Abstract
Studies have revealed that high-fat (HF) diets promote hyperglycaemia, whole-body insulin resistance and non-alcoholic fatty liver disease (NAFLD). Recently, hepatic glucagon resistance has been shown to occur in rats fed a HF diet. More precisely, diet-induced obesity (DIO) reduces the number of hepatic plasma membrane glucagon receptors (GR), which results in a diminished response to glucagon during a hyperglucagonaemic clamp. The present study was undertaken to test the hypothesis that a HF-DIO is associated with a desensitization and destruction of the hepatic GR. We also hypothesized that a single bout of endurance exercise would modify the GR cellular distribution under our DIO model. Male rats were either fed a standard (sd) or a HF diet for two weeks. Each group was subdivided into a non-exercised (Rest) and an acute exercised (EX) group. The HF diet resulted in a reduction of total hepatic GR (55%) and hepatic plasma membrane GR protein content (20%). These changes were accompanied by a significant increase in endosomal and lysosomal GR content with the feeding of a HF diet. The reduction of GR plasma membrane as well as the increase in endosomal GR was strongly correlated with an increase of PKC-α, suggesting a role of PKC-α in GR desensitization. EX increased significantly PKC-α protein content in both diets, suggesting a role of PKC-α in EX-induced GR desensitization. The present results suggest that liver lipid infiltration plays a role in reducing glucagon action in the liver through a reduction in total cellular and plasma membrane GR content. Furthermore, the GR desensitization observed in our in vivo model of HF diet-induced hepatic steatosis and in EX individuals may be regulated by PKC-α.
Physiopathologies such as liver cirrhosis, steatosis and diabetes have been shown to have deleterious effects on hepatic glucagon sensitivity and subsequently on systemic glucose metabolism. Past studies have shown and speculated that the decreased hepatic glucagon sensitivity observed in the aforementioned pathologies was associated with a reduction in total liver glucagon receptor (GR) (Charbonneau et al. 2005a; Keller et al. 1982; Orskov et al. 1991; Rao, 1995). Several factors, including increased plasma glucagon and glucose concentrations as well as elevated cAMP and oxygen concentrations (Abrahamsen et al. 1995; Burcelin et al. 1998; Jiang & Zhang, 2003), have been identified to explain a decrease in hepatic GR number under these circumstances. Recently, we reported a significant reduction in hepatic GR number in the plasma membrane of hepatic steatotic rats without any changes in circulating glucagon concentration (Charbonneau et al. 2005a). This suggests the presence of a mechanism other than homologous desensitization to explain the reduction in plasma membrane hepatic GR with liver lipid accumulation. It was postulated that hepatic lipids and/or their metabolites could play a role in the reduction of GR in the plasma membranes. Interestingly, several physiopathological conditions associated with a reduction in hepatic GR are also associated with an increase in liver lipid infiltration (Keller et al. 1982; Orskov et al. 1991; Rao et al. 1995).
Normal desensitization mechanisms in G protein-coupled receptors (GPCRs) such as the GR are initiated with plasma membrane receptors being internalized in endosomes and, thereafter, destroyed in lysosomal fractions (Lefkowitz, 1998a and b). One fundamental mechanism of GPCR regulation is receptor-homologous desensitization, which occurs rapidly after receptor occupancy by the agonist. As a result, homologous desensitization is an adaptive mechanism of the receptors that favours the regaining of receptor responsiveness after repeated stimuli over time. Following hepatic GR homologous desensitization, it is thought that the receptors are temporarily stored in the endosomes to minimize the stimulation effects of agonists (Van Ermen & Fraeyman, 1994). After cessation of the agonist stimulation, the receptors are recruited back to the plasma membrane. However, it is not known if liver lipid accumulation results in an increased internalization of the GR in endosomes. Under chronic stimulation of the receptors, internalization results in GR destruction in lysosomes (Lefkowitz, 1998a and b). It is possible that the presence of liver triglycerides could redirect normal GPCR internalization in endosomes to lysosomal compartments (Lefkowitz et al. 1998a and b). This would result in a reduction in GR availability to normal physiological processes and partly explain the lower hepatic glucose production seen during a hyperglucagonemic clamp in hepatic steatotic rats (Charbonneau et al. 2005b).
Protein kinase C is implicated in the glucagon receptor homologous and heterologous desensitization in hepatocytes in vitro. Agonist occupancy of related hormone receptors results in heterologous desensitization, with kinetics somewhat slower than those observed for homologous desensitization (Premont & Iyengar, 1988). Both homologous and heterologous glucagon receptor desensitization are independent of increases in intracellular calcium (Savage et al. 1995); receptor desensitization is also independent of cAMP activation and is induced by activators of inositol phospholipid metabolism such as protein kinase C that phosphorylate GPCR and increases the recruitment of G protein-related kinase (GRK) which initiates the desensitization mechanism (Murphy et al. 1989, 1999). It is therefore of interest to assess the effects of a high-fat diet on PKC and GRK plasma membrane recruitment in hepatic GR desensitization.
In contrast to several pathophysiological conditions (Murphy et al. 1987, 1989), chronic and acute physical exercise have been associated with increased glucagon action in the liver. Drouin et al. (1998, 2004) reported that under glucagon stimulation, trained individuals and animals showed higher hepatic glucose production than sedentary controls. Similarly, Legare et al. 2001) and Podolin et al. (2001) demonstrated that endurance training increases the number of plasma membrane hepatic GRs in rats. There is also evidence that not only chronic but also acute exercise (60 min) may enhance hepatic glucose production in response to glucagon (Pohl, 1977). Although the exact mechanism remains unknown, there is no doubt that the liver can rapidly adapt to physiological stress through modulation of GR-binding characteristics, desensitization and internalization processes. In our previous study, using a 30 min acute exercise protocol, we were unable to determine any significant effects of acute exercise on GR number and binding capacity (Charbonneau et al. 2005a). This was attributed to the short duration of the exercise protocol. In the present study, we used 60 min of acute exercise to physiologically stimulate the glucagon receptors in an attempt to determine if the dynamic response of the GR to glucagon is altered by exercise in a condition of liver lipid accumulation.
In an attempt to further characterize the interaction between liver lipid infiltration, hepatic GR distribution and exercise, the first aim of this study was to use an immunofluorescence technique against a specific rat glucagon receptor antibody to determine if a high-fat diet-induced hepatic steatosis decreases total cellular GR as it did for plasma membrane GR (Charbonneau et al. 2005a). The second aim was to test the hypothesis that, in addition to a decrease in total GR, liver lipid infiltration is also associated with an increased GR content in endosomal and lysosomal fractions. Thirdly, we assessed changes in plasma membrane PKC and GRK2 levels in our in vivo model of diet-induced hepatic steatosis to have a mechanistic insight on the GR desensitization observed in the present study. Finally, the fourth aim was to assess the effects of acute exercise on total cellular glucagon receptor distribution and on PKC and GRK2 protein levels under a condition of high-fat diet-induced hepatic steatosis.
Methods
Animal care and exercise protocol
Male Sprague-Dawley rats (Charles River, St Constant, Canada), weighing 180–200 g, were housed individually and allowed food (standard diet or high-fat diet) and water ad libitum for 2 weeks after they were received in our laboratory. The lighting schedule was such that lights were on from 0700 until 1900, and room temperature was maintained at 20–23°C. Seven days after their arrival, all rats underwent a habituation running protocol on a motor-driven rodent treadmill (Quinton Instruments, Seattle. WA, USA) consisting of three sessions over a 7-day period beginning with 15 min day−1 at 20 m min−1 and progressively increasing to 60 min day−1 at 26 m min−1 (0% grade), so that they were well accustomed to running and being handled. All animals from the exercised groups (Ex) were restrained from exercise 48 h before the experimentation. All protocols were in accordance with the directives of the Canadian Council of Animal Care and the University of Montreal Council for Animal Care.
