Abstract
Pain is regarded as a risk factor in pressure ulcer development by contributing to immobility. Pressure-induced vasodilatation (PIV) is a mechanism whereby cutaneous blood flow increases in response to progressive locally applied pressure, thereby delaying the occurrence of ischaemia and appearing to be a protective response to local pressure. When the interaction between nervous and vascular systems is deregulated, PIV, which relies on both systems, is absent. We thus hypothesized that acute pain could alter PIV. This study investigated the effects on PIV of acute pain triggered by noxious heat (50°C) applied to the tail of anaesthetized rats. To address the mechanisms underlying these effects, chronic sympathectomy was performed using guanethidine, and the plasma concentrations of pituitary adrenocorticotrophin (ACTH) and catecholamines were measured. Our results show that acute pain induces a loss of PIV associated with an increase of ACTH. Direct involvement of hypertensive effects and peripheral sympathetic nervous system are excluded in the loss of PIV, whereas the activation of brain structures that have descending inhibitory control cannot be excluded. A low dose of systemic morphine prevented this loss of PIV and maintained the ability of the cutaneous microcirculation to adapt to the applied pressure. The loss of a protective response to local pressure (PIV) induced by acute pain lends physiological support to the direct involvement of pain in pressure ulcer development. Therefore, an adequate evaluation and treatment of pain is crucial.
Although the sensation of pain alerts us to real or impending injury and triggers appropriate protective responses, pain often outlives its usefulness as a warning system and instead becomes deleterious (Julius & Basbaum, 2001). Indeed, stress induced by pain could represent a risk factor, in addition to local health of the skin, poor general health and central lesions, in the development of pressure ulcers by contributing to immobility. All these factors play pivotal roles in the development of pressure ulcers and are admitted as risk factors by Norton, Braden and Waterlow scales, the three most commonly adopted pressure ulcer risk calculators (Waterlow, 1985; Bergstrom et al. 1987; Norton, 1989).
We demonstrated that a novel relationship exists between cutaneous mechanosensitivity and vasodilatation, referred to as pressure-induced vasodilatation (PIV). Indeed, a rise in cutaneous blood flow is observed in response to locally applied pressure in healthy humans (Fromy et al. 1998), rats (Fromy et al. 2000b) and mice (Sigaudo-Roussel et al. 2004). This involves the activation of capsaicin-sensitive nerve fibres, leading to the release of neuropeptides that act at the endothelial level to synthesize and release endothelial factors to induce smooth muscle relaxation (Fromy et al. 1998, 2000b; Fizanne et al. 2004). Since this increase in cutaneous perfusion delays the occurrence of ischaemia resulting from applied pressure, PIV appears to be a protective neurovascular response to local pressure that is crucial to preserve in order to limit the risk for pressure ulcers.
Indeed, the alteration of neurovascular communication is suggested to contribute to medically important diseases (Carmeliet, 2003; Carmeliet & Tessier-Lavigne, 2005). Accordingly, interruption of the interaction between nervous and vascular systems would disrupt PIV, which relies on both systems. This would induce an inability of the cutaneous microcirculation to adapt to the applied pressure, as observed in deep anaesthesia (Fizanne et al. 2003) and diabetes (Fromy et al. 2002; Koitka et al. 2004; Sigaudo-Roussel et al. 2004; Demiot et al. 2006a). As usually described by clinicians, we showed using PIV assessment that long-term diabetes with a severe neuropathy as well as vascular dysfunction aggravated the inability of the cutaneous microcirculation to adapt to pressure (Demiot et al. 2006b), a mechanism which is very likely to be involved in diabetic foot lesions.
We thus hypothesized that acute pain could alter PIV, preventing the cutaneous microcirculation from adapting to the applied pressure, which could in the long term contribute to pressure ulcer development. The aim of the present study was to verify this hypothesis using an acute pain induction imposed on anaesthetized rats at an anatomical site distant from the local pressure application. Pain induced by a thermal noxious stimulus represents classical threatening events that activate neuronal circuits in the brain to generate a complex pattern of cardiovascular and neuroendocrine responses, via the sympathetic nervous system (SNS) and the hypothalamic–pituitary–adrenal (HPA) axis. The neuroendocrine components include the increased secretion of catecholamines (noradrenaline and adrenaline) from the SNS and adrenal medulla, the release of corticotrophin-releasing factor (CRF) and vasopressin from parvicellular neurons into the neurohypophyseal portal circulation and, seconds later, the secretion of pituitary adrenocorticotropin (ACTH), leading to secretion of glucocorticoids by the adrenal gland. Since ACTH is the major hormone regulating the synthesis and secretion of adrenal glucocorticoids, ACTH is classically used to investigate activation of the HPA axis. Noradrenaline (NA) and adrenaline (Adr) are considered as good markers for pain-induced stress linked to the SNS axis. We therefore aimed to explore the role of these different components activated by acute pain.
