial membranes (Innis et al. 1981).
Steers fed menhaden oil showed changes in both whole-body protein and glucose dynamics plus improvements in insulin signalling pathways. In terms of whole-body protein dynamics these were assessed in two different ways; first, by tracer phenylalanine kinetics in the fed state and under normal insulinaemia. Second, through the amount of amino acid that needed to be infused to maintain euaminoacidaemia during a hyperinsulinaemic clamp.
In this study, chronic adaptation to menhaden oil feeding in growing steers reduced whole-body phenylalanine ILR when assessed in a steady-fed state. This occurred despite no alteration in arterial phenylalanine concentrations and therefore a decrease in entry of phenylalanine must have occurred (Wolfe, 1992). Arterial plasma phenylalanine entry originates from either digestive absorption or protein breakdown. Although the whole-body fluxes of amino acids are influenced by absorption from the diet, this contribution is minor (< 30%) compared with inflows from protein breakdown (Harris et al. 1992). Therefore, only part of the 23% decrease in ILR will have originated from the modest 9% reduction in feed intake, with most relating to lowered protein breakdown. The net effect of a larger decrease in proteolysis than absorption will also create a more protein anabolic condition with menhaden oil infusion. This conclusion is supported by considerations of the two factors, protein synthesis and amino acid oxidation, that contribute to exit of phenylalanine from the plasma pool. For steers fed control oil, phenylalanine oxidation accounted for 13% of total ILR, but this decreased to values close to zero with menhaden oil feeding. Such markedly lowered oxidation in the presence of only a mild reduction in phenylalanine from intake would again lead to net protein anabolism. This latter statement is further supported by the unaltered whole-body protein synthesis, which sustains the hypothesis that menhaden oil feeding increases whole-body net protein anabolism through a fall in protein breakdown without altering synthesis.
Under conditions of the hyper-insulinaemic clamp, the mehaden oil infused steers required more than double (103%) the amount of mixed amino acids to prevent the hypoaminoacidaemia that occurs during insulin infusions when insulin sensitivity is enhanced (Wray-Cahen et al. 1997). Such an increase in whole-body disposal may be due to either a decline in the entry of amino acids into the plasma as a result of a marked decline in protein breakdown or an increase in the exit of amino acids from the plasma as a result of an increase in protein gain and/or elevated oxidation. Although these could not be separated during the clamp procedure, the fact that phenylalanine disposal was similar to the other amino acids plus the finding from the ILR measurements that phenylalanine oxidation was reduced indicates that the amino acids were directed towards protein anabolism. Such anabolism, if driven by increased protein synthesis would be an energy dependent response (Reeds et al. 1981), and this may be supported by the concomitant 37% increment in whole-body glucose utilization rate. Although a similar plasma insulin concentration was achieved in both groups during the hyperinsulinaemic clamp, steers treated chronically with menhaden oil had lower baseline insulin levels, likely reflecting a higher insulin sensitivity induced by treatment. This resulted in a higher insulin increment above baseline in the menhaden oil treated steers, suggesting that a higher insulin sensitivity of muscles requires less insulin to bind to its receptor to induce the insulin signalling cascade. Whether differences in insulin levels may also be a consequence of altered hepatic removal or pancreatic production remains to be clarified.
In parallel, during fed-steady state, muscle GLUT4 expression tended to increase with chronic menhaden oil feeding. This is in accordance with the augmented insulin sensitivity for glucose disposal in these animals as insulin regulation of glucose metabolism is relayed through a complex signalling cascade initiated by insulin binding to its receptor and activation of PI 3-kinase and the downstream effector, protein kinase, PKB. PKB is implicated in the stimulation of glucose uptake, and it is responsible for the induction of GLUT4 glucose transporter translocation to the sarcolemma (Borkman et al. 1993). Given the changes observed in insulin-mediated glucose disposal rates in response to menhaden oil feeding, it will be interesting to determine the amount of active GLUT4 proteins present at the muscle cell surface in future studies. Such a measurement may provide a more sensitive marker of GLUT4 regulation by LCn–3PUFA.