After termination of the assigned treatment (Rest and Ex), rats were weighed and anaesthetized with sodium-pentobarbital (40 mg kg−1 ip). After complete anaesthesia the abdominal cavity was rapidly opened following the median line of the abdomen. Thereafter, protocol A or B was carried out. For protocol A, blood was withdrawn from the vena cava (4–6 ml) and immediately after this the liver was excised and frozen in liquid nitrogen using a freeze clamp. Consequently, animals were killed by exsanguination. For protocol B (liver perfusion/digestion protocol), the animals died by exsanguination, after being perfused for approximately 30 min with collagenase and calcium solutions.
Groups and surgery
After arrival, rats were randomly assigned to one of the four experimental groups. Two groups of rats were fed with standard (SD) pellet rat chow (12.5% lipids, 62.3% carbohydrate, and 24.3% protein; kcal; Agribrands Purina Canada, Woodstock, Ontario). The other groups of rats were fed with high-fat (HF) small-pellet rat chow (42% lipids: (80% lard, 20% corn oil); 36% carbohydrate, and 22% protein (kcal); ICN Pharmaceuticals, NY, USA). The duration of the diet (2 weeks) was based on previous time-course studies (Gauthier et al. 2003, 2004) in which we observed that hepatic triglyceride content was highest 2 weeks after the initiation of the high-fat diet. Details of the diets are described elsewhere (Gauthier et al. 2004, 2006). Rats in each dietary condition were thereafter assigned either to a rested group (Rest) or an acute exercised group (Ex). On the day of experimentation, rats assigned to exercised groups ran 60 min at 26 m min−1 (representing 70% of the V̇o2max) before being killed.
The four experimental groups were duplicated and used as follows: the first four groups were assigned to plasma membrane, endosome and lysosome isolation protocol (Protocol A) along with blood sampling, while the second four groups were assigned to the perfusion protocol (Protocol B). Because it is impossible to isolate liver organelles after collagenase perfusion, a duplicate of the initial four groups was obligatory. On the morning of the experiment, any remaining food was removed from the cages 2–3 h before killing. After termination of the assigned treatment (Rest and Ex), rats were weighed and anaesthetized with sodium-pentobarbital (40 mg kg−1i.p.). After complete anaesthesia the abdominal cavity was rapidly opened following the median line of the abdomen after which protocol A or B was carried out.
Protocol A: organelle isolation, plasma and tissue data
Blood was rapidly (<45 s) drawn from the abdominal vena cava (∼4 ml) into a syringe pretreated with EDTA (15%). The fraction of blood (250 μl) to be used for glucagon determination was preserved in aprotinin (25 μl) before centrifugation. The remaining fraction of blood was also centrifuged (Eppendorf centrifuge, no. 5415), and the plasma was stored for subsequent plasma glucose, insulin, triglyceride, and free fatty acid determinations. The liver was excised and weighed. The liver median lobe was freeze clamped in liquid nitrogen and used for glycogen and triacylglycerol determinations, while 6 g of fresh liver was used for organelle isolation and liver homogenate.
Isolation of plasma membranes, endosomes and lysosomes
All sucrose solutions were prepared 24–48 h before use and their densities determined at room temperature with an Abbé refractometer. The sucrose solutions were filtered (0.22 μm for 0.25 m and 1.2 μm for 1.42 and 2.0 m solutions), the pH and density determined, and stored at 4°C. Liver samples (1.8–2 g) were weighed, added to 10 volumes of 0.25 m sucrose in a 15 ml glass disposable culture tube (0.25 m sucrose/5 mm Tris HCl, pH 7.2–7.6/0.5 and 1.0 mm MgCl2) and homogenized with a Polytron (Polyscience, model X-520) at 1000–1100 r.p.m. For liver homogenates, after homogenization and centrifugation, several aliquots of the 10 000 g supernatant fraction were isolated and frozen at −80°C until used for Western blots. For organelle isolation, the 0.25 m solution was centrifuged (25–30 ml per 50 ml plastic tube) at 280 g or 1800 r.p.m. for 5 min (Beckman GPR centrifuge). The supernatant was saved and the pellet was resuspended in 0.25 m to one-half the initial homogenate volume. The suspension was again centrifuged as above. The first and second supernatants were combined and centrifuged at 1500 g or 2600 r.p.m. for 10 min. The resulting pellets were pooled and resuspended in 1–2 ml of 0.25 m sucrose per gram of liver (initial wet weight). A sucrose gradient consisting of three sucrose phases was performed in Beckman cellulose nitrate tubes. The bottom phase consisted of a 68.4% sucrose concentration with sufficient volume to bring it to approximately twice that of the original homogenate (i.e. 10% w/v). The second phase consisted of a 2 ml 45% sucrose concentration with, finally, a 2 ml 36% sucrose concentration phase. The combined supernatants resuspended in 1–2 ml of 0.25 m sucrose were poured over the sucrose gradients and overlaid with 2–4 ml of 0.25 m sucrose. After centrifugation for 90 min at 141 000 g (41000 r.p.m) in a Beckman ultracentrifuge (model L5-50), the pellicles at the interfaces of each sucrose gradient were collected with a blunt-tipped Pasteur pipette and resuspended in 0.25 m sucrose to obtain a density of 1.05 g cm−3. The pellicle at the interface of the 36% and 0.25 m sucrose represented the lysosomal fraction, while the pellicle at the interface 45%–36% sucrose represented the endosomal fraction (Harikumar et al. 1989). The bottom interface pellicle (68%–45%), represented the plasma membranes. All of these suspensions were centrifuged at 14000 r.p.m. for 10 min, and the final pellet was resuspended in an antiprotease solution (1 ml; Mini complete, Roche Pharmaceuticals) and stored at −78°C for further analysis.
Assessment of membranes, endosomes and lysosome purity
Protein concentrations were measured according to the method of Lowry et al. (1951) using crystalline bovine serum albumin as the standard. 5′-nucleotidase (5′-ND) activity was measured at 30°C by the method of Arkesteijn, 1976) as a marker for plasma membrane. Gamma glutamyltranspeptidase (GGTP) activity was measured, as an additional marker for plasma membrane, by the method of Szasz, 1969) using l-glutamyl p-nitroanilide as substrate. Sodium, potassium-activated adenosine triphosphatase (Na,K-ATPase) activity was measured by the method of Edelman et al. (Ismail-Beigi & Edelman, 1971) with the modification of Scharschmidt et al. (1979) as a marker for blood-sinusoidal plasma membrane (PM). Glucose-6-phosphatase (G6Pase) activity was measured by the method of Harper (1963) as a marker for endoplasmic reticulum. Succinate dehydrogenase activity was measured by the method of King (1967) as a marker for mitochondria. Lysosomal and endosomal marker enzymes, arylsulphatase, N-acetyl-β-d-glucose-aminidase and cathepsin D were assayed as described by Barrett & Merrifield. (1977). Activities of 5′-nucleotidase, succinate-p-iodonitroletra-zolium violet reductase and glucose-6-phosphatase were determined according to the method of Morre (1971) as additional markers for plasma membrane, mitochondria and microsomes, respectively. Catalase was assayed as a marker enzyme for peroxisomes (Baudhuin et al. 1964).
Preparation for SDS-PAGE immunoblotting
Plasma membranes, endosomal and lysosomal fractions as well as liver homogenates were vortexed frequently for 1 h at 4°C then centrifuged at 4500 g for 1 h at 4°C. The protein concentration of the supernatant was measured using a Bradford protein assay (Bio-Rad). Plasma membranes, endosomal and lysosomal fractions and liver homogenate lysates containing 100 μg of protein were prepared for SDS-PAGE by dilution with reducing sample buffer (Laemmli) followed by 2 min immersion in near-boiling water.