Methods
Animal instrumentation
Experiments were performed in male Wistar rats weighing 270 ± 9 g. Before the experiments, animals had free access to standard laboratory food and water. Animals were housed in a regulated environment with a constant ambient temperature of 24°C. Procedures for the maintenance and use of the experimental rats were carried out in accordance with the principles of French legislation and the experiments were approved by the ethics committee for animal experimentation of the University of Angers, France.
Two days before the experiments, the hair was removed from the heads of the animals with a depilatory lotion to present a hairless area for the cutaneous blood flow measurements and local pressure application. For the experiments, animals were anaesthetized with thiopentone (50 mg kg−1 body weight, i.p.), inducing the absence of paw withdrawal to a painful stimulus and of the corneal reflex. These reflexes were tested before and after the experiment. The left femoral vein and artery were cannulated with polyethylene catheters (PE 10, Clay Adams, Albany, NY, USA) for administration of drugs and continuous blood pressure monitoring, respectively. At the end of animal preparation, the rats were placed in an incubator (MP4SI, Mediprema, Chambray-les-Tours, France) warmed to 30°C to maintain a stable cutaneous temperature throughout the experiment. A thermocouple (Anritsu Meter Co., Ltd, Tokyo, Japan) was placed on the skull near the pressure application site to control the cutaneous temperature (a maximal variation of 0.3°C was allowed within each experiment). Mean arterial blood pressure (MABP) was continuously measured using a pressure transducer (Mallinckrodt, Medica, Dublin, Ireland), and heart rate (HR) was calculated from the pulse pressure signal (by a fast fourier transform analysis). The animals were placed in the prone position and the head was on a frame.
Pain induction
Once cardiovascular and thermal parameters were stabilized (MABP, HR and cutaneous temperature), the tail of the anaesthetized rat was immersed in a water bath of 50°C for rats subjected to thermal stimulus and 30°C for control rats. In protocols 1, 2 and 3, the thermal stimulus was induced 1 min before PIV assessment and maintained throughout the experiment. In protocol 4, the thermal stimulus was induced for 3 min before decapitation.
Assessment of PIV (protocols 1, 2 and 3)
To assess PIV, a weighbridge was adapted to hold a laser Doppler probe (PF408, Periflux, Perimed, Stockholm, Sweden) connected to a laser Doppler flowmeter (PF4001 Master, Periflux, Perimed). The weighbridge was carefully equilibrated, and the probe was then positioned on the head skin to apply local pressure and simultaneously measure the cutaneous blood flow. In PIV assessment, the local pressure is below the threshold for pain perception and no pain sensation has been reported in healthy subjects (Fromy et al. 1998, 2002).
Data collection began with a 1 min basal period prior to the onset of increasing local pressure application. Local pressure was then increased progressively at a rate of 11.1 Pa s−1 through the laser Doppler probe for 15 min. The technical details of this method have been described previously (Fromy et al. 2000a). The cutaneous temperature, cutaneous blood flow and MABP were continuously recorded using a data acquisition system (MP100, Biopac, Santa Barbara, CA, USA) at a sampling frequency of 100 Hz and analysed off-line (Acqknowledge, Biopac, Paris, France). The animals included in protocols 1, 2 and 3 were killed at the end of the experiment by an i.v. overdose of thiopentone. The cutaneous blood flow and MABP values were recorded for 2 min following the killing of the rats to assess the ‘physiological zero’ of the measurements, which was subsequently subtracted from corresponding measured values.
Protocol 1: the effects of acute pain on PIV with or without morphine
Pressure-induced vasodilatation was assessed in control rats (n = 11), in rats subjected to thermal stimulus (n = 11) and in rats subjected to thermal stimulus that received morphine (2 mg kg−1, i.p., n = 11). Morphine injection was performed 15 min before the pain induction, since the maximal analgesic effect of morphine (2.5 mg kg−1, i.v.) determined using a radiant heat stimulus or a noxious pressure stimulus applied to the hindpaw appeared within 15 min in rats (Chen & Pan, 2006). In preliminary trials, we demonstrated that morphine (2 mg kg−1, i.p.) was sufficient to prevent the pain-induced ACTH increase within 5 min of administration.
Protocol 2: SNS involvement in pain effects on PIV
A permanent destruction of perivascular sympathetic nerves in rats can be induced by chronic administration of guanethidine (GNT), an adrenergic neuron blocker (Johnson & O'Brien, 1976). This long-term chemical sympathectomy provides an opportunity to identify the effects on PIV development that are linked to SNS activation induced by pain. Guanethidine-treated rats received daily GNT (50 mg kg−1) subcutaneous injections 5 days per week for 3 weeks beginning on day 7 after birth (Brauer et al. 1994).