Apart from its role in glucose uptake, PKB is also a key signalling intermediate for protein synthesis, lying upstream of mTOR, a hormone and nutrient sensing effector which controls two key translation initiation promoters, namely S6K1 and 4E binding protein (BP) of eukaryotic initiation factor eIF4E (Kimball et al. 2003). Therefore, we explored whether the expression and activation state of these intermediates could be linked with the metabolic effects of menhaden oil feeding. In accordance with our hypothesis, that oil enhanced the activation state of the insulin signalling intermediate, PKB, concomitantly promoting a higher activation of the mTOR–S6K1–4E-BP1 pathway. This more sensitive cellular mRNA translational machinery could be behind the enhanced ability of menhaden oil-fed steers, with a LCn–3PUFA content in muscle phosphatidylcholine higher than 15–18%, to use feed for tissue deposition. We hypothesize that a higher activation of the mTOR–S6K1–4E-BP1 nutrient sensing pathway can sustain rapid withdrawal of amino acids from plasma because of improved muscle insulin sensitivity and efficient direction towards assembly of proteins. It should be noted that the mTOR pathway also functions as a checkpoint for amino acid availability and is regulated by amino acid sufficiency (Tremblay et al. 2005). Thus, the hypothesis of a higher activation of mTOR and its downstream effectors by amino acids in this study is in accordance with their lacking ability to enhance phosphorylation of effectors that lie upstream of mTOR in the insulin signalling cascade, such as PKB (Suryawan & Davis, 2003). Whether LCn–3PUFA modulate the ability of amino acids to activate the mTOR pathway remains to be explored. Nevertheless, this study strongly suggests that this nutrient sensing pathway is critical to sustain efficient amino acid disposal in insulin-sensitive steers. Unfortunately, in the current study it was not possible to determine if the anabolic responses observed specifically involve changes in muscle protein synthesis or breakdown pathways. The kinetic whole-body data do not separate the responses from individual tissues and that may involve different regulation. Therefore, in future studies it will be necessary to determine whether such LCn–3PUFA mediated augmentations in PKB activity are also linked to reduced expression of degradative enzymes (e.g. atrogin-1), which may result from enhanced nuclear extrusion of the PKB-regulated transcription factor FOXO-1.
In conclusion, our data show that long-term enteral provision of LCn–3PUFA confers a higher sensitivity to insulin-regulated amino acid and glucose disposal and that these responses probably occur, in part, in skeletal muscle. In a fed steady-state, a more sensitive insulin signalling machinery was present in the skeletal muscle of menhaden oil fed steers, promoting initiation of mRNA translation and protein synthesis with concurrent reduction in whole-body amino acid oxidation, increasing the net availability of amino acids to support anabolism. These findings are pivotal to the establishment of a new functional understanding of growth regulation as this study expands our knowledge of the complex regulation of the nutrient-sensing mechanisms governing muscle metabolism. Furthermore, our findings may also pave the way for future nutritional interventions in early development, the maintenance of muscle mass, and interventions in muscle wasting situations.
Acknowledgments
This work was supported by grants from the Conseil de recherches en pêche et agriculture du Québec (CORPAQ), la Fédération des producteurs de bovins du Québec, the Canadian Institute for Health Research, and the Institute of Nutraceuticals and Functional Food, Faculty of Food Sciences and Agriculture, Laval University. A special thank is extended to Omega Protein Inc., Reedville, VA, who kindly provided the menhaden oil required in these studies. We gratefully thank Mr Richard Prince, Gaston Côté and O'Neil Fecteau for their great animal care and assistance in conducting experiments. Appreciations are also extended to Ms Line Berthiaume who performed the analyses of the fatty acid profile of muscle phospholipid fractions. Mr Gaétan Daigle is thanked for his assistance in the statistical analyses. Ms Thérèse Carbonneau – Ferme Simmental Thérèse et Claude Carbonneau Inc. and Mr Simon Marcotte –Élevage Bovins St-Gilbert are gratefully acknowledged for their great collaboration in providing us crossbred steers corresponding to our specific needs for these studies.