Quantification of glucagon receptor, PKC isoforms and GRK2 by immunoblotting
Assessment of the glucagon receptor, was conducted using standard SDS-PAGE and immunoblotting techniques. For the glucagon receptor, liver homogenates, plasma membranes, endosomes and lysosomes were electrophoresed and transferred to nitrocellulose membranes. Thereafter, they were incubated with a purified polyclonal rabbit antiserum primary antibody kindly provided by Dr Cecilia Unson from the Rockefeller University. This antibody (ST-18: against glucagon receptor residues 468–485) was assembled stepwise by the Merrifield solid-phase method (Carruthers et al. 1994) as previously described (Barany & Merrifield, 1979). Subsequently, identification using goat antirabbit IgG conjugated to horseradish peroxidase was performed (Jackson Immunoresearch). To quantify the hepatic glucagon receptor and to ensure equal lane loading, purified fusion proteins were constructed as previously described (Podolin et al. 2001) and the protein standard and sample band at 64 kDa was quantified. All gels were poured so that samples from each of the experimental groups were always electrophoresed on the same gels to ensure standardized Western blotting analysis. Glucagon receptors were visualized with chemiluminescent solutions A and B (Amersham, Alameda, CA, USA). Image capture analysis was performed on the fusion proteins to quantify GR in all membrane, endosomal, lysosomal and liver homogenate samples. The images were quantified in arbitrary density units (A.D.U.) given by the ACFA Image Pro Plus software.
Assessment of the plasma membrane PKC isoforms and GRK2 was conducted using the same standard SDS-PAGE and immunoblotting techniques described above. Plasma membranes were incubated with four rabbit polyclonal antibobies (PKC-α, -β and -ɛ and GRK2: Santa Cruz Biotechnology, CA, USA), and identification was made using goat antirabbit IgG conjugated to horseradish peroxidase (Jackson Immunoresearch). Sample bands at 80–81 kDa (PKC-α), 79–80 KDa (PKC-β), 89–96 kDa (PKC-ɛ) and 80 KDa (GRK2) The images were quantified in A.D.U. given by the image capture system previously described.
Analytical methods
Plasma glucose concentrations were determined by using a glucose analyser (Yellow Springs Intruments 2300, Yellow Springs, OH, USA). Insulin and glucagon concentrations were determined by commercially available radioimmunoassay kits (Radioassay System Laboratory, ICN Biomedicals, Costa Mesa, CA, USA; distributed by Immunocorp, Montréal, Québec, Canada). Plasma and liver triglyceride concentrations were determined by quantitative enzymatic method (Sigma Diagnostics, St. Louis, MO, USA). Free fatty acid (FFA) concentrations were determined by enzymatic colorimetric assay (Roche Diagnostics, Laval, Quebec, Canada). Liver glycogen concentrations were determined using the phenolsulphuric acid reaction (Lo et al. 1970).
Protocol B: isolation of hepatocytes
Hepatocyte isolation was performed according to the technique described by Berry et al. (1991), with minor modifications. Briefly, the portal vein and the superior vena cava were cannulated with blunted needles (18 gauge) for selective anterograde and retrograde perfusion. Both needles were positioned to rest 1 cm from the liver. The liver was perfused in open circuit in the anterograde direction at 37°C, pH 7.4, with saturated (95% O2–5% CO2) perfusion medium at a rate of 25 ml min−1 for 5 min using a peristaltic pump (Cole Parmer Instrument, Vernon Hills, IL, USA). Thereafter, collagenase (CLS-1, 262 u mg−1, Worthington Biochemical) was infused at a rate of 40 ml min−1 for 25 min to achieve liver digestion. After complete digestion, the liver was excised and transferred to a Petri dish where it was gently disrupted with forceps. The cell solution was filtered through a nylon mesh and centrifuged twice for 2 min at 600 r.p.m., and the supernatant was discarded. Finally, cells were resuspended in a Falcon tube with 8–10 ml of ice-cold perfusion medium supplemented with 1.3 mm CaCl2 and 1% BSA. The upper part of the tube was gassed for 2 min with 95% O2–5% CO2 and left on ice for 1 h before incubation. Hepatocytes were incubated at 10 mg (ml dry weight)−1 estimated from the weight of the wet-packed cells (dry weight = wet weight ÷ 3.8) in closed vials saturated with 95% O2–5% CO2 in a final volume of 2.8 ml (mm: 24 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.2 MgSO4(7H2O), and 0.05 NaHCO3, 1.3 CaCl2, pH 7.4, 37°C). The dry weight of each hepatocyte preparation was subsequently determined.
Cell viability
The exclusion of trypan blue by intact cells is a commonly used method to distinguish between intact and damaged cells (Berry et al. 1991). The test of exclusion of trypan blue was run on each individual sample to obtain a rapid estimation of the cell viability. Samples with less than 90% viability were excluded.
Immunofluorescence microscopy of isolated hepatocytes with ST-18 antibody
After hepatocyte isolation, cells were placed overnight on coverglasses pretreated with AES (3-aminopropyltriethoxy-silane, Sigma, A-3648) placed in a 15-well plate apparatus where all subsequent incubations were performed. Thereafter, the medium was aspirated and cells were washed twice with a phosphate-buffered solution (mm: 137 NaCl, 8.0 Na2HPO4, 2.7 KCl, 1.5 KH2PO4). One millilitre of 3% paraformaldehyde in buffer B (mm: 150 NaCl, 10 Na2HPO4, 2 MgCl2, pH 7.4) was poured into each well for 40 min, and cells were then washed twice in buffer B for 5 min. Cell permeabilization, was carried out by treatment with 1 ml of 0.2% Triton X-100 in buffer B for 15 min at 4°C. After washing the cells twice with buffer B for 5 min, non-specific binding sites were blocked with 1% bovine serum albumin in buffer B for 40 min at room temperature, followed by incubation at room temperature for 1 h with 0.5 μg of ST-18 antibody in 0.1% bovine serum albumin in buffer B. Three washes in 1 ml of buffer B for 10 min each were followed by incubation at room temperature with 0.5 μg of Alexa Fluor 633 goat antirabbit IgG (Molecular Probes) diluted in 0.1% bovine serum albumin in buffer B. All incubations and washes were performed subsequently in the dark. After washing three times in buffer B for 10 min, coverglasses were blotted on kimwipes and mounted on slides with Mowiol (Polyvinyl Alcohol 4–88, Fluka). All cells were viewed with a Nikon Microphot-SA epifluorescence microscope. Images were photographed at 1000× magnification with a motorized FX-35DX dark box connected to a Nikon Microflex UFX-DX photomicrographic attachment using Kodak Ektachrome ISO 400 35 mm film. The exposure time for each image was determined automatically by the photomicrographic attachment. For each group, 100 hepatocytes were selected and optical densiometry analysis was performed on each individual cell. Image capture analysis was performed on the fusion proteins to quantify GR protein content in each hepatocyte. The images were quantified in A.D.U. given by the ACFA Image Pro Plus software. Thereafter the A.D.U. of each cell was divided by its area for standardization.
Statistical analysis
All data are reported as means ± s.e.m. Statistical comparisons were performed by using a two-way ANOVA for non-repeated measures design. Newman–Keuls post hoc test was used in the event of a significant (P < 0.05) F ratio.