Assessment of PIV was then conducted 3 months after cessation of GNT administration in sympathectomized rats (n = 10) and in sympathectomized rats subjected to thermal stimulus (n = 10). In addition to the increased baseline CVC in GNT rats compared with control animals (data not shown), the efficacy of sympathectomy was tested at the end of the experiment by measuring the response to tyramine (150 μg kg−1, i.v.), which releases endogenous NA from sympathetic nerve terminals. In control rats, tyramine injection significantly increased MABP from 108 ± 3 to 146 ± 6 mmHg (P < 0.001), whereas no increase in MABP was observed in GNT-treated rats (from 101 ± 3 to 109 ± 5 mmHg), showing the efficacy of chemical sympathectomy induced by GNT.
Protocol 3: cardiovascular involvement in pain effects on PIV
Noradrenaline was continuously infused (25 μg kg −1 min−1, i.v.) to increase MABP. When MABP reached a stable value for 1 min, the local pressure was applied (n = 13, NA rats). A control group received a continuous infusion of saline (2 μl·min−1) at the same rate as the treated group (n = 11, vehicle rats).
Protocol 4: involvement of neuroendocrine components in pain effects on PIV
In anaesthetized rats, blood samples were collected by decapitation in the morning between 9.30 and 1130 am to minimize effects of the circadian rhythm. Blood samples were then centrifuged (1972 g) at 4°C for 10 min and plasma samples were stored at −80°C. Catecholamine levels were measured by HPLC (Pharmacology Laboratory, University Hospital of Angers, France) and ACTH by kit radioimmunoassay (Biochemistry Laboratory, Medical School, University of Angers, France). For the measurements of plasma ACTH, decapitation was performed in control rats (n = 5), in rats subjected to thermal stimulus without morphine (n = 5), in rats subjected to thermal stimulus with morphine (n = 5), in GNT-treated rats (n = 5) and in GNT-treated rats subjected to thermal stimulus (n = 5). For the measurements of plasma catecholamines, decapitation was performed in control rats (n = 5), in rats subjected to thermal stimulus without morphine (n = 5), in rats subjected to thermal stimulus with morphine (n = 5), in GNT-treated rats (n = 5) and in GNT-treated rats subjected to thermal stimulus (n = 5). The thermal noxious stimulus was applied to the tail for 3 min, starting 1 min before to the local pressure application of 2 min duration, which was the mean duration needed to observe the maximal increase in the cutaneous vascular conductance (CVC) in response to local pressure (PIV) in control rats.
Drugs
The drugs used in the present study were morphine hydrochloride, 1% (Lot/batch 22618; Laboratoire Renaudin, Itxassou, France), guanethidine monosulphate and tyramine, both purchased from Sigma (St Louis, MI, USA). Guanethidine was diluted with 0.1 m phosphate-buffered saline to permit accurate dosing.
Data analysis
The signals from the laser Doppler probe were averaged every 10 s to reduce instantaneous variability owing to vasomotion. Cutaneous vascular conductance was calculated as the ratio of cutaneous blood flow to MABP (expressed in a.u. mmHg−1). Results are expressed as means ± s.e.m. Baseline values were calculated as the average over the 1 min baseline period prior to the onset of increasing local pressure. Student's unpaired t test was used to evaluate the significance between two groups. Student's paired t test was used to evaluate differences resulting from locally applied pressure within a group. One-way ANOVA with Dunnett's multiple comparison test (rats without pain induction as controls) was used to test for significant differences between groups. P < 0.05 was regarded as statistically significant.
Results
All rats subjected to the thermal stimulus, even with morphine, had an increased MABP and HR at baseline compared with their respective control rats (Table 1), indicating that this stimulus was causing a central autonomic response. The cutaneous temperature was unchanged between groups.
Table 1.
Mean arterial blood pressure (MABP), heart rate (HR) and cutaneous temperature prior to local pressure application in all groups
| Protocol 1 | Protocol 2 | Protocol 3 | |||||
|---|---|---|---|---|---|---|---|
| C | P | PM | GNT | P_GNT | Vehicle | NA | |
| MABP (mmHg) | 111 ± 2 | 156 ± 3* | 151 ± 4* | 105 ± 3 | 146 ± 4** | 102 ± 3 | 155 ± 4** |
| HR (beats min−1) | 386 ± 1 | 2490 ± 8* | 466 ± 9* | 447 ± 10 | 519 ± 8** | 367 ± 4 | 435 ± 17* |
| Skin temperature (°C) | 36.3 ± 0.1 | 36.6 ± 0.1 | 36.5 ± 0.1 | 36.6 ± 0.2 | 36.8 ± 0.1 | 36.6 ± 0.2 | 36.3 ± 0.2 |
Groups are: control rats (C, n = 11), rats subjected to thermal stimulus without morphine (P, n = 11), rats subjected to thermal stimulus with 2 mg kg−1 morphine (PM, n = 11), sympathectomized rats (GNT, n = 10), GNT rats subjected to thermal stimulus (P_GNT, n = 10), rats treated with vehicle (vehicle, n = 11) and rats infused with NA (NA, n = 13).