References
- Abel S, Gelderblom WCA, Smuts CM, Kruger M. Thresholds and kinetics of fatty acid replacement in different cellular compartments in rat liver as a function of dietary n-6/n-3 fatty acid content. Prostaglandins Leukot Essent Fatty Acids. 1997;56:29–39. doi: 10.1016/s0952-3278(97)90522-6. [DOI] [PubMed] [Google Scholar]
- Adams HR, editor. Veterinary Pharmacology and Therapeutics. Ames, IA, USA: Iowa State University Press; 2001. [Google Scholar]
- Official Methods of Analysis of the A.O.A.C. Arlington, VA, USA: Association of Official Analytical Chemists; 1990. Association of Official Analytical Chemists. [Google Scholar]
- Baldwin RL, Emery RS, McNamara JP. Metabolic relationships in the supply of nutrients for milk protein synthesis: Integrative modeling. J Dairy Sci. 1994;77:2821–2836. doi: 10.3168/jds.S0022-0302(94)77222-2. [DOI] [PubMed] [Google Scholar]
- Beckett PR, Hardin DS, Davis TA, Nguyen HV, Wray-Cahen D, Copeland KC. Spectrophometric assay for measuring branched-chain amino acid concentrations: application for measuring the sensitivity of protein metabolism to insulin. Anal Biochem. 1996;240:48–53. doi: 10.1006/abio.1996.0329. [DOI] [PubMed] [Google Scholar]
- Black AL, Anand RS, Bruss ML, Brown CA, Nakagiri JA. Partitioning of amino acids in lactating cows: Oxidation to carbon dioxide. J Nutr. 1990;120:700–710. doi: 10.1093/jn/120.7.700. [DOI] [PubMed] [Google Scholar]
- Borkman M, Storlien LH, Pan DA, Jenkins AB, Chishlom DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. 1993;328:238–244. doi: 10.1056/NEJM199301283280404. [DOI] [PubMed] [Google Scholar]
- Campbell IM. Incorporation and dilution values – Their calculation in mass spectrally assayed stable isotope labeling experiments. Bioorg Chem. 1974;3:386–397. [Google Scholar]
- Guide to Care and Used of Experimental Animals. 2. Vol. 1. Ottawa, ON, Canada: Bradda Printing Services; 1993. Canadian Council on Animal Care. [Google Scholar]
- Chapman RA, Mackay K. The estimation of peroxides in fats and oils by the ferric thiocyanate method. J Am Oil Chem Soc. 1949;26:321–325. [Google Scholar]
- Chouinard PY, Corneau L, Barbano DM, Metzger LE, Bauman DE. Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J Nutr. 1999;129:1579–1584. doi: 10.1093/jn/129.8.1579. [DOI] [PubMed] [Google Scholar]
- Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71:171S–175S. doi: 10.1093/ajcn/71.1.171S. [DOI] [PubMed] [Google Scholar]
- Daveloose D, Linard A, Asfi T, Viret J, Christon R. Simultaneous changes in lipid composition, fluidity and enzyme activity in piglet intestinal brush border membrane as affected by dietary polyunsaturated fatty acid deficiency. Biochim Biophys Acta. 1993;1166:229–237. doi: 10.1016/0005-2760(93)90102-f. [DOI] [PubMed] [Google Scholar]
- Davis TA, Burrin DG, Fiorotto ML, Reeds PJ, Jahoor F. Roles of insulin and amino acids in the regulation of protein synthesis in the neonate. J Nutr. 1998;128:347S–350S. doi: 10.1093/jn/128.2.347S. [DOI] [PubMed] [Google Scholar]
- Eisemann JH, Huntington GB, Catherman DR. Insulin sensitivity and responsiveness of portal-drained viscera, liver, hindquarters, and whole body of beef steers weighing 275 or 490 kilograms. J Anim Sci. 1997;75:2084–2091. doi: 10.2527/1997.7582084x. [DOI] [PubMed] [Google Scholar]
- Else PL, Hulbert AJ. Membranes as metabolic pacemakers. Clin Exp Parmacol Physiol. 2003;30:559–564. doi: 10.1046/j.1440-1681.2003.03883.x. [DOI] [PubMed] [Google Scholar]