Results
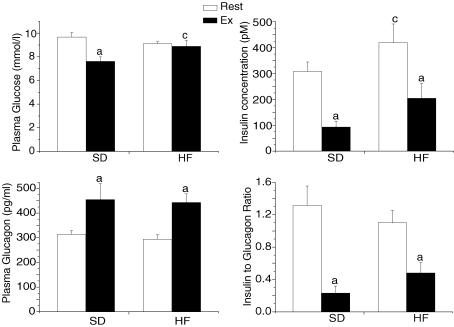
After two weeks on a high-fat diet, animals showed no differences in body and liver weight compared to the standard fed animals (Table 1). We next determined whether the feeding of a high-fat diet altered plasma glucose, insulin and glucagon concentrations (Fig. 1). For plasma glucose, we did not find any effect in the rested state; however, exercise resulted in a significantly (P < 0.05) lower plasma glucose concentrations in the standard fed group. As for plasma insulin concentrations, the HF feeding resulted in a significantly (P < 0.05) higher concentration compared to their standard fed counterparts. As expected, plasma insulin concentrations were significantly (P < 0.05) lower after an acute exercise bout in both standard and high-fat groups. Although no differences in plasma glucagon concentration were observed between the standard and high-fat-fed rats, exercise resulted in a 50% higher plasma glucagon concentration, in both dietary conditions, confirming that the exercise bout was of adequate intensity. Despite a significant (Fig. 1; P > 0.05) reduction in insulin to glucagon ratio following exercise in both dietary conditions, no significant differences were observed between the two dietary protocols in either the rested or exercised state.
Table 1.
Body weight (BW) measured at day 1 and 14 of the experimental period and relative liver weight measured at the end of the experiment
| Groups | BW upon arrival (g) | BW at day 14 (g) | Liver weight (g (kg BW)−1) |
|---|---|---|---|
| SD–Rest | 208.6 ± 2.3 | 335.9 ± 5.1 | 37.7 ± 0.9 |
| SD–Ex | 209.3 ± 1.3 | 320.3 ± 6.1 | 32.6 ± 1.3 |
| HF–Rest | 208.4 ± 5.6 | 326 ± 6.8 | 36.7 ± 1.6 |
| HF–Ex | 208.7 ± 3,0 | 315 ± 4.5 | 30.5 ± 3.4 |
Values are reported as means ± s.e.m. (n = 8–10 rats/group). SD–Rest: standard diet-rest; SD–Ex: standard diet–acute exercise; HF–Rest: high-fat diet–rest; HF–Ex: high-fat diet–acute exercise.
Figure 1. Plasma glucose, insulin and glucagon concentrations and insulin to glucagon ratio in resting (Rest) and exercised (Ex) rats after two weeks of a standard (SD) or a high-fat (HF) diet.
Values are means ± s.e.m. with n = 8–10 rats/group. aSignificantly different between Rest and Ex groups, P < 0.05. cSignificantly different from the SD-fed counterpart, P < 0.05.
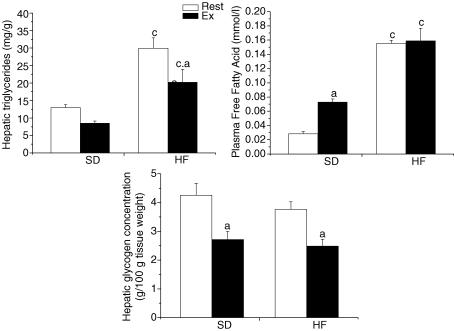
To assess whether the feeding of a high-fat diet resulted in liver and plasma lipid changes reflecting the development of non-alcoholic fatty liver disease (NAFLD), we measured hepatic triglycerides and plasma FFA content. As expected, hepatic triglyceride concentrations were significantly higher with the feeding of the high-fat diet (Fig. 2; P < 0.05). Liver triglyceride levels increased by ∼250% in the rested group following the high-fat diet. Exercise resulted in a significant decrease of hepatic triglyceride concentrations only in the HF group (P < 0.05). Predictably, the feeding of a high-fat diet resulted in a significant increase of plasma FFA concentrations in the rested and exercised groups (P < 0.05). Exercise resulted in a significant increase in FFA concentrations in the standard fed regimen (Fig. 2; P < 0.05). As for hepatic glycogen content, despite a tendency for lower glycogen concentration in the high-fat-fed animals, no differences were observed between the standard and high-fat-fed groups in both rested and exercise conditions (Fig. 2; P > 0.05). As for the exercise groups, an acute bout of exercise resulted in a lowering of hepatic glycogen concentration (Fig. 2; P > 0.05).
Figure 2. Hepatic triglyceride and glycogen concentrations and plasma free fatty acid levels in resting (Rest) and exercised (Ex) rats after two weeks of a standard (SD) or a high-fat (HF) diet.
Values are means ± s.e.m. with n = 8–10 rats/group. aSignificantly different between Rest and Ex groups, P < 0.05. cSignificantly different from the SD-fed counterpart, P < 0.05
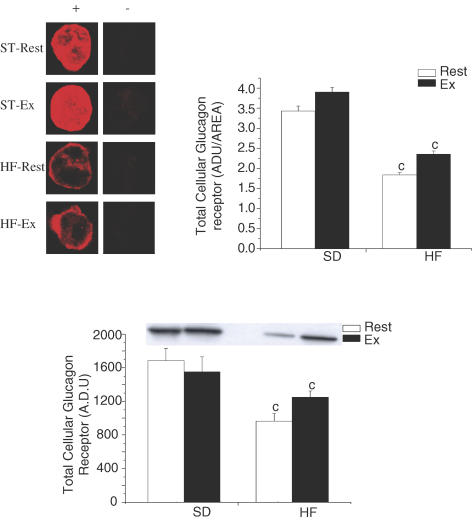
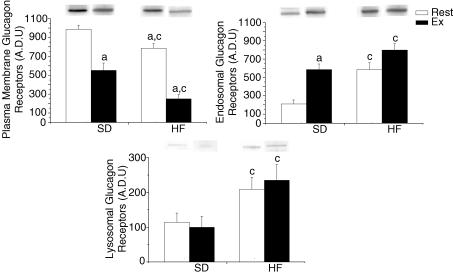
In agreement with our first hypothesis, total hepatic glucagon receptor, assessed by immonofluorescence microscopy and by Western blotting, was significantly reduced with the feeding of a high-fat diet (Fig. 3; P < 0.01). A ∼55% lower total GR was observed in all HF-fed groups (Fig. 3). To further characterize GR desensitization following the feeding of a high-fat diet, we assessed whether differences in plasma membrane, endosomal and lysosomal GR content could be observed. Confirming our previous results (11), the feeding of a high-fat diet resulted in a significant decrease in plasma membrane GR (Fig. 4; P < 0.05). In addition, exercise resulted in a significant decrease in plasma membrane GR in both standard and high-fat-fed groups (Fig. 4; P < 0.05). In accordance with our second hypothesis, GR content, in the endosomal and lysosomal fractions, was significantly increased by 300% and 100%, respectively, following the high-fat diet (Fig. 4; P < 0.05). Surprisingly, exercise had limited effects on GR content in endosomal and lysosomal fractions. The only noticeable effect of exercise was seen in the endosomal fraction of the standard fed groups, where it significantly increased GR content (Fig. 4; P < 0.05).
Figure 3. Total glucagon receptor content in hepatocytes assessed by immunofluorescence and Western blotting in resting (Rest) and exercised (Ex) rats after two weeks of a standard (SD) or a high-fat (HF) diet.