P < 0.01
P < 0.001 versus respective control rats.
Protocol 1: the effects of acute pain on PIV with or without morphine
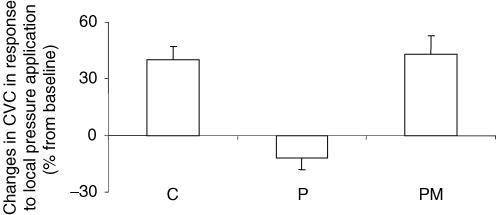
With local pressure application, CVC increased progressively from 1.07 ± 0.06 a.u. mmHg−1 at baseline to 1.48 ± 0.08 a.u. mmHg−1 (P < 0.0001) at 1.3 ± 0.1 kPa, representing a maximal percentage increase in CVC from baseline of 40 ± 7% in control rats (Fig. 1).
Figure 1. Percentage changes in CVC in response to 11.1 Pa s−1 local pressure application.
Groups are: control rats (C, n = 11) and rats subjected to thermal stimulus without morphine (P, n = 11) and with 2 mg kg−1 morphine (PM, n = 11). The percentage increase in CVC from baseline was abolished in P rats (P < 0.01) compared with C rats, whereas it was not different in PM rats.
In rats subjected to thermal stimulus without morphine, local pressure application induced a progressive decrease in CVC. Cutaneous vascular conductance decreased from 0.96 ± 0.05 a.u. mmHg−1 at baseline to 0.90 ± 0.04 a.u. mmHg−1 (P < 0.05) at 1.3 kPa, representing a percentage decrease in CVC from baseline of −12 ± 6% (Fig. 1).
In rats subjected to thermal stimulus with morphine, local pressure application induced a progressive increase in CVC. Cutaneous vascular conductance increased from 0.80 ± 0.08 a.u. mmHg−1 at baseline to 1.17 ± 0.16 a.u. mmHg−1 (P < 0.01) at 1.0 ± 0.2 kPa, representing a maximal percentage increase in CVC from baseline of 43 ± 10% (Fig. 1). The locally applied pressures corresponding to the maximal increase in CVC for control rats (1.3 ± 0.1 kPa) and for rats subjected to thermal stimulus with morphine (1.0 ± 0.2 kPa) were not different.
The percentage increase in CVC from baseline was abolished in rats subjected to thermal stimulus without morphine (P < 0.01) compared with control rats, whereas it was not different from control rats in rats subjected to thermal stimulus with morphine (Fig. 1).
Cutaneous vascular conductance prior to local pressure application was unchanged in rats subjected to thermal stimulus without morphine compared with control animals, but it was decreased in rats subjected to thermal stimulus with morphine (P < 0.05).
Protocol 2: SNS involvement in pain effects on PIV
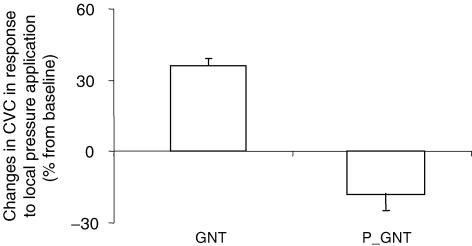
In GNT-treated rats, CVC increased progressively with local pressure application from 1.49 ± 0.15 a.u. mmHg−1 at baseline to 2.03 ± 0.21 a.u. mmHg−1 (P < 0.0001) at 1.3 ± 0.1 kPa, representing a percentage increase in CVC from baseline of 36 ± 3% (Fig. 2).
Figure 2. Percentage changes in CVC in response to 11.1 Pa s−1 local pressure application in sympathectomized rats (GNT, n = 10) and GNT-treated rats subjected to thermal stimulus (P_GNT, n = 10).
The percentage increase in CVC from baseline was abolished in P_GNT rats (P < 0.0001) compared with GNT rats.
In GNT-treated rats subjected to thermal stimulus, CVC decreased in response to local pressure application from 1.11 ± 0.17 a.u. mmHg−1 at baseline to 0.87 ± 0.13 a.u. mmHg−1 (P < 0.05) at 1.3 kPa, representing a percentage decrease in CVC from baseline of −18 ± 7% (Fig. 2).
The percentage changes in CVC from baseline were different between groups (P < 0.0001).