- Fox DG, Sniffen CJ, O'Connor JD, Russell JB, Soest PJV. A net carbohydrate and protein system for evaluating cattle diets. III. Cattle requirements and diet adequacy. J Anim Sci. 1992;70:3578–3596. doi: 10.2527/1992.70113578x. [DOI] [PubMed] [Google Scholar]
- Garlick PJ, McNurlan MA, Bark T, Lang CH, Gelato MC. Hormonal regulation of protein metabolism in relation to nutrition and disease. J Nutr. 1998;128:356S–359S. doi: 10.1093/jn/128.2.356S. [DOI] [PubMed] [Google Scholar]
- Harris PM, Skene PA, Buchan V, Milne E, Calder AG, Anderson SE, Connell A, Lobley GE. Effect of food intake on hind-limb and whole-body protein metabolism in young growing sheep: chronic studies based on arterio-venous techniques. Br J Nutr. 1992;68:389–407. doi: 10.1079/bjn19920097. [DOI] [PubMed] [Google Scholar]
- Innis SM, Clandinin MT. Dynamic modulation of mitochondrial inner-membrane lipids in rat heart by dietary fat. Biochem J. 1981;193:155–167. doi: 10.1042/bj1930155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Berthiaume L, Hadj-Tahar A, Rajput AH, Bedard PJ, Paolo TD, Julien P, Calon F. Postmortem brain fatty acid profile of levodopa-treated Parkinson disease patients and parkinsonian monkeys. Neurochem Int. 2006;48:404–414. doi: 10.1016/j.neuint.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Kamada T, Yamashita T, Baba Y, Kai M, Setoyama S, Chuman Y, Otsuji S. Dietary sardine oil increases erythrocyte membrane fluidity in diabetic patients. Diabetes. 1986;35:604–611. doi: 10.2337/diab.35.5.604. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Orellana RA, O'Connor PMJ, Suryawan A, Bush JA, Nguyen HV, Thivierge MC, Jefferson LS, Davis TA. Endotoxin induces differential regulation of mTOR-dependent signaling in skeletal muscle and liver of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E637–E644. doi: 10.1152/ajpendo.00340.2002. [DOI] [PubMed] [Google Scholar]
- Lapierre H, Bernier JF, Dubreuil P, Reynolds CK, Farmer C, Ouellet DR, Lobley GE. The effect of intake on protein metabolism across splanchnic tissues in growing beef steers. Br J Nutr. 1999;81:457–466. [PubMed] [Google Scholar]
- Lapierre H, Farmer C, Girard C, Brazeau P. Effect of age and intake on growth hormone kinetics in dairy heifers. Dom Anim Endocrinol. 1992;9:199–207. doi: 10.1016/0739-7240(92)90033-t. [DOI] [PubMed] [Google Scholar]
- Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- Liu S, Baracos VE, Quinney HA, Clandinin MT. Dietary omega-3 and polyunsaturated fatty acids modify fatty acyl composition and insulin binding in skeletal-muscle sarcolemma. Biochem J. 1994;299:831–837. doi: 10.1042/bj2990831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JIS, Sprecher H. Phospholipid fatty acid remodeling in mammalian cells. Biochim Biophys Acta. 1991;1084:105–121. doi: 10.1016/0005-2760(91)90209-z. [DOI] [PubMed] [Google Scholar]
- McCance RA, Widdowson E. Amino acid composition. In: Paul AA, Southgate DAT, editors. The Composition of Foods. New York: Elsevier/North Holland Biomedical Press; 1978. p. 418. [Google Scholar]
- Montgomery DC. Design and Analysis of Experiments. New York: John Wiley & Sons Inc; 2001. [Google Scholar]
- Muriana FJG, Ruiz-Gutierrez V. Effect of n-6 and n-3 polyunsaturated fatty acids ingestion on rat liver membrane-associated enzymes and fluidity. J Nutr Biochem. 1992;3:659–663. [Google Scholar]
- Nutrient Requirements of Beef Cattle. Washington, DC: National Academy of Sciences; 2000. National Research Council. [Google Scholar]