The upper left panel represents immunofluorescence microscopy of isolated hepatocytes with the glucagon receptor antibody ST-18 as the primary antibody. An Alexa Fluor 633 dye was used as the secondary antibody. For each primary antibody, permeabilized hepatocytes (+) are compared with non-permeabilized hepatocytes (−). Each image in the left column represent a single hepatocyte: images selected are an accurate representation of the statistical mean. For each group, 100 hepatocytes were used for densitometry calculation. Results of those calculations are given in the upper right panel of Fig. 4. The bottom panel is the result of the Western blot for total GR. Values are means ± s.e.m. with n = 8–10 rats/group. cSignificantly different from the SD-fed counterpart, P < 0.05
Figure 4. Plasma membrane, endosomal and lysosomal glucagon receptor in resting (Rest) and exercised (Ex) rats after two weeks of a standard (SD) or a high-fat (HF) diet.
Values are means ± s.e.m. with n = 8–10 rats/group. aSignificantly different between Rest and Ex groups, P < 0.05.
To determine if the feeding of a high-fat diet altered isolated plasma membrane, endosome and lysosome fraction purity, enzymatic procedures were carried on the three organelles. There was no significant difference between the marker enzyme profiles of liver membranes, endosomes and lysosomes isolated from standard and high-fat-fed animals in the rested or exercised state. (Table 2). This latter observation is in accordance with results observed in previous studies (Harikumar et al. 1989; Yamada & Lieber, 1984). Therefore, organelle purity is not an underlying factor in the changes observed in plasma membrane, endosome and lysosome GR content following a high-fat diet and/or exercise.
Table 2.
Protein recovery, enrichment of plasma membrane marker enzymes and plasma membrane, endosome and lysosome purity in organelles isolated from rats hepatocytes
| SD–Rest | SD–Ex | HF–Rest | HF–Ex | |
|---|---|---|---|---|
| Protein recovery (mg protein g−1) | 1.91 ± 0.20 | 1.84 ± 0.18 | 1.78 ± 0.15 | 1.80 ± 0.24 |
| Relative specific activity | ||||
| Membranes | ||||
| 5′-ND | 13.01 ± 0.65 | 12.68 ± 0.67 | 10.13 ± 0.75 | 9.98 ± 0.59 |
| GGTP | 5.02 ± 1.31 | 4.99 ± 1.5 | 5.60 ± 1.37 | 5.40 ± 1.42 |
| Na,K-ATPase | 16.66 ± 1.41 | 16.45 ± 1.55 | 15.96 ± 0.98 | 14.77 ± 0.99 |
| SDH | 0.18 ± 0.04 | 0.26 ± 0.06 | 0.24 ± 0.07 | 0.27 ± 0.08 |
| G6Pase | 0.83 ± 0.09 | 0.84 ± 0.08 | 0.64 ± 0.12 | 0.67 ± 0.11 |
| Lysosomes | ||||
| 5′-ND | 0.8 ± 0.09 | 1.0 ± 0.12 | 0.9 ± 0.15 | 1.1 ± 0.03 |
| Arylsulphatase | 60 ± 12 | 65 ± 21 | 63 ± 11 | 66 ± 21 |
| N-acetyl-β-glucosaminidase | 55 ± 9 | 59 ± 13 | 54 ± 9 | 53 ± 15 |
| Succinate-INT-reductase | 0.04 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.03 | 0.1 ± 0.05 |
| G6Pase | 0.07 ± 0.02 | 0.12 ± 0.02 | 0.09 ± 0.01 | 0.08 ± 0.02 |
| Endosomes | ||||
| 5′-ND | 1.2 ± 0.09 | 1.4 ± 0.07 | 1.1 ± 0.1 | 1.2 ± 0.08 |
| Arylsulphatase | 100 ± 15 | 113 ± 18 | 109 ± 10 | 102 ± 11 |
| N-acetyl-β-glucosaminidase | 98 ± 12 | 103 ± 15 | 96 ± 7 | 103 ± 14 |
| Succinate-INT-reductase | 0.4 ± 0.05 | 0.57 ± 0.1 | 0.41 ± 0.03 | 0.61 ± 0.11 |
| G6Pase | 0.3 ± 0.04 | 0.60 ± 0.07 | 0.52 ± 0.06 | 0.55 ± 0.12 |
Relative specific activity for each enzyme was calculated by dividing its specific activity in plasma membrane by its specific activity in homogenate. SD–Rest: standard diet-rest; SD–Ex: standard diet–acute exercise; HF–Rest: high-fat diet–rest; HF–Ex: high-fat diet–acute exercise; SDH: succinate deshydrogenase; INT: iodonitrotetrazolium violet.
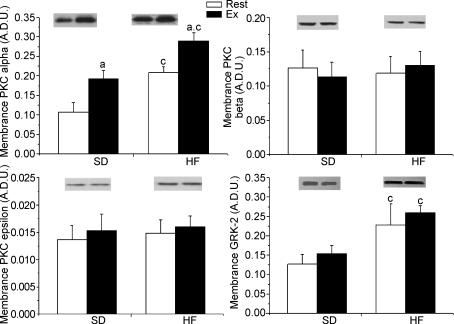
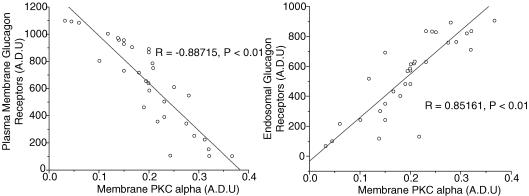
Protein kinase C seem to play a predominant role in glucagon receptor desensitization in hepatocytes (Savage et al. 1995) by phosphorylating G-related kinases (GRKs) that are responsible for initiating receptor internalization. Moreover, PKC isoforms α, β and ɛ as well as GRK2 are known to increase in liver plasma membrane during a high-fat diet and in type 2 diabetes. Therefore, to assess whether the GR desensitization observed in our high-fat diet model is linked to a PKC and GRK elevation in hepatic plasma membrane, PKC isoforms α, β and ɛ and GRK2 were measured. Two weeks of high-fat diet resulted in a higher (∼100%) PKC-α protein content in plasma membrane (Fig. 5; P < 0.05) while the protein content of isoforms β and ɛ was not affected by the dietary manipulation. A similar increase (∼83%) in GRK2 protein content in plasma membrane was observed following two weeks of high-fat feeding (Fig. 5; P < 0.05). Exercise resulted in a significant increase in plasma membrane PKC-α protein content in both standard (∼90%) and high-fat-fed (∼45%) groups (Fig. 5; P < 0.05). No effect of exercise was observed on the protein content of PKC isoforms β and ɛ and GRK2 in hepatic plasma membrane in either standard or high-fat-fed groups. Finally, to evaluate whether a relationship exists between hepatic plasma membrane PKC-α, GRK2 and GR desensitization, individual results of plasma membrane PKC and GRK2 were plotted against individual results of plasma membrane, endosomal and lysosomal GR (Fig. 6). Those comparisons revealed a negative relationship between plasma membrane GR and PKC-α (R = −0.88715, P < 0.01) and a positive relationship between endosomal GR and PKC-α (R = 0.85161, P < 0.01).
Figure 5. PKC isoforms and GRK2 protein content in hepatic plasma membrane in resting (Rest) and exercised (Ex) rats after two weeks of a standard (SD.) or a high-fat (HF) diet.
Values are means ± s.e.m. with n = 8–10 rats/group. aSignificantly different between Rest and Ex groups, P < 0.05.cSignificantly different from the SD-fed counterpart, P < 0.05.
Figure 6. Relationship between plasma membrane and endosomal GR with plasma membrane PKC-α (n = 32, P < 0.01) for all rats in all dietary and rest-exercise conditions.
Discussion
Although numerous studies have revealed that high-fat diets induce hepatic steatosis, hyperglycaemia and systemic insulin resistance, less is known about deleterious effects of such diets on hepatic glucagon sensitivity. Recent studies have shown that liver lipid infiltration can induce hepatic glucagon resistance, but nothing is known of the molecular mechanisms that regulate hepatic glucagon sensitivity. In the present study, we have demonstrated that liver lipid infiltration reduces hepatic GR content and increases hepatic plasma membrane PKC-α and GRK2. These finding can potentially explain the reduced hepatic glucagon sensitivity seen in our previous studies (Charbonneau et al. 2005a, 2005b).