Protocol 3: cardiovascular involvement in pain effects on PIV
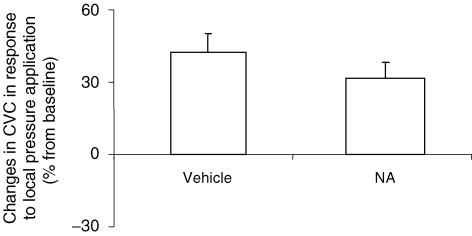
In vehicle-treated rats, CVC increased progressively with local pressure application from 0.95 ± 0.07 a.u. mmHg−1 at baseline to 1.30 ± 0.09 a.u. mmHg−1 (P < 0.001) at 1.3 ± 0.1 kPa, representing a percentage increase in CVC from baseline of 42 ± 8% (Fig. 3).
Figure 3. Percentage changes in CVC in response to 11.1 Pa s−1 local pressure application in rats infused with noradrenaline (NA, 25 μg kg−1 min−1, i.v., n = 13) and rats infused with saline at the same rate (vehicle, n = 11).
There was no significant difference between the groups.
In NA-treated rats, CVC increased progressively with local pressure application from 0.63 ± 0.05 a.u. mmHg−1 at baseline to 0.82 ± 0.06 a.u. mmHg−1 (P < 0.001) at 1.3 ± 0.1 kPa, representing a percentage increase in CVC from baseline of 32 ± 6% (Fig. 3).
The percentage changes in CVC from baseline were not different between groups (Fig. 3).
Protocol 4: involvement of neuroendocrine components in pain effects on PIV
Regarding HPA axis activation, pain induced an increase in ACTH level (Table 2), which was totally prevented by morphine. Regarding SNS activation, the catecholamines were not increased by the painful stimulus.
Table 2.
Plasma ACTH (n = 5 per group) and catecholamine levels (noradrenaline and adrenaline; n = 5 per group) from blood samples collected by decapitation
| C | P | PM | GNT | P_GNT | |
|---|---|---|---|---|---|
| ACTH (pg ml−1) | 227 ± 45 | 1175 ± 223* | 256 ± 76 | 239 ± 48 | 662 ± 81* |
| Noradrenaline (ng ml−1) | 1.2 ± 0.3 | 1.8 ± 0.6 | 1.8 ± 0.3 | 1.9 ± 0.3 | 1.8 ± 0.4 |
| Adrenaline (ng ml−1) | 12.0 ± 3.1 | 11.3 ± 2.1 | 13.8 ± 2.1 | 15.8 ± 2.9 | 13.7 ± 1.8 |
Groups are: control rats (C), rats subjected to thermal stimulus without morphine (P), rats subjected to thermal stimulus with 2 mg kg−1 morphine (PM), sympathectomized rats (GNT) and GNT-treated rats subjected to thermal stimulus (P_GNT).
P < 0.01 versus respective control rats.
Discussion
The main result of the present study was the loss of PIV induced by an acute painful stimulus, which prevented the cutaneous microcirculation from adapting to locally applied pressure. In contrast, a low dose of systemic morphine (2 mg kg−1, i.p.) was sufficient to preserve PIV in the presence of pain. The loss of PIV is consistent with a major role for HPA axis activity, as reflected by elevated ACTH levels on peripheral blood sampling, and not for SNS and cardiovascular responses to pain. The activation of neuronal circuits in the brain that have strong inhibitory control cannot be excluded as a mechanism for the loss of PIV during pain.
Our results demonstrate that acute pain led to a loss of PIV in the rats that did not receive morphine injection. During a disruption of normal homeostasis, the major endocrine response via activation of the HPA axis is a rapid secretion of ACTH, as reported in cold stress (Donnerer & Lembeck, 1990) or following the electrical stimulation of afferent Aδ and C fibres in the sciatic nerve (Donnerer & Lembeck, 1988). In the present study, the noxious heat applied to the tail triggered a measurable increase in plasma ACTH concentration, which was observed in all rats subjected to thermal stimulus except for rats that received morphine. It has been shown that exogenous ACTH application (1 nm) blocked the capsaicin-evoked release of calcitonin gene-related peptide (CGRP) from in vitro superfused adrenal capsules (Ulrich-Lai et al. 2001), mimicking an in vivo situation in which the capsaicin-sensitive fibres could be activated (such as nociception). Since PIV depends on neurotransmitters released from capsaicin-sensitive fibres, such as CGRP, VIP and pituitary adenylate cyclase-activating polypeptide (PACAP) (Fromy et al. 2000b; Fizanne et al. 2004), a modulation of the release of these neurotransmitters could explain the observation that elevated plasma ACTH concentrations triggered by pain induction to the tail were correlated with the loss of PIV on the skull. Further studies are required, however, to determine whether modulation of neurotransmitter release by ACTH is a common attribute of afferent fibres or is unique to adrenal afferents. Following morphine injection, the noxious heat applied to the tail did not increase the plasma ACTH concentration and PIV was preserved. Accordingly, six intermittent injections of morphine (6 mg kg−1 per injection, s.c.) also prevented an increase in ACTH secretion in response to water restriction in rats (Zhou et al. 1999). Therefore, morphine prevented the loss of PIV in rats subjected to thermal stimulus and also prevented an increase in ACTH, confirming the close link between PIV and ACTH level.