- Pan DA, Lillioja S, Milner MR, Kriketos AD, Baur LA, Bogardus C, Storlien LH. Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J Clin Invest. 1995;96:2802–2808. doi: 10.1172/JCI118350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram L, Nieto R, Lobley GE. Tissue sequestration of C-labeled bicarbonate [HCO3−] in fed and fasted young sheep. Comp Biochem Physiol A Mol Integr Physiol. 1999;122:323–330. doi: 10.1016/s1095-6433(99)00007-0. [DOI] [PubMed] [Google Scholar]
- Reeds PJ, Fuller MF, Cadenhead A, Lobley GE, McDonald JD. Effects of changes in the intakes of protein and non-protein energy on whole-body protein turnover in growing pigs. Br J Nutr. 1981;45:539–546. doi: 10.1079/bjn19810132. [DOI] [PubMed] [Google Scholar]
- Statistical Analysis System. Cary, NC, USA: SAS Institute Inc; 2000. SAS Institute Inc. [Google Scholar]
- Schweigert BS. The nutritional content and value of meat and meat products. In: Price JF, Schweigert BS, editors. The Science of Meat and Meat Products. Westport, CT, USA: Food & Nutrition Press Inc; 1987. p. 275. [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Storlien LH, Jenkins AB, Chishlom DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglycerides and omega-3 fatty acids in muscle phospholipids. Diabetes. 1991;40:280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- Storlien LH, Kraegen EW, Chishlom DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885–888. doi: 10.1126/science.3303333. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Davis TA. Protein-tyrosine-phosphatase 1B activation is regulated developmentally in muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E47–E54. doi: 10.1152/ajpendo.00210.2002. [DOI] [PubMed] [Google Scholar]
- Thivierge MC, Bernier JF, Dubreuil P, Lapierre H. The effect of jugular or abomasal infusion of amino acids on milk yield in lactating cows fed a protein deficient diet. Reprod Nutr Dev. 2002a;42:1–13. doi: 10.1051/rnd:2002001. [DOI] [PubMed] [Google Scholar]
- Thivierge MC, Bush JA, Suryawan AV, Nguyen H, Orellana RA, Burrin DG, Jahoor F, Davis TA. Whole body and hindlimb protein breakdown are differentially altered by feeding in neonatal piglets. J Nutr. 2005;135:1430–1437. doi: 10.1093/jn/135.6.1430. [DOI] [PubMed] [Google Scholar]
- Thivierge MC, Petitclerc D, Bernier JF, Couture Y, Lapierre H. Variations in mammary protein metabolism during the natural filling of the udder with milk over a 12-h period between two milkings: leucine kinetics. J Dairy Sci. 2002b;85:2974–2985. doi: 10.3168/jds.s0022-0302(02)74383-x. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Jacques H, Marette A. Modulation of insulin action by dietary proteins and amino acids: role of the mammalian target of rapamycin nutrient sensing pathway. Curr Opin Clin Nutr Metab Care. 2005;8:457–462. doi: 10.1097/01.mco.0000172589.55434.03. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70, S6 kinase pathway. A negative feedback leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- Vessby B, Tengblad S, Lithell H. Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia. 1994;37:1044–1050. doi: 10.1007/BF00400468. [DOI] [PubMed] [Google Scholar]
- Voelker DR. Organelle biogenesis and intracellular lipid transport in eukaryotes. Microbiol Rev. 1991;55:543–560. doi: 10.1128/mr.55.4.543-560.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetics Analysis. New York: Wiley-Liss, Inc.; 1992. [Google Scholar]
- Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acids clamps decreases with development. Am J Physiol Endocrinol Metab. 1997;273:E305–E314. doi: 10.1152/ajpendo.1997.273.2.E305. [DOI] [PubMed] [Google Scholar]