The major finding of the present investigation is that a high-fat-diet-induced liver lipid infiltration is associated with a ∼55% reduction in total cellular hepatic GR. This reduction in total GR cellular components was accompanied by a 20% lower plasma membrane level of GR protein content along with a 3- and 2-fold increase in endosomal and lysosomal GR protein content, respectively. In a previous study (Charbonneau et al. 2005a), we reported that liver lipid infiltration was associated with a reduction in plasma membrane glucagon receptor number (Bmax) by 45%. The present data confirm and extend this observation by being the first to report a decrease in total hepatic GR in an in vivo model of a high-fat-diet-induced hepatic steatosis in rats. The decrease in total GR in a state of liver lipid infiltration strongly suggests that this nutritional situation is associated either with a destruction of the GR or a decrease in GR mRNA translation. The feeding of a high-fat diet also resulted in a significant increase in the presence of the GR in microvesicular bodies. GR protein content in the endosomal and lysosomal fractions was increased by 2- and 3-fold, respectively, in our in vivo model of hepatic steatosis. This indicates that liver lipid infiltration is also associated with an increased internalization of the GR.
Our group recently reported a reduction in hepatic plasma membrane GR number (Bmax), assessed by binding assay, in an in vivo model of hepatic steatosis (Charbonneau et al. 2005a). In the present study, we used a specific rat antibody for the intracellular C-terminal of the hepatic glucagon receptor (ST-18) instead of the high-affinity binding assay, because binding assays are only useful when the radioligand has a high affinity for the receptor. If the receptor has a low affinity for the radioligand used, binding is minimized. Therefore, assessment of total and organelle receptor content is diminished. Furthermore, binding assays are based on the laws of mass action that state that all receptors are (1) equally accessible to ligands (the model ignores any states of partial binding); (2) neither ligand nor receptor are altered by binding; and (3) binding is reversible. Physiopathologies such as hepatic steatosis, diabetes, and obesity cause alterations in receptor structural integrity as well as receptor kinetics, that can affect the law of mass action (Feldman, 1972). In those conditions, changes in receptor-binding capacities by binding assays might not reflect the changes in protein content of the same receptors: receptors could have structural damage or changes that would limit binding capacities. Using immunofluorescence and immunoblotting techniques we confirmed that a ∼55% reduction in total GR cellular components was accompanied by a 20% lower plasma membrane level of GR protein content in a model of liver lipid accumulation.
In a previous study, we have observed a similar decrease in plasma membrane GR with the same dietary protocol. At that time, we hypothesized that hepatic cytosolic triglycerides could activate second messenger protein kinases such as PKC and G protein-coupled receptor kinase (GRKs), and mediate hepatic GR desensitization mechanisms in hepatic steatosis. Homologous and heterologous desensitization of the GR mediated by protein kinase C was previously observed in isolated hepatocytes treated with glucagon, vasopressin and diacylglycerol (Newlands & Houslay, 1991; Savage et al. 1995). In the present experiment, the feeding of a high-fat diet resulted in a 100% increase in plasma membrane PKC-α (Fig. 5) that was strongly correlated with the reduction of plasma membrane GR and with the increased presence of GR in endosomal fractions (Fig. 6). Therefore, the present data extend the observation made by Savage et al. (1995) and Newlands & Houslay (1991) by being the first to suggest that PKC-α might play a role in GR desensitization in an in vivo high-fat model of hepatic steatosis in rats. In past studies (Murphy et al. 1987), it was postulated that an increase in plasma membrane PKC could phosphorylate the GR and increase the recruitment of G protein-related kinase (GRK) that could initiate the GR desensitization mechanism. The present data indicates that GRK2 is increased with the feeding of a high-fat diet, but no significant relationship with the reduction of plasma membrane GR was observed. Nonetheless, it is possible that GRK2, a known GPCR phosphorylating protein, plays a role in the GR desensitization mechanism in an in vivo high-fat model of hepatic steatosis in rats.
In vivo and in vitro studies indicate that GR mRNA expression and the number of glucagon receptors expressed in the liver can be downregulated by glucagon itself (Srikant et al. 1977; Bhathena et al. 1978; Soman & Felig, 1978; Santos & Blasquez, 1982; Dighe et al. 1984; Premont & Iyengar, 1988). However, one has to remember that an increase in the expression of glucagon receptor mRNA does not necessarily signify the formation of glucagon receptors. Accordingly, it has been suggested that glucagon receptor expression is modulated at a step after mRNA formation (Watanabe et al. 1998). The mechanism involved in this glucagon-induced downregulation of its own receptors, however, has not been established. A reduction in liver glucagon receptor number in diabetes could explain in part that, under stimulation with glucagon, liver glucose production is significantly lower in diabetic patients than in healthy subjects (Orskov et al. 1991). In the present study, plasma glucagon levels were not increased by the present dietary manipulation, and therefore, cannot explain the ∼55% decrease in total glucagon receptor numbers observed in the high-fat-fed rats. GR mRNA expression has been reported to be upregulated in the presence of increasing concentrations of glucose and insulin (Abrahamsen et al. 1995; Burcelin et al. 1998; Portois et al. 1999; Svoboda et al. 1999). Similarly to plasma glucagon concentrations, plasma insulin and glucose concentrations in the present study can hardly be associated with any changes in glucagon receptor number with the high-fat feeding.
A 60 min acute bout of aerobic exercise resulted in a significant decrease in plasma membrane GR in both standard and high-fat diets, as well as increased GR content in endosomal fractions. In the present study, rats were submitted to an acute period of exercise to stimulate the hepatic GR under physiological conditions, and thereby to test the possibility that an acute glucagon secretion may alter the cellular distribution of the GR. In previous studies, chronic exercise training was shown to increase hepatic glucagon receptor density (Légaré et al. 2001), but the effect of an acute bout of exercise on GR distribution was never assessed, especially in a state of hepatic steatosis. The present results suggest that an acute bout of 60 min exercise reduces plasma membrane GR and increases GR distribution in microvesicular organelles without affecting total cellular GR. We therefore hypothesize that a 60 min bout of acute exercise results in a homologous desensitization and a redistribution of the GR without a subsequent destruction of the GR. PKC-α is significantly increased with acute exercise in both standard and high-fat diets (Fig. 4). Savage et al. (1995) previously demonstrated that PKCs could play an important role in GR homologous desensitization in vitro. Our results suggest that PKC-α might be involved in GR homologous desensitization during a 60 min acute bout of exercise. Acute exercise could potentially result in GR distribution in microvesicular bodies to protect the energy supplies of the liver from being depleted by a prolonged action of glucagon.
The present HF diet regimen in rats resulted in a 250% higher fat accumulation in liver compared to the SD-fed animals (Fig. 2). Our results corroborate those observed in previous studies (Straczkowski et al. 2001; Charbonneau et al. 2005a; Gauthier et al. 2004, 2006). The duration of the high-fat feeding in the present study was based on the observation that two weeks of high-fat feeding is adequate to cause a substantial accretion of fat inside the liver that is compatible with the development of a state of hepatic steatosis (Gauthier et al. 2003, 2006). In addition to liver lipid infiltration, the present HF diet resulted in a three-fold increase in plasma FFA concentration. In previous studies, in which hepatic steatosis was induced by high-fat diets, a substantial increase in plasma FFA was constantly observed (Straczkowski et al. 2001; Gauthier et al. 2003, 2004, 2006). It has been suggested that the increased delivery of fatty acids to the liver could result in a higher uptake by the liver, since FFA uptake mostly occurs in a concentration-dependent manner.