In addition to ACTH level, the activation of neuronal circuits in the brain triggered by the acute pain could also explain the loss of PIV, since inputs from nociceptive specific, wide-dynamic range and non-nociceptive neurons are not segregated into anatomically distinct regions (Monconduit et al. 2006). It has already been reported that dorsal column nuclei and spinal routes co-operate, rather than operate separately, to produce the many perceptions of touch and pain (Berkley & Hubscher, 1995). Consistent with this possibility is the observation that nociceptive input to the primary somatosensory cortex (S1) can modulate tactile perception, as shown by the observations that noxious heat is able to reduce the intrinsic optical imaging signal in S1 evoked by innocuous mechanical stimulation of the skin (Tommerdahl et al. 1996). The diffuse noxious inhibitory controls (DNIC) are an example of descending control of nociceptive transmission involving brain structures (Villanueva & Le Bars, 1995; Le Bars, 2002) that strongly inhibited both the A fibre- and C fibre-related activities of trigeminal convergent neurons (Dickenson et al. 1980). In addition to DNIC activation, which strongly depressed pain sensation in response painful stimuli, cortical responses to noxious stimuli can also be inhibited at the cortical level by innocuous tactile stimuli (Inui et al. 2006). Although local pressure applied to the skin was low (less than 1.5 kPa) and not perceived as painful by humans (Fromy et al. 1998, 2002), we showed that PIV was absent following chronic treatment with capsaicin (Fromy et al. 1998, 2000b), demonstrating that PIV is mediated by capsaicin-sensitive primary afferent fibres. Therefore, we cannot exclude the possibility that the noxious heat applied to the tail of the rat exerted powerful inhibitory control, blocking the activity of capsaicin-sensitive primary afferent fibres, which in turn led to the loss of PIV on the skull in the rats that endured pain without morphine. In contrast, we demonstrated that systemic administration of a low dose of morphine (2.0 mg kg−1) prevented the loss of PIV induced by the acute pain. Systemic morphine depressed ventromedial thalamus neuronal activities evoked by a thermal noxious stimulus (48°C; Monconduit et al. 2002), suggesting that morphine selectively depresses a thalamic link of widespread nociceptive inputs that arise from cutaneous polymodal nociceptors in the rat. The important role of μ-opioid receptors in the spinal cord was shown in the antinociceptive effect of systemic administration of morphine on acute nociception (Chen et al. 2005; Chen & Pan, 2006). Since systemic morphine exerts its actions by interfering with pain transmission in the central nervous system (Basbaum & Fields, 1984), the prevention of pain-induced PIV abolition by morphine strengthens the idea that the inhibitory control that led to PIV abolition in response to an acute pain exerts its effect at the central level, without excluding the possibility that peripheral opioid receptors could be involved. Although further studies will be needed to determine the brain structures involved in the inhibitory control triggered by acute pain on PIV, the present study demonstrated a broad control of PIV modulated by the central nervous system rather than a local control restricted to the periphery.
The main cardiovascular response to stress is an acute rise in systemic blood pressure to hypertensive levels, which could explain the loss of PIV in rats subjected to thermal stimulus. However, PIV was not abolished by pain in the presence of a low dose of morphine, while the rises in MABP and HR were of the same magnitude as those observed in rats subjected to a thermal stimulus without morphine. Moreover, the rises of MABP and HR resulting from NA infusion did not abolish PIV, strengthening the idea that the cardiovascular effects of pain per se are unlikely to explain the loss of PIV.
In contrast to ACTH, catecholamines were not significantly increased in rats subjected to the 3 min thermal stimulus, suggesting that activation of the SNS was not involved in the loss of PIV. Accordingly, sympathectomy, accomplished by chronic treatment with GNT, did not modify the negative effects triggered by acute pain on PIV. We verified that GNT extensively destroyed the peripheral sympathetic fibres, as indicated by the absence of a pressor response to tyramine injection in GNT-treated rats, as previously reported (Julien et al. 1990). In contrast, GNT did not affect the increase in MABP and HR in response to pain (Table 1), suggesting that adrenal medulla was not affected by the GNT treatment. Indeed, sympathetic nerves and adrenal medulla have important influences on cardiovascular function during stress and, in the absence of either, the other system may partly compensate (Barron & Van Loon, 1989). The increase in ACTH in response to acute pain in GNT-treated rats strengthens the idea that GNT did not affect the adrenal medulla and was therefore specific to peripheral sympathetic fibres. It is interesting to note that GNT-induced sympathectomy had no effect on the cutaneous vasodilatation in response to local pressure application, showing that the sympathetic noradrenergic fibres are involved neither in PIV nor in the loss of PIV under the influence of pain.
In conclusion, we report that acute pain caused a loss of PIV in rats, mediated by a neuroendocrine pathway. Indeed, pain-arousing HPA axis activity may act by an increase of ACTH to abolish PIV in anaesthetized rats. The activation of brain structures that have descending inhibitory control cannot be excluded. In contrast, direct involvement of hypertensive effects and the peripheral sympathetic nervous system are excluded in the loss of PIV induced by acute pain. Morphine prevented the loss of PIV and maintained the ability of the cutaneous microcirculation to adapt to the applied pressure. Emerging recommendations in pressure ulcer management (Reddy et al. 2003) indicate that pain represents a risk factor. The loss of a protective response to local pressure (PIV) induced by an acute pain lends physiological support to the direct involvement of pain in pressure ulcer occurrence, strengthening the view that an adequate evaluation and treatment of pain is crucial.
Acknowledgments
We thank the local animal care unit of the University of Angers for facilities.
References
- Barron BA, Van Loon GR. Role of sympathoadrenomedullary system in cardiovascular response to stress in rats. J Auton Nerv Syst. 1989;28:179–187. doi: 10.1016/0165-1838(89)90090-8. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for Predicting Pressure Sore Risk. Nurs Res. 1987;36:205–210. [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH. Are there separate central nervous system pathways for touch and pain? Nat Med. 1995;1:766–773. doi: 10.1038/nm0895-766. [DOI] [PubMed] [Google Scholar]
- Brauer MM, Lincoln J, Milner P, Sarner S, Blundell D, Passaro M, Corbacho A, Burnstock G. Plasticity of autonomic nerves: differential effects of long-term guanethidine sympathectomy on the sensory innervation of rat uterus during maturation. Int J Dev Neurosci. 1994;12:579–586. doi: 10.1016/0736-5748(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Chen YP, Chen SR, Pan HL. Systemic morphine inhibits dorsal horn projection neurons through spinal cholinergic system independent of descending pathways. J Pharmacol Exp Ther. 2005;314:611–617. doi: 10.1124/jpet.105.085563. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Blocking μ opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res. 2006;1081:119–125. doi: 10.1016/j.brainres.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Demiot C, Fromy B, Saumet JL, Sigaudo-Roussel D. Preservation of pressure-induced cutaneous vasodilation by limiting oxidative stress in short-term diabetic mice. Cardiovasc Res. 2006a;69:245–252. doi: 10.1016/j.cardiores.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Demiot C, Tartas M, Fromy B, Abraham P, Saumet JL, Sigaudo-Roussel D. Aldose reductase pathway inhibition improved vascular and C-fiber functions, allowing for pressure-induced vasodilation restoration during severe diabetic neuropathy. Diabetes. 2006b;55:1478–1483. doi: 10.2337/db05-1433. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Le Bars D, Besson JM. Diffuse noxious inhibitory controls (DNIC). Effects on trigeminal nucleus caudalis neurones in the rat. Brain Res. 1980;200:293–305. doi: 10.1016/0006-8993(80)90921-x. [DOI] [PubMed] [Google Scholar]
- Donnerer J, Lembeck F. Neonatal capsaicin treatment of rats reduces ACTH secretion in response to peripheral neuronal stimuli but not to centrally acting stressors. Br J Pharmacol. 1988;94:647–652. doi: 10.1111/j.1476-5381.1988.tb11571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnerer J, Lembeck F. Different control of the adrenocorticotropin-corticosterone response and of prolactin secretion during cold stress, anesthesia, surgery, and nicotine injection in the rat: involvement of capsaicin-sensitive sensory neurons. Endocrinology. 1990;126:921–926. doi: 10.1210/endo-126-2-921. [DOI] [PubMed] [Google Scholar]
- Fizanne L, Fromy B, Preckel MP, Sigaudo-Roussel D, Saumet JL. Effect of isoflurane on skin-pressure-induced vasodilation. J Vasc Res. 2003;40:416–422. doi: 10.1159/000072890. [DOI] [PubMed] [Google Scholar]
- Fizanne L, Sigaudo-Roussel D, Saumet JL, Fromy B. Evidence for the involvement of VPAC1 and VPAC2 receptors in pressure-induced vasodilatation in rodents. J Physiol. 2004;554:519–528. doi: 10.1113/jphysiol.2003.053835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromy B, Abraham P, Bouvet C, Bouhanick B, Fressinaud P, Saumet JL. Early decrease of skin blood flow in response to locally applied pressure in diabetic subjects. Diabetes. 2002;51:1214–1217. doi: 10.2337/diabetes.51.4.1214. [DOI] [PubMed] [Google Scholar]