Having accepted that hepatic glucagon receptor desensitization and hepatic glucagon resistance are consequences of NAFLD, one must raise the question of why this occurs. We believe that the loss of liver sensitivity to glucagon is a protective phenomenon in response to the loss of the inhibitory effect of insulin on various enzymes of gluconeogenesis and glycogenolysis in ‘early-stage’ liver lipid infiltration. Therefore, the reduced glucagon sensitivity, in the first weeks of NAFLD, would prevent a more severe hyperglycaemia brought on by a decrease in liver insulin sensitivity. However, we believe that the reduced glucagon sensitivity does not persist in ‘late-stage’ liver lipid infiltration seen in obesity, type 2 diabetes and cirrhosis. The use of glucagon and glucagon receptor antagonists in the treatment of type 2 diabetes (Mayo et al. 2003) leads us to think that the reduced hepatic glucagon sensitivity is a beneficial phenomenon only in the first instance of the development of hepatic insulin resistance and type 2 diabetes induced by a high-fat diet. Consequently, the physiological fallout of our present and previous findings is of importance: we are convinced that the hepatic glucagon receptor desensitization, and therefore the loss of hepatic glucagon sensitivity, is a forthcoming sign of more important deleterious effects on glucose metabolism resulting from the ingestion of a high-fat diet. Thus, the early detection of a reduced hepatic glucagon sensitivity would prompt the application of a convenient treatment to prevent further glucose metabolism deterioration resulting from liver lipid infiltration.
In summary, results of the present study indicate that a HF-diet-induced liver lipid infiltration results in a reduction in total cellular and plasma membrane GR, as well as an increase in endosomal and lysosomal GR. An increase in PKC-α and GRK2 in our in vivo model of HF-diet-induced hepatic steatosis strongly suggests that this second messenger protein kinase is implicated in the GR desensitization observed in the present study and the previous one (Charbonneau et al. 2005a). Although GRK2 was increased following the HF diet, we can only speculate that this second messenger might have a synergic role to play with PKC-α in GR desensitization. In addition, a 60 min acute bout of aerobic exercise reduced significantly plasma membrane GR in both standard and high-fat diets as well as increasing GR content in endosomal fractions, without affecting total cellular GR. This observation is strongly correlated with an increase in PKC-α following an acute exercise in both standard and HF diets. It would be of interest, in future studies, to inhibit PKC along with the HF diet to assess in detail the role of PKC in GR desensitization in an in vivo model of hepatic steatosis and during an acute bout of exercise.
Acknowledgments
We thank Dr André Marette and Dr Kerstin Bellhman from the centre for lipid research of the University Laval for proofreading the manuscript and for technical assistance. This work was supported by grants from Natural Sciences and Engineering Research Council of Canada (NSERC)
References
- Abrahamsen N, Lundgren K, Nishimura E. Regulation of glucagon receptor mRNA in cultured primary rat hepatocytes by glucose and cAMP. J Biol Chem. 1995;270:15853–15857. doi: 10.1074/jbc.270.26.15853. [DOI] [PubMed] [Google Scholar]
- Arkesteijn CKM. A kinetic method for serum 5′-nucleotidase using stabilized glutamate dehydrogenase. J Clin Chem Clin Biochem. 1976;14:155–158. doi: 10.1515/cclm.1976.14.1-12.155. [DOI] [PubMed] [Google Scholar]
- Barany G, Merrifield RB. A chromatographic method for the quantitative analysis of the deprotection of dithiasuccinoyl (Dts) amino acids. Anal Biochem. 1979;95:160–170. doi: 10.1016/0003-2697(79)90199-4. [DOI] [PubMed] [Google Scholar]
- Barrett AJ, Heath MF. In: Lysosomes – A Laboratory Handbook. Dingle JT, editor. Amsterdam: North Holland Publishing Co; 1977. pp. 110–126. [Google Scholar]
- Baudhuin P, Beaufay H, Li YR, Sellinger OZ, Wattiaux R, Jacques P, de Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, d-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964;92:179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MN, Edwards AM, Barritt GJ. Isolated hepatocytes, preparation, properties and applications. In: Burdon RH, van Kuippenberg PH, editors. Laboratory Techniques in Biochemistry and Molecular Biology. Amsterdam: Elsevier Science Publishers; 1991. [Google Scholar]
- Bhathena SJ, Voyles NR, Smith S, Recant L. Decreased glucagon receptors in diabetic rat hepatocytes. Evidence for regulation of glucagon receptors by hyperglucagonemia. J Clin Invest. 1978;61:1488–1497. doi: 10.1172/JCI109069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcelin R, Mrejen C, Decaux JF, De Mouzon SH, Girard J, Charron MJ. In vivo and in vitro regulation of hepatic glucagon receptor mRNA concentration by glucose metabolism. J Biol Chem. 1998;273:8088–8093. doi: 10.1074/jbc.273.14.8088. [DOI] [PubMed] [Google Scholar]
- Carruthers CJL, Unson CG, Kim HN, Sakmar TP. Synthesis and expression of a gene for the rat glucagon receptor. Replacement of an aspartic acid in the extracellular domain prevents glucagon binding. J Biol Chem. 1994;18:29321–29328. [PubMed] [Google Scholar]
- Charbonneau A, Couturier K, Gauthier MS, Lavoie JM. Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training. Int J Sports Med. 2005b;26:432–441. doi: 10.1055/s-2004-821225. [DOI] [PubMed] [Google Scholar]
- Charbonneau A, Melancon A, Lavoie C, Lavoie JM. Alterations in hepatic glucagon receptor density and in Gsalpha and Gialpha2 protein content with diet-induced hepatic steatosis: effects of acute exercise. Am J Physiol Endocrinol Metab. 2005a;289:E8–E14. doi: 10.1152/ajpendo.00570.2004. [DOI] [PubMed] [Google Scholar]
- Dighe RR, Rojas FJ, Birnbaumer L, Garber AJ. Glucagon-stimulable adenylyl cyclase in rat liver. The impact of streptozotocin-induced diabetes mellitus. J Clin Invest. 1984;73:1013–1023. doi: 10.1172/JCI111286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin R, Lavoie C, Bourque J, Ducros F, Poisson D, Chiasson JL. Increased hepatic glucose production response to glucagon in trained subjects. Am J Physiol Endocrinol Metab. 1998;274:E23–E28. doi: 10.1152/ajpendo.1998.274.1.E23. [DOI] [PubMed] [Google Scholar]
- Drouin R, Robert G, Milot M, Masicotte D, Péronnet F, Lavoie C. Swim training increases glucose output from liver perfused in situ with glucagon in fed and fasted rats. Metabolism. 2004;53:1027–1031. doi: 10.1016/j.metabol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Feldman HA. Mathematical theory of complex ligand-binding systems at equilibrium. Anal Biochem. 1972;48:317–338. doi: 10.1016/0003-2697(72)90084-x. [DOI] [PubMed] [Google Scholar]
- Gauthier MS, Couturier K, Charbonneau A, Lavoie JM. Effects of introducing physical training in the course of a 16-week high-fat diet regimen on hepatic steatosis, adipose tissue fat accumulation, and plasma lipid profile. Int J Obes Relat Metab Disord. 2004;28:1064–1071. doi: 10.1038/sj.ijo.0802628. [DOI] [PubMed] [Google Scholar]
- Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol. 2003;94:2127–2134. doi: 10.1152/japplphysiol.01164.2002. [DOI] [PubMed] [Google Scholar]
- Gauthier MS, Favier R, Lavoie JM. Time course of the development of non-alcoholic hepatic steatosis in response to high-fat diet-induced obesity in rat. Br J Nutrition. 2006;95:273–281. doi: 10.1079/bjn20051635. [DOI] [PubMed] [Google Scholar]
- Harikumar P, Darshini T, Aditidutt T, Ninjoor V. A rapid procedure for the isolation of lysosomes from kidney cortex by Percoll density gradient centrifugation. J Biosci. 1989;14:269–277. [Google Scholar]
- Harper AE. Glucose-6-phosphatase. In: Bergmeyer H-U, editor. Methods of Enzymatic Analysis. New York and London: Verlag-Chemie Academic Press; 1963. pp. 788–792. [Google Scholar]
- Ismail-Beigi F, Edelman IS. The mechanism of the calorigenic action of thyroid hormone. Stimulation of the Na+K+-activated adenosine triphosphatase activity. J Gen Physiol. 1971;57:710–722. doi: 10.1085/jgp.57.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E671–E678. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- Keller U, Sonnenberg GE, Burckhardt D, Perruchoud A. Evidence for an augmented glucagon dependence of hepatic glucose production in cirrhosis of the liver. J Clin Endocrinol Metab. 1982;54:961–968. doi: 10.1210/jcem-54-5-961. [DOI] [PubMed] [Google Scholar]
- King TE. Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods Enzymol. 1967;10:322–331. [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998a;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Pitcher J, Krueger K, Daaka Y. Mechanisms of beta-adrenergic receptor desensitization and resensitization. Adv Pharmacol. 1998b;42:416–420. doi: 10.1016/s1054-3589(08)60777-2. [DOI] [PubMed] [Google Scholar]
- Legare A, Drouin R, Milot M, Massicotte D, Peronnet F, Massicotte G, Lavoie C. Increased density of glucagon receptors in liver from endurance-trained rats. Am J Physiol Endocrinol Metab. 2001;280:E193–E196. doi: 10.1152/ajpendo.2001.280.1.E193. [DOI] [PubMed] [Google Scholar]
- Lo S, Russell JC, Taylor AW. Determination of glycogen in small tissue samples. J Appl Physiol. 1970;28:234–236. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough AC, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV Glucagon receptor family. Pharmacol Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- Morre DJ. In: Methods in Enzymology. Colowick SP, Kaplan NO, Jakoby WB, editors. New York: Academic Press; 1971. pp. 130–148. [Google Scholar]
- Murphy GJ, Gawler DJ, Milligan G, Wakelam MJO, Pyne NJ, Houslay MD. Glucagon desensitization of adenylate cyclase and stimulation of inositol phospholipid metabolism does not involve the inhibitory guanine nucleotide regulatory protein Gi, which is inactivated upon challenge of hepatocytes with glucagon. Biochem J. 1989;259:191–197. doi: 10.1042/bj2590191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, Hruby VJ, Trivedi D, Wakelam MJ, Houslay MD. The rapid desensitization of glucagon-stimulated adenylate cyclase is a cyclic AMP-independent process that can be mimicked by hormones which stimulate inositol phospholipids metabolism. Biochem J. 1987;243:39–46. doi: 10.1042/bj2430039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlands C, Houslay MD. Treatment of intact hepatocytes with synthetic diacyl glycerols mimics the ability of glucagon to cause the desensitization of adenylate cyclase. FEBS Lett. 1991;289:129–132. doi: 10.1016/0014-5793(91)81051-9. [DOI] [PubMed] [Google Scholar]
- Orskov L, Alberti KG, Mengel A, Moller N, Pedersen O, Rasmussen O, Seefeldt T, Schmitz O. Decreased hepatic glucagon responses in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1991;34:521–526. doi: 10.1007/BF00403290. [DOI] [PubMed] [Google Scholar]
- Podolin DA, Wills BK, Wood IO, Lopez M, Mazzeo RS, Roth DA. Attenuation of age-related declines in glucagon-mediated signal transduction in rat liver by exercise training. Am J Physiol Endocrinol Metab. 2001;281:E516–E523. doi: 10.1152/ajpendo.2001.281.3.E516. [DOI] [PubMed] [Google Scholar]
- Pohl SL. The glucagon receptor and its relationship to adenylate cyclase. Fed Proc. 1977;36:2115–2118. [PubMed] [Google Scholar]
- Portois L, Maget B, Tastenoy M, Perret J, Svoboda M. Identification of a glucose response element in the promoter of the rat glucagon receptor gene. J Biol Chem. 1999;274:8181–8190. doi: 10.1074/jbc.274.12.8181. [DOI] [PubMed] [Google Scholar]
- Premont R, Iyengar R. Glucagon-induced desensitization of adenylyl cyclase in primary cultures of chick hepatocytes: Evidence for multiple pathways. J Biol Chem. 1988;263:16087–16095. [PubMed] [Google Scholar]
- Rao RH. Adaptations in glucose homeostasis during chronic nutritional deprivation in rats: hepatic resistance to both insulin and glucagon. Metabolism. 1995;44:817–824. doi: 10.1016/0026-0495(95)90199-x. [DOI] [PubMed] [Google Scholar]
- Santos A, Blasquez E. Regulatory effect of glucagon on its own receptor concentrations and target-cell sensitivity in the rat. Diabetologia. 1982;22:362–371. doi: 10.1007/BF00253583. [DOI] [PubMed] [Google Scholar]
- Savage A, Zeng L, Houslay MD. A role for protein kinase C-mediated phosphorylation in eliciting glucagon desensitization in rat hepatocytes. Biochem J. 1995;1:281–285. doi: 10.1042/bj3070281. 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt BF, Keefe EB, Blankenship NM, Ockner RK. Validation of a recording spectrophotometric method for measurement of membrane-associated Mg- and NaKATPase activity. J Lab Clin Med. 1979;93:790–798. [PubMed] [Google Scholar]
- Soman V, Felig P. Regulation of the glucagon receptor by physiological hyperglucagonaemia. Nature. 1978;27(5656):829–832. doi: 10.1038/272829a0. 272. [DOI] [PubMed] [Google Scholar]
- Srikant CB, Freeman D, McCorkle K, Unger RH. Binding and biologic activity of glucagon in liver cell membranes of chronically hyperglucagonemic rats. J Biol Chem. 1977;10:7434–7438. [PubMed] [Google Scholar]
- Straczkowski M, Kowalska I, Dzienis-Straczkowska S, Kinalski M, Gorski J, Kinalska I. The effect of exercise training on glucose tolerance and skeletal muscle triacylglycerol content in rats fed with a high-fat diet. Diabetes Metab. 2001;27:19–23. [PubMed] [Google Scholar]
- Svoboda M, Portois L, Malaisse WJ. Glucose regulation of the expression of the glucagon receptor gene. Mol Genet Metab. 1999;68:258–267. doi: 10.1006/mgme.1999.2913. [DOI] [PubMed] [Google Scholar]
- Szasz GA. Kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin Chem. 1969;15:124–136. [PubMed] [Google Scholar]
- Van Ermen A, Fraeyman N. Desensitization of α1-, β- and glucagon receptors in rat hepatocytes: influence of ageing. Mech Ageing Dev. 1994;75:45–58. doi: 10.1016/0047-6374(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hayasaki H, Tamayama T, Shimada M. Histologic distribution of insulin and glucagon receptors. Braz J Med Biol Res. 1998;31:243–256. doi: 10.1590/s0100-879x1998000200008. [DOI] [PubMed] [Google Scholar]
- Yamada S, Lieber CS. Decrease in microviscosity and cholesterol content of rat liver plasma membranes after chronic ethanol feeding. J Clin Invest. 1984;74:2285–2289. doi: 10.1172/JCI111656. [DOI] [PMC free article] [PubMed] [Google Scholar]