- Fromy B, Abraham P, Saumet JL. Non-nociceptive capsaicin-sensitive nerve terminal stimulation allows for an original vasodilatory reflex in the human skin. Brain Res. 1998;811:166–168. doi: 10.1016/s0006-8993(98)00973-1. [DOI] [PubMed] [Google Scholar]
- Fromy B, Abraham P, Saumet JL. Progressive calibrated pressure device to measure cutaneous blood flow changes to external pressure strain. Brain Res Brain Res Protoc. 2000a;5:198–203. doi: 10.1016/s1385-299x(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Fromy B, Merzeau S, Abraham P, Saumet JL. Mechanisms of the cutaneous vasodilator response to local external pressure application in rats: involvement of CGRP, neurokinins, prostaglandins and NO. Br J Pharmacol. 2000b;131:1161–1171. doi: 10.1038/sj.bjp.0703685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui K, Tsuji T, Kakigi R. Temporal analysis of cortical mechanisms for pain relief by tactile stimuli in humans. Cereb Cortex. 2006;16:355–365. doi: 10.1093/cercor/bhi114. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Jr, O'Brien F. Evaluation of the permanent sympathectomy produced by the administration of guanethidine to adult rats. J Pharmacol Exp Ther. 1976;196:53–61. [PubMed] [Google Scholar]
- Julien C, Kandza P, Barres C, Lo M, Cerutti C, Sassard J. Effects of sympathectomy on blood pressure and its variability in conscious rats. Am J Physiol Heart Circ Physiol. 1990;259:H1337–H1342. doi: 10.1152/ajpheart.1990.259.5.H1337. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Koitka A, Abraham P, Bouhanick B, Sigaudo-Roussel D, Demiot C, Saumet JL. Impaired pressure-induced vasodilation at the foot in young adults with type 1 diabetes. Diabetes. 2004;53:721–725. doi: 10.2337/diabetes.53.3.721. [DOI] [PubMed] [Google Scholar]
- Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Brain Res Rev. 2002;40:29–44. doi: 10.1016/s0165-0173(02)00186-8. [DOI] [PubMed] [Google Scholar]
- Monconduit L, Bourgeais L, Bernard JF, Villanueva L. Systemic morphine selectively depresses a thalamic link of widespread nociceptive inputs in the rat. Eur J Pain. 2002;6:81–87. doi: 10.1053/eujp.2001.0308. [DOI] [PubMed] [Google Scholar]
- Monconduit L, Lopez-Avila A, Molat JL, Chalus M, Villanueva L. Corticofugal output from the primary somatosensory cortex selectively modulates innocuous and noxious inputs in the rat spinothalamic system. J Neurosci. 2006;26:8441–8450. doi: 10.1523/JNEUROSCI.1293-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton D. Calculating the risk: reflections on the Norton Scale. Decubitus. 1989;2:24–31. [PubMed] [Google Scholar]
- Reddy M, Keast D, Fowler E, Sibbald RG. Pain in pressure ulcers. Ostomy Wound Manage. 2003;49:30–35. [PubMed] [Google Scholar]
- Sigaudo-Roussel D, Demiot C, Fromy B, Koitka A, Leftheriotis G, Abraham P, Saumet JL. Early endothelial dysfunction severely impairs skin blood flow response to local pressure application in streptozotocin-induced diabetic mice. Diabetes. 2004;53:1564–1569. doi: 10.2337/diabetes.53.6.1564. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Delemos KA, Vierck CJ, Jr, Favorov OV, Whitsel BL. Anterior parietal cortical response to tactile and skin-heating stimuli applied to the same skin site. J Neurophysiol. 1996;75:2662–2670. doi: 10.1152/jn.1996.75.6.2662. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Harding-Rose CA, Guo A, Bowles WR, Engeland WC. ACTH inhibits the capsaicin-evoked release of CGRP from rat adrenal afferent nerves. Am J Physiol Regul Integr Comp Physiol. 2001;280:R137–R142. doi: 10.1152/ajpregu.2001.280.1.R137. [DOI] [PubMed] [Google Scholar]
- Villanueva L, Le Bars D. The activation of bulbo-spinal controls by peripheral nociceptive inputs: diffuse noxious inhibitory controls. Biol Res. 1995;28:113–125. [PubMed] [Google Scholar]
- Waterlow J. Pressure sores: a risk assessment card. Nurs Times. 1985;81:49–55. [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Maggos CE, Wang XM, Han JS, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenal activity and pro-opiomelanocortin mRNA levels in the hypothalamus and pituitary of the rat are differentially modulated by acute intermittent morphine with or without water restriction stress. J Endocrinol. 1999;163:261–267. doi: 10.1677/joe.0.1630261. [DOI] [PubMed] [Google Scholar]